Key Points
Unusual clinical syndromes or clusters of infections in recipients of organs from the same donor suggest donor-derived infection as a possible source of transmission
The incidence of transmission of unexpected infection by organ allografts is low, but precise data are lacking
Screening of donors for common pathogens involves both epidemiologic history and microbiological assays, and is highly effective for preventing the transmission of HIV and hepatitis B and C viruses
Donor screening for uncommon pathogens must be guided by knowledge of changes in the local epidemiology of infection
The key element in the detection of donor-derived infection is suspicion on the part of the clinicians caring for organ recipients
Application of newer microbiological techniques will increase the speed of donor screening and enhance transplant safety
Supplementary information
The online version of this article (doi:10.1038/nrneph.2014.159) contains supplementary material, which is available to authorized users.
Subject terms: End-stage renal disease, Organ transplantation, Infectious diseases, Risk factors
Each year, over 70,000 organs are transplanted worldwide. The degree of risk of transmission of infection from transplanted organs to the recipient is largely unknown and is difficult to assess for specific organs. Here, Jay A. Fishman and Paolo A. Grossi describe the major risk factors for organ donor-derived transmission of infection and discuss opportunities to reduce the incidence of such events.
Supplementary information
The online version of this article (doi:10.1038/nrneph.2014.159) contains supplementary material, which is available to authorized users.
Abstract
Organ transplantation, including of the heart, lung, kidney, liver, pancreas, and small bowel, is considered the therapy of choice for end-stage organ failure. Each year, over 70,000 organs are implanted worldwide. One donor may provide multiple organs, as well as corneas and other tissues, for multiple recipients. The degree of risk for transmission of infection carried with grafts, notably of viruses, is largely unknown and, for a specific organ, difficult to assess. The approach to microbiological screening of organ donors varies with national and regional regulations and with the availability and performance of microbiological assays used for potential donors. Transmission of both expected or common, and unexpected infections has been observed in organ transplants, generally recognized after development of clusters of infections among recipients of organs from a common donor. Other than for unusual or catastrophic events, few data exist that define the incidence and manifestations of donor-derived infections or the ideal assays to use in screening to prevent such transmissions. Absolute prevention of the transmission of donor-derived infections in organ transplantation is not possible. However, improvements in screening technologies will enhance the safety of transplantation in the future.
Supplementary information
The online version of this article (doi:10.1038/nrneph.2014.159) contains supplementary material, which is available to authorized users.
Introduction
Organ transplantation is the therapy of choice for end-stage organ failure. Each year, over 70,000 organs are transplanted worldwide. Unexpected transmission of infection from donors to recipients is infrequent, but few data exist that bear directly on the degree of risk of transmission of donor-derived infection through transplantation.1,2,3 Transplanted organs might come from donors with unknown or asymptomatic infections, or could be contaminated during procurement or implantation. Transmission of infection is facilitated by the viable cells of vascularized organs. Moreover, immunosuppressive drugs used to prevent immunological attack on the grafts, or graft rejection, may amplify the risk of infection carried by the donor organ. For example, West Nile virus (WNV) has been reported to cause invasive neurologic infection more often in immunocompromised organ recipients than in normal individuals.4,5,6,7 In immunocompromised hosts with a diminished inflammatory response, signs and symptoms of infection are reduced and the incidence of infection is often underestimated.
Reporting of suspected or documented donor-derived transmission events is required in the USA and voluntary in many other countries. Unexpected disease transmission occurs in less than 1% of solid organ recipients.8,9 By contrast, hepatitis C virus (HCV) or human immunodeficiency virus (HIV) is transmitted with screened blood products in less than one in 2–3 million units transfused.1,2,3 Screening processes for blood products allow confirmatory testing and enable discarding of potentially contaminated blood, but the limited supply of organs and the urgent need to implant them to preserve organ function after procurement from deceased donors precludes prolonged screening processes or the discarding of potentially useful grafts. Few data are available that directly assess the risk of disease transmission with organ transplants;1,2,3 such studies are difficult and costly to perform.
Microbiological screening of potential organ donors is improving with the availability of new assays such as nucleic acid testing (NAT) or advanced antibody tests. However, the availability of such assays and the proficiency of the clinical laboratories, as well as the national regulations governing reporting of adverse events and donor screening, vary worldwide.1,2,8,9,10,11,12 Some regions, including parts of South America, Africa and Asia lack requirements for reporting of adverse events in transplantation. In addition, organs are increasingly shared across international borders. Identification of clusters of infections associated with organ transplantation has resulted in reassessment of screening processes for organ donors.1,2,4,13,14,15,16,17,18,19 In this article, we review the major risk factors for organ-donor-derived transmission of infection and opportunities to reduce the incidence of such events.
Categories of donor-derived infections
Donor-derived infections can be loosely categorized into two groups: 'expected' and 'unexpected' infections (Box 1). This distinction is somewhat arbitrary. Many ubiquitous pathogens such as cytomegalovirus (CMV) can generally be detected with available microbiological assays, and pose a known, but modest risk to organ recipients. In general, such infections are treatable. Other, unexpected infections could result from the spread of known infections to new regions, such as the recent outbreaks of WNV in the USA or Chikungunya virus in Italy, for which both diagnostic assays and therapies were lacking at the time of discovery. Within national borders, geographic variation exists for endemic infections such as histoplasmosis or Chagas disease (caused by the parasite Trypanosoma cruzi).20,21 For some pathogens, screening assays might not be available in clinical laboratories, might be inaccurate (for example, assays for lymphocytic choriomeningitis virus [LCMV] or tuberculosis), too costly or too slow for routine use in donor screening or could be restricted to specialty laboratories. Screening assays for organ donors must be completed within 4–12 h of identification of the potential organ donor to maintain graft viability. Some screening data (such as blood cultures) might become available only after the organ has been implanted. Thus, the approach to the assessment and management of donor-derived infection is a function of disease epidemiology, availability of diagnostic technology, cost of screening, data communication and the degree of risk of infection that physicians and patients consider acceptable to obtain life-saving transplants that might be otherwise unavailable.
Box 1: Organ-donor-derived infectious transmissions*.
Expected ‡
• Cytomegalovirus
• Epstein–Barr virus
• HBV
• HCV
• Toxoplasma gondii
• BK polyomavirus
Unexpected
Viruses
• Adenovirus
• Herpes simplex virus
• HIV
• HBV
• HCV
• Hepatitis E virus
• Human T-cell lymphotropic virus 1 and 2
• Influenza A/B
• Lymphocytic choriomeningitis virus
• Parvovirus B19
• Rabies
• West Nile virus
Fungi
• Aspergillus spp.
• Candida spp.
• Coccidioides immitis
• Cryptococcus neoformans
• Histoplasma capsulatum
• Scopulariopsis brevicaulis
• Zygomycetes (Mucor)
Bacteria§
• Gram-negative: Pseudomonas, Acinetobacter, Legionella, Klebsiella, Ehrlichia, Serratia, Escherichia coli, Veillonella
• Gram-positive: Brucella, Enterococcus (for example, vancomycin-resistant Enterococcus), Staphylococcus spp. (for example, methicillin-resistant Staphylococcus aureus), Listeria
• Mycobacterium tuberculosis
• Nocardia spp.
• Rickettsia rickettsii (Rocky Mountain Spotted Fever)
• Treponema pallidum (Syphillis)
• Borrelia (Lyme disease)
Parasites
• Babesia microti
• Balamuthia mandrillaris
• Malaria spp.
• Naegleria fowleri
• Toxoplasma gondii
• Trypanosoma cruzi
• Schistosoma spp.
• Strongyloides stercoralis
*Cases of meningitis and septic emboli have been associated with a donor source without further characterization. Multiple malignancies have been transmitted with organ transplantation. ‡On the basis of previous knowledge of infection in the donor. §Including multi-drug resistant gram-negative infections. Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Using infected donor organs
Patients may accept organs, with informed consent, from donors with known infections. These infections are generally treatable and include bacteria (in the case of donors with pneumonia or sepsis) and viruses (for example, hepatitis B virus [HBV], HCV or HIV). Individuals infected with HIV, HBV or HCV may be offered organs from donors carrying the same virus (discussed below), given the availability of improved antiviral therapies. The availability of effective therapies for syphilis or tuberculosis can prevent transmission of Treponema pallidum or Mycobacterium tuberculosis in most recipients.
