Abstract
The 2014 to 2016 Ebola outbreak response resulted in many lessons learned about biocontainment patient care, leading to enhanced domestic capabilities for highly infectious and hazardous communicable diseases. However, additional opportunities for improvement remain. The article identifies and describes key considerations and challenges for laboratory analysis, clinical management, transportation, and personnel management during the care of patients infected with Ebola or other special pathogens. Dedication to maintaining preparedness enables biocontainment patient care teams to perform at the highest levels of safety and confidence.
Keywords: Ebola virus, Biocontainment, Biosecurity, Emerging diseases, Clinical management
Key points
-
•
Heightened infection control considerations led to lessons learned for US biocontainment care units that provided care to patients with the Ebola virus disease in 2014 to 2015.
-
•
Protocols for caring for patients with the Ebola virus or other special pathogens should ensure the safety of staff and be well-practiced through trainings and exercises.
-
•
The 2014 to 2016 Ebola outbreak response led to changes in approach and enhanced preparedness for future threats; however, additional opportunities for improvement remain.
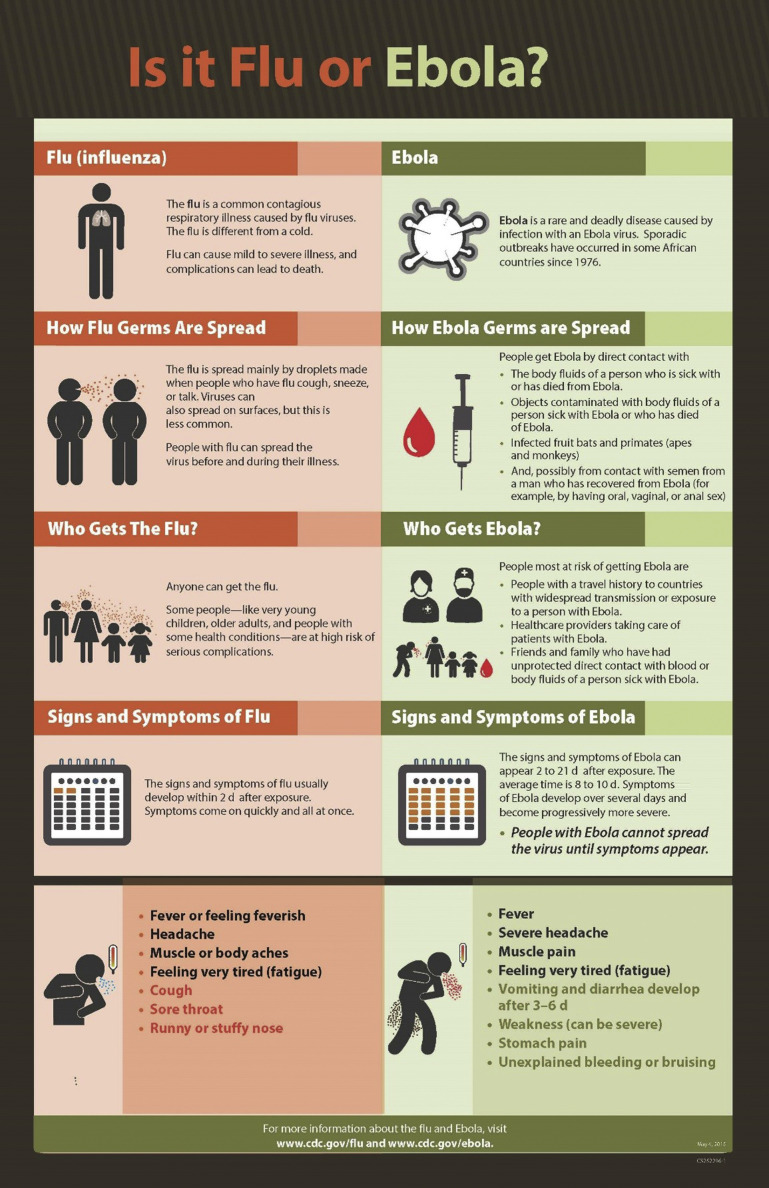
The epidemic of Ebola virus disease (EVD) in West Africa that began in 2013 was the world’s 25th known outbreak of the disease, dwarfing, in numbers of cases, the previous 24 combined. Transmitted from person to person, more than 28,000 people were ultimately infected, and more than 11,000 were killed, with the bulk of the cases in Guinea, Liberia, and Sierra Leone.1 As local health care systems struggled to respond to the large number of infected patients, health care providers from the United States and other countries responded to the outbreak in Africa through multiple aid organizations. These heroic individuals were faced with providing care to very ill patients with limited clinical resources. The hysteria over EVD in the media and among the state and local agencies responsible for making decisions regarding the monitoring and quarantine of returning health care workers were reminiscent of challenges encountered in the early days of the acquired immunodeficiency syndrome (AIDS) crisis of the 1980s.2 The media was concurrently attempting to cover the unprecedented outbreak while sharing public policy on the situation throughout the events.3 In the fall of 2014, a patient who had recently traveled from West Africa was treated at a hospital in the United States. The patient was eventually confirmed to have EVD, ultimately resulting in 2 nurses who had direct contact with the patient also acquiring the illness.4 EVD is a viral illness with some overlapping symptoms of other common and travel-related illnesses, so educating the public became critical to prevent panic and public concern regarding transmission of the virus (Fig. 1 ).
