Abstract
In this study, livestock herders in eastern Sudan were interviewed through structured questionnaire involved 14046 animals in 151 herds (87 camel herds, 51 sheep and 13 goats) from June to September of 2016 in Showak area of Gadarif State to get some epidemiological information on contagious ecthyma (CE) infection. 102 suspected cases of CE were investigated (38 sheep, 22 goats and 42 camels) by a second questionnaire focusing on age and sex of affected animals beside number and localization of the lesions. Representative tissue samples of scab lesion scrapings were collected from a total of 36 suspected sheep, goats and camels for DNA extraction to identify PPV by quantitative real-time PCR and gel-based PCR, then a PCR protocol was used to obtain DNA fragment of B2L gene from six DNAs (2 from each animal species) for sequencing. Phylogenetic tree based on nucleotide sequences was constructed and all data were analyzed statistically. Obtained result has shown morbidity rate of 23.8% and a case fatality rate of 4.7 % in overall investigated animals resulting in a significant economic loss. Within individual herd, the morbidity rate varied from 5.6 to 42.8%, while the case fatality rate ranged between 0 and 33.3%. Camels accounted for the highest case fatality rate with 6.5% compared to sheep and goats which their rates were 2.8% and 1.3%, respectively. 93% of the affected animals were young less than one-year-old. The prevalence of CE was high in the rainy season compared to winter and summer. Out of 36 scab materials collected from sheep, goats, and camels, 24 gave positive specific amplification in real-time PCR and 21 in the gel-based PCR. DNA sequencing confirmed the PCR results. All sequences had a high G + C content of 62.6–63.9%. A BLAST search also revealed that the studied sheep PPV (SPPV) isolates shared 99.08% nucleotide sequence intragroup identity, 96.88–97.27% identity with the goat PPV (GPPV) isolates and together they belong to the Orf virus (ORFV) species, while the camel PPV (CPPV) isolates are close to the Pseudocowpoxvirus (PCPV) species of the PPV genus and share 92.51–93.62 % identity with the GPPV isolates. In conclusion the present study demonstrated that the gross lesion produced by PPV in sheep, goats and camels is generally similar, yet the PPVs circulating in eastern Sudan in camels (PCPV) are genetically distinct from those affecting sheep and goats (ORFV). Contagious ecthyma in eastern Sudan causes significant morbidities and mortalities and control measures, guided by the results of this investigation ought to be implemented.
Keywords: Microbiology, Virology, Infectious disease, Veterinary medicine, Microbiology epidemiology, Field investigation, Phylogenetic characterization, Parapoxviruses, Small ruminants, Dromedary camels, East Sudan
Microbiology; Virology; Infectious disease; Veterinary medicine; Microbiology epidemiology; Field investigation; Phylogenetic characterization; Parapoxviruses; Small ruminants; Dromedary camels; East Sudan
1. Introduction
Livestock represents a basic resource and play multiple roles in the livelihoods of people in developing countries, particularly for millions of pastoralists in Africa. However, animal productivity is currently confronting many challenges, including infectious disease that causes enormous economic loss.
Parapoxviruses (PPVs) are found worldwide, affecting mainly sheep, goats, cattle and camels as well as many domestic and wild animal species. Additionally, these viruses cause skin infections in humans, red deer, reindeer, squirrel, seal, grey seal and probably other species of animals such as dogs and cats. PPV infection in sheep and goats is referred to as contagious ecthyma (CE), Orf, contagious pustular dermatitis (CPD) and scabby mouth. Different local names have been used; for instance, in the Sudan, the disease is named `abu-khoshaim or al-kolate. In camels, the PPV cause the camel CE (CCE; locally called in the Sudan al-kolate and abu-shalamboo) which has been reported in Mongolia, Kenya, Kazakhstan and Turkmenistan, Somalia, Sudan, Libya, Saudi Arabia, Bahrain and India (Khalafalla et al., 2015). PPV primarily infects epithelial cells causing severe proliferative dermatitis that is clinically manifested by the appearance of macules, papules, vesicles, pustules to rapidly growing scabs and fissured crusts. Lesions can likewise be found inside the buccal cavity and occasionally in the esophagus, abomasum and rumen (Bouznach et al., 2013).
The PPVs belong to the family Poxviridae. Currently, there are four established species within the PPV genus of the family Poxviridae; Orf virus (ORFV), the type species of the PPV genus, that causes the CE disease mainly in sheep and goats, pseudocowpoxvirus (PCPV) and bovine papular stomatitis virus (BPSV) which both infect cattle, in addition to parapoxvirus of red deer in New Zealand (PVNZ), which has only been isolated from red deer in New Zealand. BPSV and PCPV affect mainly cattle but differ from ORFVs in the site of the pox lesion, as BPSV is restricted to the muzzle and PCPV to the teat. Tentative species include the camel PPV (CPPV) that causes camel contagious ecthyma (CCE), reindeer parapoxvirus, musk ox and seal parapoxvirus (Vikøren et al., 2008).
Infection with PPVs is often regarded as endemic, but the actual impact and prevalence of the disease amongst livestock is not well understood or underestimated (Hosamani et al., 2009; Scagliarini et al., 2012). Outbreaks of CE have been regularly reported in sheep, goat, and camels to the veterinary authorities in the Sudan. CE in camels is recognized in Sudan (Khalafalla et al., 1994; Khalafalla, 2000), however, the disease in sheep and goats has not published despite its widespread occurrence. Yet, the PPVs circulating in sheep and goats in this country have not genetically analyzed and the information about these viruses that circulating in animals sharing the same pasture are still missing, specially whether a cross-species transmission exists, or the infection is caused by genetically different PPV species. Therefore, this study is considered as the first investigation on PPV infection to include three livestock species in the same time mainly; sheep, goats and dromedary camels in order to estimate the occurrence of this disease and to explore the epidemiological nature of the CE in eastern Sudan, beside trying to confirm the suspected cases by using molecular analyses and to identify the field isolates from sheep, goat, and camels to find out any phylogenetic relationships between all of them.
