Abstract
Background
Cardiac disease is an important cause of morbidity and mortality in Irish Wolfhounds (IWs), but its prevalence and clinical characteristics in North American IWs are incompletely described.
Hypothesis/Objectives
That atrial fibrillation (AF) is a diagnostic marker of echocardiographic abnormalities, and that clinical characteristics predict development of IW cardiomyopathy (IWCM). To define the prevalence of arrhythmias and echocardiographic abnormalities in North American IWs.
Animals
Six hundred and forty‐five adult IWs presented for screening examinations intended to identify familial cardiac disease.
Methods
In this retrospective cohort study, reference intervals defined based on echocardiographic data from IW classified as normal, were used to define the prevalence of structural and functional abnormalities. A logistic model was developed to identify clinical findings that predict future development of IWCM.
Results
The prevalence of AF was 8.9% (95% confidence interval [CI], 6.6‐11.2) of which 55.5% had echocardiographic abnormalities. IWCM defined by left atrial enlargement, left ventricular dilatation, and systolic dysfunction had a prevalence of 1.8% (0.72‐2.8). Positive and negative likelihood ratios for AF in the identification of IWCM were, respectively, 10.8 (7.29‐16) and 0.2 (0.06‐0.69). Multivariable logistic regression identified AF (odds ratio [OR]; 10.6, 95% CI, 2.67‐42.3) and male sex (OR; 3.8, 95% CI, 1.02‐14) as predictors of future development of IWCM.
Conclusions and Clinical Importance
Atrial fibrillation is common in North American IW. It occurs in association with structural cardiac disease but also in its absence. Irish Wolfhounds cardiomyopathy is characterized by chamber enlargement but minimally decreased ejection phase indices of myocardial function. Atrial fibrillation is a risk factor for future development of IWCM.
Keywords: atrial fibrillation, canine, cardiomyopathy, prevalence
Abbreviations
- AF
atrial fibrillation
- AFlone
AF identified in the absence of echocardiographic abnormalities
- CM
cardiomyopathy
- DCM
dilated cardiomyopathy
- %EF
ejection fraction
- ESVI
end‐systolic volume indexed
- %FS
percent fractional shortening
- IWs
Irish Wolfhounds
- IWCM
Irish Wolfhound cardiomyopathy
- LA : Ao
short‐axis left atrial dimension indexed to aortic diameter
- LAE
left atrial enlargement
- LVdil
left ventricular dilatation
- LVdys
systolic myocardial dysfunction
- LVIDd
left ventricular internal dimension at end‐diastole
- LVIDs
left ventricular internal dimension at end‐systole
- SVTA
supraventricular tachyarrhythmia
- VTA
ventricular tachyarrhythmias
1. INTRODUCTION
Cardiac disease, specifically dilated cardiomyopathy (DCM), is an important cause of morbidity and mortality in the Irish Wolfhound (IW).1, 2 Cardiomyopathy (CM) in the IW is familial and likely has a genetic basis. Although the presence of specific single‐nucleotide polymorphisms provides evidence of a genetic, probably oligogenic, basis for the disease, definitive associations of CM in IW with specific mutations have not been identified.3, 4, 5 A high prevalence of both DCM and atrial fibrillation (AF) in IW has been identified in northwestern Europe and in the United Kingdom.6, 7 In the United Kingdom, the prevalence of arrhythmias in the IW was estimated to be 22.2%; AF was the most common rhythm abnormality and was recorded in 10.5% of dogs examined.8 Based on the published data abstracted from a registry of medical records, the prevalence of AF in North American IW is 5.84%.9 Retrospective evaluation of the case records of 500 IWs presented to a veterinary hospital in Germany disclosed abnormalities in 41.8%. Dilated cardiomyopathy was diagnosed in 24.2%, of which nearly 88% had AF.7 Similarly high prevalence estimates have been more recently reported.3, 10 The development of DCM recently was evaluated in 52 IWs that had AF associated with atrial enlargement but not ventricular enlargement, and a contemporaneous control group of 52 apparently healthy IW that had sinus rhythm. Fifty percent of IW that had AF developed DCM, compared to 21% of the control group. The odds that an IW that had AF would develop DCM was 3.7 times that of IW that did not.11
The prevalence and clinical characteristics of cardiac disease in North American IW have been incompletely described. To define the prevalence and clinical characteristics of cardiac disease in apparently healthy North American IW, we retrospectively analyzed clinical data obtained from cardiac screening examinations performed at dog shows that were intended to identify familial, presumed heritable, cardiac disease. Although diagnostic confirmation of AF requires electrocardiography, the arrhythmia has characteristic features that are evident by physical examination. Furthermore, it is likely that electrocardiography has greater availability in general veterinary practice than does echocardiography. Therefore, we evaluated the diagnostic accuracy of AF for detection of echocardiographic abnormalities. From longitudinal data, we developed a logistic regression model to identify factors that might predict the future development of DCM.
2. MATERIALS AND METHODS
Between 2006 and 2014, at national and regional specialty shows in the United States and Canada, IWs were subject to screening examinations intended to identify familial cardiac disease. The Irish Wolfhound Foundation (IWF) advertised the screening clinics and assisted in recruitment of both breeding age hounds as well as retired, veteran IW. The Irish Wolfhound Club of America encourages annual screening examinations for all IWs whether or not abnormalities are identified. The IWF provided incentives by subsidizing the costs of examinations. Veteran IWs were defined as those older than 8 years of age, and those hounds were examined at no charge to the owner. The size of the North American population of IW is not known; on average, however, approximately 830 IWs were registered by the American Kennel Club (AKC) in each year of the study period (AKC End of Year Registration Statistics 2009‐2014).