Major infections resulting from use of infected organs are, in general, either due to delays in the availability of microbiological data to the transplantation team (owing to the time required for cultures to mature), delayed or incomplete communication from the testing laboratory to the clinical site, or a failure of testing (for example, improperly performed assays as has occurred for HIV and HCV infection; Figure 1).22,23
Figure 1. The 'window period' in microbiological screening of potential organ donors.
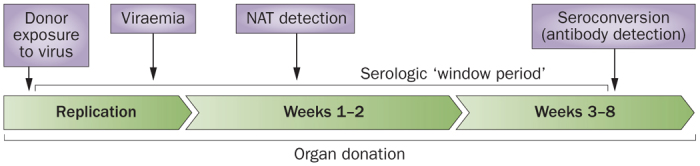
The development of an antibody response against a pathogen requires weeks to months after the initial infectious exposure. The time between the infectious exposure and the development of antibodies that can be detected by microbiological assays is called the window period. Serologic testing during this period might result in false-negative results. NAT measures viral nucleic acids, often using signal amplification techniques. Depending on the performance characteristics of the assay and the amount of virus present in the clinical specimen, NAT tends to detect infection earlier and with greater sensitivity than the corresponding serologic test. However, false-positive assays are generally more common with NAT testing. Abbreviation: NAT, nucleic acid test.
Bacterial infections
Historically, transmission of most bacterial infections has been prevented by the use of surgical prophylaxis with antibacterial therapies given around the time of transplant surgery. With the emergence of multidrug antimicrobial resistant (MDR) bacteria and yeast species, however, routine prophylaxis might fail to prevent transmission of organisms present in the donor organ or the bloodstream at the time of procurement.24,25,26,27 Increasingly common MDR organisms include extended-spectrum β-lactamase (ESBL)-producing enterobacteriaceae, carbapenem-resistant Acinetobacter baumannii (CRAB), Klebsiella pneumoniae (CRKP) and other carbapenem-resistant enterobacteriaceae (CRE). When transmitted, morbidity caused by these pathogens among organ recipients is high.28,29 Among possible risk factors for donor colonization with MDR organisms are prolonged (>7 days) stays in intensive care units, critical illness requiring vasopressor support and the need for cardiopulmonary resuscitation.30 Unfortunately, in some donors, hospitalization of 2 days is sufficient to acquire MDR infection.31
Clinical experience suggests that some respiratory-colonized CRKP-positive or CRAB-positive organ donors may be considered as candidate donors for organs other than the lungs but that close follow-up of recipients is mandatory. Similarly, if the donor has a positive urine culture for CRKP or CRAB, transplantation of their kidneys should be avoided unless the infection is eradicated. Some risk exists for unrecognized bacteraemia caused by these organisms; in the presence of MDR bacteraemia, transplantation of any organ should be avoided. The efficacy of appropriate antimicrobial treatment of the donor before organ procurement on the basis of in vitro susceptibility data, in preventing recipient infection, is not known. In general, some period of effective therapy should also be considered for the recipient after transplantation.
The results of microbiological cultures obtained at the time of organ donation might not be available until after implantation; given such delays, organ recipients may be at an increased risk of donor-derived infection.32 Rapid communication of complete microbiological susceptibility data by the organ procurement organization to the clinical centre is essential to guide management. Such recipient infections may not be recognized as donor-derived and may be more resistant to therapy in the immunocompromised host, potentially contributing to organ loss (for example, mycotic aneurysm of the graft blood vessels) or death. Thus, when possible, infections should be identified and fully treated in the donor before procurement.6,10,14,32,33,34,35,36 Decisions regarding the use of organs that might be infected with antimicrobial-resistant pathogens must be made on an individual basis.
Transplant tourism
Potential organ recipients are increasingly travelling abroad to reduce the cost of, and waiting time for, transplantation. Donor screening in some regions is limited; in countries where payment for organ donation is legal, donors may tend to come from low socioeconomic strata and endemic infections such as tuberculosis, malaria, Chagas disease and leishmaniasis might be more common than in unpaid or deceased donors. In case series from Turkey, Canada and California, many renal recipients who travelled abroad for organs developed severe infections, often requiring hospitalization.37,38,39,40 Travellers who are organ donors or so-called 'transplant tourists' might become colonized with highly resistant strains of 'superbugs' including vancomycin-resistant staphylococci and MDR enterobacteriaceae. Among these newer isolates, a carbapenemase enzyme called New Delhi Metallo-β-lactamase-1 (NDM-1), which makes bacteria resistant to a wide range of antimicrobial agents, was first identified in 2008 in India, and has since been detected worldwide.41,42 This resistance trait compromises the efficacy of almost all β-lactam antimicrobials, including the carbapenems. Therapeutic options for infections with this type of resistant bacteria are largely limited to colistin, tigecycline and fosfomycin. Although this carbapenemase constitutes a critical medical issue, no NDM-1 infection has yet been reported in the transplant setting.
Highly resistant strains of bacteria are thought to emerge under antimicrobial pressure in hospitals and in animals used for meat consumption, and via contamination of fruit, vegetables and farmed seafood. These bacteria have been observed in patients and travellers from China, India, Southeast Asia, the Middle East, the Balkans, Australia and other regions.37,38,39,40 Knowledge of specific infections that exist in regions that provide transplant services might guide patient evaluation. Transplant tourists might also provide a vector for the transmission of new MDR organisms to medical institutions. Consequently, horizontal transmission of MDR pathogens to other patients could occur, which could lead to institution-wide outbreaks of infection. Stringent infection control measures to control the spread of MDR organisms should be implemented whenever such resistant pathogens are recognized.
Viral infections
The risk for transmission of HBV from infected donors is illustrative of the potential organ-specificity of pathogen transmission and the importance of vaccination in transplant populations. Transmission of HBV is common in the absence of prophylaxis based on available data for liver transplant recipients. In a recent review, the rate of de novo HBV (DNH) infection without prophylaxis was 58% (81 of 140 patients) in HBV non-immune recipients, but was only 18% (6 of 34 patients) in previously vaccinated individuals, 14% (5 of 35 patients) in isolated recipients who were anti-HBcAg-positive (that is, positive for antibody to HBV core antigen), and 4% (3 of 70 patients) in naturally immune recipients.43 This experience has produced a series of preventive strategies to prevent DNH in HBV surface antigen (HBsAg)-negative recipients. A 2007 survey revealed that indefinite antiviral therapy was commonly employed, with lamivudine as the preferred antiviral agent; antivirals were variably supplemented with HBV hyperimmune globulin (HBIG).44 Although entecavir and tenofovir have supplanted lamivudine in the treatment of chronic HBV infection and often for prophylaxis of liver transplant recipients from anti-HBcAg-positive donors, a new study using a Markov model has now demonstrated that lamivudine remains the most cost-effective option for preventing DNH in the context of anti-HBcAg-positive donors.45,46
In heart and kidney transplant recipients, the limited available data suggest that the risk of HBV transmission using organs from anti-HBcAg-positive donors is low, with or without post-transplant antiviral prophylaxis.47 The available studies are often limited by the absence of baseline data on the serologic status (presence of anti-HBs and HBsAg) and immunosuppressive regimens of donors and recipients that may affect transmission risk. Early studies in heart transplant recipients without prophylaxis did not reveal any HBV transmission in >80 patients, some of whom were likely vaccinated before transplant.47 Use of these donor hearts should be considered safe and may help to augment the available donor pool.47 Of interest, HBV seropositivity in the donor or recipient may be associated with an increased risk of cardiac allograft vasculopathy.48
The risk of transmission of HBV from an anti-HBcAg-positive, HBsAg-negative donor to a HBV-naive kidney recipient varies from 0% to 27%.49 The definition of transmission is highly variable and includes acquisition of anti-HBsAg and anti-HBcAg; most studies did not require acquisition of HBsAg or detection of HBV DNA in case definition. The risk of transmission is, again, influenced by recipient HBV immunity and possibly by prophylaxis.50 In a review of nine studies with 1,385 evaluable HBsAg-negative recipients of kidneys from anti-HBcAg-positive donors, new HBV serologic markers were observed in only 45 (3.2%) patients and the rate of HBsAg acquisition was only 0.28% (four patients) with no evidence of symptomatic hepatitis.51 This study did not explore the influence of recipient anti-HBsAg status or use of prophylactic therapy. Patient or graft outcomes were not worse among patients with HBsAg acquisition or evidence of anti-HBsAg or anti-HBcAg seroconversion.