2. Materials and methods
2.1. Study area
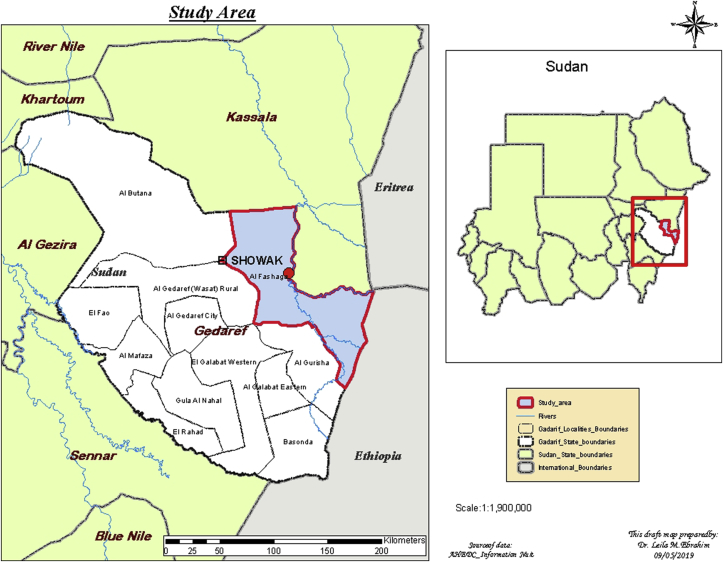
The present study was performed during the period from June to September 2016 in Showak area of eastern Sudan, which is in Gedarif State at Latitude14.4° and Longitude 35.8° in decimal degrees and extends eastwards to the Eritrean and Ethiopian borders (Figure 1). Sheep, goats, and camels are commingling in the study area particularly in the rainy season (June-September).
Figure 1.
Map of the Sudan showing the study location; Showak area in Gedarif State of eastern Sudan, June to September 2016 (Blue color).
Farmers around Showak area practice a mixed crop-livestock farming system, where open grazing pattern is used during the rainy season to early winter and after that the crop residues are used as animal feed for the rest of the year. All investigated animals are of indigenous breeds.
2.2. Questionnaire survey
The study involved two types of questionnaires. The first one was a pre-tested structured questionnaire (Table 1) aiming to estimate the occurrence of contagious ecthyma in sheep, goats, and camels was conducted by personal interviews with 151 livestock (51 sheep, 13 goats and 87 camels) randomly selected herders/owners. The study was carried out with a convenience sampling technique based on the availability of animal herders and willingness to participate in our study. All respondents were livestock herders accompanying their animals that have been met during the field visits or at Showak livestock market. The collected data were recorded on an Excel spreadsheet. The second questionnaire was addressed to 102 cases of PPV infection (38 in sheep, 22 in goats and 42 in camels) focusing on age and sex of affected animals and the number and localization of the lesion (Table 2).
Table 1.
Domains of the questionnaire used to collect data on the epidemiology of PPV infection in sheep, goats and camels, in Showak area of eastern Sudan, 2016.
| Principle | Subject |
|---|---|
| A | Herd size |
| B | Species of animal |
| C | Frequency of disease occurrence in the past 10 years |
| D | Source of disease |
| E | Season of disease occurrence |
| F | Deaths due to the disease |
| G | Treatment of affected animals |
| H | Human lesion encountered |
Table 2.
Domains of the questionnaire used to collect data on Contagious Ecthyma disease picture in some of the affected herds in Showak area of eastern Sudan, 2016.
| Principle | Subject |
|---|---|
| 1 | Age group of affected animals |
| 2 | Sex of affected animals |
| 3 | localization of the lesion in affected animals |
2.3. Statistical analyses
Statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 22.0., IBM Corp, Armonk, NY, USA). Chi-square test with Fisher's exact test was used to compare variables. P values of less than 0.05 were regarded as statistically significant.
2.4. Disease diagnosis and sample collection
During the period of the investigation, field visits were conducted to collect samples and data on the structured questionnaire. 102 herds were investigated; gross necropsy examination and tissue sample collection were performed for the laboratory diagnosis. Lab. diagnosis was restricted to a limited number of cases from which samples were available. Representative tissue samples of scab lesion scrapings were collected from a total of 36 sheep, goats and camelss showing suspected clinical signs of poxvirus infection.
2.5. Ethics statement
Samples were collected from sick animal during disease outbreaks for laboratory confirmation and no animal experiment was performed. All experiments were approved by the University of Khartoum Institutional Ethical committee. Consent for sample collection and photographing was verbally obtained from animal owners.
2.6. Tissue homogenization and DNA extraction
About 3 g of tissue samples were collected from each animal and placed in a labeled sterile universal tube. A 20% suspension was made of the scab material in phosphate buffered saline (PBS), pH 7.2 supplemented with antibiotics and antifungal, then mechanically homogenized using a mechanical homogenizer (TissueRuptor, Qiagen, Hilden, Germany) and centrifuged at 1500 g for 10 min at 4 °C. Total viral DNA was extracted from supernatants using a QiaAmp DNA Mini Kit (Cat.no. 51304, Qiagen, Hilden, Germany) according to the manufacturer's instructions.
2.7. Polymerase chain reaction (PCR)
To check its PPV identity each extracted DNA was first tested using the quantitative real-time PCR assay as described earlier (Nitsche et al., 2006). The amplification was carried out on a Mx3000P qPCR system (Agilent Technologies, USA) using Brilliant II QPCR Master Mix (Agilent Technologies, USA). Secondly, with DNA of known PPV identity, a PCR protocol was used to obtain DNA fragment for sequencing. This PCR assay targets the commonly used B2L gene (open reading frame 011) which is a homologue of vaccinia virus Copenhagen (VACV) gene F13L encoding the major envelope antigen p37K (Sullivan et al., 1994). PCR amplification was done using a forward primer (5′-TTAATTTATTGGCTTGCAGAACTCCGAGCGC-3′) and reverse primer (5′-ATGTGGCCGTTCTCCTCCATC-3′) that amplify 1200 bp DNA sequence (Guo et al., 2004). The PCR products were then checked in purified agarose gel and purified using QIAquick PCR Purification Kit (cat. no. 28106, Qiagen, Hilden, Germany) and then sent for sequencing. Sequencing was completed using the BigDye® Terminator v3.1 cycle sequencing kit and each primer pairs. Nucleotide positions were confirmed based on two independent sequencing reactions in both directions.
The DNA from ORFV D1701 reference strain (kindly provided by Prof. Dr. Matthias Buettner, Leipzig University, Germany) and DNA extracted from scabs from camels with contagious ecthyma (Khalafalla et al., 2015) were used as positive controls, beside that a double distilled water and DNA extracted from a camel pox virus (CMLV) were used as a negative controls.
