Abstract
Background
In metabolically stable horses, alpha‐2‐agonists suppress insulin secretion with transient hyperglycemia and rebound hyperinsulinemia. In horses with insulin dysregulation (ID), the effect of alpha‐2‐agonists has not been investigated; however, both the alpha‐2‐agonist‐induced suppression of insulin secretion and rebound hyperinsulinemia could have clinical relevance.
Hypothesis/Objectives
In horses with ID, alpha‐2‐agonists will alter insulin and glucose dynamics.
Animals
Seven horses with ID and 7 control horses.
Methods
In this randomized crossover study, xylazine hydrochloride (1.1 mg/kg) or detomidine hydrochloride (30 μg/kg) were administered IV, and blood was collected for glucose and insulin concentrations at 0, 15, 30, 45, 60, 90, 120, 150, 180, and 300 minutes after administration. Horses received each drug in a random order with a 24‐hour washout period between drugs. Percent change in glucose and insulin concentrations was compared between groups, drugs, and over time with P < .05 considered significant.
Results
A significant time‐dependent effect of both alpha‐2‐agonists on glucose and insulin concentrations in control and ID horses was identified (P = .01 for all comparisons). There was no significant effect of sedative selection and endocrine status on blood glucose concentration in either group; however, in ID horses, xylazine administration resulted in severe rebound hyperinsulinemia whereas detomidine administration did not (P = .02).
Conclusions and Clinical Importance
Alpha‐2‐agonists have a significant effect on glucose and insulin concentrations in horses. In ID horses, detomidine could minimize hyperinsulinemia when compared to xylazine.
Keywords: detomidine, endocrinology, equine, metabolism, pancreas, xylazine
Abbreviations
- ID
insulin dysregulation
- OGT
oral glucose test
- OST
oral sugar test
1. INTRODUCTION
Xylazine and detomidine are commonly used in horses, alone or in combination with other sedatives or analgesics, for many types of procedures because they provide acceptable levels of both sedation and analgesia.1, 2 The choice between xylazine and detomidine in equine practice mainly stems from clinician's preference and duration of the procedure for which sedation is required, as detomidine induces a longer sedation. 3
Although convenient to use in everyday practice, alpha‐2‐agonists alter glucose and insulin dynamics. 4 In metabolically stable animals, alpha‐2‐agonist administration results in a decrease in insulin secretion from the pancreatic beta cells and, to a much lesser extent, an increase in glucagon secretion from pancreatic alpha cells. 5 This causes a transient hyperglycemia and, as blood glucose is the main driver for insulin secretion in horses, a compensatory hyperinsulinemic response develops with blood glucose normalization.3, 6 The duration and the extent of each phase vary tremendously with the alpha‐2‐agonist used with more pronounced effects with romifidine compared to xylazine. 4 In critically ill horses, hyperglycemia is associated with complications and a poor outcome; however, in metabolically fragile horses, alpha‐2‐agonist‐induced perturbation of glucose and insulin dynamics could have more dramatic effects than those described in other equids. 7
Horses with insulin dysregulation (ID), a key element of equine metabolic syndrome, have blood glucose concentrations in the higher values of reference ranges suggesting that an alpha‐2‐agonist‐induced hyperglycemia could be more pronounced in those horses. 8 In addition, some horses with ID are more prone to hyperinsulinemia suggesting that, on 1 hand, alpha‐2‐agonists could help blunt a hyperinsulinemic peak, whereas, on the other hand, the compensatory insulin response could be more severe. 9 Hyperinsulinemia is linked with the development of laminitis; therefore, if alpha‐2‐agonist‐induced sedation could result in prolonged hyperinsulinemia, it could potentially contribute to the development of a laminitic episode in horses with ID. 10
Taken together, these data suggest that horses with ID could experience different glucose and insulin responses after alpha‐2‐agonist‐induced sedation than control horses and that these responses could increase their risk of laminitis. The aim of this study is therefore to describe how horses with ID respond to alpha‐2‐agonists and to evaluate the differential effects of the 2 most commonly used alpha‐2‐agonists in equine practice, xylazine hydrochloride and detomidine hydrochloride.
