Abstract
Staghorn calculi comprise a unique subset of complex kidney stone disease. Percutaneous nephrolithotomy (PCNL) is the gold standard treatment for staghorn stones. Despite continuous refinements to the technique and instrumentation of PCNL, these stones remain a troublesome challenge for endourologists and are associated with a higher rate of perioperative complications than that for non-staghorn stones. Common and notable intraoperative complications include bleeding, renal collecting system injury, injury of visceral organs, pulmonary complications, thromboembolic complications, extrarenal stone migration, and misplacement of the nephrostomy tube. Postoperative complications include infection and urosepsis, bleeding, persistent nephrocutaneous urine leakage, infundibular stenosis, and death. In this review, we report recommendations regarding troubleshooting measures that can be used to identify and characterize these complications. Additionally, we include information regarding management strategies for complications associated with PCNL for staghorn calculi.
Keywords: Percutaneous nephrolithotomy, Staghorn, Complications, Management, Urolithiasis
1. Introduction
Staghorn calculi comprise complete and partial forms. Complete staghorn stones occupy the renal pelvis and the caliceal system, or more than 80% of the renal collecting system, while partial stones occupy the renal pelvis and at least two calices [1]. According to current guidelines, large-volume and staghorn stones should be managed with percutaneous nephrolithotomy (PCNL) [2,3].
Despite recent refinements to the technique and instrumentation of PCNL for the treatment of staghorn calculi, the number of PCNL procedures remained stable over the years and these stones are still a troublesome challenge for endourologists and are associated with a higher rate of perioperative complications than that for non-staghorn disease [4]. Furthermore, a study by the Clinical Research Office of the Endourological Society (CROES) revealed that staghorn patients treated with PCNL can expect a stone free rate of 56.9% compared with 82.5% for non-staghorn patients [5]. In addition, a rarely mentioned but important aspect of staghorn stone management is the potential for inadequate communication between the urologist and patient regarding complications and stone-free expectations. If clear communication regarding the challenging nature of staghorn stone management is lacking, the result may well be a complicated course of treatment with medico-legal litigation [6]. The use of scoring systems [[7], [8], [9]], audio-visual information and educational brochures can aid in physician-patient communication in the informed decision making process and can help to appropriately manage patient expectations [[10], [11], [12]].
In understanding and anticipating the potential for complications associated with PCNL for staghorn stones, one must consider variables for the patient, the stone, and the renal anatomy. Regarding the patient, fragility index scores, comorbid status, and obesity must be taken into account. As for the stone and the anatomy, computed tomography (CT) imaging is the gold standard imaging modality [13] and can provide information on stone characteristics including skin-to-stone distance [7], vicinity of visceral organs [14], stone stereolithometry, stone burden, stone density [9,15], angles between the entry point and stone branches [16], and width of the infundibular neck at the calyx of entry [17]. In some cases, it is prudent to obtain the CT scan with the patient positioned as they will be during the operation (i.e. prone vs. supine) [18].
2. Complications overview
All complications are reported according to the Clavien-Dindo classification system [19]. The complication rate for PCNL is as high as 83%, but many of these are minor complications [20]. Major complications require timely diagnosis and proper treatment and occur at a rate between 1.1% and 7.0% [21].
Complications may be divided into intra- and postoperative complications. Intraoperative complications include the following: Bleeding, renal collecting system injury, injury of visceral organs, pulmonary complications, thromboembolic complications, extrarenal stone migration, and misplacement of the nephrostomy tube.
Postoperative complications include infection and urosepsis, bleeding, persistent nephrocutaneous urine leakage, infundibular stenosis, and death.
In this review, we did not consider failure to achieve stone-free status as a complication since it is related to the efficacy of the PCNL.
3. Intraoperative complications
3.1. Bleeding
3.1.1. Troubleshooting
Staghorn calculi are significantly associated with intraoperative bleeding [22]. This is largely due to larger tract size [23] and multiple punctures needed to achieve stone-free status. Moreover, the use of rigid nephroscopes to reach the calices occupied by the stone may injure the renal parenchyma and caliceal necks, resulting in increased bleeding [24]. Blood loss during the procedure often goes unnoticed, and surgeon-estimated blood loss often underestimates true blood loss [25].
The use of hemostatic agents and sealants in PCNL is a viable option; however, these products have not yet demonstrated proven efficacy [26]. If during PCNL the visual field is obscured due to bleeding, attempts should be made to restore vision by increasing irrigation flow, as an adequate field of view is strongly related to achieving stone-free status. However, if the patient develops signs of hypotension, then the procedure should be aborted and immediate active management initiated.
3.1.2. Management
For vision restoration, Amplatz sheath manipulation may tamponade small parenchymal vessels. If sheath manipulation does not restore vision, then tranexamic acid can be used. Kumar et al. [27] reported that the use of tranexamic acid during PCNL improved the visual field and was associated with a mean reduction in operative time of 22 min and a mean reduction in irrigation fluid volume of 5.5 L. If tranexamic acid is used, then one should consider inserting a double-J ureteral stent, as lysis of clots in the pelvicaliceal system takes longer than usual and may result in obstruction.
In cases of continuous brisk venous bleeding, it is better to end the procedure by placing a large bore (>18 Ch) nephrostomy tube and clamping it for at least 1 h, allowing the bleeding sites to tamponade. In cases of brisk arterial bleeding and signs of hypotension, a Kaye tamponade balloon catheter should be inflated up to 36 Ch in the renal parenchyma, and the patient should undergo immediate angioembolization. Aside from these extreme cases, most of the bleeding that is related to PCNL can be managed with supportive treatment, and less than 1.5% of patients require angioembolization [28,29]. If angioembolization fails, then hemorrhage can be controlled by partial nephrectomy or renorrhaphy as a last resort [30].
Options for reducing the risk of bleeding complications include the use of miniaturized instruments [31] and transpapillary anatomical puncture [32].
3.2. Renal collecting system injury
3.2.1. Troubleshooting
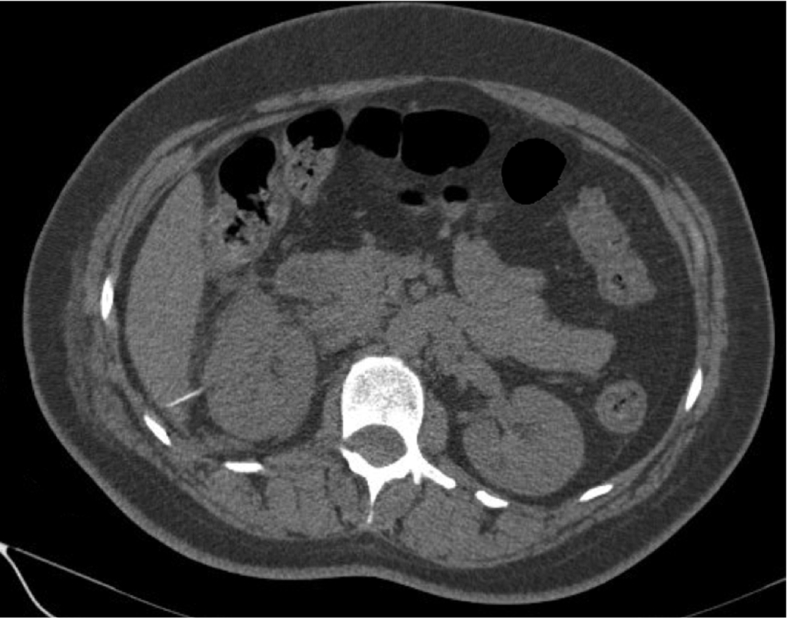
Renal collecting system injury during PCNL occurs in up to 8% of patients. Usually, it is due to too far dilation, improper choice of a guidewire, or overzealous use of rigid nephroscopy. The resultant extravasation and absorption of irrigation fluid can lead to electrolyte abnormalities, mental status changes, or intravascular volume overload [33]. Intraoperative signs of renal collecting system injury include contrast extravasation on fluoroscopy, direct visualization of perinephric structures or fat, abnormal hemodynamic parameters, and a decrease in irrigation fluid drainage [34]. Postoperative enhanced CT may reveal signs of urine leakage (Fig. 1).