2.8. BLAST and phylogenetic analysis
The biologically correct sequences of the six PPV isolates were subjected to basic local alignment search, then compared with the nucleotide sequence in the GenBank database (Table 3) using the online BLASTN program on the NCBI website. Phylogenetic tree based on nucleotide sequences was constructed using the Neighbor-Joining method in MEGA7 (Kumar et al., 2016). The significance of all deduced phylogenetic trees was verified by bootstrap analysis of 1000 replicates. Nucleotide sequences of the six viruses were deposited in the GenBank under accession numbers MN701771 to MN701775 and MN970156.
Table 3.
Information on PPV sequences used for the phylogenetic analysis of the B2L gene in comparison to virus isolates collected from eastern Sudan, 2016.
| No | Species | Virus identification | Host | origin | GenBank accession # | Reference |
|---|---|---|---|---|---|---|
| 1 | ORFV | D1701 | Reindeer | Finland | AY453654.1 | Tikkanen et al. (2004) |
| 2 | ORFV | Korea 09 | Goat | Korea | GQ328006.1 | Oem et al. (2009) |
| 3 | ORFV | Hoping | Goat | Taiwan | EU935106.1 | Chan et al. (2009) |
| 4 | ORFV | B044 | Goat | Germany | KF478798.1 | Friederichs et al. (2014) |
| 5 | ORFV | Assam | Goat | India | JQ040300.1 | Bora et al. (2012) |
| 6 | ORFV | Xinjiang | Sheep | China | JN565694 | Li et al. (2013) |
| 7 | ORFV | IN-Jodhpur | Dromedary | India | GQ390365 | Nagarajan et al. (2010) |
| 8 | ORFV | ORF3/2007 | Sheep | Turkey | KC491191.1 | Unpublished |
| 9 | ORFV | ORF/2011/7-18 | Goat | Korea | JX968992.1 | Unpublished |
| 10 | ORFV | NE1 | Goat | Brazil | JN613810 | Unpublished |
| 11 | ORFV | Gondar_zuria/O04/2013 | Goat | Ethiopia | KT438530 | Gelaye et al. (2016) |
| 12 | ORFV | ATARC/O02/2010 | Sheep | Ethiopia | KT438524 | Gelaye et al. (2016) |
| 13 | ORFV | Amba_Giorgis/C02/2012 | goat | Ethiopia | KT438518 | Gelaye et al. (2016) |
| 14 | ORFV | Arero/04/2013 | Dromedary | Ethiopia | KU645548 | Gelaye et al. (2016) |
| 15 | ORFV | Arero/02/2013 | Dromedary | Ethiopia | KU645546 | Gelaye et al. (2016) |
| 16 | ORFV | Hordha/01/2011 | Dromedary | Ethiopia | KU645563 | Gelaye et al. (2016) |
| 17 | PCPV | F94.848R | Reindeer | Finland | AY453661.1 | Tikkanen et al. (2004) |
| 18 | PCPV | B074 | Man | Germany | KF478803.1 | Friederichs et al. (2014) |
| 19 | PCPV | SA 98 | Dromedary | Saudi Arabia | EF555793.1 | Abubakr et al. (2007) |
| 20 | PCPV | BH 1 | Dromedary | Bahrain | EF555792.1 | Abubakr et al. (2007) |
| 21 | PCPV | BH 3 | Dromedary | Bahrain | EF555791.1 | Abubakr et al. (2007) |
| 22 | PCPV | SD-V4 | Dromedary | Sudan | KR231664 | Khalafalla et al. (2015) |
| 23 | PCPV | SD-V8 | Dromedary | Sudan | KR231665 | Khalafalla et al. (2015) |
| 24 | PCPV | SD-V13 | Dromedary | Sudan | KR231666 | Khalafalla et al. (2015) |
| 25 | PCPV | SD-V20 | Dromedary | Sudan | KR231667 | Khalafalla et al. (2015) |
| 26 | PCPV | SD-K1 | Dromedary | Sudan | KR231669 | Khalafalla et al. (2015) |
| 27 | PCPV | SD-K2 | Dromedary | Sudan | KR231670 | Khalafalla et al. (2015) |
| 28 | PCPV | SD-K3 | Dromedary | Sudan | KR231671 | Khalafalla et al. (2015) |
| 29 | PCPV | YG2828 | Cattle | Japan | LC230119.1 | Ohtani et al. (2017) |
| 30 | BPSV | V660 | Cattle | Germany | KF478805.1 | Friederichs et al. (2014) |
| 31 | BPSV | Aomori | Cattle | Japan | AB044797.1 | Inoshima et al. (2001) |
| 32 | BPSV | Iwate | Cattle | Japan | AB538385 | Oem et al. (2009) |
3. Results
3.1. Field observations
In the three-animal species, the gross lesion is proliferative beginning with pustules developed around the mouth on the upper and lower lips followed by papular elevations and scab formation. In some cases, the lesion involves nostrils, around eyes, ears, and leg. Clinical signs also included salivation, emaciation, and sometimes death especially in young animals. Different degrees of severity of the infection were observed in the three-animal species. However, the disease in camels is more severe than in sheep or goats due to a higher case fatality rate.
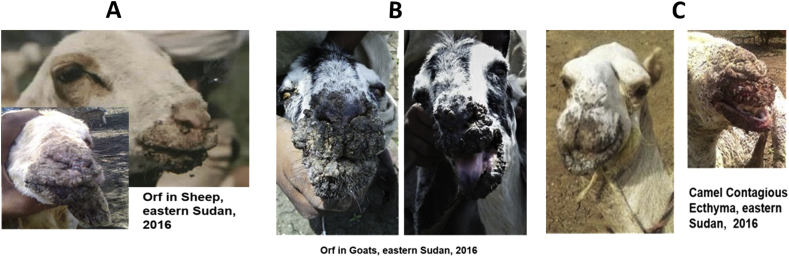
In sheep and goats, the disease affects mostly young animals, but some adults are also involved. Affected sheep and goats had proliferative crusted gross lesions covered by dark, friable fissured crusts, which sometimes extend to the nostrils and ears (Figure 2, panels A and B). These lesions can be dry or moist and are painful, bleed easily and often have a foul smell. In two sheep and one goat, we observed reddish-brown lesions on the gum of the oral cavity. One sheep that exhibited feet lesion around the coronets was suffering from chronic pneumonia too.