Fig. 1.
Is it Flu or Ebola?
(From CDC. Available at: https://www.cdc.gov/vhf/ebola/pdf/is-it-flu-or-ebola.pdf. Accessed October 8, 2018.)
The Ebola virus is an enveloped RNA virus classified in the Filoviridae family.5 The typical transmission pattern of the Ebola virus through direct contact with blood, secretions, or tissues of infected humans or nonhuman primates was described in a consensus paper in 2002.6 At that time, outbreaks of EVD had typically been limited to remote areas of Africa and were controlled without the use of airborne precautions. Despite this, the consensus statement specifically suggested the use of N95 respirators or powered air purifying respirators for respiratory protection to protect health care workers in light of several situations in which transmission could not be fully explained.6 At the time, the knowledge of EVD and the other viral hemorrhagic fevers was largely limited to primate studies and learnings from outbreak responses.
Recognizing emerging infectious disease threats, key leaders in the United States developed consensus around the establishment of biocontainment units in 2006, detailing aspects such as provision of clinical care, infection control standards, facility design, and ethical issues for high level isolation.7 Early learnings from the Nebraska Biocontainment Unit were also outlined in the literature and included design priorities, unit management, and challenges in transporting patients.8 Moreover, the classification of hemorrhagic fever viruses as category A bioweapon agents6 creates challenges for laboratory recognition and clinical management, specifically specimen shipment and waste handling.9 Unique regulations and heightened infection control considerations led to many lessons learned for US biocontainment care units that provided patient care to patients with EVD.
The recent West African outbreak was caused by Zaire ebolavirus; however, other members of the genus Ebolavirus, namely Bundibugyo virus and Sudan virus, would be expected to share transmission modes, produce similar disease, and require similar infection control precautions. The same can be said for the viruses of the genus Marburgvirus (along with ebolaviruses, members of the family Filoviridae): Marburg and Ravn. In addition, other viral hemorrhagic fever viruses, such as Lassa, Junin, Machupo, Sabia, Guanarito, and Crimean-Congo, would be expected to produce disease that, although potentially variable in presentation, would also require meticulous attention to personal protection and infection control guidance.
In addition to the viral hemorrhagic fevers, several other human diseases are known to be highly infectious, highly contagious, and highly hazardous. As such, patients harboring these diseases are considered to be candidates for care in biocontainment units. Included among these are a group of severe acute respiratory diseases thought capable of transmission via droplet nuclei (and thus requiring airborne precautions), namely severe acute respiratory syndrome, Middle East respiratory syndrome, and certain novel, avian, and prepandemic strains of influenza.
Beyond the viral hemorrhagic fevers and severe acute respiratory diseases, several other diseases pose infection control challenges and potentially warrant biocontainment care. Smallpox, monkeypox, Nipah, Hendra, pneumonic plague, and extensively drug-resistant tuberculosis are among these. Although these diseases differ in their clinical manifestations and modes of transmission, and although the infection control modalities and personal protective equipment (PPE) ensembles used to protect against them may also differ, they share a high degree of hazard and communicability. As such, it is imperative that the health care worker caring for patients with these diseases be exceedingly well-trained and practiced in the use of these modalities and ensembles.