Figure 1.
Enhanced CT scan demonstrating massive urine leakage through renal collecting system defect. L, left; R, right. CT, computed tomography.
3.2.2. Management
Endoscopic-guided renal access has been shown to decrease the complications associated with too far dilation. Khan and colleagues [35] reported their experience with endoscopic-guided access using an approach that allows for precise insertion of the needle and placement of the sheath, reducing the risk of complications associated with tract dilation. However, in patients with staghorn stones flexible ureteroscope may not reach the calyx of interest. Minor extravasation associated with small renal collecting system injury typically does not require early cessation of the procedure, but large disruptions, including perforation of the renal pelvis, require prompt cessation and adequate drainage via a nephrostomy tube, ureteral stent or percutaneous drain [33]. Drainage should be kept in place for at least 5–7 days. The use of sealants to close the renal collecting system defect is not recommended [36].
3.3. Injury of visceral organs during PCNL
3.3.1. Liver
3.3.1.1. Troubleshooting
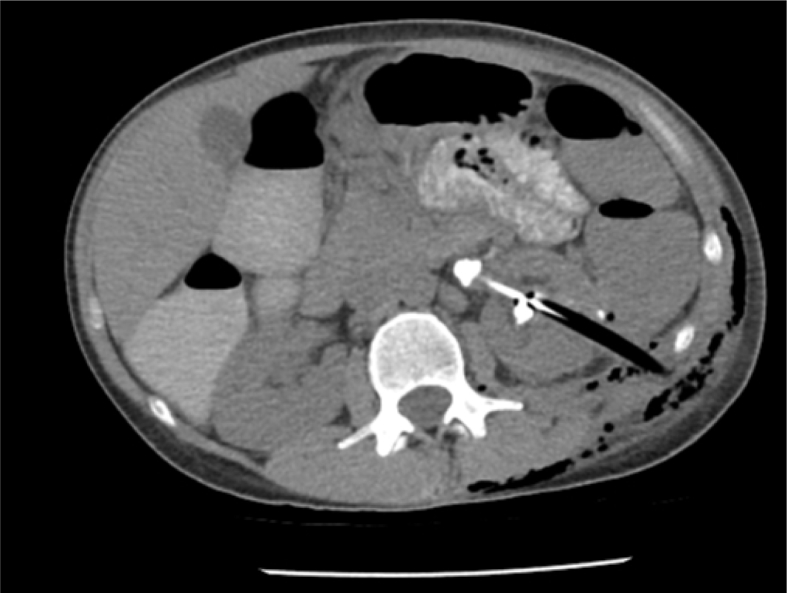
Liver injury during PCNL is a rare complication and may go unnoticed. A higher risk of liver injury is associated with right-sided supracostal (superior to the 11th rib) percutaneous renal access anterior to the posterior axillary line. Hepatomegaly also increases the risk of liver injury during percutaneous access [21]. Postoperatively, patients with a liver injury may describe an unusual burning sensation at the right flank. A CT scan can reveal the route of injury to the liver (Fig. 2) [37]. In order to prevent liver injury ultrasound scanning of the puncturing site can be utilized [38].
Figure 2.
Axial CT scan demonstrating liver injury. The nephrostomy tube traverses the liver. CT, computed tomography.
3.3.1.2. Management
If the patient is hemodynamically stable, no exploration is needed. Conservative measures include close monitoring and coagulant agents as needed [37]. Prolonged nephrostomy drainage to ensure proper healing of the injured site is also recommended [21]. Based on our experience, however, removal of the nephrostomy tube and placement of a ureteral stent are superior to placement of a nephrostomy tube alone. In addition, a Foley catheter must be inserted for adequate urinary drainage. Follow-up ultrasound or CT scan is recommended if there is a concern for the formation of a biloma [39].
3.3.2. Spleen
3.3.2.1. Troubleshooting
Significant injuries to the spleen are rare and have not been encountered even in large series [40], but the true incidence of spleen injury could be underestimated because of poor documentation [41]. The spleen lies obliquely along the long axis of the 10th rib on the left side, in close proximity to the anterolateral aspect of the left kidney [42]. Thus, left-sided PCNL with 10th intercostal access and/or splenomegaly are associated with a higher risk of splenic injury. Splenic trauma should be suspected in any patient who is undergoing left-sided PCNL with high intercostal access and who is hemodynamically unstable in the absence of significant intraoperative blood loss [40]. In a hemodynamically stable patient, abdominal CT scanning can characterize the injury. The diagnosis of splenic injury is challenging due to the variability of symptoms and signs, which can occur immediately or several days after the procedure.
3.3.2.2. Management
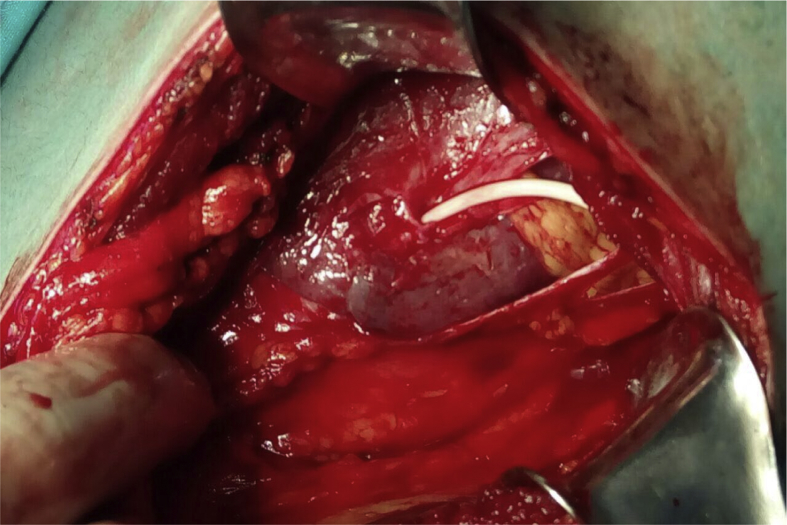
If splenic injury is diagnosed on postoperative CT scan and the patient is hemodynamically stable, strict bed rest is recommended. One may also consider leaving the nephrostomy tube in place to tamponade the bleeding and induce fibrosis, thereby decreasing the risk of catastrophic bleeding [43]. If the patient is hemodynamically unstable, then live-saving splenorraphy or splenectomy is mandated (Fig. 3). Hemostatic fibrin glue can be used to increase the chance of preserving the spleen [44]. In order to minimize the risk of splenic injury during percutaneous access, percutaneous puncture between the posterior axillary line and the spine and US scanning of the puncture site is recommended [45].
Figure 3.
Image demonstrating injury to the spleen. The nephrostomy tube traverses the spleen. Image obtained after open laparotomy. Image courtesy of Shaduri Vano.
3.3.3. Colon
3.3.3.1. Troubleshooting
Colonic perforation is a rare complication of percutaneous kidney surgery, and it is reported in fewer than 1% of cases [21]. The position of the colon is usually anterior or anterolateral to the lateral renal border, but retrorenal positioning of the colon is found in approximatively 0.6% of the general population [46]. A retrorenal colon is more frequently found on the left side and is most likely to be situated near the inferior kidney pole [47]. A retrorenal colon and/or a puncture that is too far lateral are two risk factors for colonic injury during PCNL [48].
The diagnosis of colonic injury is generally made on the post-PCNL nephrostogram after placing the nephrostomy tube. The success rate of conservative therapy is 86% if the diagnosis is made perioperatively or postoperatively before the removal of the nephrostomy tube; however, the success rate decreases by 50% if the diagnosis is delayed and the nephrostomy tube is removed prior to identifying the injury [49].
