Abstract
Viral infections can influence both the development and the severity of asthma. In early life, viral infections can either increase or, remarkably, decrease the risk of subsequent asthma. In children and adults with existing asthma, viral respiratory infections frequently cause acute airway obstruction and wheezing. This article discusses the influence of viral infections on mechanisms of virus-induced airway inflammation in relationship to the development, persistence and severity of asthma.
Main
The relationship between viral infections, wheezing illnesses and asthma is a close one in all age groups. It has long been appreciated that certain viral infections in infancy, for example those caused by respiratory syncytial virus (RSV), cause inflammation and obstruction of the small airways (bronchioles), which lead to coughing and wheezing. It has also been proposed that infants who contract RSV bronchiolitis are at an increased risk of developing asthma later in life. By contrast, recent epidemiological studies indicate that not all viral infections increase the risk of asthma and, in fact, some may actually reduce the risk of developing allergic diseases and asthma.
In older children and adults with established asthma, common colds, which are relatively mild illnesses for most people, can cause severe pulmonary problems. In fact, up to 80% of exacerbations of asthma in children and about half of such episodes in adults are caused by viral infections, most of which are attributable to rhinoviruses1,2.
This article will consider the relationship between viral infections, virus-induced inflammation, and the development and activity of asthma. The pathogenesis of asthma will be reviewed, and current concepts pertaining to how viral infections may either increase or decrease the subsequent risk of asthma will be discussed. Finally, the potential uses of antiviral and anti-inflammatory medicines for the prevention and treatment of asthma will be considered.
Asthma pathogenesis
Asthma is a disease characterized by chronic airway inflammation and a heightened responsiveness of the airways to irritants and, in most cases, allergens3 (FIG. 1). The chronic inflammation in asthma is a pleiotropic picture that usually includes an increase in the number of activated T cells, which predominantly secrete T helper (TH) 2-like cytokines such as interleukin 4 (IL-4), IL-5 and IL-13. This pattern of cytokine secretion, together with the secretion of epithelial-derived chemokines such as eotaxin (CCL11) and RANTES (regulated on activation, normal T cells expressed and secreted; CCL5), promotes the recruitment and activation of eosinophils and mast cells, which contribute to chronic airway inflammation and hyperresponsiveness of the airway to a variety of nonspecific stimuli. These factors, together with chronic structural changes to the airway and increased production of mucus by goblet cells, increase the risk of intermittent episodes of acute obstruction of airflow through the small airways of the lung in response to irritants (such as tobacco, smoke and air pollution), allergens and acute viral infections.
Figure 1. Airway inflammation in asthma.
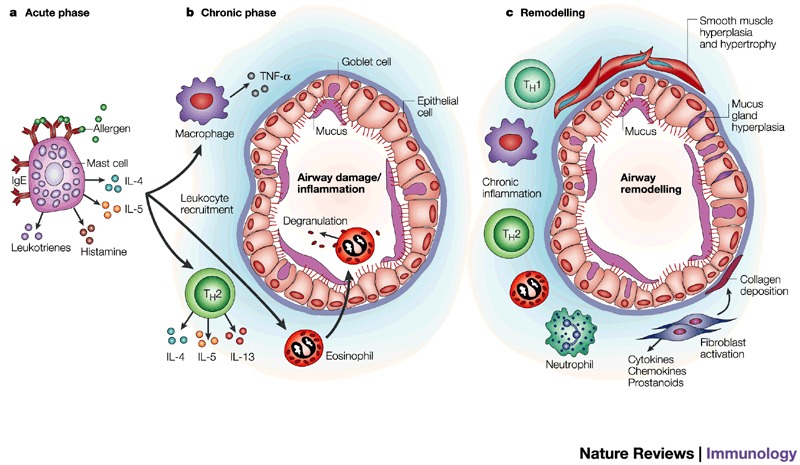
Asthma is a complex and interactive process involving many cell types, mediators and target-organ responses. Furthermore, the process can involve a progression from acute events (a) such as allergen-induced activation of mast cells to release pro-inflammatory cytokines and mediators, leading to acute bronchoconstriction and airway obstruction, to chronic inflammation (b) characterized by activation of T helper (TH) 2 cells and macrophages, and recruitment and degranulation of eosinophils. The changes in the airway cause not only airflow obstruction but also an increase in airway responsiveness. Finally, in some subjects, there is a further progression of the inflammatory changes towards airway remodelling (c). The remodelling changes can lead to permanent alterations in the airway architecture such that obstructive events are irreversible. IgE, immunoglobulin E; IL-4, interleukin 4; TH1, T helper 1 cells; TNF-α, tumour necrosis factor α.
The pattern of inflammation with viral infections and its effect on asthma appears to be distinct from that associated with allergen activation. Exposure to allergens causes airway eosinophilia, whereas viral infections elicit neutrophilic responses4,5. Furthermore, in contrast to allergen-induced wheezing, virus-induced wheezing illnesses respond incompletely to standard asthma therapy such as administration of bronchodilators and inhaled corticosteroids6. These observations highlight the need to understand better the pathogenesis of virus-induced wheezing and exacerbations of asthma and, consequently, to enable the development of new therapeutic approaches to these common illnesses.
