Abstract
Background
Hepatocellular carcinoma is a disease of great concern. Surgery is the treatment of choice, but there is still a high recurrence rate after resection.
Objectives
To determine the benefits and harms of neoadjuvant and adjuvant therapies compared to surgery alone or surgery and placebo/supportive therapy after curative resection for operable hepatocellular carcinoma.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, Chinese Biomedical Database, and US National Cancer Institute's Physician's Data Query Trials Database until 2005. References of the identified trials were also searched for identifying further trials.
Selection criteria
Randomised and quasi‐randomised trials that compared hepatocellular carcinoma patients who were given and not given neoadjuvant/adjuvant therapy as a supplement to curative liver resection.
Data collection and analysis
Data were extracted independently by two authors and discrepancies resolved by consensus. The survival and disease‐free survival curves were compared using their one, two, three, four, and five‐year survival rates, median survival times, and the result of the significance tests (P‐values).
Main results
A total of 12 randomised trials were identified, totaling 843 patients. The size of the randomised clinical trials ranged from 30 to 155 patients. Both preoperative (neoadjuvant) and postoperative (adjuvant), systemic and locoregional (+/‐ embolisation), chemo‐ and immunotherapy interventions were tested. Treatment regimens and patients selected were not comparable, so no pooling was done. Only one regimen using preoperative transcatheter arterial chemoembolisation with doxorubicin was similar in two trials. Four of the twelve trials reported survival benefit at five years when given adjuvant or neoadjuvant therapy. Disease‐free survival was reported in nine trials, and the estimated hazard ratios show that disease‐free survival was significant in two trials at five years. These two trials had not shown a survival advantage, but the recurrence was significantly lower in patients given adjuvant or neoadjuvant therapy. The highest toxicity rate was in a trial using oral 1‐hexylcarbamoyl 5‐fluorouracil which resulted in 12 out of 38 patients being withdrawn from the trial because of adverse events.
Authors' conclusions
There is no clear evidence for efficacy of any of the adjuvant and neo‐adjuvant protocols reviewed, but there is some evidence to suggest that adjuvant therapy may be beneficial offering prolonged disease‐free survival. In order to detect a realistic treatment advantage, larger trials with lower risk of systematic error will have to be conducted.
Plain language summary
Not enough evidence to show if anti‐cancer drugs before and after surgery increase survival in liver cell cancer patients
Hepatocellular carcinoma, the commonest primary cancer of the liver is the sixth most common cancer in the world. According to the World Health Organization, most cases of hepatocellular carcinoma occur in Asia and Africa, however recent reports suggest that the incidence of primary liver cancer is also increasing in several developed countries, mainly in the Unites States and Europe. In Southeast Asia and Africa, hepatocellular carcinoma is predominantly associated with hepatitis B virus infection, whereas in Western countries and Japan it is associated with infection due to hepatitis C virus.
For hepatocellular carcinoma, surgery is the main form of treatment, but it is only possible for a small proportion of those afflicted. Even after curative resection, recurrence is common and is the main cause of death. Adjuvant (that is, chemotherapy after surgery) and neo‐adjuvant therapy (that is, chemotherapy before surgery) are thus attempted to try to improve outcomes.
This review sets on to determine the efficacy and adverse events of different neoadjuvant therapies (drug given before) versus adjuvant therapies (drug given after) compared to surgery alone, or surgery and placebo or supportive therapy when given to improve relapse and survival rates for operable hepatocellular carcinoma. A total of 12 randomised trials were identified, totaling 843 patients. The size of the randomised clinical trials ranged from 30 to 155 patients. Nine of the twelve trials reported no survival benefit from adjuvant therapy. Two trials reported a significant difference for survival and four studies for disease‐free survival for the treatment group, but the results of one of the trials on both its groups were very poor when compared to other trials. Two of the trials that did not report any absolute survival advantage reported statistically significant differences in disease‐free survival. The highest toxicity rate was in a trial using oral 1‐hexylcarbamoyl 5‐ fluorouracil which resulted in 12 out of 38 patients being withdrawn from the trial because of adverse events.
In conclusion, this review found insufficient evidence to show that adjuvant and neo‐adjuvant therapy increase survival from hepatocellular carcinoma, but there is limited evidence to suggest that neoadjuvant or adjuvant therapy may be useful for disease‐free survival.
Background
Hepatocellular carcinoma, the commonest primary cancer of the liver is an important disease entity and is the sixth most common cancer in the world, with more than half a million new cases reported annually (Okuda 1997; Lovet 2008). According to the World Health Organization, most cases of hepatocellular carcinoma occur in Asia. East Asia particularly have a very high incidence (over 20 cases per 100,000 population). Another region of concern is sub‐Saharan Africa, and particularly the western region of Africa (Gomaa 2008). The incidence of primary liver cancer is also increasing in the Unites States as well as in high‐income Euroepan countries (El‐Serag 2004; Forner 2006; Lovet 2008). In Southeast Asia and Africa, hepatocellular carcinoma is predominantly associated with hepatitis B virus infection, whereas in Western countries and Japan it is associated with infection due to hepatitis C virus. Other risk factors include toxic (alcohol and aflatoxins), metabolic (diabetes and non‐alcoholic fatty liver disease, hereditary haemochromatosis), and immune‐related (primary biliary cirrhosis and autoimmune hepatitis).
Curative treatments currently in use include surgical (either hepatic resection or liver transplantation), or local ablation, either radiofrequency or percutaneous ethanol injection (Lovet 2008; Lee 1982; Okuda 1985). Surgical resection remains one of the treatment modalities that offers the possibility of long‐term survival for patients with a non‐cirrhotic liver or selected patients with hepatic cirrhosis and well preserved hepatic function or who are unsuitable for liver transplantation (Lee 1982; Okuda 1985; NCCN 2008), but less than 10% of patients are operable at presentation (Oon 1980; Lai 1981; Lee 1982; Okuda 1985; Wang 1991).
Even after resection, the rate of disease recurrence is extremely high, and recurrence of complications is up to 70% of the cases at 5 years (Huguet 1994; Lai 1994; Forner 2006 ). Some studies on resected clinical hepatocellular carcinoma report a one‐year survival of around 55% (48% to 81%) and three‐year survival of around 30% (24% to 48%) (Nagourney 1987; Okuda 1987). Distant metastases occur locally in the remaining liver. It is generally believed that recurrences arise not because of inadequate resection, but because of pre‐existing microscopic tumour foci that are undetected by imaging modalities or because of malignant cells that have been disseminated during surgical manipulation (Finkelstein 2003). This general belief is the motivation for attempting neoadjuvant (pre‐operative) and adjuvant (post‐operative) therapies to supplement surgery.
This review was first published in 1999 in the Cochrane Database of Systematic Reviews (CDSR) and the authors concluded that 'there was no clear evidence for efficacy of any of the adjuvant and neo‐adjuvant protocols reviewed, but there was some evidence to suggest that adjuvant therapy might be beneficial for prolonged disease‐free survival (Chan 1999). Conclusions from the review showed that clinical trial results published until 1999 did not show a clear picture on the effectiveness of neoadjuvant and adjuvant therapies in patients who underwent liver resection. Subsequently, a review was published in 2002 (Schwartz 2002) concluding that 'systemic and hepatic‐artery chemotherapy or chemoembolisation have not been shown to improve overall or disease‐free survival after resection of hepatocellular carcinoma'. Therefore, the objective of undertaking this update was to review the emerging status of evidence after 1999 (after the publication of the Cochrane review), to determine the effectiveness and safety of neoadjuvant and adjuvant therapies before or after liver resection in hepatocellular carcinoma patients.
Objectives
To determine the benefits and harms of different neoadjuvant/adjuvant therapies compared to liver resection alone, or liver resection and placebo or supportive therapy when these therapies are given to improve relapse and survival rates for operable hepatocellular carcinoma.
Methods
Criteria for considering studies for this review
Types of studies
All randomised clinical trials.
Types of participants
Participants with previously untreated and histologically confirmed hepatocellular carcinoma who underwent liver resection with curative intent. Excluded were patients with concurrent malignancies that had been diagnosed either prior to or at the time of liver resection and patients with previous liver resection for another pathology, or liver transplantation.
Types of interventions
All neoadjuvant/adjuvant measures to surgery that had been evaluated in randomised clinical trials or controlled clinical studies against surgery alone or surgery and placebo/supportive therapy. Excluded were nutritional support interventions (amino acids, minerals, and vitamins) given after surgery. Studies without a non‐active treatment group were excluded.
Types of outcome measures
1. One, two, three, four, and five‐year survival rates, median survival times, and the result of significance tests (Log‐rank or Wilcoxon) for the difference between survival curves. 2. All adverse events.
Search methods for identification of studies
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded (Royle 2003), Chinese Biomedical Database, and US National Cancer Institute's Physician's Data Query Trials Database until 2005. We have given the search strategies and the time span of the searches in Appendix 1. References of the identified trials were also searched for identifying further trials. Non‐English language papers were considered where translation was possible.
Data collection and analysis
Selection of trials A pool of abstracts of potentially relevant trials were first screened for neoadjuvant and adjuvant randomised clinical trials and controlled clinical trials. Three authors, ES‐YC, PK‐HC, and SM, independently screened the full text of selected trials to confirm eligibility, assess quality, and extract data. Discrepancies were resolved by discussion and consensus.