2. MATERIALS AND METHODS
2.1. Horses
The study population consisted of adult horses that had been donated to either institution for problems unrelated to their gastrointestinal or endocrine systems. Horses were divided into 2 groups depending on the results of an oral glucose test (OGT) or oral sugar test (OST) and a 2‐step insulin sensitivity test as previously described.11, 12, 13, 14 Briefly, for the OGT, horses were fasted overnight for 10 hours, 0.75 g/kg of d‐glucose was administered through a nasogastric tube, and blood samples were collected at 0 and 120 minutes to measure serum insulin. 11 For the OST, horses received 15 mL/100 kg of concentrated light corn syrup (equivalent to 158 mg/kg of glucose) PO after a 10‐hour fast, and blood was collected 0 and 90 minutes after corn syrup administration. 12 For the 2‐step insulin sensitivity test, horses were administered 0.1 IU/kg of regular insulin IV, and blood samples were collected at 0 and 30 minutes to measure blood glucose. 13 A diagnosis of ID was made when a horse had both hyperinsulinemia as per the OGT or OST (serum insulin >80 μIU/mL or > 60 μIU/mL, respectively) and peripheral tissue insulin resistance as per the 2‐step insulin sensitivity test (decrease in blood glucose <50%). 15 Horses having both hyperinsulinemia and peripheral tissue insulin resistance were included in the ID group, horses having neither hyperinsulinemia nor peripheral tissue insulin resistance were included in the control group and horses having only 1 positive test were excluded. All the horses were determined to be healthy by physical examination and none had clinical signs consistent with pituitary pars intermedia dysfunction, although endogenous ACTH concentrations were not measured.
2.2. Procedures
After acclimatization, an IV catheter was placed in the left jugular vein of each horse. Sixty minutes after catheter placement, horses were administered xylazine hydrochloride (Rompun [100 mg/mL], Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, Shawnee, Kansas, 1.1 mg/kg) or detomidine hydrochlorideb (Dormosedan [10 mg/mL], Zoetis Inc, Parsippany, Troy Hills, New Jersy, 30 μg/kg) by IV injections in the right jugular vein. The alpha‐2‐agonist administered was randomly selected (coin toss). Blood samples were then collected through the IV catheter at 0, 15, 30, 45, 60, 90, 120, 150, 180, and 300 minutes after alpha‐2‐agonist injection and placed in either an ethylenediaminetetraacetic acid (EDTA) containing tube (Institution 1) or in a plain tube (Institution 2). Plasma was collected immediately, whereas blood was allowed to clot for 45 minutes at room temperature, centrifuged, and serum was extracted. Depending on the institution, insulin was measured using a radioimmunoassay (Institution 1) or a chemiluminescent assay (Institution 2) previously validated in horses.16, 17 Blood glucose was measured using a glucohexokinase colorimetric assay as previously described. 14 A 24‐hour washout period was observed before the same procedure was followed with the other alpha‐2‐agonist.
In order to limit variability, all the procedures were performed in summer (July‐August for Institution 1 and December‐January for Institution 2). Horses were kept in stalls and had access to a mixed alfalfa and grass and water ad libitum before and after the experiments; however, they only had access to water during the experimental procedures. All the procedures were approved by both institutional Animal Ethics Committees.
2.3. Data analysis
Horses were grouped by insulin regulation status and by alpha‐2‐agonist administered (control or ID and xylazine or detomidine, respectively) and compared with P < .05 considered statistically significant. Normal distribution was determined by the Shapiro‐Wilk test. Means ± SDs were reported for normally distributed data, and medians (range) were reported for data that did not follow normal distribution. Baseline glucose and insulin concentrations were compared between groups and between experiments using an unpaired or paired t test, or a Mann‐Whitney U test or a Wilcoxon signed‐rank test depending on comparison of interest and distribution. As different insulin assays were used between institutions, percent change in insulin and glucose concentrations induced by alpha‐2‐agonist administration were calculated using baseline as reference. Percent change was then compared by means of a 2‐way repeated‐measures analysis of variance and a Tukey post hoc test when relevant. Statistical analysis was performed using commercially available statistical software (Prism, GraphPad Software Inc, La Jolla, California).
