Abstract
Background
A clinical diagnosis (CDx) of pancreatitis includes evaluation of clinical signs, abdominal ultrasound (AUS), and pancreatic lipase. However, practitioners are using AUS to diagnose pancreatitis and are using AUS severity to guide decisions. The validity of this is unknown.
Objectives
To determine whether (1) there is a correlation between AUS, specific canine pancreatic lipase (Spec cPL) assay, and CDx; (2) individual AUS abnormalities correlate more closely with CDx than others; (3) AUS severity mirrors clinical severity indices; (4) changes in AUS can be used as a marker for changes in Spec cPL or CDx; and (5) the sensitivity and specificity of AUS for pancreatitis.
Animals
One hundred fifty‐seven dogs.
Methods
In this retrospective case study, inclusion criteria were signs of gastrointestinal, pancreatic disease, or both, in addition to having a Spec cPL and AUS performed within 30 hours. Information extracted from the records included bloodwork, Spec cPL, AUS images/clips, and severity of ultrasonographic findings.
Results
AUS was weakly correlated with Spec cPL (r s = .0178, P = .03) and moderately correlated with CDx (r s = .379, P = <.001). Pancreatic size (r s = .285, P = <.001), echogenicity (r s = .365, P = <.001), and mesenteric echogenicity (r s = .343, P = <.001) were correlated with CDx. Change in AUS was not correlated with Spec cPL or CDx changes. When pancreatic enlargement, echogenicity, or altered mesenteric echogenicity were required for a diagnosis, the sensitivity and specificity were 89% (95% confidence interval [CI] 71.8, 97.7) and 43% (95% CI 34.0, 51.6). When all 3 criteria were required, the sensitivity and specificity were 43% (95% CI 24.5, 62.8) and 92% (95% CI 85.3, 95.7).
Conclusions
AUS should not be used in isolation to diagnose pancreatitis and is a poor indicator of severity.
Keywords: diagnostic imaging, dogs, lipase, pancreas, quantitative, severity score, UPASS
Abbreviations
- APPLE
acute patient physiologic and laboratory evaluation
- AUS
abdominal ultrasound
- CAPS
canine acute pancreatitis severity
- CDx
clinical diagnosis
- CI
confidence interval
- iCa
ionized calcium
- Spec cPL
specific canine pancreatic lipase
- UPASS
ultrasonographic pancreatic assessment severity score
1. INTRODUCTION
Histopathology is the gold standard for the diagnosis of pancreatitis1, 2; however, it is rarely performed due to its invasive nature and inherent limitations, including the potential to miss localized lesions or subclinical pancreatitis.3, 4 Given these limitations, several recent studies have utilized a variety of data including clinicopathologic abnormalities, pancreatic ultrasound, and pancreatic lipase concentration (specific canine pancreatic lipase [Spec cPL]) as a surrogate gold standard for pancreatitis in dogs.5, 6, 7, 8, 9, 10 Spec cPL is frequently utilized as it has the greatest sensitivity (21%‐71%) and specificity (100%) for detection of histopathologic‐confirmed pancreatitis.11
Ultrasonographic findings consistent with acute pancreatitis include pancreatic enlargement, hypoechoic regions within the pancreas, increased echogenicity of the surrounding mesentery, altered pancreatic echotexture, and dilation of the pancreatic or biliary duct.12, 13, 14, 15 Pancreatic cyst‐like lesions also occur in the subacute phase of pancreatitis.16 Other nonspecific changes include abdominal effusion, ileus, gastric wall thickening, and lateral displacement of the duodenum.13, 15, 17 Anecdotally veterinary practitioners might use abdominal ultrasound (AUS) in conjunction with clinical signs and supportive clinical pathology screening, in the absence of a quantitative pancreatic lipase assay, for the diagnosis of pancreatitis and assessment of disease severity. In addition, although prior studies have documented potential ultrasonographic abnormalities seen with pancreatitis, the individual contribution of each abnormality to a CDx of pancreatitis in dogs has not been evaluated.
It has also been suggested that repeat AUS examinations can be used to monitor response to treatment, but in the absence of scientific study this remains controversial.12 Clinical severity indices have been validated but it is currently unknown if ultrasonographic assessment of the severity of pancreatitis mirrors these indices.18, 19 Therefore, the value of repeat AUS for monitoring of routine pancreatitis cases is unknown.
Although AUS is the imaging method of choice for pancreatitis, it is highly dependent on the skill and experience of the ultrasonographer and the equipment available.20 Thus, ultrasonographer skill and ultrasound technology must be considered when evaluating prior studies. The sensitivity of AUS for detection of severe fatal pancreatitis was reported to be 68% in 1 study over 20 years ago.13 Changes in ultrasound technology over the years might have increased the sensitivity, and caution has been advised to prevent the overinterpretation of ultrasonographic findings.20
There were 5 objectives of this study. Firstly to determine if a correlation exists between AUS findings, pancreatic lipase concentration, and a CDx of pancreatitis in dogs. Secondly to determine if individual ultrasonographic abnormalities correlated more closely with a CDx of pancreatitis than others. Thirdly, to determine whether ultrasonographic assessment of the severity of pancreatitis mirrors clinical severity indices and fourthly to determine if changes in the ultrasonographic assessment of severity over time, in the same dog, mirror a change in Spec cPL concentration or change in CDx. The final objective was to calculate the sensitivity and specificity of modern AUS for the diagnosis of pancreatitis in dogs.