Viruses and asthma development
Studies to determine the effect of viral infections on the development of asthma have yielded controversial and conflicting results. Collectively, however, the variability in the studies' results may be attributable to several factors, including the specific viral pathogen, the severity of the illness, the anatomical location of the illness and the age of the affected individual. The following sections review data related to the positive and negative influences of viral infections on the development of asthma.
Do viruses increase the risk of developing asthma? There is a close association between RSV infections and wheezing syndromes in infants. Although nearly all infants are infected with RSV during the first 3 years of life, only a subset of infants develop wheezing illnesses, and so other risk factors, including reduced pulmonary function7,8 and genetic factors9, are involved.
It has long been recognized that children who develop wheezing with RSV infection are at an increased risk of recurrent wheezing and possibly asthma, and it is interesting that childhood asthma and hospitalizations due to RSV infection have increased in prevalence over the past two decades10,11. Most of the risk factors for developing asthma after bronchiolitis in infancy relate to other signs or indicators of allergy, such as atopic dermatitis, food allergy or allergic rhinitis12,13,14. Furthermore, there is evidence that certain patterns of immune response to the infection, including eosinophilic inflammation in airway secretions15,16,17, reduced interferon-γ (IFN-γ) production from peripheral blood mononuclear cells (PBMC) ex vivo18, and increased ex vivo PBMC IL-10 secretion during convalescence19, are associated with an increased risk of recurrent wheezing, and possibly asthma. In animal models of infection with a related paramyxovirus, episodic lower airway obstruction after Sendai virus infection has been related to reduced production of IFN-γ20, and it is likely that additional immunological risk factors for lower airway effects of respiratory virus infections in humans will be identified. Although lower airway infections with RSV seem to increase the risk of recurrent wheezing in childhood21,22, this effect disappears by the age of 13 years21, indicating the increasing importance of other risk factors for asthma later on in childhood.
The key to understanding the relationship between viral infections in infancy, allergy (and the associated immune abnormalities) and asthma will be to determine the temporal sequence of these events and the interplay between existing gene-susceptibility factors and the introduction of the viral infection (Fig. 2). Do severe viral infections in infancy affect the development of the immune system and so modify the subsequent risk of allergy or asthma, or are there pre-existing abnormalities in the lung or the immune response that cause more severe respiratory manifestations of viral infections and increase the risk for allergy and asthma?
Figure 2. Possible relationships between respiratory syncytial virus infection, wheezing in infancy, and asthma.
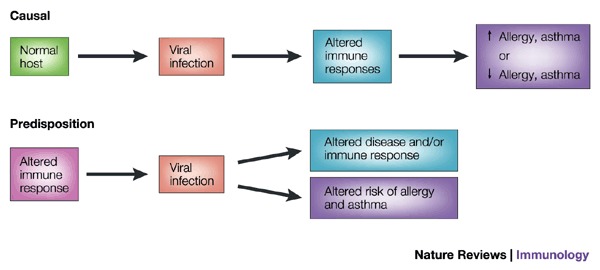
Respiratory syncytial virus (RSV) bronchiolitis is associated with an increased risk of recurrent wheezing and asthma in childhood, but the nature of this association has not yet been clearly defined. It is possible that this is a causal relationship, and that RSV bronchiolitis alters immune responses or lung development to promote allergy or asthma. Alternately, RSV infection could be an early stimulus for wheezing in children who have a predisposition for allergies and asthma. These possibilities are not mutually exclusive, and are currently under investigation in longitudinal clinical studies.
Do viruses protect against asthma? Some viral infections might actually protect against the development of allergies and asthma. This controversial idea, termed the 'hygiene hypothesis', was first suggested by David Strachan, who noted that the risk of developing allergies and asthma is inversely related to the number of children in the family23 — a result that has been replicated in several subsequent studies24. This finding has led to speculation that infectious diseases, which are more likely to be transmitted in large families, could modulate the development of the immune system in such a way as to reduce the chances of developing allergies. This hypothesis implies that the immune system is skewed towards a TH2-like response pattern at birth25. According to the theory, each viral infection would provide a stimulus for the development and/or activation of TH1-like immune responses. The result of this repetitive stimulation would be to change the polarization of the TH system away from a TH2 overexpression and thus reduce the risk of developing allergies (Fig. 3).
Figure 3. The 'hygiene hypothesis'.
