ABSTRACT
Cells and tissues sense, respond to and translate mechanical forces into biochemical signals through mechanotransduction, which governs individual cell responses that drive gene expression, metabolic pathways and cell motility, and determines how cells work together in tissues. Mechanotransduction often depends on cytoskeletal networks and their attachment sites that physically couple cells to each other and to the extracellular matrix. One way that cells associate with each other is through Ca2+-dependent adhesion molecules called cadherins, which mediate cell–cell interactions through adherens junctions, thereby anchoring and organizing the cortical actin cytoskeleton. This actin-based network confers dynamic properties to cell sheets and developing organisms. However, these contractile networks do not work alone but in concert with other cytoarchitectural elements, including a diverse network of intermediate filaments. This Review takes a close look at the intermediate filament network and its associated intercellular junctions, desmosomes. We provide evidence that this system not only ensures tissue integrity, but also cooperates with other networks to create more complex tissues with emerging properties in sensing and responding to increasingly stressful environments. We will also draw attention to how defects in intermediate filament and desmosome networks result in both chronic and acquired diseases.
KEY WORDS: Mechanotransduction, Cadherin, Cytoskeleton, Cell–cell adhesion, Desmosome, Intermediate filaments
Summary: A review of the roles of intermediate filaments and desmosomes in mechanobiology, highlighting their integration with other cytoskeletal and adhesive systems.
Introduction
Cells and tissues are primarily regulated by two main types of stimuli: chemical and physical. The advent of modern molecular biology has accelerated our understanding of biochemical influences over biology, but these signals are insufficient to explain the complexity of life. More recently, it has become increasingly apparent how pervasive the effects of physical signals are in essentially all aspects of cell and tissue biology. For example, mechanical forces play fundamental roles in cell and tissue growth, differentiation, morphogenesis, tissue repair and even in disease states, including cancer, pulmonary disease, muscular dystrophy and cardiomyopathies (Mammoto et al., 2013; Jaalouk and Lammerding, 2009; Barnes et al., 2017; Lampi and Reinhart-King, 2018).
Cells and tissues both sense and respond to mechanical forces through a process called mechanotransduction, whereby mechanical forces are translated into biochemical signals. This process depends on cytoskeletal networks that physically couple cells to their extracellular environment and to other cells within tissues through adhesive junctions. The cytoskeleton of animal cells comprises three main filamentous networks: filamentous actin (F-actin), microtubules and intermediate filaments (IFs) (Fig. 1A). These cytoskeletal networks are in continuous communication through physical linkages and signaling crosstalk, cooperating to regulate cell behaviors, such as cell migration and division, as well as governing cell mechanics (Fig. 1B) (Chang and Goldman, 2004; Huber et al., 2015). Furthermore, mechanical forces are sensed and transmitted by adhesive complexes that form links to either the extracellular environment or other cells, and intracellularly interact with the cytoskeleton (Schwartz and DeSimone, 2008; De Pascalis et al., 2018). Linkage of IFs and F-actin to the extracellular substrate is provided by hemidesmosomes and focal adhesions, while linkage of IFs and F-actin at sites of cell–cell contact is provided by desmosomes and adherens junctions (Fig. 2), respectively.
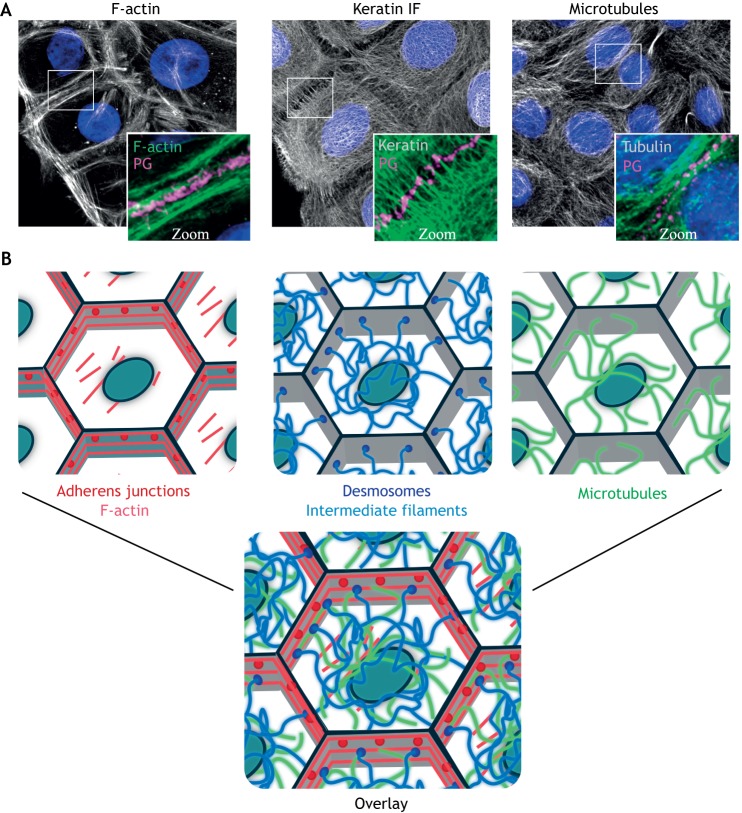
Fig. 1.
Architecture of the three main cytoskeletal systems. (A) Immunofluorescence staining shows the organization of the F-actin, keratin IF and microtubule cytoskeletons in human epidermal keratinocytes. Plakoglobin (PG) is used to show regions of cell–cell contact and nuclei are shown in blue with DAPI. (B) Schematic representations of the indicated filamentous cytoskeletons and associated cell–cell adhesive complexes, as well as an overlay to illustrate their interconnected nature. Gray outlines represent cell–cell junctional areas and ovals represent nuclei. Images supplied by the laboratory of K.J.G.
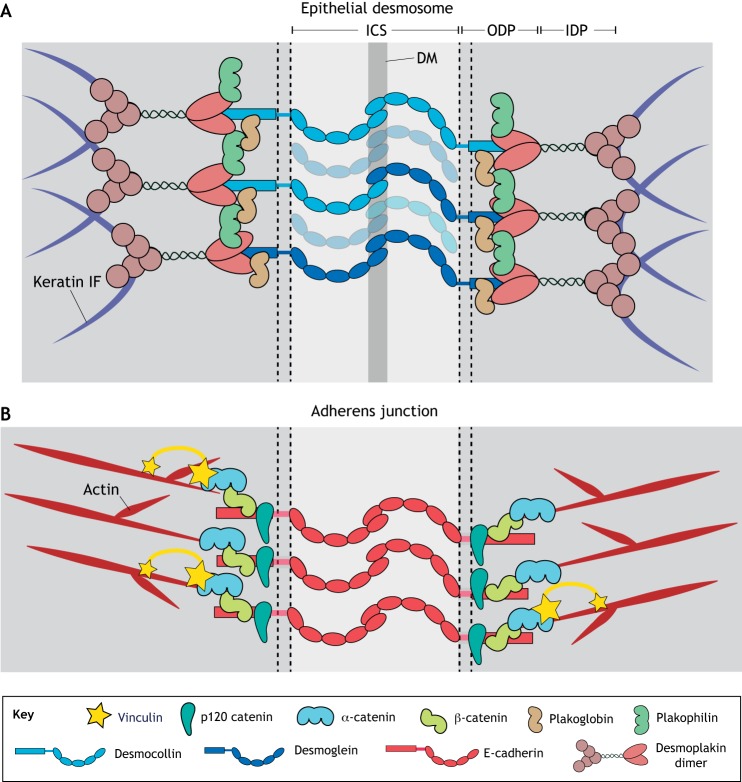
Fig. 2.
Major desmosome and adherens junction components. (A) Transmembrane desmosomal cadherins form extracellular interactions between adjacent cells. The cytoplasmic domains of these cadherins bind to the armadillo proteins plakophilin and plakoglobin and together bind desmoplakin, which anchors the desmosome to the IF cytoskeleton. (B) Adherens junctions contain classical transmembrane cadherins that facilitate cell–cell interactions owing to interactions between their extracellular domains. Intracellularly, classical cadherins interact with the armadillo protein β-catenin and catenin family proteins, including p120 catenin and α-catenin. Linkage to the actin cytoskeleton is mediated through α-catenin, as well as vinculin, which is recruited to the junction under tension. ICS, intercellular space; IDP, inner dense plaque; ODP, outer dense plaque; DM, dense midline.
While defining mechanical properties of individual IFs has been a research focus for decades, less is known about how IFs and their plasma membrane connections are integrated with other cytoskeletal elements to control cell and tissue mechanics and signaling. Here, we provide a summary of our current understanding of the mechanobiology of the IF-based adhesive network, with a focus on discussing the emerging functions of desmosomes and their integration with other cytoskeleton–plasma membrane networks. We highlight evidence that, like IFs, desmosomes not only play a role in tissue integrity but actively contribute to cellular mechanotransduction pathways.
IFs regulate cell mechanics and are mechanosensitive
IFs were the last of the three major cytoskeletal elements confirmed as distinct entities (Lazarides, 1980; Oshima, 2007). They are vital to the integrity of tissues that require mechanical resilience, such as muscle and stratified epithelia (Vassar et al., 1991; Galou et al., 1997). Loss of or aberrant gain of IF function is linked to a wide array of mechanically associated human diseases, including myopathies, skin blistering and fragility, and neurogenerative disorders (Lane, 2006; Omary et al., 2004). Thus, IFs are prime candidates to play roles in mechanobiology and transduction, but how they perform these roles in an integrated fashion with the other cytoskeletal components is poorly understood.
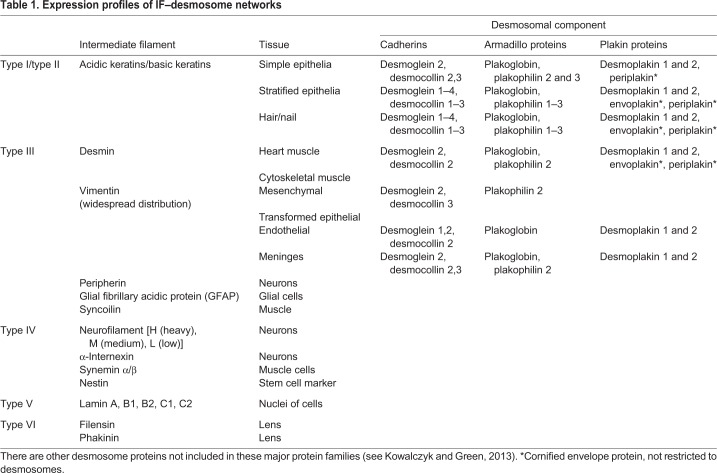
There are 70 genes encoding IF proteins (Herrmann et al., 2009), some with multiple splice forms (Hol et al., 2003). There are six main groups, or ‘types’, of IF, based on sequence similarity, and these are expressed in tissue-specific patterns, namely, type I/II keratins, type III vimentin/desmin, type IV neurofilaments, type V nuclear lamins and type VI lens filaments (Table 1). This Review will focus on cytoplasmic IFs.