Figure 2.
Gross lesion caused by Parpoxvirus infection in sheep, goats and camels in eastern Sudan, 2016
In camels, all affected animals were less than one year old. The gross lesion is like that seen in sheep and goats, but in most cases starts with a swelling of the face that may extend to the neck (Figure 2, panel C).
3.2. The questionnaire
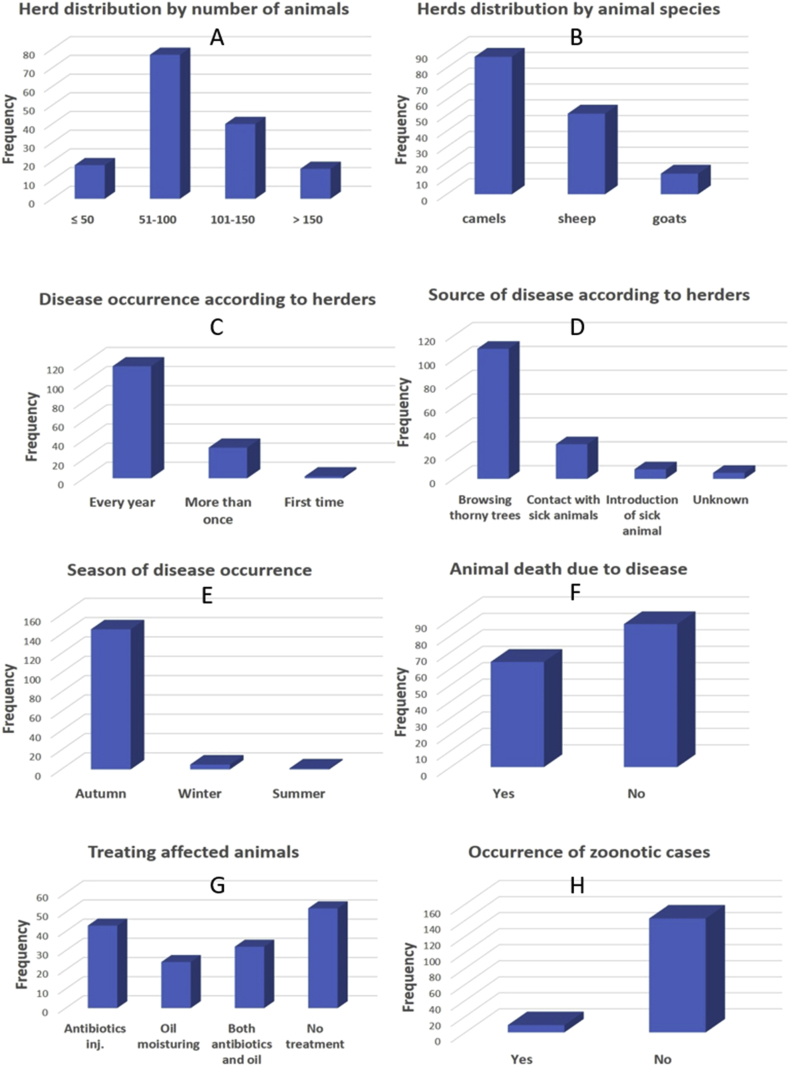
The questionnaire involved 14046 animals in 151 herds (87 camel herds, 51 sheep and 13 goats). Figure 3 shows the distribution of investigated animals by herd size, species, frequency of disease occurrence, source of infection, the season of disease occurrence, death of affected animals, treatment of affected animals and zoonotic infection related to the disease.
Figure 3.
Epidemiology of Contagious Ecthyma in Eastern Sudan in 2016. Distribution of studied animals by number, species, season, deaths, treatment and zoonotic transmission of the disease.
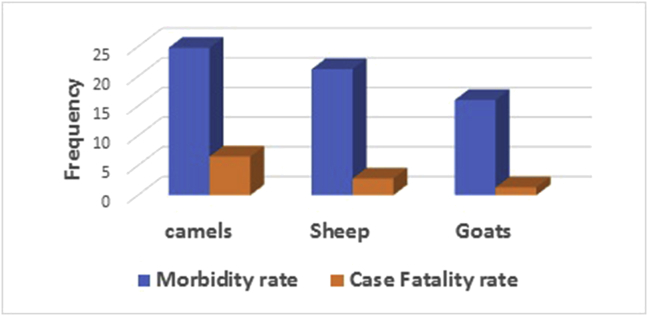
Overall 3340 cases that were recorded during the season of 2016, 157 animals died due to the disease; (morbidity rate 23.8%, 4.7 % case fatality rate). Within individual herd, the morbidity rate varied from 5.6 to 42.8%, while the case fatality rate ranged between 0 and 33.3%. Camels accounted for the highest case fatality rate of 6.5% compared to 2.8 in sheep and 1.3 in goats (Figure 4).
Figure 4.
Morbidity and case fatality rates due to PPV infection in small ruminants and camels in eastern Sudan, 2016
Generally, CE in the study area occurs every year in the rainy season, 93% of the affected animals were young less than one year old, higher prevalence in the rainy season compared to winter and summer, browsing thorny acacia trees were the main sources of the disease, most of the herders in the study area use to treat their animals when affected by CE, majority of the herders (98%) reported no zoonotic cases with few cases of zoonotic transmission from sheep to hands of herders.
3.3. Laboratory diagnosis
Out of 36 scab materials collected from sheep, goats, and camels, 24 gave positive specific amplification in real-time PCR and 21 in the gel-based PCR. The PCR detect PPV infection in all the three-animal species. The B2L gene fragment from six DNAs (2 from each animal species) was selected for sequencing. DNA sequencing confirmed the PCR results. All sequences have a high G + C content of 62.6–63.9%.