3. RESULTS
3.1. Horses
Fourteen horses met the inclusion criteria, including 8 mares and 6 geldings, 9 from Institution 1 and 5 from Institution 2. The mean age was 16 ± 4 years, the mean body weight was 490 ± 100 kg, and the median body condition score was 5 ranging from 4 to 8 on a 9‐point scale. Seven horses were included in the ID group and 7 horses were included in the control group (Table S1). There was no significant difference regarding sex distribution (P = .59), age (P = .67), body weight (P = .93), or body condition score (P = .10) between groups. There was no significant difference in baseline glucose concentrations between the control group and the ID group (5.5 ± 0.1 versus 5.7 ± 0.2 mmol/L, respectively, P = .34); however, baseline insulin concentration (obtained by radioimmunoassay or chemiluminescent assay depending on institution) in the ID group was significantly higher than in the control group (26.94 μIU/mL [1.4‐138.0] versus 10.14 μIU/mL [1.4‐28.2], respectively, P = .01).
3.2. Glucose
In the control group, there was a significant effect of alpha‐2‐agonist administration on glucose concentration (P = .01); however, there was no significant effect of the type of alpha‐2‐agonist administered (P = .47). Administration of alpha‐2‐agonists induced an increase in glucose concentration significantly above baseline from 30 minutes post‐injection up to 60 minutes for xylazine hydrochloride and up to 150 minutes for detomidine hydrochloride (Figure 1A).
Figure 1.
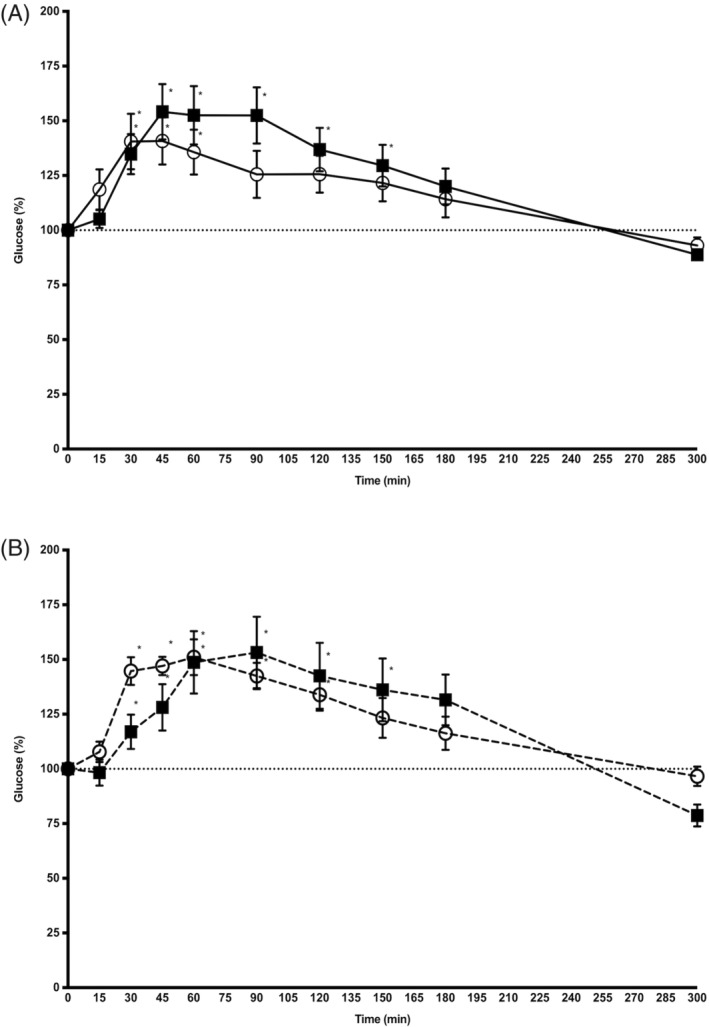
Mean (±SD) glucose concentration (percentage of baseline) after IV administration of xylazine hydrochloride (open circles) and detomidine hydrochloride (black squares) in 7 control horses (solid line, panel A) and 7 insulin dysregulated horses (dashed line, panel B). *P < .05 versus baseline
In the ID group, there was a significant effect of alpha‐2‐agonist administration on glucose concentration (P = .01); however, there was no significant effect of the type of alpha‐2‐agonist administered (P = .74) Administration of alpha‐2‐agonists induced an increase in glucose concentration significantly above baseline from 30 minutes postinjection up to 120 minutes for xylazine hydrochloride and up to 180 minutes for detomidine hydrochloride (Figure 1B).