Table 1.
Expression profiles of IF–desmosome networks
Cytoplasmic IFs play an important role in protecting cells from stress, and mutations in IF proteins are associated with human diseases that manifest downstream of multiple types of stress (Toivola et al., 2010). An example is epidermolysis bullosa simplex (EBS), a blistering disease caused by mutated keratin 5 or 14 (Coulombe et al., 1991; Russell et al., 2004; Stephens et al., 1995). One type of stress that can induce blistering is mechanical force. Stretching cells expressing EBS mutant keratins has been reported to induce IF network fragmentation and disassembly of their anchoring adhesive structures (Russell et al., 2004). In this case, the morphology of IF networks comprising EBS mutant keratin is similar to controls prior to stretch (Russell et al., 2004), suggesting that force can act as a mechanotrigger for disease progression. However, EBS keratin IF fragmentation has also been reported to occur in the absence of external mechanical triggers (Kitajima et al., 1989). In lung alveolar epithelial cells, keratin IFs disassemble in response to shear stress generated by continuous liquid laminar flow, through protein kinase C-mediated phosphorylation of keratin 8 (Ridge et al., 2005). In contrast, stretching does not affect keratin phosphorylation, suggesting various types of force affect IF organization differently.
In addition to their protective role, cytoplasmic IFs, along with F-actin and microtubules, are involved in cellular responses to force. For example, cells respond to applied cyclical stretching by reorienting their cell body axis approximately perpendicular to the applied load (Buck, 1980). Reorientation depends on the magnitude of the applied stretch, with higher magnitudes inducing further reorientation (Wang et al., 1995; Takemasa et al., 1997). Interestingly, cytoskeletal reorganization precedes cell body reorientation, suggesting this is an active adaptation mechanism (Zielinski et al., 2018). Strain-induced reorientation occurs for all three major cytoskeletal systems, with distinctive timing: F-actin reorients first, followed by microtubules and then IFs (Zielinski et al., 2018; Kreplak and Fudge, 2007). F-actin is remodeled such that filament bundles orient perpendicular to the applied cyclical force (Takemasa et al., 1997; Iba and Sumpio, 1991; Chen et al., 2013). While microtubules reorient, cell reorientation does not depend on microtubules (Goldyn et al., 2010; Wang et al., 2001) but on F-actin (Iba and Sumpio, 1991; Goldyn et al., 2010) and, occasionally, cooperation between F-actin and IFs.
For example, epithelial keratin IFs reorient when cells are exposed to stretch (Kreplak and Fudge, 2007) (Fig. 3). These changes in IFs are coordinated with alterations in F-actin and contractile signaling, as loss of keratin 18 in MDCK cells suppresses force-induced actin stress fiber reinforcement and alignment (Fujiwara et al., 2016). Evidence suggests that cells use adaptive reorientation to minimize passively stored elastic energy, thereby reducing the mechanical load on cytoskeletal components (Livne et al., 2014).
Fig. 3.
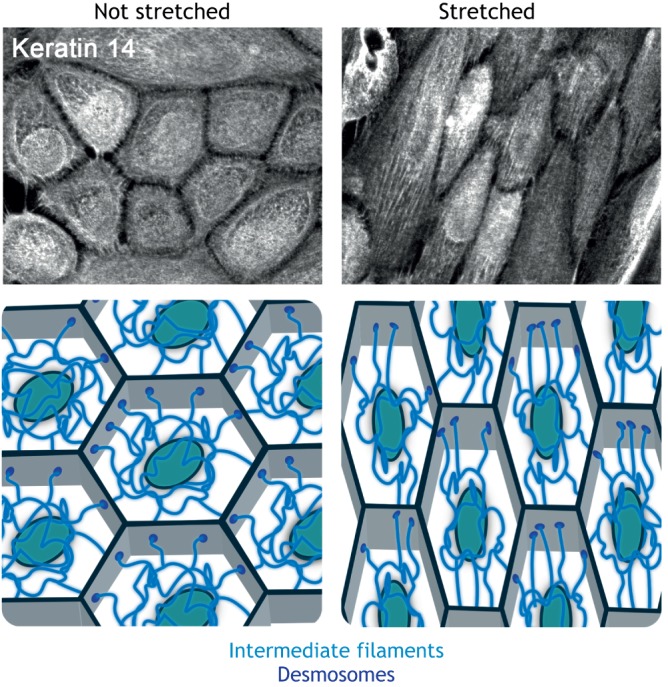
Changes to the IF cytoskeleton under external force. Mechanical stretch induces alignment of the IF network. Neonatal epidermal keratinocytes were incubated with 1.2 mM Ca2+-containing medium overnight to induce the formation of robust cell–cell junctions. Cell monolayers were then subjected to cyclical stretch for 24 h, fixed, and stained for keratin 14 to demonstrate reorganization of the IF cytoskeleton. Control cells were not stretched. Images supplied by the laboratory of K.J.G. A schematic representation of the reorganization of the IF cytoskeleton that occurs upon application of mechanical stimulation is also shown.
Another example is desmin, the major muscle IF protein. Desmin mutations underlie several myopathies and cardiomyopathies (Hnia et al., 2015; van Tintelen et al., 2009). Myoblasts, like most cells, respond to cyclical stretch by elongating and reorienting their cell bodies. Myoblasts expressing a p.D399Y desmin mutation (associated with myofibrillar myopathy) exhibit reduced elongation and spread area in response to cyclical stretch (Leccia et al., 2013). Moreover, p.D399Y desmin altered the ability of cyclical stretch to induce myoblast reorientation, suggesting that IFs play a role in what is considered, in most cases, a predominantly actin-driven process. However, in this case it is unclear whether the effects of the desmin IF system on cell reorientation involve the actin cytoskeleton.
The ability of IFs to regulate cell mechanical properties, respond to force and regulate force-sensitive cell behaviors could be mediated through direct mechanosensing mechanisms, for which experimental evidence is limited, and/or a combination of crosstalk mechanisms with other mechanosensitive cytoskeletal systems, including F-actin and microtubules. In the next sections, we discuss the physical properties that underlie the unique mechanics of the IF network, the mechanisms that link IFs with the F-actin and microtubule systems through the early stages of assembly or within mature networks, and IF-mediated mechanosignaling.
Mechanical properties of IF networks
The mechanical properties of the IF cytoskeleton differ from those of F-actin and microtubules. IFs assemble into polymers that are highly elastic and exhibit strain stiffening upon deformation (Janmey et al., 1991; Leterrier et al., 1996; Charrier and Janmey, 2016; Gardel et al., 2008). IFs are uniquely resilient to pulling forces and, unlike F-actin and microtubules, individual IFs can be stretched to multiple times their original length without breaking (Kreplak et al., 2005, 2008; Guzmán et al., 2006). Moreover, they exhibit nonlinear tensile properties; in this way, they are better capable of resisting force at higher tensile loads. These properties indicate that IFs can provide support to cells and tissues that would not be possible with other cytoskeletal types.
IFs are highly flexible. Persistence length is a mechanical property reflecting polymer stiffness. Larger values indicate higher stiffness and shorter more flexibility. Of the cytoskeletal systems, the persistence length of microtubules is the largest, at a few millimeters, indicating their high stiffness (Gittes et al., 1993). F-actin has a persistence length of 10–20 μm and filaments are more flexible than microtubules (Gittes et al., 1993). IFs are the most flexible with the smallest persistence length, typically less than 2 μm (Block et al., 2015). Thus, compared with F-actin and especially microtubules, individual IFs would most easily deform under small compressive forces. However, rheology experiments have shown that networks of IFs can resist applied forces. Like individual filaments, networks of IFs are less rigid at low strain, and stiffen to become more rigid at higher strains (Janmey et al., 1991; Köster et al., 2015; Wagner et al., 2007).
IF networks have profound effects on the mechanical rigidity of even individual cells, as their loss generally results in cells becoming less rigid (Charrier and Janmey, 2016). A modest decrease in stiffness occurs in vimentin-null fibroblasts when small forces are applied to the cell surface (Mendez et al., 2014). Deletion of vimentin in mesenchymal stem cells has the opposite effect, stiffening the cell cortex, presumably because stiffer cytoskeletal fibers such as F-actin are upregulated (Sharma et al., 2017). Consistent with the high degree of strain stiffening in IF networks, softening due to loss of vimentin is more evident at large cell deformations, especially in response to compression (Mendez et al., 2014). Similarly, loss of desmin has little effect on the stiffness of the myocyte cortex at small deformations, but is important for whole-cell stiffness at large strains (Charrier et al., 2018).
Epidermal keratins, in contrast, have a larger effect on cortical stiffness (Seltmann et al., 2013), perhaps due to their relatively high abundance. In addition, keratin IF networks are anchored to the plasma membrane through desmosomes. There is also a cortical meshwork of keratin IFs that might contribute to cell mechanics (Quinlan et al., 2017). Thus, while keratin IFs are highly flexible and not stiff in single cells, they can still have a large impact on overall cell stiffness. Keratin IFs, as well as other IFs, are attached at regions of cell–substrate contact and cell–cell contact, and are highly integrated with other filamentous cytoskeletal networks. How cooperation with other cytoskeletal networks and anchorage to adhesive complexes affect the mechanical properties of the IF network and thus the mechanical properties of cells is not well understood. It has been shown recently, however, that modulation of the linkage between IFs and desmosomes alters cell stiffness in human epithelial cells (Broussard et al., 2017). A mutation in desmoplakin that strengthens its interaction with IF increases stiffness, whereas a mutant that prevents this interaction reduces stiffness (Broussard et al., 2017). The capability of the mutants to affect stiffness required cell–cell contact, suggesting that anchorage of the IF network to desmosomes plays a role in the ability of IFs to regulate cell mechanics. These effects on cell stiffness were mediated at least in part through crosstalk with the actin cytoskeleton (Broussard et al., 2017), suggesting the possibility that actomyosin-generated forces are counterbalanced and/or resisted by an anchored IF system.
IF network assembly and its relationship with the F-actin and microtubule networks
In vitro, IF proteins can self-assemble into filaments without the aid of other co-factors (Steinert et al., 1981; Herrmann et al., 2002). IF assembly properties vary by their type and are dependent on in vitro experimental conditions (Herrmann et al., 2004). However, in living cells, IFs are integrated with the F-actin and microtubule systems from the earliest stages of network assembly (Chang and Goldman, 2004; Weber and Bement, 2002).