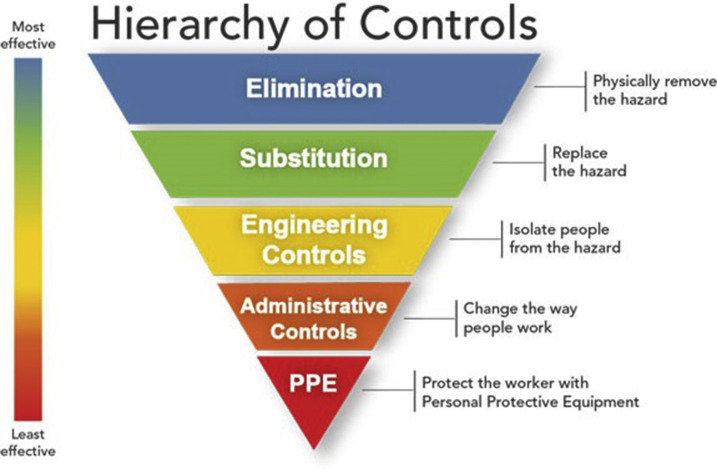
Moreover, although the concerning pathogens over time may change or be unknown, the basic modes of transmission will remain the same. The Centers for Disease Control and Prevention’s National Institute for Occupational Safety and Health developed a hierarchy of controls designed to prevent injury and illness in the workplace (Fig. 2 ). The hierarchy can be used to guide the development of protocols aimed at protecting health care workers from occupational hazards and exposure. It suggests a ranking of effectiveness with elimination and substitution described as the most effective because they direct removal or replacement of the hazard. This is followed by engineering controls, to isolate people from the hazard, and administration controls, which change the way people work through training or protocol development. PPE is at the bottom of the hierarchy and described as the least effective control. This is not because PPE is ineffective but as a control measure it means the hazard has not been eliminated or replaced, and protection is required.10 PPE guidelines have been in place in the realm of health care–associated infection and isolation care with little modification for over a decade.11 Despite this guidance, the lack of standards for design and performance of gowns in particular came to light after the experience of treating patients with EVD in 2014.12 The Nebraska Biocontainment Unit has described the protocols used for protection of health care workers for EVD care in the literature.13 The initial standards and procedures set by the hospitals that cared for patients with Ebola in the United States eventually became a guidance resource for managing patients with EVD worldwide.14
Fig. 2.
Hierarchy of controls.
(From National Institute for Occupational Safety and Health. Available at: https://www.cdc.gov/niosh/topics/hierarchy/default.html. Accessed on October 8, 2018.)
Recognition
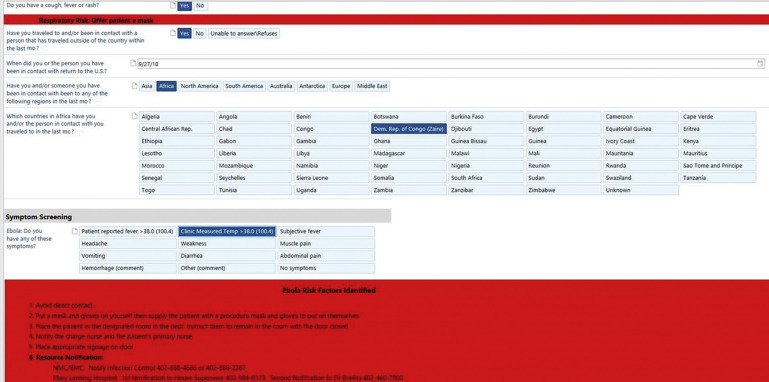
When a disease outbreak occurs, public health and health care systems need to work together to monitor for the emergence of cases and remain vigilant. Because EVD cases were occurring in West Africa in 2014, travel screening was initiated at designated international airports with follow-up from the local public health department for active monitoring of fever or other symptoms.15 Travel screening was also implemented at health care facilities across the country because an ill patient might present for medical care through the emergency department, ambulatory clinic, or other point of entry to a health care facility.16 Quick front desk assessment was encouraged to include travel history, travel location, timeframe, and symptoms. This action allowed prompt identification and isolation when appropriate until further discernment could be made to drive laboratory testing decisions. Integrating this strategy into the electronic health record hard wired the process and alerted other caregivers if travel and symptom screening triggered an alert. Creation of consistent work flow and process tools for use by caregivers at all entry areas allowed for consistency in whom to contact, the type of isolation needed, the location of PPE, and various other duties or actions to take (Fig. 3 ).
Fig. 3.
Triage Ebola: Nebraska Medicine.