Assessment of methodological quality We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and The Cochrane Hepato‐Biliary Group Module (Gluud 2008). Due to the risk of overestimation of intervention effects in randomised trials with unclear or inadequate methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), we assessed the influence of methodological quality of the trials on the trial results by evaluating the reported randomisation and follow‐up procedures in each trial. If information was not available in the published trial, we contacted the authors in order to assess the trials correctly. We assessed the following components:
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice was considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names, or admittance numbers were used for the allocation of patients. Due to the small number of clinical trials on operable hepatocellular carcinoma, quasi‐randomised studies were included in the review.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, or sealed envelopes.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding
Adequate, if the trial was described as single or double‐blind, and the method of blinding involved identical placebo or active drug.
Unclear, if the trial was described as blinded, but the method of blinding was not described.
Not performed, if the trial was not single or double‐blind.
Follow‐up
Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
Inadequate, if the number or reasons for dropouts and withdrawals were not described.
ITT Analysis
Trial participants should be analysed in the groups to which they were randomised regardless of which (or how much) treatment they actually received, and regardless of other protocol irregularities, such as ineligibility.
All participants should be included regardless of whether their outcomes were actually collected.
Other criteria, which were considered were:
Sample size calculations reported in studies
Comparability between treatment groups based on baseline characteristics reported.
Data synthesis We conducted the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2008). We used the software package RevMan 5 (RevMan 2008) provided by The Cochrane Collaboration for analysis. For trials, which did not report the hazard ratio (HR) or had insufficient information for it to be estimated, several surrogate measures characterising the survival experience of each group in a trial were extracted from the published survival curves and tabulated [Cumulative survival rates reported using Kaplan‐Meir method and the significance of differences between groups assessed by log‐rank test and Wilcoxon tests]. Trials for which there was sufficient information, HR was calculated by using approximation methods as described by Parmar 1998. Pooling of results was not done because we judged a priori that the studies were too heterogenous and because of the lack of a suitable summary estimate of effect, ie, the HR).
The calculated hazard ratios (HR) were entered as generic inverse variance data on a logarithmic scale to produce the forest plot.
Results
Description of studies
A total of 12 studies were included totaling 843 participants, of which one was a Chinese language article (Yunxue 1999) identified from the Chinese Biomedical Database. One study was reported initially as a brief communication and then published again in the following years with complete data (Nishiguchi 2005). The total number of patients in the studies ranged from 30 (Nishiguchi 2005) to 155 (Takayama 2000). However, only three studies reported sample size calculations (Lai 1998; Lau 1999; Takayama 2000). One study (Lau 1999) with 30 participants was stopped prematurely after an interim analysis was done showing that there was a significant improvement in the disease‐free survival outcome. The polyprenoic acid study of Muto 1996 and interferon study of Ikeda 2000 were excluded because the control and treatment groups comprised an unknown/known proportion of curative surgical resection and percutaneous ethanol injection patients (the authors clearly favour the latter in their discussion). It was also primarily concerned with the prevention of onset of a second primary tumour and not absolute survival, whereas the objective of the studies included in this review was to improve the absolute survival after the resection of the original primary. Two other trials, which were excluded from the review, were Chinese language articles (Bao 2001; Li 2002). They did not meet our inclusion criteria (See Characteristics of excluded studies).
Apart from the fact that participants had hepatocellular carcinoma regarded as curatively resectable and had reasonable liver function tests, Child A/B (Child 1964), Okuda I/II (Okuda 1984), the entry criteria varied and were not easily compared. Three studies (Yamamoto 1996; Yamasaki 1996; Nishiguchi 2005) specified participants with small tumours (2 to 5 cm), one (Wu 1995) specified participants with only large tumours (more than 10 cm), one study included tumours ranging from less than 5 cm to more than 10 cm (Yunxue 1999), and the rest did not place any restriction on tumour size.
All studies compared a surgery‐only group against surgery combined with chemotherapy that was given either preoperatively (Wu 1995; Yamasaki 1996; Yunxue 1999), postoperatively (Izumi 1994; Yamamoto 1996; Ono 1997; Lai 1998; Lau 1999; Takayama 2000; Nishiguchi 2005), or pre‐ and postoperatively (Lygidakis 1995; Lygidakis 1996). Izumi et al (Izumi 1994) was the only study with two adjuvant groups: with and without gelatin sponge embolisation for participants having good and poor liver function results (following surgery), respectively. Both Lygidakis' studies (Lygidakis 1995; Lygidakis 1996) used slightly different, but complex regimens of locoregional chemo‐ and immunotherapy before and after surgery. The connection between these two studies is unclear, but they appear to be independent studies. We contacted its authors for clarification on 17th April 2007, but have not received an answer until now. Lygidakis is the first and only common author, but the later study (91 patients, two authors) does not cite the earlier one (40 patients, four authors).
Only the two neoadjuvant studies of Wu 1995 and Yamasaki 1996 had similar interventions. Both used locoregional chemoembolisation (specifically transcatheter arterial chemoembolisation) with doxorubicin. Wu 1995 reported an average delay until surgery in the neoadjuvant group of about four weeks, and Yamasaki 1996 did not report this figure. The other neoadjuvant study Yunxue 1999 used transcatheter chemoembolisation with a combination of two or three interventions of either (mitomycin C) MMC 10 mg, 5Fu50 mg to 200 mg, EPIR 6 mg to 10 mg, or Carcbo platin 300 mg to 500 mg. The mean time to surgery was 47.2±52.8 days (shortest was two weeks and the longest was 11 months).
Among the pure adjuvant trials, Izumi 1994 used transcatheter arterial chemoembolisation (TACE) in patients with good liver function, lipiodol, doxorubicin, mitomycin followed by getain sponge cubes or chemotherapy without embolisation, lipiodol, doxorubicin and mitomycin, Lai 1998 used a combination of systemic epirubicin and locoregional cisplatin, Yamamoto 1996 used oral 1‐hexylcarbamoyl 5‐fluoracil (HCFU), Ono 1997 used systemic and locoregional epirubicin and oral HCFU, Lau 1999 used 1850 MBq lipiodol‐Iodine‐131, Takayama 2000 used adoptive immunotherapy, and Nishiguchi 2005 used interferon‐alpha. Eight of the studies (Izumi 1994; Wu 1995; Yamamoto 1996; Ono 1997; Lai 1998; Lau 1999;Takayama 2000;Nishiguchi 2005) mentioned that patients with recurrent cancers were given additional therapy independent of their randomised treatment.
Absolute and disease‐free (recurrence‐free) survival experience in all studies were reported using Kaplan‐Meier survival curves, except for two trials (Lygidakis 1995; Yunxue 1999). Lygidakis 1995 reported only mean survival times and survival percentages at some unspecified time, and Lygidakis 1996 reported only the absolute survival curves. Yunxue 1999 reported only two‐year survival rate and time to recurrence. Yamamoto 1996 reported survival curves separately stratified for clinical stage I and II of liver cirrhosis (LCSG Japan 1992). Lau 1999 and Takayama 2000 studies reported hazard ratios (HR) in addition to the Kaplan‐Meier survival curves. Lau 1999 study, reported HRs of overall survival and disease‐free survival of interim analysis and after the completion of the study. Only eight studies reported survival and recurrence rates at five years.
All studies, except Yamasaki 1996 and Yunxue 1999, reported adverse events (see Characteristics of included studies). The most notable number and severity of adverse events were seen in Yamamoto 1996 (oral HCFU) where 12 out of 38 participants in the adjuvant group stopped treatment. Interestingly, the Ono 1997 study, which also used oral HCFU, did not report such severe adverse events. In Takayama 2000 study, 59% of the patients had adverse events of grade 1 or 2 and were treated as outpatients, of which five patients had more than one adverse event. None of the patients had pulmonary or renal symptoms, or any sign of infection, hepatic functional deterioration, or immune disorder. Treatment adherence was also satisfactory (97%). In Nishiguchi 2005 study, treatment could not be completed in 3 patients because of depression, severe general fatigue, and renal abscess. Studies, which looked at the effectiveness of nutrition before and after surgery on survival and recurrence rates, were excluded from this review.
Risk of bias in included studies
All the studies were described as randomised, but exceptionally few details were given. All 12 trials were randomised clinical trials, but only four trials are of high quality, ie, assessed as trials having low risk of bias, based on adequate concealment of allocation. Yamasaki 1996, Ono 1997, and Lai 1998 reported using sealed envelopes, while Yamamoto 1996 used central office randomisation (Characteristics of included studies).
Of all the trials, only two trials were blinded. In Nishiguchi 2005 study, investigators and the radiologists responsible to check the recurrence were blinded, while in Takayama 2000 study only the radiologists were blinded to the treatment allocation.
Patient numbers were generally small, ranging from 15 to 79 per intervention group. Lai 1998, Lau 1999, and Takayama 2000 were the only three studies to justify the sample size used by an explicit statement of the expected treatment effect, power, and significance level. In all studies, both groups appeared reasonably balanced for other measured variables.
The best criteria for adequacy of resection seemed to be that used by Ono 1997, Lai 1998, and Nishiguchi 2005. These three studies used the criterion negative by imaging one month after surgery. Participants who qualified were then randomised to no further therapy or adjuvant therapy. This approach is obviously only applicable to post‐operative regimens.
Two studies reported interval estimates of treatment effect (Lau 1999; Takayama 2000). The exception Lygidakis 1995 did not provide any survival curves and only used an inappropriate statistic ‐ the mean survival time to compare the treatments. This study also reported survival percentages but at unspecified time points. This was in contrast with the reporting in Lygidakis 1996, which did provide the survival curves. However, in this study, the authors measured the time until death from the time of surgery in both groups. This, however, has the potential for introducing bias because the adjuvant treatment in this study actually started prior to surgery. In Nishiguchi 2005 trial, recurrence rates and cumulative survival rates were reported until the study end point, from operation until death, which was seven years and six months. Yunxue 1999 study reported survival and time to recurrence only at two years.