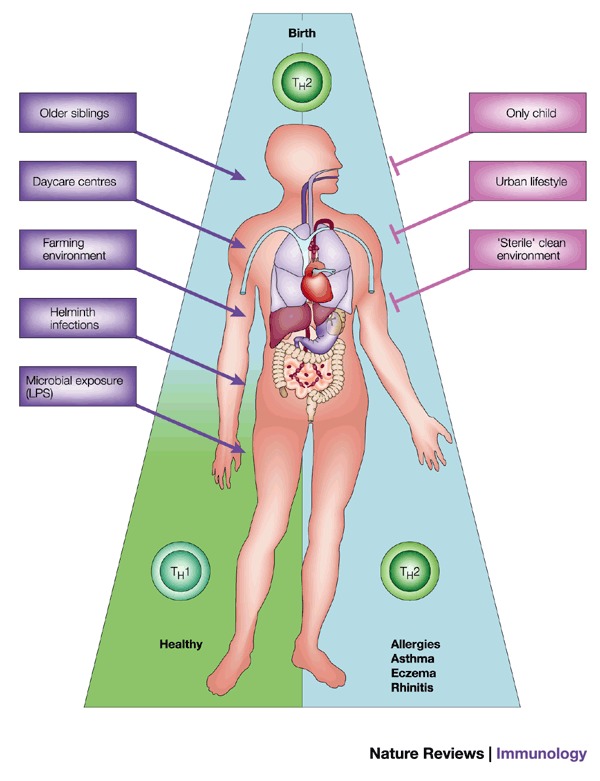
According to this theory, the immune system at birth is immature and is skewed towards T helper (TH) 2-like cytokine production. Certain stimuli, such as infections with helminths or viruses (contracted from siblings or peers in day care centres), can help immunological development towards a healthy balance of TH1 and TH2-like cytokine responses. In the absence of these stimuli (that is, children who do not have contact with other children, or who live in relatively 'sterile' urban environments), the immature TH2-like pattern of cytokine production persists, leading to an increased risk of asthma and other atopic diseases. LPS, lipopolysaccharide.
Some of the early reports suggesting that contracting a single infectious disease, such as measles, can protect against allergies and asthma26 have not been confirmed27. However, the hygiene hypothesis is supported by studies that show an inverse relationship between attendance at day care centres, where exposure to viral infections is quite high, and subsequent rates of allergy and asthma development28. However, it is not only the type of infection that is important, but also the route of exposure, as food-borne or enteric infections may have greater effects on allergy than respiratory infections29. Finally, it may not be the infections per se that are important, but rather exposure to microbes or microbial products in the environment. This concept is supported by the finding that exposure to a farming environment in early childhood, and/or having exposure to higher levels of lipopolysaccharide (LPS), is associated with reduced rates of allergy and asthma development30,31,32,33, Collectively, these findings indicate that repeated exposure to common infectious diseases and prolonged exposure to microbial products may protect against the development of allergies and asthma. If this concept is confirmed, determining the relevant mechanisms involved could lead to an entirely new approach to the prevention of these common problems.
Viruses and exacerbations of asthma
Clinical studies conducted in children and adults with asthma have provided conclusive evidence that infections caused by respiratory viruses are important precipitants for acute episodes of airway obstruction and wheezing. In recent studies using reverse transcription polymerase chain reaction (RT-PCR)-based diagnostic assays, the rhinovirus has emerged as the most important cause of these acute episodes1,2, and infection with this common cold virus can produce severe wheezing34.
In fact, hospitalization rates for asthma in a community parallel the prevalence of common colds35. In addition to rhinoviruses, several other viral respiratory infections (for example, seasonal infections with influenza, coronavirus or RSV) can also provoke acute asthma2,34,35.
Understanding how respiratory viruses such as RSV and influenza virus can affect lower airway physiology is reasonably straightforward because these organisms infect and injure lower airway tissues. The rhinovirus has traditionally been considered to be an upper airway pathogen, as early studies indicated that it replicates better at 33–35 °C than at body temperature, and its injurious effects on airway tissue are limited. However, whether it induces acute asthma by infecting lower airway tissues is controversial. Direct measurements, however, have shown that temperatures in the proximal lower airways are conducive to rhinovirus replication, and only exceed 35 °C in the periphery of the lung36. Moreover, rhinovirus seems to replicate equally well in cultured epithelial cells derived from either upper or lower airway epithelium37, and has been detected in lower airway cells and secretions after experimental inoculation38,39. Finally, inoculation of healthy volunteers with rhinovirus produces lower airway inflammatory changes, including increases in neutrophils40, lymphocytes41,42 and intercellular adhesion molecule 1 (ICAM-1)43. In addition, rhinovirus infection has been found to cause small increases in lower airway eosinophils in some40,41, but not all42, studies. Remaining areas of investigation include determining how much virus is present in the lower airway and establishing whether viral replication in the lower airway is a sufficient stimulus to provoke exacerbations of asthma.
To begin to address these questions, studies have been performed using the techniques of bronchoscopy and experimental viral inoculation to determine pro-inflammatory effects of viral infections, and potential interactions between viral infections and existing airway inflammation in asthma. These investigations have shown that infections with rhinovirus can enhance both the immediate (histamine release) and late-phase (eosinophil recruitment) airway responses to allergen challenge44,45. In addition, individuals with respiratory allergies may develop greater changes in airway responsiveness after experimental inoculation with rhinovirus46.
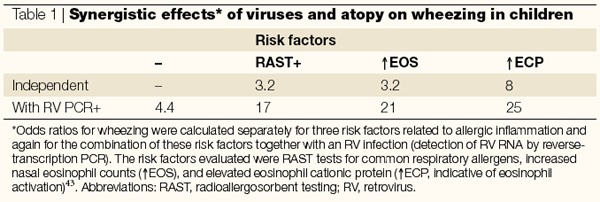
Other interactions between allergy and the effects of common cold infections in the lower airways have been observed in clinical studies involving infections occurring naturally. In these studies, risk factors for developing acute wheezing episodes were ascertained in children who presented to an emergency department47. Individual risk factors for developing wheezing included detection of a respiratory virus, most commonly rhinoviruses, positive allergen-specific immunoglobulin E as detected by radioallergosorbent testing (RAST), and either eosinophilia or evidence of eosinophil degranulation in nasal secretions. Remarkably, the presence of virus in nasal secretions and indications of allergy (eosinophilic inflammation or positive RAST testing) synergistically increased the odds ratio for wheezing (Table 1). When considered together with findings from studies of experimental infection, these observations provide convincing evidence that people with either respiratory allergies or eosinophilic airway inflammation are at increased risk of the lower airway effects of viral infections.