IF assembly and the actin cytoskeleton
In Xenopus egg extracts, IFs are associated with spontaneously polymerizing F-actin (Weber and Bement, 2002). When F-actin assembly is prevented, keratin IFs form aggregates rather than filaments. In mammalian cells, keratin IF precursors often assemble at the cell cortex near F-actin-rich focal adhesions (Kölsch et al., 2009; Windoffer et al., 2006). The keratin IF precursors move alongside F-actin toward the cell center before incorporating into the peripheral keratin IF network (Windoffer et al., 2006; Kölsch et al., 2009). Disruption of focal adhesion function through depletion of talin decreases the amount of keratin IFs in the cell periphery (Windoffer et al., 2006). Keratin IF precursors still form upon F-actin perturbation but fail to move toward the cell center (Kölsch et al., 2009). In this context, disruption of the microtubule network does not affect the centripetal movement of keratin IF precursors (Kölsch et al., 2009). Interestingly, there appears to be a feedback loop from IFs to focal adhesions, as vimentin IFs can affect cell–matrix contacts (Bhattacharya et al., 2009; Tsuruta and Jones, 2003).
IF assembly and microtubules
Numerous links exist between IFs and the microtubule cytoskeleton. Immunofluorescence staining suggests that the distribution of vimentin IFs and microtubules is similar (Ball and Singer, 1981). Because the vimentin IF network is more stable than the microtubule network, its structure can act as a template for the reassembly of newly forming microtubules (Gan et al., 2016). On the other hand, microtubule depolymerization or disruption of microtubule-based motors induces vimentin IF reorganization (Gyoeva and Gelfand, 1991; Helfand et al., 2002; Hookway et al., 2015; Goldman, 1971). Interestingly, after cell division, the IF cytoskeleton reassembles by severing and annealing (Hookway et al., 2015), which is a unique assembly mechanism among cytoskeletal structures. The cellular distribution of IFs is also regulated by microtubule-dependent transport via kinesin and dynein motors. A recent study showed that both keratin and vimentin IFs are nonconventional kinesin-1 cargoes, in that they do not require kinesin light chains for association or transport (Robert et al., 2019). Furthermore, both keratin and vimentin IFs interact with the same kinesin heavy-chain tail domain, suggesting that different IFs use similar mechanisms for microtubule transport (Robert et al., 2019). Neuronal IFs associate with both F-actin and microtubule systems for transport; here, dynein and kinesin mediate bi-directional transport of neurofilaments (Shea and Flanagan, 2001) and myosin Va controls the distribution and local density of neurofilaments (Alami et al., 2009; Rao et al., 2002).
Although it is clear that F-actin and microtubules play a role in IF network organization, a direct effect on in vivo IF assembly has not been experimentally shown. Instead, in vivo assembly of IFs is generally associated with adhesive complexes that contain an apparent assembly and/or nucleation function. These sites include focal adhesions, as indicated above, and desmosomes (Schwarz et al., 2015; Moch et al., 2020). A potential model would be that F-actin and microtubules are important for delivering soluble IF components (e.g. unit length filaments) to these sites of active assembly via, for example, motor proteins.
It is not well understood why the interdependence among cytoskeletal components as they assemble into higher order networks is so important. Many studies reporting the importance of the F-actin and microtubule networks in mechanobiology depend on results from gain- or loss-of-function experiments, without an examination of the consequences on the IF system. Therefore, the extent to which observed alterations in cell mechanics might also depend on an intact and properly networked IF cytoskeleton is unclear. Since these networks are intimately interconnected, it is important to consider the effects of experimental manipulations on the cytoskeleton in a more comprehensive manner.
Physical and functional links between the IF, F-actin and microtubule networks
Cytoskeletal filaments are linked to each other (e.g. IF–IF) or to other filament systems (e.g. IF–F-actin) through direct binding or intermediary crosslinking molecules. Both types of linkage affect the mechanical properties of the cytoskeleton and are important for controlling organelle positioning and formation of cell adhesion sites. Examples of direct physical connections are divalent cation crosslinks (Lin et al., 2010), and it has been shown that direct crosslinking of in vitro co-polymerized networks of vimentin IF and F-actin governs their strength (Jensen et al., 2014). Intermediary cross-linking molecules include filamin A (an F-actin crosslinker) and fimbrin (an actin-bundling protein, also known as plastin 1), which have distinct sites for binding IFs (Kim et al., 2010a,b; Correia et al., 1999), and plakins such as plectin (see below). In addition to their structural links with other cytoskeletal elements, IFs regulate chemical signaling pathways that control cellular functions, including growth, survival and motility (Kim and Coulombe, 2007; Kim et al., 2006; Vijayaraj et al., 2010; Schmitt et al., 2019). In this section, we will review how IFs interact physically and functionally with F-actin and microtubules.
Particularly versatile crosslinkers are found in the plakin/spectraplakin family of proteins, which evolved to link cytoskeletal elements to plasma membrane structures and each other (Zhang et al., 2017; Leung et al., 2001). The plakin family member plectin contains side arms that crosslink IFs, F-actin and microtubules to integrate these filament systems throughout cells (Svitkina et al., 1996). Plectin can regulate cell mechanics, including long distance stress propagation through signaling mediators such as RhoA, which is discussed in more detail below (Na et al., 2009).
In astrocytes, the IF network composed of vimentin, GFAP and nestin, in conjunction with plectin, promotes actomyosin-driven treadmilling of adherens junctions during collective migration (De Pascalis et al., 2018). At the same time, IFs reduce the mechanical coupling of focal contacts with actomyosin, thereby restricting traction forces to the front of cell sheets and driving collective cell migration (De Pascalis et al., 2018). In U2OS cells, plectin couples the movement of vimentin IFs with that of actin transverse arcs (Jiu et al., 2015). Retrograde flow of actin induces the rearward flow of vimentin IFs toward the nucleus. At the same time, the retrograde flow of the actin arcs is restricted by the interaction with vimentin IFs, controlling lamellipodial protrusions and cell migration. Loss of plectin specifically affects transverse arcs and has no effect on vimentin IFs or actin stress fiber organization (Jiu et al., 2015). In this way, it appears the IF network could, in some ways, act in a similar manner to the clutch model in matrix-anchoring focal adhesions (Craig et al., 2015). This suggests that physical engagement between the F-actin and IF networks, mediated through linker proteins such as plectin, allows actomyosin-generated forces to work against the IF network to drive cell morphogenetic behaviors including cell migration.
Members of the plakin family also associate with microtubules. Interestingly, the plectin 1c isoform in keratinocytes operates as a destabilizer of microtubules (Valencia et al., 2013). Microtubules in plectin 1c-deficient keratinocytes resist depolymerization induced by nocodazole, exhibit increased acetylation and are less dynamic. Valencia et al. propose that the SH3 domain of plectin antagonizes MAP-promoted stabilization of microtubules, thereby promoting microtubule disassembly in proximity of IFs. Consequently, mechanically driven cell behaviors are altered, resulting in increased migration velocity, decreased migration directionality, reduced cell growth rates and changes in cell shape (Valencia et al., 2013).
The actomyosin network is the main force-generating machinery of the cell (Ananthakrishnan and Ehrlicher, 2007), and by controlling actomyosin organization and function, IFs can modulate cell behavior. Vimentin IFs control mesenchymal cell plasticity and cancer cell migration at least in part through modulation of actomyosin (Battaglia et al., 2018). Vimentin expression is increased in many carcinomas and its overexpression correlates with tumor growth and invasion (Satelli and Li, 2011; Dmello et al., 2018). Vimentin knockout in U2OS cells increases actin stress fiber assembly and contractility (Jiu et al., 2017), resulting in reduced motility. This phenotype is rescued by wild-type vimentin, but not a mutant that is unable to form unit length filaments, suggesting that intact vimentin IFs are required.
In fibroblasts, vimentin facilitates F-actin rearrangements by activating RhoA through the mechanosensitive focal adhesion kinase (FAK, also known as PTK2) (Gregor et al., 2014). However, RhoA activation can also be mediated via the microtubule-associated guanine nucleotide exchange factor GEF-H1 (also known as ARHGEF2), as shown in U2OS cells (Jiu et al., 2017). Here, loss of vimentin triggers phosphorylation and activation of GEF-H1, activating RhoA and promoting stress fiber assembly. Therefore, multiple mechanisms exist through which vimentin modulates F-actin-based structures to control cell migration (Battaglia et al., 2018).
Keratin IFs also interact with the Rho pathway to mediate mechanical signaling. In rat H4 hepatoma cells, keratin 8 knockdown decreases cell stiffness and alters F-actin organization through the modulation of Rho–ROCK signaling (Bordeleau et al., 2012). In MDCK cells, the RhoA GEF Solo binds to keratin IFs (Fujiwara et al., 2016). Loss of Solo (also known as ARHGEF40) results in the keratin 8 and 18 IFs and F-actin network being disorganized, and loss of either Solo or keratin 18 suppresses the ability of tensile force to activate RhoA (Fujiwara et al., 2016).
Cytoskeletal networks are attached to the cell membrane through adhesive complexes, and these structures are increasingly recognized for their mechanosensing and transducing roles (Charras and Yap, 2018; Sun et al., 2016). It is therefore critical to consider the role of IF-anchoring complexes in mechanobiology, as discussed below.
Cell–cell junctions regulate cell mechanics and respond to mechanical forces
The desmosome–IF system comprises numerous tissue- and differentiation-specific proteins (Table 1). In humans, there are seven desmosomal cadherin genes (Rübsam et al., 2018; Herrmann et al., 2009). Desmosome dysfunction caused by mutations, autoimmune antibodies and bacterial toxins leads to human disorders of the skin, hair and heart, and epithelial cancers (Garrod and Chidgey, 2008; Stahley and Kowalczyk, 2015; Celentano and Cirillo, 2017; Mahoney et al., 2010). The unique physical properties of IFs and the prominent role of desmosomes and IFs in tissues that experience considerable mechanical input (e.g. heart and skin) place the desmosome–IF system in a prime position to regulate mechanobiology. Here, we discuss the emerging roles of desmosomal proteins in regulating cell mechanics and mechanosignaling.
Cell–cell junctions are critical for force sensing in epithelia
As discussed above, IFs reorient and/or reorganize in response to stretch and modulate stretch-induced cell reorientation. These observations raise the possibility that forces affect their desmosomal anchors, potentially revealing cryptic binding sites (as is the case for focal adhesion proteins) to reinforce IF–desmosome connections and/or recruit other signaling proteins. Supporting this idea, molecular dynamic simulations indicate that force application in desmoplakin reveals a potential SH3-domain-binding site, suggesting a putative mechanotransduction mechanism (Daday et al., 2017). However, whether desmosomes are indeed mechanotransducers in cells or play a role in force-sensing cell behavior is unknown.
To begin to understand the roles of IF-anchored desmosomes in force sensing, it is important to consider that this would occur in association with F-actin-based adhesive mechanosensing mechanisms. For example, in single adherent cells, reorientation in response to stretch requires focal adhesion proteins (Chen et al., 2013, 2012), as focal adhesions link the forces exerted from the substrate to the actin cytoskeleton within cells. However, less is known about reorientation in monolayers, where cells are connected through cell–cell contacts. In fact, epithelial cells remodel focal adhesions upon cell–cell contact and focal adhesion proteins, such as vinculin, relocalize from focal adhesions to cell–cell contacts (Hodivala and Watt, 1994; Twiss and de Rooij, 2013). This corresponds with a switch from cells predominantly exerting cell–substrate forces to cadherin-based cell–cell forces. Increased force on cell–cell adherens junctions results in the recruitment of vinculin to a protein binding interface present on α-catenin that is exposed upon mechanical stretch (Yonemura et al., 2010; le Duc et al., 2010; Yao et al., 2014; Kim et al., 2015) and this recruitment is responsible for force-induced cell reorientation (Noethel et al., 2018).