Only Yamamoto 1996, Ono 1997,Lai 1998,Lau 1999,Yunxue 1999,Takayama 2000, and Nishiguchi 2005 did ITT. Yamamoto 1996 also did a treatment‐received analysis, not ITT, after excluding 16 out of 67 participants for a variety of reasons.
Effects of interventions
One‐, two‐, and three‐year survival rates, median survival times, and the result of significance tests for the difference between treatment groups Two studies reported confidence interval of treatment effect (Lau 1999; Takayama 2000). Therefore, for all other studies we have tabulated the reported or approximate (estimated from the published graphs) point estimates of the one‐, two‐ three‐, four‐, and five‐year survival probabilities, disease‐free survival probabilities, median survival time, and median disease‐free survival for each treatment group, and the reported P‐value of the significance test used to compare the two treatment curves (except for Lygidakis 1995 in which no curves were given) (Analysis 1.1; Analysis 1.2).
1.1. Analysis.
Comparison 1 Neoadjuvant and adjuvant therapy versus surgery for operable HCC, Outcome 1 Survival and median survival time.
| Survival and median survival time | |||||||
|---|---|---|---|---|---|---|---|
| Study | Survival % year 1 | Survival % year 2 | Survival % year 3 | Survival % year 4 | Survival % year 5 | Median survival | Survival p‐value |
| Izumi 1994 | Surgery: 81% Adjuvant: 87% | Surgery: 65% Adjuvant: 70% | Surgery: 53% Adjuvant: 57% | Surgery: 44% Adjuvant: 52% | Surgery: 30% Adjuvant: 52% | Surgery: 3.3 years Adjuvant: 4.0 years | p = 0.5327 (Adjuvant: doxorubicin + mitomycin +/‐ transarterial embolization) |
| Lai 1998 | Surgery: 95% Adjuvant: 75% | Surgery: 90% Adjuvant: 65% | Surgery: 65% Adjuvant: 65% | Surgery: 65% Adjuvant: 55% | Not given | Surgery: not reached Adjuvant: not reached | p = 0.10 (Adjuvant: epirubicin+cisplatin) |
| Lau 1999 | Surgery: 88% Adjuvant: 92% | Surgery: 60% Adjuvant: 88% | Surgery: 48% Adjuvant 88% | Surgery: 40% Adjuvant: 78% | Surgery: 40% Adjuvant: 78% | Median overall survival: HR 3.5 (95% CI 0.7‐17.5) months, p=0.01. Median disease‐free survival: HR 4.3 (95% CI 1.2‐15.6) months., p=0.01. | P = 0.039 (Adjuvant: Lipiodol‐iodine‐131) |
| Lygidakis 1995 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Lygidakis 1996 | Surgery: 55% Adjuvant: 80% | Surgery: 15% Adjuvant: 55% | Surgery: 15% Adjuvant: 55% | Surgery: 15% Adjuvant: 45% | Surgery: not given Adjuvant: 45% | Surgery: 1.0 year Adjuvant: 3.0 years | P = 0.0042 (Neoadjuvant/adjuvant: chemo+immunotherapy) |
| Nishiguchi 2005 | Surgery: 93.3% Adjuvant: 100% | Surgery: 86.7% Adjuvant: 100% | Surgery: 80% Adjuvant: 93.3% | Surgery: 60% Adjuvant: 86.7% | Surgery: 46% Adjuvant: 75% | Surgery: 4.2 years Adjuvant: not reached | p=0.041 (Adjuvant: interferon‐alpha) |
| Ono 1997 | Surgery: 96% Adjuvant: 93% | Surgery: 85% Adjuvant: 75% | Surgery: 82% Adjuvant: 72% | Surgery: 58% Adjuvant: 45% | Surgery: 58% Adjuvant: 33% | Surgery: not reached Adjuvant: 3.5 years | P = 0.14357 (Adjuvant: epirubicin+oral HCFU) |
| Takayama 2000 | Surgery: 98% Adjuvant: 99% | Surgery: 85% Adjuvant: 92% | Surgery: 75% Adjuvant: 90% | Surgery: 68% Adjuvant: 75% | Surgery: 62% Adjuvant: 68% | Surgery: 6.2 years Adjuvant: not reached | P = 0.09 (Adjuvant: immunotherapy‐autologus lymphocytes) |
| Wu 1995 | Surgery: 95% Adjuvant: 70% | Surgery: 80% Adjuvant: 45% | Surgery: 60% Adjuvant: 30% | Surgery: 60% Adjuvant: 30% | Surgery: 60% Adjuvant: 30% | Surgery: 9.5 years Adjuvant: 1.5 years | P = 0.03 (from time of tumour detection) (Neoadjuvant: doxorubicin+TACE) |
| Yamamoto 1996 | Stage I: Surgery: 80% Stage I: Adjuvant: 90% Stage II: Surgery: 80% Stage II: Adjuvant: 90% | Stage I: Surgery: 75% Stage I: Adjuvant: 80% Stage II: Surgery: 55% Stage II: Adjuvant: 70% | Stage I: Surgery: 70% Stage I: Adjuvant: 75% Stage II: Surgery: 55% Stage II: Adjuvant: 60% | Stage I: Surgery: 52% Stage I: Adjuvant: 75% Stage II: Surgery: 55% Stage II: Adjuvant: 30% | Not given | Stage I: Surgery: 4.5 years Stage I: Adjuvant: not reached Stage II: Surgery: not reached Stage II: Adjuvant: 3.5 years | STAGE I: P = 0.08 STAGE II: P = 0.77 (Adjuvant: oral 1‐hexylcarbamoyl 5‐fluorouracil (HCFU)) |
| Yamasaki 1996 | Surgery: 95% Adjuvant: 95% | Surgery: 90% Adjuvant: 95% | Surgery: 85% Adjuvant: 85% | Surgery: 76% Adjuvant: 81% | Surgery: 60% Adjuvant: 63% | Surgery: 5.5 years Adjuvant: 5.5 years | Not significant (Neoadjuvant: doxorubicin+TACE) |
| Yunxue 1999 | No data | Surgery: 82.8% Neoadjuvant: 90.6% | No data | No data | No data | No data | P < 0.05 |
1.2. Analysis.
Comparison 1 Neoadjuvant and adjuvant therapy versus surgery for operable HCC, Outcome 2 Disease‐free survival.
| Disease‐free survival | |||||||
|---|---|---|---|---|---|---|---|
| Study | Survival % year 1 | Survival % year 2 | Survival % year 3 | Survival % year 4 | Survival % year 5 | Median survival | Survival p‐value |
| Izumi 1994 | Surgery: 43% Adjuvant: 65% | Surgery: 22% Adjuvant: 55% | Surgery: 12% Adjuvant: 32% | Surgery: 6% Adjuvant: 25% | Surgery: 0% Adjuvant: 6% | Surgery: 1.3 years Adjuvant: 2.3 years | p = 0.0237 (Adjuvant: doxorubicin + mitomycin +/‐ transarterial embolization) |
| Lai 1998 | Surgery: 69% Adjuvant: 50% | Surgery: 53% Adjuvant: 36% | Surgery: 48% Adjuvant: 18% | Surgery: 48% Adjuvant: 6% | Not given | Surgery: 2.5 years Adjuvant: 1.0 year | p = 0.04 (Adjuvant: epirubicin+cisplatin) |
| Lau 1999 | Surgery: 60% Adjuvant: 86% | Surgery: 38% Adjuvant: 75% | Surgery: 38% Adjuvant: 75% | Surgery: 38% Adjuvant: 75% | Surgery: 38% Adjuvant: 50% | Surgery: 1.8 years Adjuvant: not reached | p=0.037 (Adjuvant: lipiodol‐iodine‐131) |
| Nishiguchi 2005 | Surgery: 72% Adjuvant: 92% | Surgery: 60% Adjuvant: 67% | Surgery: 20% Adjuvant: 67% | Surgery: 20% Adjuvant: 67% | Surgery: 20% Adjuvant: 67% | Surgery: 2.6 years Adjuvant: not reached | p=0.055 (adjuvant: interferon‐alpha) |
| Ono 1997 | Surgery: 89% Adjuvant: 68% | Surgery: 70% Adjuvant: 45% | Surgery: 42% Adjuvant: 32% | Surgery: 28% Adjuvant: 32% | Surgery: 0% Adjuvant: 32% | Surgery: 2.5 years Adjuvant: 2.0 years | p = 0.07302 (Adjuvant: epirubicin+oral HCFU) |
| Takayama 2000 | Surgery: 65% Adjuvant: 85% | Surgery: 48% Adjuvant: 70% | Surgery: 33% Adjuvant: 48% | Surgery: 28% Adjuvant: 42% | Surgery: 22% Adjuvant: 40% | Surgery: 2.0 years Adjuvant: 3.0 years | p=0.008 (Adjuvant: immunotherapy) |
| Wu 1995 | Surgery: 70% Adjuvant: 65% | Surgery: 55% Adjuvant: 45% | Surgery: 50% Adjuvant: 40% | Surgery: 50% Adjuvant: 32% | Surgery: 45% Adjuvant: 20% | Surgery: 3.0 years Adjuvant: 1.5 years | p = 0.27 (Neoadjuvant: doxorubicin+TACE) |
| Yamamoto 1996 | Stage I: Surgery: 80% Stage I: Adjuvant: 90% Stage II: Surgery: 75% Stage II: Adjuvant: 70% | Stage I: Surgery: 60% Stage I: Adjuvant: 70% Stage II: Surgery: ‐ Stage II: Adjuvant: 30% | Stage I: Surgery: 30% Stage I: Adjuvant: 70% Stage II: Surgery: ‐ Stage II: Adjuvant: 15% | Stage I: Surgery: 30% Adjuvant: 50% Stage II: Not given | Stage I: Surgery: 19% Adjuvant: 50% Stage II: Not given | Stage I: Surgery: 2.0 years Stage I: Adjuvant: 5.5 years Stage II: Surgery: 1.5 years Stage II: Adjuvant: 1.5 years | STAGE I: p = 0.04 STAGE II: p = 1.00 (Adjuvant: oral 1‐hexylcarbamoyl 5‐fluorouracil (HCFU)) |
| Yamasaki 1996 | Surgery: 85% Adjuvant: 85% | Surgery: 60% Adjuvant: 65% | Surgery: 40% Adjuvant: 55% | Surgery: 35% Adjuvant: 48% | Surgery: 30% Adjuvant: 40% | Surgery: 2.0 years Adjuvant: 3.0 years | Not significant (Neoadjuvant: doxorubicin+TACE) |
Four studies, which had sufficient information (Izumi 1994; Ono 1997; Lai 1998; Yunxue 1999; Nishiguchi 2005), hazard ratios for death and recurrence were calculated by using approximate methods described by Parmar 1998 et al and presented in Analysis 1.3, Analysis 1.4, Analysis 1.5, Analysis 1.6. The hazard ratios calculated by approximate methods are plotted as inverse variance data, but pooling of the data was not possible because of the non‐comparability of the studies. The main reason was that the therapies used were different in all the studies. Due to the inconsistencies in the reported and approximate hazard ratios calculated, readers should interpret the forest plot data with caution.