Table 1.
Synergistic effects* of viruses and atopy on wheezing in children
Effects of virus-induced inflammation
The epithelial cell is a focal point in the pathogenesis of viral respiratory infections because it serves as the host cell for viral replication, and it can also initiate innate immune responses (Fig. 4). Damage to the epithelial cells can disturb airway physiology through several different pathways. For example, epithelial oedema and shedding, together with mucus production, can cause airway obstruction and wheezing. The immune response to the virus, including cell recruitment and activation, and secretion of pro-inflammatory cytokines and mediators (for example, leukotrienes, kinins and prostenoids), can also contribute to the pathogenesis of respiratory infections. For viruses such as rhinoviruses, which infect relatively few cells in the airway, it may be the primary mechanism that causes airway symptoms and lower airway dysfunction48,49. Virus-induced cytokine production and inflammatory cell activation are likely to be instrumental in the development of neurogenic inflammatory responses, and these combined factors may increase bronchoconstriction in response to allergens or irritants.
Figure 4. Mechanisms of virus-induced airway inflammation.
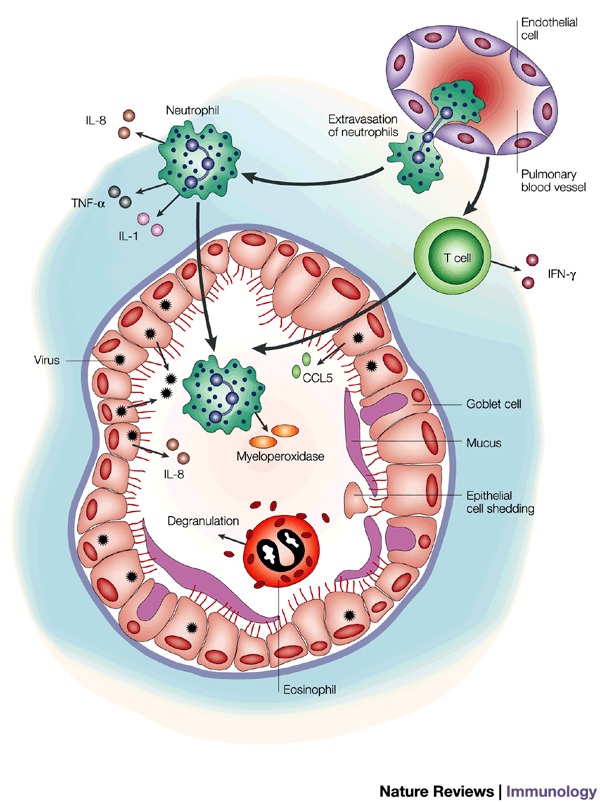
Viral replication in airway epithelial cells induces the secretion of chemokines such as interleukin (IL)-8 and RANTES (regulated on activation, normal T cell expressed and secreted); (CCL5), which recruit mononuclear cells and neutrophils into the airway. The recruited leukocytes, together with pre-existing eosinophils in allergic airways, contribute to the inflammatory milieu through the secretion of additional cytokines and mediators. Epithelial cells that are damaged by viral infection or airway inflammation are shed and, together with transudated plasma proteins and secreted mucus, contribute to airway obstruction. IFN-γ, interferon γ; TNF-α, tumour necrosis factor α.
Viral replication can affect cell-surface receptors, resulting in the initiation of immune responses. For example, it has recently been shown that monocyte and macrophage cytokine responses to the RSV fusion (F) protein are mediated by Toll-like receptor 4 (TLR4) and CD14, and that TLR4-deficient mice have prolonged viral shedding50. These findings raise the possibility that the TLR family of receptors, which has previously been associated with innate immune responses to bacteria, also participate in antiviral responses. By contrast, binding of ultraviolet-inactivated virus to ICAM-1, the receptor for 90% of rhinoviruses, is a relatively weak epithelial cell stimulus37,51,52. In addition to effects on surface receptors, virus-induced oxidative stress may be an important stimulus for the production of chemokines53,54.
Replication of single-stranded RNA viruses such as rhinoviruses, RSV and influenza virus leads to the production of double-stranded RNA (dsRNA), which is a potent stimulus for anti-viral innate immune responses. dsRNA activates intracellular signalling proteins such as the dsRNA-dependent protein kinase (PKR) and 2′,5′-oligoadenylate synthase55. Activation of PKR has been linked to nuclear factor κB (NF-κB) activation, and the generation of pro-inflammatory and antiviral cytokines. Moreover, dsRNA activation of PKR induces antiviral activity through the generation of nitric oxide, inhibition of cellular translation and activation of RNase L55,56,57,58. In turn, many viruses have evolved mechanisms to inhibit these pathways to increase the probability of viral replication55. So, host-cell recognition of dsRNA seems to be an important pathway for the initiation of multiple pro-inflammatory and antiviral pathways within the cell. Whether innate antiviral responses to viral infection are impaired in asthma is clearly of interest; however, it has not yet been determined.