3.4. Phylogenetic analysis
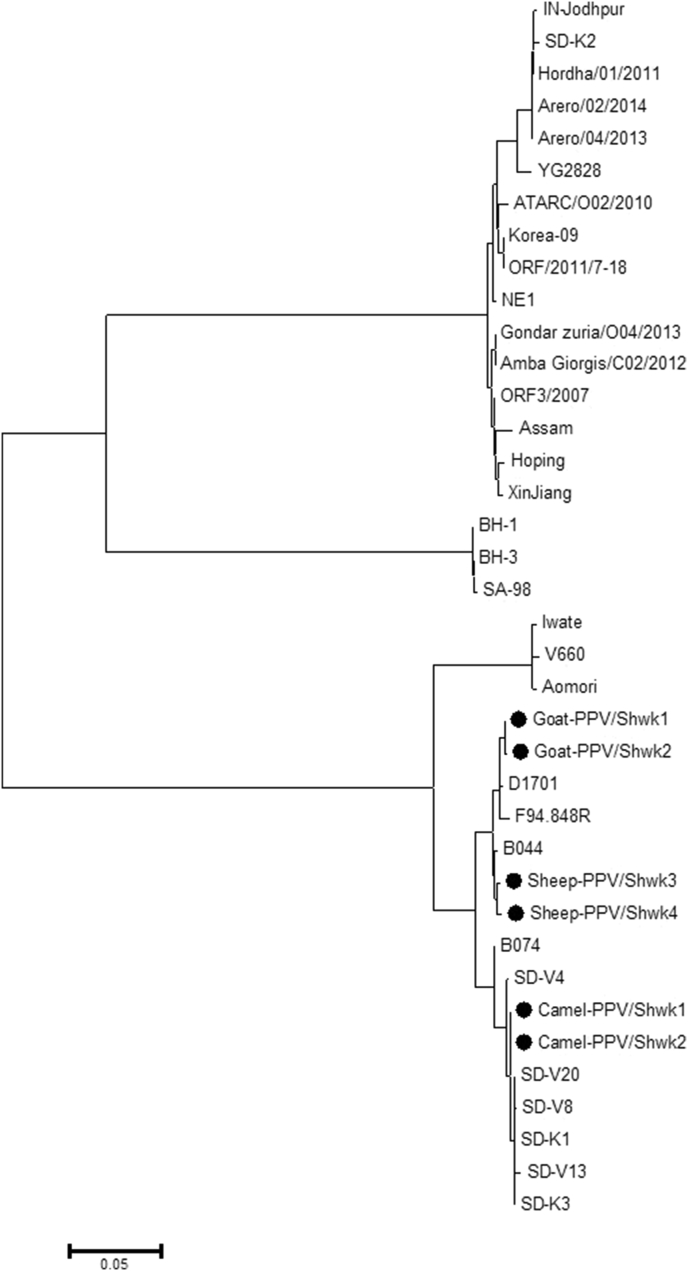
A BLAST search revealed that the studied sheep PPV (SPPV) and goat PPV (GPPV) isolates belong to the ORFV species, while the camel PPV (CPPV) isolates are close to the PCPV species of the PPV genus (Figure 5). The SPPV isolates shared 99.08% nucleotide sequence intragroup identity, 96.88–97.27% identity with the GPPV isolates, yet 92.51–93.62 % identity with the CPPV isolates (Table 4).
Figure 5.
Phylogenetic analysis of 38 PPV nucleotide sequences based on B2L gene. The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree with the sum of branch length = 0.92508009 is shown. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. The significance of all deduced phylogenetic trees was verified by bootstrap analysis of 1000 replicates Evolutionary analyses were conducted in MEGA7 (Kumar et al., 2016). Black circles = PPV isolates from this study in eastern Sudan.
Table 4.
Percent Identity Matrix - created by Clustal 2.1 for sheep (SPPV), goats (GPPV) and camel (CPPV) parapoxviruses collected from eastern Sudan, 2016
| SPPV/Shwk1 | SPPV/Shwk2 | GPPV/Shwk1 | GPPV/Shwk2 | CPPV/Shwk1 | CPPV/Shwk2 | |
|---|---|---|---|---|---|---|
| SPPV/Shwk1 | 100 | 99.08 | 96.88 | 96.88 | 92.77 | 92.51 |
| SPPV/Shwk2 | 99.08 | 100 | 97.19 | 97.27 | 93.62 | 93.27 |
| GPPV/Shwk1 | 96.88 | 97.19 | 100 | 99.67 | 93.16 | 92.71 |
| GPPV/Shwk2 | 96.88 | 97.27 | 99.67 | 100 | 93.12 | 92.52 |
| CPPV/Shwk1 | 92.77 | 93.62 | 93.16 | 93.12 | 100 | 99.27 |
| CPPV/Shwk2 | 92.51 | 93.27 | 92.71 | 92.52 | 99.27 | 100 |
4. Discussion
For a long time, contagious ecthyma caused by PPV has been neglected because of the general belief that it is a mild self-limiting disease. This is the first field and lab investigation on PPV infection in three livestock species; sheep, goats and dromedary camels. The virus causes proliferative exanthematous dermatitis characterized by pustules and scabs found mainly in the mouth of affected animals. Of note, the lesions in camels are confined to the head, in some cases; the edema extends to the neck, while in sheep and goats the lesion involves the head, also found on legs in some cases. However, no lesion was observed on the teat of lactating animals, though ORFV is known to cause such kind of lesions (Nandi et al., 2011).
The disease was diagnosed tentatively based on these clinical signs, besides lab confirmation was also obtained by PCR in some cases supported by genetic characterization. In sheep and goats, the disease affected mostly young animals, but some adults are also involved, whereas all affected camels were young less than one year of age. Affected sheep, goats and camels had proliferative crusted gross lesions covered by dark, friable fissured crusts, which sometimes extend to nostrils and ears. These clinical signs generally resemble those described for CE in sheep and goats (Nandi et al., 2011; Spyrou and Valiakos, 2015; Maganga et al., 2016), camels (Khalafalla et al., 2015; Munz et al., 1986) and other species of animals (Tryland et al., 2018).
In the present study livestock herders in Showak area of eastern Sudan were interviewed to get some epidemiological information on the CE infection. Herders who are familiar with the disease, had a good knowledge of the clinical signs, can differentiate it from similar infections such as sheep/goat pox and camelpox and don't regularly seek medical assistance or notify veterinary authorities. The result of the questionnaire has shown a morbidity rate of 23.8% and a case fatality rate of 4.7 % in the overall investigated animals (sheep, goats, and camels) causing a significant economic loss. Of note, camels accounted for the highest case fatality rate of 6.5% compared to 2.8 in sheep and 1.3 in goats. In a previous investigation, we reported a relatively higher case fatality rate of 13% in camels affected by contagious ecthyma also in eastern Sudan (Khalafalla and Mohamed, 1997). In sheep and goats, the morbidity and mortality rates recorded in the present study are considered high, as generally the mortality rate of CE is usually low, although increased rates of 15–90% have been reported due to secondary bacterial infections (Gumbrell and McGregor, 1997; Spyrou and Valiakos, 2015; Zhao et al., 2010). Besides, the mortality rate is dependent on several factors including age and status of the animals' immune system as well as environmental cleanliness or hygiene (Bala et al., 2018). Another reason for morality could be starvation due to inability of young nursing lambs and kids to suckle because of the mouth lesions (Scagliarini et al., 2012).