2. MATERIALS AND METHODS
2.1. Case selection and data collection
Cases were identified by searching the medical records at the authors' institution between June 2014 and June 2019. Criteria for inclusion in the study were dogs with clinical signs of gastrointestinal disease (eg, vomiting, diarrhea, anorexia, abdominal pain, lethargy, or a combination of these signs), in addition to having a Spec cPL and either a focal pancreatic ultrasound or a full AUS examination performed within 30 hours of each other. Thirty hours were selected to allow for emergency cases to be enrolled in the study. No other inclusion criteria were required.
2.2. Spec cPL assay
Serum was submitted to a commercial laboratory (Texas A&M University, Gastrointestinal Laboratory, College Station, Texas) for the assessment of Spec cPL concentration at the time of sample collection. The Spec cPL is a diagnostic test that utilizes an ELISA for the quantification of canine pancreatic lipase. The Spec cPL assay is highly sensitive and specific for the diagnosis of clinical pancreatitis in dogs.5, 7, 21
2.3. Ultrasonographic pancreatic assessment severity score
Abdominal ultrasound examinations were performed by either a radiology resident‐in‐training, under the supervision of a board‐certified veterinary radiologist, or directly by a board‐certified veterinary radiologist. Ultrasonographic still images and video clips were later evaluated by a single‐board certified veterinary radiologist (A.M.L.), who was blinded to case history and the results of diagnostic testing, including the Spec cPL assay. A single individual was used to retrospectively assess the images to prevent interobserver variation from impacting the data. The still images and video clips were assessed for evidence of pancreatic enlargement, echogenicity, and echotexture. Pancreatic enlargement was determined via comparison with previously published reference intervals, whereas pancreatic echotexture was a subjective measure as utilized in previous literature.22, 23, 24, 25 The echogenicity of the pancreas was assessed via comparison with internal landmarks such as the surrounding mesentery, kidneys, spleen, and liver. The echogenicity of the surrounding mesentery and the presence of peripancreatic free fluid were also assessed. The results of this retrospective evaluation were then used to assign an ultrasonographic pancreatic assessment severity score (UPASS), from 0 to 7, as outlined in Table 1. The higher the UPASS, the greater the ultrasonographic evidence of pancreatitis and the greater the ultrasonographic severity of pancreatitis. Additional abnormalities such as pancreatic cysts or pseudocysts, pancreatic nodules, and pancreatic masses were recorded; however, they were not included in the UPASS. Twelve dogs had both an initial assessment and a later assessment by AUS; the change in UPASS for these dogs was calculated for further analysis. Two ultrasound machines were used during this retrospective study: Esaote Biosound MyLab 50 (Esaote North America Inc, Fishers, Indiana) and the Logiq s8 ultrasound machine (GE Healthcare, Madison, Wisconsin).
Table 1.
Ultrasonographic pancreatic assessment severity score (UPASS)
| Component of the PASS | Assigned score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Pancreatic size | Normal | Enlarged | |
| Pancreatic echogenicity | Normal | Hyperechoic | Hypoechoic |
| Pancreatic echotexture | Homogenous | Heterogenous | |
| Echogenicity of surrounding mesentery | Normal | Hypoechoic | Hyperechoic |
| Peripancreatic free fluid | No | Yes | |
Note: Table 1 denotes the components of the UPASS and their relative contributions to the UPASS. The UPASS ranges from 0 to 7, and the higher the UPASS the greater the ultrasonographic evidence of pancreatitis. Pancreatic enlargement was determined by comparison with the previously published reference intervals.25 Pancreatic echotexture was subjectively assessed, and pancreatic echogenicity was determined by comparison to internal markers such as the kidney, liver, and spleen,
2.4. Determination of a CDx of pancreatitis (clinical gold standard)
A CDx of pancreatitis was assigned to each dog by 1 of the authors after assessment of a comprehensive panel of information including the evaluation of the history, physical examination findings, clinicopathological data, and imaging findings of each dog at each visit.
2.5. Clinical severity indices
In recent years, a number of clinical severity indices have been developed and validated for dogs admitted to intensive care units and in dogs with acute pancreatitis. Two such clinical severity indices include the acute patient physiologic and laboratory evaluation (APPLE) score and the canine acute pancreatitis severity (CAPS) score.18, 19 Both the APPLE and CAPS scores were retrospectively calculated for each dog based on data collected at admission, with minor modification due to typical practices at the author's institution. For the modified CAPS, total calcium was utilized in place of ionized calcium (iCa) when the iCa was unavailable for review. For the modified APPLE score, lactate was included if performed at the time of presentation. If insufficient data were available, the modified APPLE and modified CAPS scores were not calculated for that visit (see Figure 1).
Figure 1.
