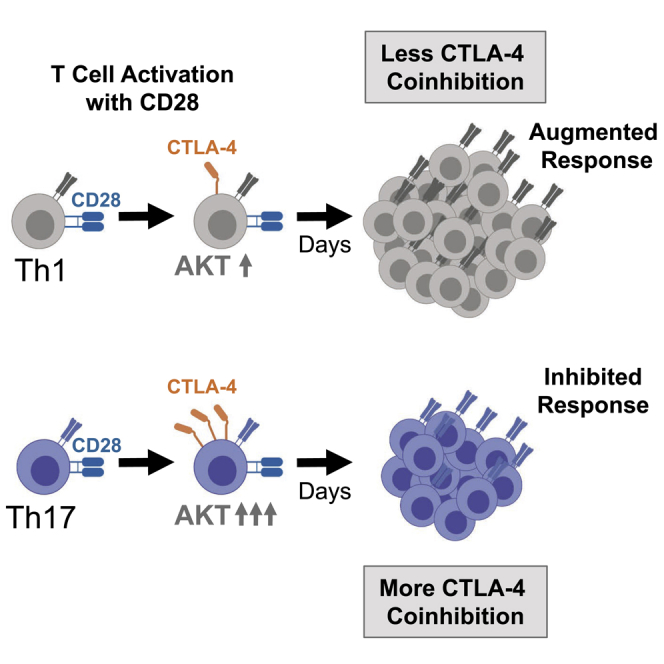
Summary
Previous work has demonstrated that Th17 memory cells but not Th1 cells are resistant to CD28/CTLA-4 blockade with CTLA-4 Ig, leading us to investigate the individual roles of the CD28 and CTLA-4 cosignaling pathways on Th1 versus Th17 cells. We found that selective CD28 blockade with a domain antibody (dAb) inhibited Th1 cells but surprisingly augmented Th17 responses. CD28 agonism resulted in a profound increase in CTLA-4 expression in Th17 cells as compared with Th1 cells. Consistent with these findings, inhibition of the CD28 signaling protein AKT revealed that CTLA-4 expression on Th17 cells was more significantly reduced by AKT inhibition relative to CTLA-4 expression on Th17 cells. Finally, we found that FOXO1 and FOXO3 overexpression restrained high expression of CTLA-4 on Th17 cells but not Th1 cells. This study demonstrates that the heterogeneity of the CD4+ T cell compartment has implications for the immunomodulation of pathologic T cell responses.
Subject Areas: Molecular Mechanism of Behavior, Immunology, Immune Response
Graphical Abstract
Highlights
-
•
CD28 blockade resulted in augmentation of human Th17 cells relative to Th1 cells
-
•
Th17 polarized mice exhibited graft rejection in the presence of CD28 blockade
-
•
A significant portion of Th17 cell CTLA-4 expression was induced by CD28 ligation
-
•
Overexpression of FOXO1 or FOXO3 inhibited Th17 cell CTLA-4 expression
Molecular Mechanism of Behavior; Immunology; Immune Response
Introduction
CD4+ T helper (Th) cells differentiate into subsets that can both provide immunity against distinct classes of microbes and mediate pathogenic immune responses, including autoimmunity and transplant rejection. A variety of cosignaling receptors are recognized to play distinct roles in the activation and differentiation of specific Th subsets. The prototypic cosignaling pathway on T cells is CD28/CTLA-4, in which costimulatory CD28 and coinhibitory CTLA-4 receptors on T cells compete for the same ligands, CD80 and CD86, on antigen-presenting cells. Recently the cosignaling requirements of Th17 cells have garnered clinical interest, as this population is the target of a number novel therapeutics for autoimmune diseases such as multiple sclerosis (MS), inflammatory bowel disease (IBD), and systemic lupus erythematosus (SLE) (McGeachy and Cua, 2008, Sallusto and Lanzavecchia, 2009, Tsokos, 2011, Miossec et al., 2009).
Clinical CD28/CTLA-4 blockade with CTLA-4 Ig (abatacept and its close derivative belatacept) is used to treat autoimmune disease and to prevent graft rejection following renal transplantation. Interestingly, Th17 cells are resistant to CD28/CTLA-4 blockade with CTLA-4 Ig in vitro relative to Th1 cells (Krummey et al., 2014a, Bouguermouh et al., 2009), and CTLA-4 Ig and its derivatives have shown limited efficacy in clinical trials of MS, IBD, and SLE (Merrill et al., 2010, Sandborn et al., 2012, Linsley and Nadler, 2009). Investigations involving the CD28 pathway on Th17 cells in several experimental systems have demonstrated variable effects of CD28 on Th17 cells relative to Th1 cells (de Wit et al., 2011, Santarlasci et al., 2012, Paulos et al., 2010). Recently, our group showed that human and murine Th17 cells express significantly more CTLA-4 than Th1 cells (Krummey et al., 2014a, Krummey et al., 2014b). However, mechanistic explanation of these observations and their relationship to Th17 cell resistance to CTLA-4 Ig is lacking.