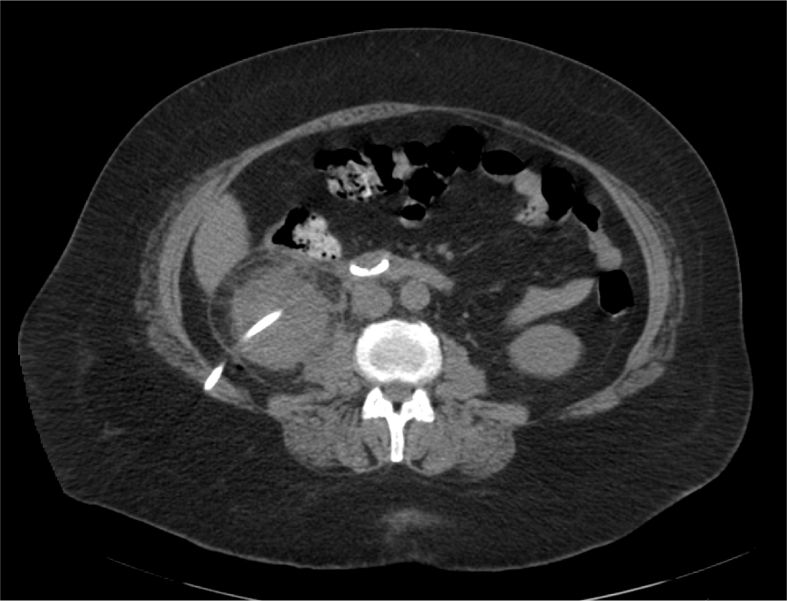
Colon perforation should be suspected if the patient develops unexplained fever or has intraoperative or immediate postoperative diarrhea or hematochezia, signs of peritonitis, or passage of gas or feces through the nephrostomy tract [49]. Abdominal CT is the best diagnostic tool to detect perforation of the colon by the nephrostomy tube (Fig. 4) [50]. In order to prevent colonic injury, ultrasound scanning of the puncturing site can be utilized [38].
Figure 4.
Axial CT scan demonstrating colonic injury. The nephrostomy tube traverses the left colon. CT, computed tomography.
3.3.3.2. Management
After the diagnosis of colonic perforation is made, if it is extraperitoneal, the first step is separation of the nephro-colic communication. A ureteral stent must be inserted, and the nephrostomy tube must be repositioned into the retroperitoneal space under fluoroscopic guidance [51]. In addition, a Foley catheter must be inserted to relieve the pressure in the urinary system. Conservative therapies include total parenteral nutrition, holding oral intake, and administration of broad-spectrum antibiotics. If a colostogram or retrograde urogram performed after 5–7 days does not exhibit extravasation or communication between the colon and collecting system, the Foley catheter may be removed and the nephrostomy tube withdrawn. A colostomy may be required in cases of colocutaneous fistula formation. In cases of intraperitoneal colonic perforation, peritonitis, sepsis, or failure of conservative management, open surgical intervention is warranted, and a colostomy is often required [48,51].
3.3.4. Gallbladder
3.3.4.1. Troubleshooting
Gallbladder injury during PCNL is a rare but potentially fatal complication, necessitating early diagnosis. As a result of challenges in making the diagnosis, gallbladder injury is often detected after the development of biliary peritonitis [21]. The combination of medial, right-sided percutaneous renal access in the setting of a distended gallbladder that lies in close proximity to the right kidney increases the risk of gallbladder injury, especially in thin individuals [52]. Aspiration of greenish fluid from the puncture needle is a clue that biliary tract injury has occurred. A patient who develops peritoneal signs in the postoperative period should also be evaluated for gallbladder injury [53].
3.3.4.2. Management
There are nine case reports in the literature regarding gallbladder injury that occurred during PCNL. All but one needed cholecystectomy [21]. The single case report that employed conservative management reported that the leak of bile from the gallbladder subsided and spontaneous closure of the perforation occurred. This may have occurred as a result of the use of a two-piece diamond tip needle, which is supposedly less traumatic than other conventional needles [52].
In the postoperative period, close clinical and ultrasound monitoring are required for patients with a suspected gallbladder injury. If the patient's clinical status deteriorates, then immediate cholecystectomy must be performed, preferably prior to the development of sepsis. Performing the initial caliceal puncture under combined ultrasonography and fluoroscopy guidance can possibly aid in avoiding gallbladder puncture and bile spillage [53].
3.3.5. Small bowel injury
3.3.5.1. Troubleshooting
Given the intraperitoneal location of the small bowel, it is generally a safe distance from the kidneys, and the risk of injury to the small intestine during PCNL is extremely low [54]. Injuries to the small bowel usually occur in right-sided PCNL cases during percutaneous access tract puncture or dilation [55]. The second and third portions of the duodenum are most commonly injured, as they are the portions of the small bowel located closest to the lower pole of the right kidney (Fig. 5) [48].
Figure 5.
Axial CT scan demonstrating small bowel injury. The nephrostomy tube resides in the second part of the duodenum. CT, computed tomography.
Usually, injury occurs due to over-advancement of a needle or an instrument. Intraoperative nephrostography at the end of the operation usually reveals any injury to the small bowel. Conventional postoperative CT may fail to diagnose small intestinal injuries in a tubeless PCNL [56]. The output of amber-color fluid from the nephrostomy tube should raise suspicion for a small bowel injury and should prompt analysis of the output fluid for bilirubin.
3.3.5.2. Management
Among published cases of small intestinal injuries during PCNL, the most common management strategy was exploratory laparotomy with repair of the damaged portion of the bowel [21]. However, conservative therapies can be considered if the diagnosis has been made in the early perioperative period.
To date, there are three cases in which conservative management of small bowel injury was successful. Two of these cases involved injury to the jejunum, and management consisted of prolonged fasting and total parenteral nutrition for 10 and 21 days, respectively [55,57]. The other case described successful endoscopic management of a duodenal injury that occurred after nephrostomy drainage of a lower pole hematoma. After removal of the percutaneous drain from the duodenum and endoscopic repair of the duodenal wall, the authors were able to remove the nephrostomy drain without the need for a periduodenal drain. The patient was subsequently managed with 3 days of bowel rest with a nasogastric tube in place [54].
3.4. Pulmonary complications
3.4.1. Troubleshooting
Pneumothorax, hydrothorax, hemothorax, and urinothorax are uncommon complications of PCNL, with an overall incidence of less than 2% [37]. Studies evaluating the incidence of pleural complications showed that supracostal (superior to the 12th rib) punctures [58,59] and left-sided procedures [60] are associated with a higher incidence of thoracic events. Chest complications have a reported incidence of 10% and 25% when access is obtained superior to the 12th and 11th rib, respectively [61]. Preoperative CT imaging is essential for operative planning, as it allows surgeons to determine the optimal location for percutaneous access and to identify the relationship of the pleura with the planned point of entry [62]. In order to avoid pleural injury during supracostal access renal displacement technique may be utilized [63]. If supracostal access is used, then it is prudent to obtain a postoperative chest radiograph in the recovery unit in order to assess for pneumothorax [64].
3.4.2. Management
In the case of a small-volume, asymptomatic pneumothorax or hydrothorax, the patient may be observed; if the patient becomes unstable or shows signs of pulmonary compromise, an immediate thoracic referral may be required [65]. Rarely, a nephropleural fistula can manifest anytime from the conclusion of the procedure to 2 weeks postoperatively; signs and symptoms may include continued thoracostomy tube drainage and shortness of breath. Diagnosis is typically made by retrograde pyelography and treatment is often successful with dual drainage of the pleural space and urinary system [66].
3.5. Thromboembolic complications
3.5.1. Troubleshooting
Deep venous thrombosis (DVT) has been reported in 1%–3% of patients undergoing PCNL [67]. DVT should be suspected in patients who present with leg swelling, pain, warmth, and erythema [68]. Usually, duplex ultrasound is sufficient for early diagnosis of DVT [69].
3.5.2. Management
Anticoagulation is typically required with the goal of preventing propagation and embolism. The use of anticoagulation may increase the risk of bleeding in the immediate postoperative period. In patients with mature nephrostomy tracts or in patients with minimal postoperative bleeding who are closely monitored, anticoagulation is safely tolerated [65].
3.6. Pulmonary embolism (PE)
3.6.1. Troubleshooting
PE is an extremely rare but serious complication of PCNL with potentially fatal consequences. This complication is most often associated with pressurized air pyelography [[70], [71], [72]] or patients with a pre-pulmonary atrioventricular shunt such as a septal heart defect [73,74]. Patients may present with hypoxemia, bradycardia, a decrease in end-tidal carbon dioxide, or cardiopulmonary arrest. Intraoperative diagnosis is made with echocardiography [65].