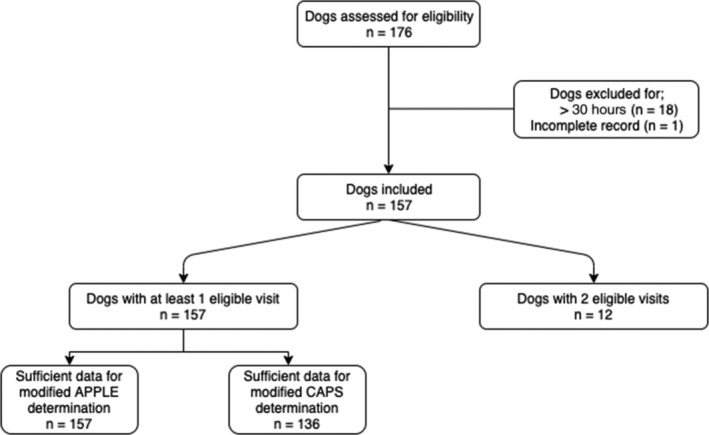
Flow diagram documenting case enrollment. Cases were identified by searching the medical records at the author's institution between June 2014 and June 2019 for dogs that had clinical signs of gastrointestinal/pancreatic disease, in addition to having an AUS and Spec cPL performed during the same visit. Dogs were subsequently excluded if the AUS and Spec cPL occurred >30 hours apart (n = 18) or if the medical record was incomplete (n = 1)
2.6. Sensitivity and specificity of AUS for detection of clinical pancreatitis
The sensitivity and specificity of AUS for detection of pancreatitis were determined via comparison to a CDx of pancreatitis. The sensitivity and specificity were calculated under 3 sets of conditions. The first scenario was when only 1 of the following criteria was required for the diagnosis of pancreatitis, pancreatic enlargement, abnormal pancreatic echogenicity, or an abnormal mesenteric echogenicity. Secondly, when 2 of the criteria were required and thirdly when all 3 criteria were required for a diagnosis of pancreatitis. These components were chosen, as they were statistically significant when correlating individual components of the UPASS to CDx.
2.7. Statistical analysis
Due to the nonparametric nature of the data, Spearman's rank correlation was used to assess associations among different variables using PROC CORR in SAS for Windows v9.4 (SAS Institute, Inc, Cary, North Carolina). Spearman's rank correlation coefficients of UPASS with Spec cPL, CAPS, APPLE, and CDx were determined. Spearman's rank correlation coefficients of the individual components of the UPASS (pancreatic size, echogenicity, echotexture, echogenicity of the surrounding mesentery, and the presence of peripancreatic free fluid) with Spec cPL and CDx were also determined. In addition, Spearman's rank correlation coefficients of the change in UPASS, in the 12 dogs that had both an initial assessment and a later assessment by AUS with the change in Spec cPL and change in CDx were determined. Sensitivity, specificity, and 95% confidence intervals (CIs) for ultrasonographic detection of clinical pancreatitis were calculated using MedCalc, version 19.1. An alpha level of .05 was used to determine statistical significance for all statistical tests. Specific canine pancreatic lipase concentrations <30 μg/L were recorded as 29.9 μg/L, and Spec cPL concentrations >1000 μg/L were recorded as 1000.1 μg/L for statistical analysis.
3. RESULTS
3.1. Animals
A total of 176 client‐owned dogs were initially identified; however, 19 dogs were subsequently excluded as the AUS and Spec cPL were performed >30 hours apart (n = 18) or incomplete medical records (n = 1), leaving 157 dogs, 12 of which were assessed on a second visit, for analysis (see Figure 1). Eighty‐one were female (74 spayed females and 7 intact females), and 76 were male (65 neutered males and 11 intact males). The median age of dogs enrolled was 9 years (range, 4 months to 17 years and 6 months). The median weight of dogs enrolled was 8.8 kg (range, 1.02‐60.0 kg).
3.2. Spec cPL assay results
All samples were able to be measured. The median Spec cPL concentration was 140 μg/L (range, <30 to >2000 μg/L). Ninety‐nine samples had a Spec cPL concentration <200 μg/L. Thirty‐three samples had a Spec cPL concentration between 201 and 399 μg/L. Thirty‐seven animals had a Spec cPL concentration ≥400 μg/L.
3.3. Pancreatic ultrasound findings and UPASS
All 169 ultrasound examinations, from the 157 enrolled dogs, were evaluated for findings consistent with pancreatitis. The right limb of the pancreas was identified in all cases. The left limb of the pancreas was identified in 137 of the 169 ultrasound examinations (81.1%), and the body of the pancreas was identified in 158 of the 169 (93.4%) ultrasound examinations. On initial examination, 46 of the 157 (29.3%) ultrasound examinations had evidence of pancreatic enlargement. Thirty‐five of the 157 (22.3%) had a hyperechoic pancreas and 60 (38.2%) had a hypoechoic pancreas. Eighty‐six of the 157 (54.8%) ultrasound examinations had a heterogenous pancreas. Six of the 157 (3.8%) had evidence of peripancreatic free fluid. One (0.6%) had a hypoechoic mesentery surrounding the pancreas, whereas 43 (27.4%) of the ultrasound examinations had a hyperechoic mesentery surrounding the pancreas. Two ultrasound examinations (1.2%) had evidence of pancreatic cysts or pseudocysts, 1 examination (0.6%) had evidence of a pancreatic nodule, and 1 examination (0.6%) had evidence of a pancreatic mass. Unfortunately, fine needle aspirates of the pancreas were not performed in any of these cases. The median UPASS was 2 (range, 0‐6). Of which, 19.5% (33/169) of ultrasound examinations had a UPASS of 0, 20.1% (34/169) had a UPASS of 1, 15.4% (26/169) had a UPASS of 2, 14.2% (24/169) had a UPASS of 3, 14.8% (25/169) had a UPASS of 4, 5.9% (10/169) had a UPASS of 5, and 10.1% (17/169) had a UPASS of 6. No ultrasound examinations had a UPASS of 7.