Given the donor organ shortage, the temptation to 'stretch the limits' on donation is almost irresistible. Unresolved issues include the optimal duration of therapy for donors with bacteraemia or tuberculosis and with meningitis or encephalitis of unknown aetiology, and the optimal prophylaxis for organs from donors who are infected with HBV, HCV, human herpesvirus 8 (HHV-8) or human T-cell lymphotropic virus (HTLV).6,20,21,32,33,34,35,36 When organs are used from infected donors, microbiologic follow-up of the recipients is essential. On the basis of risk factors identified in the donor, re-testing of the recipient of 'increased risk' donor organs is now mandated in the USA and elsewhere (at baseline, 1 month and 3 months post-transplantation) for HIV, HCV and HBV, and recommended for syphilis. Most experts also recommend a long period of monitoring (6 and 12 months) and storage of samples of donor and recipient blood, plasma and cells for future testing. National guidelines are needed for use of organs from donors with known infections.6
Unexpected and novel infections
Unexpected transmission of donor-derived infection is estimated to occur in less than 1% of solid organ transplant recipients.9 Clusters of infections, including M. tuberculosis, fungi, herpes simplex virus (HSV), HHV-8, LCMV, rabies virus, T. cruzi, microsporidiosis, HIV and HCV, have been found in multiple recipients who have received organs from a single donor (Box 1).2,4,10,13,14,15,17,18,19,21,52,53,54,55,56 These infections are most often caused by pathogens that may be amplified in the immunosuppressed host but are asymptomatic or masked in the normal organ donor by other clinical syndromes such as trauma or stroke. Individual transmission events might not be recognized unless clinical manifestations develop. For example, donor-derived chronic hepatitis E virus infection transmission was identified in immunosuppressed solid organ transplant recipients during evaluation for clinical hepatitis in Europe and the USA.57,58 In the absence of systems to automate the detection and reporting of post-transplantation infections among clinical centres and procurement organizations, recognition of these events is dependent on clinical suspicion by the transplantation teams.
Confirmation of donor-derived-infection transmission events in organ recipients, notably in the face of unusual pathogens, requires specialized clinical laboratories and epidemiologic investigation by public health authorities. International consensus definitions of donor-derived infections have been developed.59 Testing donors for all potential pathogens is impossible as assays for all organisms do not exist and such requirements would substantially delay transplantation. Clinical recognition of unusual clinical syndromes in clusters of organ recipients from single donors in the USA and Australia resulted in the development of new NAT assays for LCMV and related viruses. Application of nonspecific, high-throughput sequencing tools with the capacity to detect any unusual nucleic acid sequences in the blood was diagnostic of a new arenavirus in a cluster of fatal transplant-associated infections when standard microbiological techniques had failed.18,52
Currently, anti-HIV-1 or anti-HIV-2 reactive status in potential donors is a contraindication for organ donation in all European countries and was only recently legalized for HIV-positive recipients in the USA. Three HIV-infected donors have been used inadvertently after false-negative testing or erroneous reporting, resulting in transmission of the virus into uninfected recipients.23,60,61 Although most disease transmissions have involved deceased donors, recent unexpected transmissions of HIV and HCV have shown that recipients of organs from living donors may also be at risk.22,57 Donor-derived HIV transmissions have generally had bad outcomes; however, three patients who received organs from an HIV-infected donor in an Italian cluster, including two kidney transplant recipients, are still alive with undetectable HIV-RNA, good CD4+ T-cell counts and functioning grafts more than 7 years after transplantation (P. Grossi, unpublished work).
Transmissions of donor-derived HTLV have been reported. The natural history of HTLV-1 transmission from donor to recipient is unknown given suboptimal screening platforms and the lack of long-term follow-up. The only definitive cases of transmission of solid-organ donor-derived HTLV-1-associated disease emerged from Spain.62,63 Seronegative recipients of two kidneys and one liver developed HTLV-1-associated myelopathy within 2 years of transplantation with virus of identical sequence to that of the donor.62,63 The donor had asymptomatic, congenital infection. No donor-derived HTLV-1-associated deaths have been reported. On the basis of data from countries with a low prevalence of HTLV-1, such as Europe and the USA, and owing to the current shortage of donor organs, assessment of every donor for HTLV-1 is no longer considered to be essential. Current screening methods cannot differentiate between HTLV-1 and HTLV-2 infections and false-positive screening assays are common.64 The utility of HTLV-1 screening is uncertain in the absence of long-term follow-up data; such screening can only be recommended for endemic areas and in endemic populations.
Over the past several years, emerging pathogens such as WNV have been identified as sources of donor-derived infections. Transmission of WNV through organ transplantation was described in 2002 in four recipients of organs from a single donor.4 This route of transmission is rare; screening of organ donors for WNV is not routine and remains controversial. A long turnaround time for results, unproven test performance, and limited test availability have made such routine testing impractical. Furthermore, transmission of WNV has occurred in the absence of detectable viraemia or seroconversion in organ donors.65,66 Low viral loads in blood and a short duration of viraemia in normal individuals present major problems for nucleic acid amplification testing. The kidney may be a site of prolonged WNV replication and shedding in animals and humans.67,68,69 Thus, urine samples could be more appropriate than blood for WNV testing in organ donors in the future.70 Prospective studies are needed to verify the utility of routine WNV testing in urine (or blood) of organ donors.
Rabies causes acute encephalitis that is nearly uniformly fatal in unvaccinated hosts. Although the virus is present in animal reservoirs, infection in humans is rare in the USA and Europe. In two clusters of rabies virus transmission through organ transplantation attributed to a bat and a canine rabies virus variant, all non-vaccinated recipients developed initially unrecognized rabies symptoms within 6 weeks of transplantation and died.71,72 These observations suggest a high rabies infectivity rate and abbreviated incubation period in unvaccinated immunosuppressed recipients of solid organs.73
Similar issues with disease recognition have occurred with recent clusters of primary amoebic meningoencephalitis (PAM) caused by the free-living amoeba. Three transplant-associated clusters of encephalitis caused by the amoebae Balamuthia mandrillaris have demonstrated a risk associated with solid organ transplantation from donors with PAM.74,75 Persons of Hispanic ethnicity may be disproportionately affected.76 Unrecognized skin lesions may precede encephalitis by months to years.
As the pool of foreign donors increases, unusual infections such as malaria, tuberculosis, Chagas disease, or strongyloidiasis represent a growing threat to transplant recipients. In the USA, endemic fungi (such as Histoplasma in the mid-west and Coccidioides in the south-west regions) represent a notable risk, and routine prophylaxis is applied to many recipients, notably those of lung transplants. The differential diagnosis of fever or encephalitis within the first weeks after transplantation should include donor-derived pathogens.
Organ donor screening
Candidate organ donors are screened for potentially transmissible infections using medical and social histories (for example, past infections, travel history, animal and environmental exposures, sexual contacts and intravenous drug abuse) and laboratory assays (Box 2). A physical examination is performed and available microbiological data are reviewed.1,2,4,13,14,15,16,17,18,19,55 Blood and urine cultures are obtained. Organ preservation fluids are cultured in some countries, although the clinical relevance of such data is unclear.
Standards for screening for transmissible infections vary worldwide and are limited by the availability and cost of specialized assay platforms (such as NAT) or by the epidemiological trends of different infections. In general, the deceased donor's medical and social history is obtained from relatives whose information is often incomplete. Thus, organ procurement organizations depend on a battery of microbiological tests (Box 2) to define the transmission risks of many common infections. Evaluation of the performance of many of the assays in the relevant populations (organ donors and recipients) has not been possible. The presence of some infections, such as HTLV-1 or HIV, preclude organ donation in some countries or regions.77 The list of donor exclusion criteria might change abruptly based on outbreaks, such as those of severe acute respiratory syndrome (commonly known as SARS), WNV and Chikungunya virus. Other donor infections such as untreated meningitis or 'sepsis' are considered relative contraindications to organ donation in that organs may be used based on the clinical situation and the potential recipient's willingness to accept some, unknown, level of increased risk of infection. All data on these infections are considered in the context of the urgency in the need of the potential recipient and the availability of therapies to treat the infection should transmission occur.