Furthermore, CE lesions that extend to eyelids, accompanied by bacterial infection common in camels lead to blindness and calve losses during the seasonal herd movement resulting in mortality rates that can reach 9% (Khalafalla, 2000). These findings are relevant for management purposes and provide evidence for the endemic nature of the disease which may jeopardize the animal productivity in the studied area, particularly of camels.
Regarding the frequency of disease occurrence in their herds during the last 10 years, a large portion of the responders reported that the disease occurs every year in the rainy season compared to more than once or first time with statistical significance. These findings provide evidence that contagious ecthyma is endemic in the Sudan in accordance with its enzootic nature among small ruminants in Asia, Africa, and some other parts of the world (Nandi et al., 2011; Bala et al., 2018).
In the camels, this result concurs with our previous studies in Sudan showing that the disease is endemic occurring every rainy season (Khalafalla, 2000; Khalafalla and Mohamed, 1997).
As to the age of affected animals, 93% of the affected animals were young less than one-year-old. This observation confirms reports by other authors (Scagliarini et al., 2012; Bala et al., 2018). Localization of the lesion revealed the mouth as the most frequent site followed by the whole head and in a few cases, the infection extends to the feet in accordance with the previous observations (Scagliarini et al., 2012). Both age and localization of the lesion are significantly related to the occurrence of the disease (P < 0.05). Even though males were found more affected by CE compared with females, no statistically significant association was observed (P > 0.05).
Analysis of the prevalence of contagious ecthyma based on the season of the year showed a higher prevalence in the rainy season compared to winter and summer. The statistical association demonstrated that the season strongly affects the prevalence of the disease (P < 0.0005). This finding is in line with the findings of earlier field observation (Scagliarini et al., 2012).
Concerning the source of CE infection, results demonstrated that most herders refer to browsing thorny acacia trees as the source of the disease with some herders point to contact with sick animals and few of them believe the infection is brought via the introduction of a new animal to their herds. Those who incriminated the browsing of thorny trees they even deny any involvement of any pathogen. Such believe was previously reported from Kazakhstan (Buchnev et al., 1987), Sudan (Khalafalla et al., 1994) and South Africa (Scagliarini et al., 2012). We previously showed that the severity of the CE infection is associated with the topography of the area where camels are grazing as areas with an abundance of acacia trees, showed relatively higher morbidity and mortality rates (Khalafalla, 2000). Skin abrasions of the lips caused by browsing acacia trees damage the lips permitting the transmission of the virus and were viewed as the major predisposing factor to CCE (Buchnev et al., 1987; Khalafalla and Mohamed, 1997). Similarly, CE in sheep and goats occurs during grazing and through the abrasions developed on the lips, nostrils, and mouth by the dried foods (Spyrou and Valiakos, 2015).
Most of the herders in the study area treat their animals when affected by CE using a variety of different medications. Treatment varies from modern ones which involving giving injectable antibiotics, to traditional ones which consisting of oil dressing on affected areas or mixed antibiotics with oil dressing. Of note, no commercial or auto-vaccines were used by the farmers. In a similar field study, it has been reported that most sheep and goat herders (61.8%) treat their animals against CE (Scagliarini et al., 2012). The treatment consisted of the application of petroleum jelly (Vaseline) or machine oil, copper sulphate and zinc preparations, but only a minority of farmers used antibiotics. Accordingly, treatment of CE cases by the application of antibiotics or oil dressing of the lesion is highly recommended as a supportive disease management strategy and for preventing subsequent secondary microbial infection. The traditional animal health care or the ethnoveterinary medicine has a long history that gives minimal effort options for pastoralists, particularly those living in remote regions. Various specific and supportive traditional therapies including poring warm water and hot branding of the head have been utilized by camel herders in Suleiman mountains of Pakistan to treat contagious ecthyma in camels (Raziq et al., 2010). These ethnoveterinary practices need to be investigated and documented.
Another finding in the present study was that most of the herders reported no zoonotic cases because of handling and treating affected animals. However, few respondents reported zoonotic transmission from sheep to the hands of herders who treated them. This finding points that CE in camels and goats isn't zoonotic in eastern Sudan and only the disease in sheep seems zoonotic.
Sheep-to-humans and goats-to-humans transmissions of ORFV have been documented in the literature. For instance, Nougairede et al. (2013) reported cases of Orf in humans that were transmitted from sheep and Scagliarini et al. (2012) described a case of human infection caused by a goat bite to a veterinarian. However, a search of the literature revealed no single camel-to-human transmission of the PPV and field observations and investigations, including the present study show that CE of camels, which is caused by PCPV isn't zoonotic and the general belief that all PPVs are known to be zoonotic (Friederichs et al., 2014) should be updated.
The PPV genome is a linear double-stranded DNA characterized by an unusually high GC content. Recently, an increasing number of genomic data including partial and whole genome sequences are used to define PPV species (Friederichs et al., 2014; Hautaniemi et al., 2011). The open reading frame (ORF) 011 (B2L gene) and the PPV orthologue of the vaccinia virus Copenhagen (VACV) gene F13L, can be used to encodes the major envelope antigen p37K preferred target gene in order to generate PCR amplified DNA fragments for sequence analysis and comparison of PPV (Friederichs et al., 2014). In the present study, we have used this gene to generate a phylogenetic tree for PPV DNA representatives of the three species.