3.4. Correlation between UPASS, Spec cPL concentration, and CDx
Spearman's rank correlation coefficients were calculated between ultrasonographic assessment of the severity of pancreatitis, Spec cPL concentration, and CDx. The ultrasonographic assessment of severity of pancreatitis was determined by the UPASS. In the 12 dogs that received multiple evaluations, only the first set of data was included in statistical analysis. Spearman's rank correlation coefficient between UPASS and Spec cPL was 0.0178 (P = .03), indicating a weak positive correlation between ultrasonographic evidence of pancreatitis and pancreatic lipase concentration. Spearman's rank correlation coefficient between UPASS and CDx was 0.379, (P = <.001), indicating a moderate correlation between ultrasonographic assessment of the severity of pancreatitis and the CDx.
3.5. Correlation between components of the UPASS, Spec cPL, and CDx
Spearman's rank correlation coefficient was calculated between Spec cPL and each component of the UPASS to determine if any individual abnormalities on AUS correlated more closely with Spec cPL concentration than others. Although, pancreatic size (r s = .0176, P = .83), pancreatic echotexture (r s = −.00256, P = .75), the presence of peripancreatic free fluid (r s = −.0297, P = .71), and the echogenicity of the surrounding mesentery (r s = .153, P = .06) were not correlated with Spec cPL. Spearman's rank correlation documented a weak‐to‐moderate positive correlation between pancreatic echogenicity and Spec cPL concentration (r s = .248, P = .0017) (see Table 2). Spearman's rank correlation coefficient was also calculated between the CDx and each component of the UPASS, to determine if any individual ultrasonographic abnormalities correlated more closely with a diagnosis of pancreatitis. Pancreatic size (r s = .285, P = <.001), pancreatic echogenicity (r s = .365, P = <.001), and echogenicity of the surrounding mesentery (r s = .343, P = <.001) were significantly associated with a diagnosis of pancreatitis. A significant correlation could not be established for pancreatic echotexture (r s = .0556, P = .49) or the presence of peripancreatic free fluid (r s = −.0929, P = .25) (see Table 3).
Table 2.
Correlation between AUS indicators of pancreatitis and Spec cPL concentration in dogs
| Pancreatic size | Pancreatic echogenicity | Pancreatic echotexture | Echogenicity of surrounding mesentery | Presence of peripancreatic free fluid | UPASS | |
|---|---|---|---|---|---|---|
| r s value | .0176 | .248 | −.00256 | .153 | −.0297 | .0178 |
| P value | .83 | .0017* | .75 | .06 | .71 | .03* |
Note: Table 2 denotes Spearman's rank correlation coefficient (r s value) and statistical significance (P value) for the correlation between ultrasonographic findings and severity of pancreatitis, as determined by Spec cPL concentration in a dog with clinical signs of gastrointestinal/pancreatic disease. An alpha level of .05 was used to determine statistical significance.
Statistically significant value.
Table 3.
Correlation between AUS indicators of pancreatitis and CDx in dogs
| pancreatic size | Pancreatic echogenicity | Pancreatic echotexture | Echogenicity of surrounding mesentery | Presence of peripancreatic free fluid | UPASS | |
|---|---|---|---|---|---|---|
| r s value | .285 | .365 | .0556 | .343 | −.0929 | .379 |
| P value | <.001* | <.001* | .49 | <.001* | .25 | <.001* |
Note: Table 3 denotes Spearman's rank correlation coefficient (r s value) and statistical significance (P value) for the correlation between ultrasonographic findings and a CDx of pancreatitis. An alpha level of .05 was used to determine statistical significance.
Statistically significant value.
3.6. Correlation between UPASS and clinical severity indices
A significant association between the ultrasonographic assessment of severity of pancreatitis and either of the 2 modified clinical severity indices was not identified in our study (APPLE, r s = .138, P = .11 and CAPS, r s = . 101, P = .21).
3.7. Association between changes in UPASS, Spec cPL, and CDx
Spearman's rank correlation coefficient was used to determine if a change in UPASS between 2 AUS would mirror a change in Spec cPL concentration in the 12 dogs which had repeat testing performed. A statistically significant relationship between change in UPASS and change in Spec cPL between paired visits could not be established (r s = .220, P = .49). Spearman's rank correlation coefficient was also used to determine if a change in UPASS between 2 AUS mirrored a change in CDx. A statistically significant relationship between change in UPASS and change in CDx was not established (r s = .138, P = .67).