Following the initial viral replication in epithelial cells, large amounts of virus are released into airway secretions and, presumably, into the surrounding lung tissues. At this point, it is likely that mononuclear cells are activated by high titres of virus to secrete pro-inflammatory cytokines such as IL-1, IL-8, tumour necrosis factor α (TNF-α) and IFN-γ59,60,61,62. These cytokines can, in turn, activate other cells in the airway environment and are also potent inducers of adhesion molecule expression. Together with chemokines generated by epithelial cells, this response provides a potent stimulus for the recruitment of inflammatory cells, which consist principally of neutrophils and T cells.
It is likely that products of neutrophil activation contribute to the obstruction of the airways and cause lower airway symptoms, and this concept is supported by studies showing increased neutrophils in the lower airways of infants with recurrent wheezing63,64. In clinical studies, the quantity of neutrophils, IL-8 or the neutrophil activation product myeloperoxidase are closely correlated with the severity of respiratory symptoms65,66,67. Evidence that activated neutrophils, through the release of the potent secretagogue elastase, can upregulate mucus secretion, is of particular interest68. Although viral infections generally do not cause marked increases in airway eosinophils, changes in eosinophil cationic protein, which can be released by activated eosinophils or neutrophils69, correlate with disturbed airway physiology47,70,71. These findings indicate that neutrophils, eosinophils and their activation products contribute to airway obstruction, virus-induced wheezing and exacerbations of asthma.
Lymphocytes are recruited into the upper and lower airways during the early stages of a viral respiratory infection, and innate and adaptive immune responses may limit the extent of infection and clear virus-infected epithelial cells. This is consistent with reports of severe viral respiratory infections in immunocompromised patients72.
In animal models of respiratory viral infection, cellular immune responses and patterns of cytokine production clearly relate to the outcome of respiratory infections. This same concept has been tested in rhinovirus infections in humans. Responses of peripheral blood mononuclear cells to virus in vitro were assessed in a group of volunteers, who were then inoculated with rhinovirus 16 (RV16)59. Volunteers who had strong IFN-γ responses to virus shed less virus during the peak of the cold. Moreover, an evaluation of cytokine patterns (IFN-γ /IL-5 mRNA ratio) in sputum during the acute illness revealed that volunteers with a stronger TH1 response tended to have reduced symptom scores and more rapid viral clearance65. Similarly, during naturally acquired influenza infections, high IFN-γ concentrations in nasal secretions at the time of diagnosis are associated with a greater reduction in viral shedding on the following day73. Collectively, these findings indicate that the virus-induced cellular immune response, and IFN-γ production in particular, may influence the clinical and virological outcomes of respiratory viral infections.
Summary and therapeutic implications
Viral infections are a major cause of wheezing and exacerbations of asthma in all age groups and, unfortunately, current treatment options are not very effective, impractical due to high cost or have significant side-effects. The close epidemiological relationship between viral infections and wheezing indicates that more effective therapeutic strategies — either to prevent certain viral infections or moderate the severity of these infections — could have a major influence on the prevention or management of asthma. If children are too 'healthy' in infancy, however, will this have the unintended effect of disturbing immune system development to promote allergy and asthma? This is unlikely because the most compelling data to support the hygiene hypothesis indicate that exposure to microbial products, and not infections themselves, may have the greatest effects on immune system development and protection from allergies and asthma.
Our current understanding of the pathogenesis of virus-induced wheezing and asthma indicates that there are several opportunities for the development of new treatment strategies. The first possibility is either to prevent viral infections or use antiviral medicines to treat infections at the onset of the illness. Obviously, vaccination against influenza has proven to be cost-effective, albeit underused, and RSV is a prime target for vaccine development because of the considerable morbidity and mortality associated with RSV infections. In addition to active vaccination, passive infusion of neutralizing monoclonal antibody to RSV (palivizumab) is used to prevent severe infections in high-risk infants74, although this costly therapy is not practical for general use.
Conceptually, antiviral agents could be used to treat viral infections early in the course of the illness to prevent wheezing and pulmonary complications. The problem with this approach is that, once viral respiratory infections are recognized, much of the viral replication has already occurred and, consequently, there is likely to be a limited potential for antiviral agents to affect the course of the illness. Thus, neuramidase inhibitors, which have potent antiviral activity in vitro, have only modest effects on clinical influenza, and are only effective if used early in the course of the illness75. The neuramidase inhibitors are most effective when given prophylactically, and they have been shown to prevent ∼80% of clinically apparent cases of the flu76. Although this approach has no advantage over the less costly alternative of immunizing for the prevention of influenza, vaccination for rhinoviruses is not feasible due to the large number of serotypes. Therefore, the prophylactic use of an antiviral medication may be a reasonable strategy for preventing asthma exacerbations in affected individuals.