3.6.2. Management
If PE is suspected, the PCNL should be immediately terminated, and the patient should be placed in the left lateral decubitus position. A central venous line or puncture can be performed in order to attempt aspiration of the air bubble. Patients may need close postoperative cardiopulmonary monitoring, inotropic support, intensive supportive care, and, in cases with cerebral involvement (Fig. 6), hyperbaric oxygen therapy [73]. In order to mitigate the risk of PE, we recommend against the use of air pyelography during PCNL.
Figure 6.
Magnetic resonance imaging depicting ischemic region in thoracic cord.
3.7. Extrarenal stone migration
3.7.1. Troubleshooting
Extrarenal stone migration is a relatively rare and benign complication of PCNL. It is important, however, to document if a stone moves outside of the renal collecting system [65]. Extrarenal stone migration usually occurs due to the application of excessive pressure of the probe onto the stone, the existence of a perforation in the collecting system, or the use of an improper technique of stone extraction with an Amplatz sheath. Intraoperative pyelography and renal ultrasound are capable of locating the stone outside of the pelvicaliceal system.
3.7.2. Management
As long as the stone is not infected and fragment-associated inflammation does not obstruct the urinary tract, treatment is usually not necessary. Endoscopic retrieval of fragments outside of the urinary tract should not be attempted, as this may only enlarge the perforation [65]. Intraperitoneal [75] and pleural migration [76] of stones has been reported. These rare cases required laparoscopy and thoracosopy in order to prevent peritoneal and thoracic complications.
3.8. Misplacement of nephrostomy tube
3.8.1. Troubleshooting
Intravenous misplacement of a nephrostomy tube is a very rare complication. To date, 13 cases are published. This complication usually occurs at the stage of access tract dilation. The error is usually obvious, causing massive hemorrhage and necessitating immediate placement of a nephrostomy tube and abortion of the procedure [77].
3.8.2. Management
Most hemodynamically stable patients may be managed conservatively with strict bed rest, intravenous antibiotics, and CT or fluoroscopy-guided nephrostomy tube withdrawal. Notably, nephrostomy tube removal should take place in the operating room with the vascular team ready to intervene if needed. Open surgery can be used as an alternative treatment [78].
4. Postoperative complications
4.1. Infection and urosepsis
4.1.1. Troubleshooting
Urinary tract infections after PCNL occur in up to 37% of patients [79] and may lead to urosepsis in 0.3%–7.6% of cases [80]. Urosepsis is a life-threatening condition with a mortality rate of 66%–80% [20,81]. Infection is of particular concern in the treatment of staghorn calculi, which have an infectious origin in up to 68% of cases [82]. Additionally, the complex configuration of staghorn stones may necessitate multiple access tracts with standard sheath sizes (>24 Ch), which is associated with bleeding and an increased likelihood of blood transfusion. Both an increased number of access tracts as well as perioperative blood transfusion are significantly associated with postoperative infection [83]. Some studies have also identified diabetes mellitus [84] and female sex [85] as risk factors for postoperative infectious complications.
Significant postoperative leukocytosis and fever are a cause for concern and should prompt initiation of antibiotic therapy. Lactate and procalcitonin levels may be useful for detecting a significant postoperative infection [65].
4.1.2. Management
All patients with a urinary tract infection must be treated with appropriate antibiotics prior to PCNL in order to eradicate urinary tract bacteria. Patients with a staghorn stone presumed to be of infectious origin should be treated with antibiotics for at least 1 week prior to the procedure [65]. In “low risk” patients with negative preoperative urine cultures and without any urinary drains perioperative antibiotic prophylaxis seems sufficient [86]. If purulent urine is encountered at the time of percutaneous access, a nephrostomy tube should be placed and the PCNL should be aborted. The nephrostomy tube output should be sent for culture and the patient should be placed on broad-spectrum antibiotic therapy and observed until the culture results are known [20]. In order to minimize the risk of post-PCNL sepsis rate in patients with infection risk factors (history of LUT reconstruction, recurrent UTI) preoperative nephrostomy placement 7–10 days before PCNL with specific antibiotic treatment might be prudent [87].
During PCNL of an infectious stone, it is prudent to send a stone specimen for culture since bacteria in the stone and in the urine may differ [88]. Patients should be monitored closely for clinical signs or symptoms of infection, including vital signs, laboratory panel changes, and alterations in mental status. When urosepsis is suspected, the patient should immediately be transferred to the intensive care unit for aggressive fluid and antibiotic management as well as supportive measures including steroids and/or pressors. Patients who do not respond to these methods may be experiencing alternative pathology, including unrecognized injuries (which can be diagnosed by additional imaging) or fungal infections (especially in immunocompromised or diabetic patients or those with prolonged urinary drainage tubes) [89].
4.2. Postoperative bleeding
4.2.1. Troubleshooting
Early postoperative bleeding (<24 h) usually exists in the following forms: Bleeding through nephrostomy tube, access tract bleeding upon removal of a nephrostomy tube, and perinephric hemorrhage, which should be suspected in cases of clear urine with dropping blood counts and flank pain [65]. CT imaging can detect large perinephric hematoma formation. Delayed postoperative bleeding (1–3 weeks) after PCNL is a rare complication (1.2%) [90,91] and usually is secondary to arteriovenous fistula or pseudoaneurysm formation.
4.2.2. Management
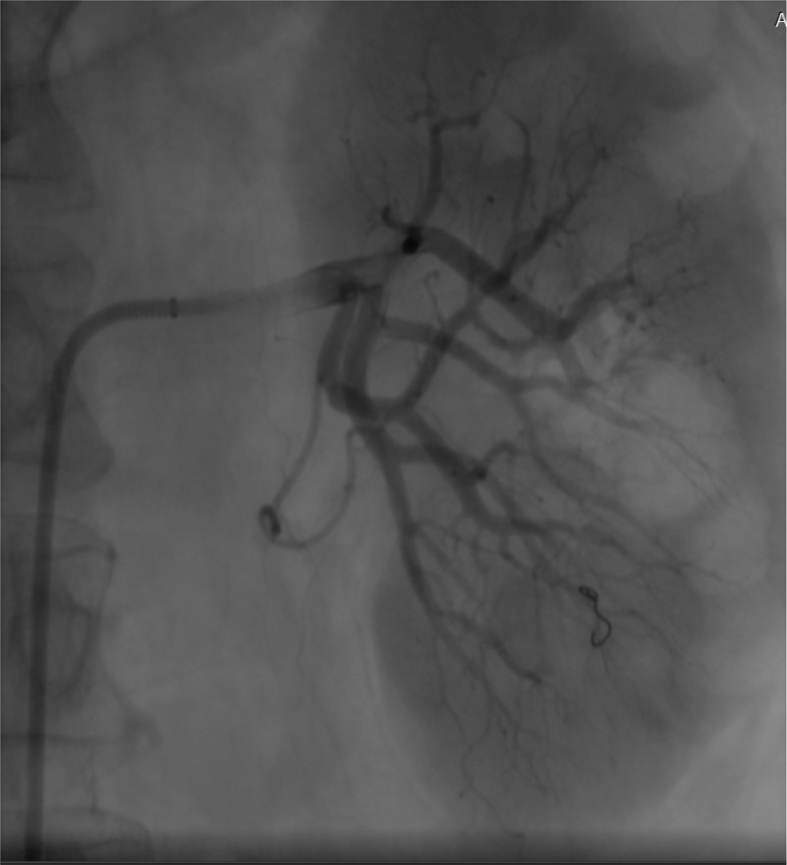
Venous bleeding through nephrostomy tube can be stopped by tube clamping for 1–2 h [92], where bleeding from the access tract can be controlled by digital tamponade [65] or by placing one's fist over the patient's back and another over the patient's abdomen and applying pressure [93]. Perinephric hemorrhage is usually self-limiting and can be managed conservatively with bed rest and transfusion as needed. Uncontrollable hemorrhage with hemoglobin drop (>4 g/dL) and/or clinical signs of hemodynamic instability should be an indication for emergent intervention [94]. Delayed bleeding should raise concern for arteriovenous fistula or pseudoaneurysm, where the patient will require angiography with embolization, which is usually sufficient to control the bleed (Fig. 7). If conservative measures as well as selective angioembolization fail, open surgical exploration with vascular repair or nephrectomy may be used as a last resort [65,93].