The screening examination consisted of physical examination, electrocardiography, and echocardiography. Some IWs were examined multiple times, at different screening clinics, whereas others were examined only once. Only IWs that were examined more than once and that were free of IWCM—defined by the presence of left ventricular dilatation (LVdil), left ventricular systolic myocardial dysfunction and left atrial enlargement (LAE)—at the time of initial examination, provided data that were included in a logistic model intended to identify characteristics that predict future development of IWCM. For those that were examined multiple times, there was no specific schedule for follow‐up. Although owners and agents were encouraged to subject IW to repeated examination, follow‐up was at their prerogative. When distinct electrocardiographic or echocardiographic abnormalities were identified, owners and agents were encouraged to seek veterinary care. For IW in this category, medications were administered but doses and drugs were not systematically recorded.
2.1. Physical examination
Focused cardiac physical examinations were performed on all dogs and included cardiac and thoracic auscultation, femoral arterial pulse palpation and precordial palpation. Presence or absence of murmur and arrhythmia was also recorded. Heart rates of IW without arrhythmias were determined by physical examination, but neither the method—palpation of arterial pulse or auscultation—nor the time interval during which heart rate was evaluated was standardized.
2.2. Electrocardiography
For classification of cardiac rhythm, electrocardiograms were recorded whereas unsedated IWs were standing (EK 10, Burdick Inc, Milton, Wisconsin; True ECG‐3, DRE, Louisville, Kentucky). One minute was the minimum recording duration, but the duration of recording was increased if abnormalities were identified. All ECGs were evaluated by 1 of 2 board‐certified veterinary cardiologists. For IW with pathologic arrhythmia, heart rate was determined from a 6‐second epoch.
Abnormalities of cardiac rhythm were described as follows: supraventricular tachyarrhythmia (SVTA) was defined by the presence of any number of supraventricular premature complexes, couplets, or periods of tachycardia but excluded AF; AF; AF identified in the absence of the echocardiographic abnormalities described below (AFlone); ventricular tachyarrhythmia (VTA), defined by the presence of any number of ventricular premature complexes, couplets, or periods of tachycardia.
2.3. Echocardiography
Echocardiography was performed by 1 of 2 board‐certified veterinary cardiologists while unsedated IWs were standing (Vivid I, GE Healthcare, Chicago, IL, USA). Minimal restraint by the owner/handler or veterinary assistant was utilized. Standard views were obtained from both the right parasternal and left apical transducer sites12 by using a multifrequency (1.5‐4 MHz) phased‐array ultrasound probe. Electrocardiography was not simultaneously recorded. For M‐mode examination, the beam was directed by the appearance of right parasternal short‐axis images; these measurements were obtained: left ventricular internal dimension at end‐diastole (LVIDd), left ventricular internal dimension at end‐systole (LVIDs), interventricular septal thickness at end‐diastole, interventricular septal thickness at end‐systole, left ventricular posterior wall thickness at end‐diastole, left ventricular posterior wall thickness at end‐systole.13 Left ventricular fractional shortening (%FS) was calculated. Measurements were obtained from 3 consecutive cardiac cycles and averaged. Left atrial and aortic measurements were obtained from the right parasternal short axis basilar view, and the left atrial dimension was indexed to aortic diameter (LA : Ao).14 Color flow and spectral Doppler interrogation of all inflow and outflow tracts was performed, and when semiquantitative evaluation revealed greater than trace valvular regurgitation, it was considered to be abnormal. This finding excluded the subject from the subsample of echocardiographically normal IW used to define reference intervals, but valvular regurgitation was not further quantified nor systematically recorded.
IWs were considered to be normal if physical examination failed to disclose abnormalities and subjective echocardiographic evaluation revealed normal cardiac structure and function in the absence of pathologic flow disturbances. Reference intervals for echocardiographic variables were defined based on data obtained from IWs that were classified as normal during the initial examination, and this assessment was based on subjective evaluation by an experienced echocardiographer, not on a priori assumptions regarding numerical echocardiographic data. Although valvular incompetence was not quantified, its presence was noted; if valvular incompetence was identified, the subject was excluded from the reference sample.
Values outside these reference intervals were used to estimate the point prevalence of echocardiographic structural and functional abnormalities. Specifically, left ventricular chamber dilatation (LVdil) was identified when LVIDd exceeded the upper limit of the 95% reference interval, and left ventricular systolic myocardial dysfunction (LVdys) was identified when LVIDs exceeded the upper limit of the 95% reference interval. Cardiomyopathy was present when both these criteria were met in the absence of LA enlargement. Left atrial enlargement was defined by an LA : Ao that exceeded the upper limit of the reference interval. We defined “IWCM” by the findings of both LVdil and LVdys together with LAE. This classification was based only on numerical echocardiographic data and intended to demonstrate the spectrum of abnormalities, not to imply that CM and IWCM are different disorders. The diagnostic accuracy of the finding of AF for the detection of echocardiographic abnormalities was evaluated. A logistic model was developed to define the predictive value of clinical findings including the presence of AF for future development of IWCM.
2.4. Statistical analysis
Analyses were performed by using commercially available computer software (SAS, ver 9.2, Carey, NC; R Core Team (2013). R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org). Reference intervals for echocardiographic variables were derived from data obtained only from IWs that were classified as normal during the first examination. The relationship between echocardiographic variables and body size was first evaluated by linear regression. The response variable was the echocardiographic dimension, and the explanatory variable was bodyweight. In keeping with published literature, the same procedure was repeated for log10‐transformed echocardiographic variables and bodyweights.15 Based on coefficients of determination (R2), body size explained less than 10% of the variation in all linear echocardiographic variables with the exception of LVIDd, for which body size accounted for 17.7% of variation. This was the case whether regression was performed by using raw data, or data that had been subject to log10 transformation. Consistent with published literature,16, 17 the relationship between echocardiographic variables and body size was considered to be clinically inconsequential and therefore 95% reference intervals, and the 90% confidence intervals (CIs) were calculated nonparametrically by using the Horn method of outlier detection.18
Abnormal cardiac structure and function were defined by variables outside of these intervals, and the prevalence of abnormal echocardiographic findings was the proportion of abnormal echocardiograms identified during first examinations.