In this study, we sought to understand the potential link between the observation that Th17 cells are relatively resistant to CTLA-4 Ig and the differential expression of CTLA-4 on Th1 versus Th17 cells. We utilized an anti-CD28 domain antibody (dAb) to selectively inhibit CD28 on Th1 versus Th17 cells, which revealed that Th1 cells are susceptible, whereas Th17 cells are resistant to CD28 blockade. This effect was mimicked by pharmacologic AKT inhibition, which revealed that Th17 cell activation is relatively resistant to AKT inhibition compared with Th1 cells. We found that the mechanism underlying this resistance is the fact that agonism of CD28 strongly induced CTLA-4 expression on Th17 but not Th1 cells and that the transcription factors FOXO1 and FOXO3 controlled high expression of CTLA-4 on Th17 cells. This report reveals a critical difference in the CD28 pathway on Th1 versus Th17 cells that results in disparate responses to immunomodulation.
Results
Human Th17 Cells Are Resistant to Selective CD28 Blockade
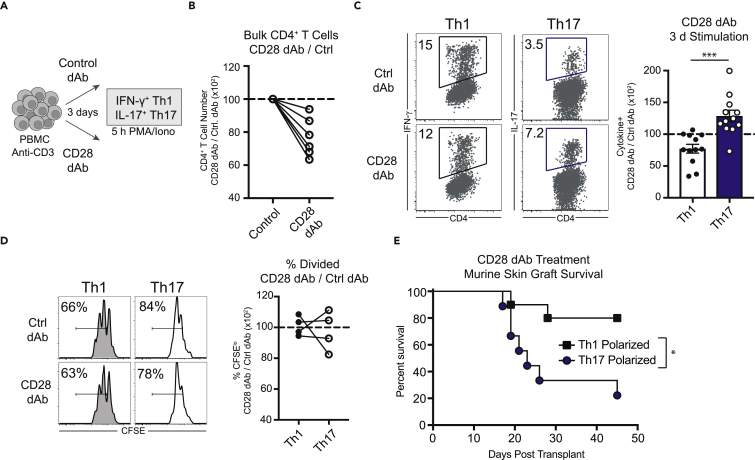
We have previously shown that human Th17 cells are more resistant to the CD28/CTLA-4 blocker CTLA-4 Ig (belatacept) relative to Th1 cells (Krummey et al., 2014a). We questioned whether differences in CD28 versus CTLA-4 signals were primarily responsible for this observation. Using a novel anti-CD28 domain antibody (dAb), which comprises a Vκ single antigen-binding site and a mutated “silent” Fc domain (lulizumab [Suchard et al., 2014]), we selectively inhibited CD28 signals during polyclonal stimulation with anti-CD3 (Figure 1A) (see Transparent Methods section within Supplemental Information). Anti-CD28 dAb treatment of bulk CD4+ T cell cultures resulted in reduced numbers of CD4+ T cells after 3 days (Figure 1B). The frequency of Th1 cells within those cultures (as measured by IFN-γ secretion following restimulation with PMA/ionomycin) was significantly inhibited by CD28 blockade (Figure 1C), whereas the frequency of Th17 cells was not inhibited by CD28 blockade (Figure 1C). These results suggest that CD28 signaling leads to distinct functional outcomes in Th1 versus Th17 cells.
Figure 1.
Th17 Cells Are Resistant to Selective CD28 Blockade
(A) Schematic of peripheral blood mononuclear cell (PBMC) stimulation and assessment of Th1 and Th17 populations.
(B) Frequency of bulk human CD4+ T cells after 3 days of culture with anti-CD3 and blocking anti-CD28 domain antibody (dAb), normalized to control dAb.
(C) Left panel, representative flow cytometry data depicting frequencies of human Th1 and Th17 cells following 3 days in culture in the presence of either control or anti-CD28 dAb (left). Right panel, summary data depicting the ratio of the frequency of Th1 and Th17 cells in anti-CD28 dAb cultures relative to control. See also Figure S1.
(D) Representative flow cytometry and summary data depicting CFSE dilution of human Th1 and Th17 cells after 3 days in culture following stimulation with anti-CD3 in the presence of anti-CD28 dAb or control dAb.
(E) Graft survival of mice containing donor-reactive cells polarized to either Th1 or Th17 and treated with anti-CD28 dAb. See also Figure S2.
(B–D) Each data point in the summary data represents an individual human donor. (E) Data shown represent 9–10 mice/group compiled from two independent experiments (p = 0.015). Statistical analysis performed using (C) Student's t test (two-tailed) and (E) log rank (Mantel-Cox) test. Summary data depict mean ± SEM. dAb, domain antibody. All summary data depict the mean ± standard deviation. Significance is defined as ∗p < 0.05, ∗∗∗p < 0.001.