Several antiviral agents have been developed for the treatment of rhinovirus infection, including soluble ICAM-1 and capsid-binding agents, which either hinder rhinovirus binding to cellular receptors or inhibit uncoating of the virus to release RNA inside the cell77,78. In addition, inhibitors of rhinovirus 3C protease reduce rhinovirus replication in vitro79. Some of these compounds have been tested in clinical trials and have shown antiviral activity.
Another potential therapeutic approach to respiratory viral infections in asthma is to inhibit pro-inflammatory immune responses induced by the virus, either by targeting specific signalling pathways or pro-inflammatory cytokines or mediators. In fact, this approach has proven to be effective, as systemic administration of glucocorticoids at the first sign of a cold can reduce acute airway obstruction and the risk of hospitalization for acute exacerbations of asthma80. It should be noted that oral corticosteroids are ineffective at reducing wheezing caused by RSV infection in infants, indicating that there are differences in the pathogenesis of these two clinical syndromes. New findings regarding the immunopathogenesis of viral respiratory illnesses indicate that it may be possible to inhibit virus-induced immune responses that are pro-inflammatory, without blocking antiviral effectors, such as IFN secretion and CD8+ T-cell-mediated responses, which are needed to clear the virus infection. Although evidence of a cause-and-effect relationship between specific cells or mediators and viral respiratory illness is at present circumstantial, viral induction of IL-8 and neutrophilic inflammation has been most closely associated with clinical symptoms, making this pathway a promising therapeutic target. In the absence of a valid animal model for rhinovirus-induced exacerbations of asthma, testing this hypothesis awaits the development of inhibitors of IL-8 and other pro-inflammatory pathways that are suitable for human clinical trials.
Asthma is clearly a complex collection of clinical phenotypes that have in common the propensity to develop acute episodes of airway obstruction and wheezing. This concept is supported by genetic studies, which have provided compelling evidence that as many as 20 genes may be involved in conferring increased risk of asthma81. Given the clinical and genetic diversity of this disorder, it is likely that viral respiratory infections in infancy and later in life could produce different effects depending on the genetic and environmental factors that led to the disease. Current and future studies to identify the functional significance of gene-by-environment interactions are likely to yield new insights into the relationship between viral infections, wheezing and asthma.
Biographies
James Gern is a Pediatric Allergist and Clinical Immunologist at the Morris Institute for Respiratory Research at the University of Wisconsin-Madison. The focus of his work is to determine mechanisms by which rhinoviruses activate epithelial cell immune responses, and to determine the relationship of virus-induced inflammation to the pathogenesis of virus-induced exacerbations of asthma.
William W. Busse is the Charles E. Reed Professor of Medicine and Head of the Allergy and Immunology Section at the University of Wisconsin Medical School, Madison, Wisconsin. His research interests include mechanisms of eosinophilic inflammation in asthma and rhinovirus provocation of asthma. In the latter area, his focus has been on how respiratory viruses such as rhinovirus enhance existing inflammation in asthma.
Related links
DATABASES
References
- 1.Johnston SL, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busse WW, Lemanske RF., Jr Asthma. N. Engl. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Harb M, et al. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur. Respir. J. 1999;14:139–143. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- 5.Pizzichini MM, et al. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am. J. Respir. Crit. Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- 6.Reddel H, et al. Differences between asthma exacerbations and poor asthma control. Lancet. 1999;353:364–369. doi: 10.1016/S0140-6736(98)06128-5. [DOI] [PubMed] [Google Scholar]
- 7.Martinez FD, et al. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 8.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N. Engl. J. Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 9.Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55:1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Measuring childhood asthma prevalence before and after the 1997 redesign of the National Health Interview Survey — United States. Morbidity Mortality Weekly Rep.49, 908–911 (2000). [PubMed]
- 11.Shay DK, et al. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am. J. Respir. Crit. Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 13.Clough JB, et al. Can we predict which wheezy infants will continue to wheeze? Am. J. Respir. Crit. Care Med. 1999;160:1473–1480. doi: 10.1164/ajrccm.160.5.9807019. [DOI] [PubMed] [Google Scholar]
- 14.Reijonen TM, Kotaniemi-Syrjanen A, Korhonen K, Korppi M. Predictors of asthma three years after hospital admission for wheezing in infancy. Pediatrics. 2000;106:1406–1412. doi: 10.1542/peds.106.6.1406. [DOI] [PubMed] [Google Scholar]
- 15.Ehlenfield DR, Cameron K, Welliver RC. Eosinophilia at the time of respiratory syncytial virus bronchiolitis predicts childhood reactive airway disease. Pediatrics. 2000;105:79–83. doi: 10.1542/peds.105.1.79. [DOI] [PubMed] [Google Scholar]
- 16.Koller DY, et al. High levels of eosinophilic cationic protein in wheezing infants predict the development of asthma. J. Allergy Clin. Immunol. 1997;99:752–756. doi: 10.1016/S0091-6749(97)80007-3. [DOI] [PubMed] [Google Scholar]