Organ donors and candidate recipients are screened using serologic, antibody-based assays or NAT for common or expected infectious agents (Box 2) and results from these tests are used to guide deployment of preventative strategies and prophylaxis to reduce the risk of recipient infection. The use of NAT for the detection of viral replication (DNAemia or RNAemia) is a complement to serologic tests that measure antibodies derived from prior exposures or vaccination. A positive serology or antibody test is considered a marker of life-long, latent infection with pathogens, for example, the herpes family of viruses such as CMV, Epstein–Barr virus, HSV, or varicella zoster virus (commonly known as chickenpox). Seropositivity indicates risk of viral reactivation in the setting of immunosuppression. Similarly, the greatest risk of infection with a virus such as CMV is in a seronegative recipient who receives an organ from a seropositive donor (with latent or active infection), which produces a primary infection in a host without prior immunity. Such recipients can either be monitored for viral reactivation using a nucleic acid or antigen detection assay, or receive prophylactic antiviral therapy to prevent the emergence of disease. Although antiviral antibodies can develop following acute infections, such responses are delayed for at least 3–8 weeks after exposure (called the 'window period'; defined as the time between exposure to infection and serological detection of antibodies. See below and Figure 1). Thus, in the immunocompromised host, serologic testing is often not useful for the diagnosis of acute infection. NAT usually involves hybridization of primers or probes to detect a specific sequence in circulating viral nucleic acids during active infection and uses various techniques such as PCR to amplify the generated signal. As a result, when performed properly, NAT sensitivity and specificity are generally high, but the very high sensitivity may produce false-positive results that may confound interpretation.
BK virus (BKV)-associated nephropathy (also called polyomavirus-associated nephropathy) is a common source of renal allograft dysfunction and loss after kidney transplantation.78,79 This syndrome results from BKV replication in the allograft. In a large series, the origin of BKV replication in kidney transplant recipients was investigated by analysing viral sequences of living matched donor–recipient pairs by quantitative molecular assays.80 In all 20 donor–recipient pairs, the sequence of the viral VP1 typing region was identical in the donor and the recipient after transplantation, consistent with donor derivation of viral infection, although other sources of infection might be considered. Importantly, BKV seroprevalence in the general population is too high to exclude seropositive donors from kidney donation. Most centres have adopted post-transplantation monitoring for BK viraemia using NAT assays; preventative strategies that are based on serologic testing might merit study.
Local epidemiology can dictate additional testing requirements. Serologic testing for endemic fungi (such as Histoplasma, Coccidioides and Paracoccidioides spp.) and parasites (such as Strongyloides stercoralis and T. cruzi) is used as a basis for monitoring or prophylaxis in the organ recipient. In lung donors, sputum cultures might provide evidence of fungal colonization (for example, by Aspergillus spp.) and suggest enhanced antifungal prophylaxis is needed in the recipient.
The quality of microbiological screening is dependent on national standards for clinical laboratories in terms of assay validation, proficiency testing, and the recording and communication of results.16 Laboratories that infrequently perform specific assays might lack the required competencies for donor screening. Interpretation of serologic test results must be considered in the context of the specific donor conditions. For example, intensive fluid resuscitation with crystalloid or colloid solutions might lead to haemodilution and thereby to false-negative tests, whereas resuscitation with blood products might lead to false-positive tests, thereby misleading clinical decision-making.81 In individuals who have undergone fluid resuscitation, NAT testing may be preferred to serology. However, NAT testing is not available in all regions given that a high financial investment is required to establish and maintain test proficiency and staffing for round-the-clock availability. Moreover, incorrect data recording, and miscommunication or delayed test results have resulted in transmission of donor-derived infection2,3 as well as unnecessary organ disposal.1,2,82 These factors highlight the need for more accurate and faster assays, and the rapid and complete evaluation and communication of data.
Increased-risk donors
In 2013, the US Public Health Service Guidelines were developed to establish an evidence-based approach to the screening of organ donors with potential for carrying an increased risk for the transmission of infection with HIV, HCV and HBV to transplant recipients.3 These new guidelines also evaluated the limited evidence regarding the optimal deployment of NAT in donor screening for these viruses. Previous guidelines established epidemiologic risk factors for HIV on the basis of the medical and social histories of the donor.83 The updated guidelines are described in Box 3.3,84,85 Potential donors are screened for social risk factors (such as injection drug abuse, sexual promiscuity, incarceration or sexual contacts between men) that may increase the risk of donor-derived infection with HBV, HCV or HIV. The guidelines recommend universal testing of deceased and living organ and vessel conduit (spare vessel segments) donors for HBV, HCV and HIV. Routine testing of donors not known to carry an increased risk (Box 3) also includes use of more sensitive assays for HIV and HCV, such as NAT, which reduce the likelihood of infection transmission compared with serologic testing that could miss active infection in the window period (Figure 1).84,85 The guideline recommends “a robust informed consent discussion between the transplant candidate (or medical decision maker) and the clinician” so that the recipient understands the potential risks and benefits associated with accepting or rejecting individual organs including those from “PHS [public health service] increased risk of disease transmission” or virus-positive organ donors.3 Microbiological follow-up of such recipients is mandatory; storage of serum and cells from such donor–recipient pairs would be useful if recipients develop infectious syndromes or if improved assays (or new pathogens) are identified in the future.
The development of guidelines, as well as clinical experience with the transmission of HIV, HCV and HBV in transplantation, have been instructive in respect to the development of screening strategies. The lack of prospective data on the risk of disease transmission hampers discussions of risk both in terms of the required sensitivity of the assays deployed for screening and in terms of discussions with prospective recipients. Routine use of NAT in donor screening requires development of consensus around the optimal assays, approaches to assay validation and proficiency maintenance, education regarding data evaluation, and improved mechanisms for the rapid communication of laboratory data to clinical centres and organ procurement organizations. Development of effective antiviral therapies for HCV and HIV infections will alter discussions regarding the risk of transmission of infection in critically ill individuals with organ failure. These observations contrast with the lack of transmission data and lack of routine, validated and rapid diagnostic tools, and effective therapies for uncommon infections such as WNV or LCMV.
Box 3: Criteria for organ donors with an increased risk of infection*.
• People who have had sex with a person known or suspected to have HIV, HBV or HCV infection in the past 12 months
• Men who have had sex with men in the preceding 12 months
• Women who have had sex with a man with a history of sex with men in the preceding 12 months
• People who have had sex in exchange for money or drugs in the preceding 12 months
• People who have had sex with a person who had sex in exchange for money or drugs in the preceding 12 months
• People who have had sex with a person who injected drugs by intravenous, intramuscular or subcutaneous route for nonmedical reasons in the preceding 12 months
• A child who is less than 18 months of age and born to a mother known to be infected with, or at an increased risk of, HIV, HBV or HCV infection
• A child who has been breastfed within the preceding 12 months by a mother who is known to be infected with, or at an increased risk of, HIV infection
• People who have injected drugs by intravenous, intramuscular or subcutaneous routes for nonmedical reasons in the preceding 12 months
• People who have been in lockup, jail, prison or a juvenile correctional facility for more than 72 consecutive hours in the preceding 12 months
• People who have been newly diagnosed with, or have been treated for, syphilis, gonorrhoea, Chlamydia or genital ulcers in the preceding 12 months
• People who have been on haemodialysis in the preceding 12 months should be identified as being at increased risk of recent HCV infection only
*Criteria defined by the 2013 US Public Health Service Guidelines.3 Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus. Republished with permission of The Association of Schools and Programs of Public Health © United States Public Health Service. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Reports 128, 247–343 (2013); permission conveyed through Copyright Clearance Center, Inc.