1.3. Analysis.
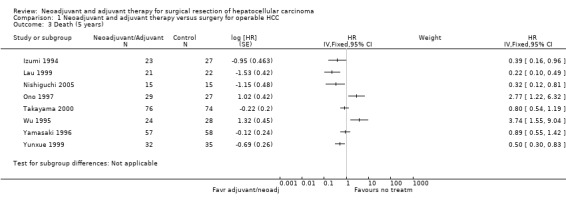
Comparison 1 Neoadjuvant and adjuvant therapy versus surgery for operable HCC, Outcome 3 Death (5 years).
1.4. Analysis.
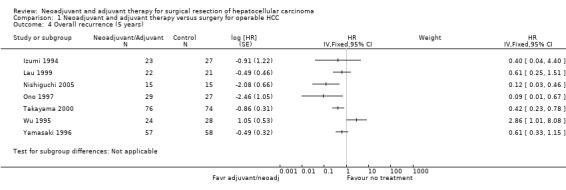
Comparison 1 Neoadjuvant and adjuvant therapy versus surgery for operable HCC, Outcome 4 Overall recurrence (5 years).
1.5. Analysis.
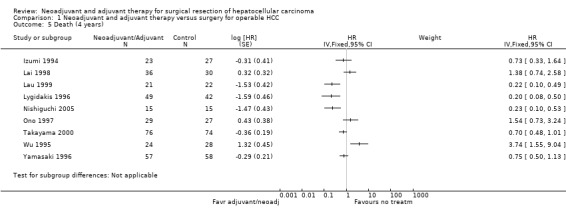
Comparison 1 Neoadjuvant and adjuvant therapy versus surgery for operable HCC, Outcome 5 Death (4 years).
1.6. Analysis.
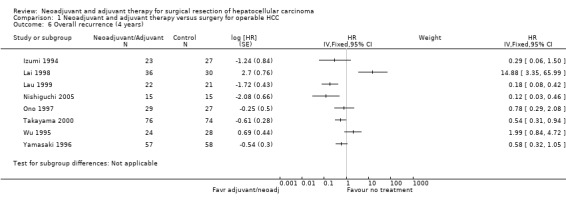
Comparison 1 Neoadjuvant and adjuvant therapy versus surgery for operable HCC, Outcome 6 Overall recurrence (4 years).
In four studies, risk of death at five years was significantly reduced in patients who had either neoadjuvant or adjuvant therapy (Izumi 1994; Lau 1999; Yunxue 1999; Nishiguchi 2005). On the other hand, two studies showed significant increase in the risk of death; Wu 1995, Analysis 1.3, (HR 3.74, 95% CI 1.55 to 9.04) and Ono 1997, Analysis 1.3, (HR 2.77, 95% CI 1.22 to 6.32). Overall, recurrence showed more consistent results across studies favouring neoadjuvant and adjuvant treatment groups with patients who underwent operation alone. A significant reduction in recurrence compared to the operation alone group at five years was, however, observed only in three studies (Ono 1997; Takayama 2000; Nishiguchi 2005) (Analysis 1.4). Only one study, Wu 1995 reported a significant increase in risk of recurrence compared to the surgical group (HR 2.86, 95% CI 1.01 to 8.08).
At four years, the risk of death decreased significantly in three studies (Lygidakis 1996 (HR 0.20, 95% CI 0.08 to 0.50); Nishiguchi 2005 (HR 0.23, 95% CI 0.10 to 0.53); Lau 1999 (HR 0.22, 95% CI 0.10 to 0.49)) in patients who underwent adjuvant/neoadjuvant therapy compared to patients who did not have any therapy. A similar reduction in recurrence was observed in three studies (Lau 1999; Takayama 2000; Nishiguchi 2005). Lai 1998 study, however, reported a significant increase in recurrence in patients who underwent therapy in addition to operation (HR 14.88, 95% CI 3.35 to 65.99), and the results were significant.
The reported results and the calculated hazard ratios for death and recurrence varied in some studies due to inconsistency in statistical significance (Izumi 1994; Ono 1997). In Izumi 1994, it was reported that cumulative survival rates were not different between groups, but the approximation method used by us showed statistical significance (graph 1.03 (HR 0.39 95% CI 0.16 to 0.96)) and in Ono 1997 study, the difference in survival rates and recurrence rates were reported to be insignificant between the two groups, but by the approximation method it is found to be significant Analysis 1.3 (HR 2.77, 95% CI 1.22 to 6.32); Analysis 1.4 (HR 0.0.09, 95% CI 0.01 to 0.67)) at five years.
Although Lau 1999 study reported HR of survival and disease‐free survival at interim analysis, we used only data reported at the end of the study. Adverse events
The highest toxicity rate was in a trial using oral 1‐hexylcarbamoyl 5‐fluorouracil (Yamamoto 1996) which resulted in 12 out of 38 (31.5%) participants stopping because of adverse events: neuropathy, liver dysfunction, exanthema and diarrhoea. Unspecified treatments were given to all patients. Another trial, which used postoperative hepatic arterial infusion chemotherapy with epirubicin and oral 1‐hexylcarbamoyl 5‐fluorouracil, reported three severe adverse events (atrial fibrillation, severe appetite loss) resulting in withdrawal from the trial (Ono 1997). Seven participants experienced side‐effects: nausea, vomiting, alopecia. Other studies also reported severe and minor adverse events (Characteristics of included studies).
Discussion
This review analyses 12 randomised clinical trials of neoadjuvant/adjuvant therapy versus no such therapy for the improvement of survival as a supplement to curative resection of non‐metastatic hepatocellular carcinoma. Various regimens ranging from the relatively simple (Yamamoto 1996) to the complex (Lygidakis 1995; Lygidakis 1996), covering neoadjuvant and adjuvant, systemic and locoregional (with and without embolisation), chemo‐ and immunotherapy were tried. None were comparable in terms of both the participants recruited and the regimen used. The most similar were the neoadjuvant TACE‐epirubicin trials of Wu 1995 and Yamasaki 1996. However, the patients in Wu 1995 had large tumours of at least 10 cm, whereas the Yamasaki 1996 participants had tumours 2 cm to 5 cm in diameter.
Neoadjuvant therapy, targeted at the tumour (locoregional) either via the portal vein or hepatic artery, may reduce the tumour mass and destroy microscopic tumour foci. However, the overall delay in performing surgery may eventually prove to be detrimental. The much poorer performance of the neoadjuvant group (three‐year survival: 40% versus 60%) in the Wu 1995 study is probably attributable to a combination of delayed surgery and also the large tumours these patients had. On the other hand, Yamasaki 1996 used a similar protocol, but on patients with smaller tumours. They had much better three‐year survival results in both groups compared to Wu 1995 (surgery: 85% versus 60%; neoadjuvant: 85% versus 40%), but no details on the extent of operative delay were given in Yamasaki 1996 study. Locoregional adjuvant therapy is given in the hope of destroying tumour cells released by surgery and any pre‐existing microscopic foci in the remaining liver, while systemic therapy is given to destroy any undetected distant metastases.
Most studies reported survival curves except Lygidakis 1995. Only two studies (Lygidakis 1996; Lau 1999) reported a statistically significant absolute survival advantage for the neoadjuvant/adjuvant group. No studies except for Lai 1998; Lau 1999; and Takayama 2000 specified a priori level of clinical significance. In the case of the Lai 1998, the result was not significant either clinically or statistically. Wu 1995 found a difference favouring the surgery‐only group (for reasons already described). Both Izumi 1994 and Yamamoto 1996 (stage I participants) reported significant disease‐free survival differences for adjuvant therapy, but no difference was seen for absolute survival. The recent Lau 1999 and Takayama 2000 studies reported a statistically and clinically significant disease‐free survival in patients treated with lipiodol‐iodine‐131 and immunotherapy, respectively at four‐years. In Takayama 2000 study, overall survival rate did not differ significantly between the groups, but the patients in Lau 1999 study had a significant increase in overall survival at four and five years, which is also statistically and clinically relevant.