Crosstalk between cell–cell adhesive complex assembly and cytoskeletal remodeling
Both the assembly of cell–cell junctions and the mechanisms regulating F-actin and IF-based junctional interdependence are important for understanding their mechanical contributions. Initial adherens junction formation is required for desmosome assembly (Lewis et al., 1994). The underlying mechanisms are not well understood, but plakoglobin, which interacts with the cytoplasmic tails of both classical and desmosomal cadherins, is important (Lewis et al., 1997). Moreover, adherens junctions and desmosomes are mutually dependent. While complete loss of desmoplakin in mouse results in keratin IF disorganization and lethality (Gallicano et al., 1998), conditional ablation of desmoplakin in the epidermis results in an impaired maturation of adherens junctions (Vasioukhin et al., 2001). Additionally, it has been proposed that junctional E-cadherin recruits desmoglein-2 (Dsg2) through a direct cis-interaction, thereby initiating desmosome assembly (Shafraz et al., 2018).
Desmosomes participate in active cytoskeletal rearrangements. During mouse epidermal differentiation, desmoplakin facilitates the reorganization of the microtubule network through the recruitment of centrosomal proteins, such as ninein, Lis1 (also known as PAFAH1B1) and Ndel1, to desmosomes (Lechler and Fuchs, 2007; Sumigray et al., 2011). Loss of Lis1 results in decreased desmosomal stability and is associated with defective epidermal barrier function (Sumigray et al., 2011). In the skin-blistering disease pemphigus, autoantibodies against Dsgs cause desmosome disruption and keratinocyte dissociation, induce elasticity changes in keratinocytes, and severely alter both the keratin IF and F-actin cytoskeletons (Vielmuth et al., 2018; Vielmuth et al., 2015). Desmoplakin knockout in keratinocytes induces defects in cortical F-actin cytoskeleton assembly after initiation of cell–cell contact (Hatsell and Cowin, 2001; Vasioukhin et al., 2001). Interestingly, in the mouse gut, loss of desmoplakin causes defects in the shape and length of F-actin-rich microvilli (Sumigray and Lechler, 2012). Moreover, loss of plakophilins 1, 2 or 3 in mouse or human keratinocytes alters cortical F-actin organization (Godsel et al., 2010; Keil et al., 2016), indicating that multiple desmosomal proteins are important for cortical F-actin rearrangements.
Desmosomal cadherins have recently been shown to modulate the distribution of the F-actin-nucleating Arp2/3 complex, typically linked to classical cadherins. Arp2/3 is recruited to E-cadherin-based contacts in simple epithelia to generate a high-tension region near the apical-lateral surface that is actomyosin dependent (Verma et al., 2004; Wu et al., 2015). This high-tension region relies on a WAVE2–Arp2/3 complex that is recruited to cadherin cytoplasmic tails by binding to cortactin (Verma et al., 2012; Han et al., 2014). The roles of Arp2/3 in the morphogenesis of stratified epithelia, such as the epidermis, appear to be more complex, as loss of various Arp2/3 components has yielded somewhat conflicting results (Zhou et al., 2013; van der Kammen et al., 2017). However, we found that during cell fate specification in epidermal basal cells, Dsg1, a desmosomal cadherin only found in complex stratified epithelia, recruits cortactin and Arp2/3 to cell–cell interfaces. This promotes active F-actin rearrangements and decreases apical tension, as well as tension on E-cadherin (Nekrasova et al., 2018). Dsg1-dependent remodeling of cortical F-actin, as well as altered membrane tension, promotes delamination (detachment from the basal layer to form a second cell layer) through a process that is similar to extrusion in simple epithelia (Nekrasova et al., 2018). Importantly, ectopic expression of Dsg1 in simple epithelial MDCK cells is sufficient to induce the formation of a second cell layer by basal cells escaping the monolayer (Nekrasova et al., 2018). We have also shown that these processes require the interaction of Dsg1 with the dynein light chain Tctex-1 (also known as DYNLT1), highlighting the interrelatedness of the cytoskeletal systems in promoting mechanically driven cell behaviors (Nekrasova et al., 2018).
The effect of IFs on desmosomes
Mechanical stresses at desmosomes are largely considered to arise because of external forces on tissues, and the flexible elements within the desmosome are thought to be able to absorb forces without damage (Ai-Jassar et al., 2013). However, stresses at the desmosome also arise from cell-generated forces. In this context, signaling and mechanical resistance may both be important, for instance during epithelial morphogenesis or wound healing. Epithelial remodeling necessitates rearrangements of cellular adhesive structures, but at the same time induces cell–cell forces that are propagated over a multicellular scale (Trepat et al., 2009; Tambe et al., 2011; Weber et al., 2012; Barry et al., 2015). This process likely involves the integrative function of the desmosome–IF system in modulating or harnessing tissue-level forces. However, the extent to which mechanical forces generated during epithelial remodeling directly affect the dynamics of desmosomes or their linkage to the IF cytoskeleton is largely unknown.
Using a scratch-wound migration assay, desmosomes have been shown to assemble at lateral junctions and grow and mature while moving rearward (Roberts et al., 2011). In this context, desmosome dynamics are initially F-actin dependent and only later become associated with IFs (Roberts et al., 2011). These findings are reminiscent of work showing de novo cell–cell contact initiates the formation of desmoplakin–plakophilin cytoplasmic particles that are translocated to cell–cell junctions in an F-actin-dependent manner (Godsel et al., 2005). Homeostatic desmoplakin dynamics, however, depend on the association with IFs (Godsel et al., 2005). In contrast, desmosomal cadherin trafficking employs microtubule-based motors, with Dsg2 and desmocollin 2 utilizing distinct kinesin motors (Nekrasova et al., 2011). However, it is currently unknown whether and how force directly affects desmosome dynamics and/or assembly.
IF–cell-surface connectors as mechanosensors
Although direct evidence that vertebrate desmosomes are mechanotransducers is currently lacking, studies of invertebrate IF-anchoring components support their role as critical regulators of morphogenesis and potential mechanotransducers. For example, VAB-10 is a component of C. elegans adhesion complexes that link IFs to the extracellular matrix, separating the epithelial layer from the underlying muscle. This linker protein is a member of the spectraplakin family, which ultimately gave rise to vertebrate plakins, including desmoplakin. VAB-10 is required for morphogenetic events including cell elongation and establishment of planar cell polarity involving Par3 localization and the actin cytoskeleton in epidermal cells (Gillard et al., 2019). At a mechanistic level, it has been proposed that these hemidesmosome-like attachments act as mechanotransducers, whereby C. elegans muscle tension induces conformational changes in proteins like VAB-10 to activate a signaling cascade involving GIT-1, PIX-1 and PAK-1 that promotes IF phosphorylation and junction maturation (Zhang et al., 2011). Whether this type of mechanosensitive pathway occurs at other IF-anchoring structures, like desmosomes in mammals, is an interesting question for future research.
Individual desmosomal proteins outside of desmosomes have been shown to respond to force and regulate mechanosensitive cell behavior in Xenopus. In the mesendoderm of this vertebrate model system, force applied to C-cadherin induces polarized migration (Weber et al., 2012). C-cadherin is a classical cadherin but, in this context, it interacts with the IF cytoskeleton through plakoglobin. Tension on the cadherin results in reorganization of the keratin IF network through plakoglobin, which is recruited to cadherin-containing adhesions upon force application. Loss of either keratin 8 or plakoglobin renders the cells unable to respond to tugging forces. In a sheet of migrating cells, local tugging forces on trailing cell–cell contacts use this mechanism to polarize and direct collective migration, which is likely important during embryogenesis and/or morphogenesis and wound healing (Weber et al., 2012). Interestingly, desmoplakin is required for not only epidermal integrity but also morphogenesis in Xenopus (Bharathan and Dickinson, 2019). Desmoplakin has been shown to promote apical cell expansion during epidermal stratification in Xenopus, which is known to be regulated by mechanical forces (Sedzinski et al., 2016; Sedzinski et al., 2017). Finally, inhibiting desmosome function through either loss of desmoplakin or uncoupling the desmosome–IF connection results in impaired apoptotic cell extrusion in MDCK cell monolayers due to alterations in actomyosin contractility (Thomas et al., 2020).
With their prominent expression in tissues that experience large amounts of mechanical stress, it is not surprising that a number of desmosomal proteins, including desmoplakin, Dsg2, plakophilin and plakoglobin, are molecular targets in arrhythmogenic cardiomyopathy (AC) (Kannankeril et al., 2006; Asimaki et al., 2007; Pilichou et al., 2006; Bauce et al., 2005; Norman et al., 2005), often referred to as a disease of the desmosome. AC model studies have provided insight into how forces regulate desmosomal proteins. Both plakophilin and plakoglobin are recruited to cell–cell junctions of cardiac myocytes upon application of fluid shear stress, whereas AC-associated mutants of plakophilin and plakoglobin fail to be recruited (Hariharan et al., 2014). Moreover, expression of a mutant plakoglobin also suppressed force-induced junctional recruitment of N-cadherin, suggesting that desmosomal proteins can influence the mechanoresponses of adherens junctions (Hariharan et al., 2014).
Force distribution within cell adhesion and cytoskeletal networks
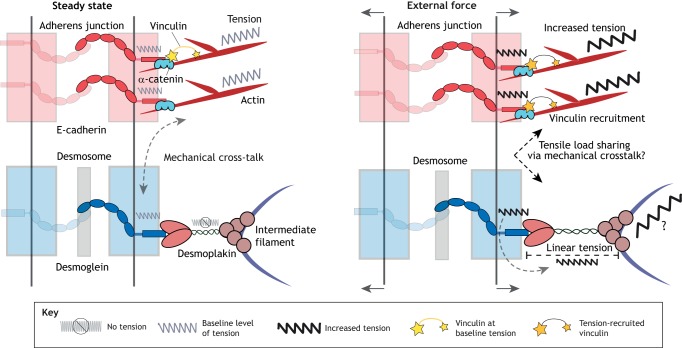
FRET-based tension biosensors have shown that classical cadherins, including E-cadherin (Borghi et al., 2012) and VE-cadherin (Conway et al., 2013), are under tension within adherens junctions. Recently, Dsg2 was found to be under a small amount of tension, especially in contracting cardiac myocytes (Baddam et al., 2018). However, a desmoplakin tension sensor study suggested desmosomes are only under tension upon externally applied load (Price et al., 2018); under these conditions, part of this load may be borne by the IF cytoskeleton. The lack of measurable homeostatic tension in desmoplakin could suggest that any tensile load on desmosomal cadherins is dissipated over multiple desmoplakin molecules, as desmoplakin functions as a dimer. Another possibility is that tension experienced by the desmosomal cadherin is offloaded onto IFs or another cytoskeletal system, perhaps F-actin, through an unknown mechanism (Fig. 4). Plakoglobin and plakophilin are potential candidates to mediate this transfer of tension as they interact with adherens junction components including the classical cadherins and members of the catenin family (Nieset et al., 1997; Lewis et al., 1997; Kowalczyk et al., 1998; Goossens et al., 2007).