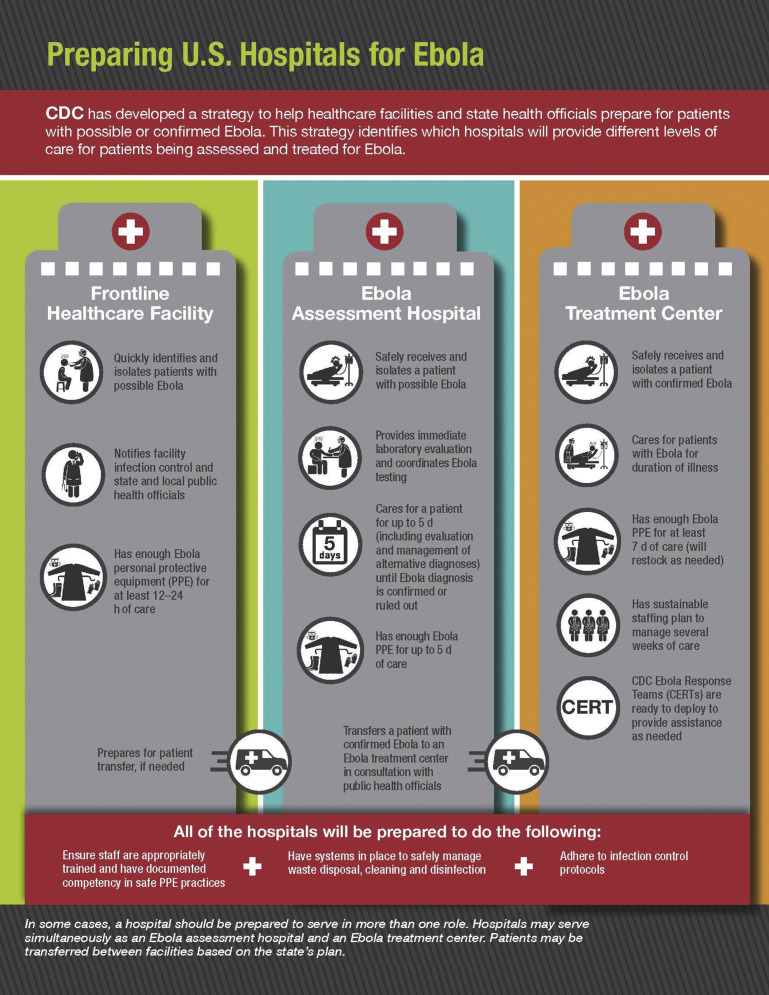
The Centers for Disease Control and Prevention and the Health and Human Services Assistant Secretary for Preparedness and Response (ASPR) developed a tiered approach to preparedness for EVD (Fig. 4 ), which includes regional Ebola and other special pathogen treatment centers, state designated EVD treatment centers, state designated EVD assessment hospitals, and frontline health care facilities.15 Frontline health care facilities include any facility not otherwise designated as a higher tier and also include emergency departments and other urgent care settings that are expected to safely and effectively identify potential cases, isolate those individuals in a timely manner, and inform the appropriate public health officials (Fig. 5 ). Ebola assessment hospitals are expected to care for patients under investigation until a diagnosis can be confirmed or ruled out. If confirmed, a patient would be transferred to a regional or state designated Ebola treatment center, which is expected to provide care for the duration of the patient’s illness.15
Fig. 4.
Preparing U.S. Hospitals for Ebola.
(From CDC. Available at: https://www.cdc.gov/vhf/ebola/pdf/preparing-hospitals-ebola-P.pdf. Accessed October 8, 2018.)
