Abstract
Background
Upper gastrointestinal (GI) ulceration and bleeding in critically ill dogs can cause severe anemia and increase morbidity. Acid suppressants using proton pump inhibitors or histamine‐2 receptor blockers administered IV is commonly recommended.
Hypothesis/Objectives
To evaluate the efficacy of IV administered esomeprazole, pantoprazole, and famotidine constant rate infusion (CRI) on increasing the intragastric pH of dogs. We hypothesized that esomeprazole and famotidine CRI would provide superior acid suppression compared to pantoprazole and reach pH goals for the treatment of GI bleeding.
Animals
Nine healthy research Beagles.
Methods
Randomized, 3‐way crossover. Dogs received pantoprazole or esomeprazole at 1 mg/kg IV q12h and famotidine with a loading dose of 1 mg/kg followed by 8 mg/kg IV CRI daily for 3 consecutive days. The intragastric pH was recorded at baseline and for 72 hours of treatment. The mean pH and the mean percentage time (MPT) the intragastric pH was ≥3 or ≥4 were compared among and within treatment groups.
Results
Significant increases in mean pH (P < 0.0001), MPT ≥3 (P < 0.001), and MPT ≥4 (P = 0.0006) were noted over time with all 3 treatments. The time effect did not differ by treatment for mean pH, MPT ≥3, and MPT ≥4 (P = .29, .56, and .37, respectively); however, only esomeprazole and famotidine CRI achieved the goals established for the treatment of gastroduodenal ulceration in people.
Conclusions and Clinical Importance
Famotidine CRI and esomeprazole might be superior acid suppressants compared to standard doses of pantoprazole for the first 72 hours of treatment.
Keywords: bravo monitoring, canine, histamine‐2 receptor antagonist, proton pump inhibitor
Abbreviations
- CRI
constant rate infusion
- GI
gastrointestinal
- MPT
mean percentage time
- PPI
proton pump inhibitor
1. INTRODUCTION
Gastrointestinal (GI) bleeding in critically ill dogs can result in severe anemia and an increase in treatment cost and morbidity.1 An important adjunctive treatment in the treatment of upper GI bleeding is to promote a favorable environment for clot formation and stabilization, which requires inhibition of gastric acid secretion. Increasing the gastric pH ≥3 for 75% of the day and pH ≥4 for 67% of the day are the goals for the adjunctive treatment of duodenal ulceration and esophagitis, respectively, in people,2, 3 whereas gastric acid suppression >pH 6 is thought to optimize clot formation in the presence of severe upper GI hemorrhage.4, 5 According to a sequential endoscopic study in humans with bleeding peptic ulcers, the risk for ulcer rebleeding is highest during the first 72 hours of admission, with most rebleeding occurring within the first 24 hours.6 Thus, administration of drugs that are quick in onset and provide potent gastric acid suppression are desired for a minimum of 3 days to promote mucosal healing and hemostasis.
Gastric acid suppression using IV administered gastric acid suppressants like proton pump inhibitors (PPIs, e.g., pantoprazole, esomeprazole) or histamine‐2 receptor antagonists (e.g., famotidine) is commonly recommended in critically ill dogs with documented upper GI bleeding. Standard doses (1 mg/kg IV q12h) of pantoprazole are more effective at increasing intragastric pH compared to standard doses of famotidine in humans7 and likely in dogs but might not provide superior gastric acid suppression compared to other acid suppressants.8 Identification of a more effective IV administered acid suppressant is of considerable interest for the treatment of upper GI ulceration and bleeding in critically ill dogs.
Esomeprazole might provide superior acid suppression compared to other PPIs.9 Esomeprazole dramatically increases gastric pH in dogs,10 but a comparative analysis with other gastric acid suppressants is not available. A constant rate infusion (CRI) of famotidine at a higher total daily dose also provides potent acid suppression in healthy dogs.11 A comparative study of these IV administered acid suppressants is warranted in dogs.
Our study objective was to compare standard intermittent bolus injections of esomeprazole and pantoprazole to a CRI of famotidine to determine which of these methods provides superior gastric acid suppression. We hypothesized that esomeprazole and famotidine CRI would significantly increase gastric pH compared to pantoprazole and meet the pH goals for treatment of esophagitis and duodenal ulceration as defined in people.
