Abstract
Background
In dogs with protein‐losing enteropathy (PLE), data on the clinical characteristics of food‐responsive PLE (FR‐PLE) remain scarce.
Objective
To determine the clinical characteristics of FR‐PLE in dogs responsive to ultralow‐fat diet (ULFD) management.
Animals
Thirty‐three dogs diagnosed with PLE based on standard diagnostic criteria.
Methods
Retrospective review of medical records. Clinical findings were compared between dogs with FR‐PLE (FR‐PLE group) and those with immunosuppressant‐responsive PLE (IR‐PLE) or nonresponsive PLE (NR‐PLE) (IR/NR‐PLE group). The area under the curve (AUC) of a receiver operating characteristic curve was used to evaluate the ability of factors to differentiate the FR‐PLE and IR/NR‐PLE groups. Survival time was compared between the FR‐PLE and IR/NR‐PLE groups.
Results
Twenty‐three dogs responded to ULFD management and were diagnosed with FR‐PLE. The canine chronic enteropathy clinical activity index (CCECAI) was significantly lower in the FR‐PLE group than in the IR/NR‐PLE group (P < .001). The AUC of CCECAI for differentiating the FR‐PLE group was 0.935 (95% confidence interval [CI], 0.845‐1.000) with an optimal cutoff value of 8 (sensitivity, 0.826; specificity, 0.889). Survival times were significantly longer in the FR‐PLE group (median, not reached) than in the IR/NR‐PLE group (median, 432 days; P < .001).
Conclusions and Clinical Importance
Dogs that respond to ULFD management and are diagnosed with FR‐PLE are expected to have a favorable prognosis. Clinical scores, specifically the CCECAI, could be useful for differentiating FR‐PLE from IR‐PLE or NR‐PLE.
Keywords: canine, CCECAI, chronic enteropathy, CIBDAI, clinical disease, immunosuppressant, intestinal lymphangiectasia, ultralow‐fat diet
Abbreviations
- ALB
plasma albumin concentration
- AUC
area under the curve
- BUN
blood urea nitrogen
- CCECAI
canine chronic enteropathy clinical activity index
- CI
confidence interval
- CIBDAI
canine inflammatory bowel disease activity index
- CRE
creatinine
- CRP
C‐reactive protein
- FR‐PLE
food‐responsive PLE
- GLB
globulin
- IL
intestinal lymphangiectasia
- IR‐PLE
immunosuppressant‐responsive PLE
- NR‐PLE
nonresponsive PLE
- PARR
polymerase chain reaction for antigen receptor gene rearrangement
- PCR
polymerase chain reaction
- PLE
protein‐losing enteropathy
- ROC
receiver operating characteristic
- ULFD
ultralow‐fat diet
- WSAVA
World Small Animal Veterinary Association
1. INTRODUCTION
Protein‐losing enteropathy (PLE) is a syndrome characterized by excessive loss of albumin from the gut mucosa.1 Common diseases related to PLE in dogs include chronic enteritis, infectious diseases, intestinal lymphoma and intestinal lymphangiectasia (IL), and the clinical presentations of these diseases are similar.1 Although histopathological evaluation of the gastrointestinal tract by endoscopic or full‐thickness biopsy generally is recommended to determine the cause of PLE and select appropriate treatments, therapeutic trials including dietary treatment are applied in some cases.2, 3 These cases may include dogs with severe hypoalbuminemia and expected anesthetic risk.
There is limited information on the effect of diet on dogs with PLE because these dogs typically show severe clinical signs and are expected to have a guarded prognosis with rapid progression.4, 5 An exception is management using a fat‐restricted diet for dogs with primary IL.3, 4, 6, 7 A fat‐restricted diet, especially homemade ultralow‐fat diet (ULFD), has been recommended for dogs with primary IL for many years, and it has been believed to decrease intestinal lymphatic pressure by decreasing fat absorption from intestinal mucosa.6 The fat content of an ULFD made from chicken breast and white potato is 0.35 g/100 kcal,7 whereas that of a conventional low‐fat dry diet is approximately 2 g/100 kcal.8 A study showed that dogs with IL refractory to prednisolone or dependent on high‐dose prednisolone exhibited improvements in total protein and albumin concentrations and clinical signs after an ULFD were introduced.7 Another study indicated that an ULFD (fat content, 0.31 g/100 kcal) or a low‐fat diet (fat content, 1.86‐2.56 g/100 kcal) as the sole treatment is a potential treatment strategy in Yorkshire Terriers with suspected PLE.3 Although a low‐fat diet alone has been shown to be effective for some dogs with PLE,3 the effect of ULFD generally is considered to be better than that of a low‐fat diet for dogs with IL because of its strict fat restriction and high palatability.6, 7 These data indicated that dietary management, specifically an ULFD, is a preferable treatment choice for dogs with PLE.4
Recently, dogs with PLE that respond to dietary interventions have been classified as having food‐responsive PLE (FR‐PLE).4 Differentiating FR‐PLE from other types of PLE, such as immunosuppressant‐responsive PLE (IR‐PLE) or nonresponsive PLE (NR‐PLE),4 may be clinically important, especially in terms of initiating dietary management. Dietary management has several advantages, including elimination of unnecessary use of glucocorticoids or other immunosuppressants, which are associated with various adverse effects. Nevertheless, the decision to carry out dietary intervention for PLE is difficult because of the scarcity of information available on the clinical characteristics of FR‐PLE in dogs and the unpredictable response of PLE patients to dietary management. Therefore, the purpose of our study was to clarify the clinical characteristics of dogs with FR‐PLE in comparison with those of dogs with IR‐PLE or NR‐PLE. The hypothesis was that dogs with less severe clinical signs would be responsive to an ULFD and that, among dogs with PLE, prognoses would be better in those with FR‐PLE than in those with IR‐PLE or NR‐PLE.
2. MATERIALS AND METHODS
2.1. Study design
A retrospective review of the medical records of dogs with PLE was conducted between June 2013 and July 2018 in a veterinary teaching hospital.