Categorical variables from IW with abnormal findings were compared to those without abnormal findings by chi square test or Fisher's exact test as appropriate based on expected cell counts. Continuous variables were compared by unpaired T tests or by Mann‐Whitney tests depending on the distribution of the data.
The diagnostic accuracy of the finding of AF for the identification of abnormal echocardiographic findings was evaluated through calculation of sensitivity, specificity, likelihood ratios, and predictive values.
A logistic regression model was developed to identify characteristics evident at the first examination that might predict the future development of echocardiographic abnormalities. Presence/absence of AF, presence/absence of AFlone, presence/absence of myocardial dysfunction, and sex were candidate explanatory variables. A stepwise procedure for variable selection was used. The association between candidate variables was evaluated by Fisher's exact text.
The distribution of the data or residuals was graphically evaluated to ensure that the assumptions of all statistical tests were met. Alpha was set to a value of .05. Continuous data are expressed as mean ± SD or median (range) as appropriate based on data distribution. Proportions are expressed as counts and percentages.
3. RESULTS
Between 2006 and 2014, data from 925 examinations of 645 different IWs were acquired. Of the 645 IWs, 480 were examined only once; the remainder was examined between 2 and 9 times during the study period. Echocardiographic classification of IW was based only on quantitative definitions derived from reference intervals. The number of IWs that were considered normal on this basis exceeds the size of the reference sample (n = 413) because there were IWs that had minor mitral or aortic valve regurgitation that was presumed to be hemodynamically inconsequential based on recorded chamber size. The numbers of IWs that were normal and the numbers that exhibited selected abnormalities are shown in Figure 1.
Figure 1.
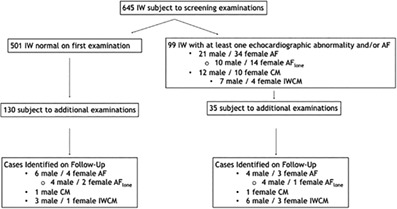
A flow chart that schematically provides the numbers of IWs subject to screening examinations intended to identify heritable cardiac disease, and the numbers of selected echocardiographic abnormalities identified during the first and during follow‐up examinations. Six hundred and forty‐five IWs initially were screened but because of missing data, 600 could be classified as normal or not. In cases identified on follow‐up, those classified as CM were ones that were not later classified as IWCM. AF, atrial fibrillation; AFlone, lone atrial fibrillation; CM, cardiomyopathy; IWs, Irish Wolfhounds; IWCM, IW cardiomyopathy
The median (range) of age at the time of the initial examination was 3.04 (1‐11.43) years, mean body weight was 64.8 ± 9.1 kg, and 253 (39.2%) IWs were male. Systolic murmurs were identified in 23 of 645 IWs. Murmur intensity ranged from grade 1/6 to grade 4/6; 2/6 was the modal value. Median heart rate of IW at first examination was 120 bpm (72‐240). Because the method for heart rate acquisition was not standardized, these data were not subject to further analysis.
Descriptive statistics and proposed reference intervals for LVIDd, LVIDs, %FS, and LA : Ao are presented in Table 1. Because of missing observations, the numbers of IW that provided data for references intervals were not the same for all variables. Of the 413 IWs (61% female) that provided data for development of reference intervals, 107 of these were subject to 1 or more additional examinations; abnormalities were not identified after initial examination of 82 of these IWs. These 82 IWs had a median of 2 (2‐6) examinations and the median time from the first to last examination was 2.7 years (0.5‐6.5).
Table 1.
Proposed echocardiographic reference intervals for North American IWs
| Echocardiographic variable | n | Median | Range | 95% Reference interval | 90% CI about lower limit | 90% CI about upper limit |
|---|---|---|---|---|---|---|
| LVIDd (cm) | 413 | 5.33 | 4.2‐6.5 | 4.44‐6.01 | 4.32‐4.58 | 5.94‐6.17 |
| LVIDs (cm) | 413 | 3.3 | 1.96‐4.31 | 2.64‐4.04 | 2.55‐2.7 | 3.98‐4.16 |
| %FS | 413 | 37 | 21‐53 | 26.8‐48.8 | 26‐28 | 47.6‐50.3 |
| LA : Ao | 399 | 1.11 | 0.76‐1.43 | 0.88‐1.36 | 0.84‐0.9 | 1.32‐1.39 |
Note: Because of missing observations, the numbers of IW that provided data for calculation of reference intervals were not the same for all variables.
Abbreviations: CI, confidence interval; %FS, percent fractional shortening; IW, Irish Wolfhound; LA : Ao, short‐axis left atrial dimension indexed to aortic diameter; LVIDd, left ventricular internal dimension at end‐diastole; LVIDs, left ventricular internal dimension at end‐systole.
The point prevalence of each of the defined rhythm abnormalities was in the range of 1%‐8.9%. Atrial fibrillation was the most prevalent of the arrhythmias; 44.5% of hounds with AF had AFlone. Statistically significant sex predispositions for abnormal cardiac rhythm were not identified (all P > .05). Except for the case of VTA (P = .46), hounds affected by arrhythmia were significantly older than unaffected hounds (all P < .05). Prevalence, associated 95% CIs, as well as descriptions of the age and sex of affected and unaffected IWs are presented in Table 2.
Table 2.