3.8. Sensitivity and specificity of AUS for detection of clinical pancreatitis
Three significant variables were included in the determination of sensitivity and specificity of AUS for clinical pancreatitis: pancreatic enlargement, pancreatic echogenicity, and the echogenicity of the surrounding mesentery. When only 1 of the criteria was required, the sensitivity and specificity of AUS for detection of pancreatitis was 89% (95% CI: 71.8%‐97.7%) and 43% (95% CI: 34.0%‐51.6%). When 2 criteria were required, the sensitivity and specificity were 78% (95% CI: 57.7%‐91.4%) and 69% (95% CI: 60.3%‐76.8%), respectively. When all 3 criteria were required, the sensitivity and specificity were 43% (95% CI: 24.5%‐62.8%) and 92% (95% CI: 85.3%‐95.7%), respectively.
4. DISCUSSION
In this retrospective study, we evaluated the correlation between ultrasonographic evidence of pancreatitis, pancreatic lipase concentration, clinical severity indices, and a CDx of pancreatitis in 157 client‐owned dogs. The results of this study highlight a discrepancy between AUS and pancreatic lipase concentrations in the diagnosis of pancreatitis in dogs, similar to previously reported.36 This study also highlights a discrepancy between the ultrasonographic assessment of severity of pancreatitis and the validated clinical severity indices with only minor modifications. We also report the diagnostic sensitivity and specificity of modern AUS for the detection of pancreatitis in dogs using a variety of criteria.
Ultrasonographic evidence of pancreatitis, as determined by UPASS, had only a weak positive correlation with pancreatic lipase concentration (r s = .0178, P = .03), and a moderate correlation with a CDx of pancreatitis in dogs (r s = .379, P = <.001). The weak‐to‐moderate correlation indicates that clinicians should not assume that ultrasonographic evidence of pancreatitis is indicative of an above reference interval Spec cPL or indicative of a CDx of pancreatitis. Therefore, the authors recommend that a quantitative pancreatic lipase assay should be performed in addition to an AUS in all dogs being assessed for pancreatitis. One potential explanation for the lower correlation between UPASS and Spec cPL than between UPASS and CDx is that primary nonpancreatic diseases and steroid treatment have been associated with an above reference interval Spec cPL concentration, although the clinical relevance of these increases are often unknown.21, 26, 27, 28, 29, 30, 31, 32
When evaluating individual components of the UPASS, this study noted that pancreatic echotexture and peripancreatic free fluid were not significantly correlated with a CDx of pancreatitis. In contrast, a significant relationship between pancreatic size, pancreatic echogenicity, and echogenicity of the surrounding mesentery with a CDx of pancreatitis was established. Thus, pancreatic echotexture and peripancreatic free fluid might be poor indicators of pancreatitis, when compared to measures of echogenicity and pancreatic size in dogs. Pancreatic enlargement and development of a hypoechoic pancreas occur due to the accumulation of interstitial edema in association with pancreatic inflammation.14 Despite the statistical significance, the correlation between pancreatic echogenicity and a CDx of pancreatitis was only moderate (r s = .349, P = <.001). This is likely due to the fact that diseases other than pancreatitis cause pancreatic edema, including hypoalbuminemia and portal hypertension.33 A significant correlation between peripancreatic free fluid and a diagnosis of pancreatitis was not established, this is also likely due to the many different etiologies for abdominal free fluid in dogs including, but not limited to, portal hypertension and hypoalbuminemia. The hyperechoic mesentery associated with pancreatitis, as demonstrated in this study and prior studies, might be due to extension of inflammation beyond the pancreas due to release of proinflammatory cytokines and fat saponification.33 Pancreatic echotexture was also not significantly associated with a CDx of pancreatitis. This finding might instead, be related to disease severity, as an amorphous appearance to the pancreas is typically associated with pancreatic necrosis and hemorrhage.33 Pancreatic echotexture is also a very subjective measurement when compared to pancreatic size, which has established reference intervals, and pancreatic echogenicity which can be compared to internal markers such as the liver, kidneys, and spleen.
The correlation between the ultrasonographic assessment of severity of pancreatitis, as measured by UPASS, and the validated clinical severity indices (with minor modifications) was evaluated. The first clinical severity index assessed was the APPLE, which was previously validated for use in dogs presenting to an intensive care unit and was independent of primary diagnosis.18 In the prior study, both the APPLEfull and APPLEfast scores were validated; however, a modified APPLEfull score was utilized in this study, as the APPLEfull score had a greater specificity than the APPLEfast score in the prior study.18 The APPLEfull score was retrospectively applied to the cases, with a minor modification in that lactate was not included if it was not performed at the time of presentation. No other variables were excluded. The second clinical severity index evaluated was the CAPS score, which was recently validated in dogs with acute pancreatitis.19 Again, this score system was applied with minor modification, in that total calcium was utilized if an iCa was not performed. At the authors' institution, an iCa is typically performed only if the dog is displaying clinical signs of hypocalcemia or there is an abnormal total calcium concentration. We did not document a significant correlation between the ultrasonographic assessment of severity of pancreatitis and either of the 2 modified clinical severity indices, suggesting that AUS is a poor indicator of the severity of pancreatitis. It is therefore recommended that clinical severity indices are calculated in all cases of acute pancreatitis, especially when determination of clinical severity is considered important. Future studies should prospectively evaluate the relationship between UPASS and clinical severity indices, without any modifications, to fully determine the use of AUS in the assessment of severity of pancreatitis.