Lygidakis 1995 reported only mean survival time and not survival curves. This is an inappropriate measure of treatment effect because of the skewed nature of the distribution of survival times. They also reported and tested the difference in proportion of disease‐free participants, but without defining the time point. This is also inappropriate because the difference in follow‐up times between the two groups is not accounted for in such a non‐survival analysis. In contrast, the Lygidakis 1996 study did report absolute survival curves, and the study found a statistically significant difference for absolute survival, favouring the neoadjuvant/adjuvant group. However, this apparent superiority is deceptive because both group have markedly poorer survival rates when compared to other studies. The surgery‐only group had a very low three‐year survival rate (15% versus a range of 55% to 85% in the other trials), and the 55% three‐year survival rate of the neoadjuvant/adjuvant therapy group was not superior to the survival rates of the surgery‐only group in any of the other trials. This conclusion stands, even after accounting for the fact that survival time was shortened by 40 days (treatment actually started 40 days earlier) for the adjuvant group because of the authors' decision to measure death from time of surgery. The quality of life of patients was reported as satisfactory, but no other details were given.
All studies reported survival rate at four or five years, except for Yunxue 1999, which reported survival rate at two years. The results show that the risk of death was reduced significantly in the TACE group (HR 0.50, 95% CI 0.30 to 0.83). The time to recurrence at two years was also longer in TACE group compared to the operations group (mean 7.09±3.27 months versus 4.29±3.29 months).
Except for Lai 1998; Lau 1999; and Takayama 2000 trials, no other study reported sample size computations. This is of concern since small samples mean that not only small, but clinically significant effects could be missed, but as well the protective effect of randomisation against bias from unmeasured confounders is much reduced. Although Lau 1999 study had anticipated to recruit 120 patients at the beginning of the study, an interim analysis was done after 30 patients, and in view of the significant improvement in disease‐free survival, the study was stopped prematurely after recruitment of 43 patients. If the study had adhered to the original sample size calculation, effectiveness of lipiodol‐iodine‐131 might have been much clearer and certain than now. In particular, eight of the studies reported a policy of treatment of recurrent disease by whatever means deemed appropriate. If this re‐treatment did in fact have a beneficial effect on survival and both groups were balanced for type and proportion of re‐treated patients, then this would have the effect of making the two groups more similar, and thus of making it harder to detect an effect of neoadjuvant/adjuvant therapy. However, if there was a significant chance imbalance between the groups of retreated patients (due to small sample size), then this policy could have biased the result in either direction.
Five of the studies did on‐treatment analysis rather than ITT analysis. Exclusions were for postoperative surgical death and refusal or inability to take neoadjuvant/adjuvant therapy. The Yamamoto 1996 study did both ITT and on‐treatment analysis. This study was unusual for the large number of exclusions: 16 out of 67 participants ‐ eight for non‐curative resection (histopathology) and eight for protocol violations (all from the adjuvant group). The resulting P‐values from the ITT analysis were ten times larger than that of the on‐treatment analysis.
Only Yamamoto 1996 attempted to further control for confounding by analytical means ‐ stratification of patients according to degree of liver dysfunction. Several other possible prognostic confounders, such as pathological tumour stage, tumour capsule, time between adjuvant therapy and surgery, fraction of participants who completed therapy, tumour size, multiplicity, and liver function, might also be usefully controlled by a multivariate analysis.
Authors' conclusions
Implications for practice.
Clinicians should be aware that there is no strong evidence that any of the reviewed neoadjuvant or adjuvant regimens prolong survival or disease‐free survival after curative resection for hepatocellular carcinoma. Only a few small trials have been done, and none have been independently replicated. There is limited evidence to show that adjuvant therapies, lipiodol‐iodine 131 and immunotherapy may have potential benefits on disease‐free survival for liver cancer patients with operable hepatocellular carcinoma. Oral HCFU may be associated with frequent adverse events.
Implications for research.
One could argue that the time is not ripe for phase III trials and that more early phase trials are needed to define new treatments with better potential efficacy. If limited to current therapies, it is unlikely that a treatment benefit as large as a 50% increase in the three‐year survival rate is realistically achievable. Therefore, larger, well‐designed randomised clinical trials and reported according to the CONSORT guidelines (www.consort‐statement.org) are needed in order to detect realistically achievable treatment benefits, which are still of clinical significance. The potential benefits of lipiodol and immunotherapies are encouraging, but more studies are needed to confirm the effectiveness. Quality of life would be an important additional outcome to estimate in view of the fact that presently no demonstrable superiority exists for neo‐adjuvant/adjuvant therapy, but that its administration may be accompanied by objectionable adverse events and would definitely incur additional risks and expense.
What's new
| Date | Event | Description |
|---|---|---|
| 22 September 2008 | New search has been performed | Evidence from new studies added. |
| 13 April 2008 | New citation required and conclusions have changed | Substantive amendment. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 22 September 2008 | Amended | Converted to new review format. |
Notes
Miny Samuel joined the team of authors in Sept 2004 and took the lead on updating the review.
Acknowledgements
The Cochrane Hepato‐Biliary Group for editorial and literature search support. Tai Bee Choo, a former author, for her helpful suggestions on the protocol and first draft of the review. ES‐YC acknowledge National Medical Research Council of Singapore and SM acknowledge Research Triangle Institute‐Health Solutions, UK for their support.
We are grateful to the editors, C Gluud and Dimitrinka Nikolova for their unprecedented support throughout the review process.
Appendices
Appendix 1. Search Strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | September 2005. | *adjuvant AND (((hepatocellular OR 'liver cell') AND carcinoma) OR HCC) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 3, 2005. | #1 MeSH descriptor Chemotherapy, Adjuvant explode all trees in MeSH products #2 MeSH descriptor Neoadjuvant Therapy explode all trees in MeSH products #3 neoadjuvant* or adjuvant* in All Fields in all products #4 (#1 OR #2 OR #3) #5 MeSH descriptor Carcinoma, Hepatocellular explode all trees in MeSH products #6 ((hepatocellular or liver cell) and carcinoma) or HCC in All Fields in all products #7 (#5 OR #6) #8 (#4 AND #7) |
| MEDLINE (WinSPIRS 5.0) | 1950 to September 2005. | #1 explode "Chemotherapy‐Adjuvant"/ all subheadings #2 explode "Neoadjuvant‐Therapy"/ all subheadings #3 neoadjuvant* or adjuvant* #4 #1 or #2 or #3 #5 explode "Carcinoma‐Hepatocellular"/ all subheadings #6 ((hepatocellular or liver cell) and carcinoma) or HCC #7 #5 or #6 #8 #4 and #7 #9 random* or blind* or placebo* or meta‐analysis #10 #8 and #9 |
| EMBASE | 1980 to September 2005. | #1 explode "adjuvant‐therapy"/ all subheadings #2 neoadjuvant* or adjuvant* #3 #1 or #2 #4 explode "liver‐cell‐carcinoma"/ all subheadings #5 ((hepatocellular or liver cell) and carcinoma) or HCC #6 #4 or #5 #7 #3 and #6 #8 random* or blind* or placebo* or meta‐analysis #9 #7 and #8 |
| Science Citation Index EXPANDED (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | 1945 to September 2005. | #1 TS=(neoadjuvant* OR adjuvant) #2 TS=(((hepatocellular OR 'liver cell') AND carcinoma) OR HCC) #3 #2 AND #1 #4 TS=(random* or blind* or placebo* or meta‐analysis) #5 #4 AND #3 |
| Chinese Biomedical Database | 1978‐2003 | #1 Cancer, Liver cell #2 Liver #3 Hepatocellular carcinoma #4 Surgical operation #5 Resection #6 Surgery #7 Surgical resection #8 #1 or #2 or #3 #9 #4 or #5 or #6 #10 #8 and #9 #11 Randomized controlled trial #12 Random allocation #13 #11 or #12 #14 #10 and #13 This strategy was translated from Chinese to English. |
| US National Cancer Institute's Physician's Data Query Trials Database | September 2005 | Type of Cancer= Liver cancer Stage/Subtype of Cancer = Localised resectable cancer Type of trial = treatment |
Data and analyses
Comparison 1. Neoadjuvant and adjuvant therapy versus surgery for operable HCC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival and median survival time | Other data | No numeric data | ||
| 2 Disease‐free survival | Other data | No numeric data | ||
| 3 Death (5 years) | 8 | HR (Fixed, 95% CI) | Subtotals only | |
| 4 Overall recurrence (5 years) | 7 | HR (Fixed, 95% CI) | Subtotals only | |
| 5 Death (4 years) | 9 | HR (Fixed, 95% CI) | Subtotals only | |
| 6 Overall recurrence (4 years) | 8 | HR (Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Izumi 1994.
| Methods | Randomised. Generation of the allocation sequence: not clear. Allocation concealment: not clear. Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: no. Two patients originally randomised to the adjuvant group seem to have been reallocated to the surgery group. Sample size calculations: no. | |
| Participants | 50 patients with invasive Hepatocellular Carcinoma with vascular invasion and/or intrahepatic metastasis diagnosed by computed tomography (CT), and ultrasonography (US). | |
| Interventions | (1) Surgery: 25 patients randomised, but 27 treated by lobectomy, sub‐ and segmentectomy, and major wedge resection. (2) Adjuvant: 25 patients randomised, but 23 treated, hepatic arterial bolus infusion 21 to 84 days postoperatively (postop) (mean 38.4) either by (a) transcatheter arterial chemoembolisation (TACE) for 7 patients with good liver function (hepaplastin > 60%) lipiodol 3 ml/m2, doxorubicin 20 mg/m2, mitomycin C 10 mg/m2 followed by gelatin sponge cubes (Gelfoam, Upjohn) or (b) chemotherapy without embolisation for the remaining 16 patients, lipiodol 2 ml/m2, doxorubicin 20 mg/m2, mitomycin C 10 mg/m2. |
|
| Outcomes | Follow‐up bimonthly for first year and hence every three‐monthly by alpha‐fetoprotein (AFP), US, CT, angiography. 1. Survival: survival curves. 2. Disease‐free survival: survival curves. | |
| Notes | Groups comparable at baseline. Radicality of resection judged by intraoperative US, all macroscopic tumour removed, no histopathology discussed. Transient fever or nausea after adjuvant therapy. Repeat TACE, systemic chemotherapy and resection were performed on patients with recurrence. Hazard ratio (HR) not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lai 1998.