2.2. Cases
The medical records of all dogs that underwent upper gastrointestinal endoscopy with or without lower gastrointestinal endoscopy in our hospital were reviewed. The inclusion criterion for our study was the diagnosis of PLE. The criteria for the diagnosis of PLE were hypoalbuminemia (<2.6 g/dL) with no evidence of other causes of hypoalbuminemia based on physical examination, CBC, serum biochemistry, fecal examination, urinalysis, radiography, and abdominal ultrasonography. Dogs with concurrent disorders were excluded from the study. Dogs lost to follow‐up within 2 weeks of admission also were excluded from the study. Dogs diagnosed with intestinal neoplasia, such as lymphoma based on histopathology, also were excluded. Informed written consent from all dog owners was obtained for data collection and usage in the study.
The definitive diagnoses of the dogs with PLE were determined based on the treatment response, and the identified dogs were divided into 2 groups: dogs with FR‐PLE (FR‐PLE group) and dogs with IR‐PLE or NR‐PLE (IR/NR‐PLE group). Dogs that responded to ULFD as an initial treatment were placed in the FR‐PLE group. The response to ULFD was evaluated based on clinical signs according to previously established scoring systems, which include the canine inflammatory bowel disease activity index (CIBDAI)9 and the canine chronic enteropathy clinical activity index (CCECAI),10 and plasma albumin concentration (ALB). The CIBDAI and CCECAI scores were retrospectively calculated based on individual criteria extracted from the medical records. If dogs showed improvement in the CIBDAI (≤3) or CCECAI (≤3) score after the ULFD intervention, they were considered to have FR‐PLE. For dogs with CIBDAI (≤3) and CCECAI (≤3) score on the initial visit, improvements in the ALB score based on the CCECAI scoring system10 were considered to be responsive (FR‐PLE). Dogs with FR‐PLE were further divided into complete responders and partial responders based on the ALB or a requirement for additional prednisolone during the clinical course. The complete responders were defined as those that achieved normal ALB (≥2.6 g/dL) and did not require additional prednisolone, whereas partial responders were defined as those with partial improvement in ALB (not reaching 2.6 g/dL) or requirement for additional prednisolone during the clinical course. Other dogs were classified as IR‐PLE or NR‐PLE according to their response to immunosuppressant drugs.
The ULFD was formulated based on a previous study.7 The formula included 1 part chicken breast without skin and 2 parts white potato without skin or rice (all of the ingredients were boiled). When dogs showed improvement in ALB after initiation of the ULFD, a low‐fat (Royal Canin GI low fat with fat content of 2.03 g/100 kcal or Hill's i/d low fat with fat content of 2.3 g/100 kcal) or hydrolyzed (Royal Canin Anallergenic with fat content of 4.25 g/100 kcal) dry canine diet was added gradually to the ULFD to prevent secondary nutritional hyperparathyroidism and deficiencies in vitamins and minerals from the long‐term feeding of the ULFD.
2.3. Histopathology
Tissue samples were obtained from the stomach, proximal duodenum, and distal duodenum for all dogs and from the ileum for some dogs (n = 11) by endoscopy. Ileal tissue collection was performed by attending clinicians on the basis of the clinical signs and ultrasonographic findings in each case. During endoscopy, at least 6 mucosal samples were collected from each previously noted segment of the gastrointestinal tract.11 Histopathological examination was conducted by an American College of Veterinary Pathologists board‐certified pathologist using a scoring system according to histopathologic standards established in the World Small Animal Veterinary Association (WSAVA) guidelines.12 Polymerase chain reaction for antigen receptor gene rearrangement (PARR) was performed based on clinical findings and histopathological analysis on a case by case basis. Immunohistochemistry was not performed in any cases in our study.
2.4. Data collection
The following information was collected from the medical records: breed, age, weight, sex, ALB, plasma concentrations of globulin (GLB), blood urea nitrogen (BUN), creatinine (CRE) and C‐reactive protein (CRP), clinical signs, previous treatments (dietary or prednisolone management), date of first visit, date of intestinal endoscopy, date of death, and cause of death. The severity of clinical signs was evaluated by using the CIBDAI and CCECAI scoring systems.9, 10 Follow‐up information was collected up to October 2018 from the medical records or from communications with the referring hospitals.
Ultrasonographic images of the small intestine were reviewed for each case, and the following findings were extracted: the presence of hyperechoic intestinal mucosal striations defined as multiple clear intramucosal hyperechoic lines, mesenteric lymphadenopathy defined as the size of a mesenteric lymph node >5 mm, and loss of layering defined as indistinguishable intestinal layers.13 The presence of ascites also was evaluated.
Endoscopic scores were retrospectively evaluated by using still images based on the previously described simple endoscopic scoring system in which the absence (score 0) or presence (score 1) of friability, granularity, erosions, and lymphatic dilatation of the duodenum was assessed.14 These images were evaluated by an experienced endoscopist, and scores were summarized with maximum scores of 4.
2.5. Statistical analysis
Data distribution was analyzed by using the Kolmogorov‐Smirnov test. Baseline variables were compared by using Fisher's exact test for categorical variables and the Student t test or Mann‐Whitney U test for continuous variables with Bonferroni correction for multiple comparisons. Comparisons between findings for ALB and CIBDAI and CCECAI scores obtained before and after ULFD treatment were performed by using the paired t test (CIBDAI) or Wilcoxon signed‐rank test (ALB and CCECAI). The area under the curve (AUC) of receiver operating characteristic (ROC) curves was used to evaluate the ability of each factor to differentiate the FR‐PLE group from the IR/NR‐PLE group. Survival time was compared between groups by using the Kaplan‐Meier product‐limit method and the log‐rank test. Survival time was defined as the time between the date of the first visit and the date of death or censoring.
All statistical analyses were conducted by using EZR, which is a graphical user interface for R.15 P values <.05 were considered statistically significant (P < .004 after Bonferroni correction).
3. RESULTS
A flowchart of the inclusion criteria for the study is shown in Figure 1. During the study period, 57 dogs were suspected to have PLE and underwent gastrointestinal endoscopy. Of these 57 dogs, 33 were included in the study. Eight dogs were excluded from the study because of concurrent disorders. An additional 8 dogs were excluded from the study because of a lack of follow‐up information. Four dogs diagnosed with large cell lymphoma and 4 dogs diagnosed with small cell lymphoma also were excluded. The median time from first visit to intestinal endoscopy was 19 days (range, 0 [on first visit] to 416 days).