Some authors have recommended the use of repeat AUS examinations for monitoring response to treatment and resolution of pancreatic inflammation in dogs.12 However, little scientific research has been performed in this area. We did not document a significant correlation between change in the ultrasonographic assessment of severity of pancreatitis, as measured by UPASS and change in Spec cPL concentration (r s = .220, P = .49) or change in CDx (r s = .138, P = .67) in 12 dogs that had both diagnostics performed twice. Power was likely limited by the low number of cases with more than 1 episode of paired AUS and Spec cPL readings; however, an estimation of correlation between these parameters had not previously been reported and was much lower than anticipated. The correlation established in this study could be used for future power calculations. Larger prospective studies evaluating the benefit of repeat AUS examinations in dogs with pancreatitis are required, and until such data are available, caution is advised when utilizing AUS alone to monitor and guide treatment in dogs with pancreatitis. Repeat AUS examinations are, however, always indicated to monitor for complications when dogs are not responding to treatment or develop uncontrolled or worsening abdominal pain.
The sensitivity and specificity of AUS for the detection of clinical pancreatitis in dogs vary depending on the definition of ultrasonographic evidence of pancreatitis. We utilized 3 different ultrasonographic features that were significantly correlated with a diagnosis of pancreatitis during initial analysis: pancreatic size, pancreatic echogenicity, and echogenicity of the surrounding mesentery. When only 1 criteria was required for a diagnosis of pancreatitis the sensitivity was high (89%) and the specificity was modest (43%). If 2 criteria were required, the sensitivity and specificity were both moderate at 78% and 69%, respectively. If pancreatic enlargement, a hypoechoic pancreas and a hyperechoic mesentery were all required, the specificity was excellent at 92%, but the sensitivity was significantly reduced at 42%. The sensitivity and specificities calculated in the current study differ from those reported 20 years ago which might be associated with advances in technology, as previously suspected, or due to differences in definitions for ultrasonographic evidence of pancreatitis.13, 20 The presence of pancreatic enlargement in conjunction with an abnormal pancreatic echogenicity and a hyperechoic surrounding mesentery in an equivocal case of pancreatitis (based on history, physical examination, and a quantitative pancreatic lipase assay) might increase the probability that the dog has clinically relevant pancreatitis. However, if only 1 of the findings is present, it reduces the probability that the dog has clinically relevant pancreatitis. Consequently, whereas ultrasound examination does not provide a definitive diagnosis of pancreatitis, it can assist in classification of equivocal cases. One potential explanation for this finding is that systemic disease might cause a small number or reactive/secondary changes to the pancreas, such as a hyperechoic mesentery in septic peritonitis, whereas multiple focal pancreatic abnormalities are rarely seen in systemic nonpancreatic disease. Necropsy studies have also documented the presence of peripancreatic fat necrosis in the absence of severe pancreatic inflammation.34 Although sensitivity and specificity are reported throughout this manuscript, positive and negative predictive values are also clinically relevant. However, they are dependent on the prevalence of disease in the test population, which varies between institutions. Therefore, veterinarians should consider the prevalence of disease in their test population when interpreting test results.
As pancreatitis has relatively nonspecific clinical signs, abdominal imaging is important to rule out other differential diagnoses. Although the diagnostic performance of AUS and its correlation with Spec cPL noted in this study appear relatively poor, unless stringent criteria are applied, it still plays a key role in the diagnostic approach to dogs with clinical signs of gastrointestinal/pancreatic disease. Abdominal ultrasound also allows for the evaluation of potential complicating factors such as extrahepatic biliary duct obstruction and potentially vascular thrombosis. Veterinarians should continue to use AUS as part of a clinical examination as it is likely to provide the most useful information when combined with a quantitative pancreatic lipase assay. The limitations of each diagnostic test must be considered when assigning a CDx of pancreatitis.