Fig. 4.
Contributions of junctional and cytoskeletal proteins to cellular mechanics. At steady state (left), F-actin and the associated classical cadherins are under a baseline level of tension at the adherens junction. In addition, at steady state, desmosomal cadherins are under low tension, while desmoplakin experiences little to no tension. It is not known why the low tensile forces on desmosomal cadherins fail to be propagated to the IF cytoskeleton, but this could be explained through a mechanical crosstalk with the tensile F-actin-based adhesive system. When an external force is applied (right), the tensile load is shared between adherens junctions and desmosomes. Increased tension is ‘felt’ by the classical cadherins and F-actin at the adherens junction, leading to increased recruitment of vinculin, thereby facilitating mechanotransduction. When an external force is applied, the desmosomal cadherins propagate tensile forces to desmoplakin and potentially the IF network in order for cells and tissues to resist large mechanical loads.
Keratin IFs can bear tensile loads but also display characteristics that are consistent with experiencing compressive forces. For example, in SCC-25 cells exposed to >40% stretch amplitudes, keratin IFs in the cell periphery undergo a large extension, suggesting they are under tensile loads (Fois et al., 2013). When stretch is released, however, keratin IFs adopt a tortuous morphology, suggesting they experience compressive forces (Fois et al., 2013). This morphology can be abrogated by interfering with keratin 18 phosphorylation, the actomyosin cytoskeleton or, to a lesser degree, microtubule function (Fois et al., 2013).
In the specialized cell–cell junction known as the area composita, in cardiomyocytes, adherens junctions and desmosomes are highly intermixed (Franke et al., 2006; Borrmann et al., 2006). Plakophilin 2 and particularly the interaction between plakophilin 2 and αT-catenin is essential for the formation of these mixed junctions (Pieperhoff et al., 2008; van Hengel et al., 2013). This would be a useful system to examine the mechanical crosstalk between these typically distinct junctional complexes. Moreover, it would be interesting to examine the mechanical forces experienced by desmoplakin in cardiac myocytes, as it is possible that desmosomes are only under high amounts of force in those tissues that endure large mechanical stimulation.
Conclusions and future directions
On the long timescale of evolutionary history, cytoplasmic IFs and desmosomes are relatively new structures. Cytoplasmic IFs arose from nuclear lamins, acquiring new cytoplasmic roles in early metazoan lineages where they contribute to tissue stability (Wickstead and Gull, 2011; Hering et al., 2016), while desmosomes appeared later in vertebrates (Rübsam et al., 2018). Prior to the emergence of desmosomes, intervening lineages began anchoring cytoplasmic IFs through spectraplakin family proteins to confer additional stability on tissues (Gally et al., 2016). Later, in vertebrates, the plakin family adopted use of the IF-binding plakin domains to create the plakin family, which includes the obligate IF anchor in desmosomes, desmoplakin.
The large diversity in composition and tissue and/or differentiation-dependent expression patterns of desmosome and IF-based networks suggests that their overlay onto the less-diverse and evolutionarily older F-actin- and microtubule-based systems may have facilitated new mechanisms for organisms to achieve complexity in tissue architecture and the ability to respond to new challenges. Since roles for F-actin and microtubules in sensing and responding to mechanical forces are well established, future work is likely to increase our understanding of IFs in this context, especially given their unique high extensibility and strain-stiffening properties. We know that IFs are well-suited to bearing tensile loads, but is there a role for anchored IF networks in tissue jamming and/or bearing compressive forces? Future studies of desmosomes will identify new mechanosensitive proteins and mechanotransduction pathways that regulate important biological processes like cell migration, differentiation and tissue morphogenesis. Additionally, new force-sensing biosensors that are better able to differentiate between tension and compression will allow us to map out force distributions throughout the integrated cytoskeletal systems. Currently, there are limited biochemical and genetic tools for examining IF function in vivo, which has been a barrier to investigation. Discovering new ways to specifically alter IF function, such as currently unavailable pharmacological agents, would allow the ‘dissecting out’ of specific functions given that the interconnected nature of the cytoskeletal and adhesive components has made this goal particularly challenging.
Acknowledgements
We have endeavored to be as comprehensive as possible in our coverage of the work in these diverse fields and regret any omissions due to space limitations. We thank members of the laboratory of K.J.G. for helpful discusions contributing to the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Work in the authors laboratories is funded by the National Institutes of Health (NIH) (K01 AR075087 to J.A.B., R01 AR041836, R37 AR43380 and R01 CA228196 to K.J.G., R03 AR068096 and R35 GM119617 to D.E.C., and P01 GM096971 to P.A.J.); the J.L. Mayberry endowment to K.J.G.; the American Heart Association (AHA) (19POST34370124 to A.J. and 18POST33960144 to H.Z.); and a Multi-University Research Initiative through the Air Force Office of Scientific Research (AFOSR-FA9550-15-1-0009) and National Science Foundation (NSF) DMR-1408901 to H.D.E. Deposited in PMC for release after 12 months.
References
- Ai-Jassar C., Bikker H., Overduin M. and Chidgey M (2013). Mechanistic basis of desmosome-targeted diseases. J. Mol. Biol. 425, 4006-4022. 10.1016/j.jmb.2013.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alami N. H., Jung P. and Brown A (2009). Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. J. Neurosci. 29, 6625-6634. 10.1523/JNEUROSCI.3829-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan R. and Ehrlicher A (2007). The forces behind cell movement. Int. J. Biol. Sci. 3, 303-317. 10.7150/ijbs.3.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimaki A., Syrris P., Wichter T., Matthias P., Saffitz J. E. and Mckenna W. J. (2007). A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 81, 964-973. 10.1086/521633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddam S. R., Arsenovic P. T., Narayanan V., Duggan N. R., Mayer C. R., Newman S. T., Abutaleb D. A., Mohan A., Kowalczyk A. P. and Conway D. E. (2018). The desmosomal cadherin desmoglein-2 experiences mechanical tension as demonstrated by a FRET-based tension biosensor expressed in living cells. Cells 7, E66 10.3390/cells7070066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball E. H. and Singer S. J. (1981). Association of microtubules and intermediate filaments in normal fibroblasts and its disruption upon transformation by a temperature-sensitive mutant of Rous sarcoma virus. Proc. Natl. Acad. Sci. USA 78, 6986-6990. 10.1073/pnas.78.11.6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J. M., Przybyla L. and Weaver V. M. (2017). Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci. 130, 71-82. 10.1242/jcs.191742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. K., Wang N. and Leckband D. E. (2015). Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. J. Cell Sci. 128, 1341-1351. 10.1242/jcs.159954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia R. A., Delic S., Herrmann H. and Snider N. T. (2018). Vimentin on the move: new developments in cell migration. F1000Research 7, F1000 Faculty Rev-1796 10.12688/f1000research.15967.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauce B., Basso C., Rampazzo A., Beffagna G., Daliento L., Frigo G., Malacrida S., Settimo L., Danieli G., Thiene G. et al. (2005). Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur. Heart J. 26, 1666-1675. 10.1093/eurheartj/ehi341 [DOI] [PubMed] [Google Scholar]
- Bharathan N. K. and Dickinson A. J. G. (2019). Desmoplakin is required for epidermal integrity and morphogenesis in the Xenopus laevis embryo. Dev. Biol. 450, 115-131. 10.1101/464370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R., Gonzalez A. M., DeBiase P. J., Trejo H. E., Goldman R. D., Flitney F. W. and Jones J. C. R. (2009). Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength. J. Cell Sci. 122, 1390-1400. 10.1242/jcs.043042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J., Schroeder V., Pawelzyk P., Willenbacher N. and Köster S (2015). Physical properties of cytoplasmic intermediate filaments. Biochim. Biophys. Acta 1853, 3053-3064. 10.1016/j.bbamcr.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Bordeleau F., Myrand Lapierre M.-E., Sheng Y. and Marceau N (2012). Keratin 8/18 regulation of cell stiffness-extracellular matrix interplay through modulation of Rho-mediated actin cytoskeleton dynamics. PLoS ONE 7, e38780-e38780. 10.1371/journal.pone.0038780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi N., Sorokina M., Shcherbakova O. G., Weis W. I., Pruitt B. L., Nelson W. J. and Dunn A. R. (2012). E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc. Natl. Acad. Sci. USA 109, 12568-12573. 10.1073/pnas.1204390109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann C. M., Grund C., Kuhn C., Hofmann I., Pieperhoff S. and Franke W. W. (2006). The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur. J. Cell Biol. 85, 469-485. 10.1016/j.ejcb.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Broussard J. A., Yang R., Huang C., Nathamgari S. S. P., Beese A. M., Godsel L. M., Hegazy M. H., Lee S., Zhou F., Sniadecki N. J. et al. (2017). The desmoplakin-intermediate filament linkage regulates cell mechanics. Mol. Biol. Cell 28, 3156-3164. 10.1091/mbc.e16-07-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck R. C. (1980). Reorientation response of cells to repeated stretch and recoil of the substratum. Exp. Cell Res. 127, 470-474. 10.1016/0014-4827(80)90456-5 [DOI] [PubMed] [Google Scholar]
- Celentano A. and Cirillo N (2017). Desmosomes in disease: a guide for clinicians. Oral Dis. 23, 157-167. 10.1111/odi.12527 [DOI] [PubMed] [Google Scholar]
- Chang L. and Goldman R. D. (2004). Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 5, 601-613. 10.1038/nrm1438 [DOI] [PubMed] [Google Scholar]