Figure 1.
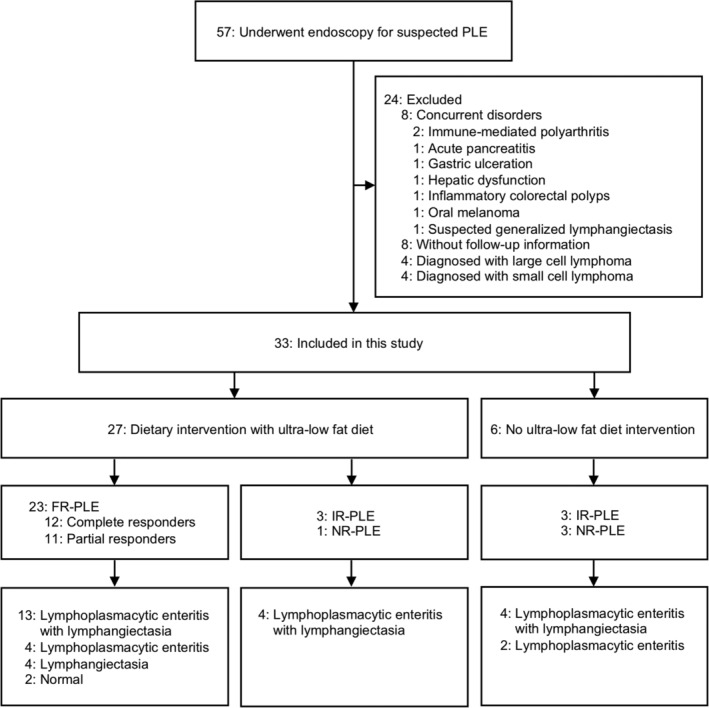
A flowchart of the case descriptions. FR‐PLE, food‐responsive PLE; IR‐PLE, immunosuppressant‐responsive PLE; NR‐PLE, nonresponsive PLE; PLE, protein‐losing enteropathy
Dietary management using ULFD was conducted on 27 of the 33 dogs with PLE. Among the dogs treated with the ULFD, 23 of the dogs responded, with an increase of ALB from a median of 1.5 g/dL (range, 0.9‐2.3) to a median of 2.3 g/dL (range, 1.4‐3.6; P < .001), an improvement of CIBDAI from a median of 3 (range, 0‐8) to a median of 1 (range, 0‐3; P < .001), or an improvement of CCECAI from a median of 5 (range, 2‐10) to a median of 2 (range, 0‐4; P < .001). These 23 dogs were included in the FR‐PLE group. Among these 23 dogs, 3 dogs had decreased appetite (slightly decreased in all 3 dogs), 5 dogs experienced vomiting (mild in 2 dogs and moderate in 3 dogs) and 13 dogs had soft feces or diarrhea (slightly soft in 5 dogs, very soft in 4 dogs, and watery diarrhea in 4 dogs) at the first visit. Four dogs had none of the clinical signs listed in CIBDAI and showed only hypoalbuminemia with or without ascites (CCECAI was 2, 3, 4, and 6 in these 4 dogs). The median duration until the dogs responded to ULFD was 15 days (range, 6‐32 days). When the response to ULFD was confirmed, a low‐fat (Royal Canin GI low fat or Hill's i/d low fat) or hydrolyzed (Royal Canin Anallergenic) dry canine diet was gradually added to the ULFD in all but 1 dog, which was the most recently admitted dog and only received the ULFD toward the end of the study (32 days after initiation of the ULFD). The median duration from initiation of the ULFD to the addition of the dry diet was 47 days (range, 0 [together with ULFD] to 212 days). Fifteen of the 23 dogs classified as FR‐PLE achieved normal ALB (≥2.6 g/dL) after initiation of the ULFD. However, 3 of these 15 dogs experienced recurrence of hypoalbuminemia when the percentage of dry food was increased, and prednisolone treatment subsequently was added in these dogs at dosages of 0.93, 1.0, and 1.1 mg/kg/day. These 3 dogs responded well to the adjunctive prednisolone treatment and achieved normal ALB. The other 12 dogs remained in complete remission during their clinical course without prednisolone treatment except 1 dog in which prednisolone was tapered after the initiation of the ULFD but continued until final follow‐up (91 days after referral to our hospital). Eight of the 23 dogs classified as FR‐PLE experienced a partial response. Six of these dogs were treated with prednisolone (dosage range, 0.73‐2.0 mg/kg/day) because mild hypoalbuminemia persisted even after a response to the ULFD occurred. These 6 dogs had normal ALB after prednisolone treatment was added. Two dogs with partial response continued to be treated by dietary management only according to the owners' preference, but clinical signs in these dogs remained controlled throughout their clinical course. Overall, 12 dogs were considered complete responders in that they achieved normal ALB and did not require additional prednisolone, whereas the other 11 dogs were considered partial responders in that they exhibited partial improvement in ALB or required adjunctive prednisolone treatment. The median dosage of prednisolone for partial responders was 1.1 mg/kg/day (range, 0.73‐2.0 mg/kg/day). Among the dogs in the FR‐PLE group, 7 previously participated in dietary trials that had included a low‐fat diet (3 dogs), a hydrolyzed diet (1 dog), a novel antigen diet (1 dog), a high‐fiber diet (1 dog), or a combination diet (1 dog). Seven dogs were treated with prednisolone (range, 0.40‐2.4 mg/kg/day) before referral to our hospital, but this treatment resulted in no improvement. Prednisolone was completely withdrawn in 4 of these dogs and tapered in 1 dog by the final follow‐up after they were fed the ULFD. Two dogs required treatment with a higher dosage of prednisolone (from 0.40 to 0.93 mg/kg and from 0.5 to 1.1 mg/kg) after achieving a partial response with the ULFD. Based on histopathology, 17 of the 23 dogs showed various degrees of lacteal dilatation, whereas the other 6 dogs did not show lacteal dilatation (Figure 1).