Figure 7.
Super selective embolization of a bleeding artery following percutaneous nephrolithotomy for staghorn stone.
4.3. Postoperative persistent nephrocutaneous leakage
4.3.1. Troubleshooting
The percutaneous access tract normally closes within 6–12 h of nephrostomy tube removal [95]. Urinary leakage persisting >24 h after nephrostomy tube removal called prolonged and usually needs treatment [96]. The incidence of prolonged nephrocutaneous leakage from the tract is between 1.5% and 4.6% [37]. It is advisable to obtain a low-dose CT scan to evaluate for stone fragments in the ureter that may be causing obstruction.
4.3.2. Management
The insertion of a ureteral stent is helpful in the majority of cases of prolonged urinary leakage [37]. In addition, a Foley catheter may be inserted for 24 h in order to relieve pressure in the urinary system and promote anterograde drainage of urine [97].
4.4. Infundibular stenosis
4.4.1. Troubleshooting
Postoperative infundibular stenosis is a rare complication (2%) of PCNL associated with protracted inflammatory processes of the renal urothelium that presumably induce fibrosis, scarring and gradual obliteration of the infundibular lumen. The radiographic hallmark of infundibular stenosis is a dilated calyx or calices with a normal renal pelvis. Among factors predisposing to infundibular stenosis are staghorn configuration of the stone and a nephroscope size that is disproportionately large compared to the diameter of the infundibulum, which can lead to rupture of the latter [98].
4.4.2. Management
The treatment approach for infundibular stenosis is usually endoscopic (either antegrade or retrograde), although the reported success rate is only 60%–80% [99]. Among methods of treatment are cold knife excision [100], laser ablation [101], balloon dilation [102] or endoscopic formation of a new infundibulum [103]. If none of these methods are successful, one may consider observation provided that the patient is asymptomatic and does not exhibit renal function deterioration [98]. Alternative strategies included permanent stenting, excision of the dilated portion of the kidney, or heminephrectomy [104].
4.5. Mortality
Mortality as a consequence of PCNL is an extremely rare complication. One cohort of 1 406 patients had a 0.2% mortality rate. Mortality rate is directly associated with both Charlson Comorbidity Index (CCI) [105] and operation duration [106]. Sepsis is the leading cause of PCNL-associated mortality. Risk factors for mortality include multiple comorbidities and spinal cord injury [106]. High-risk patients should receive proper preoperative counseling regarding their risk and should be informed of conservative treatment options [65].
5. Conclusion
PCNL is a safe procedure with a low incidence of major complications. However, patients with staghorn calculi are at an increased risk of experiencing intraoperative and postoperative complications, including bleeding, infection, and infundibular stenosis. Most complications can be managed conservatively, though a minority of complications may require surgical intervention. In all patients undergoing PCNL, appropriate perioperative measures should be taken in order to minimize the risk of preventable complications, and this is especially true for patients with staghorn calculi.
Author contributions
Study concept and design: Nariman Gadzhiev, Vigen Malkhasyan.
Data acquisition: Gagik Akopyan, Vigen Malkhasyan.
Data analysis and interpretation: Nariman Gadzhiev, Sergei Petrov.
Drafting of manuscript: Zhamshid Okhunov, Francis Jefferson.
Critical revision of the manuscript: Sergei Petrov, Zhamshid Okhunov.
Final approval of the version: Nariman Gadzhiev, Zhamshid Okhunov.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Mishra S., Bhattu A.S., Sabnis R.B., Desai M.R. Staghorn classification: platform for morphometry assessment. Indian J Urol. 2014;30:80–83. doi: 10.4103/0970-1591.124212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turk C., Petrik A., Sarica K., Seitz C., Skolarikos A., Straub M. 2019. EAU guidelines on urolithiasis.https://uroweb.org/guideline/urolithiasis/ [Accessed 2 July 2019] [Google Scholar]
- 3.Assimos D., Krambeck A., Miller N.L., Monga M., Murad M., Nelson C. 2019. American Urological Association (AUA) guideline—surgical management of stones.https://www.auanet.org/guidelines/kidney-stones-surgical-management-guideline [Accessed 13 May 2019] [Google Scholar]
- 4.Oberlin D.T., Flum A.S., Bachrach L., Matulewicz R.S., Flury S.C. Contemporary surgical trends in the management of upper tract calculi. J Urol. 2015;193:880–884. doi: 10.1016/j.juro.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Desai M., De Lisa A., Turna B., Rioja J., Walfridsson H., D'Addessi A. The clinical research office of the endourological society percutaneous nephrolithotomy global study: staghorn versus nonstaghorn stones. J Endourol. 2011;25:1263–1268. doi: 10.1089/end.2011.0055. [DOI] [PubMed] [Google Scholar]
- 6.Kee J.W.Y., Khoo H.S., Lim I., Koh M.Y.H. Communication skills in patient-doctor interactions: learning from patient complaints. Heal Prof Educ. 2018;4:97–106. [Google Scholar]
- 7.Okhunov Z., Friedlander J.I., George A.K., Duty B.D., Moreira D.M., Srinivasan A.K. S.T.O.N.E. nephrolithometry: novel surgical classification system for kidney calculi. Urology. 2013;81:1154–1159. doi: 10.1016/j.urology.2012.10.083. [DOI] [PubMed] [Google Scholar]
- 8.Thomas K., Smith N.C., Hegarty N., Glass J.M. The Guy's stone score—grading the complexity of percutaneous nephrolithotomy procedures. Urology. 2011;78:277–281. doi: 10.1016/j.urology.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Gadzhiev N., Brovkin S., Grigoryev V., Dmitriev V., Baketin P., Obidnyak V. Are we ready to predict percutaneous nephrolithotomy (PCNL) stone-free failure? J Clin Urol. 2016;9:11–18. [Google Scholar]
- 10.Anderson O.A., Wearne I.M.J. Informed consent for elective surgery—what is best practice? J R Soc Med. 2007;100:97–100. doi: 10.1258/jrsm.100.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bach T., Behrendt M., Tanidir Y., Cornford P., Sun Y., Van Poppel H. Harnessing new media tools in patient information. Eur Urol. 2018;74:685–687. doi: 10.1016/j.eururo.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Becker B., Gadzhiev N., Netsch C., Popiolek M., Pisarev A.V., Obidnyak V. A new mobile application for kidney stone patients. J Urol. 2018;199:e295. [Google Scholar]
- 13.Xiang H., Chan M., Brown V., Huo Y.R., Chan L., Ridley L. Systematic review and meta-analysis of the diagnostic accuracy of low-dose computed tomography of the kidneys, ureters and bladder for urolithiasis. J Med Imaging Radiat Oncol. 2017;61:582–590. doi: 10.1111/1754-9485.12587. [DOI] [PubMed] [Google Scholar]
- 14.Ganpule A.P., Vijayakumar M., Malpani A., Desai M.R. Percutaneous nephrolithotomy (PCNL) a critical review. Int J Surg. 2016;36:660–664. doi: 10.1016/j.ijsu.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Anastasiadis A., Onal B., Modi P., Turna B., Duvdevani M., Timoney A. Impact of stone density on outcomes in percutaneous nephrolithotomy (PCNL): an analysis of the clinical research office of the endourological society (CROES) PCNL global study database. Scand J Urol. 2013;47:509–514. doi: 10.3109/21681805.2013.803261. [DOI] [PubMed] [Google Scholar]