| Methods | Randomised. Generation of the allocation sequence: not clear. Allocation concealment: sealed envelopes. Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: yes. Sample size calculations: yes. | |
| Participants | 76 patients (53 men and 13 women, mean age 53.3 years (range 28 to 78 years)), without residual disease by imaging 1 month after curative resection. | |
| Interventions | 1. Surgery: 36 patients by referenced technique (Lai 1995) . 2. Adjuvant: 30 patients, six‐eight weeks postoperative systemic chemotherapy of IV epirubicin 40 mg/m twice at six‐week intervals (max eight courses). Additionally, three bimonthly courses of slow hepatic arterial infusion chemotherapy using iodised oil 10 ml and cisplatin 10 mg either via a subcutaneous port or femoral artery catheterization. |
|
| Outcomes | 1. Survival: survival curves from day of operation. 2. Diseases‐free survival: survival curves from day of operation. | |
| Notes | Groups comparable at baseline. Radicality of resection judged by US, angiography and histopathology, all randomised were negative for disease one month after surgery. Adverse events in six patients in adjuvant group, three had local complications (extravasation cellulitis (2), severe epigastric pain and gastric necrosis) after TACE with subcutaneous port and three others with atrial fibrillation, leukopenia, and alopecia. Frequency and manner of follow‐up not described. TACE, systemic chemotherapy and resection were performed on 40 patients with recurrence. Sample size calculation based on recurrence rate at 3rd postoperative year of 70% (surgery) versus 35% (adjuvant) with 5% type I error and 20% type II error (2‐tailed). No HRs reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lau 1999.
| Methods | Randomised. Generation of the allocation sequence: computer generated numbers. Allocation concealment: not clear. Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: yes. Sample size calculations: yes. | |
| Participants | 43 patients with histologically proven hepatocellular carcinoma, aged 17 to 75 years who had undergone curative resection and had recovered within 6 weeks of operation with a Karnofsky performance score of 70 or higher. | |
| Interventions | 1. Surgery: 22 patients were randomised. 2. Adjuvant: 21 patients were randomised. Patients received I‐lipiodol within 2 weeks of randomisation. On the day of treatment, the patient underwent selective cannulation of the hepatic artery by Selinger technique, which was performed by the same interventional radiologist who administered I‐lipiodol. 1850 MBq of I‐lipiodol was then given by the oncologist over 5 min into the hepatic artery, under fluoroscopic control. | |
| Outcomes | 1. Disease free and overall survival. | |
| Notes | Had histologically confirmed hepatocellular carcinoma, stage of the disease was classified by Okuda staging (gradesI‐III). Curative resection was defined as a clear resection margin (>1cm) on pathological examination. I‐lipiodol had no significant toxic effects. An interim analysis was done in this study when 30 patients were entered and followed‐up for 2 years. A significant improvement in disease‐free survival was reported and the trialists stopped the trial prematurely after the recruitment of 43 patients. Adverse events was reported to be minimal but no details were given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lygidakis 1995.
| Methods | Randomised. Generation of the allocation sequence: not clear. Allocation concealment: not clear. Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: no, four patients (two per group) who died during first 30 days after surgery excluded. Sample size calculations: no. | |
| Participants | 40 patients with hepatocellular carcinoma, tumour mass <50% of liver surface, Okuda stage I or II, no extrahepatic disease. | |
| Interventions | 1. Surgery: 20 patients, extended right, right and left hemihepatectomies. 2. Adjuvant/Neoadjuvant: 20 patients, 50 days preoperative, hepatic arterial infusion chemotherapy with lipiodol 10 ml, 58 % urographin 2 ml, mitomycin C 30 mg, farmorubicin 70 mg, leukovorin 100 mg, 5‐fluorouracil 750 mg, gamma‐interferon 100mcg. Chemotherapy repeated 20 days later followed by 5 daily immunotherapy courses by arterial infusion of lipiodol 3 ml, 58 % urographin 0.5 ml, proleukin 1 ml, gamma‐interferon 100 mcg directed to liver space occupied by tumour. Surgical resection done 30 days later (extended right, right and left hemihepatectomies). four weeks later, 10 daily immunotherapy courses given followed ten days later by the chemotherapy regimen as a bolus infusion. This postoperative immunotherapy‐chemotherapy combination was repeated every three months for the first year for a total of four courses. If lipiodol retention > 48 hrs after last course, treatment continued till no longer retained. In second year postoperative course given every six months and once for the third year. |
|
| Outcomes | Follow‐up four‐ monthly by blood tests, AFP, CT for surgery group and on 10th day after each postoperative course for adjuvant group. 1. Survival: reported only mean survival times and survival fractions, but not standardized to a defined period. 2. Intra‐hepatic recurrence fraction, not standardized to a defined period. | |
| Notes | Groups comparable at baseline. Radicality of resection judged by histopathology (>= 1 cm tumour‐free margin), no data given. Moderate fever in all on adjuvant group. No survival curves reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lygidakis 1996.
| Methods | Randomised. Generation of the allocation sequence: not clear. Allocation concealment: not clear. Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: no, six post‐op (30 days) deaths excluded. Sample size calculations: no. | |
| Participants | 91 patients with hepatocellular carcinoma, tumour mass < 60% of liver surface, Okuda stage I or II, no extrahepatic disease. | |
| Interventions | 1. Surgery: 42 patients, hepatectomies and segmentectomies. 2. Neoadjuvant/Adjuvant: 49 participants. (i) 40 days preoperative (preoperative), chemoembolisation via portal vein with lipiodol 15 ml, urographin 5 ml, mitomycin C 0.2 mg/kg body wt (bw), carboplatin 0.5 mg/kg bw, Novantrone 0.8 mg/kg bw, gelfoam. (ii) 30 days preoperative, hepatic arterial chemotherapy with lipiodol 15 ml & urographin 5 ml emulsion of mitomycin C 0.2 mg/kg bw, carboplatin 1.5 mg/kg bw and Novantrone 0.2 mg/kg bw. (iii) 20 days preoperative, repeat of (ii). (iv) Surgery on day 40. (v) 1 month postoperative, hepatic arterial bolus chemotherapy with (ii), followed 15 days later by a 10‐day course of lipiodol 1.5 ml & urographin 0.5 ml emulsion of interleukin‐2 (Proleukin, Chiron) 1 ml, and gamma‐interferon 100 mcg 0.5 ml. (vi) Repeat of (v) every three months for first year, every four months for second year, every six months for third and fourth year. |
|
| Outcomes | Follow‐up after each course with complete screening test. 1. Survival: survival curves. | |
| Notes | Groups comparable at baseline. Radicality of resection criteria not given. Quality of life measured, no details given. Adverse events were minimal and effectively treated with paracetamol or indomethacin. HR not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nishiguchi 2005.
| Methods | Randomised. Generation of the allocation sequence: random number tables. Allocation concealment: not clear. Blinding: yes. Investigators and radiologists responsible for recording tumour recurrence. Follow‐up: adequate Intention‐to‐treat analysis: yes. Sample size calculations: no. | |
| Participants | 30 patients (all men) with curative resection of hepatocellular carcinoma and mean age 61.9 ± 5.8 years in interferon group and 60.0 ± 4.8 in control group. | |
| Interventions | 1. Surgery: 15 patients were randomised and did not receive any postoperative treatment up to the detection of recurrence. 2. Adjuvant: 15 patients were randomised. Patients received 6 MIU interferon intramuscularly every day for 2 weeks, then 3 times weekly for 14 weeks and twice weekly for 88 weeks. Treatment was started 5 to 15 weeks after resection, except for one patient it was delayed for 7 months. |
|
| Outcomes | Survival: time of resection until death. Recurrence: time of resection until recurrence of the tumour. | |
| Notes | This study was an update of the trial results published by Kubo in 2001 and Kubo 2002. Groups were comparable at baseline. Curative resection was defined as complete resection of all macroscopic disease with no requirement for 1 cm tumour‐free margin. Interferon administration was not completed in 3 patients because of adverse events like depression and general fatigue. No HR reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ono 1997.
| Methods | Randomised. Generation of the allocation sequence: not clear. Allocation concealment: sealed envelopes. Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: yes. Sample size calculations: no. | |
| Participants | 57 patients without residual disease 1 month after curative resection, Child's grade A or B. | |
| Interventions | 1. Surgery: 27 patients, major hepatectomy (25%) and minor hepatectomy (75%). 2. Adjuvant: 29 patients, one month postoperative hepatic arterial infusion chemotherapy with epirubicin 40 mg/m2, then IV epirubicin 40 mg/m2 every three months for two years. Additionally, oral 1‐hexylcarbamoyl 5‐fluorouracil (HCFU) 300mg daily at one month postoperative for 24 months. | |
| Outcomes | Follow‐up every two weeks for first six months postoperative, thereafter at monthly intervals by US, CT, and angiography. 1. Survival: survival curves. 2. Disease‐free survival: survival curves. | |
| Notes | Groups comparable at baseline. Radicality of resection judged by intraoperative and postoperative imaging, histopathology (margin >= 5mm). Repeat TACE or resection on patients with recurrence. Severe adverse events caused three withdrawals from adjuvant group (atrial fibrillation, severe appetite loss (2)), seven patients with minor side‐effects (nausea, vomiting, alopecia). HR not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Takayama 2000.