The other dogs in the study included 6 with IR‐PLE and 4 with NR‐PLE. Four dogs did not respond to ULFD management and were histopathologically diagnosed with lymphoplasmacytic enteritis with lymphangiectasia. Three of these 4 dogs responded to immunosuppressant treatment (prednisolone 1.1 mg/kg/day, prednisolone 1.2 mg/kg/day, and prednisolone 1.77 mg/kg/day with cyclosporine 4.2 mg/kg/day). The ULFD was not used in 6 dogs, and immunosuppressant treatment was immediately commenced in these dogs. Two of these 6 dogs previously underwent dietary trials that included a low‐fat diet (1 dog) or a digestive support diet (1 dog). These 6 dogs were histopathologically diagnosed with lymphoplasmacytic enteritis with lymphangiectasia (4 dogs) or lymphoplasmacytic enteritis (2 dogs). Among the dogs with IR‐PLE, 3 dogs responded to treatment with only prednisolone (range, 1.1‐1.3 mg/kg/day), whereas the other 3 dogs required other immunosuppressants combined with prednisolone (prednisolone 1.8 mg/kg/day with cyclosporine 4.2 mg/kg/day, prednisolone 2.0 mg/kg/day with cyclosporine 7.4 mg/kg/day, or prednisolone 1.8 mg/kg/day with cyclosporine 6.2 mg/kg/day, azathioprine 2.0 mg/kg/day, mycophenolate mofetil 26 mg/kg/day, or chlorambucil 6.5 mg/m2/day). Four dogs with NR‐PLE showed no response to any of the previously mentioned treatments.
The PARR was performed in 6 dogs (1 with FR‐PLE, 2 with IR‐PLE, and 3 with NR‐PLE). One dog with FR‐PLE, 1 dog with IR‐PLE, and 1 dog with NR‐PLE were negative, whereas 1 dog with IR‐PLE had clonal rearrangements of lymphocyte antigen receptor genes for B‐cells, and 2 dogs with NR‐PLE had clonal rearrangements of lymphocyte antigen receptor genes for T‐cells.
The FR‐PLE group included 4 Boston Terriers, 3 Miniature Dachshunds, 3 Chihuahuas, 2 Papillons, 2 Toy Poodles, 2 Yorkshire Terriers, 1 Bernese Mountain Dog, 1 Italian Greyhound, 1 Jack Russell Terrier, 1 Japanese Spitz, 1 Miniature Schnauzer, 1 Mongrel, and 1 Welsh Corgi. In the FR‐PLE group, all except 1 dog (Bernese Mountain Dog) were small breed dogs. The IR/NR‐PLE group included 5 Miniature Dachshunds, 1 French Bulldog, 1 Boston Terrier, 1 Papillon, 1 Yorkshire Terrier, and 1 Welsh Corgi. All dogs in the IR/NR‐PLE group were small breed dogs.
Signalment, laboratory findings, ultrasonographic findings, and endoscopic scores were compared between the FR‐PLE and IR/NR‐PLE groups (Table 1). The FR‐PLE group was significantly younger (mean age, 7.5 versus 10.4 years old; P < .001) and had lower CIBDAI scores (median score, 3 versus 10; P < .001) and CCECAI scores (median score, 5 versus 11; P < .001) than those found in the IR/NR‐PLE group. Intestinal hyperechoic mucosal striations were detected by ultrasonography in 22 of 23 dogs (95.7%) in the FR‐PLE group and in 8 of 10 dogs (80%) in the IR/NR‐PLE group. Mesenteric lymphadenopathy was detected in 3 of 23 dogs (13.0%) in the FR‐PLE group and 6 of 10 dogs (60%) in the IR/NR‐PLE group. Loss of intestinal layering was detected in only 1 dog that was in the IR/NR‐PLE group. Ascites was detected in 17 of 23 dogs (73.9%) in the FR‐PLE group and 7 of 10 dogs (70.0%) in the IR/NR‐PLE group. Endoscopic scores were not significantly different between groups. Because age and CIBDAI and CCECAI scores were significantly different between the FR‐PLE and IR/NR‐PLE groups, these variables were used to plot the ROC curve. The AUCs for age, CIBDAI, and CCECAI to differentiate the FR‐PLE and IR/NR‐PLE groups were 0.843 (95% CI, 0.698‐0.989), 0.928 (95% CI, 0.836‐1.000), and 0.935 (95% CI, 0.845‐1.000), respectively (Table 2). Although no significant differences were found among the AUCs of these 3 variables (age versus CIBDAI, P = 0.59; age versus CCECAI, P = 0.51; CIBDAI versus CCECAI, P = 0.74), the highest accuracy was detected for CCECAI, which had an optimal cutoff value of 8, a sensitivity of 0.826, and a specificity of 0.889.
Table 1.
Baseline signalment, laboratory findings, ultrasonographic findings, and endoscopic scores for both groups
| Variable | FR‐PLE | n | IR/NR‐PLE | n | P valuea |
|---|---|---|---|---|---|
| Mean age, years (SD) | 7.5 (±1.7) | 23 | 10.4 (±2.3) | 10 | <.001b |
| Median weight, kg (range) | 4.9 (1.9‐48.6) | 23 | 4.5 (2.2‐9.0) | 10 | .33c |
| Female, number (%) | 11 (47.8) | 23 | 3 (30.0) | 10 | .46d |
| Median CIBDAI, (range) | 3 (0‐8) | 23 | 10 (4‐17) | 9 | <.001c |
| Median CCECAI, (range) | 5 (2‐10) | 23 | 11 (6‐18) | 9 | <.001c |
| Mean ALB, g/dL (SD) | 1.5 (±0.31) | 23 | 1.7 (±0.47) | 10 | .22b |
| Mean GLB, g/dL (SD) | 2.3 (±0.32) | 23 | 2.4 (±0.47) | 10 | .47b |
| Median BUN, mg/dL (range) | 14.1 (6.2‐37) | 23 | 11.5 (5.1‐58.8) | 10 | .37c |
| Mean CRE, mg/dL (SD) | 0.8 (±0.36) | 23 | 0.4 (±0.16) | 10 | .004b |
| Median CRP, mg/dL (range) | 0.35 (0.0‐2.3) | 23 | 1.5 (0.2‐11.0) | 10 | .005c |
| Hyperechoic intestinal mucosal striations, number (%) | 22 (95.7) | 23 | 8 (80.0) | 10 | .21d |
| Mesenteric lymphadenopathy, number (%) | 3 (13.0) | 23 | 6 (60.0) | 10 | .011d |
| Loss of intestinal layering, number (%) | 0 (0.0) | 23 | 1 (10.0) | 10 | .30d |
| Presence of ascites, number (%) | 17 (73.9%) | 23 | 7 (70.0) | 10 | 1d |
| Median endoscopic score, (range) | 2 (0‐3) | 23 | 1.5 (1–3) | 10 | 0.79c |
Abbreviations: ALB, plasma albumin concentration; BUN, blood urea nitrogen; CCECAI, canine chronic enteropathy clinical activity index; CIBDAI, canine inflammatory bowel disease activity index; CRE, creatinine; CRP, C‐reactive protein; FR‐PLE, food‐responsive PLE; GLB, globulin; IR/NR‐PLE, immunosuppressant‐responsive/nonresponsive PLE; PLE, protein‐losing enteropathy.