2. MATERIALS AND METHODS
2.1. Study animals
The study protocol was approved by the Institutional Animal Care and Use Committee. Nine adult healthy purpose‐bred Beagle dogs from a research colony at the University of Tennessee were enrolled in the study (4 intact females, 5 castrated males), all approximately 2 years of age and weighing 7.6‐11.4 kg (median 9.5 kg). The number of dogs was selected based on a sample size calculation using a study comparing the effects of intermittent oral dosing of PPIs in humans on intragastric pH.12 By assuming a conservative within‐subject correlation (0.25) and power equal to 0.8, 7 dogs were needed to detect a clinically important 20% change in mean percentage time (MPT) intragastric pH >4. An additional 2 dogs were enrolled to account for the potential for study dropout. All dogs were deemed healthy based on a lack of a history of GI disease (eg, vomiting, diarrhea, anorexia), a normal physical examination, recent laboratory diagnostics (CBC, serum biochemistry, urinalysis, and a centrifugal fecal examination) obtained on day 0 of the study. Dogs were maintained in a closed colony and receive monthly preventatives containing an anthelmintic. Dogs were excluded from the study if they developed inappetence, characterized by consuming <50% of their meal on more than 3 consecutive occasions, weight loss >10% of their body mass, >3 episodes of vomiting in a 24‐hour period, or diarrhea characterized by a Purina fecal score >5 for more than a 48‐hour period. Dogs were fed their normal commercial dry food diet (Purina ONE Smartblend Lamb & Rice Formula, Nestlé Purina PetCare Company, St. Louis, Missouri) twicedaily and given water ad libitum throughout the study.
2.2. pH monitor placement
On the morning of day 0 of each treatment period, the morning meal was withheld and the dogs were sedated using a combination of butorphanol (0.3‐0.4 mg/kg IV) (Torbugesic 10 mg/mL injection; Fort Dodge Animal Health, FortDodge, Iowa) and dexmedetomidine (4‐6 μg/kg IV) (Dexdomitor 0.5 mg/mL injection; Orion Pharma, Espoo, Finland) for digital radiology‐assisted placement of the Bravo pH capsule. Following sedation, a cephalic IV catheter was placed and intragastric pH capsules were adhered to the dog's gastric mucosa as previously described.11, 13 The sedation was then reversed with atipamezole (0.04‐0.06 mg/kg IM) after pH capsule placement. (Antisedan 5 mg/mL injection; Orion Pharma, Espoo, Finland).
2.3. Study design
A randomized, 3‐way open‐label crossover study design was performed. A random number generator was used to determine the treatment order for each dog. Dogs initially received 0.9% saline IV every 6 hours for the first 24 hours to obtain baseline intragastric data and thereafter until study completion to ensure catheter patency. All dogs then received either twicedaily IV injections of pantoprazole (Pantoprazole sodium 40 mg/vial; Wyeth Pharmaceuticals, LLC, Philadelphia, Pennsylvania) or esomeprazole (Esomeprazole magnesium 40 mg/vial; AstraZeneca LP, Wilmington, Delaware) at 1.0 mg/kg q12h, prepared according to the manufacturer's instructions and diluted with 0.9% saline to a concentration of 10 mg/mL for esomeprazole and 4 mg/mL for pantoprazole, or famotidine (Famotidine 200 mg/20 mL injection, Westward Pharmaceuticals, LLC, Eatontown, New Jersey) dosed at 1.0 mg/kg IV as a 1‐time, loading bolus dose, followed by continuous infusion of 8.0 mg/kg/day and diluted with 0.9% saline to a concentration of 1.0 mg/mL. Each drug was administered for 3 consecutive days. A 10‐day washout period separated the treatment groups. All treatments were administered 30 minutes prior to a meal. Clinical signs, including general attitude, number of vomiting episodes, number of daily defecations, and fecal score were recorded every 6 hours. Fecal scores were graded using a standardized fecal scoring system (Fecal Scoring System, Nestlé Purina PetCare Company).