The 'window period'
Serological testing for infections has been highly effective in donor screening. However, seroconversion requires the elaboration of antibodies against a specific pathogen and could be delayed for several weeks after infectious exposure. This window period is likely to result in a false-negative test result and could result in inadvertent transmission of infection (Figure 1).22,86,87,88,89,90,91,92,93,94,95,96,97 NAT assays detect viral nucleic acids directly or following signal amplification with greater sensitivity and specificity than does serologic testing. As a result, the window period for HIV detection using NAT is reduced from 22–180 days (by using antibody screens) to 5.6–10.2 days—a minimum 12 day reduction in potential recipient exposure using NAT instead of serology (Figure 1). Similar reductions in the window period are observed for HCV and HBV detection (Table 1).86,87,88,89,90,91,92,93,94,95,96,97,98 The 'residual risk' of infectious transmission in the face of false-negative serologic and NAT testing is estimated to be low for HIV and HCV (Table 2). However, in outbreaks of WNV, false-negative results were detected for both serologic and NAT assays, suggesting that dual testing is beneficial for some untreatable infections.
Table 1. Estimated 'window period' for viral testing*.
| Virus | Time to positive result on serology after infectious exposure (days) | Time to positive result on NAT after infectious exposure (days) | Approximate reduction in window period by NAT (days) |
|---|---|---|---|
| HIV | 22 (up to 180) | 5.6–10.2 | 12 |
| HCV | 38–94 | 6.1–8.7 | 30 |
| HBV | 38.3–49.7 | 20.4–25.7 | 12 |
*The window period for each assay depends on the specific assay performed.
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; NAT, nucleic acid test.
Table 2. Risk of HIV or HCV infection per 10,000 increased-risk donors84,85,97.
| Increased-risk donor criteria | HIV | HCV | ||
|---|---|---|---|---|
| Serology alone | Serology plus NAT | Serology alone | Serology plus NAT | |
| Men who have sex with men | 8.3 | 3.4 | 36.0 | 3.8 |
| Nonmedical intravenous, intramuscular or subcutaneous drug use | 12.9 | 5.3 | 350.0 | 37.8 |
| Individuals with haemophilia | 0.05 | 0.02 | 0.46 | 0.05 |
| Persons who have had sex in exchange for money or drugs | 2.9 | 1.2 | 107.8 | 11.5 |
| Partners with any of the above risk factors | 2.7 | 1.1 | 126.2 | 13.5 |
| Individuals who have been exposed to blood or blood products from a person with HIV or HCV | 1.3 | 0.5 | 22.0 | 2.3 |
| Incarcerated individuals | 1.5 | 0.6 | 68.6 | 7.3 |
Abbreviations: HCV, hepatitis C virus; NAT, nucleic acid test.
Clinical laboratories in screening
The quality of laboratory testing is an essential component in the safety paradigm for transplantation and is dependent on the assay performed, the quality of the specimen tested and laboratory techniques.1,2 The assay may lack the required sensitivity or specificity or may be improperly performed, the specimen may become degraded or contaminated in handling, or the data may be erroneously recorded or communicated. Internal quality controls for laboratory procedures are essential and the quality, performance characteristics and limitations of assays must be tracked by organ procurement organizations. The cost of the infrastructure required and the need to assure the maintenance of proficiency may dictate the use of regional or validated 'central testing laboratories' in organ donor screening. Assay data may not be comparable between laboratories that use different commercial tests or laboratory-developed (so-called 'home brew') reagents. New tests developed in the face of a changing epidemiology may lack validation in large clinical populations. False-positive and false-negative tests could occur as a result. Validation of new assays is difficult, as validation studies in deceased or living donor populations, and for rare pathogens (such as rabies and LCMV), are almost impossible to perform.
Introduction of a new assay into the screening panel requires consideration of whether the assay has been validated and can be performed in the relevant clinical laboratories. For example, even in endemic regions, introduction of serologic tests for hepatitis E has been problematic owing to sensitivity issues and difficulties in performing RNA—NAT reliably. Such assays may be used to allocate or discard organs. Tests for life-threatening or untreatable diseases require assays that optimize sensitivity. Thus, confirmatory tests and internal controls must be employed to detect false-positive or false-negative tests.
Box 2: Screening organ donors*.
Assays to assess risk of infection ‡
• Cytomegalovirus antibody serology
• EBV antibody panel (EBV viral capsid antigen ± early antigen and nuclear antigen antibody levels)
• Herpes simplex virus serology
• Varicella-zoster virus serology
• Syphilis assays§ (non-treponemal and treponemal testing [rapid plasma reagin plus Treponema pallidum haemagglutination assay, T. pallidum particle agglutination assay or fluorescent treponemal antibody assay])
• Toxoplasma serology (notably in cardiac donors)
Common donor exclusion criteria ||
• Presence of HIV antibody in serology and NAT
• Positive result on HBV serologies, including HBsAg, core antibody, surface antibody and hepatitis δ antigen and/or antibody in HBsAg-positive donors
• Presence of hepatitis C virus antibody in serology and NAT
• Presence of human T-cell lymphotropic virus 1 or 2 antibodies¶
• Untreated/positive blood cultures (all organs), untreated/positive urine cultures (kidney donors), or positive sputum cultures (lung donors)—organs may be used for infections not considered to pose a risk to the recipient
*Many procurement organizations supplement these tests with additional assays based on local epidemiology and/or use nucleic acid-based assays. These additional assays might include serological tests for Histoplasma and Coccidioides species and for Strongyloides and Trypanosoma cruzi. Donor microbiological data are reviewed. ‡Performed in both the donor and recipient §May be treated in donor and/or recipient. ||May not be applicable in appropriate recipients. ¶No longer required in the USA based on assay performance. Abbreviations: EBV, Epstein Barr virus; HBsAg, HBV surface antigen; HBV, hepatitis B virus; NAT, nucleic acid test.
Conclusions
A broad array of organisms may be transmitted with the living cells of transplanted organs. Donor screening has improved with the availability of sensitive microbiological assays that target common pathogens. The incidence of infectious diseases resulting from donor-derived infection is low, although exact data are lacking and some infections are probably not detected. Because immunocompromised recipients develop diseases of unusual severity with infections of low native virulence, they are sentinels for new or unusual infections in the community. This patient population has been especially affected by, for example, inadvertent transmission of HCV, WNV and Chagas disease in the absence of highly sensitive testing. The key element in the detection of donor-derived infection remains suspicion on the part of the clinicians who care for organ recipients. The presentations of donor-derived infections have often been obscure, including unexplained and culture-negative encephalitis, weakness, fever, thrombocytopenia, lymphocytosis, erythema of surgical incisions and graft dysfunction. Both organ procurement organizations and public-health authorities should be engaged in the evaluation of such incidents. Microbiological identification of donor-derived pathogens in transplant recipients is important to enable appropriate therapy for affected individuals, and to recognize outbreaks of infectious diseases in immunocompromised hosts before these pathogens affect the broader population.
Testing protocols must be adapted to the known or changing epidemiology of disease and incorporate new technologies for microbiological screening. Screening technologies may incorporate new high-throughput genetic sequencing for new sequences in circulation or multiplexed assays targeting many specific pathogens simultaneously in a single assay.99,100 Identification of potential pathogens after organ implantation also has important implications for the need for rapid communication of microbiological data or clinical suspicion between clinical laboratories, clinical centres, procurement organizations and public-health authorities. Prospective research is required to clarify the incidence of disease transmission and the optimal panels of microbiological assays for donor screening.
Improved coordination of information must be facilitated between public-health authorities, clinical centres, patients and tissue and organ procurement groups. Standards for investigation and reporting, terminology and data sharing of potential transmission events will enhance the safety of organ transplantation worldwide.
Review criteria
We obtained all papers that were used in the development of USPHS 2013 guidelines and original articles published between 1975 and 2014 and indexed in MEDLINE and PubMed based on the search terms “donor-derived”, “transmission”, “organ transplantation” and “infection”, alone and in combination. All articles identified were full-text papers. We also searched the reference lists of identified articles for further relevant papers.