Estimated point prevalence of abnormalities of cardiac rhythm, structure, and function in North American IWs
| Prevalence (%) | 95% CI | Sex (%M) | P value | Median age (affected/unaffected) | P value | |
|---|---|---|---|---|---|---|
| AF | 55/618 (8.9) | 6.6‐11.2 | 38.1 | 0.88 | 5.58/2.92 | 0.0001 |
| AFlone | 24/600 (4) | 2.4‐5.6 | 41.7 | 0.81 | 5.42/3 | 0.0002 |
| SVTA | 6/618 (1) | 0.2‐1.7 | 50 | 0.68 | 6.23/3.01 | 0.04 |
| VTA | 16/618 (2.6) | 1.3‐3.8 | 37.5 | 0.89 | 3.67/3.01 | 0.46 |
| LVdil | 38/645 (5.9) | 4.1‐7.7 | 52.6 | 0.08 | 5.74/3 | 0.0001 |
| LVdys | 39/645 (6) | 4.2‐7.9 | 51.2 | 0.12 | 5.95/2.99 | 0.0001 |
| LAE | 44/627 (7) | 5‐9 | 43.2 | 0.6 | 5.2/2.99 | 0.0001 |
| CM | 11/627 (1.8) | 0.72‐2.8 | 45.5 | 0.76 | 5.13/3.02 | 0.03 |
| IWCM | 11/627 (1.8) | 0.72‐2.8 | 63.6 | 0.12 | 5.95/3.01 | 0.0003 |
Note: Because of missing observations, the numbers of IW that provided data for estimation of prevalence were not the same for all abnormalities.
Abbreviations: AF, atrial fibrillation; AFlone, AF identified in the absence of echocardiographic abnormalities; CI, confidence interval; CM, cardiomyopathy; IW, Irish Wolfhound; IWCM, Irish Wolfhound cardiomyopathy; LAE, left atrial enlargement; LVdil, left ventricular dilatation; LVdys, systolic myocardial dysfunction; SVTA, supraventricular tachyarrhythmia exclusive of AF; VTA, ventricular tachycarrhythmias.
Prevalence estimates of LVdil, LVdys, and LAE were close to 6%. The prevalence of CM was 1.7% as was the prevalence of IWCM. Of hounds with LAE, 64% had AF and of those with IWCM, 81.8% had AF. Left atrial enlargement was identified in 51% of IW with AF. Statistically significant sex predispositions for echocardiographic abnormalities were not detected (all P > .05), but hounds affected by echocardiographic abnormalities were older than those that were not (all P < .0003). Prevalence, CIs, and associations between echocardiographic abnormalities and age and sex are presented in Table 2. Because of missing observations, the numbers of IW that provided data for estimation of prevalence were not the same for all abnormalities. Prevalence of AF and IWCM in arbitrarily defined age categories is shown by Figure 2.
Figure 2.
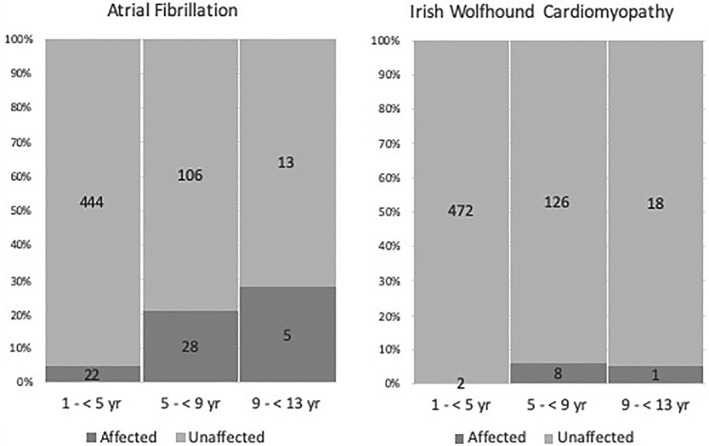
Age‐dependent prevalence of atrial fibrillation (left) and Irish Wolfhound cardiomyopathy (right). The x‐axis consists of ordinal categories of age and the y‐axis represents prevalence expressed as percent. Numerical values are the absolute counts of affected and unaffected Irish Wolfhounds in each age category
AF was relatively prevalent in this cohort generally and observed in the majority of hounds with LAE but was also observed in the absence of echocardiographic abnormalities. Therefore, statistical indices that describe diagnostic accuracy of the finding of AF for the detection of LAE, CM, and IWCM varied widely (Table 3). In general, there was modest sensitivity, high specificity and high negative predictive values of AF for the identification of echocardiographic abnormalities. The values obtained for likelihood ratios reflect moderate or marked effects on pretest probability of disease.19
Table 3.
Statistical indices of diagnostic accuracy of the finding of atrial fibrillation for the detection of echocardiographic abnormalities in North American IWs
| Sensitivity (95% CI) | Specificity (95% CI) | +Likelihood ratio | –Likelihood ratio | +Predictive valuea | –Predictive valuea | |
|---|---|---|---|---|---|---|
| LAE | 0.64 (0.48‐0.78) | 0.95 (0.93‐0.97) | 13.1 (8.52‐20.15) | 0.38 (0.26‐0.57) | 0.51 (0.37‐0.65) | 0.97 (0.95‐0.98) |
| CM | 0.5 (0.28‐0.71 | 0.93 (0.9‐0.95) | 6.77 (4.09‐11.23) | 0.54 (0.36‐0.82) | 0.2 (0.1‐0.33) | 0.98 (0.97‐0.99) |
| IWCM | 0.82 (0.52‐0.95) | 0.92 (0.9‐0.94) | 10.8 (7.29‐16) | 0.2 (0.06‐0.69) | 0.16 (0.09‐0.28) | 0.996 (0.987‐0.999) |
Abbreviations: CI, confidence interval; CM, cardiomyopathy; IW, Irish Wolfhound; IWCM, Irish Wolfhound cardiomyopathy; LAE, left atrial enlargement.
These predictive values are relevant only to the sample of IWs described in this report.
During the course of the study, a total of 24 different hounds met criteria that defined IWCM; 11 of these were identified during the initial examination, 13 additional cases were identified after the initial examination. Twenty‐one (87.5%) of these IWs had AF. Descriptive statistics for the echocardiographic variables obtained from these IWs are reported in Table 4.
Table 4.
Echocardiographic variables of North American IWs with IWCM
| Age (yr) | LVIDd (cm) | LVIDs (cm) | %FS | LA : Ao |
|---|---|---|---|---|
| 7.14 (3.17‐11.51) | 6.92 (6.03‐8.3) | 4.67 (4.12‐6.53) | 31 (16‐45) | 1.9 (1.4‐2.7) |
Note: Age refers to age of Irish Wolfhound when IWCM was first identified. Values are expressed as median (range).