2.4. Intragastric pH monitoring
Intragastric pH were recorded continuously for 96 hours after capsule placement starting on day 0 and continuing through 3 treatment days or until the capsule detached if detachment occurred before day 3. Telemetric data were transferred to a corresponding receiver that was placed within 3 ft of the dog and remained with the dog throughout the treatment period. pH data were uploaded to a commercial computer software system (Polygram Net Software, Given Imaging, Yoqneam, Israel) every 24 hours. The receiver was then reset after the data were uploaded, and the same receiver was used to capture the next 24 hours of data. Right lateral radiographs were taken to confirm the pH capsule was still within the stomach if early gastric detachment and passage was suspected based on a rapid and sustained increase in the intragastric pH > 4.
2.5. Statistical analysis
A 3 treatment, 3 sequence, crossover design with repeated measures was performed to evaluate mean intragastric pH, MPT that intragastric pH was ≥3, and MPT that intragastric pH was ≥4.14, 15 Each response measure was analyzed with repeated‐measures mixed model analysis of variance (ANOVA) to evaluate for treatment, time, the treatment by time interaction, and carryover effects. Unstructured Kronecker product variance/covariance structures were incorporated into each model. In each analysis, post hoc was developed to test for within‐day and between‐treatment differences. A Shapiro‐Wilk test for normality and QQ plots were used to evaluate normality of ANOVA residuals. Levene's equality of variances test was used to evaluate equality of treatment variances. All statistical assumptions regarding normality and equality of variances were met and no transformations were required. Statistical significance was defined as P < .05. Statistical analysis was performed using commercial software (SAS software, version 9.4, Cary, North Carolina; Release TS1M5). Figures were created using commercially available software (Prism8, GraphPad Software, San Diego, California).
3. RESULTS
3.1. pH capsule placement
A total of 32 capsules were deployed during the study. Fifteen of 32 capsules remained in place for the entirety of the 96‐hour study period. Five capsules failed and required re‐deployment on day 0 of the study (n = 1 for esomeprazole, n = 2 famotidine, n = 1 pantoprazole). Capsule failure was attributed to inadequate vacuum suction (n = 4) or failure of the receiver to appropriately sync once the capsule was placed (n = 1). On 3 occasions, the pH capsule detached on day 2 (n = 1 esomeprazole, n = 2 pantoprazole). On 12 occasions, the pH capsule detached and exited the stomach on day 3 before the end of the monitoring period (n = 3 esomeprazole, n = 6 famotidine, n = 3 pantoprazole). Data from these dogs were not included in the treatment comparisons on days in which the data were not available. All remaining pH capsules placed the previous treatment week had passed through the feces before placement of new capsules.
3.2. Intragastric pH recording
The mean intragastric pH and the MPT intragastric pH were ≥3 and ≥4 for each treatment group and are graphically depicted in Figures 1, 2, 3, respectively. Tests for carryover effects were incorporated into each model, and no significant carryover effects were identified. Significant increases were observed over time for mean pH, MPT intragastric pH ≥3, and pH ≥4 (P < .0001, P = .001, and P < .0006), respectively, across all treatment groups. Post hoc tests revealed mean pH, MPT intragastric pH ≥3, and pH ≥4 significantly differed with higher values on days 1, 2, and 3 compared to baseline (P ≤ .001, for all comparisons). No significant differences were observed between days 2 and 3 for mean pH, MPT intragastric pH ≥3, and pH ≥4 (P = .59, P = .90, P = .60, respectively). No significant differences were observed for mean pH, MPT intragastric pH ≥3, and pH ≥4 for treatment (P = .77, P = .56, P = .96, respectively) or the treatment‐by‐time interaction (P = .29, P = .56, P = .37). The MPT intragastric pH ≥5 and ≥6 were also evaluated (Figures 4 and 5) but not statistically compared because of the potential for a type 1 error with a small sample size and multiple statistical comparisons.
Figure 1.