Biographies
Jay A. Fishman is a Professor of Medicine at Harvard Medical School, Director of the Transplant Infectious Diseases and Compromised Host Program at the Massachusetts General Hospital (MGH), and Associate Director of the MGH Transplant Center. Dr. Fishman received his undergraduate degree (magna cum laude) from the University of Pennsylvania and his MD from the Johns Hopkins University School of Medicine. He completed residency training in Internal Medicine and Fellowships in Infectious Disease and Molecular Biology and Genetics at MGH and Harvard Medical School. Dr. Fishman is an internationally recognized expert in infectious diseases in individuals with abnormal immune systems including organ and stem-cell-transplant recipients and has trained many of the international leaders in this field. At the MGH, he directs a leading training program in the infectious diseases of organ and stem-cell-transplant recipients. His research laboratory is investigating infections related to the development of swine as organ donors for humans (xenotransplantation) and the molecular biology of viruses in transplantation. He has a special interest in molecular diagnostics and biotechnology, transplant virology, and in medical education. He has held leadership roles in multiple not-for-profit organizations including service as President of the American Society of Transplantation and at the international Transplantation Society (Transplant Infectious Disease and International Xenotransplanation Sections), and the Board of Directors of the International Immunocompromised Host Society. He is a frequent contributor at national and international symposia. He has received career achievement awards from both the American Society of Transplantation and the international Transplantation Society. He is the 2014 recipient of the Senior Achievement Award in Clinical Transplantation of the American Society of Transplantation.
Paolo A. Grossi is Professor of Infectious Diseases at the University of Insubria in Varese, Italy. He received his medical degree from the University of Milano, Italy. He attended the residency programme on Infectious Diseases at the University of Pavia, Italy, where he also received his post-doctoral degree on Preventive and Community Medicine. Since the beginning of his career, he has performed clinical and research activities at the Infectious Diseases Department of the University of Pavia. In 1993, he became a visiting professor at the University of Pittsburgh, Pennsylvania, where he remained until the end of 1994. Since 2000, he has been Professor of Infectious Diseases and Director of the residency programmes in Infectious Diseases and in Hygiene and Preventive Medicine at the University of Insubria, Varese, Italy. Since February 2001, he has been Director of the Infectious and Tropical Diseases Department of the Ospedale di Circolo and Fondazione Macchi of Varese, Italy, where, starting in April 2009, he was Director of the Department of Transplantation. Furthermore, since 1999, he has been the advisor for all infectious-diseases-related problems at the Italian National Centre for Transplantation in Italy and has covered the role of 'second opinion' for all organ donors with potentially transmissible infectious diseases. Since 2001, he has been a member of the Steering Committee of the programme of liver, kidney, kidney–pancreas, lung and heart transplantation in HIV-positive individuals in Italy. He is a member of the steering committee of the ESCMID Study Group on infections in Immunocompromised Hosts (ESGICH). In 2005, he joined the ISMETT/UPMC, a centre for organ transplantation located in Palerma Italy, within a research agreement with the University of Insubria. In April 2013, he was elected co-Chair of the ID Council of the International Society of Heart and Lung Transplantation. In July 2013, he was nominated member of the WHO International Health Regulations Roster of Experts, as an expert in Essential Health Technologies (Transplantation Services).
PowerPoint slides
Author Contributions
Both authors researched the data, wrote the article, contributed to discussions of the content and edited and/or reviewed the manuscript before submission.
Competing interests
J.A.F. has served as an expert reviewer for the United States Public Health Service Guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Reports 128, 247–343 (2013). P.A.G. declares no competing interests.
References
- 1.Fishman JA, Greenwald MA, Grossi PA. Transmission of infection with human allografts: essential considerations in donor screening. Clin. Infect. Dis. 2012;55:720–727. doi: 10.1093/cid/cis519. [DOI] [PubMed] [Google Scholar]
- 2.Grossi PA, Fishman JA. Donor-derived infections in solid organ transplant recipients. Am. J. Transplant. 2009;9(Suppl. 4):S19–S26. doi: 10.1111/j.1600-6143.2009.02889.x. [DOI] [PubMed] [Google Scholar]
- 3.United States Public Health Service. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep.128, 247–343 (2013). [DOI] [PMC free article] [PubMed]
- 4.Iwamoto M. Transmission of West Nile virus from an organ donor to four transplant recipients. N. Engl. J. Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Davila P. Transmission of tropical and geographically restricted infections during solid-organ transplantation. Clin. Microbiol. Rev. 2008;21:60–96. doi: 10.1128/CMR.00021-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanni Costa A, Grossi P, Gianelli Castiglione A, Grigioni WF. Quality and safety in the Italian donor evaluation process. Transplantation. 2008;85:S52–S56. doi: 10.1097/TP.0b013e31816c2f05. [DOI] [PubMed] [Google Scholar]
- 7.Kumar D. A seroprevalence study of West Nile virus infection in solid organ transplant recipients. Am. J. Transplant. 2004;4:1883–1888. doi: 10.1111/j.1600-6143.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 8.Ison MG. Donor-derived disease transmission events in the United States: data reviewed by the OPTN/UNOS Disease Transmission Advisory Committee. Am. J. Transplant. 2009;9:1929–1935. doi: 10.1111/j.1600-6143.2009.02700.x. [DOI] [PubMed] [Google Scholar]
- 9.Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. Am. J. Transplant. 2011;11:1123–1130. doi: 10.1111/j.1600-6143.2011.03493.x. [DOI] [PubMed] [Google Scholar]
- 10.Fishman JA. Infection in solid-organ transplant recipients. N. Engl. J. Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 11.Davis JA, Horn DL, Marr KA, Fishman JA. Central nervous system involvement in cryptococcal infection in individuals after solid organ transplantation or with AIDS. Transpl. Infect. Dis. 2009;11:432–437. doi: 10.1111/j.1399-3062.2009.00424.x. [DOI] [PubMed] [Google Scholar]
- 12.Fishman JA, Greenwald MA, Kuehnert MJ. Enhancing transplant safety: a new era in the microbiologic evaluation of organ donors? Am. J. Transplant. 2007;7:2652–2654. doi: 10.1111/j.1600-6143.2007.02023.x. [DOI] [PubMed] [Google Scholar]
- 13.Delmonico FL. Cadaver donor screening for infectious agents in solid organ transplantation. Clin. Infect. Dis. 2000;31:781–786. doi: 10.1086/314000. [DOI] [PubMed] [Google Scholar]
- 14.Delmonico FL, Snydman DR. Organ donor screening for infectious diseases: review of practice and implications for transplantation. Transplantation. 1998;65:603–610. doi: 10.1097/00007890-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Fischer SA, Avery RK. Screening of donor and recipient prior to solid organ transplantation. Am. J. Transplant. 2009;9(Suppl. 4):S7–S18. doi: 10.1111/j.1600-6143.2009.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fishman JA. Rapporto dell'OMS sulla valutazione del programma nazionale trapianti in Italia [Italian] Trapianti. 2008;12:37–53. [Google Scholar]
- 17.Kiberd BA, Forward K. Screening for West Nile virus in organ transplantation: a medical decision analysis. Am. J. Transplant. 2004;4:1296–1301. doi: 10.1111/j.1600-6143.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 18.Palacios G. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008;358:991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- 19.Wilck M, Fishman JA. The challenges of infection in transplantation: donor-derived infections. Curr. Opin. Organ. Transplant. 2005;10:301–306. doi: 10.1097/01.mot.0000183245.66967.b3. [DOI] [Google Scholar]