We performed the same assay with Carboxyfluorescein succinimidyl ester (CFSE)-labeled peripheral blood mononuclear cells (PBMCs) to assess the impact of anti-CD28 dAb on proliferation in Th1 versus Th17 cells. The presence of anti-CD28 dAb resulted in less proliferation of the total CD4+ T cells in culture (Figure S1). Interestingly, Th1 versus Th17 cells proliferated to similar degrees in the presence of the anti-CD28 dAb versus control dAb (Figure 1D). This suggests that the ability of the anti-CD28 dAb to inhibit Th17 cells occurs at the level of activation and does not affect cells once they begin to proliferate.
Murine Th17 Cells Mediate Graft Rejection in the Presence of CD28 Blockade
To assess the impact of CD28 blockade on Th17 cells in vivo, we utilized an antigen-specific model of skin graft rejection that relies on CD4+ T cells polarized to Th1 or Th17 in vivo (Krummey et al., 2014b). In this model, graft survival times in Th1-polarized versus Th17-polarized mice treated with control dAb were not significantly different (Th1, 15 days; Th17, 17 days) (Figure S2). In contrast, we observed a differential effect of anti-CD28 dAb on graft survival in Th1 versus Th17-polarized mice. The majority of Th1-polarized mice maintained their grafts long-term (Figure 1E), whereas the majority of Th17-polarized animals rejected their grafts by day 30 (MST 23 d, Figure 1E). These results demonstrate that the presence of Th17 immunity confers resistance to CD28 blockade in vivo, similarly to our observations of in vitro stimulated of human T cells.
CTLA-4 Expression on Human Th17 Cells Is Uniquely Sensitive to AKT
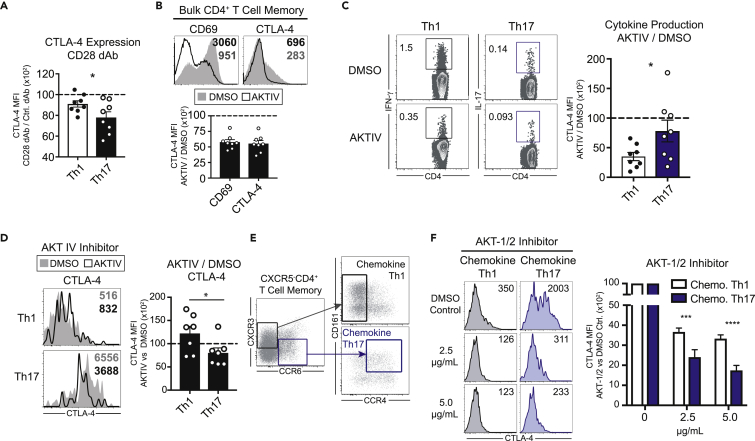
CTLA-4 expression has been shown to be dependent on CD28 signals in bulk T cells (Finn et al., 1997, Walunas et al., 1994, Walunas et al., 1996, Lindsten et al., 1993, Krummel and Allison, 1995), leading us to hypothesize that differential sensitivity to CD28-mediated AKT signals in Th1 versus Th17 cells could result in the CD28 blockade-resistant phenotype observed in Th17 cells relative to Th1 cells. In support of this, we found that CD28 blockade with anti-CD28 dAbs resulted in a greater inhibition of CTLA-4 on Th17 cells relative to Th1 cells (Figure 2A).
Figure 2.
CTLA-4 Expression on Human Th17 Cells Is Uniquely Sensitive to AKT Inhibition
(A) Expression of CTLA-4 on Th1 versus Th17 cells following activation with anti-CD3 and blocking anti-CD28 dAb or control dAb.
(B) Expression of CD69 and CTLA-4 on human bulk CD4+ memory T cells activation with anti-CD3/anti-CD28 in the presence or absence of the AKT inhibitor AKTIV.
(C) Frequency of Th1 versus Th17 memory cells activation with anti-CD3/anti-CD28 in the presence or absence of the AKT inhibitor AKTIV.
(D) Expression of CTLA-4 on human Th1 versus Th17 memory T cells activation with anti-CD3/anti-CD28 in the presence or absence of the AKT inhibitor AKTIV.
(E) Gating strategy for identifying CXCR3hiCCR4lo “chemokine” Th1 cells or CCR6hiCCR4hiCD161hi “chemokine” Th17 cells. See also Figure S3.
(F) Expression of CTLA-4 on chemokine Th1 or Th17 cells following activation with anti-CD3/anti-CD28 mAbs in the presence of the AKT inhibitor AKT-1/2. Summary data depict six individual donors.