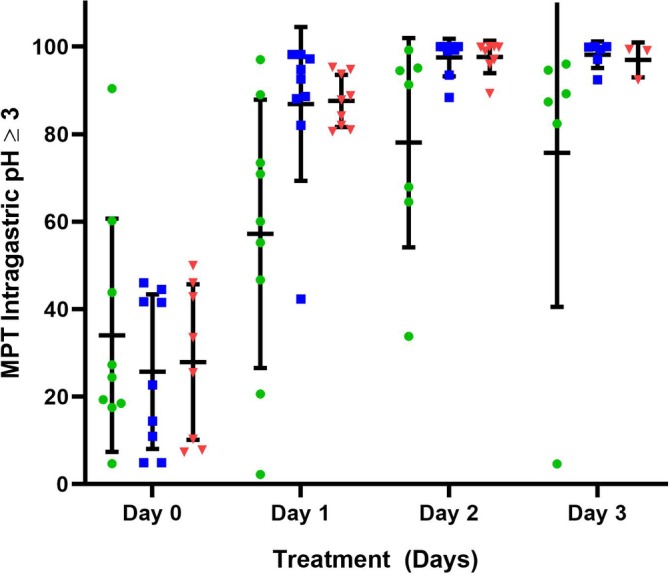
The mean percentage time (MPT) intragastric pH ≥3 for all dogs receiving 1 mg/kg q12h esomeprazole, pantoprazole, and 8 mg/kg/day famotidine constant rate infusion (CRI) on treatment days 1‐3. Horizontal and vertical lines represent the mean and standard deviations, respectively. Individual dog data are represented by green circles (pantoprazole), blue squares (esomeprazole), and red triangles (famotidine CRI). Significant increases in MPT intragastric pH ≥3 were noted over time (P = .001) with all 3 treatments. No significant treatment (P = .56) or treatment‐by‐time interaction (P = .56) were observed
Figure 2.
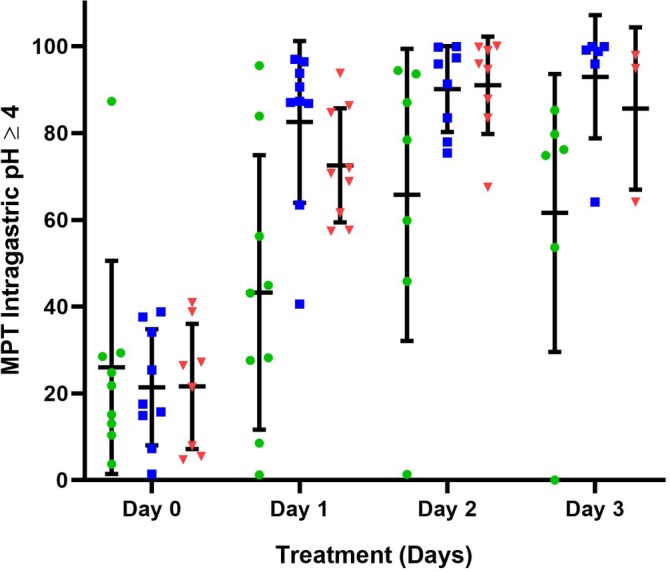
The mean percentage time (MPT) intragastric pH ≥4 for all dogs receiving 1 mg/kg q12h esomeprazole, pantoprazole, and 8 mg/kg/day famotidine constant rate infusion (CRI) on treatment days 1‐3. Horizontal and vertical lines represent the mean and standard deviations, respectively. Individual dog data are represented by green circles (pantoprazole), blue squares (esomeprazole), and red triangles (famotidine CRI). Significant increases in MPT intragastric pH ≥4 were noted over time (P = .001) with all 3 treatments. No significant treatment (P = .96) or treatment‐by‐time interaction (P = .37) were observed
Figure 3.
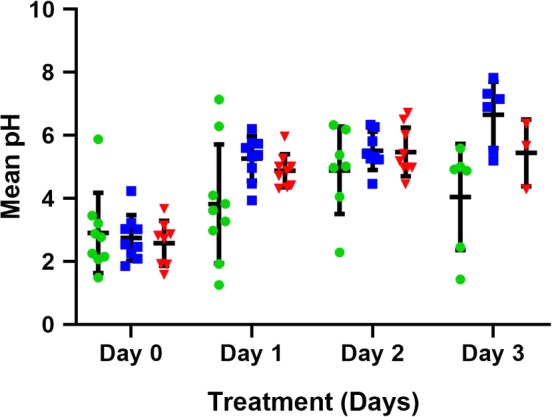
The mean pH for all dogs receiving 1 mg/kg q12h esomeprazole, pantoprazole, and 8 mg/kg/day famotidine constant rate infusion (CRI) on treatment days 1‐3. Horizontal and vertical lines represent the mean and standard deviations, respectively. Individual dog data are represented by green circles (pantoprazole), blue squares (esomeprazole), and red triangles (famotidine CRI). Significant increases in mean pH were noted over time with all 3 treatments (P < .001). No significant treatment (P = .77) or treatment‐by‐time interaction (P = .29) were observed
Figure 4.