- Charras G. and Yap A. S. (2018). Tensile forces and mechanotransduction at cell-cell junctions. Curr. Biol. 28, R445-r457. 10.1016/j.cub.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Charrier E. E. and Janmey P. A. (2016). Mechanical properties of intermediate filament proteins. Methods Enzymol. 568, 35-57. 10.1016/bs.mie.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier E. E., Montel L., Asnacios A., Delort F., Vicart P., Gallet F., Batonnet-Pichon S. and Hénon S (2018). The desmin network is a determinant of the cytoplasmic stiffness of myoblasts. Biol. Cell 110, 77-90. 10.1111/boc.201700040 [DOI] [PubMed] [Google Scholar]
- Chen B., Kemkemer R., Deibler M., Spatz J. and Gao H (2012). Cyclic stretch induces cell reorientation on substrates by destabilizing catch bonds in focal adhesions. PLoS ONE 7, e48346 10.1371/journal.pone.0048346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Pasapera A. M., Koretsky A. P. and Waterman C. M. (2013). Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proc. Natl. Acad. Sci. USA 110, E2352-E2361. 10.1073/pnas.1221637110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway D. E., Breckenridge M. T., Hinde E., Gratton E., Chen C. S. and Schwartz M. A. (2013). Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23, 1024-1030. 10.1016/j.cub.2013.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia I., Chu D., Chou Y.-H., Goldman R. D. and Matsudaira P (1999). Integrating the actin and vimentin cytoskeletons: Adhesion-dependent formation of fimbrin-vimentin complexes in macrophages. J. Cell Biol. 146, 831-842. 10.1083/jcb.146.4.831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S. and Fuchs E (1991). Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell 66, 1301-1311. 10.1016/0092-8674(91)90051-Y [DOI] [PubMed] [Google Scholar]
- Craig E. M., Stricker J., Gardel M. and Mogilner A (2015). Model for adhesion clutch explains biphasic relationship between actin flow and traction at the cell leading edge. Phys. Biol. 12, 035002-035002. 10.1088/1478-3975/12/3/035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daday C., Kolšek K. and Gräter F (2017). The mechano-sensing role of the unique SH3 insertion in plakin domains revealed by molecular Dynamics simulations. Sci. Rep. 7, 11669 10.1038/s41598-017-11017-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascalis C., Pérez-González C., Seetharaman S., Boëda B., Vianay B., Burute M., Leduc C., Borghi N., Trepat X. and Etienne-Manneville S (2018). Intermediate filaments control collective migration by restricting traction forces and sustaining cell-cell contacts. J. Cell Biol. 217, 3031-3044. 10.1083/jcb.201801162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmello C., Sawant S., Chaudhari P. R., Dongre H., Ahire C., D'souza Z. C., Charles S. E., Rane P., Costea D. E., Chaukar D. et al. (2018). Aberrant expression of vimentin predisposes oral premalignant lesion derived cells towards transformation. Exp. Mol. Pathol. 105, 243-251. 10.1016/j.yexmp.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Fois G., Weimer M., Busch T., Felder E. T., Oswald F., von Wichert G., Seufferlein T., Dietl P. and Felder E (2013). Effects of keratin phosphorylation on the mechanical properties of keratin filaments in living cells. FASEB J. 27, 1322-1329. 10.1096/fj.12-215632 [DOI] [PubMed] [Google Scholar]
- Franke W. W., Borrmann C. M., Grund C. and Pieperhoff S (2006). The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur. J. Cell Biol. 85, 69-82. 10.1016/j.ejcb.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Ohashi K., Mashiko T., Kondo H. and Mizuno K (2016). Interplay between Solo and keratin filaments is crucial for mechanical force-induced stress fiber reinforcement. Mol. Biol. Cell 27, 954-966. 10.1091/mbc.E15-06-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicano G. I., Kouklis P., Bauer C., Yin M., Vasioukhin V., Degenstein L. and Fuchs E (1998). Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J. Cell Biol. 143, 2009-2022. 10.1083/jcb.143.7.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally C., Zhang H. and Labouesse M (2016). Functional and genetic analysis of VAB-10 Spectraplakin in Caenorhabditis elegans. Methods Enzymol. 569, 407-430. 10.1016/bs.mie.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Galou M., Gao J., Humbert J., Mericskay M., Li Z., Paulin D. and Vicart P (1997). The importance of intermediate filaments in the adaptation of tissues to mechanical stress: evidence from gene knockout studies. Biol. Cell 89, 85-97. 10.1111/j.1768-322X.1997.tb00997.x [DOI] [PubMed] [Google Scholar]
- Gan Z., Ding L., Burckhardt C. J., Lowery J., Zaritsky A., Sitterley K., Mota A., Costigliola N., Starker C. G., Voytas D. F. et al. (2016). Vimentin intermediate filaments template microtubule networks to enhance persistence in cell polarity and directed migration. Cell Systems 3, 252 10.1016/j.cels.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel M. L., Kasza K. E., Brangwynne C. P., Liu J. and Weitz D. A. (2008). Chapter 19: mechanical response of cytoskeletal networks. Methods Cell Biol. 89, 487-519. 10.1016/S0091-679X(08)00619-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D. and Chidgey M (2008). Desmosome structure, composition and function. Biochim. Biophys. Acta 1778, 572-587. 10.1016/j.bbamem.2007.07.014 [DOI] [PubMed] [Google Scholar]
- Gillard G., Nicolle O., Brugière T., Prigent S., Pinot M. and Michaux G (2019). Force transmission between three tissues controls bipolar planar polarity establishment and morphogenesis. Curr. Biol. 29, 1360-1368.e4. 10.1016/j.cub.2019.02.059 [DOI] [PubMed] [Google Scholar]
- Gittes F., Mickey B., Nettleton J. and Howard J (1993). Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 120, 923-934. 10.1083/jcb.120.4.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsel L. M., Hsieh S. N., Amargo E. V., Bass A. E., Pascoe-Mcgillicuddy L. T., Huen A. C., Thorne M. E., Gaudry C. A., Park J. K., Myung K. et al. (2005). Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin. J. Cell Biol. 171, 1045-1059. 10.1083/jcb.200510038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsel L. M., Dubash A. D., Bass-Zubek A. E., Amargo E. V., Klessner J. L., Hobbs R. P., Chen X. and Green K. J. (2010). Plakophilin 2 couples actomyosin remodeling to desmosomal plaque assembly via RhoA. Mol. Biol. Cell 21, 2844-2859. 10.1091/mbc.e10-02-0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D. (1971). The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J. Cell Biol. 51, 752-762. 10.1083/jcb.51.3.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldyn A. M., Kaiser P., Spatz J. P., Ballestrem C. and Kemkemer R (2010). The kinetics of force-induced cell reorganization depend on microtubules and actin. Cytoskeleton (Hoboken, N.J.) 67, 241-250. 10.1002/cm.20439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens S., Janssens B., Bonné S., De Rycke R., Braet F., van Hengel J. and van Roy F. (2007). A unique and specific interaction between αT-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J. Cell Sci. 120, 2126-2136. 10.1242/jcs.004713 [DOI] [PubMed] [Google Scholar]
- Gregor M., Osmanagic-Myers S., Burgstaller G., Wolfram M., Fischer I., Walko G., Resch G. P., Jorgl A., Herrmann H. and Wiche G (2014). Mechanosensing through focal adhesion-anchored intermediate filaments. FASEB J. 28, 715-729. 10.1096/fj.13-231829 [DOI] [PubMed] [Google Scholar]
- Guzmán C., Jeney S., Kreplak L., Kasas S., Kulik A. J., Aebi U. and Forró L (2006). Exploring the mechanical properties of single vimentin intermediate filaments by atomic force microscopy. J. Mol. Biol. 360, 623-630. 10.1016/j.jmb.2006.05.030 [DOI] [PubMed] [Google Scholar]
- Gyoeva F. K. and Gelfand V. I. (1991). Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature 353, 445-448. 10.1038/353445a0 [DOI] [PubMed] [Google Scholar]
- Han S. P., Gambin Y., Gomez G. A., Verma S., Giles N., Michael M., Wu S. K., Guo Z., Johnston W., Sierecki E. et al. (2014). Cortactin scaffolds Arp2/3 and WAVE2 at the epithelial zonula adherens. J. Biol. Chem. 289, 7764-7775. 10.1074/jbc.M113.544478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan V., Asimaki A., Michaelson J. E., Plovie E., Macrae C. A., Saffitz J. E. and Huang H (2014). Arrhythmogenic right ventricular cardiomyopathy mutations alter shear response without changes in cell-cell adhesion. Cardiovasc. Res. 104, 280-289. 10.1093/cvr/cvu212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell S. and Cowin P (2001). Deconstructing desmoplakin. Nat. Cell Biol. 3, E270-E272. 10.1038/ncb1201-e270 [DOI] [PubMed] [Google Scholar]
- Helfand B. T., Mikami A., Vallee R. B. and Goldman R. D. (2002). A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J. Cell Biol. 157, 795-806. 10.1083/jcb.200202027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering L., Bouameur J.-E., Reichelt J., Magin T. M. and Mayer G (2016). Novel origin of lamin-derived cytoplasmic intermediate filaments in tardigrades. eLife 5, e11117-e11117. 10.7554/eLife.11117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H., Wedig T., Porter R. M., Lane E. B. and Aebi U (2002). Characterization of early assembly intermediates of recombinant human keratins. J. Struct. Biol. 137, 82-96. 10.1006/jsbi.2002.4466 [DOI] [PubMed] [Google Scholar]
- Herrmann H., Kreplak L. and Aebi U (2004). Isolation, characterization, and in vitro assembly of intermediate filaments. Methods Cell Biol. 78, 3-24. 10.1016/S0091-679X(04)78001-2 [DOI] [PubMed] [Google Scholar]
- Herrmann H., Strelkov S. V., Burkhard P. and Aebi U (2009). Intermediate filaments: primary determinants of cell architecture and plasticity. J. Clin. Invest. 119, 1772-1783. 10.1172/JCI38214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnia K., Ramspacher C., Vermot J. and Laporte J (2015). Desmin in muscle and associated diseases: beyond the structural function. Cell Tissue Res. 360, 591-608. 10.1007/s00441-014-2016-4 [DOI] [PubMed] [Google Scholar]
- Hodivala K. J. and Watt F. M. (1994). Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J. Cell Biol. 124, 589-600. 10.1083/jcb.124.4.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol E. M., Roelofs R. F., Moraal E., Sonnemans M. A. F., Sluijs J. A., Proper E. A., de Graan P. N. E., Fischer D. F. and van Leeuwen F. W. (2003). Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol. Psychiatry 8, 786-796. 10.1038/sj.mp.4001379 [DOI] [PubMed] [Google Scholar]
- Hookway C., Ding L., Davidson M. W., Rappoport J. Z., Danuser G. and Gelfand V. I. (2015). Microtubule-dependent transport and dynamics of vimentin intermediate filaments. Mol. Biol. Cell 26, 1675-1686. 10.1091/mbc.E14-09-1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber F., Boire A., López M. P. and Koenderink G. H. (2015). Cytoskeletal crosstalk: when three different personalities team up. Curr. Opin. Cell Biol. 32, 39-47. 10.1016/j.ceb.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Iba T. and Sumpio B. E. (1991). Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc. Res. 42, 245-254. 10.1016/0026-2862(91)90059-K [DOI] [PubMed] [Google Scholar]