Statistical analyses performed using (C and D) Student's t test (two-tailed) or (F) two-way ANOVA with Tukey's multiple comparison test. Summary data depict mean ± SEM. Significance is defined as ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
We next investigated whether intracellular signals downstream of CD28, which are transmitted through PI3K-Akt-mTOR axis (Hedrick et al., 2012, Powell et al., 2012), could inhibit CTLA-4 expression. We treated cells with an AKT phosphorylation inhibitor (AKT IV) (Kau et al., 2003, Lee et al., 2009) or DMSO vehicle control and activated them in the presence of CD3/CD28 monoclonal antibodies (mAbs). AKT inhibition significantly reduced both CD69 and CTLA-4 expression on bulk CD4+ populations compared with vehicle control (Figure 2B). AKT inhibition resulted in a relatively greater inhibition of IFN-γ-producing Th1 cells relative to IL-17-producing Th17 cells (Figure 2C). On the other hand, AKT inhibition resulted in a relatively less fold reduction of CTLA-4 expression on cytokine-producing Th1 cells relative to Th17 cells (Figure 2D).
In addition to defining Th1 and Th17 cells by cytokine production, surface markers have also been used to define these populations (Maggi et al., 2010, Annunziato et al., 2007, Singh et al., 2016). Following stimulation with anti-CD3/anti-CD28, the majority of cytokine-producing Th1 cells were CXCR3hiCCR6lo and CCR4lo (Figures S3A and S3B). Th17 cells, in contrast, were CCR6hiCXCR3lo and CCR4hiCD161hi (Figures S3A and S3B). We explored differences in CTLA-4 expression between these “chemokine” Th1 and Th17 cells identified using these alternate definitions. Similar to Th17 cells defined by their IL-17 secreting ability, chemokine Th17 cells expressed significantly higher levels of CTLA-4 than chemokine Th1 cells (Figure S3C). In chemokine Th1 and Th17 cells, we found that AKT inhibition with an additional AKT phosphorylation inhibitor (AKT-1/2) (Zhao et al., 2008) resulted in greater fold reduction of CTLA-4 expression on Th17 cells relative to Th1 cells (Figures 2D–2F). Together, these results demonstrate that Th17 cells are uniquely sensitive to CD28/AKT signaling, as blockade of this pathway results in both diminished CTLA-4 expression and a greater efficiency of activation relative to Th1 cells. This interpretation is supported by the finding that CD28 blockade results in less activation, as measured by cytokine production, of Th1 cells compared with Th17 after 3-day culture (Figure 1B).
Human Th17 CTLA-4 Expression Is Dependent on CD28 Signals
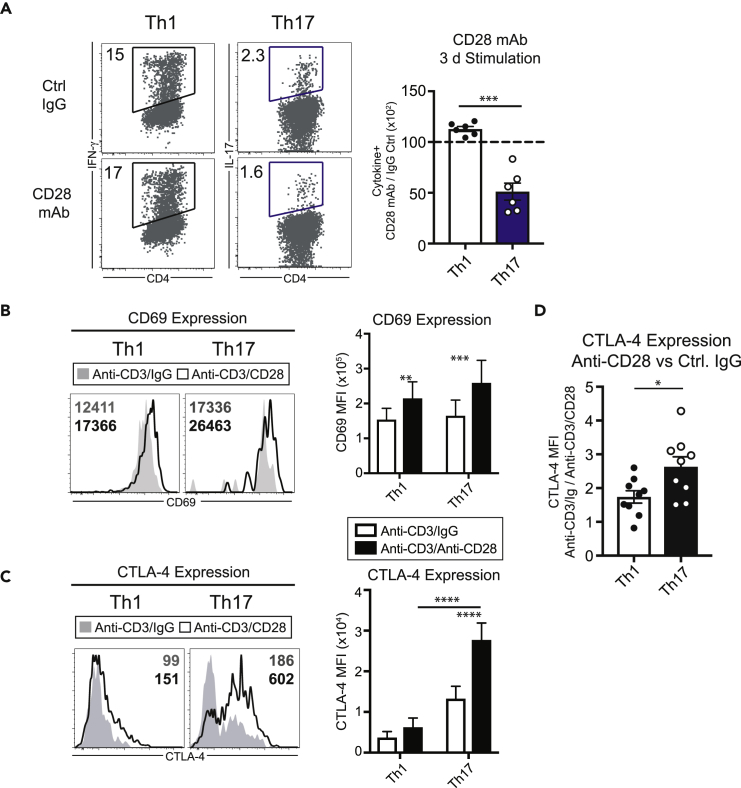
To directly assess the functional impact of CD28 agonism on Th1 and Th17 cells, we stimulated T cells in the presence of anti-CD28 mAb or control IgG. We found that, although CD28 agonism resulted in a modest increase in the frequency of Th1 cells (Figure 3A), it resulted in a significant decrease in the frequency of Th17 cells (Figure 3A).
Figure 3.
High Th17 CTLA-4 Expression Is Dependent on CD28 Signals
(A) Representative flow cytometry and summary data of frequency of Th1 versus Th17 cells after 3 days in culture with anti-CD3 and agonistic anti-CD28 mAb or control IgG.