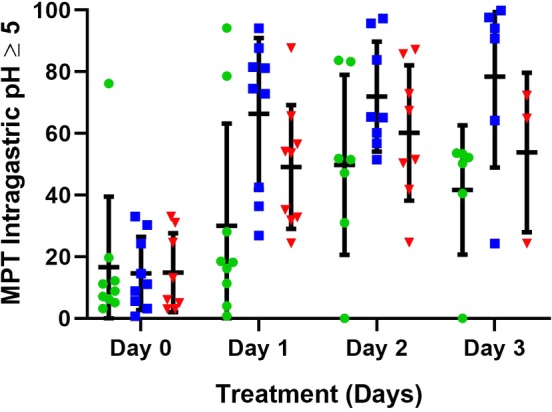
The mean percentage time (MPT) intragastric pH ≥5 for all dogs receiving 1 mg/kg q12h esomeprazole, pantoprazole, and 8 mg/kg/day famotidine constant rate infusion (CRI) on treatment days 1‐3. Horizontal and vertical lines represent the mean and standard deviations, respectively. Individual dog data are represented by green circles (pantoprazole), blue squares (esomeprazole), and red triangles (famotidine CRI)
Figure 5.
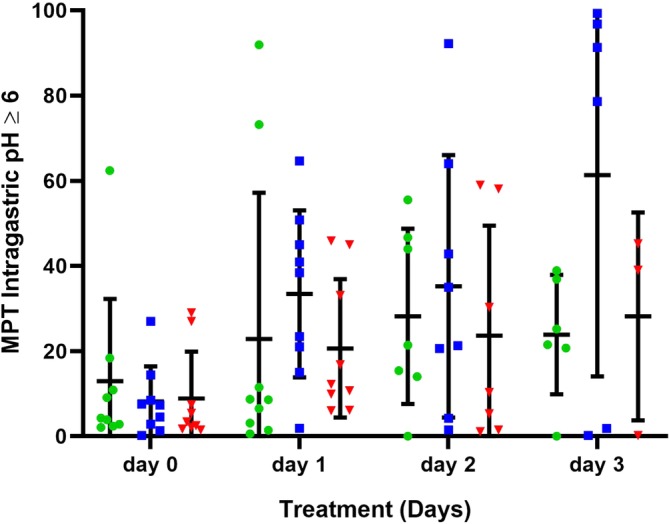
The mean percentage time (MPT) intragastric pH ≥6 for all dogs receiving 1 mg/kg q12h esomeprazole, pantoprazole, and 8 mg/kg/day famotidine constant rate infusion (CRI) on treatment days 1‐3. Horizontal and vertical lines represent the mean and standard deviations, respectively. Individual dog data are represented by green circles (pantoprazole), blue squares (esomeprazole), and red triangles (famotidine CRI)
3.3. Adverse effects
No dogs were excluded from the study. No changes in activity were noted. All dogs consumed 100% of their meals on all baseline and treatment days during all 3 treatments. The total number of vomiting episodes for all days was 4, 2, and 2 for the famotidine CRI, esomeprazole, and pantoprazole treatment groups, respectively. The majority of these vomiting episodes happened on treatment days 2‐3. None of these vomiting episodes occurred immediately after medicating. The mean ± standard deviations for fecal scores for the famotidine group, esomeprazole group, and pantoprazole group for all treatment days were 2.3 ± 0.5, 2.4 ± 0.48, and 2.4 ± 0.5, respectively. There were no significant associations with treatment groups and number of vomiting episodes, fecal score, or appetite.