- 17.Reijonen TM, et al. Nasopharyngel eosinophil cationic protein in bronchiolitis — relation to viral findings and subsequent wheezing. Pediatr. Pulmonol. 1997;24:35–41. doi: 10.1002/(SICI)1099-0496(199707)24:1<35::AID-PPUL6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Renzi PM, et al. Cellular immunity is activated and a TH-2 response is associated with early wheezing in infants after bronchiolitis. J. Pediatr. 1997;130:584–593. doi: 10.1016/S0022-3476(97)70243-9. [DOI] [PubMed] [Google Scholar]
- 19.Bont L, et al. Monocyte IL-10 production during respiratory syncytial virus bronchiolitis is associated with recurrent wheezing in a one-year follow-up study. Am. J. Respir. Crit. Care Med. 2000;161:1518–1523. doi: 10.1164/ajrccm.161.5.9904078. [DOI] [PubMed] [Google Scholar]
- 20.Sorkness RL, Castleman WL, Kumar A, Kaplan MR, Lemanske RF., Jr Prevention of chronic postbronchiolitis airway sequelae with IFN-γ treatment in rats. Am. J. Respir. Crit. Care Med. 1999;160:705–710. doi: 10.1164/ajrccm.160.2.9810002. [DOI] [PubMed] [Google Scholar]
- 21.Stein RT, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 22.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 23.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Mutius E. The environmental predictors of allergic disease. J. Allergy Clin. Immunol. 2000;105:9–19. doi: 10.1016/S0091-6749(00)90171-4. [DOI] [PubMed] [Google Scholar]
- 25.Prescott SL, et al. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 26.Shaheen SO, et al. Measles and atopy in Guinea-Bissau. Lancet. 1996;347:1792–1796. doi: 10.1016/S0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- 27.Paunio M, et al. Measles history and atopic diseases: a population based cross-sectional study. JAMA. 2000;283:343–346. doi: 10.1001/jama.283.3.343. [DOI] [PubMed] [Google Scholar]
- 28.Ball TM, et al. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N. Engl. J. Med. 2000;343:538–543. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 29.Matricardi PM, et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ. 2000;320:412–417. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Von Ehrenstein OS, et al. Reduced risk of hay fever and asthma among children of farmers. Clin. Exp. Allergy. 2000;30:187–193. doi: 10.1046/j.1365-2222.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 31.Gereda JE, Leung DY, Liu AH. Levels of environmental endotoxin and prevalence of atopic disease. JAMA. 2000;284:1652–1653. doi: 10.1001/jama.284.13.1647. [DOI] [PubMed] [Google Scholar]
- 32.von Mutius E, et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin. Exp. Allergy. 2000;30:1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 33.Riedler J, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 34.Atmar RL, et al. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 35.Johnston SL, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time trend analysis. Am. J. Respir. Crit. Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 36.McFadden ER, Jr, et al. Thermal mapping of the airways in humans. J. Appl. Physiol. 1985;58:564–570. doi: 10.1152/jappl.1985.58.2.564. [DOI] [PubMed] [Google Scholar]
- 37.Schroth MK, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 38.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally-induced infection. Am. J. Respir. Crit. Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos NG, et al. Rhinoviruses infect the lower airways. J. Infect. Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 40.Jarjour NN, et al. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J. Allergy Clin. Immunol. 2000;105:1169–1177. doi: 10.1067/mai.2000.106376. [DOI] [PubMed] [Google Scholar]
- 41.Fraenkel DJ, et al. Lower airway inflammation during rhinovirus colds in normal and in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 42.Grunberg K, et al. Rhinovirus-induced airway inflammation in asthma. Effect of treatment with inhaled corticosteroids before and during experimental infection. Am. J. Respir. Crit. Care Med. 2001;164:1816–1822. doi: 10.1164/ajrccm.164.10.2102118. [DOI] [PubMed] [Google Scholar]
- 43.Grünberg K, et al. Experimental rhinovirus 16 infection increases intercellular adhesion molecule-1 expression in bronchial epithelium of asthmatics regardless of inhaled steroid treatment. Clin. Exp. Allergy. 2000;30:1015–1023. doi: 10.1046/j.1365-2222.2000.00854.x. [DOI] [PubMed] [Google Scholar]
- 44.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J. Clin. Invest. 1994;94:2200–2208. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemanske RF, Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J. Clin. Invest. 1989;83:1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gern JE, Calhoun WJ, Swenson C, Shen G, Busse WW. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am. J. Respir. Crit. Care Med. 1997;155:1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 47.Rakes GP, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 48.Arruda E, et al. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J. Infect. Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 49.Hendley JO. The host response, not the virus, causes the symptoms of the common cold. Clin. Infect. Dis. 1998;26:847–848. doi: 10.1086/513921. [DOI] [PubMed] [Google Scholar]