When comparing control and ID horses, no significant effect of endocrine status on xylazine hydrochloride‐induced or detomidine hydrochloride‐induced changes in glucose concentration was identified.
3.3. Insulin
In the control group, both alpha‐2‐agonists affected insulin concentration (P = .01), but there was no difference on insulin release between drugs (P = .40). Administration of alpha‐2‐agonists induced an initial decrease in insulin concentration below baseline; however, this effect did not reach statistical significance. This initial decrease was followed by an increase in insulin concentration for both alpha‐2‐agonists; however, this increase was only significantly above baseline for xylazine hydrochloride at 120 minutes (P = .03, Figure 2A).
Figure 2.
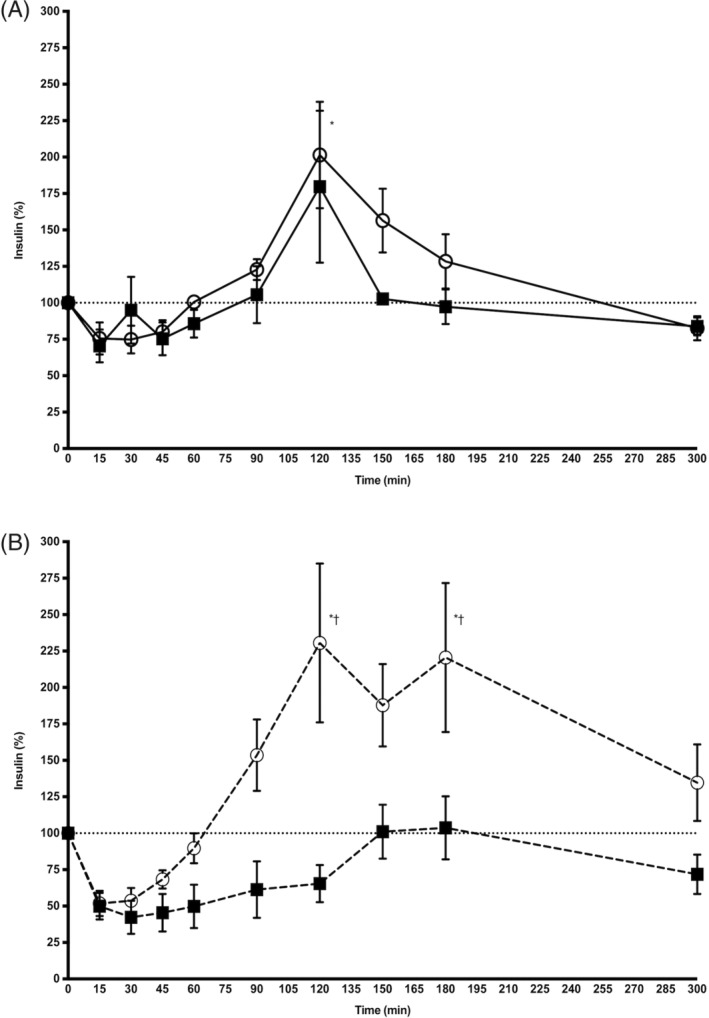
Mean (± SD) insulin concentration (percentage of baseline) after IV administration of xylazine hydrochloride (open circles) and detomidine hydrochloride (black squares) in 7 control horses (solid line, panel A) and 7 insulin dysregulated horses (dashed line, panel B). *P < .05 versus baseline; †P < .05 xylazine versus detomidine
In the ID group, there was a significant effect of alpha‐2‐agonist administration on insulin concentration (P = .01) and a significant effect of the type of alpha‐2‐agonists administered (P = .02). Xylazine hydrochloride administration induced an initial decrease in insulin concentration; however, this effect did not reach statistical significance. This initial decrease was followed by a marked increase, significantly above baseline at 120 and 180 minutes (P = .01 for both comparisons). Administration of detomidine hydrochloride induced a decrease in insulin concentration, below baseline with no subsequent increase above baseline; however, none of these effects did reach statistical significance (Figure 2B).