P values of <.004 were statistically significant after Bonferroni correction.
Student t test.
Mann‐Whitney U test.
Fisher's exact test.
Table 2.
Optimal cutoffs and AUC between the FR‐PLE and IR/NR‐PLE groups
| Variable | Cutoff | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|
| Age, years | 9.1 | 0.843 (0.698–0.989) | 0.826 | 0.800 |
| CIBDAI | 5 | 0.928 (0.836–1.000) | 0.913 | 0.778 |
| CCECAI | 8 | 0.935 (0.845–1.000) | 0.826 | 0.889 |
Abbreviations: AUC, area under the curve; CCECAI, canine chronic enteropathy clinical activity index; CI, confidence interval; CIBDAI, canine inflammatory bowel disease activity index; CRP, C‐reactive protein; FR‐PLE, food‐responsive PLE; IR/NR‐PLE, immunosuppressant‐responsive/nonresponsive PLE; PLE, protein‐losing enteropathy.
During the study period, 8 dogs died (2 in the FR‐PLE group and 6 in the IR/NR‐PLE group). Survival time was compared between these 2 groups (Figure 2) and showed that the median survival time of the FR‐PLE group was not reached, with a median follow‐up period of 575 days. The median survival time of the IR/NR‐PLE group was 432 days and was significantly shorter than that found in the FR‐PLE group (P < .001). The causes of death in the IR/NR‐PLE group were related to the disease in 5 dogs and unrelated to the disease in 1 dog (PO melanoma), whereas cause of death in the FR‐PLE group was unrelated to the diseases in both dogs (cardiogenic pulmonary edema and acute neurological signs of unknown cause; no necropsy was performed in either case).
Figure 2.
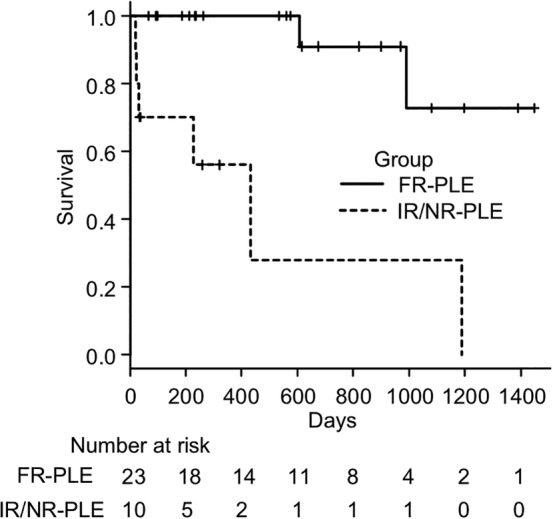
Survival curves for the FR‐PLE (solid line) and IR/NR‐PLE groups (dashed line). The median survival time was not reached in the group and was significantly longer in the FR‐PLE group than in the IR/NR‐PLE group (432 days, P < .001). FR‐PLE, food‐responsive PLE; IR/NR‐PLE, immunosuppressant‐responsive/nonresponsive PLE
Among the 27 dogs with ULFD management (23 responders in the FR‐PLE group and 4 nonresponders in the IR/NR‐PLE group), CCECAI scores were lower in the responders (median score, 5; range, 2‐10) than in the nonresponders (median score, 11; range, 10‐18; P = .007; Figure 3). Similarly, CIBDAI scores were lower in the responders (median score, 3; range, 0‐8) than in the nonresponders (median score, 10; range, 5‐17; P = .015; Figure 3). However, no significant difference was found in CCECAI scores between the complete responders (median score, 5; range, 2‐10) and partial responders (median score, 5; range, 2‐10; P = 1; Figure 4) and no significant difference was found in CIBDAI scores between complete responders (median score, 4; range, 0‐8) and partial responders (median score, 2; range, 0‐5; P = .23; Figure 4), nor in age, weight, ALB, GLB, CRP, and ultrasonographic findings (P > .05).
Figure 3.
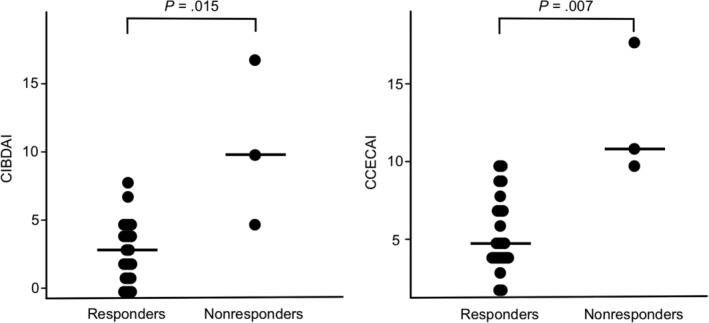
Comparison of CIBDAI and CCECAI scores between responders (n = 23) and nonresponders (n = 3) to ULFD management. CIBDAI and CCECAI scores were lower in the responders than in the nonresponders. The horizontal lines indicate the median value. CECAI, canine chronic enteropathy clinical activity index; CIBDAI, canine inflammatory bowel disease activity index
Figure 4.