- 20.Schwartz BS, Paster M, Ison MG, Chin-Hong PV. Organ donor screening practices for Trypanosoma cruzi infection among US organ procurement organizations. Am. J. Transplant. 2011;11:848–851. doi: 10.1111/j.1600-6143.2011.03436.x. [DOI] [PubMed] [Google Scholar]
- 21.Chin-Hong PV. Screening and treatment of Chagas disease in organ transplant recipients in the United States: recommendations from the Chagas in transplant working group. Am. J. Transplant. 2011;11:672–680. doi: 10.1111/j.1600-6143.2011.03444.x. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. HIV Transmitted from a living organ donor---New York City, 2009. MMWR Morb. Mortal. Wkly Rep.60, 297–301 (2011). [PubMed]
- 23.Ison MG. Transmission of human immunodeficiency virus and hepatitis C virus from an organ donor to four transplant recipients. Am. J. Transplant. 2011;11:1218–1225. doi: 10.1111/j.1600-6143.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 24.Ariza-Heredia EJ. Outcomes of transplantation using organs from a donor infected with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae. Transpl. Infect. Dis. 2012;14:229–236. doi: 10.1111/j.1399-3062.2012.00742.x. [DOI] [PubMed] [Google Scholar]
- 25.van Delden C, Blumberg EA. Multidrug resistant gram-negative bacteria in solid organ transplant recipients. Am. J. Transplant. 2009;9(Suppl. 4):S27–S34. doi: 10.1111/j.1600-6143.2009.02890.x. [DOI] [PubMed] [Google Scholar]
- 26.van Duin D, van Delden C. Multidrug-resistant gram-negative bacteria infections in solid organ transplantation. Am. J. Transplant. 2013;13(Suppl. 4):31–41. doi: 10.1111/ajt.12096. [DOI] [PubMed] [Google Scholar]
- 27.Lubbert C. Colonization of liver transplant recipients with KPC-producing Klebsiella pneumoniae is associated with high infection rates and excess mortality: a case-control analysis. Infection. 2014;42:309–316. doi: 10.1007/s15010-013-0547-3. [DOI] [PubMed] [Google Scholar]
- 28.Bergamasco MD. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl. Infect. Dis. 2012;14:198–205. doi: 10.1111/j.1399-3062.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Control. Carbapenemase-Producing Bacteria in Europe: Interim Results from the European Survey on Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Project 2013[online], (2013)
- 30.Wu TJ, Lee CF, Chou HS, Yu MC, Lee WC. Suspect the donor with potential infection in the adult deceased donor liver transplantation. Transplant. Proc. 2008;40:2486–2488. doi: 10.1016/j.transproceed.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Martins N. Severe infection in a lung transplant recipient caused by donor-transmitted carbapenem-resistant Acinetobacter baumannii. Transpl. Infect. Dis. 2012;14:316–320. doi: 10.1111/j.1399-3062.2011.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh N. Impact of donor bacteremia on outcome in organ transplant recipients. Liver Transpl. 2002;8:975–976. doi: 10.1053/jlts.2002.0080975. [DOI] [PubMed] [Google Scholar]
- 33.Freeman RB. Outcome of transplantation of organs procured from bacteremic donors. Transplantation. 1999;68:1107–1111. doi: 10.1097/00007890-199910270-00008. [DOI] [PubMed] [Google Scholar]
- 34.D'Albuquerque LA. Liver transplantation from deceased donors serologically positive for Chagas disease. Am. J. Transplant. 2007;7:680–684. doi: 10.1111/j.1600-6143.2007.01663.x. [DOI] [PubMed] [Google Scholar]
- 35.Lumbreras C. Clinical significance of donor-unrecognized bacteremia in the outcome of solid-organ transplant recipients. Clin. Infect. Dis. 2001;33:722–726. doi: 10.1086/322599. [DOI] [PubMed] [Google Scholar]
- 36.Satoi S. The use of liver grafts from donors with bacterial meningitis. Transplantation. 2001;72:1108–1113. doi: 10.1097/00007890-200109270-00022. [DOI] [PubMed] [Google Scholar]
- 37.Canales MT, Kasiske BL, Rosenberg ME. Transplant tourism: outcomes of United States residents who undergo kidney transplantation overseas. Transplantation. 2006;82:1658–1661. doi: 10.1097/01.tp.0000250763.52186.df. [DOI] [PubMed] [Google Scholar]
- 38.Gill J. Transplant tourism in the United States: a single-center experience. Clin. J. Am. Soc. Nephrol. 2008;3:1820–1828. doi: 10.2215/CJN.02180508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad GV, Shukla A, Huang M, D'A Honey RJ, Zaltzman JS. Outcomes of commercial renal transplantation: a Canadian experience. Transplantation. 2006;82:1130–1135. doi: 10.1097/01.tp.0000241072.03400.11. [DOI] [PubMed] [Google Scholar]
- 40.Sever MS. Outcome of living unrelated (commercial) renal transplantation. Kidney Int. 2001;60:1477–1483. doi: 10.1046/j.1523-1755.2001.00951.x. [DOI] [PubMed] [Google Scholar]
- 41.Dortet L, Poirel L, Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in gram-negative bacteria. Biomed. Res. Int. 2014;2014:249856. doi: 10.1155/2014/249856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yong D. Characterization of a new metallo-β-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skagen CL, Jou JH, Said A. Risk of de novo hepatitis in liver recipients from hepatitis-B core antibody-positive grafts—a systematic analysis. Clin. Transplant. 2011;25:E243–E249. doi: 10.1111/j.1399-0012.2011.01409.x. [DOI] [PubMed] [Google Scholar]
- 44.Perrillo R. Hepatitis B virus prevention strategies for antibody to hepatitis B core antigen-positive liver donation: a survey of North American, European, and Asian-Pacific transplant programs. Liver Transpl. 2009;15:223–232. doi: 10.1002/lt.21675. [DOI] [PubMed] [Google Scholar]
- 45.Chang MS. Prevention of de novo hepatitis B in recipients of core antibody-positive livers with lamivudine and other nucleos(t)ides: a 12-year experience. Transplantation. 2013;95:960–965. doi: 10.1097/TP.0b013e3182845f97. [DOI] [PubMed] [Google Scholar]
- 46.Wright AJ, Fishman JA, Chung RT. Lamivudine compared with newer antivirals for prophylaxis of hepatitis B core antibody positive livers: a cost-effectiveness analysis. Am. J.Transplant. 2014;14:629–634. doi: 10.1111/ajt.12598. [DOI] [PubMed] [Google Scholar]
- 47.Mawhorter SD, Avery RK. Can donors with prior hepatitis be safely used for heart transplantation? J. Heart Lung Transplant. 2006;25:805–813. doi: 10.1016/j.healun.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Haji SA. Donor or recipient hepatitis B seropositivity is associated with allograft vasculopathy. J. Heart Lung Transplant. 2006;25:294–297. doi: 10.1016/j.healun.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Ouseph R. Review of the use of hepatitis B core antibody-positive kidney donors. Transplant. Rev. (Orlando) 2010;24:167–171. doi: 10.1016/j.trre.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Pilmore HL, Gane EJ. Hepatitis B-positive donors in renal transplantation: increasing the deceased donor pool. Transplantation. 2012;94:205–210. doi: 10.1097/TP.0b013e31824e3db4. [DOI] [PubMed] [Google Scholar]
- 51.Mahboobi N, Tabatabaei SV, Blum HE, Alavian SM. Renal grafts from anti-hepatitis B core-positive donors: a quantitative review of the literature. Transpl. Infect. Dis. 2012;14:445–451. doi: 10.1111/j.1399-3062.2012.00782.x. [DOI] [PubMed] [Google Scholar]
- 52.Fischer SA. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 2006;354:2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- 53.Fishman JA, Strong DM, Kuehnert MJ. Organ and tissue safety workshop 2007: advances and challenges. Cell Tissue Bank. 2009;10:271–280. doi: 10.1007/s10561-008-9114-z. [DOI] [PubMed] [Google Scholar]
- 54.Grossi P, Strong DM. Tissue and Cell Donation: an Essential Guide. 2009. [Google Scholar]
- 55.Avery RK. Recipient screening prior to solid-organ transplantation. Clin. Infect. Dis. 2002;35:1513–1519. doi: 10.1086/344777. [DOI] [PubMed] [Google Scholar]
- 56.Pietrosi G. Primary and reactivated HHV8 infection and disease after liver transplantation: a prospective study. Am. J.Transplant. 2011;11:2715–2723. doi: 10.1111/j.1600-6143.2011.03769.x. [DOI] [PubMed] [Google Scholar]
- 57.Kamar N. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 58.Schlosser B. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J. Hepatol. 2012;56:500–502. doi: 10.1016/j.jhep.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 59.Garzoni C, Ison MG. Uniform definitions for donor-derived infectious disease transmissions in solid organ transplantation. Transplantation. 2011;92:1297–1300. doi: 10.1097/TP.0b013e318236cd02. [DOI] [PubMed] [Google Scholar]