| Methods | Randomised. Generation of the allocation sequence: used permuted blocks. Allocation concealment: not clear. Blinding: yes. Radiologists were blinded. Follow‐up: adequate. Intention‐to‐treat analysis: yes. Sample size calculations: yes. | |
| Participants | 155 patients aged 18 to 80 years, with histologically confirmed hepatocellular carcinoma and who underwent curative resection. | |
| Interventions | 1. Surgery: 76 patients were randomised but only 74 met the inclusion criteria. 2. Adjuvant: 79 were randomised but only 76 met the inclusion criteria. Patients received autologous lymphocytes (immunotherapy) intravenously at weeks 2, 3, 4, 12 and 24 weeks after surgery. This schedule was designed to transfer sufficient cells. |
|
| Outcomes | 1. Time to first recurrence. 2. Recurrence free survival. 3. Disease‐free survival. 4. Overall survival. | |
| Notes | Groups were similar at baseline. The treatment was given not later than 1 week after surgery. 62 adverse events developed in 45 patients, all of which were grade 1 or 2 and self limiting. The most common was fever (47%) followed by headache (4%), nausea (4%), dizziness (1%), itching (1%) and tachycardia (1%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wu 1995.
| Methods | Randomised. Generation of the allocation sequence: not clear Allocation concealment: not clear Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculations: no. Not ITT, three postoperative deaths (within 30 days) excluded. | |
| Participants | 52 patients with large (diameter >= 10 cm) tumour, resectable (not diffuse bilobar, advanced cirrhosis, distant metastasis or main portal thrombi) and functional reserve ‐ Child's grade A or B. | |
| Interventions | 1. Surgery: 28 patients (classical or extended major hepatectomies for cirrhotics and Couinaud segment resection (Couinaud 1954) for non‐cirrhotics). 2. Neoadjuvant TACE: 24 patients, preoperative hepatic arterial infusion of doxorubicin 20 to 30 mg, lipiodol 20 to 30 ml and Spongostan film particles. Second course four to six weeks later, the number of courses per subject (max of six) decided on an individual basis. |
|
| Outcomes | Follow‐up every three‐six months by AFP, CT, chest X‐ray, US. 1. Survival: survival curves for survival from time of tumour detection and from time of operation. 2. Disease‐free survival: survival curves. | |
| Notes | Groups comparable at baseline. Radicality of resection judged by histopathology, ˜50% patients <1cm disease‐free margin. Repeat TACE or resection was performed on patients with hepatic recurrence and systemic chemotherapy with 5‐fluorouracil, cisplatin, and doxorubicin on those with extrahepatic recurrence. Minor adverse events in 22 patients of adjuvant group ‐ fever, abdominal pain, upper gastrointestinal bleeding, transient ascites, acute cholecystitis. HR not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yamamoto 1996.
| Methods | Randomised. Generation of the allocation sequence: not clear. Allocation concealment: post‐op central office telephone randomisation. Blinding: no. Follow‐up: treatment given until adverse events appeared. Intention‐to‐treat analysis: yes. Sample size calculations: no. | |
| Participants | 67 patients with UICC (see Notes) stage II hepatocellular carcinoma, stratified into three clinical stages of liver dysfunction. | |
| Interventions | 1. Surgery: 38 patients, curative resection as defined by the Liver Cancer Study Group of Japan (1992). 2. Adjuvant: 38 patients, two to four weeks postoperative twice daily oral HCFU 200 mg for as long as possible till severe adverse events appeared. |
|
| Outcomes | Follow‐up at least bimonthly with US and six‐monthly by CT, further investigations if
recurrence suspected.
1. Survival: survival curves stratified for stage. 2. Disease‐free survival: survival curves stratified for stage. |
|
| Notes | Groups comparable at baseline. Radicality of resection judged by histopathology (Union Internationale Contre le Cancer (UICC) stage II curative criteria), none analysed had <1cm disease‐free margin. 16 patients were treated as withdrawn in the effectiveness (not ITT) analysis (eight for non‐curative resection and eight from adjuvant group for protocol violation). HCFU stopped in 12 patients because of adverse events ‐ neuropathy, liver dysfunction, exanthema, diarrhoea. Unspecified treatments were performed on all patients with recurrence. HR not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yamasaki 1996.
| Methods | Randomised. Generation of the allocation sequence: not clear. Allocation concealment: sealed envelopes. Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: 18 patients (seven surgery, 11 adjuvant) excluded. Sample size calculations: no. | |
| Participants | 115 male patients, <= 65 years with resectable hepatocellular carcinoma tumours from 2 to 5 cm in diameter diagnosed by imaging modalities, without severe cirrhosis, Indocyanin Green 15 min retention time (ICG R15) < 40%, serum bilirubin <=1.5 mg/ml, Okuda stage I. | |
| Interventions | 1. Surgery: 58 patients randomised, but 47 treated by limited resection, sub‐ and segmentectomy and lobectomy. 2. Adjuvant TACE: 57 patients randomised, but 50 treated, preoperative hepatic arterial infusion of doxorubicin 20 mg in urografin 2.5 ml and lipiodol 5 ml followed by gelatin sponge cubes 1 to 3 mm in urografin. |
|
| Outcomes | 1. Survival: survival curves and five‐year survival rate. 2. Disease‐free survival: survival curves and 5‐yr disease‐free survival rate. | |
| Notes | Groups comparable at baseline. Radicality of resection judged by histopathology, no data given. Frequency or manner of follow‐up not reported. No discussion of adverse events. HR not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yunxue 1999.
| Methods | Randomised. Generation of the allocation sequence: not clear. Allocation concealment: no. Blinding: no. Follow‐up: adequate. Intention‐to‐treat analysis: yes. Sample size calculations: no. | |
| Participants | TACE group: 29 male and 3 female patients with a mean age of 44.2 years. Alpha‐Fetoprotein was > 200ng/ml in 22 patients and < 200 ng/ml in 10 patients. Tumour size was >10 cm in 14 patients, 5 to 10 cm in 14 patients and < 5 cm in 4 patients. Surgery Alone: 31 male and 4 female patients with a mean age of 46.3 years. Alpha‐Fetoprotein was > 200ng/ml in 25 patients and < 200 ng/ml in 10 patients. Tumour size was >10 cm in 9 patients, 5 to 10 cm in 22 patients and < 5 cm in 4 patients. |
|
| Interventions | 1. Surgery: 35 patients were operated. 2. Adjuvant: 32 patients were given preoperative transcatheter hepatic arterial chemoembolisation with MMC1‐10mg, 5Fu50 to 2000mg, EPIR 6 to 10mg, Carboplatin 300 to 500 mg; either in combination of 2 or 3 treatments. |
|
| Outcomes | Recurrence at original site; Recurrence at other sites; Time to recurrence; Two‐year survival rate. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
AFP = alpha‐fetoprotein. CT = computerised tomography. HCFU = 1‐hexylcarbamoyl 5‐fluorouracil. HR ‐ hazard ratio. ICG‐R15 ‐ Indocyanin Green 15 min retention rate. ITT ‐ intention‐to‐treat. TACE ‐ transcatheter arterial chemoembolisation. US ‐ ultrasonography.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bao 2001 | A randomised trial of 40 patients which compared the effectiveness of hydroxycamptothecin and mateling versus Cis‐Platinum diamminedichloride (PDD) and 5‐Fluorouracil postoperatively (after curative resection of hepatocellular carcinoma) as arterial infusion. |
| Ikeda 2000 | A small trial of 20 patients with surgical resection of HCC or percutaneous ethanol injection therapy were randomised to a control or adjuvant therapy group. Ethanol injection therapy is not a generally accepted method and therefore the patients does not meet the inclusion criteria for this review. We will try to contact the authors to obtain more information about the outcomes of patients who underwent resection. |
| Li 2002 | A randomised trial of 45 patients which compared the effectiveness of route of administration of chemotherapy. The comparison was postoperative intraarterial and intraportal chemotherapy versus intraarterial chemotherapy alone. |
| Muto 1996 | Control group comprised an unknown proportion of surgical resection and percutaneous ethanol injection cases. Concerned with prevention of onset of second primary tumours and not absolute survival from secondary tumours of the resected primary. |
Differences between protocol and review
Differences between previously published and present review versions
In Chan ES‐Y, Chow PK‐H, Tai B‐C, Machin D, Soo K‐C. Neoadjuvant and adjuvant therapy for operable hepatocellular carcinoma. Cochrane Database of Systematic Reviews 1999, Issue 3. Art. No.: CD001199. DOI: 10.1002/14651858.CD001199 we concluded that there was no clear evidence for efficacy of any of the adjuvant and neo‐adjuvant protocols reviewed, but there was some evidence to suggest that adjuvant therapy might be beneficial for prolonged disease‐ free survival. In order to detect a realistic treatment advantage, larger trials would have to be conducted. In this present updated version, unlike the statement in our previous review version, benefit for disease‐free survival is not clear with neoadjuvant or adjuvant therapy. We could include six new reports on studies published until March 2006. Of these, one trial was published in triplicate. Two studies were interim reports of Nishiguchi 2005 trial. The conclusions drawn from all the trials show a change in the evidence from the earlier report. There is limited evidence to suggest that neoadjuvant or adjuvant therapy may be useful for disease‐free survival.