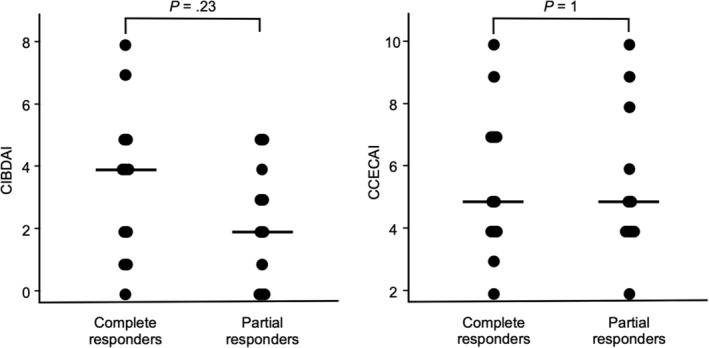
Comparison of CIBDAI and CCECAI scores between complete responders (n = 12) and partial responders (n = 11) to ULFD management. There was no significant difference in CIBDAI and CCECAI scores between the complete responders and partial responders. The horizontal lines indicate the median value. CCECAI, canine chronic enteropathy clinical activity index; CIBDAI, canine inflammatory bowel disease activity index
4. DISCUSSION
Our results suggest that age and CIBDAI and CCECAI scores at the time of diagnosis of PLE may be useful for predicting patient responsiveness to an ULFD. In our study, the dogs with FR‐PLE had a more favorable prognosis than those with IR‐PLE or NR‐PLE.
We attempted to evaluate clinical findings in FR‐PLE in dogs by including all dogs that were diagnosed with PLE in our hospital and that also underwent endoscopic biopsies. Although some dogs in the study did not have normal ALB or required adjunctive prednisolone after achieving a partial response to ULFD, we classified these dogs as FR‐PLE (partial responders) rather than IR‐PLE because a partial response to a dietary intervention may benefit dogs with PLE in terms of its potentially sparing effect on immunosuppressant drug use. The sparing effect of the dietary intervention may result in a decrease in the dose of prednisolone required to achieve a complete response and eliminate the concomitant use of other immunosuppressants. In fact, no dogs with partial response in our study required the concomitant use of other immunosuppressants to achieve normal ALB concentrations.
Our results show that CCECAI scores were significantly lower in the FR‐PLE group than in the IR/NR‐PLE group. According to the ROC analysis, CCECAI scores are useful for discriminating between the FR‐PLE and IR/NR‐PLE groups. In addition, among the 27 dogs subjected to dietary interventions, CCECAI scores were significantly lower in responders than in nonresponders, indicating that CCECAI scores are a possible indicator that predicts the response to an ULFD in dogs with PLE. However, only 4 dogs in our study were nonresponders. These results support the idea that dietary intervention may be beneficial for dogs with PLE if the dogs have less severe clinical signs.4 Based on our results and those of a previous study,3 at least 2 weeks of a dietary trial can be recommended in these cases. On the other hand, CCECAI scores were not significantly different between complete responders and partial responders, indicating that differentiating these populations using CCECAI scores would be difficult. However, in our study, a complete responder was defined as a dog that achieved and maintained normal ALB (>2.6 g/dL). This is a relatively strict criterion because an improvement in CCECAI score (CCECAI ≤3) without normal ALB can be considered a treatment success.3 In this population of dogs with PLE, the goal of treatment was to achieve normal ALB, and prednisolone therefore was commonly used for dogs with even mild hypoalbuminemia. It is not clear whether these dogs actually required the adjunctive prednisolone. In fact, 2 partial responders were clinically controlled by the final follow‐up (212 and 1449 days) without prednisolone (ALB concentrations at the final follow‐up were 2.1 and 2.4 g/dL, respectively). Moreover, 3 dogs initially had achieved a complete response with ULFD treatment but showed recurrence of hypoalbuminemia when the percentage of dry food was increased, prompting adjunctive prednisolone treatment. We chose to administer adjunctive prednisolone treatment in these 3 dogs instead of using a 100% ULFD in terms of the nutritional disadvantage associated with a 100% ULFD. These dogs possibly could have been managed using a nutritionally balanced 100% ULFD that has been suggested previously.6
Interestingly, the median CIBDAI score even before dietary intervention in the FR‐PLE group indicated clinically unimportant disease, suggesting that this clinical score is not useful in some cases in dogs with PLE. In fact, 1 dog was considered to have clinically unimportant disease by CIBDAI (score 0) but moderate disease by CCECAI (score 6). Therefore, it may be important to calculate both CIBDAI and CCECAI scores for dogs with PLE.
Our results also indicated age as a useful indicator for predicting patient responsiveness to an ULFD. A previous study showed that dogs diagnosed with IL tend to be younger than those with chronic enteropathy, small cell lymphoma, or large cell lymphoma.5 Considering that dogs with primary IL tend to be young and responsive to ULFD,5, 7 we speculate that the dogs with FR‐PLE in our study in fact may have had primary IL even though not every responder showed lacteal dilatation on histopathology. Considering the limitations of endoscopic tissue biopsy samples for the diagnosis of IL, structural lesions might have been present in the deeper mucosa or distal jejunum, or might have been artificially altered during the collection, which may have led to an underestimation of IL.6, 16, 17, 18, 19 These findings are supported by the fact that the majority of the dogs in the FR‐PLE group in our study (22 of the 23, 95.7%) had intestinal hyperechoic mucosal striations. A previous study indicated that almost all dogs with intestinal hyperechoic mucosal striations have lacteal dilatation based on histopathology.13 However, 8 of the 10 (80.0%) dogs in the IR/NR‐PLE group in our study also had intestinal hyperechoic mucosal striations, indicating that our ultrasonographic finding was not specific for IL. Therefore, intestinal hyperechoic mucosal striations alone cannot be used to select dogs for ULFD management, although using it in combination with other variables, such as age and CCECAI score, may be useful.
Other ultrasonographic findings investigated in our study included mesenteric lymphadenopathy, loss of intestinal layering, and the presence of ascites. Mesenteric lymphadenopathy was detected in only 3 dogs (13.0%) in the FR‐PLE group, consistent with a previous study that showed that mesenteric lymphadenopathy was not commonly detected in dogs with intestinal mucosal striations.13 Interestingly, all 3 dogs were partial responders. Although no significant difference was found in the presence of mesenteric lymphadenopathy between complete and partial responders, this may indicate more severe inflammation and the necessity of adjunctive prednisolone treatment. In contrast, mesenteric lymphadenopathy was detected in 6 dogs (60.0%) in the IR/NR‐PLE group, consistent with a previous study in which 41% of dogs with chronic enteropathy had enlarged abdominal lymph nodes.20 No dogs among the responders showed loss of intestinal layering in our study, which is similar to a previous study in which no dogs with intestinal mucosal striations showed loss of intestinal layering, except 1 dog with a lipogranuloma.13 These findings suggested that mesenteric lymphadenopathy and loss of intestinal layering are not common findings in dogs with FR‐PLE or primary IL.