- Jaalouk D. E. and Lammerding J (2009). Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10, 63-73. 10.1038/nrm2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Euteneuer U., Traub P. and Schliwa M (1991). Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 113, 155-160. 10.1083/jcb.113.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. H., Morris E. J., Goldman R. D. and Weitz D. A. (2014). Emergent properties of composite semiflexible biopolymer networks. BioArchitecture 4, 138-143. 10.4161/19490992.2014.989035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiu Y., Lehtimäki J., Tojkander S., Cheng F., Jäälinoja H., Liu X., Varjosalo M., Eriksson J. E. and Lappalainen P (2015). Bidirectional interplay between vimentin intermediate filaments and contractile actin stress fibers. Cell Rep. 11, 1511-1518. 10.1016/j.celrep.2015.05.008 [DOI] [PubMed] [Google Scholar]
- Jiu Y., Peränen J., Schaible N., Cheng F., Eriksson J. E., Krishnan R. and Lappalainen P (2017). Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J. Cell Sci. 130, 892-902. 10.1242/jcs.196881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannankeril P. J., Bhuiyan Z. A., Darbar D., Mannens M. M., Wilde A. A. M. and Roden D. M. (2006). Arrhythmogenic right ventricular cardiomyopathy due to a novel plakophilin 2 mutation: wide spectrum of disease in mutation carriers within a family. Heart Rhythm 3, 939-944. 10.1016/j.hrthm.2006.04.028 [DOI] [PubMed] [Google Scholar]
- Keil R., Rietscher K. and Hatzfeld M (2016). Antagonistic regulation of intercellular cohesion by plakophilins 1 and 3. J. Invest. Dermatol. 136, 2022-2029. 10.1016/j.jid.2016.05.124 [DOI] [PubMed] [Google Scholar]
- Kim S. and Coulombe P. A. (2007). Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 21, 1581-1597. 10.1101/gad.1552107 [DOI] [PubMed] [Google Scholar]
- Kim S., Wong P. and Coulombe P. A. (2006). A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 441, 362-365. 10.1038/nature04659 [DOI] [PubMed] [Google Scholar]
- Kim H., Nakamura F., Lee W., Hong C., Pérez-Sala D. and Mcculloch C. A. (2010a). Regulation of cell adhesion to collagen via beta 1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp. Cell Res. 316, 1829-1844. 10.1016/j.yexcr.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Kim H., Nakamura F., Lee W., Shifrin Y., Arora P. and McCulloch C. A. (2010b). Filamin A is required for vimentin-mediated cell adhesion and spreading. Am. J. Physiol. Cell Physiol. 298, C221-C236. 10.1152/ajpcell.00323.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.-J., Zheng S., Sun J., Muhamed I., Wu J., Lei L., Kong X., Leckband D. E. and Wang Y (2015). Dynamic visualization of alpha-catenin reveals rapid, reversible conformation switching between tension states. Curr. Biol. 25, 218-224. 10.1016/j.cub.2014.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima Y., Inoue S. and Yaoita H (1989). Abnormal organization of keratin intermediate filaments in cultured keratinocytes of epidermolysis bullosa simplex. Arch. Dermatol. Res. 281, 5-10. 10.1007/BF00424265 [DOI] [PubMed] [Google Scholar]
- Kölsch A., Windoffer R. and Leube R. E. (2009). Actin-dependent dynamics of keratin filament precursors. Cell Motility 66, 976-985. 10.1002/cm.20395 [DOI] [PubMed] [Google Scholar]
- Köster S., Weitz D. A., Goldman R. D., Aebi U. and Herrmann H (2015). Intermediate filament mechanics in vitro and in the cell: from coiled coils to filaments, fibers and networks. Curr. Opin. Cell Biol. 32, 82-91. 10.1016/j.ceb.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A. P. and Green K. J. (2013). Structure, function, and regulation of desmosomes. Prog. Mol. Biol. Transl. Sci. 116, 95-118. 10.1016/B978-0-12-394311-8.00005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A. P., Navarro P., Dejana E., Bornslaeger E. A., Green K. J., Kopp D. S. and Borgwardt J. E. (1998). VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J. Cell Sci. 111, 3045-3057. [DOI] [PubMed] [Google Scholar]
- Kreplak L. and Fudge D (2007). Biomechanical properties of intermediate filaments: from tissues to single filaments and back. BioEssays 29, 26-35. 10.1002/bies.20514 [DOI] [PubMed] [Google Scholar]
- Kreplak L., Bär H., Leterrier J. F., Herrmann H. and Aebi U (2005). Exploring the mechanical behavior of single intermediate filaments. J. Mol. Biol. 354, 569-577. 10.1016/j.jmb.2005.09.092 [DOI] [PubMed] [Google Scholar]
- Kreplak L., Herrmann H. and Aebi U (2008). Tensile properties of single desmin intermediate filaments. Biophys. J. 94, 2790-2799. 10.1529/biophysj.107.119826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi M. C. and Reinhart-King C. A. (2018). Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 10, eaao0475 10.1126/scitranslmed.aao0475 [DOI] [PubMed] [Google Scholar]
- Lane E. B. (2006). Keratin Intermediate Filaments and Diseases of the Skin. Intermediate Filaments. Boston, MA: Springer US. [Google Scholar]
- Lazarides E. (1980). Intermediate filaments as mechanical integrators of cellular space. Nature 283, 249-256. 10.1038/283249a0 [DOI] [PubMed] [Google Scholar]
- le Duc Q., Shi Q., Blonk I., Sonnenberg A., Wang N., Leckband D. and De Rooij J. (2010). Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 189, 1107-1115. 10.1083/jcb.201001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leccia E., Batonnet-Pichon S., Tarze A., Bailleux V., Doucet J., Pelloux M., Delort F., Pizon V., Vicart P. and Briki F (2013). Cyclic stretch reveals a mechanical role for intermediate filaments in a desminopathic cell model. Phys. Biol. 10, 016001 10.1088/1478-3975/10/1/016001 [DOI] [PubMed] [Google Scholar]
- Lechler T. and Fuchs E (2007). Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J. Cell Biol. 176, 147-154. 10.1083/jcb.200609109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Käs J., Hartwig J., Vegners R. and Janmey P. A. (1996). Mechanical effects of neurofilament cross-bridges. Modulation by phosphorylation, lipids, and interactions with F-actin. J. Biol. Chem. 271, 15687-15694. 10.1074/jbc.271.26.15687 [DOI] [PubMed] [Google Scholar]
- Leung C. L., Liem R. K. H., Parry D. A. D. and Green K. J. (2001). The plakin family. J. Cell Sci. 114, 3409-3410. [DOI] [PubMed] [Google Scholar]
- Lewis J. E., Jensen P. J. and Wheelock M. J. (1994). Cadherin function is required for human keratinocytes to assemble desmosomes and stratify in response to calcium. J. Invest. Dermatol. 102, 870-877. 10.1111/1523-1747.ep12382690 [DOI] [PubMed] [Google Scholar]
- Lewis J. E., Wahl J. K. III, Sass K. M., Jensen P. J., Johnson K. R. and Wheelock M. J. (1997). Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J. Cell Biol. 136, 919-934. 10.1083/jcb.136.4.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-C., Broedersz C. P., Rowat A. C., Wedig T., Herrmann H., Mackintosh F. C. and Weitz D. A. (2010). Divalent cations crosslink vimentin intermediate filament tail domains to regulate network mechanics. J. Mol. Biol. 399, 637-644. 10.1016/j.jmb.2010.04.054 [DOI] [PubMed] [Google Scholar]
- Livne A., Bouchbinder E. and Geiger B (2014). Cell reorientation under cyclic stretching. Nat. Commun. 5, 3938 10.1038/ncomms4938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney M. G., Müller E. J. and Koch P. J. (2010). Desmosomes and desmosomal cadherin function in skin and heart diseases-advancements in basic and clinical research. Dermatol. Res. Pract. 2010 10.1155/2010/725647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T., Mammoto A. and Ingber D. E. (2013). Mechanobiology and developmental control. Annu. Rev. Cell Dev. Biol. 29, 27-61. 10.1146/annurev-cellbio-101512-122340 [DOI] [PubMed] [Google Scholar]
- Mendez M. G., Restle D. and Janmey P. A. (2014). Vimentin enhances cell elastic behavior and protects against compressive stress. Biophys. J. 107, 314-323. 10.1016/j.bpj.2014.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch M., Schwarz N., Windoffer R. and Leube R. E. (2020). The keratin–desmosome scaffold: pivotal role of desmosomes for keratin network morphogenesis. Cell. Mol. Life Sci. 77, 543-558. 10.1007/s00018-019-03198-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S., Chowdhury F., Tay B., Ouyang M., Gregor M., Wang Y., Wiche G. and Wang N (2009). Plectin contributes to mechanical properties of living cells. Am. J. Physiol. Cell Physiol. 296, C868-C877. 10.1152/ajpcell.00604.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasova O. E., Amargo E. V., Smith W. O., Chen J., Kreitzer G. E. and Green K. J. (2011). Desmosomal cadherins utilize distinct kinesins for assembly into desmosomes. J. Cell Biol. 195, 1185-1203. 10.1083/jcb.201106057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasova O., Harmon R. M., Broussard J. A., Koetsier J. L., Godsel L. M., Fitz G. N., Gardel M. L. and Green K. J. (2018). Desmosomal cadherin association with Tctex-1 and cortactin-Arp2/3 drives perijunctional actin polymerization to promote keratinocyte delamination. Nat. Commun. 9, 1053 10.1038/s41467-018-03414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieset J. E., Redfield A. R., Jin F., Knudsen K. A., Johnson K. R. and Wheelock M. J. (1997). Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J. Cell Sci. 110, 1013-1022. [DOI] [PubMed] [Google Scholar]
- Noethel B., Ramms L., Dreissen G., Hoffmann M., Springer R., Rübsam M., Ziegler W. H., Niessen C. M., Merkel R. and Hoffmann B (2018). Transition of responsive mechanosensitive elements from focal adhesions to adherens junctions on epithelial differentiation. Mol. Biol. Cell 29, 2317-2325. 10.1091/mbc.E17-06-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman M., Simpson M., Mogensen J., Shaw A., Hughes S., Syrris P., Sen-Chowdhry S., Rowland E., Crosby A. and Mckenna W. J. (2005). Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 112, 636-642. 10.1161/CIRCULATIONAHA.104.532234 [DOI] [PubMed] [Google Scholar]
- Omary M. B., Coulombe P. A. and McLean W. H. I. (2004). Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 351, 2087-2100. 10.1056/NEJMra040319 [DOI] [PubMed] [Google Scholar]
- Oshima R. G. (2007). Intermediate filaments: a historical perspective. Exp. Cell Res. 313, 1981-1994. 10.1016/j.yexcr.2007.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieperhoff S., Schumacher H. and Franke W. W. (2008). The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur. J. Cell Biol. 87, 399-411. 10.1016/j.ejcb.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Pilichou K., Nava A., Basso C., Beffagna G., Bauce B., Lorenzon A., Frigo G., Vettori A., Valente M., Towbin J. et al. (2006). Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation 113, 1171-1179. 10.1161/CIRCULATIONAHA.105.583674 [DOI] [PubMed] [Google Scholar]