Abbreviations: %FS, percent fractional shortening; IWCM, Irish Wolfhound cardiomyopathy; LA : Ao, short‐axis left atrial dimension indexed to aortic diameter.
Of 26 IWs in which AF was identified in the absence of IWCM at any examination, and were subject to follow‐up, 7 developed IWCM and the median time to identification of IWCM was 1.04 years (0.56‐6.5). Of the 19 that did not develop IWCM, the duration between first and last examinations was 2.01 (0.52‐5.32) years. Four of 15 IWs with lone AF later developed CM or IWCM; all these 4 IWs developed IWCM. The 4 that developed IWCM were followed for 1.51 (0.99‐8.02) years, and the 11 that did not develop CM or IWCM were followed for 2.01 (0.52‐5.01) years.
There were 12 IWs in which CM was identified and were subject to serial examinations; 4 later developed IWCM. These 4 IWs were followed for a median (range) of 3.17 (1.00‐5.01) years. The 8 that were not known to develop IWCM were followed for 1.22 (0.56‐3.34) years.
A logistic model was developed to identify characteristics evident at first examination that predicted later development of IWCM. Candidate variables were: AF, AFlone, LVdys, and sex. Other than for AF and AFlone, statistically significant associations between candidate variables were not detected; AF and AFlone were associated by design as AFlone is a subset of AF. Stepwise multivariable regression identified AF and sex as significant independent predictors of future development of IWCM; the odds ratio (OR) for AF was 10.6 (95% CI, 2.7‐42.3.1) and the OR for male sex was 3.8 (95% CI, 1.02‐14).
4. DISCUSSION
We retrospectively evaluated electrocardiographic and echocardiographic records of IWs that were presented for screening examinations intended to identify heritable cardiac disease. From normative data, we developed reference intervals and estimated the prevalence of cardiac disease in a large, relatively unselected population of apparently healthy IW. Cardiac disease is an important cause of morbidity and mortality in IW1 and the study reported here is 1 of the most extensive surveys of cardiac rhythm and echocardiographic characteristics of IW to date.3
Others, in the United Kingdom and in continental Europe, have proposed echocardiographic reference intervals for the IW breed, and those intervals are very similar to those reported here. Specifically, 3 different investigations have yielded upper limits of reference intervals for LVIDd that were between 5.89 and 6.1 cm,6, 16, 20 whereas we defined an upper limit of 6.01 cm. We propose that the upper limit for the reference interval for the LVIDs of North American IWs is 4.04 cm. Previously published upper limits for this variable are between 4.08 and 4.5 cm.6, 16, 20 These normative data are most closely matched with the published report that included the largest number of subjects.16 More recently, age‐dependent cardiac remodeling in IW was investigated.21 In male, but not female, IW serial examination revealed statistically significant, age‐dependent increases in left ventricular dimensions. Reference intervals were not presented, but based on the data presented, the upper limits for LVIDd and LVIDs in males/females were respectively 60.8/57.1 and 42.1/40.6 mm. These intervals, which did not take into account sex, were similar, differing by 3 mm or less. In that study, the lower limit of the reference interval derived from data obtained from the oldest IW, was 22.8% which is somewhat lower that limit for these data which was 26.8%.The minor differences between these findings and those of others might relate to interoperator variation, differences in the methods of calculation and perhaps to differences between populations.
We identified AF in 8.9% of apparently healthy IW presented for screening examinations at North American dog shows. This figure is similar, but somewhat higher than the prevalence of 5.84% estimated from registry data.9 In 44.5% of these IWs, AF was detected in the absence of echocardiographic abnormalities. The prevalence of other arrhythmias—ventricular arrhythmias, supraventricular arrhythmias excluding AF—was less than 3%. Sex predispositions for arrhythmias were not identified, but IWs with SVTA or AF were older than those without those findings. It seems likely, based on this, that the incidence of these arrhythmias is age dependent, although that cannot be definitively stated given that the prevalence estimates were obtained from cross‐sectional data. In the investigation that is most similar to this study, the prevalence of AF was 11.6%.8 Of IW affected by CM in another report, the proportion with AF was 83.3%.7 Amongst IW that we classified as IWCM, 87.5% had AF. To our knowledge, the prevalence of AFlone in apparently healthy IW has not previously been reported.