- 16.Guglielmetti G.B., Danilovic A., Torricelli F.C.M., Coelho R.F., Mazzucchi E., Srougi M. Predicting calyceal access for percutaneous nephrolithotomy with computed tomography multiplanar reconstruction. Clinics (Sao Paulo) 2013;68:892–895. doi: 10.6061/clinics/2013(06)27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koopman S.G., Fuchs G. Management of stones associated with intrarenal stenosis: infundibular stenosis and caliceal diverticulum. J Endourol. 2013;27:1546–1550. doi: 10.1089/end.2013.0186. [DOI] [PubMed] [Google Scholar]
- 18.Marchini G.S., Berto F.C.G., Vicentini F.C., Shan C.J., Srougi M., Mazzucchi E. Preoperative planning with noncontrast computed tomography in the prone and supine position for percutaneous nephrolithotomy: a practical overview. J Endourol. 2015;29:6–12. doi: 10.1089/end.2014.0299. [DOI] [PubMed] [Google Scholar]
- 19.de la Rosette J.J., Opondo D., Daels F.P., Giusti G., Serrano A., Kandasami S.V. Categorisation of complications and validation of the Clavien score for percutaneous nephrolithotomy. Eur Urol. 2012;62:246–255. doi: 10.1016/j.eururo.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 20.Michel M.S., Trojan L., Rassweiler J.J. Complications in percutaneous nephrolithotomy. Eur Urol. 2007;51:899–906. doi: 10.1016/j.eururo.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Öztürk H. Gastrointestinal system complications in percutaneous nephrolithotomy: a systematic review. J Endourol. 2014;28:1256–1267. doi: 10.1089/end.2014.0344. [DOI] [PubMed] [Google Scholar]
- 22.Turna B., Nazli O., Demiryoguran S., Mammadov R., Cal C. Percutaneous nephrolithotomy: variables that influence hemorrhage. Urology. 2007;69:603–607. doi: 10.1016/j.urology.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Cheng F., Yu W., Zhang X., Yang S., Xia Y., Ruan Y. Minimally invasive tract in percutaneous nephrolithotomy for renal stones. J Endourol. 2010;24:1579–1582. doi: 10.1089/end.2009.0581. [DOI] [PubMed] [Google Scholar]
- 24.Akman T., Binbay M., Sari E., Yuruk E., Tepeler A., Akcay M. Factors affecting bleeding during percutaneous nephrolithotomy: single surgeon experience. J Endourol. 2011;25:327–333. doi: 10.1089/end.2010.0302. [DOI] [PubMed] [Google Scholar]
- 25.Stoller M.L., Wolf J.S., St Lezin M.A. Estimated blood loss and transfusion rates associated with percutaneous nephrolithotomy. J Urol. 1994;152:1977–1981. doi: 10.1016/s0022-5347(17)32283-8. [DOI] [PubMed] [Google Scholar]
- 26.Wang J., Zhang C., Tan G., Yang B., Chen W., Tan D. The use of adjunctive hemostatic agents in tubeless percutaneous nephrolithotomy: a meta-analysis. Urolithiasis. 2014;42:509–517. doi: 10.1007/s00240-014-0717-5. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S., Randhawa M.S., Ganesamoni R., Singh S.K. Tranexamic acid reduces blood loss during percutaneous nephrolithotomy: A prospective randomized controlled study. J Urol. 2013;189:1757–1761. doi: 10.1016/j.juro.2012.10.115. [DOI] [PubMed] [Google Scholar]
- 28.Kessaris D.N., Bellman G.C., Pardalidis N.P., Smith A.G. Management of hemorrhage after percutaneous renal surgery. J Urol. 1995;153:604–608. doi: 10.1097/00005392-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Lee K.L., Stoller M.L. Minimizing and managing bleeding after percutaneous nephrolithotomy. Curr Opin Urol. 2007;17:120–124. doi: 10.1097/MOU.0b013e328010ca76. [DOI] [PubMed] [Google Scholar]
- 30.Aminsharifi A., Irani D., Eslahi A. Massive hemorrhage after percutaneous nephrolithotomy: saving the kidney when angioembolization has failed or is unavailable. Int J Surg. 2014;12:872–876. doi: 10.1016/j.ijsu.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Ghani K.R., Andonian S., Bultitude M., Desai M., Giusti G., Okhunov Z. Percutaneous nephrolithotomy: update, trends, and future directions. Eur Urol. 2016;70:382–396. doi: 10.1016/j.eururo.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 32.Campobasso D., Ferretti S., Frattini A. Papillary puncture: still a good practice. World J Urol. 2019;37:573–574. doi: 10.1007/s00345-018-2527-9. [DOI] [PubMed] [Google Scholar]
- 33.Taylor E., Miller J., Chi T., Stoller M.L. Complications associated with percutaneous nephrolithotomy. Transl Androl Urol. 2012;1:223–228. doi: 10.3978/j.issn.2223-4683.2012.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skolarikos A., de la Rosette J. Prevention and treatment of complications following percutaneous nephrolithotomy. Curr Opin Urol. 2008;18:229–234. doi: 10.1097/MOU.0b013e3282f46afc. [DOI] [PubMed] [Google Scholar]
- 35.Khan F., Borin J.F., Pearle M.S., McDougall E.M., Clayman R.V. Endoscopically guided percutaneous renal access: "Seeing is believing". J Endourol. 2006;20:451–455. doi: 10.1089/end.2006.20.451. [DOI] [PubMed] [Google Scholar]
- 36.Yu C., Xu Z., Long W., Longfei L., Feng Z., Lin Q. Hemostatic agents used for nephrostomy tract closure after tubeless PCNL: a systematic review and meta-analysis. Urolithiasis. 2014;42:445–453. doi: 10.1007/s00240-014-0687-7. [DOI] [PubMed] [Google Scholar]
- 37.Kallidonis P., Panagopoulos V., Kyriazis I., Liatsikos E. Complications of percutaneous nephrolithotomy. Curr Opin Urol. 2016;26:88–94. doi: 10.1097/MOU.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 38.Chu C., Masic S., Usawachintachit M., Hu W., Yang W., Stoller M. Ultrasound-guided renal access for percutaneous nephrolithotomy: a description of three novel ultrasound-guided needle techniques. J Endourol. 2016;30:153–158. doi: 10.1089/end.2015.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omar M., Monga M., Noble M. Iatrogenic hepatic subcapsular biloma following PCNL: diagnosis and management. Can Urol Assoc J. 2015;9:E397–E399. doi: 10.5489/cuaj.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah H.N., Hegde S.S., Mahajan A.P., Sodha H., Shah R., Bansal M. Splenic injury: rare complication of percutaneous nephrolithotomy: report of two cases with review of literature. J Endourol. 2007;21:919–922. doi: 10.1089/end.2006.0451. [DOI] [PubMed] [Google Scholar]
- 41.Cassar K., Munro A. Iatrogenic splenic injury. J R Coll Surg Edinb. 2002;47:731–741. [PubMed] [Google Scholar]
- 42.Wein A.J. Saunders:Elsevier; 2007. Campbell-Walsh urology ninth edition review; pp. 3–32. [Google Scholar]
- 43.Carey R.I., Siddiq F.M., Guerra J., Bird V.G. Conservative management of a splenic injury related to percutaneous nephrostolithotomy. JSLS. 2006;10:504–506. [PMC free article] [PubMed] [Google Scholar]
- 44.Canby-Hagino E.D., Morey A.F., Jatoi I., Perahia B., Bishoff J.T. Fibrin sealant treatment of splenic injury during open and laparoscopic left radical nephrectomy. J Urol. 2000;164:2004–2005. [PubMed] [Google Scholar]
- 45.Osman M., Wendt-Nordahl G., Heger K., Michel M.S., Alken P., Knoll T. Percutaneous nephrolithotomy with ultrasonography-guided renal access: experience from over 300 cases. BJU Int. 2005;96:875–878. doi: 10.1111/j.1464-410X.2005.05749.x. [DOI] [PubMed] [Google Scholar]
- 46.Sherman J.L., Hopper K.D., Greene A.J., Johns T.T. The retrorenal colon on computed tomography: a normal variant. J Comput Assist Tomogr. 1985;9:339–341. doi: 10.1097/00004728-198503000-00021. [DOI] [PubMed] [Google Scholar]