- 60.Parry J. Taiwan transplant team blamed for HIV positive organ blunder. BMJ. 2011;343:d6523. doi: 10.1136/bmj.d6523. [DOI] [PubMed] [Google Scholar]
- 61.Villa E, Nanni Costa A. HIV-positive organs used for transplant in Italy due to human error. Euro. Surveill. 2007;12:E0703081. doi: 10.2807/esw.12.10.03150-en. [DOI] [PubMed] [Google Scholar]
- 62.Toro C, Rodes B, Poveda E, Soriano V. Rapid development of subacute myelopathy in three organ transplant recipients after transmission of human T-cell lymphotropic virus type I from a single donor. Transplantation. 2003;75:102–104. doi: 10.1097/00007890-200301150-00019. [DOI] [PubMed] [Google Scholar]
- 63.Zarranz JJ, Rouco I, Gomez-Esteban JC, Corral J. Human T lymphotropic virus type I (HTLV-1) associated myelopathy acquired through a liver transplant. J. Neurol. Neurosurg. Psychiatry. 2001;71:818. doi: 10.1136/jnnp.71.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaul DR. Donor screening for human T-cell lymphotrophic virus 1/2: changing paradigms for changing testing capacity. Am. J. Transplant. 2010;10:207–213. doi: 10.1111/j.1600-6143.2009.02867.x. [DOI] [PubMed] [Google Scholar]
- 65.Kusne S, Smilack J. Transmission of West Nile virus by organ transplantation. Liver Transpl. 2005;11:239–241. doi: 10.1002/lt.20350. [DOI] [PubMed] [Google Scholar]
- 66.Singh N, Levi ME. Arenavirus and West Nile virus in solid organ transplantation. Am. J. Transplant. 2013;13(Suppl. 4):361–371. doi: 10.1111/ajt.12128. [DOI] [PubMed] [Google Scholar]
- 67.Komar N. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murray K. Persistent infection with West Nile virus years after initial infection. J. Infect. Dis. 2010;201:2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tonry JH. West Nile virus detection in urine. Emerg. Infect. Dis. 2005;11:1294–1296. doi: 10.3201/eid1108.050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barzon L. Excretion of West Nile virus in urine during acute infection. J. Infect. Dis. 2013;208:1086–1092. doi: 10.1093/infdis/jit290. [DOI] [PubMed] [Google Scholar]
- 71.Bronnert J, Wilde H, Tepsumethanon V, Lumlertdacha B, Hemachudha T. Organ transplantations and rabies transmission. J. Travel Med. 2007;14:177–180. doi: 10.1111/j.1708-8305.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- 72.Srinivasan A. Transmission of rabies virus from an organ donor to four transplant recipients. N. Engl. J. Med. 2005;352:1103–1111. doi: 10.1056/NEJMoa043018. [DOI] [PubMed] [Google Scholar]
- 73.Vora NM. Raccoon rabies virus variant transmission through solid organ transplantation. JAMA. 2013;310:398–407. doi: 10.1001/jama.2013.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention. Notes from the field: transplant-transmitted Balamuthia mandrillaris—Arizona, 2010. MMWR Morb. Mortal. Wkly Rep.59, 1182 (2010). [PubMed]
- 75.Centers for Disease Control and Prevention. Balamuthia mandrillaris transmitted through organ transplantation—Mississippi, 2009. Morbidity and Mortality Weekly Report (MMWR).59, 1165–1170 (2010). [PubMed]
- 76.Schuster FL. Under the radar: Balamuthia amebic encephalitis. Clin. Infect. Dis. 2009;48:879–887. doi: 10.1086/597260. [DOI] [PubMed] [Google Scholar]
- 77.Huang RC, Fishman JA. Screening of deceased organ donors: no easy answers. Transplantation. 2011;91:146–149. doi: 10.1097/TP.0b013e3181ffb9bb. [DOI] [PubMed] [Google Scholar]
- 78.Hirsch, H. H. et al. European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin. Microbiol. Infect.10.1111/1469-0691.12538. [DOI] [PubMed]
- 79.Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am. J. Transplant. 2013;13(Suppl. 4):179–188. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 80.Schmitt C. Donor origin of BKV replication after kidney transplantation. J. Clin. Virol. 2014;59:120–125. doi: 10.1016/j.jcv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Eastlund T. Hemodilution due to blood loss and transfusion and reliability of cadaver tissue donor infectious disease testing. Cell Tissue Bank. 2000;1:121–127. doi: 10.1023/A:1010120115451. [DOI] [PubMed] [Google Scholar]
- 82.Humar A. Nucleic acid testing (NAT) of organ donors: is the 'best' test the right test? A consensus conference report. Am. J. Transplant. 2010;10:889–899. doi: 10.1111/j.1600-6143.2009.02992.x. [DOI] [PubMed] [Google Scholar]
- 83.US Public Health Service. PHS guideline for preventing transmission of HIV through transplantation of human tissue and organs. MMWR Morb. Mortal. Wkly Rep.43, 1–17 (1994).
- 84.Kucirka LM. Risk of window period hepatitis-C infection in high infectious risk donors: systematic review and meta-analysis. Am. J. Transplant. 2011;11:1188–1200. doi: 10.1111/j.1600-6143.2011.03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kucirka LM. Risk of window period HIV infection in high infectious risk donors: systematic review and meta-analysis. Am. J. Transplant. 2011;11:1176–1187. doi: 10.1111/j.1600-6143.2010.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiebig EW. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 87.Kolk DP. Significant closure of the human immunodeficiency virus type 1 and hepatitis C virus preseroconversion detection windows with a transcription-mediated-amplification-driven assay. J. Clin. Microbiol. 2002;40:1761–1766. doi: 10.1128/JCM.40.5.1761-1766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao F. The risk of HIV, HBV, HCV and HTLV infection among musculoskeletal tissue donors in Australia. Am. J. Transplant. 2007;7:2723–2726. doi: 10.1111/j.1600-6143.2007.02012.x. [DOI] [PubMed] [Google Scholar]
- 89.Yao F. Comparison of the risk of viral infection between the living and nonliving musculoskeletal tissue donors in Australia. Transplant Int. 2008;21:936–941. doi: 10.1111/j.1432-2277.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 90.Biswas R. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion. 2003;43:788–798. doi: 10.1046/j.1537-2995.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 91.Busch MP. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion. 2005;45:254–264. doi: 10.1111/j.1537-2995.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- 92.Busch MP. Time course of detection of viral and serologic markers preceding human immunodeficiency virus type 1 seroconversion: implications for screening of blood and tissue donors. Transfusion. 1995;35:91–97. doi: 10.1046/j.1537-2995.1995.35295125745.x. [DOI] [PubMed] [Google Scholar]
- 93.Kleinman SH, Busch MP. Assessing the impact of HBV NAT on window period reduction and residual risk. J. Clin. Virol. 2006;36(Suppl. 1):S23–S29. doi: 10.1016/S1386-6532(06)80005-3. [DOI] [PubMed] [Google Scholar]
- 94.Kleinman SH. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: implications for transfusion transmission and donor screening. Transfusion. 2003;43:696–704. doi: 10.1046/j.1537-2995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 95.Pillonel J, Saura C, Courouce AM. Prevalence of HIV, HTLV, and hepatitis B and C viruses in blood donors in France, 1992–1996 [French] Transfus. Clin. Biol. 1998;5:305–312. doi: 10.1016/S1246-7820(98)85001-5. [DOI] [PubMed] [Google Scholar]
- 96.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N. Engl. J. Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 97.Ellingson K, Seem D, Nowicki M, Strong DM, Kuehnert MJ. Estimated risk of human immunodeficiency virus and hepatitis C virus infection among potential organ donors from 17 organ procurement organizations in the United States. Am. J. Transplant. 2011;11:1201–1208. doi: 10.1111/j.1600-6143.2011.03518.x. [DOI] [PubMed] [Google Scholar]
- 98.Zou S, Dodd RY, Stramer SL, Strong DM. Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N. Engl. J. Med. 2004;351:751–759. doi: 10.1056/NEJMoa032510. [DOI] [PubMed] [Google Scholar]
- 99.Cheval J. Evaluation of high-throughput sequencing for identifying known and unknown viruses in biological samples. J. Clin. Microbiol. 2011;49:3268–3275. doi: 10.1128/JCM.00850-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dunbar SA. Applications of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin. Chim. Acta. 2006;363:71–82. doi: 10.1016/j.cccn.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