We defined the presence of chamber enlargement and myocardial dysfunction based on echocardiographic criteria. The prevalence of LVdil, LVdys, or LAE was close to 6%, whereas the prevalence of CM—ventricular dilatation together with myocardial dysfunction—and IWCM—CM associated with LAE—were both 1.8%. Considerably higher prevalence estimates of echocardiographic abnormalities have been reported, but those estimates were from a hospital population.7 More recently, data obtained from IW recruited mainly through cardiovascular screening clinics in Belgium, Germany, and the Netherlands were reported. Of 1018 IW that had cardiovascular examinations performed, DCM was diagnosed in 25.5%; the prevalence of DCM in male IW was 33.5% and in females, 19.4%.3 In this study, AF was identified in echocardiographically normal IW, 64% of those with LAE had AF, and 51% of IW with AF had LAE. The relatively high prevalence of AF in IW generally and the still higher prevalence in IW with LAE is perhaps unsurprising and is consistent with both laboratory findings and clinical observation. In vitro, a critical mass of myocardium is required to sustain the arrhythmia of fibrillation, and the results of in silico experiments suggest a “fibrillation number,” analogous to the Reynolds number of hydraulics, which relates myocardial mass, action potential, and conduction velocity and defines the tendency to sustain fibrillation.22, 23
We chose to define systolic myocardial dysfunction in terms of LVIDs, rather than through calculation of ejection phase indices of ventricular performance such as fractional shortening (%FS) or ejection fraction (%EF). Both of %EF and %SF are dimensionless indices that represent a surrogate measure of stroke volume expressed as proportion of ventricular size. As such, they are highly dependent on loading conditions.24 Furthermore, because they are ratios, they are subject to propagation of error.25 Although end‐systolic indices of myocardial function are not independent of load, they are subject to fewer influences than are ejection phase indices. Left ventricular internal dimension at end‐systole depends on afterload, contractility, and body size but, in contrast to ejection phase indices, is largely independent of preload.24 The estimated end‐systolic volume indexed (ESVI) to body surface area has been applied to the diagnosis of cardiac dysfunction in IW and might have diagnostic utility.26 However, as reported in literature that relates to IW, end‐systolic volume ESVI was estimated from linear, M‐mode derived left ventricular dimensions, not based on areas or measured volumes.26 Furthermore, a volume estimate indexed to body surface area is dimensionally inconsistent; a linear relationship between volume and surface area is unlikely and inconsistent with allometric principles.15
The echocardiographic characteristics of cases we classified as IWCM differ somewhat from those described for other familial cardiomyopathies in dogs. All had echocardiographic evidence of chamber enlargement and systolic myocardial dysfunction but %FS of most affected IW was within the reference interval. The entity that we described as IWCM is characterized by left atrial and left ventricular enlargement, AF in most and normal or mildly lethargic ejection phase indices of myocardial function. Although the latter finding is possibly surprising, it is consistent with the observations of others. In a publication that addressed the use of echocardiography in the diagnosis of DCM in IW, the mean (SD) %FS of 33 IWs reported to have “occult DCM” was 25.6 (4.5); assuming the data were normally distributed, 50% of the affected IW had a %FS that exceeded the upper limit of the reference interval from the same investigation.26 In a smaller study, normative data are presented which suggest a lower limit of 18.8 for the reference interval for %FS. In the subsample of IW that developed congestive heart failure, the mean %FS was 30.6; none of the dogs had %FS that was subnormal based on the reference interval from the same publication.6 The echocardiographic appearance of myocardial disease described here, and by others, might be a unique characteristic of IWCM. Certainly, it differs from the echocardiographic abnormalities that are typical of the familial CM observed in Doberman Pinschers. In dogs of that breed, marked decreases in systolic ventricular performance are the rule; in 1 investigation of clinically occult DCM, the mean %FS was approximately 14%,27 and in dogs with congestive heart failure, %FS is often in the single digits.28 Comparison of this description of IWCM to that of other familial cardiomyopathies in dogs is hampered by the paucity of publications, and the fact that the range of %FS in some studies has been constrained by use of low %FS, typically less than 25, as an inclusion criterion. These data do not explain the finding of minimally decreased ejection phase indices in IWCM. Possibly, IWCM alters ventricular geometry in a way that results in the development of substantive secondary mitral regurgitation, which might serve to preserve %FS. And indeed, these data do not exclude the possibility that mitral regurgitation related to primary valvular disease contributes to the pathogenesis of the entity that we defined as iwCM. Additionally, AF is prevalent in IW and possibly the prodrome of IWCM suggesting it might have a pathogenetic role. Atrial fibrillation not only leads to LAE,29 but there has been recent recognition of the phenomenon of “atrial functional mitral regurgitation”, mitral regurgitation that results from AF.30
We evaluated the diagnostic accuracy of the electrocardiographic finding of AF for the identification of echocardiographic abnormalities. In general, AF is a diagnostically specific marker of echocardiographic chamber enlargement and dysfunction. However, the diagnostic accuracy of AF is modest because sensitivity varied but was less than 83% for the echocardiographic abnormalities that we considered. In the context of this study, sensitivity and specificity reflect the proportions of the study population that do, or do not have confirmed echocardiographic abnormalities. From the perspective of the clinician, however, it is likelihood ratios and predictive values that are more useful because they provide information regarding the probability of a disease presence, given a test result. And in fact, positive likelihood ratios for AF in the detection of echocardiographic were in the range that reflects a moderate or large increase in the “post‐test probability of disease”.19 The post‐test probability of disease takes into account the likelihood ratio and disease prevalence. For these data, the prevalence of IWCM was 1.8% and a positive likelihood ratio of 10.8 suggests that the probability that a randomly selected individual with AF has IWCM is approximately 20%.19 The negative likelihood ratio of 0.2 provides a post‐test probability of approximately 0.2%; meaning, that in the absence of AF there is 0.2% probability that the dog has IWCM. These estimates are consistent with the generally low positive predictive values, and highnegative predictive values. The negative predictive value of AF in the detection of IWCM was 0.996, meaning that there is a .04% probability that an IW that does not have AF has IWCM. Predictive values depend on disease prevalence and those calculated here are only relevant to the screening population which presumably, has a lower prevalence of CM than does the hospital population.
The implications of a diagnosis of subclinical AF in IW have been subject to conjecture. It has been observed that subclinical AF is a common antecedent to the development of congestive heart failure in IW6 and an investigation of the efficacy of drug treatment in IW that had preclinical DCM, AF, or both was recently published.17 The study design of the latter was presumably predicated on the assumption that AF is a precursor to the development of DCM. Accordingly, we developed a logistic regression model to identify predictors of the development of IWCM. By multivariable analysis, sex, and presence of AF were statistically significant predictors of later development of IWCM; the odds that a male IW will develop IWCM is 3.8, but based on these data, the odds that an IW with AF will develop IWCM is 10.6 times greater than an IW that does not have AF. The latter finding is evidence that AF represents an important independent risk for the development of IWCM. These odds are similar to, but somewhat greater than those recently reported from Europe in which the odds that an IW with AF were to develop CM were 3.7 (95% CI, 1.6‐8.88).10 From these data, it is not possible to determine whether this relationship results from the deleterious effect of chronic AF, or if AF is commonly the first manifestation of a diffuse myocardial disease. We determined that the median time from recognition of AF to identification of IWCM was 1.04 years with a range of 0.56‐6.5. This duration might be somewhat greater than the result of a study in which all IWs with AF developed CM within 3 years.5 However, these data must be considered in the context small sample size—only 7 of IWs with AF subject to follow‐up developed IWCM—and, the fact that follow‐up examinations were at the prerogative of IW owners and not conducted according to a protocol.