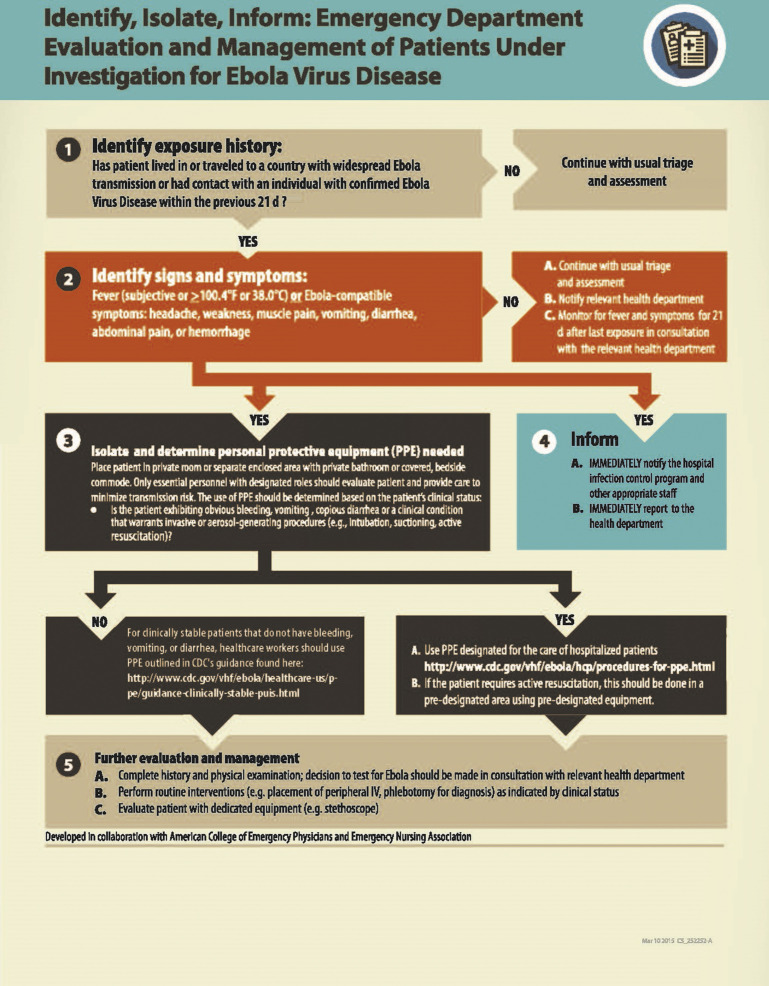
Fig. 5.
Identify, isolate, inform: emergency department evaluation and management of patients under investigation for Ebola virus disease.
(From CDC. Available at: https://www.cdc.gov/vhf/ebola/pdf/ed-algorithm-management-patients-possible-ebola.pdf. Accessed on October 8, 2018.)
Laboratory resources are a critical element of both a recognition and management plan for patients with EVD. Public health laboratories in the Laboratory Response Network are authorized to perform the molecular screening test for EVD.17 Risk assessment is a critical element of developing a laboratory plan for EVD testing, and the Association of Public Health Laboratories has developed a template for this process.18 Laboratories use a combination of point-of-care testing and compact analyzers that fit into a biosafety cabinet, as well as large automated closed-tube analyzers in core testing facilities.19 When preparing a laboratory to support the care of patients with EVD, a specialized and trained team should be assembled, and a safe space identified to perform the testing. A test menu should be established. The laboratory team should train together and use a buddy system to maintain safety while performing testing. The laboratorians should practice routine skills and run laboratory test controls in the space designated for EVD testing to develop muscle memory for the different skills and clinical challenges that they will face when working with specimens in PPE that is not used in everyday practice. Practicing these skills allows for problem-solving for unique challenges. Successful clinical management of a patient under investigation or a confirmed patient with EVD will depend on a collaborative and connected relationship between the physician team, the nursing team, and the clinical laboratorians, with a strong connection to public health laboratories throughout the process.
Management
If a patient is not identified in close proximity to an assessment or treatment center, they may need to be transported from another state in the region, or even from another country outside the United States, to receive care at a regional or state designated treatment center.15 This type of transport requires emergency medical service (EMS) provided by trained professionals to support transportation of the patient. After the 2014 to 2016 EVD outbreak, federal funding became available to advance preparedness to respond to and effectively manage EVD or other infectious pathogens. One of the requests for all states was to create a concept of operations (CONOPs) to include, at a minimum, a plan to coordinate air or ground transport within the state and region. Direction, control, and coordination are described in detail to guide an event response. Anticipated key participating agencies must agree to the process that is outlined. These plans should also specifically discuss financing and how the plan itself is maintained through training and exercises. A critical element in preparedness planning includes exercising the plan to allow for refinement and ensuring the operational capability of a written plan. Forming a training and exercise planning committee can provide a venue to increase engagement across all partners and further develop working relationships among key partners. During the activation of a regional network, some transports may require multiple care teams and, therefore, exchanges of team members, as well as refueling for successful extended ground transports. Extended ground transports should be defined by the designated EMS transport service and the state health department in the state’s CONOPs. Consideration should be given to the condition of the patient, the length of time staff can remain in PPE, and the distance the EMS vehicle can travel before needing to refuel. This type of coordinated trip requires advance planning and creative thinking to identify safe locations to perform these tasks. Many regions are exercising their regional plans and working through these challenges. These types of exercises to advance regional planning focus on the communication processes, patient care handoffs and the importance of the connection between EMS and nursing. Clinical updates during the transport and at the point of transition are critical to continuous quality care of the patient.