On the phylogenetic tree the SPPV and the GPPVs grouped in one sub-branch together with other ORFV from goats and reindeer, while the CPPVs clustered close to PCPVs from cattle and other Sudanese CPPVs published earlier (Figure 5). However, the studied isolates share the same main branch of the tree comprising mainly PPV from Europe and Africa while the other branch involves PPVs mostly from Asia with some Sudanese CPPVs. The results provided evidence for close relationships between sheep and goat PPVs and a genetic variation of the camel PPV (CPPV). Two previous studies have pointed out that the CPPV infection (camel contagious ecthyma; CCE) can be caused by ORFV (Khalafalla et al., 2015; Li et al., 2013). This is what has been anticipated by the gross lesions which resemble lesions induced by ORFV in sheep and goats, but not PCPV or BPSV, because camels in Africa and Asia commonly share the same pasture with sheep, and goats and seldom with cattle. To this end, this investigation affirms that the PPV that cause the disease in camels in eastern Sudan is genetically distinct from that cause the disease in sheep and goats and support the previous finding which failed to experimentally infect sheep and goats with an isolate of CPPV (Abu-Elzein et al., 2004).
According to De La Concha-Bermejillo et al. (2003) natural cross-infection of orf between sheep and goats can occur, but the experimental transmission of infection from one species to another may not be successful. Explanations behind the failure of cross-infection of ORFV between sheep and goats, despite the genetic similarity could be the host factors as certain breeds of sheep and goats are more susceptible to the disease (Nandi et al., 2011).
5. Conclusion
The present study demonstrated that the gross lesion produced by PPV in sheep, goats, and camels is generally similar, yet the PPVs circulating in eastern Sudan in camels (PCPV) are genetically distinct from those affecting sheep and goats (ORFV). The investigation helps in our understanding of the diversity of PPV strains in Sudan and their association with other strains globally. Contagious ecthyma in eastern Sudan causes significant morbidities and mortalities and control measures, guided by the results of this investigation ought to be implemented. Further studies should focus on whole-genome sequencing of these PPVs which is anticipated to bring out a better understanding of the origin of the CPPV. There is also a need to investigate and document the ethnoveterinary practices employed by the herders for treating their sick animals.
Declarations
Author contribution statement
Abdelmalik Ibrahim Khalafalla: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ahmed Eisa Elhag: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hassan Zackaria Ali Ishag: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Mr. Abdo Bushara, the Camel Research Center, University of Khartoum, Showak Station for the valuable help in the field work. Our thanks also go to Dr. Leila M Ebrahim, Ministry of Animal Resources, Sudan for drawing the map.
References
- Abu-Elzein E., Housawi F., Al-Afaleq A., Ramadan R., Gameel A., Al-Gundi O. Clinico-pathological response of dromedary camels and sheep to cross-experimental infection with two virulent orf viruses originating from camels and sheep. J. Camel Pract. Res. 2004;11:15–19. [Google Scholar]
- Abubakr M.I., Abu-Elzein E.M.E., Housawi F.M., Abdelrahman A.O., Fadlallah M.E., Nayel M.N., Adam A.S., Moss S., Forrester N.L., Coloyan E., Gameel A. Pseudo cowpox virus: the etiological agent of contagious ecthyma (Auzdyk) in camels (Camelus dromedarius) in the Arabian Peninsula. Vector Borne Zoonotic Dis. 2007;7(2):257–260. doi: 10.1089/vbz.2006.0627. [DOI] [PubMed] [Google Scholar]
- Bala J.A., Balakrishnan K.N., Abdullah A.A., Mohamed R., Haron A.W., Jesse F.F.A., Noordin M.M., Mohd-Azmi M.L. The re-emerging of orf virus infection: a call for surveillance, vaccination and effective control measures. Microb. Pathog. 2018;120:55–63. doi: 10.1016/j.micpath.2018.04.057. [DOI] [PubMed] [Google Scholar]
- Bora D.P., Barman N.N., Das S.K., Bhanuprakash V., Yogisharadhya R., Venkatesan G., Kumar A., Rajbongshi G., Khatoon E., Chakraborty A., Bujarbaruah K.M. Identification and phylogenetic analysis of orf viruses isolated from outbreaks in goats of Assam, a northeastern state of India. Virus Gene. 2012;45(1):98–104. doi: 10.1007/s11262-012-0740-y. [DOI] [PubMed] [Google Scholar]
- Bouznach A., Hahn S., Stram Y., Menasherov S., Edery N., Shicaht N., Kenigswald G., Perl S. Case report: contagious ecthyma-Deviations in the anatomical appearance of lesions in an outbreak in lambs in Israel. Isr. J. Vet. Med. 2013;68:246–251. [Google Scholar]
- Buchnev K., Tulepbaev S.Z., Sansyzbaev A. Revue Scientifique et Technique de l'OIE (France); 1987. Infectious Diseases of Camels in the USSR. [DOI] [PubMed] [Google Scholar]
- Chan K.W., Hsu W.L., Wang C.Y., Yang C.H., Lin F.Y., Chulakasian S., Wong M.L. Differential diagnosis of orf viruses by a single-step PCR. J. Virol. Methods. 2009;160:85–89. doi: 10.1016/j.jviromet.2009.04.025. [DOI] [PubMed] [Google Scholar]
- De La Concha-Bermejillo A., Guo J., Zhang Z., Waldron D. Severe persistent orf in young goats. J. Vet. Diagn. Invest. 2003;15:423–431. doi: 10.1177/104063870301500504. [DOI] [PubMed] [Google Scholar]
- Friederichs S., Krebs S., Blum H., Wolf E., Lang H., von Buttlar H., Büttner M. Comparative and retrospective molecular analysis of Parapoxvirus (PPV) isolates. Virus Res. 2014;181:11–21. doi: 10.1016/j.virusres.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Gelaye E., Achenbach J.E., Jenberie S., Ayelet G., Belay A., Yami M., Loitsch A., Grabherr R., Diallo A., Lamien C.E. Molecular characterization of orf virus from sheep and goats in Ethiopia, 2008. Virol. J. 2016;13:34. doi: 10.1186/s12985-016-0489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbrell R., McGregor D. Outbreak of severe fatal orf in lambs. Vet. Rec. 1997;141:150–151. doi: 10.1136/vr.141.6.150. [DOI] [PubMed] [Google Scholar]