The results of this study must be considered in the context of its limitations. The data were evaluated retrospectively and inevitably there were missing observations. The examinations were conducted in the suboptimal circumstances of dog shows, were necessarily brief, and histories, including administration of medications and prior illnesses or non‐cardiac comorbidities, were inconsistently available or recorded. Initial examinations of the IW included in this study occurred over the course of 8 years and it is possible that the epidemiological characteristics of heart disease in IW changed during that period. Irish Wolfhounds that are presented to screening clinics represent a relatively unselected population of “asymptomatic” animals, but the study sample is not free of bias. Many IW presented to these screening examinations were sexually intact animals of breeding age. Furthermore, identification of abnormalities including AF might lead to withdrawal from this population, or conversely, to a tendency for greater scrutiny so it is possible that the importance of AF, or other potential risk factors, has been biased by the voluntary nature of follow‐up. The results of this study can only be used to make inferences regarding the North American population of apparently healthy IW, which might also be strength. However, we did not evaluate pedigrees, or consider importations of IW from other geographical areas. Interoperator variability might have contributed to measurement variation as 2 investigators collected echocardiographic data. Furthermore, assessment of left atrial size was only through evaluation of the short‐axis left atrial dimension indexed to aortic diameter. This method is less repeatable than use of a single end‐systolic atrial dimension as was used in a recent investigation of AF in IW, and it is likely insensitive relative to other methods such volume estimation.11, 31, 32 Recently published longitudinal data provide evidence of age‐ and sex‐dependent cardiac remodeling, but there was no practical way to account for this in this data set.21 The use of the term AFlone has been questioned, but this is largely because of improved understanding of the noncardiac conditions which predispose to AF in people.33 Here, it was simply used to refer to AF in the absence of echocardiographic abnormalities, but we concede that affected IW might have had undetected, non‐cardiac disorders that played a role in the pathogenesis of AF. It was not practical to quantify the magnitude of valvular regurgitation by using approaches such as a mitral regurgitation severity score or proximal isovelocity surface area which might have provided insights into the pathophysiology of IWCM. Although the authors acknowledge these limitations, they do not undermine the important results of this study.
We have reported the results of a large survey of cardiac rhythm and echocardiographic characteristics of North American IW. Atrial fibrillation has prevalence near 10%. This arrhythmia is common in IWs that have echocardiographic abnormalities but also observed in its absence. Irish Wolfhounds cardiomyopathy characterized by left atrial and left ventricular enlargement associated with systolic myocardial dysfunction, but minimally lethargic ejection phase indices of ventricular function might be a unique, breed‐specific disease entity. Most IWs that fit this description have AF. Based on longitudinal data, AF is risk‐factor for the development of IWCM.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed because the investigation consisted of retrospective evaluation of clinical data.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of Dr. Stephen Werre who provided statistical consultation, the Irish Wolfhound Foundation, Irish Wolfhound Club of America, Irish Wolfhound Club of Canada, Irish Wolfhound Association—Delaware Valley, Potomac Valley Irish Wolfhound Club, and all their members and hounds. These data were presented, in part, at the 2015 ACVIM Forum, Indianapolis, Indiana.
Tyrrell WD Jr., Abbott JA, Rosenthal SL, Dentino M, Abrams F. Echocardiographic and electrocardiographic evaluation of North American Irish Wolfhounds. J Vet Intern Med. 2020;34:581–590. 10.1111/jvim.15709
William D. Tyrrell, Jr. and Jonathan A. Abbott contributed equally to this study.
Funding information Irish Wolfhound Foundation
REFERENCES
- 1. Egenvall A, Bonnett BN, Hedhammar A, Olson P. Mortality in over 350,000 insured Swedish dogs from 1995 to 2000: II. Breed‐specific age and survival patterns and relative risk for causes of death. Acta Vet Scand 2005;46(3):121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleming JM, Creevy KE, Promislow DEL. Mortality in North American dogs from 1984 to 2004: an investigation into age‐, size‐, and breed‐related causes of death. J Vet Intern Med 2011;25 10.1111/j.1939-1676.2011.0695.x [DOI] [PubMed] [Google Scholar]
- 3. Distl O, Vollmar AC, Broschk C, Hamann H, Fox PR. Complex segregation analysis of dilated cardiomyopathy (DCM) in Irish Wolfhounds. Heredity. 2007;99 10.1038/sj.hdy.6801024 [DOI] [PubMed] [Google Scholar]
- 4. Philipp U, Vollmar A, Häggström J, Thomas A, Distl O. Multiple loci are associated with dilated cardiomyopathy in irish wolfhounds. PLoS One 2012;7(6):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simpson S, Dunning MD, Brownlie S, Patel J, Godden M, Cobb M, et al Multiple genetic associations with irish wolfhound dilated cardiomyopathy. Biomed Res Int 2016;2016 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brownlie SE, Cobb MA. Observations on the development of congestive heart failure in Irish wolfhounds with dilated cardiomyopathy. J Small Anim Pract 1999;40(8):371–377. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10476524 [DOI] [PubMed] [Google Scholar]