4. DISCUSSION
In this study, we compared the efficacy of IV administered esomeprazole, pantoprazole, and a CRI of famotidine to increase the intragastric pH of healthy dogs to determine which acid suppressant might be most efficacious for treatment of upper GI ulceration and bleeding in critically ill dogs that require parenterally administered medications. We found no significant differences among the treatment groups. The optimal degree of acid suppression for the treatment of duodenal ulceration and esophagitis in people is to increase the intragastric pH ≥3 for approximately 18 hours or 75% of the day and pH ≥4 for approximately 16 hours or 67%, respectively.2, 3 As no goals have been established for companion animals and goals for the treatment of gastric ulceration are largely undefined, these goals have been adopted as measures of acid suppressant efficacy in dogs and were used, in addition to reporting of mean gastric pH, in our study for comparative analysis. On treatment day 1, esomeprazole and famotidine CRI had excellent gastric acid suppression, maintaining an MPT intragastric pH ≥3 of 80% and 87% and MPT intragastric pH ≥4 of 84% and 73%, respectively. Pantoprazole did not achieve the clinical goals for acid suppression on day 1 with an MPT intragastric pH ≥3 and ≥4 of 58% and 43%, respectively. On treatment days 2‐3, esomeprazole and famotidine CRI continued to outperform pantoprazole, maintaining an MPT intragastric pH ≥3 and ≥4 above 85%. Pantoprazole again failed to meet the clinical goals for the treatment of acid‐related injury on subsequent treatment days with an MPT intragastric pH ≥4 of 66% and 62% on treatment days 2 and 3, respectively. Although additional study is needed in dogs with upper GI bleeding, our findings lead us to believe that esomeprazole and famotidine CRI provide superior gastric acid suppression compared to pantoprazole. In previous studies, both parenterally and PO administered esomeprazole formulations have proven to provide potent acid suppression in dogs.10 And, in a recent report, a lower dose of IV administered esomeprazole (0.5 mg/kg q 12 hours) was still effective in raising gastric pH in dogs compared to 1.0 mg/kg q12h.16 Orally administered esomeprazole was superior to other PO administered FDA‐approved PPIs in mucosal erosion healing and heartburn relief in people.17 Moreover, although no definitive conclusions can be drawn based on our findings, we did note that esomeprazole had the highest MPT intragastric pH ≥5 and ≥6, 2 variables that are critical for gastric mucosal hemostasis in the face of severe coagulation impairment. These preliminary findings lead us to believe that esomeprazole is the superior choice when aggressive acid suppression is desired in critically ill dogs with upper GI hemorrhage. Additionally, intermittent IV administered injections of esomeprazole also afford a lower cost to the client and less concern for drug incompatibility in hospitalized dogs with 1 single lumen IV catheter compared to a famotidine CRI. In a recent meta‐analysis, intermittent IV administered PPI injections were comparable to CRI PPI treatment for reduction of ulcer rebleeding in humans.18
A diminished acid suppressing effect can be observed with PO administered famotidine over time in dogs,13 but we cannot comment as to whether this occurs after an IV administered CRI of famotidine as can occur in people with IV administered ranitidine.19 In a previous study10 and in the current study, a diminished efficacy over time was not observed; however, medications were only administered for 72 hours. Moreover, in the current study, only 3 dogs receiving famotidine CRI had a capsule in place at the end of the 72‐hour monitoring period. Thus, we can only conclude that an IV administered CRI of famotidine is likely to be effective for up to 72 hours.
Our study was limited by a small sample size that likely contributed to the high degree of variability among dogs. We evaluated a population of healthy dogs with no evidence of GI disease based on normal physical examination and laboratory findings. The response noted in these individual dogs might not reflect the response of dogs with upper GI ulceration or bleeding.
In conclusion, famotidine CRI and esomeprazole might be superior acid suppressants compared to pantoprazole in the first 72 hours of treatment. These findings provide a platform for further investigation and treatment of upper GI ulceration and bleeding in dogs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The IACUC at The University of Tennessee approved the protocol for this study (Approval #2634‐0718).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank Tammy Moyers, Gina Galyon, Missy Smith, Gordon Conklin, Jackie Bellan, Maggie Gulley, Skylar Bowers, Rachel Feuerstein, Christina Zukas, Becky Smith, Andrew Teague, Eric Hilton, Derek Vanderhoff and Kristin Watts for their technical support. This project was supported by The Companion Animal Fund (CAF.2018.04). This subject was presented at the 2019 ACVIM Forum, Phoenix, Arizona. Drs. Odunayo and Tolbert contributed equally to this work and should be considered co‐corresponding authors.