Compared to the IR/NR‐PLE group, the FR‐PLE group had longer survival times, indicating that differentiating between these 2 groups of dogs with PLE based on responsiveness to the ULFD is important in predicting the prognosis. Because the FR‐PLE group had significantly lower CIBDAI and CCECAI scores than those found in the IR/NR‐PLE group, the longer survival time of the FR‐PLE group may be attributed to the severity of the disease, which has been shown to be associated with prognosis in dogs with PLE.5, 21 In addition, other factors, such as body weight, BUN, ALB, and PARR could be associated with survival time.5, 8, 22, 23, 24, 25, 26 Therefore, further study is needed to evaluate the effect of ULFD itself on the prognosis of dogs with PLE. The ALB of the dogs in our study was not significantly different between the groups despite the significant difference between the survival times. Previous studies performed in dogs with PLE showed that ALB is not associated with survival.5, 22, 24 However, other studies that included dogs with chronic enteropathy or Yorkshire Terriers with PLE have reported that hypoalbuminemia is related to a poor prognosis.10, 25, 27 This difference may be attributed to differences in the inclusion criteria. Based on these results and previous studies, ALB alone does not seem to predict prognosis of different groups of dogs with PLE.
Our study had several limitations. First, we could include only a relatively small number of cases because the study was conducted at a single center. Therefore, further evaluation using a larger number of cases is necessary to verify these findings. Second, because of the retrospective study design, diagnostic procedures and treatment choices differed in each case. The CIBDAI and CCECAI scores were calculated retrospectively, possibly resulting in some bias. Because ileal biopsies were performed in only 33% of dogs in our study, disease processes in the ileum different from those in the duodenum (including localized lymphoma) could not be completely ruled out. Furthermore, lymphoma was diagnosed based on histopathology and not immunohistochemistry and PARR in our study. Considering the limitations of histopathology for the diagnosis of lymphoma,28 lymphoma could not be completely excluded especially in 3 dogs that were PARR positive, although 51% of dogs with chronic enteritis showed positive results for PARR in a previous study.29 In addition, functional biomarkers for enteropathy in dogs, such as fecal and serum concentrations of alpha‐1 proteinase inhibitor, folate, and cobalamin,30 which are possibly useful for determining responsiveness to ULFD, were not measured in most dogs in our study. Serum cobalamin concentrations were measured in only 2 dogs (180 pg/mL in 1 dog with FR‐PLE and 195 pg/mL for the other dog with IR‐PLE; reference interval, 230‐900 pg/mL). Moreover, not all dogs underwent ULFD management in our study, and therefore the responsiveness to the ULFD for 6 dogs in the IR/NR‐PLE group was not determined. These dogs might have responded to the ULFD if given concurrently with symptomatic treatment such as antiemetic medication or an ULFD formulation designed for assisted feeding. Because of the retrospective design, initiating dietary intervention in all cases was difficult in our study. A prospective and multicenter study to confirm the effectiveness of an ULFD targeting dogs with PLE is needed. Finally, serum bile acid concentrations were not measured in all dogs in the study, but hepatic dysfunction was considered unlikely in these dogs based on routine serum biochemical analysis (ie, normal glucose, BUN, and total bilirubin concentrations and normal liver enzyme activity). Likewise, because both the urine protein: creatinine ratio and urine dipstick protein test were not available for 2 dogs in our study, protein‐losing nephropathy was not completely ruled out for these dogs. In addition, because serum cortisol concentrations were measured in only 2 dogs (basal cortisol concentrations of 3 and 4.1 μg/dL), atypical hypoadrenocorticism could not be fully excluded for other dogs, although other findings, including abdominal ultrasonography, did not support a diagnosis of hypoadrenocorticism in any of the dogs. However, abdominal ultrasonography is not completely sensitive diagnostically for hypoadrenocorticism.31, 32, 33 This is important especially among dogs that respond to prednisolone treatment. Thus, the coexistence of hepatic dysfunction, protein‐losing nephropathy, and atypical hypoadrenocorticism was not completely ruled out in this population of dogs.
In conclusion, an ULFD may be beneficial for dogs with PLE. Dogs that respond to ULFD management and are diagnosed with FR‐PLE are expected to have a favorable prognosis. Age and clinical scores, such as CCECAI, may be useful for differentiating FR‐PLE from IR‐PLE or NR‐PLE. Further validation of our findings should be carried out in a prospective study including a larger number of dogs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
We thank Dr. Yumiko Kagawa, an American College of Veterinary Pathologists board‐certified pathologist, for her help with the interpretation of the histopathological findings.