(B) Expression of CD69 on Th1 versus Th17 cells activated with beads coated in anti-CD3/isotype control IgG or anti-CD3/agonistic anti-CD28 mAb.
(C) Expression of CTLA-4 on Th1 versus Th17 cells activated with beads coated in anti-CD3 or anti-CD3/anti-CD28.
(D) Expression of CTLA-4 on Th1 versus Th17 cells activated with anti-CD3/IgG relative to anti-CD3/agonistic anti-CD28. See also Figure S4.
(A and D) Each data point represents an individual human donor. (B) Summary data represent seven individual human donors. (C) Summary data represent nine individual human donors. Statistical analyses performed using (A and D) Student's t-test (two-tailed) or (B and C) two-way ANOVA with Sidak's multiple comparison test. Summary data depict mean ± SEM. mAb, monoclonal antibody. Significance is defined as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Given the findings that Th17 CTLA-4 expression is relatively dependent on AKT signaling (Figures 2D and 2F) and that the frequency of Th17 cells is reduced in the presence of strong CD28 cosignaling (Figure 3A), we hypothesized that coinhibitory CTLA-4 expression on Th17 cells could be driven by CD28 signaling. To address this question, we purified CD4+ T cells to remove CD80/86-bearing APCs within peripheral blood leukocytes and stimulated CD4+ T cells in the presence of beads coated in either anti-CD3 or anti-CD3/CD28 mAb. We found that Th1 and Th17 memory cells were similarly activated by anti-CD3 stimulation as measured by CD69 expression (Figure 3B). CD28 agonism, however, resulted in slightly greater CD69 expression on both Th1 and Th17 cells (Figure 3B). CTLA-4 expression was slightly higher on Th17 cells compared with Th1 cells in the presence of CD3 stimulation alone, whereas the expression of CTLA-4 was dramatically increased on Th17 cells by CD28 agonism (Figure 3C). In contrast, Th1 memory cells did not significantly upregulate CTLA-4 following CD28 agonism (Figure 3C). Thus, CD28 agonism resulted in a relatively greater fold increase in the expression (MFI) of CTLA-4 in Th17 versus Th1 cells (Figure 3D). CD28 agonism did not significantly alter the expression of 2B4, TIGIT, TIM-3, or PD-1 (Figure S4). Thus, these results demonstrate that CD28 stimulation results in different levels of CTLA-4 upregulation on Th1 versus Th17 cells.
FOXO1 and FOXO3 Control CTLA-4 Expression in Human Th17 Cells
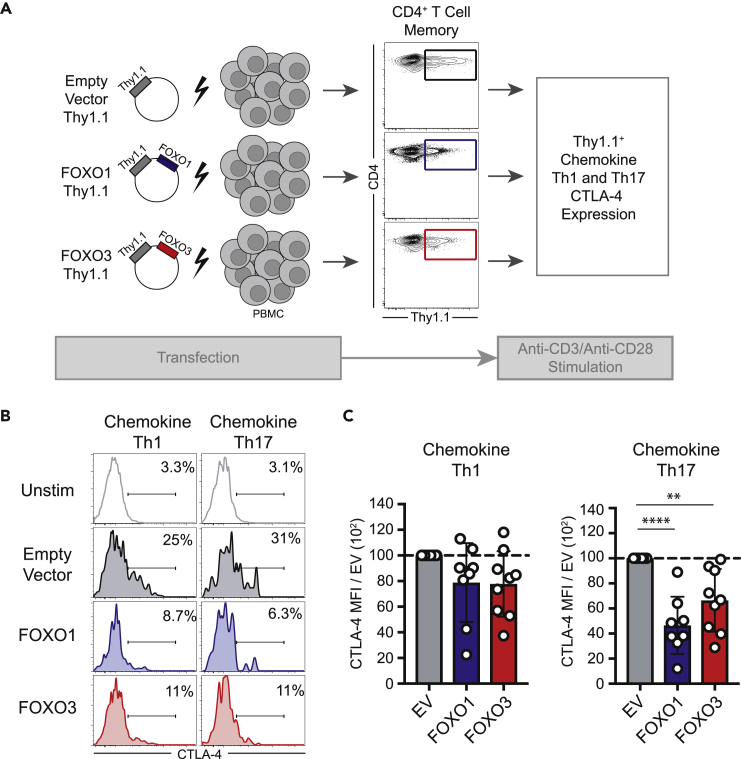
We next assessed whether the terminal signaling component of the CD28/AKT pathway, the transcription factors FOXO1 and FOXO3, impacts CTLA-4 expression in Th1 versus Th17 cells. We overexpressed FOXO1 or FOXO3 by transfecting human PBMC with vectors containing FOXO1 or FOXO3 along with the surface maker Thy1.1 as a tag to allow for detection of transfected cells (Figure 4A). CD4+ T cells were transfected with FOXO1 and FOXO3 vectors (or empty vector control) and stimulated with anti-CD3/anti-CD28 mAbs, and transfected Th1 and Th17 cells were identified by gating on Thy1.1 and CXCR3hiCCR6lo and CCR4lo or CCR6hiCXCR3lo and CCR4hiCD161hi, respectively. Results indicated that neither FOXO1 nor FOXO3 overexpression significantly impacted CTLA-4 expression on Thy1.1+ chemokine Th1 cells relative to empty vector Th1 controls (Figures 4B and 4C). Among Thy1.1+ chemokine Th17 cells, however, overexpression of either FOXO1 or FOXO3 resulted in significant inhibition of CTLA-4 expression (Figures 4B and 4C). These results demonstrate that overexpression of either FOXO1 or FOXO3 is sufficient to repress CTLA-4 expression in human Th17 cells.