Kuhl A, Odunayo A, Price J, et al. Comparative analysis of the effect of IV administered acid suppressants on gastric pH in dogs. J Vet Intern Med. 2020;34:678–683. 10.1111/jvim.15718
Funding information The Companion Animal Fund, Grant/Award Number: CAF.2018.04
Contributor Information
Adesola Odunayo, Email: aodunayo@utk.edu.
M. Katherine Tolbert, Email: ktolbert@cvm.tamu.edu.
REFERENCES
- 1. Waldrop JE, Rozanski EA, Freeman LM, et al. Packed red blood cell transfusions in dogs with gastrointestinal hemorrhage: 55 cases (1999–2001). J Am Anim Hosp Assoc. 2003;39:523‐527. [DOI] [PubMed] [Google Scholar]
- 2. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345‐351. [DOI] [PubMed] [Google Scholar]
- 3. Bell NJ, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro‐oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59‐67. [DOI] [PubMed] [Google Scholar]
- 4. Green FW Jr, Kaplan MM, Curtis LE, et al. Effect of acid and pepsin on blood coagulation and platelet aggregation: a possible contributor to prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74:38‐43. [PubMed] [Google Scholar]
- 5. Cheng HC, Sheu BS. Intravenous proton pump inhibitors for peptic ulcer bleeding: clinical benefits and limits. World J Gastrointest Endosc. 2011;3:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lau JY, Barkun A, Fan DM, et al. Challenges in the management of acute peptic ulcer bleeding. Lancet. 2013;381:2033‐2043. [DOI] [PubMed] [Google Scholar]
- 7. Mathews S, Reid A, Tian C, et al. An update on the use of pantoprazole as a treatment for gastroesophageal reflux disease. Clin Exp Gastroenterol. 2019;3:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tolbert K, Odunayo A, Howell R, Peters E, Reed A. Efficacy of intravenous administration of combined acid suppressants in healthy dogs. J Vet Intern Med. 2015;29:556‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindberg P, Keeling D, Fryklund J, et al. Esomeprazole enhanced bio‐availability, specificity for the proton pump and inhibition of acid secretion. Aliment Pharmacol Ther. 2003;17:481‐488. [DOI] [PubMed] [Google Scholar]
- 10. Hwang JH, Jeong JW, Song GH, et al. Pharmacokinetics and acid suppressant efficacy of esomeprazole after intravenous, oral, and subcutaneous administration to healthy beagle dogs. J Vet Intern Med. 2017;31:743‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedges K, Odunayo A, Price JM, et al. Evaluation of the effect of a famotidine continuous rate infusion on intragastric pH in healthy dogs. J Vet Intern Med. 2019;33:1988‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Çelebi A, Aydın D, Kocaman O, et al. Comparison of the effects of esomeprazole 40 mg, rabeprazole 20 mg, lansoprazole 30 mg, and pantoprazole 40 mg on intragastrıc pH in extensive metabolizer patients with gastroesophageal reflux disease. Turk J Gastroenterol. 2016;27:408‐414. [DOI] [PubMed] [Google Scholar]
- 13. Tolbert MK, Graham A, Odunayo A, et al. Repeated famotidine administration results in a diminished effect on intragastric pH in dogs. J Vet Intern Med. 2017;31:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Senn S. Cross‐Over Trials in Clinical Research. 2nd ed. West Sussex, England: Wiley; 2002. [Google Scholar]
- 15. Brown H. Applied Mixed Models in Medicine. 3rd ed. Chichester, England: Wiley; 2015. [Google Scholar]
- 16. Seo DH, Lee JB, Hwang JH, et al. Pharmacokinetics and pharmacodynamics of intravenous esomeprazole at 2 different dosages in dogs. J Vet Intern Med. 2019;33(2):531‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li M, Li Q, Sun M, et al. Comparative effectiveness and acceptability of the FDA licensed proton pump inhibitors for erosive esophagitis: a PRISMA‐compliant network meta‐analysis. Medicine. 2017;96(39):8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sachar H, Vaidya K, Laine L. Intermittent vs continuous proton pump inhibitor therapy for high‐risk bleeding ulcers: a systematic review and meta‐analysis. JAMA Intern Med. 2014;174:1755‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merki HS, Wilder‐Smith CH. Do continuous infusions of omeprazole and ranitidine retain their effect with prolonged dosing? Gastroenterology. 1994;106:60‐64. [DOI] [PubMed] [Google Scholar]