The decision to transport a patient with EVD is difficult. The risk involved in caring for the patient increases on leaving the relative safety of a controlled clinical care environment in a hospital setting for the fluid, less stable setting of ground or air transportation vehicles. As such, transportation may require clinical management that operates under altered standards of care. As a precaution for any potential generation of aerosols that may affect the driver during ground transport, air handling in the transport vehicle is set to prevent the circulation of air, with the ventilation fan put on high in the driver’s compartment to establish a positive-pressure environment.20 Decisions on the amount of barrier protection required for a ground vehicle transport and for the preparation of the patient should be made well in advance. Some teams use 6-mil plastic sheeting to create these barriers in the patient care area,19 whereas others have identified prefabricated enclosures or have purchased purpose-built ambulances. If barriers are used, materials will add to the waste burden on arrival to the destination. Cooperative patients may tolerate an impervious suit and mask, whereas others may be unresponsive or unable to adequately control bodily fluids and require a more reliable containment device or wrap.20 Many of these same challenges exist in the aircraft environment, with additional concerns related to the safety regulations for flight. Limitations exist on the type of chemical disinfectants that can be used so as not to compromise airworthiness (eg, not deteriorate aircraft components), and materials for restraint, such as seat belts or airline seats, are difficult to decontaminate. Moreover, certain electronic equipment routinely used in a ground vehicle or hospital environment may be prohibited on an aircraft due to interference of wireless services. Also, medical equipment must be deemed safe-to-fly by the appropriate authority. Guidance is available on many of these issues specific to the air medical transport of patients with EVD.21 The high-risk nature of such transports demands clear lines of communication between transport teams and referring and receiving facilities, including frequent, accurate updates by transporting EMS teams on clinical status. Communicating the estimated time of arrival and patient information helps to establish receiving teams’ expectations to facilitate timely, successful patient transitions. On arrival at the receiving hospital, a team of isolation unit staff, wearing appropriate PPE and trained in decontamination protocols, should meet the ground transport vehicle. Any fluid spills should be immediately decontaminated, followed by a full decontamination of the intrafacility route on patient placement. The full decontamination process is performed for community reassurance, especially in situations in which the transport occurs in public spaces.
After the patient is safely transported to the hospital, providing care to a patient with confirmed EVD requires more resources than a typical hospitalized patient owing to the rigorous safety controls and the specialized team required to successfully care for a patient in a biocontainment unit.21 Protocols should be developed that direct the management of the unit and ensure the safety of staff, and these should be practiced through trainings and exercises. Protocols are refined based on feedback from the staff, which promotes ownership of the plans. Protocols should also be reviewed by an interprofessional team with expertise in all facets of the process. For example, a provider-down protocol directs the plan to get an ill or incapacitated health care worker out of the patient care room, out of PPE, and provide them with the required medical care. Other protocols might include staff monitoring or symptom surveillance, spill cleanup, and care of the deceased patient.
The disease severity of the patients with EVD cared for in the United States and Europe ranged from moderate to critical, with some patients developing complications that resulted in death.22 Caring for a patient with EVD requires nursing staff be skilled and proficient in the care of patients for all stages of their illness. Some biocontainment units use nursing staff from many specialties to include critical care, medical-surgical, pediatrics, procedural services, and others to obtain a skill-mix, whereas other teams are made up solely of critical care nurses. Respiratory therapists (RTs) and patient care technicians can provide additional support. Education and training of the team is an ongoing cycle and consists of PPE donning and doffing, operating specialized equipment in the unit, practicing hands-on clinical skills while in PPE, infection control training, and education on infectious diseases and current outbreaks. A training and exercise plan should include quarterly drills with other agencies or departments within the hospital and the community.
Staffing is best explained with a case study. During the activations in 2014 to 2016, when caring for a single EVD patient, the Nebraska Biocontainment Unit operated with 6 staff members on the day shift and 5 staff members on the night shift. Each shift included at least 3 registered nurses. If the patient was in need of respiratory care, an RT was also present. Each staff member was assigned a staffing role at the beginning of each shift with specific tasks and everyone rotated through the roles, remaining within their scope of practice. The 3 registered nurses assigned to patient care worked directly at the patient’s bedside in 4-hour shifts. Generally, 1 registered nurse was in the patient care room but this number increased in critically ill patients who required intensive care. Staffing depends on multiple factors, such as the number of patients that can be cared for, patient acuity, and the physical structure of a biocontainment unit.