In the current study, all images were retrospectively evaluated by a single‐board certified radiologist blinded to the results of the history, physical examination findings, clinicopathological data, and the Spec cPL assay. This approach can be considered both an advantage and disadvantage, as it eliminates the effect of interobserver variability on interpretation of results. However, as AUS is highly dependent on the skill of the ultrasonographer, the results reported here might be different than those obtained by individuals without advanced training in abdominal ultrasonography.20
A major limitation of our study is its retrospective nature. Ultrasonographic still images and video clips might represent only the most severe lesions, rather than giving a fair representation of the whole pancreas. This might have influenced subjective measures of pancreatitis such as echotexture. In addition, this retrospective evaluation was dependent on the quality of the AUS images collected and their interpretation. Furthermore, interpretation might be more challenging for less experienced sonographers and this may influence the results of this study. Although, every effort was made to minimize the subjective nature of ultrasonographic assessment of the pancreas by using previously utilized reference intervals where available, and by comparing pancreatic echogenicity to internal markers, pancreatic ultrasound is, by its nature, inherently subjective. Pancreatic assessment on AUS might be affected by transducer frequency, echogenicity of surrounding tissues, and angle of interrogation among other factors. Future studies should consider the use of more objective measures of pancreatic disease, for example, the use of computer software to evaluate echogenicity. However, despite specific uses in human medicine, no such studies have been performed in veterinary medicine, and to the authors' knowledge, the software has not been adapted for use in veterinary species.35
A further potential limitation is the lack of histopathology in the cases evaluated. Despite histopathology being the historical gold standard, it has many limitations as previously discussed, and many recent studies have utilized a clinical gold standard for the diagnosis of pancreatitis.5, 6, 7, 8, 9, 10
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Cridge H, Sullivant AM, Wills RW, Lee AM. Association between abdominal ultrasound findings, the specific canine pancreatic lipase assay, clinical severity indices, and clinical diagnosis in dogs with pancreatitis. J Vet Intern Med. 2020;34:636–643. 10.1111/jvim.15693
REFERENCES
- 1. Steiner JM. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract. 2003;33(5):1181‐1195. [DOI] [PubMed] [Google Scholar]
- 2. Xenoulis PG, Suchodolski JS, Steiner JM. Chronic pancreatitis in dogs and cats. Compend Contin Educ Vet. 2008;30(3):166‐180. [PubMed] [Google Scholar]
- 3. Newman S, Woosley K, Barton L, et al. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med. 2010;18:488‐493. [DOI] [PubMed] [Google Scholar]
- 4. Pratschke KM, Ryan J, Mcalinden A, et al. Pancreatic surgical biopsy in 24 dogs and 19 cats: postoperative complications and clinical relevance of histological findings. J Small Anim Pract. 2015;56(1):60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCord K, Morley P, Armstrong J, et al. A multi‐institutional study evaluating the diagnostic utility of the Spec cPL™ and SNAP® cPL™ in clinical acute pancreatitis in 84 dogs. J Vet Intern Med. 2012;26(4):888‐896. [DOI] [PubMed] [Google Scholar]
- 6. Graca R, Messick J, McCullough S, Barger A, Hoffmann W. Validation and diagnostic efficacy of a lipase assay using the substrate 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6'methyl resorufin)‐ester for the diagnosis of acute pancreatitis in dogs. Vet Clin Pathol. 2005;34(1):39‐43. [DOI] [PubMed] [Google Scholar]
- 7. Cridge H, MacLeod AG, Pachtinger GE, et al. Evaluation of SNAP cPL, Spec cPL, VetScan cPL rapid test, and precision PSL assays for the diagnosis of clinical pancreatitis in dogs. J Vet Intern Med. 2018;32(2):658‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gori E, Lippi I, Guidi G, Perondi F, Pierini A, Marchetti V. Acute pancreatitis and acute kidney injury in dogs. Vet J. 2019;245:77‐81. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen L, Holm J, Rozanski E, Meola D, Price LL, Laforcade A. Multicenter investigation of hemostatic dysfunction in 15 dogs with acute pancreatitis. J Vet Emerg Crit Care. 2019;29(3):264‐268. [DOI] [PubMed] [Google Scholar]
- 10. Okanishi H, Nagata T, Nakane S, Watari T. Comparison of initial treatment with and without corticosteroids for suspected acute pancreatitis in dogs. J Small Anim Pract. 2019;60(5):298‐304. [DOI] [PubMed] [Google Scholar]
- 11. Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas‐specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med. 2011;25(6):1241‐1247. [DOI] [PubMed] [Google Scholar]
- 12. Hecht S, Henry G. Sonographic evaluation of the normal and abnormal pancreas. Clin Tech Small Anim Pract. 2007;22(3):115‐121. [DOI] [PubMed] [Google Scholar]
- 13. Hess RS, Saunders HM, Van Winkle TJ, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986‐1995). J Am Vet Med Assoc. 1998;213(5):665‐670. [PubMed] [Google Scholar]