When comparing control and ID horses, no significant effect of endocrine status on xylazine hydrochloride‐induced (P = .34, Figure 3A) or detomidine hydrochloride‐induced (P = .08, Figure 3B) changes in insulin concentration was identified.
Figure 3.
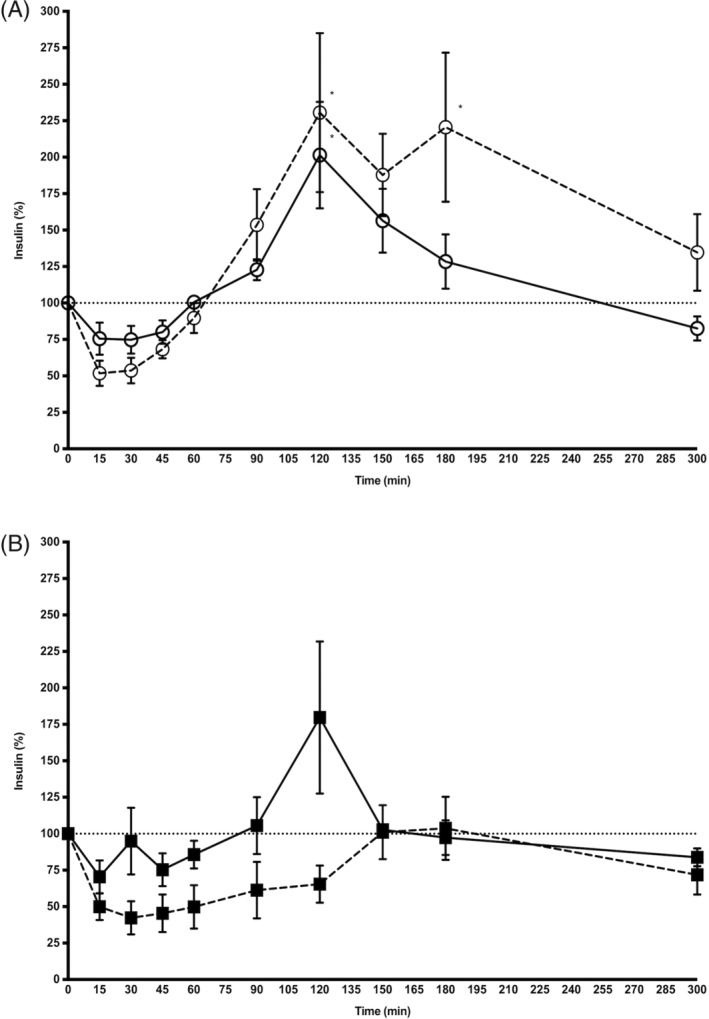
Mean (±SD) insulin concentration (percentage of baseline) after IV administration of xylazine hydrochloride (open circles, panel A) and detomidine hydrochloride (black squares, panel B) in 7 control horses (solid line) and 7 insulin dysregulated horses (dashed line). *P < .05 versus baseline. To facilitate the reader's evaluation, insulin data are presented per group (Figure 2) and drug (Figure 3)
4. DISCUSSION
The main results of this study are (a) administration of alpha‐2‐agonists results in a significant increase in blood glucose concentration in both control and ID horses; and (b) administration of alpha‐2‐agonists results in the suppression of insulin secretion in both control and ID horses; however, in horses administered xylazine hydrochloride, this initial decrease is followed by a marked and significant increase in insulin secretion.