Procedures in a biocontainment unit must be performed in a controlled and coordinated manner to enhance safety of the patient and health care workers. Central venous catheter insertion is performed to facilitate phlebotomy and provide intravenous access for medications and fluids. Intubation is performed using rapid sequence induction to reduce the creation of aerosols, patient movement, or reflexes such as coughing. The use of a video laryngoscope allows the physician to distance themselves from the patient’s airway.23 Patients with EVD commonly develop copious amounts of diarrhea that can lead to dehydration and this alone or in combination with sepsis sequelae can result in acute kidney injury, so considerations and planning to provide dialysis or continuous renal replacement therapy (CRRT) is essential.23 In regard to renal replacement, processes for cleaning the equipment, ensuring safety of the lines, and treating the effluent must be developed.22 Studies investigating the effluent from CRRT found no viral material present, suggesting the virus does not cross the dialyzer membrane24; however, precautions are still advised.
Because no treatment of EVD approved by the US Food and Drug Administration is available, various investigational therapeutic agents were used in the management of patients with EVD during the 2014 to 2016 outbreak. Investigational therapeutics were administered to people who experienced a high-risk exposure, as well as patients with confirmed EVD. Unfortunately, the uncontrolled nature of the open-label use in most instances and the limited numbers of individuals treated with experimental therapeutics, as well as the use of multiple concomitant investigational products simultaneously, made generalization of the data challenging.25, 26 In response to this unmet need, the Special Pathogens Research Network (SPRN) was organized after the 2014 to 2016 outbreak to proactively develop a centralized infrastructure to initiate research protocols and collect meaningful data. Sponsored by ASPR, this network of 10 regional treatment centers supported by centralized research resources such as a biorepository, data repository, case report forms, and a central rapid response institutional review board, will help to efficiently and systematically launch research protocols in the next outbreak. This readiness is further ensured by routine regional and national exercises involving the SPRN and the 10 regional centers.
The 2014 to 2016 Ebola outbreak response resulted in many lessons learned, leading to changes in approach and preparedness; however, additional opportunities for improvement remain. Key challenges in laboratory analysis, waste management, clinical management, research, and workforce issues persist.27 Multidisciplinary teamwork is critical to address the unique problem solving challenges of caring for patients infected with Ebola or other special pathogens, and dedication to maintenance of preparedness allow these teams able to perform at the highest levels of safety and confidence.28
Footnotes
Disclosure Statement: The authors report no conflicts of interest and have nothing to disclose.
References
- 1.WHO Ebola Response Team After Ebola in West Africa: unpredictable risks, preventable epidemics. N Engl J Med. 2016;375:587–596. doi: 10.1056/NEJMsr1513109. [DOI] [PubMed] [Google Scholar]
- 2.Gonsalves G., Staley P. Panic, paranoia, and public health: the AIDS epidemic’s lessons for Ebola. N Engl J Med. 2014;371:2348–2349. doi: 10.1056/NEJMp1413425. [DOI] [PubMed] [Google Scholar]
- 3.Sell T.K., Boddie C., McGinty E.E., et al. News media coverage of US Ebola policies: implications for communication during future infectious disease threats. Prev Med. 2016;93:15–120. doi: 10.1016/j.ypmed.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Liddell A.M., Davey R.T., Mehta A.K., et al. Characteristics and clinical management of a cluster of 3 patients with Ebola virus disease, including the first domestically acquired cases in the United States. Ann Intern Med. 2015;163:81–91. doi: 10.7326/M15-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiley M.P., Bowen E.T., Eddy G.A., et al. Filoviridae: a taxonomic home for Marburg and Ebola viruses? Intervirology. 1982;18:24–32. doi: 10.1159/000149300. [DOI] [PubMed] [Google Scholar]
- 6.Borio L., Inglesby T., Peters C.J., et al. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 7.Smith P.W., Anderson A.O., Christopher G.W., et al. Designing a biocontainment unit to care for patients with serious communicable diseases: a consensus statement. Biosecur Bioterror. 2006;4:351–365. doi: 10.1089/bsp.2006.4.351. [DOI] [PubMed] [Google Scholar]
- 8.Beam E.L., Boulter K.C., Freihaut F., et al. The Nebraska experience in biocontainment patient care. Public Health Nurs. 2010;27:140–147. doi: 10.1111/j.1525-1446.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 9.Hewlett A.L., Varkey J.B., Smith P.W., et al. Ebola virus disease: preparedness and infection control lessons learned from two biocontainment units. Curr Opin Infect Dis. 2015;28:343–348. doi: 10.1097/QCO.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institute for Occupational Safety and Health Hierarchy of controls. 2018. https://www.cdc.gov/niosh/topics/hierarchy/default.html Available at: Accessed October 15, 2018.