- 7. Vollmar AC. The prevalence of cardiomyopathy in the Irish wolfhound: a clinical study of 500 dogs. J Am Anim Hosp Assoc 2000;36(2):125–132. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10730622 [DOI] [PubMed] [Google Scholar]
- 8. Brownlie SE. An electrocardiographic survey of cardiac rhythm in Irish wolfhounds. Vet Rec 1991;129(21):470–471. http://www.ncbi.nlm.nih.gov/pubmed/1763469 [DOI] [PubMed] [Google Scholar]
- 9. Westling J, Westling W, Pyle RL. Epidemiology of atrial fibrillation in the dog. Int J Appl Res Vet Med 2008;6(3):151–154. [Google Scholar]
- 10. Vollmar C, Vollmar A, Keene BW, Fox PR, Reese S, Kohn B. Dilated cardiomyopathy in 151 Irish Wolfhounds: characteristic clinical findings, life expectancy and causes of death. Vet J. 2019;245:15–21. Available from: 10.1016/j.tvjl.2018.12.018 [DOI] [PubMed] [Google Scholar]
- 11. Vollmar C, Vollmar A, Keene B, Fox PR, Reese S, Kohn B. Irish wolfhounds with subclinical atrial fibrillation: progression of disease and causes of death. J Vet Cardiol 2019;24:48–57. 10.1016/j.jvc.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 12. Thomas WP, Gaber CE, Jacobs GJ, Kaplan PM, Lombard CW, Moise NS, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7(4):247–252. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8246215 [DOI] [PubMed] [Google Scholar]
- 13. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58(6):1072–1083. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=709763 [DOI] [PubMed] [Google Scholar]
- 14. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14(4):429–435. [DOI] [PubMed] [Google Scholar]
- 15. Cornell CC, Kittleson MD, della Torre P, Häggström J, Lombard CW, Pedersen HD, et al Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18(3):311–321. [DOI] [PubMed] [Google Scholar]
- 16. Vollmar AC. Echocardiographic measurements in the Irish wolfhound: reference values for the breed. J Am Anim Hosp Assoc 1999;35(4):271–277. 10.5326/15473317-35-4-271 [DOI] [PubMed] [Google Scholar]
- 17. Vollmar AC, Fox PR. Long‐term outcome of Irish Wolfhound dogs with preclinical cardiomyopathy, atrial fibrillation, or both treated with pimobendan, benazepril hydrochloride, or methyldigoxin monotherapy. J Vet Intern Med 2016;30(2):553–559. 10.1111/jvim.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horn PS, Feng L, Li Y, Pesce AJ. Effect of outliers and nonhealthy individuals on reference interval estimation. Clin Chem 2001;47(12):2137–2145. http://www.clinchem.org/content/47/12/2137.abstract [PubMed] [Google Scholar]
- 19. Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet 365(9469):1500–1505. http://www.sciencedirect.com/science/article/B6T1B-4G10642-14/2/ec02b1263de22e3da10241df8a19c9ed [DOI] [PubMed] [Google Scholar]
- 20. Koch J, Pedersen HD, Jensen AL, Flagstad A. M‐mode echocardiographic diagnosis of dilated cardiomyopathy in giant breed dogs. Zentralbl Veterinarmed A 1996;43(5):297–304. [DOI] [PubMed] [Google Scholar]
- 21. Brungs A, Vollmar A, Reese S, Poulsen Nautrup C. Echocardiographic indices of age‐ and gender‐dependent cardiac remodeling over the adult lifespan in Irish Wolfhounds. J Vet Cardiol 2018;20(5):307–318. 10.1016/j.jvc.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 22. Garrey WE. The nature of fibrillatory contraction of the heart—its relation to tissue mass and form. Am J Physiol 1914;33(3):397–414. [Google Scholar]
- 23. Hwang M, Park J, Lee YS, Park JH, Choi SH, Shim EB, et al Fibrillation number based on wavelength and critical mass in patients who underwent radiofrequency catheter ablation for atrial fibrillation. IEEE Trans Biomed Eng 2015;62(2):673–679. [DOI] [PubMed] [Google Scholar]
- 24. Carabello BA, Spann JF. The uses and limitations of end‐systolic indexes of left ventricular function. Circulation 1984;69(5):1058–1064. [DOI] [PubMed] [Google Scholar]
- 25. Holmes DT, Buhr KA. Error propagation in calculated ratios. Clin Biochem 2007;40(9–10):728–734. [DOI] [PubMed] [Google Scholar]
- 26. Vollmar AC. Use of echocardiography in the diagnosis of dilated cardiomyopathy in Irish wolfhounds. J Am Anim Hosp Assoc 1999;35(4):279–283. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10416770 [DOI] [PubMed] [Google Scholar]
- 27. O'Grady MR, O'Sullivan ML, Minors SL, Horne R. Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers. J Vet Intern Med 2009;23(5):977–983. http://www.ncbi.nlm.nih.gov/pubmed/19572914 [DOI] [PubMed] [Google Scholar]
- 28. O'Grady MR, Minors SL, O'Sullivan ML, Horne R. Effect of pimobendan on case fatality rate in Doberman Pinschers with congestive heart failure caused by dilated cardiomyopathy. J Vet Intern Med 2008;22(4):897–904. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18537880 [DOI] [PubMed] [Google Scholar]
- 29. Zatuchni J. Atrial enlargement as a consequence of atrial fibrillation. Circulation 1991;83(4):1458 http://www.ncbi.nlm.nih.gov/pubmed/1826477 [PubMed] [Google Scholar]
- 30. Silbiger JJ. Mechanistic insights into atrial functional mitral regurgitation: far more complicated than just left atrial remodeling. Echocardiography 2019;36(1):164–169. [DOI] [PubMed] [Google Scholar]
- 31. Visser LC, Ciccozzi MM, Sintov DJ, Sharpe AN. Echocardiographic quantitation of left heart size and function in 122 healthy dogs: a prospective study proposing reference intervals and assessing repeatability. J Vet Intern Med 2019:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wesselowski S, Borgarelli M, Bello NM, Abbott J. Discrepancies in Identification of left atrial enlargement using left atrial volume versus left atrial‐to‐aortic root ratio in dogs. J Vet Intern Med 2014;28(5):1527–1533. 10.1111/jvim.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wachtell K. Atrial fibrillation, maybe it is not so lone? J Am Coll Cardiol 2009;53(1):30–31. http://www.sciencedirect.com/science/article/B6T18-4V8B39S-7/2/07ae2c38f0a3f2572b52b5f25296cfd4 [DOI] [PubMed] [Google Scholar]