- 14. Nyland TG, Mulvany MH, Strombeck DR. Ultrasonic features of experimentally induced, acute pancreatitis in the dog. Vet Radiol. 1983;24(6):260‐266. [Google Scholar]
- 15. Nyland TG, Mattoon JS, Herrgesell EJ, Wisner ER. Pancreas In: Nyland TG, Mattoon J, eds. Small animal diagnostic ultrasound. 2nd ed. Philadelphia, PA: W.B. Saunders Company; 2002:144‐157. [Google Scholar]
- 16. Rutgers C, Herring DS, Orton EC. Pancreatic pseudocyst associated with pancreatitis in a dog: ultrasonographic diagnosis. J Am Anim Hosp Assoc. 1985;21:411‐416. [Google Scholar]
- 17. Murakami M, Heng HG, Lim CK, et al. Ultrasonographic features of presumed gastric wall edema in 14 dogs with pancreatitis. J Vet Intern Med. 2019;33(3):1260‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayes G, Mathews K, Doig G, et al. The acute patient physiologic and laboratory evaluation (APPLE) score: a severity of illness stratification system for hospitalized dogs. J Vet Intern Med. 2010;24:1034‐1047. [DOI] [PubMed] [Google Scholar]
- 19. Fabrès V, Dossin O, Reif C, et al. Development and validation of a novel clinical scoring system for short‐term prediction of death in dogs with acute pancreatitis. J Vet Intern Med. 2019;33(2):499‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Washabau RJ. Pancreas. In: Washabau RJ, Day MJ, eds. St. louis, MO: Elsevier Saunders; 2013:799‐848. [Google Scholar]
- 21. Haworth MD, Hosgood G, Swindells KL, Mansfield CS. Diagnostic accuracy of the SNAP and spec canine pancreatic lipase tests for pancreatitis in dogs presenting with clinical signs of acute abdominal disease. J Vet Emerg Crit Care. 2014;24(2):135‐143. [DOI] [PubMed] [Google Scholar]
- 22. French JM, Twedt DC, Rao S, Marolf AJ. Computed tomographic angiography and ultrasonography in the diagnosis and evaluation of acute pancreatitis in dogs. J Vet Intern Med. 2019;33(1):79‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Granger LA, Hilferty M, Francis T, Steiner JM, Gaschen L. Variability in the ultrasonographic appearance of the pancreas in healthy dogs compared to dogs with hyperadrenocorticism. Vet Radiol Ultrasound. 2015;56(5):540‐548. [DOI] [PubMed] [Google Scholar]
- 24. Rademacher N, Schur D, Gaschen F, Kearney M, Gaschen L. Contrast‐enhanced ultrasonography of the pancreas in healthy dogs and in dogs with acute pancreatitis. Vet Radiol Ultrasound. 2016;57(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 25. Penninck DG, Zeyen U, Taeymans ON, Webster CR. Ultrasonographic measurement of the pancreas and pancreatic duct in clinically normal dogs. Am J Vet Res. 2013;74:433‐437. [DOI] [PubMed] [Google Scholar]
- 26. Trehy MR, Batchelor D, Noble PJ, et al. Serum pancreas‐specific lipase concentrations in dogs with upper gastrointestinal foreign bodies. J Vet Intern Med. 2014;28:711‐744. [Google Scholar]
- 27. Kalli IV, Adamama‐Moraitou KK, Patsika MN, et al. Prevalence of increased canine pancreas‐specific lipase concentrations in young dogs with parvovirus enteritis. Vet Clin Pathol. 2017;46(1):111‐119. [DOI] [PubMed] [Google Scholar]
- 28. Kathrani A, Steiner JM, Suchodolski J, et al. Elevated canine pancreatic lipase immunoreactivity concentration in dogs with inflammatory bowel disease is associated with a negative outcome. J Small Anim Pract. 2009;50(3):126‐132. [DOI] [PubMed] [Google Scholar]
- 29. Mawby DI, Whittemore JC, Fecteau KA. Canine pancreatic‐specific lipase concentrations in clinically healthy dogs and dogs with naturally occurring hyperadrenocorticism. J Vet Intern Med. 2014;28(4):1244‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohta H, Morita T, Yokoyama N, et al. Serial measurement of pancreatic lipase immunoreactivity concentration in dogs with immune‐mediated disease treated with prednisolone. J Small Anim Pract. 2017;58(6):342‐347. [DOI] [PubMed] [Google Scholar]
- 31. Han D, Choi R, Hyun C. Canine pancreatic‐specific lipase concentrations in dogs with heart failure and chronic mitral valvular insufficiency. J Vet Intern Med. 2015;29(1):180‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schueler RO, White G, Schueler RL, Steiner JM, Wassef A. Canine pancreatic lipase immunoreactivity concentrations associated with intervertebral disc disease in 84 dogs. J Small Anim Pract. 2018;59(5):305‐310. [DOI] [PubMed] [Google Scholar]
- 33. Penninck DG, D'Anjou MA. Pancreas In: Penninck DG, D'Anjou MA, eds. Atlas of Small Animal Ultrasonography. 2nd ed. Ames, IA: Wiley Blackwell; 2015:309‐330. [Google Scholar]
- 34. Mansfield CS, Anderson GA, O'Hara AJ. Association between canine pancreatic‐specific lipase and histologic exocrine pancreatic inflammation in dogs: assessing specificity. J Vet Diagn Invest. 2012;24(2):312‐318. [DOI] [PubMed] [Google Scholar]
- 35. Soder RB, Baldisserotto M, Da Silva VD. Computer‐assisted ultrasound analysis of liver echogenicity in obese and normal‐weight children. Am J Roentgenol. 2009;192(5):201‐205. [DOI] [PubMed] [Google Scholar]
- 36. Kook PH, Kohler N, Hartnack S, et al. Agreement of serum spec cPL with the 1,2‐o‐dilauryl‐rac‐glycero glutaric acit‐(6′methylresorufin) ester (DGGR) lipase assay and pancreatic ultrasonography in dogs with suspected pancreatitis. J Vet Intern Med. 2014;28:863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]