In this study, alpha‐2‐agonists induced hyperglycemia in both groups regardless of the alpha‐2‐agonist used. Although the magnitude of the increase was similar in all conditions with glucose concentrations reaching on average 150% of baseline, the effect was more prolonged in horses from the ID groups. This prolonged hyperglycemia could be explained by the presence of peripheral tissue insulin resistance in ID horses. 18 Nevertheless, considering that there was no significant difference in baseline glucose between groups, these increases of similar magnitude suggest a saturable mechanism present in both control and ID horses.
Two potential mechanisms responsible for this saturable effect are urinary spilling and rebound insulin secretion, both maintaining glucose concentrations within a narrow range. The urinary spilling threshold in horses is estimated to be between 8.9 and 10.0 mmol/L. 19 Assessment of glycosuria was beyond the scope of this study; however, considering that the mean blood glucose concentration was above 8.5 mmol/L at the hyperglycemic peak, urinary spilling is a likely cause of the saturable effect observed. Another possible mechanism for glucose regulation in a narrow range is rebound insulin secretion. Blood glucose is responsible for about 70% of insulin secretion in horses. 6 In both groups, alpha‐2‐agonists initially induced a mild decrease in serum insulin followed by an increase of variable amplitude. Although there was no obvious parallelism between the glucose and the insulin curves, the presence of hyperglycemia followed by hyperinsulinemia could suggest a delayed yet adequate overall pancreatic beta cell response. 20 In ID horses, after xylazine hydrochloride injection, after a mild decrease, insulin concentration showed a steady increase, doubling baseline value by 120 minutes whereas after detomidine hydrochloride injection, insulin concentration slowly returned to baseline at 150 minutes without exceeding baseline. The different amplitudes between the glucose and the insulin curves in horses with ID could suggest an erratic pancreatic response to alpha‐2‐agonists or a more complex regulation mechanism of pancreatic beta cell alpha‐2‐adrenergic receptors such as a greater role of pancreatic alpha cell alpha‐2‐adrenergic receptors in those horses, as documented in rats. 5 Determination of glucagon concentrations was beyond the scope of this study; however, this dual effect of alpha‐2‐agonists on pancreatic alpha and beta cells would require further investigation. These data also confirm the results of other studies regarding glucose being a poorly sensitive and poorly repeatable marker when investigating ID in horses.6, 18
The second main finding of this study is the different insulin response observed in ID horses receiving detomidine compared to xylazine. As mentioned above, after xylazine hydrochloride administration, insulin concentration in ID horses more than doubled, whereas after detomidine hydrochloride administration, insulin concentration did not rebound. The observed difference between xylazine and detomidine could be explained by a different affinity for alpha‐2‐adrenergic receptors. Detomidine has a 100‐fold higher affinity for alpha 2‐adrenergic receptors compared to xylazine, suggesting that, as observed in this study, a better binding between detomidine and pancreatic beta cells alpha‐2‐adrenergic receptors could be expected resulting in a longer inhibition of insulin secretion and an absence of insulin rebound. 21
The immediate consequence of these results could be that careful selection of alpha‐2‐agonists might be warranted in horses with ID as using detomidine might limit the degree of hyperinsulinemia. This could be beneficial in some specific cases and limit the risk of potential iatrogenic lamellar damage and associated laminitis. 10
Another possible consequence of these results could be that alpha‐2‐agonists, and especially detomidine, could be investigated as potential targets to decrease insulin secretion in horses with ID. In dogs and humans with type‐2 diabetes, alpha‐2‐antagonists have been investigated as potential targets to stimulate insulin secretion from pancreatic beta cell islets. 22 That being said, alpha‐2‐agonists also have significant influence over many functions such as the cardiovascular system, and as such, their use must be carefully deliberated upon when considering administration to patients. 23 Inhibition of insulin secretion via stimulation of alpha‐2 receptors on pancreatic beta cells might provide a means of controlling insulin secretion; however, further research is required to document the appropriateness of alpha‐2‐agonist treatment.