- Guo J., Rasmussen J., Wünschmann A., de La Concha-Bermejillo A. Genetic characterization of orf viruses isolated from various ruminant species of a zoo. Vet. Microbiol. 2004;99:81–92. doi: 10.1016/j.vetmic.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Hautaniemi M., Vaccari F., Scacliarini A., Laaksonen S., Huovilainen A., McInnes C.J. Analysis of deletion within the reindeer pseudocowpoxvirus genome. Virus Res. 2011;160:326–332. doi: 10.1016/j.virusres.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Hosamani M., Scagliarini A., Bhanuprakash V., McInnes C.J., Singh R.K. Orf: an update on current research and future perspectives. Expert Rev. Anti-infect. Ther. 2009;7:879–893. doi: 10.1586/eri.09.64. [DOI] [PubMed] [Google Scholar]
- Inoshima Y., Murakami K., Yokoyama T., Sentsui H. Genetic heterogeneity among parapoxviruses isolated from sheep, cattle and Japanese serows (Capricornis crispus) Arch. Virol. 2001:1215–1220. doi: 10.1099/0022-1317-82-5-1215. [DOI] [PubMed] [Google Scholar]
- Khalafalla A.I., Agab H., Abbas B. An outbreak of contagious ecthyma in camels (Camelus dromedarius) in eastern Sudan. Trop. Anim. Health Prod. 1994;26:253–254. doi: 10.1007/BF02240398. [DOI] [PubMed] [Google Scholar]
- Khalafalla A.I. Camel contagious ecthyma: risks in young calves. Revue d'élevage et de médecine vétérinaire des pays tropicaux. 2000;53:173–176. [Google Scholar]
- Khalafalla A.I., El-Sabagh I.M., Al-Busada K.A., Al-Mubarak A.I., Ali Y.H. Phylogenetic analysis of eight sudanese camel contagious ecthyma viruses based on B2L gene sequence. Virol. J. 2015;12:124. doi: 10.1186/s12985-015-0348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafalla A.I., Mohamed M. Epizootiology of camel contagious ecthyma in Eastern Sudan. Revue d'élevage et de médecine vétérinaire des pays tropicaux. 1997;50:99–103. [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhu X., Zheng Y., Wang S., Liu Z., Dou Y., Li H., Cai X., Luo X. Phylogenetic analysis of two Chinese orf virus isolates based on sequences of B2L and VIR genes. Arch. Virol. 2013;158:1477–1485. doi: 10.1007/s00705-013-1641-7. [DOI] [PubMed] [Google Scholar]
- Maganga G.D., Relmy A., Bakkali-Kassimi L., Ngoubangoye B., Tsoumbou T., Bouchier C., N’Dilimabaka N., Leroy E.M., Zientara S., Berthet N. Molecular characterization of orf virus in goats in Gabon, central Africa. Virol. J. 2016;13:79–84. doi: 10.1186/s12985-016-0535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz E., Schillinger D., Reimann M., Mahnel H. Electron microscopical diagnosis of ecthyma contagiosum in camels (Camelus dromedarius) first report of the disease in Kenya. J. Vet. Med. Ser. B. 1986;33:73–77. doi: 10.1111/j.1439-0450.1986.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Nagarajan G., Ghorui S.K., Kumar S., Pathak K.M. Complete nucleotide sequence of the envelope gene of pseudocowpox virus isolates from Indian dromedaries (Camelus dromedarius) Arch. Virol. 2010;155(10):1725–1728. doi: 10.1007/s00705-010-0784-z. [DOI] [PubMed] [Google Scholar]
- Nandi S., De U.K., Chowdhury S. Current status of contagious ecthyma or orf disease in goat and sheep—a global perspective. Small Rumin. Res. 2011;96:73–82. [Google Scholar]
- Nitsche A., Büttner M., Wilhelm S., Pauli G., Meyer H. Real-time PCR detection of parapoxvirus DNA. Clin. Chem. 2006;52:316–319. doi: 10.1373/clinchem.2005.060335. [DOI] [PubMed] [Google Scholar]
- Oem J.K., Roh I.S., Lee K.H., Lee K.K., Kim H.R., Jean Y.H., Lee O.S. Phylogenetic analysis and characterization of Korean orf virus from dairy goats: case report. Virol. J. 2009;6:167. doi: 10.1186/1743-422X-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani A., Yokoyama A., Narushige H., Inoshima Y. First isolation and genetic characterization of pseudocowpox virus from cattle in Japan. Virol. J. 2017;14:172. doi: 10.1186/s12985-017-0840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raziq A., de Verdier K., Younas M. Research Ethnoveterinary treatments by dromedary camel herders in the Suleiman Mountainous Region in Pakistan: an observation and questionnaire study. J. Ethnobiol. Ethnomed. 2010;6:16. doi: 10.1186/1746-4269-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagliarini A., Piovesana S., Turrini F., Savini F., Sithole F., McCrindle C.M. Orf in South Africa: endemic but neglected. Onderstepoort J. Vet. Res. 2012;79:1–8. doi: 10.4102/ojvr.v79i1.499. [DOI] [PubMed] [Google Scholar]
- Spyrou V., Valiakos G. Orf virus infection in sheep or goats. Vet. Microbiol. 2015;181:178–182. doi: 10.1016/j.vetmic.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Sullivan J.T., Mercer A.A., Fleming S.B., Robinson A.J. Identification and characterization of an orf virus homologue of the vaccinia virus gene encoding the major envelope antigen p37K. Virology. 1994;202:968–973. doi: 10.1006/viro.1994.1420. [DOI] [PubMed] [Google Scholar]
- Tikkanen M.K. Recent isolates of parapoxvirus of Finnish reindeer (Rangifer tarandus tarandus) are closely related to bovine pseudocowpox virus. J. Gen. Virol. 2004;85:1413–1418. doi: 10.1099/vir.0.79781-0. [DOI] [PubMed] [Google Scholar]
- Tryland M., Beckmen K.B., Burek-Huntington K.A., Breines E.M., Klein J. Orf virus infection in Alaskan mountain goats, Dall’s sheep, muskoxen, caribou and Sitka black-tailed deer. Acta Vet. Scand. 2018;60:12. doi: 10.1186/s13028-018-0366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikøren T., Lillehaug A., Åkerstedt J., Bretten T., Haugum M., Tryland M. A severe outbreak of contagious ecthyma (orf) in a free-ranging musk ox (Ovibos moschatus) population in Norway. Vet. Microbiol. 2008;127:10–20. doi: 10.1016/j.vetmic.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Zhao K., Song D., He W., Lu H., Zhang B., Li C., Chen K., Gao F. Identification and phylogenetic analysis of an Orf virus isolated from an outbreak in sheep in the Jilin province of China. Vet. Microbiol. 2010;142:408–415. doi: 10.1016/j.vetmic.2009.10.006. [DOI] [PubMed] [Google Scholar]