Nagata N, Ohta H, Yokoyama N, et al. Clinical characteristics of dogs with food‐responsive protein‐losing enteropathy. J Vet Intern Med. 2020;34:659–668. 10.1111/jvim.15720
REFERENCES
- 1. Dossin O, Lavoué R. Protein‐losing enteropathies in dogs. Vet Clin North Am Small Anim Pract. 2011;41:399‐418. [DOI] [PubMed] [Google Scholar]
- 2. Willard MD. Malabsorption In: Washabau RJ, Day MJ, eds. Canine and Feline Gastroenterology. St. Louis, MO: Elsevier; 2013:678‐683. [Google Scholar]
- 3. Rudinsky AJ, Howard JP, Bishop MA, Sherding RG, Parker VJ, Gilor C. Dietary management of presumptive protein‐losing enteropathy in Yorkshire terriers. J Small Anim Pract. 2017;58:103‐108. [DOI] [PubMed] [Google Scholar]
- 4. Dandrieux JRS. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J Small Anim Pract. 2016;57:589‐599. [DOI] [PubMed] [Google Scholar]
- 5. Nakashima K, Hiyoshi S, Ohno K, et al. Prognostic factors in dogs with protein‐losing enteropathy. Vet J. 2015;205:28‐32. [DOI] [PubMed] [Google Scholar]
- 6. Peterson PB, Willard MD. Protein‐losing enteropathies. Vet Clin North Am Small Anim Pract. 2003;33:1061‐1082. [DOI] [PubMed] [Google Scholar]
- 7. Okanishi H, Yoshioka R, Kagawa Y, Watari T. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J Vet Intern Med. 2014;28:809‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Craven MD, Washabau RJ. Comparative pathophysiology and management of protein‐losing enteropathy. J Vet Intern Med. 2019;33:383‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291‐297. [DOI] [PubMed] [Google Scholar]
- 10. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700‐708. [DOI] [PubMed] [Google Scholar]
- 11. Jergens AE, Willard MD, Allenspach K. Maximizing the diagnostic utility of endoscopic biopsy in dogs and cats with gastrointestinal disease. Vet J. 2016;214:50‐60. [DOI] [PubMed] [Google Scholar]
- 12. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the world small animal veterinary association gastrointestinal standardization group. J Comp Pathol. 2008;138(SUPPL. 1):1‐43. [DOI] [PubMed] [Google Scholar]
- 13. Sutherland‐Smith J, Penninck DG, Keating JH, et al. Ultrasonographic intestinal hyperechoic mucosal striations in dogs are associated with lacteal dilation. Vet Radiol Ultrasound. 2007;48:51‐57. [DOI] [PubMed] [Google Scholar]
- 14. Slovak JE, Wang C, Sun Y, et al. Development and validation of an endoscopic activity score for canine inflammatory bowel disease. Vet J. 2015;203:290‐295. [DOI] [PubMed] [Google Scholar]
- 15. Kanda Y. Investigation of the freely available easy‐to‐use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lecoindre A, Lecoindre P, Cadoré JL, et al. Focal intestinal lipogranulomatous lymphangitis in 10 dogs. J Small Anim Pract. 2016;57:465‐471. [DOI] [PubMed] [Google Scholar]
- 17. Watson VE, Hobday MM, Durham AC. Focal intestinal lipogranulomatous lymphangitis in 6 dogs (2008‐2011). J Vet Intern Med. 2014;28:48‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meschter CL, Rakich PM, Tyler DE. Intestinal lymphangiectasia with lipogranulomatous lymphangitis in a dog. J Am Vet Med Assoc. 1987;190:427‐430. [PubMed] [Google Scholar]
- 19. Van Kruiningen HJ, Lees GE, Hayden DW, et al. Lipogranulomatous lymphangitis in canine intestinal lymphangiectasia. Vet Pathol. 1984;21:377‐383. [DOI] [PubMed] [Google Scholar]
- 20. Gaschen L, Kircher P, Stüssi A, et al. Comparison of ultrasonographic findings with clinical activity index (CIBDAI) and diagnosis in dogs with chronic enteropathies. Vet Radiol Ultrasound. 2008;49:56‐64. [DOI] [PubMed] [Google Scholar]
- 21. Kathrani A, Sánchez‐Vizcaíno F, Hall EJ. Association of chronic enteropathy activity index, blood urea concentration, and risk of death in dogs with protein‐losing enteropathy. J Vet Intern Med. 2019;33:536‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allenspach K, Rizzo J, Jergens AE, Chang YM. Hypovitaminosis D is associated with negative outcome in dogs with protein losing enteropathy: a retrospective study of 43 cases. BMC Vet Res. 2017;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gianella P, Lotti U, Bellino C, et al. Clinicopathologic and prognostic factors in short‐ and long‐term surviving dogs with protein‐losing enteropathy. Schweiz Arch Tierheilkd. 2017;159:163‐169. [DOI] [PubMed] [Google Scholar]
- 24. Equilino M, Théodoloz V, Gorgas D, et al. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein‐losing enteropathy. J Am Vet Med Assoc. 2015;246:91‐99. [DOI] [PubMed] [Google Scholar]
- 25. Simmerson SM, Armstrong PJ, Wünschmann A, Jessen CR, Crews LJ, Washabau RJ. Clinical features, intestinal histopathology, and outcome in protein‐losing enteropathy in Yorkshire terrier dogs. J Vet Intern Med. 2014;28:331‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dandrieux JR, Noble PJ, Scase TJ, et al. Comparison of a chlorambucil‐prednisolone combination with an azathioprine‐prednisolone combination for treatment of chronic enteropathy with concurrent protein‐losing enteropathy in dogs: 27 cases (2007‐2010). J Am Vet Med Assoc. 2013;242:1705‐1174. [DOI] [PubMed] [Google Scholar]
- 27. Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995‐2002). J Small Anim Pract. 2004;45:336‐342. [DOI] [PubMed] [Google Scholar]
- 28. Carrasco V, Rodríguez‐Bertos A, Rodríguez‐Franco F, et al. Distinguishing intestinal lymphoma from inflammatory bowel disease in canine duodenal endoscopic biopsy samples. Vet Pathol. 2015;52:668‐675. [DOI] [PubMed] [Google Scholar]
- 29. Hiyoshi S, Ohno K, Uchida K, et al. Association between lymphocyte antigen receptor gene rearrangements and histopathological evaluation in canine chronic enteropathy. Vet Immunol Immunopathol. 2015;165:138‐144. [DOI] [PubMed] [Google Scholar]
- 30. Heilmann RM, Steiner JM. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J Vet Intern Med. 2018;32:1495‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kook PH, Grest P, Raute‐Kreinsen U, Leo C, Reusch CE. Addison's disease due to bilateral adrenal malignancy in a dog. J Small Anim Pract. 2010;51:333‐336. [DOI] [PubMed] [Google Scholar]
- 32. Wenger M, Mueller C, Kook PH, Reusch CE. Ultrasonographic evaluation of adrenal glands in dogs with primary hypoadrenocorticism or mimicking diseases. Vet Rec. 2010;167:207‐210. [DOI] [PubMed] [Google Scholar]
- 33. Hoerauf A, Reusch C. Ultrasonographic evaluation of the adrenal glands in six dogs with hypoadrenocorticism. J Am Anim Hosp Assoc. 1999;35:214‐218. [DOI] [PubMed] [Google Scholar]