Only 1 dosage of each drug was tested, and the amounts given are the label dosages in horses for each product. Because alpha‐2‐agonists have dose‐dependent effects, it is possible that insulin response to higher or lower dosages would have been different and that at doses commonly used in practice, xylazine and detomidine would have had similar effects. 1 Another limitation of this study was the use of different assays to measure insulin concentration. It has been well described that, as most insulin assays are immunoassays, the amount of immunoreactive insulin detected by each assay can vary and that a simple correction factor cannot be used to easily compare concentrations obtained with 1 assay with concentrations obtained with another assay.17, 24 Therefore, this limitation was addressed by using the percent change in insulin concentration rather than the actual measured insulin concentration. An additional limitation was the use of a 24‐hour washout period between alpha‐2‐agonist testing periods. Because of the long‐acting detomidine, a longer washout period between measurements might have been better for increased accuracy. A final limitation of the study was the number of horses included; with a higher number of horses, some of the changes might have become statistically significant.
In summary, this study indicates that administration of alpha‐2‐agonists results in an initial decrease in blood insulin in both control and ID horses; followed by a marked increase in insulin secretion with xylazine. Further research is required to investigate the repeatability of these findings and the potential clinical application of alpha‐2‐agonists to ameliorate hyperinsulinemia in horses with ID.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The University of Queensland and Purdue University Animal Ethics Committees approved all procedures. The University of Queensland Animal Ethics certificates are SVS/475/17 and SVS/481/18.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1 Results of the oral sugar test (OST) or oral glucose test (OGT) and 2‐step insulin sensitivity test in 7 control horses and 7 horses with insulin dysregulation (ID)
ACKNOWLEDGMENTS
The authors thank The University of Queensland Summer Research Scholarship. This article was presented in part as a research poster abstract at the 3rd International Equine Endocrinology Summit in Coral Gables, Florida, January 2017.
Kritchevsky JE, Muir GS, Leschke DHZ, Hodgson JK, Hess EK, Bertin F‐R. Blood glucose and insulin concentrations after alpha‐2‐agonists administration in horses with and without insulin dysregulation. J Vet Intern Med. 2020;34:902–908. 10.1111/jvim.15747
Funding information John & Mary Kibble Trust
REFERENCES
- 1. Rohrbach H, Korpivaara T, Schatzmann U, Spadavecchia C. Comparison of the effects of the alpha‐2 agonists detomidine, romifidine and xylazine on nociceptive withdrawal reflex and temporal summation in horses. Vet Anaesth Analg. 2009;36:384‐395. [DOI] [PubMed] [Google Scholar]
- 2. England GC, Clarke KW, Goossens L. A comparison of the sedative effects of three alpha 2‐adrenoceptor agonists (romifidine, detomidine and xylazine) in the horse. J Vet Pharmacol Ther. 1992;15:194‐201. [DOI] [PubMed] [Google Scholar]
- 3. Grimsrud KN, Ait‐Oudhia S, Durbin‐Johnson BP, et al. Pharmacokinetic and pharmacodynamic analysis comparing diverse effects of detomidine, medetomidine, and dexmedetomidine in the horse: a population analysis. J Vet Pharmacol Ther. 2015;38:24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ringer SK, Schwarzwald CC, Portier K, Mauch J, Ritter A, Bettschart‐Wolfensberger R. Blood glucose, acid‐base and electrolyte changes during loading doses of alpha(2)‐adrenergic agonists followed by constant rate infusions in horses. Vet J. 2013;198:684‐689. [DOI] [PubMed] [Google Scholar]
- 5. Hirose H, Maruyama H, Ito K, Kido K, Koyama K, Saruta T. Effects of alpha 2‐ and beta‐adrenergic agonism on glucagon secretion from perfused pancreata of normal and streptozocin‐induced diabetic rats. Metabolism. 1993;42:1072‐1076. [DOI] [PubMed] [Google Scholar]