- 47.LeRoy A.J., Williams H.J., Bender C.E., Segura J.W., Patterson D.E., Benson R.C. Colon perforation following percutaneous nephrostomy and renal calculus removal. Radiology. 1985;155:83–85. doi: 10.1148/radiology.155.1.3975424. [DOI] [PubMed] [Google Scholar]
- 48.Traxer O. Management of injury to the bowel during percutaneous stone removal. J Endourol. 2009;23:1777–1780. doi: 10.1089/end.2009.1553. [DOI] [PubMed] [Google Scholar]
- 49.Öztürk H. Treatment of colonic injury during percutaneous nephrolithotomy. Rev Urol. 2015;17:194–201. [PMC free article] [PubMed] [Google Scholar]
- 50.Gerspach J.M., Bellman G.C., Stoller M.L., Fugelso P. Conservative management of colon injury following percutaneous renal surgery. Urology. 1997;49:831–836. doi: 10.1016/s0090-4295(97)00237-9. [DOI] [PubMed] [Google Scholar]
- 51.Seitz C., Desai M., Häcker A., Hakenberg O.W., Liatsikos E., Nagele U. Incidence, prevention, and management of complications following percutaneous nephrolitholapaxy. Eur Urol. 2012;61:146–158. doi: 10.1016/j.eururo.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Patil N.A., Kundargi V.S., Patil S.B., Biradar A.N., Desai A.S. Conservative management of accidental gall bladder puncture during percutaneous nephrolithotomy. Cent Eur J Urol. 2014;67:191–192. doi: 10.5173/ceju.2014.02.art15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yadav S., Singh A., Singh P. Biliary peritonitis following percutaneous nephrolithotomy: minimally invasive management. Indian J Urol. 2015;31:251–253. doi: 10.4103/0970-1591.159656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gadzhiev N., Gorelov D., Smirnov A., Al-Shukri S., Petrov S. Novel approach for endoscopic management of duodenal injury during perirenal infected haematoma drainage after shock-wave lithotripsy. Case Rep Urol. 2018;2018:2020572. doi: 10.1155/2018/2020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Assiri M., Binsaleh S., Libman J., Anidjar J. Jejunal perforation during percutaneous nephrolithotrypsy. SciWorld J. 2005;5:496–499. doi: 10.1100/tsw.2005.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Begliomini H., Mattos D., Jr. Bowel perforation during percutaneous renal surgery. Int Braz J Urol. 2002;28:533–535. [PubMed] [Google Scholar]
- 57.Marquesine Paul G., Slongo L.E., Rocha L.C. Enterocutaneous fistula as a complication of percutaneous nephrolithotomy in patients with previous bariatric surgery: case report and bibliographic review. Arch Esp Urol. 2010;63:460–464. [PubMed] [Google Scholar]
- 58.Lojanapiwat B., Prasopsuk S. Upper-pole access for percutaneous nephrolithotomy: comparison of supracostal and infracostal approaches. J Endourol. 2006;20:491–494. doi: 10.1089/end.2006.20.491. [DOI] [PubMed] [Google Scholar]
- 59.Tefekli A., Esen T., Olbert P.J., Tolley D., Nadler R.B., Sun Y.H. Isolated upper pole access in percutaneous nephrolithotomy: a large-scale analysis from the CROES percutaneous nephrolithotomy global study. J Urol. 2013;189:568–573. doi: 10.1016/j.juro.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 60.Palnizky G., Halachmi S., Barak M. Pulmonary complications following percutaneous nephrolithotomy: a prospective study. Curr Urol. 2013;7:113–116. doi: 10.1159/000356260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sourial M.W., Francois N., Box G.N., Knudsen B.E. Supracostal access tubeless percutaneous nephrolithotomy: minimizing complications. World J Urol. 2019:1429–1433. doi: 10.1007/s00345-018-2518-x. [DOI] [PubMed] [Google Scholar]
- 62.NG C.S., Herts B.R., Streem S.B. Percutaneous access to upper pole renal stones: role of prone 3-dimensional computerized tomography in inspiratory and expiratory phases. J Urol. 2005;173:124–126. doi: 10.1097/01.ju.0000146792.69885.1b. [DOI] [PubMed] [Google Scholar]
- 63.Lezrek M., Bazine K., Ammani A., Asseban M., Kassmaoui E.H., Qarro A. Needle renal displacement technique for the percutaneous approach to the superior calix. J Endourol. 2011;25:1723–1726. doi: 10.1089/end.2010.0721. [DOI] [PubMed] [Google Scholar]
- 64.Webb D.R. Webb DR. Percutaneous renal surgery. Springer International Publishing; Switzerland: 2016. Complications of PCNL; pp. 169–185. [Google Scholar]
- 65.Wollin D.A., Preminger G.M. Percutaneous nephrolithotomy: complications and how to deal with them. Urolithiasis. 2018;46:87–97. doi: 10.1007/s00240-017-1022-x. [DOI] [PubMed] [Google Scholar]
- 66.Lallas C.D., Delvecchio F.C., Evans B.R., Silverstein A.D., Preminger G.M., Auge B.K. Management of nephropleural fistula after supracostal percutaneous nephrolithotomy. Urology. 2004;64:241–245. doi: 10.1016/j.urology.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 67.Segura J.W., Patterson D.E., LeRoy A.J., Williams H.J., Barrett D.M., Benson R.C. Percutaneous removal of kidney stones: review of 1,000 cases. J Urol. 1985;134:1077–1081. doi: 10.1016/s0022-5347(17)47633-6. [DOI] [PubMed] [Google Scholar]
- 68.Wells P.S., Hirsh J., Anderson D.R., Lensing A.W., Foster G., Kearon C. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345:1326–1330. doi: 10.1016/s0140-6736(95)92535-x. [DOI] [PubMed] [Google Scholar]
- 69.Shahrour W., Andonian S. Ambulatory percutaneous nephrolithotomy: initial series. Urology. 2010;76:1288–1292. doi: 10.1016/j.urology.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Pyron C.L., Segal A.J. Air embolism: a potential complication of retrograde pyelography. J Urol. 1983;130:125–126. doi: 10.1016/s0022-5347(17)50990-8. [DOI] [PubMed] [Google Scholar]
- 71.Parikh G.P., Sonde S.R., Kadam P. Venous air embolism: A complication during percutaneous nephrolithotomy. Indian J Urol. 2014;30:348–349. doi: 10.4103/0970-1591.128510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Usha N. Air embolism—a complication of percutaneous nephrolithotripsy. Br J Anaesth. 2003;91:760–761. doi: 10.1093/bja/aeg630. [DOI] [PubMed] [Google Scholar]
- 73.Chahal D., Ruzhynsky V., McAuley I., Sweeney D., Sobkin P., Kinahan M. Paradoxical air embolism during percutaneous nephrolithotomy due to patent foramen ovale: case report. Can Urol Assoc J. 2015;9:658–660. doi: 10.5489/cuaj.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhowmick S., Sarma K., Kayal A.K., Basumatary L.J. Cerebrospinal air embolism following percutaneous nephrolithotomy: gravitational gradient effect. Neurol India. 2014;62:221–222. doi: 10.4103/0028-3886.132444. [DOI] [PubMed] [Google Scholar]
- 75.Diri A., Karakan T., Resorlu M., Kabar M., Germiyanoglu C. Intraperitoneal stone migration during percutaneos nephrolithotomy. Arch Ital Urol Androl. 2014;86:293–294. doi: 10.4081/aiua.2014.4.293. [DOI] [PubMed] [Google Scholar]
- 76.Lezrek M., Tazi H., Slimani A., Asseban M., Bazine K., Kasmaoui E.H. Stone migration in the pleural cavity: an unusual complication of percutaneous renal surgery. Eur Urol Suppl. 2014;13:eV8. [Google Scholar]