Figure 4.
FOXO1 and FOXO3 Control CTLA-4 Expression in Human Th17 Cells
(A) Schematic of the experimental design in which vectors containing a Thy1.1 tag and either FOXO1 or FOXO3 were used to overexpress FOXO1 or FOXO3 in Th1 versus Th17 cells via transient transfection.
(B) Bulk CD4+ T cells were transfected with FOXO1- or FOXO3-containing vectors (or empty vector controls) and were stimulated with anti-CD3/agonistic anti-CD28. Expression of CTLA-4 on Thy1.1+ chemokine Th1 or Th17 cells was assessed.
(C) Summary data of the expression of CTLA-4 (MFI) on chemokine Th1 versus Th17 cells following transfection as in (B) relative to the CTLA-4 MFI in empty vector control Th1 or Th17 cells, respectively. Each data point represents an individual human donor.
Statistical analysis performed using one-way ANOVA with Dunnett's multiple comparisons test. Summary data depict mean ± SEM. Significance is defined as ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Discussion
Although Th17 cells have been shown to be resistant to CD28/CTLA-4 blockade with CTLA-4 Ig both in vitro and in vivo by our group and others, it was not clear from these studies whether this resistance is mediated through CD28, CTLA-4, or another signaling pathway. Here we provide evidence that CTLA-4 expression is more sensitive to CD28/AKT signaling in Th17 as compared with Th1 cells, resulting in stronger induction of this coinhibitory pathway following costimulatory CD28 signaling. Consistent with this finding, in a murine model of Th1 or Th17 polarized effector cells, Th17 polarized mice were not protected from graft rejection in the presence of CD28 blockade, providing an in vivo correlation with in vitro studies of human T cells. Thus, this work demonstrates how differences in the amount of CD28-dependent CTLA-4 expression serves as a feedback loop to fine-tune Th1 and Th17 cell responses.
The role for CD28-dependent CTLA-4 expression on Th17 cells offers a nuanced understanding of classic work defining this cosignaling pathway. Seminal studies demonstrated that CD28 signals are required for optimal Ctla4 gene expression (Teft et al., 2006, Finn et al., 1997, Walunas et al., 1994, Walunas et al., 1996, Lindsten et al., 1993, Krummel and Allison, 1995). It is important to note that these studies were conducted on bulk murine CD4+ or CD8+ T cell populations or T cell lines, in contrast to the primary human CD4+ T cell subsets investigated in this study. Our findings provide evidence that subtle differences in sensitivity to the CD28 pathway can result in profound functional differences on specific CD4+ T helper subsets, which cannot be appreciated in evaluation of bulk T cell populations. As evidence of this distinction, although we found that CD28 blockade inhibited the proliferation of bulk CD4+ T cells, we did not find a difference in the number of cell divisions among Th1 or Th17 cells treated with anti-CD28 dAb. This supports the notion that changes in the frequency of Th1 and Th17 cells under CD28 blockade reflects differences in activation rather than proliferation, in contrast to the impact of CD28 blockade on the proliferation of bulk CD4+ T cells.
This study supports a role for cell intrinsic coinhibition by CTLA-4, as our data provide evidence that CD28-dependent CTLA-4 acts to diminish the number of polarized Th17 cells that become activated to produce cytokine or enter the cell cycle, thus paradoxically reducing the number and frequency of Th17 cells after CD28 ligation. Although multiple functional roles have recently been ascribed to CTLA-4, including cell extrinsic mechanisms (Corse and Allison, 2012, Wang et al., 2012, Qureshi et al., 2011), our results are consistent with previous findings that CTLA-4 signaling inhibits activation without inducing apoptosis (Krummel and Allison, 1995, Walunas et al., 1996). However, it is important to note that our results do not exclude the possibility of additional cell extrinsic functions of CTLA-4 on Th17 cells.