- 50.Kurt-Jones EA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Z, et al. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor κ B-dependent transcriptional activation. J. Clin. Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanders SP, Siekierski ES, Porter JD, Richards SM, Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J. Virol. 1998;72:934–942. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaul P, Biagioli MC, Singh I, Turner RB. Rhinovirus-induced oxidative stress and interleukin-8 elaboration involves p47-phox but is independent of attachment to intercellular adhesion molecule-1 and viral replication. J. Infect. Dis. 2000;181:1885–1890. doi: 10.1086/315504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casola A, et al. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. Role in viral-induced interferon regulatory factor activation. J. Biol. Chem. 2001;276:19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 55.Williams BRG. PKR: a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 56.Uetani K, et al. Central role of double-stranded RNA-activated protein kinase in microbial induction of nitric oxide synthase. J. Immunol. 2000;165:988–996. doi: 10.4049/jimmunol.165.2.988. [DOI] [PubMed] [Google Scholar]
- 57.Sanders SP, et al. Rhinovirus infection induces expression of type 2 nitric oxide synthase in human respiratory epithelial cells in vitro and in vivo. J. Allergy Clin. Immunol. 2001;107:235–243. doi: 10.1067/mai.2001.112028. [DOI] [PubMed] [Google Scholar]
- 58.Terenzi F, et al. The antiviral enzymes PKR and RNase L suppress gene expression from viral and non-viral based vectors. Nucleic Acids Res. 1999;27:4369–4375. doi: 10.1093/nar/27.22.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parry DE, et al. Rhinovirus-induced peripheral blood mononuclear cell responses and outcome of experimental infection in allergic subjects. J. Allergy Clin. Immunol. 2000;105:692–698. doi: 10.1067/mai.2000.104785. [DOI] [PubMed] [Google Scholar]
- 60.Gern JE, et al. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J. Immunol. 1996;156:621–627. [PubMed] [Google Scholar]
- 61.Johnston SL, Papi A, Monick MM, Hunninghake GW. Rhinoviruses induce interleukin-8 mRNA and protein production in human monocytes. J. Infect. Dis. 1997;175:323–329. doi: 10.1093/infdis/175.2.323. [DOI] [PubMed] [Google Scholar]
- 62.Levandowski RA, Horohov DW. Rhinovirus induces natural killer-like cytotoxic cells and interferon alpha in mononuclear leukocytes. J. Med. Virol. 1991;35:116–120. doi: 10.1002/jmv.1890350208. [DOI] [PubMed] [Google Scholar]
- 63.Stevenson EC, et al. Bronchoalveolar lavage findings suggest two different forms of childhood asthma. Clin. Exp. Allergy. 1997;27:1027–1035. doi: 10.1111/j.1365-2222.1997.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 64.Krawiec ME, et al. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am. J. Respir. Crit. Care Med. 2001;163:1338–1343. doi: 10.1164/ajrccm.163.6.2005116. [DOI] [PubMed] [Google Scholar]
- 65.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am. J. Respir. Crit. Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 66.Turner RB, Weingand KW, Yeh CH, Leedy DW. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin. Infect. Dis. 1998;26:840–846. doi: 10.1086/513922. [DOI] [PubMed] [Google Scholar]
- 67.Grünberg K, et al. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin. Exp. Allergy. 1997;27:36–45. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cardell LO, Agusti C, Takeyama K, Stjarne P, Nadel JA. LTB(4)-induced nasal gland serous cell secretion mediated by neutrophil elastase. Am. J. Respir. Crit. Care Med. 1999;160:411–414. doi: 10.1164/ajrccm.160.2.9808117. [DOI] [PubMed] [Google Scholar]
- 69.Sur S, et al. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukoc Biol. 1998;63:715–722. doi: 10.1002/jlb.63.6.715. [DOI] [PubMed] [Google Scholar]
- 70.Garofalo R, Kimpen JLL, Welliver RC, Ogra PL. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J. Pediatr. 1992;120:28–32. doi: 10.1016/S0022-3476(05)80592-X. [DOI] [PubMed] [Google Scholar]
- 71.Grünberg K, et al. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1997;156:609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- 72.Malcolm E, Arruda E, Hayden FG, Kaiser L. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J. Clin. Virol. 2001;21:9–16. doi: 10.1016/S1386-6532(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 73.Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J. Med. Virol. 2001;64:262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 74.Meissner HC, et al. Safety and pharmacokinetics of an intramuscular monoclonal antibody (SB209763) against respiratory syncytial virus (RSV) in infants and young children at risk for severe RSV disease. Antimicrob. Agents Chemother. 1999;43:1183–1188. doi: 10.1128/AAC.43.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Treanor JJ, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 76.Hayden FG, et al. Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group. N. Engl. J. Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 77.Rotbart HA. Pleconaril treatment of enterovirus and rhinovirus infections. Infect. Med. 2000;17:488–490. [Google Scholar]
- 78.Huguenel ED, et al. Prevention of rhinovirus infection in chimpanzees by soluble intercellular adhesion molecule-1. Am. J. Respir. Crit. Care Med. 1997;155:1206–1210. doi: 10.1164/ajrccm.155.4.9105055. [DOI] [PubMed] [Google Scholar]
- 79.Dragovich PS, et al. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 3. Structure–activity studies of ketomethylene-containing peptidomimetics. J. Med. Chem. 1999;42:1203–1212. doi: 10.1021/jm980537b. [DOI] [PubMed] [Google Scholar]
- 80.Brunette MG, Lands L, Thibodeau LP. Childhood asthma: prevention of attacks with short-term corticosteroid treatment of upper respiratory tract infections. Pediatrics. 1988;81:624–629. [PubMed] [Google Scholar]
- 81.Weiss ST. Association studies in asthma genetics. Am. J. Respir. Crit. Care Med. 2001;164:2014–2015. doi: 10.1164/ajrccm.164.11.2110043b. [DOI] [PubMed] [Google Scholar]