- 77.Fu W., Yang Z., Xie Z., Yan H. Intravenous misplacement of the nephrostomy catheter following percutaneous nephrostolithotomy: two case reports and literature review. BMC Urol. 2017;17:43. doi: 10.1186/s12894-017-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ge G., Wang Z., Wang M., Li G., Xu Z., Wang Y. Inadvertent insertion of nephrostomy tube into the renal vein following percutaneous nephrolithotomy: a case report and literature review. Asian J Urol. 2020;7:64–67. doi: 10.1016/j.ajur.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh P., Yadav S., Singh A., Saini A.K., Kumar R., Seth A. Systemic inflammatory response syndrome following percutaneous nephrolithotomy: assessment of risk factors and their impact on patient outcomes. Urol Int. 2016;96:207–211. doi: 10.1159/000441954. [DOI] [PubMed] [Google Scholar]
- 80.Olvera-Posada D., Tailly T., Alenezi H., Violette P.D., Nott L., Denstedt J.D. Risk factors for postoperative complications of percutaneous nephrolithotomy at a tertiary referral center. J Urol. 2015;194:1646–1651. doi: 10.1016/j.juro.2015.06.095. [DOI] [PubMed] [Google Scholar]
- 81.O'Keeffe N.K., Mortimer A.J., Sambrook P.A., Rao P.N. Severe sepsis following percutaneous or endoscopic procedures for urinary tract stones. Br J Urol. 1993;72:277–283. doi: 10.1111/j.1464-410x.1993.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 82.Viprakasit D.P., Sawyer M.D., Herrell S.D., Miller N.L. Changing composition of staghorn calculi. J Urol. 2011;186:2285–2290. doi: 10.1016/j.juro.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 83.Lai W.S., Assimos D. Factors associated with postoperative infection after percutaneous nephrolithotomy. Rev Urol. 2018;20:7–11. doi: 10.3909/riu0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu C., Zhang X., Liu Y., Wang P. Prevention and treatment of septic shock following mini-percutaneous nephrolithotomy: a single-center retrospective study of 834 cases. World J Urol. 2013;31:1593–1597. doi: 10.1007/s00345-012-1002-2. [DOI] [PubMed] [Google Scholar]
- 85.Kumar S., Bag S., Ganesamoni R., Mandal A.K., Taneja N., Singh S.K. Risk factors for urosepsis following percutaneous nephrolithotomy: role of 1 week of nitrofurantoin in reducing the risk of urosepsis. Urol Res. 2012 Feb;40:79–86. doi: 10.1007/s00240-011-0386-6. [DOI] [PubMed] [Google Scholar]
- 86.Chew B.H., Miller N.L., Abbott J.E., Lange D., Humphreys M.R., Pais V.M. A randomized controlled trial of preoperative prophylactic antibiotics prior to percutaneous nephrolithotomy in a low infectious risk population: a Report from the EDGE consortium. J Urol. 2018;200:801–808. doi: 10.1016/j.juro.2018.04.062. [DOI] [PubMed] [Google Scholar]
- 87.Benson A.D., Juliano T.M., Miller N.L. Infectious outcomes of nephrostomy drainage before percutaneous nephrolithotomy compared to concurrent access. J Urol. 2014;192:770–774. doi: 10.1016/j.juro.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Margel D., Ehrlich Y., Brown N., Lask D., Livne P.M., Lifshitz D.A. Clinical implication of routine stone culture in percutaneous nephrolithotomy—a prospective study. Urology. 2006;67:26–29. doi: 10.1016/j.urology.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Gautam G., Singh A.K., Kumar R., Hemal A.K., Kothari A. Beware! Fungal urosepsis may follow endoscopic intervention for prolonged indwelling ureteral stent. J Endourol. 2006;20:522–524. doi: 10.1089/end.2006.20.522. [DOI] [PubMed] [Google Scholar]
- 90.Keoghane S.R., Cetti R.J., Rogers A.E., Walmsley B.H. Blood transfusion, embolisation and nephrectomy after percutaneous nephrolithotomy (PCNL) BJU Int. 2013;111:628–632. doi: 10.1111/j.1464-410X.2012.11394.x. [DOI] [PubMed] [Google Scholar]
- 91.Richstone L., Reggio E., Ost M.C., Seideman C., Fossett L.K., Okeke Z. First Prize (tie): hemorrhage following percutaneous renal surgery: characterization of angiographic findings. J Endourol. 2008;22:1129–1135. doi: 10.1089/end.2008.0061. [DOI] [PubMed] [Google Scholar]
- 92.Srivastava A., Singh K.J., Suri A., Dubey D., Kumar A., Kapoor R. Vascular complications after percutaneous nephrolithotomy: are there any predictive factors? Urology. 2005;66:38–40. doi: 10.1016/j.urology.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 93.Ganpule A.P., Shah D.H., Desai M.R. Postpercutaneous nephrolithotomy bleeding. Curr Opin Urol. 2014;24:189–194. doi: 10.1097/MOU.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 94.Jinga V., Dorobat B., Youssef S., Radavoi G.D., Braticevici B., Filipoiu F. Transarterial embolization of renal vascular lesions after percutaneous nephrolithotomy. Chirurgia (Bucur) 2013;108:521–529. [PubMed] [Google Scholar]
- 95.Tefekli A., Altunrende F., Tepeler K., Tas A., Aydin S., Muslumanoglu A.Y. Tubeless percutaneous nephrolithotomy in selected patients: a prospective randomized comparison. Int Urol Nephrol. 2007;39:57–63. doi: 10.1007/s11255-006-9040-6. [DOI] [PubMed] [Google Scholar]
- 96.Ansari H., Tomar V., Yadav S.S., Agarwal N. Study of predictive factors affecting the prolonged urinary leakage after percutaneous nephrolithotomy. Urol Ann. 2016;8:60–65. doi: 10.4103/0974-7796.164856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andonian S., Okhunov Z., Shapiro E.Y., Smith A.D., Okeke Z. Diagnostic utility and clinical value of postpercutaneous nephrolithotomy nephrostogram. J Endourol. 2010;24:1427–1430. doi: 10.1089/end.2010.0173. [DOI] [PubMed] [Google Scholar]
- 98.Parsons J.K., Jarrett T.W., Lancini V., Kavoussi L.R. Infundibular stenosis after percutaneous nephrolithotomy. J Urol. 2002;167:35–38. [PubMed] [Google Scholar]
- 99.Goel A., Goel A., Dalela D. Excluded calyx following percutaneous nephrolithotomy: a rare complication. Indian J Surg. 2013;75:56–58. doi: 10.1007/s12262-012-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang T.K., Park Y.H. Endoscopic infundibulotomy in tuberculous renal infundibular stricture. J Urol. 1994;151:852–854. doi: 10.1016/s0022-5347(17)35104-2. [DOI] [PubMed] [Google Scholar]
- 101.Kim H.L., Gerber G.S. Use of ureteroscopy and holmium:yttrium-aluminum-garnet laser in the treatment of an infundibular stenosis. Urology. 2000;55:129–131. doi: 10.1016/s0090-4295(99)00385-4. [DOI] [PubMed] [Google Scholar]
- 102.Bruner B., Ashley R., Leibovich B., Blute M., LeRoy A. Case report: percutaneous catheter management of persistent urine leak due to iatrogenic infundibular stenosis after partial nephrectomy. J Endourol. 2009;23:37–41. doi: 10.1089/end.2008.0291. [DOI] [PubMed] [Google Scholar]
- 103.Mues A.C., Landman J., Gupta M. Endoscopic management of completely excluded calices: a single institution experience. J Endourol. 2010;24:1241–1245. doi: 10.1089/end.2009.0663. [DOI] [PubMed] [Google Scholar]
- 104.Sungur M., Caliskan S., Lokman U. Hydrocalyx: Uncommon complication of percutaneous nephrolithotomy. 2018;28:974–975. doi: 10.29271/jcpsp.2018.12.974. [DOI] [PubMed] [Google Scholar]
- 105.Unsal A., Resorlu B., Atmaca A.F., Diri A., Goktug H.N.G., Can C.E. Prediction of morbidity and mortality after percutaneous nephrolithotomy by using the Charlson comorbidity index. Urology. 2012;79:55–60. doi: 10.1016/j.urology.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 106.Whitehurst L., Jones P., Somani B.K. Mortality from kidney stone disease (KSD) as reported in the literature over the last two decades: a systematic review. World J Urol. 2018:1–18. doi: 10.1007/s00345-018-2424-2. [DOI] [PubMed] [Google Scholar]