This study provides a link between functional data involving CD28/CTLA-4 blockade of Th1 and Th17 cells and investigations of FOXO1/FOXO3-mediated CTLA-4 expression (Powell et al., 2012). Mouse models of FOXO1 and dual FOXO1/FOXO3 germline deletion have diminished CTLA-4 expression on CD4+ populations and established that FOXO1 and FOXO3 can bind to the Ctla4 promoter (Kerdiles et al., 2010, Ouyang et al., 2010, Kim et al., 2013). In a study of the role of common gamma chain-induced T cell cytokine production, Il-17 production by human Th17 cells is uniquely reliant on AKT, PI3K, and FOXO1 (Wan et al., 2011). However, a specific connection between AKT and CTLA-4 expression on human Th17 cells has not previously been shown. Further investigation is needed to uncover the transcriptional mechanism that enables FOXO3 to selectively repress Ctla4 expression in Th17 cells, such as the possibility that epigenetic changes at the Ctla4 locus play a role in these findings.
As clinical immunomodulatory drugs become more targeted to specific pathways on pathologic T cells, understanding the effect of these agents on individual T cell subsets is critical. This work provides an important example of how manipulation of a single receptor can have profound functional implications for shaping T cell responses in autoimmune disease and organ transplantation.
Limitations of the Study
Although we made efforts to evaluate the cell-intrinsic impact of CD28/CTLA-4 signals on CD4+ T cells, a number of the in vitro assays and our in vivo experiments necessarily included the presence of additional cells. Thus, we cannot definitively rule out that the impact of CD28 blockade on Th1 and Th17 cells was partially the result of interaction with additional cell type(s). One additional limitation of the study is the difficulty in dissecting signaling mechanisms in primary human cells. More specifically, although we were able to show a differential impact of CD28 signaling and AKT inhibition on Th17 cells relative to Th17 cells, uncovering the precise differences in the signaling cascades that leads to these functional differences will require additional studies.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors thank Dr. Aneesh K. Mehta and Shannon H. Bonds for assistance, PBMC donors, and Bristol-Myers Squibb for domain antibodies. This work was supported by the Roche Organ Transplant Research Foundation (M.L.F.), R01 AI104699 (M.L.F.), K99 AI146271 (S.M.K.), T32 AI007610-11 (S.M.K.), T32 GM08169-23 (S.M.K.), T32A1070081 (S.M.K.), F30 DK098928-01 (S.M.K.), and the Transplant Immunology Research Network (S.M.K.). Molecular cloning was generously provided by Dr. Oskar Laur and the Emory Integrated Genomics Core, which is subsidized by the Emory University School of Medicine and is one of the Emory Integrated Core Facilities. Additional support was provided by the Georgia Clinical & Translational Science Alliance of the National Institutes of Health (UL1TR002378).
Author Contributions
Conceptualization, S.M.K. and M.L.F.; Methodology, S.M.K., C.R.H., and M.L.F.; Investigation, S.M.K., C.R.H., and D.L.; Formal Analysis, S.M.K.; Writing the manuscript – Original Draft, S.M.K.; Writing – Reviewing & Editing, M.L.F; Funding Acquisition, M.L.F.; Supervision, M.L.F.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100912.
Supplemental Information
References
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Fili L., Ferri S., Frosali F. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouguermouh S., Fortin G., Baba N., Rubio M., Sarfati M. CD28 co-stimulation down regulates Th17 development. PLoS One. 2009;4:e5087. doi: 10.1371/journal.pone.0005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corse E., Allison J.P. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J. Immunol. 2012;189:1123–1127. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- de Wit J., Souwer Y., van Beelen A.J., de Groot R., Muller F.J., Klaasse Bos H., Jorritsma T., Kapsenberg M.L., de Jong E.C., van Ham S.M. CD5 costimulation induces stable Th17 development by promoting IL-23R expression and sustained STAT3 activation. Blood. 2011;118:6107–6114. doi: 10.1182/blood-2011-05-352682. [DOI] [PubMed] [Google Scholar]
- Finn P.W., He H., Wang Y., Wang Z., Guan G., Listman J., Perkins D.L. Synergistic induction of CTLA-4 expression by costimulation with TCR plus CD28 signals mediated by increased transcription and messenger ribonucleic acid stability. J. Immunol. 1997;158:4074–4081. [PubMed] [Google Scholar]