Contributions of authors
EC: review team co‐ordinator, searched and appraised literature, wrote first drafts of the protocol and review, and updated the review. PC: searched and appraised the literature, helped write the protocol and review, and updated the review. DM: helped write the protocol and review. SKC: helped write the protocol and review. SM: updated the review.
Sources of support
Internal sources
National Cancer Center, Singapore.
NMRC Clinical Trials & Epidemiology Research Unit, Singapore.
Dept of General Surgery, Singapore General Hospital, Singapore.
Research Triangle Institute‐Health Solutions, UK.
External sources
No sources of support supplied
Declarations of interest
We certify that we currently have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of this review (e.g., employment, consultancy, stock ownership, honoraria, expert testimony).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Izumi 1994 {published data only}
- Izumi R, Shimizu S, Iyobe T, Ii T, Yagi M, Matsui O, et al. Postoperative adjuvant hepatic arterial infusion of lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology 1994;20:295‐301. [MEDLINE: ] [PubMed] [Google Scholar]
Lai 1998 {published data only}
- Lai ECS, Lo CM, Fan ST, Liu CL, Wong J. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma. Archives of Surgery 1998;133:183‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lau 1999 {published data only}
- Lau WY, Leung TWT, Ho SKW, Chan M, Machin D, Lau J, et al. Adjuvant intra‐arterial lipidol‐iodine‐131 for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet 1999;353:797‐801. [DOI] [PubMed] [Google Scholar]
Lygidakis 1995 {published data only}
- Lygidakis NJ, Pothoulakis J, Konstantinidou AE, Spanos H. Hepatocellular carcinoma: surgical resection versus surgical resection combined with pre‐ and post‐operative locoregional immunotherapy‐cemotherapy. A prospective randomised study. Anticancer Research 1995;15(2):543‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Lygidakis 1996 {published data only}
- Lygidakis NJ, Tsiliakos S. Multidisciplinary management of hepatocellular carcinoma. Hepato‐Gastroenterology 1996;43(12):1611‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Nishiguchi 2005 {published data only}
- Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Kinoshita H. Randomised clinical trial of long‐term outcome after resection of hepatitis C virus‐related hepatocellular carcinoma by postoperative interferon therapy. The British Journal of Surgery 2002;89:418‐22. [DOI] [PubMed] [Google Scholar]
- Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Yamazaki O, et al. Effects of long‐term postoperative interferon‐alpha therapy on intrahepatic recurrence after resection of hepatitis C virus‐related hepatocellular carcinoma. Annals of Internal Medicine 2001;134:963‐7. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Tamori A, Kubo S. Effect of long‐term postoperative interferon therapy on intrahepatic recurrence and survival rate after resection of hepatitis C virus‐related hepatocellular carcinoma. Intervirology 2005;48:71‐5. [DOI] [PubMed] [Google Scholar]
Ono 1997 {published data only}
- Ono T, Nagasue N, Kohno H, Hayashi T, Uchida M, Yukaya H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Seminars in Oncology 1997;24(2 Suppl 6):S6‐18‐S6‐25. [MEDLINE: ] [PubMed] [Google Scholar]
Takayama 2000 {published data only}
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000;356:802‐7. [DOI] [PubMed] [Google Scholar]
Wu 1995 {published data only}
- Wu CC, Ho YZ, Lin Ho W, Wu TC, Liu TJ, P'eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. The British Journal of Surgery 1995;82(1):122‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamamoto 1996 {published data only}
- Yamamoto M, Arii S, Sugahara K, Tobe T. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. The British Journal of Surgery 1996;83(3):336‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamasaki 1996 {published data only}
- Yamasaki S, Hasegawa H, Kinoshita H, Furukawa M, Imaoka S, Takahashi K, et al. A prospective randomized trial of the preventive effect of pre‐operative transcather arterial embolization against recurrence of hepatocellular carcinoma. Japanese Journal of Cancer Research 1996;87(2):206‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yunxue 1999 {published data only}
- Yunxue H, Kangsun Z, Zaibo J. The significance of preoperative TACE in preventing postoperative recurrence of primary hepatic carcinoma. Journal of JiLin Medical College 1999;19(2):22‐3. [Google Scholar]
References to studies excluded from this review
Bao 2001 {published data only}
- Bao LQ, Peng YM, Yuan HY. Clinic study on the role of hydroxycamptothecin and mateling postoperative arterial infusion in the prevention of recurrence after curative resection for human hepatocellular carcinoma. Cancer Research on Prevention and Treatment 2001;28(4):303‐5. [Google Scholar]
Ikeda 2000 {published data only}
- Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor ‐ a prospective randomized study of hepatitis C virus‐related liver cancer. Hepatology 2000;32:282‐32. [DOI] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li Q, Hao X, Zhang Z, Song T, Hao J, Ma W, et al. Intraportal chemotherapy for hepatocellular carcinoma patients with tumor thrombosis of the portal vein. Chinese Journal of General Surgery 2002;17(4):209‐10. [Google Scholar]
Muto 1996 {published data only}
- Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki K, et al. Prevention of second primary tumours by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. The New England Journal of Medicine 1996;334(24):1561‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Child 1964
- Child CG III, Tourcotte JG. Surgery and portal hypertension. In: Child CG editor(s). The Liver and Portal Hypertension. 3rd Edition. Philadelphia, Pennsylvania: WB Saunders, 1964:1‐85. [Google Scholar]
El‐Serag 2004
- El‐Serag HB . Hepatocellular carcinoma: recent trends in the United States. . Gastroenterology 2004;127(5 Suppl 1):S27‐34. [DOI] [PubMed] [Google Scholar]
Finkelstein 2003
- Finkelstein SD, Marsh W, Demetris AJ, Swalsky PA, Sasatomi E, Bonham A, Subotin M, Dvorchik I. Microdissection‐based allelotyping discriminates de novo tumor from intrahepatic spread in hepatocellular carcinoma. Hepatology 2003;37(4):871‐9. [DOI] [PubMed] [Google Scholar]
Forner 2006
- Forner A, Hessheimer AJ, Isabel Real M, Bruix J. Treatment of hepatocellular carcinoma. Crit Rev Oncol Hematol 2006;60(2):89‐98. [DOI] [PubMed] [Google Scholar]
Gluud 2008
- Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als‐Nielsen B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2008, Issue 3. Art. No.: LIVER.
Gomaa 2008
- Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor‐Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol 2008;21(14 (27)):4300‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Colloboration, 2008. Available from www.cochrane‐handbook.org.
Huguet 1994
- Huguet C, Stipa F, Gavelli A. Primary hepatocellular cancer: Western experience. In: Blumgart L editor(s). Surgery of the Liver and Biliary Tract. London: Churchill Livingstone, 1994:1365‐9. [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lai 1981
- Lai CL, Lam KC, Wong KP, Wu PC, Todd D. Clinical features of hepatocellular carcinoma: review of 211 patients in Hong Kong. Cancer 1981;47(11):2746‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lai 1994
- Lai E, Wong J. Hepatocellular carcinoma: the Asian experience. In: Blumgart L editor(s). Surgery of the Liver and Biliary Tract. London: Churchill Livingstone, 1994:1349‐63. [Google Scholar]
LCSG Japan 1992
- Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer (In Japanese). 3rd edition. 3rd Edition. Tokyo: Kanehara, 1992:20‐6. [Google Scholar]
Lee 1982
- Lee NW, Wong J, Ong GB. The surgical management of primary carcinoma of the liver. World Journal of Surgery 1982;6(1):66‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lovet 2008
- Llovet JM, Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ, Panel of Experts in HCC‐Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698‐711. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Nagourney 1987
- Nagourney DM, Adson M. Major hepatic resections for hepatoma in the West. In: Wanebo HJ editor(s). Hepatic and Biliary Cancer. New York: Marcel Dekker, 1987:167‐85. [Google Scholar]
NCCN 2008
- National Comprehensive Cancer Network. Hepatobiliary Cancers. www.nccn.org 2008; Vol. V.2.
Okuda 1984
- Okuda K, Obata H, Nakajima Y, Ohtsuki T, Okazaki N, Ohnishi K. Prognosis of primary HCC. Hepatology 1984;4:3S‐6S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okuda 1985
- Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56(4):918‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okuda 1987
- Okuda K, Ryu M, Tobe T. Surgical management of hepatoma, the Japanese experience. In: Wanebo HJ editor(s). Hepatic and Biliary Cancer. New York: Marcel Dekker, 1987:219‐38. [Google Scholar]
Okuda 1997
- Okuda K. Epidemiology. In: Livaraghi Makuuchi M, Buscarini L editor(s). Diagnosis and Treatment of Hepatocellular Carcinoma. London: Greenwich Medical Media, 1997:3‐15. [Google Scholar]
Oon 1980
- Oon CJ, Okuda K, Pang R. Primary hepatocellular carcinoma in the Asian Pacific region. Annals of the Academy of Medicine, Singapore 1980;9(2):190‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Academy of Medicine 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schwartz 2002
- Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol 2002;3(10):593‐603. [DOI] [PubMed] [Google Scholar]
Wang 1991
- Wang TL, Yap IL, Tan YO. Hepatocellular carcinoma‐a case series of 104 patients. Annals of the Academy of Medicine, Singapore 1991;20(2):215‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Chan 1999
- Chan ES‐Y, Chow PK‐H, Tai B‐C, Machin D, Soo K‐C. Neoadjuvant and adjuvant therapy for operable hepatocellular carcinoma. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001199] [DOI] [PubMed] [Google Scholar]