- 11.Centers for Disease Control and Prevention Protecting healthcare personnel. 2016. https://www.cdc.gov/hai/prevent/ppe.html Available at: Accessed October 15, 2018.
- 12.Kilinc Balci F.S. Isolation gowns in health care settings: Laboratory studies, regulations and standards, and potential barriers of gown selection and use. Am J Infect Control. 2016;44:104–111. doi: 10.1016/j.ajic.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beam E.L., Schwedhelm S., Boulter K., et al. Personal protective equipment processes and rationale for the Nebraska Biocontainment Unit during the 2014 activations for Ebola virus disease. Am J Infect Control. 2016;44:340–342. doi: 10.1016/j.ajic.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Guidance on personal protective equipment (PPE) to be used by healthcare workers during management of patients with confirmed Ebola or persons under investigation (PUIs) for Ebola who are clinically unstable or have bleeding, vomiting, or diarrhea in U.S. hospitals, including procedures for donning and doffing PPE. 2015. https://www.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance.html Available at: Accessed October 15, 2018.
- 15.Koonin L.M., Jamieson D.J., Jernigan J.A., et al. Systems for rapidly detecting and treating persons with Ebola virus disease – United States. MMWR Morb Mortal Wkly Rep. 2015;64:222–225. [PMC free article] [PubMed] [Google Scholar]
- 16.Schwedhelm S., Swanhorst J., Watson S., et al. ED Ebola triage algorithm: a tool and process for compliance. J Emerg Nurs. 2015;41(2):165–169. doi: 10.1016/j.jen.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Wadman M.C., Schwedhelm S.S., Watson S., et al. Emergency department processes for the evaluation and management of persons under investigation for Ebola virus disease. Ann Emerg Med. 2015;66:306–314. doi: 10.1016/j.annemergmed.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Association of Public Health Laboratories Template for public health laboratory risk assessment for Ebola Virus Disease (EVD) testing. https://www.aphl.org/programs/preparedness/Documents/APHL-Template.pdf Available at: Accessed July 3, 2018.
- 19.Herstein J.J., Iwen P.C., Jelden K.C., et al. U. S. High-level isolation unit clinical laboratory capabilities update. J Clin Microbiol. 2018;56 doi: 10.1128/JCM.01608-17. [pii:e01608-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isakov A., Miles W., Gibbs S., et al. Transport and management of patients with confirmed or suspected Ebola virus disease. Ann Emerg Med. 2015;66:297–305. doi: 10.1016/j.annemergmed.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Guidance on Air Medical Transport (AMT) for patients with Ebola Virus Disease (EVD) https://www.cdc.gov/vhf/ebola/clinicians/emergency-services/air-medical-transport.html Available at: Accessed August 1, 2018.
- 22.Uyeki T.M., Mehta A.K., Davey R.T., et al. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med. 2016;374:636–646. doi: 10.1056/NEJMoa1504874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasa A., Schwedhelm M., Johnson D. Critical care for the patient with Ebola Virus Disease: the Nebraska perspective. J Intensive Crit Care. 2015;1:1–5. [Google Scholar]
- 24.Connor M.J., Kraft C., Mehta A.K., et al. Successful delivery of RRT in Ebola virus disease. J Am Soc Nephrol. 2015;26:31–37. doi: 10.1681/ASN.2014111057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraft C.S., Hewlett A.L., Koepsell S., et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola Virus Disease in the United States. Clin Infect Dis. 2015;61:496–502. doi: 10.1093/cid/civ334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong K.K., Davey R.T., Hewlett A.L., et al. Use of postexposure prophylaxis after occupational exposure to Zaire ebolavirus. Clin Infect Dis. 2016;63:376–379. doi: 10.1093/cid/ciw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer D., Sell T.K., Schoch-Spana M., et al. Lessons from the domestic Ebola response: Improving health care system resilience to high consequence infectious diseases. Am J Infect Control. 2018;46:533–537. doi: 10.1016/j.ajic.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwedhelm S., Beam E.L., Morris R.D., et al. Reflections on interprofessional team-based clinical care in the ebola epidemic: The Nebraska Medicine experience. Nurs Outlook. 2015;63:27–29. doi: 10.1016/j.outlook.2014.11.019. [DOI] [PubMed] [Google Scholar]