- Hedrick S.M., Hess Michelini R., Doedens A.L., Goldrath A.W., Stone E.L. FOXO transcription factors throughout T cell biology. Nat. Rev. Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau T.R., Schroeder F., Ramaswamy S., Wojciechowski C.L., Zhao J.J., Roberts T.M., Clardy J., Sellers W.R., Silver P.A. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- Kerdiles Y.M., Stone E.L., Beisner D.R., McGargill M.A., Ch'en I.L., Stockmann C., Katayama C.D., Hedrick S.M. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Sklarz T., Banks L.B., Gohil M., Waickman A.T., Skuli N., Krock B.L., Luo C.T., Hu W., Pollizzi K.N. Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nat. Immunol. 2013;14:611–618. doi: 10.1038/ni.2607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [see comments] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummey S.M., Cheeseman J.A., Conger J.A., Jang P.S., Mehta A.K., Kirk A.D., Larsen C.P., Ford M.L. High CTLA-4 expression on Th17 cells results in increased sensitivity to CTLA-4 coinhibition and resistance to belatacept. Am. J. Transplant. 2014;14:607–614. doi: 10.1111/ajt.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummey S.M., Floyd T.L., Liu D., Wagener M.E., Song M., Ford M.L. Candida-elicited murine Th17 cells express high Ctla-4 compared with Th1 cells and are resistant to costimulation blockade. J. Immunol. 2014;192:2495–2504. doi: 10.4049/jimmunol.1301332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kanatsu-Shinohara M., Morimoto H., Kazuki Y., Takashima S., Oshimura M., Toyokuni S., Shinohara T. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell. 2009;5:76–86. doi: 10.1016/j.stem.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Lindsten T., Lee K.P., Harris E.S., Petryniak B., Craighead N., Reynolds P.J., Lombard D.B., Freeman G.J., Nadler L.M., Gray G.S. Characterization of CTLA-4 structure and expression on human T cells. J. Immunol. 1993;151:3489–3499. [PubMed] [Google Scholar]
- Linsley P.S., Nadler S.G. The clinical utility of inhibiting CD28-mediated costimulation. Immunol. Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- Maggi L., Santarlasci V., Capone M., Peired A., Frosali F., Crome S.Q., Querci V., Fambrini M., Liotta F., Levings M.K. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur. J. Immunol. 2010;40:2174–2181. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Cua D.J. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Merrill J.T., Burgos-Vargas R., Westhovens R., Chalmers A., D'cruz D., Wallace D.J., Bae S.C., Sigal L., Becker J.C., Kelly S. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- Miossec P., Korn T., Kuchroo V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Ouyang W., Beckett O., Ma Q., Paik J.H., Depinho R.A., Li M.O. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat. Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- Paulos C.M., Carpenito C., Plesa G., Suhoski M.M., Varela-Rohena A., Golovina T.N., Carroll R.G., Riley J.L., June C.H. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci. Transl. Med. 2010;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.D., Pollizzi K.N., Heikamp E.B., Horton M.R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., Baker J., Jeffery L.E., Kaur S., Briggs Z. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur. J. Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Colombel J.F., Sands B.E., Rutgeerts P., Targan S.R., Panaccione R., Bressler B., Geboes K., Schreiber S., Aranda R. Abatacept for Crohn's disease and ulcerative colitis. Gastroenterology. 2012;143:62–69.e4. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Santarlasci V., Maggi L., Capone M., Querci V., Beltrame L., Cavalieri D., D'aiuto E., Cimaz R., Nebbioso A., Liotta F. Rarity of human T helper 17 cells is due to retinoic acid orphan receptor-dependent mechanisms that limit their expansion. Immunity. 2012;36:201–214. doi: 10.1016/j.immuni.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Singh D., Henkel M., Sendon B., Feng J., Fabio A., Metes D., Moreland L.W., Mcgeachy M.J. Analysis of CXCR5(+)Th17 cells in relation to disease activity and TNF inhibitor therapy in Rheumatoid Arthritis. Sci. Rep. 2016;6:39474. doi: 10.1038/srep39474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchard S.J., Davis P.M., Kansal S., Stetsko D.K., Brosius R., Tamura J., Schneeweis L., Bryson J., Salcedo T., Wang H. A monovalent anti-human CD28 domain antibody antagonist: preclinical efficacy and safety. J. Immunol. 2014;191:4599–4610. doi: 10.4049/jimmunol.1300470. [DOI] [PubMed] [Google Scholar]
- Teft W.A., Kirchhof M.G., Madrenas J. A molecular perspective of CTLA-4 function. Annu. Rev. Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- Tsokos G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Walunas T.L., Bakker C.Y., Bluestone J.A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q., Kozhaya L., Elhed A., Ramesh R., Carlson T.J., Djuretic I.M., Sundrud M.S., Unutmaz D. Cytokine signals through PI-3 kinase pathway modulate Th17 cytokine production by CCR6+ human memory T cells. J. Exp. Med. 2011;208:1875–1887. doi: 10.1084/jem.20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.J., Kenefeck R., Wardzinski L., Attridge K., Manzotti C., Schmidt E.M., Qureshi O.S., Sansom D.M., Walker L.S. Cutting edge: cell-extrinsic immune regulation by ctla-4 expressed on conventional T cells. J. Immunol. 2012;189:1118–1122. doi: 10.4049/jimmunol.1200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Robinson R.G., Barnett S.F., Defeo-Jones D., Jones R.E., Hartman G.D., Huber H.E., Duggan M.E., Lindsley C.W. Development of potent, allosteric dual Akt1 and Akt2 inhibitors with improved physical properties and cell activity. Bioorg. Med. Chem. Lett. 2008;18:49–53. doi: 10.1016/j.bmcl.2007.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.