- 6. de Laat MA, McGree JM, Sillence MN. Equine hyperinsulinemia: investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol Endocrinol Metab. 2016;310:E61‐E72. [DOI] [PubMed] [Google Scholar]
- 7. Bertin FR, Ruffin‐Taylor D, Stewart AJ. Insulin dysregulation in horses with systemic inflammatory response syndrome. J Vet Intern Med. 2018;32:1420‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46:103‐112. [DOI] [PubMed] [Google Scholar]
- 9. Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ, American College of Veterinary Internal Medicine . Equine metabolic syndrome. J Vet Intern Med. 2010;24:467‐475. [DOI] [PubMed] [Google Scholar]
- 10. de Laat MA, McGowan CM, Sillence MN, et al. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42:129‐135. [DOI] [PubMed] [Google Scholar]
- 11. de Laat MA, Sillence MN. The repeatability of an oral glucose test in ponies. Equine Vet J. 2017;49:238‐243. [DOI] [PubMed] [Google Scholar]
- 12. Schuver A, Frank N, Chameroy KA, Elliott SB. Assessment of insulin and glucose dynamics by using an oral sugar test in horses. J Equine Vet. 2014;34:465‐470. [Google Scholar]
- 13. Bertin FR, Sojka‐Kritchevsky JE. Comparison of a 2‐step insulin‐response test to conventional insulin‐sensitivity testing in horses. Domest Anim Endocrinol. 2013;44:19‐25. [DOI] [PubMed] [Google Scholar]
- 14. Bertin FR, Taylor SD, Bianco AW, Sojka‐Kritchevsky JE. The effect of fasting duration on baseline blood glucose concentration, blood insulin concentration, glucose/insulin ratio, oral sugar test, and insulin response test results in horses. J Vet Intern Med. 2016;30:1726‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertin FR, de Laat MA. The diagnosis of equine insulin dysregulation. Equine Vet J. 2017;49:570‐576. [DOI] [PubMed] [Google Scholar]
- 16. Bertin FR, Pader KS, Lescun TB, Sojka‐Kritchevsky JE. Short‐term effect of ovariectomy on measures of insulin sensitivity and response to dexamethasone administration in horses. Am J Vet Res. 2013;74:1506‐1513. [DOI] [PubMed] [Google Scholar]
- 17. Warnken T, Huber K, Feige K. Comparison of three different methods for the quantification of equine insulin. BMC Vet Res. 2016;12:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eiler H, Frank N, Andrews FM, Oliver JW, Fecteau KA. Physiologic assessment of blood glucose homeostasis via combined intravenous glucose and insulin testing in horses. Am J Vet Res. 2005;66:1598‐1604. [DOI] [PubMed] [Google Scholar]
- 19. Toth F, Frank N, Elliott SB, et al. Optimisation of the frequently sampled intravenous glucose tolerance test to reduce urinary glucose spilling in horses. Equine Vet J. 2009;41:844‐851. [DOI] [PubMed] [Google Scholar]
- 20. Pratt SE, Geor RJ, McCutcheon LJ. Repeatability of 2 methods for assessment of insulin sensitivity and glucose dynamics in horses. J Vet Intern Med. 2005;19:883‐888. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz DD, Clark TP. Affinity of detomidine, medetomidine and xylazine for alpha‐2 adrenergic receptor subtypes. J Vet Pharmacol Ther. 1998;21:107‐111. [DOI] [PubMed] [Google Scholar]
- 22. Fagerholm V, Haaparanta M, Scheinin M. Alpha‐2‐adrenoceptor regulation of blood glucose homeostasis. Basic Clin Pharmacol Toxicol. 2011;108:365‐370. [DOI] [PubMed] [Google Scholar]
- 23. Ringer SK, Schwarzwald CC, Portier KG, Ritter A, Bettschart‐Wolfensberger R. Effects on cardiopulmonary function and oxygen delivery of doses of romifidine and xylazine followed by constant rate infusions in standing horses. Vet J. 2013;195:228‐234. [DOI] [PubMed] [Google Scholar]
- 24. Carslake HB, Pinchbeck GL, McGowan CM. Evaluation of a chemiluminescent immunoassay for measurement of equine insulin. J Vet Intern Med. 2017;31:568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Results of the oral sugar test (OST) or oral glucose test (OGT) and 2‐step insulin sensitivity test in 7 control horses and 7 horses with insulin dysregulation (ID)