- Price A. J., Cost A. L., Ungewiss H., Waschke J., Dunn A. R. and Grashoff C (2018). Mechanical loading of desmosomes depends on the magnitude and orientation of external stress. Nat. Commun. 9, 5284 10.1038/s41467-018-07523-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Schwarz N., Windoffer R., Richardson C., Hawkins T., Broussard J. A., Green K. J. and Leube R. E. (2017). A rim-and-spoke hypothesis to explain the biomechanical roles for cytoplasmic intermediate filament networks. J. Cell Sci. 130, 3437-3445. 10.1242/jcs.202168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. V., Engle L. J., Mohan P. S., Yuan A. D., Qiu D. K., Cataldo A., Hassinger L., Jacobsen S., Lee V. M.-Y., Andreadis A. et al. (2002). Myosin Va binding to neurofilaments is essential for correct myosin Va distribution and transport and neurofilament density. J. Cell Biol. 159, 279-289. 10.1083/jcb.200205062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge K. M., Linz L., Flitney F. W., Kuczmarski E. R., Chou Y. H., Omary M. B., Sznajder J. I. and Goldman R. D. (2005). Keratin 8 phosphorylation by protein kinase C delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J. Biol. Chem. 280, 30400-30405. 10.1074/jbc.M504239200 [DOI] [PubMed] [Google Scholar]
- Robert A., Tian P., Adam S. A., Kittisopikul M., Jaqaman K., Goldman R. D. and Gelfand V. I. (2019). Kinesin-dependent transport of keratin filaments: a unified mechanism for intermediate filament transport. FASEB J. 33, 388-399. 10.1096/fj.201800604R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. J., Pashaj A., Johnson K. R. and Wahl J. K. III (2011). Desmosome dynamics in migrating epithelial cells requires the actin cytoskeleton. Exp. Cell Res. 317, 2814-2822. 10.1016/j.yexcr.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsam M., Broussard J. A., Wickström S. A., Nekrasova O., Green K. J. and Niessen C. M. (2018). Adherens junctions and desmosomes coordinate mechanics and signaling to orchestrate tissue morphogenesis and function: an evolutionary perspective. Cold Spring Harb. Perspect Biol. 10, a029207 10.1101/cshperspect.a029207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Andrews P. D., James J. and Lane E. B. (2004). Mechanical stress induces profound remodelling of keratin filaments and cell junctions in epidermolysis bullosa simplex keratinocytes. J. Cell Sci. 117, 5233-5243. 10.1242/jcs.01407 [DOI] [PubMed] [Google Scholar]
- Satelli A. and Li S (2011). Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 68, 3033-3046. 10.1007/s00018-011-0735-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M., Sinnberg T., Nalpas N. C., Maass A., Schittek B. and Macek B. (2019). Quantitative proteomics links the intermediate filament nestin to resistance to targeted BRAF inhibition in melanoma cells. Mol. Cell. Proteomics 18, 1096-1109. 10.1074/mcp.RA119.001302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A. and DeSimone D. W. (2008). Cell adhesion receptors in mechanotransduction. Curr. Opin. Cell Biol. 20, 551-556. 10.1016/j.ceb.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N., Windoffer R., Magin T. M. and Leube R. E. (2015). Dissection of keratin network formation, turnover and reorganization in living murine embryos. Sci. Rep. 5, 9007 10.1038/srep09007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedzinski J., Hannezo E., Tu F., Biro M. and Wallingford J. B. (2016). Emergence of an apical epithelial cell surface In Vivo. Dev. Cell 36, 24-35. 10.1016/j.devcel.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedzinski J., Hannezo E., Tu F., Biro M. and Wallingford J. B. (2017). RhoA regulates actin network dynamics during apical surface emergence in multiciliated epithelial cells. J. Cell Sci. 130, 420-428. 10.1242/jcs.194704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltmann K., Fritsch A. W., Kas J. A. and Magin T. M. (2013). Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc. Natl. Acad. Sci. USA 110, 18507-18512. 10.1073/pnas.1310493110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafraz O., Rübsam M., Stahley S. N., Caldara A. L., Kowalczyk A. P., Niessen C. M. and Sivasankar S. (2018). E-cadherin binds to desmoglein to facilitate desmosome assembly. Elife 7, e37629 10.7554/eLife.37629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Bolten Z. T., Wagner D. R. and Hsieh A. H. (2017). Deformability of human mesenchymal stem cells is dependent on vimentin intermediate filaments. Ann. Biomed. Eng. 45, 1365-1374. 10.1007/s10439-016-1787-z [DOI] [PubMed] [Google Scholar]
- Shea T. B. and Flanagan L. A. (2001). Kinesin, dynein and neurofilament transport. Trends Neurosci. 24, 644-648. 10.1016/S0166-2236(00)01919-6 [DOI] [PubMed] [Google Scholar]
- Stahley S. N. and Kowalczyk A. P. (2015). Desmosomes in acquired disease. Cell Tissue Res. 360, 439-456. 10.1007/s00441-015-2155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Cabral F., Gottesman M. M. and Goldman R. D. (1981). In vitro assembly of homopolymer and copolymer filaments from intermediate filament subunits of muscle and fibroblastic cells. Proc. Natl. Acad. Sci. USA 78, 3692-3696. 10.1073/pnas.78.6.3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens K., Zlotogorski A., Smith L., Ehrlich P., Wijsman E., Livingston R. J. and Sybert V. P. (1995). Epidermolysis bullosa simplex: a keratin 5 mutation is a fully dominant allele in epidermal cytoskeleton function. Am. J. Hum. Genet. 56, 577-585. [PMC free article] [PubMed] [Google Scholar]
- Sumigray K. D. and Lechler T (2012). Desmoplakin controls microvilli length but not cell adhesion or keratin organization in the intestinal epithelium. Mol. Biol. Cell 23, 792-799. 10.1091/mbc.e11-11-0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumigray K. D., Chen H. and Lechler T (2011). Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J. Cell Biol. 194, 631-642. 10.1083/jcb.201104009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Guo S. S. and Fässler R (2016). Integrin-mediated mechanotransduction. J. Cell Biol. 215, 445-456. 10.1083/jcb.201609037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T. M., Verkhovsky A. B. and Borisy G. G. (1996). Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J. Cell Biol. 135, 991-1007. 10.1083/jcb.135.4.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemasa T., Sugimoto K. and Yamashita K (1997). Amplitude-dependent stress fiber reorientation in early response to cyclic strain. Exp. Cell Res. 230, 407-410. 10.1006/excr.1996.3428 [DOI] [PubMed] [Google Scholar]
- Tambe D. T., Hardin C. C., Angelini T. E., Rajendran K., Park C. Y., Serra-Picamal X., Zhou E. H., Zaman M. H., Butler J. P., Weitz D. A. et al. (2011). Collective cell guidance by cooperative intercellular forces. Nat. Mater. 10, 469-475. 10.1038/nmat3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Ladoux B. & Toyama Y. (2020). Desmosomal junctions govern tissue integrity and actomyosin contractility in apoptotic cell extrusion. Curr. Biol. 30, 682-690.e5. 10.1016/j.cub.2020.01.002 [DOI] [PubMed] [Google Scholar]
- Toivola D. M., Strnad P., Habtezion A. and Omary M. B. (2010). Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 20, 79-91. 10.1016/j.tcb.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X., Wasserman M. R., Angelini T. E., Millet E., Weitz D. A., Butler J. P. and Fredberg J. J. (2009). Physical forces during collective cell migration. Nat. Phys. 5, 426 10.1038/nphys1269 [DOI] [Google Scholar]
- Tsuruta D. and Jones J. C. R. (2003). The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J. Cell Sci. 116, 4977-4984. 10.1242/jcs.00823 [DOI] [PubMed] [Google Scholar]
- Twiss F. and De Rooij J. (2013). Cadherin mechanotransduction in tissue remodeling. Cell. Mol. Life Sci. 70, 4101-4116. 10.1007/s00018-013-1329-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia R. G., Walko G., Janda L., Novacek J., Mihailovska E., Reipert S., Andrä-Marobela K. and Wiche G (2013). Intermediate filament-associated cytolinker plectin 1c destabilizes microtubules in keratinocytes. Mol. Biol. Cell 24, 768-784. 10.1091/mbc.e12-06-0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kammen R., Song J.-Y., de Rink I., Janssen H., Madonna S., Scarponi C., Albanesi C., Brugman W. and Innocenti M (2017). Knockout of the Arp2/3 complex in epidermis causes a psoriasis-like disease hallmarked by hyperactivation of transcription factor Nrf2. Development 144, 4588-4603. 10.1242/dev.156323 [DOI] [PubMed] [Google Scholar]
- van Hengel J., Calore M., Bauce B., Dazzo E., Mazzotti E., De Bortoli M., Lorenzon A., Li Mura I. E., Beffagna G., Rigato I. et al. (2013). Mutations in the area composita protein alphaT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 34, 201-210. 10.1093/eurheartj/ehs373 [DOI] [PubMed] [Google Scholar]
- van Tintelen J. P., Van Gelder I. C., Asimaki A., Suurmeijer A. J., Wiesfeld A. C., Jongbloed J. D., Van Den Wijngaard A., Kuks J. B., Van Spaendonck-Zwarts K. Y., Notermans N. et al. (2009). Severe cardiac phenotype with right ventricular predominance in a large cohort of patients with a single missense mutation in the DES gene. Heart Rhythm 6, 1574-1583. 10.1016/j.hrthm.2009.07.041 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bowers E., Bauer C., Degenstein L. and Fuchs E (2001). Desmoplakin is essential in epidermal sheet formation. Nat. Cell Biol. 3, 1076-1085. 10.1038/ncb1201-1076 [DOI] [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K. and Fuchs E (1991). Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell 64, 365-380. 10.1016/0092-8674(91)90645-F [DOI] [PubMed] [Google Scholar]
- Verma S., Shewan A. M., Scott J. A., Helwani F. M., Den Elzen N. R., Miki H., Takenawa T. and Yap A. S. (2004). Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J. Biol. Chem. 279, 34062-34070. 10.1074/jbc.M404814200 [DOI] [PubMed] [Google Scholar]
- Verma S., Han S. P., Michael M., Gomez G. A., Yang Z., Teasdale R. D., Ratheesh A., Kovacs E. M., Ali R. G. and Yap A. S. (2012). A WAVE2–Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol. Biol. Cell 23, 4601-4610. 10.1091/mbc.e12-08-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielmuth F., Waschke J. and Spindler V (2015). Loss of desmoglein binding is not sufficient for keratinocyte dissociation in pemphigus. J. Investig. Dermatol. 135, 3068-3077. 10.1038/jid.2015.324 [DOI] [PubMed] [Google Scholar]
- Vielmuth F., Spindler V. and Waschke J. (2018). Atomic force microscopy provides new mechanistic insights into the pathogenesis of pemphigus. Frontiers in Immunology 9, 485 10.3389/fimmu.2018.00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraj P., Kroeger C., Reuter U., Hartmann D. and Magin T. M. (2010). Keratins regulate yolk sac hematopoiesis and vasculogenesis through reduced BMP-4 signaling. Eur. J. Cell Biol. 89, 299-306. 10.1016/j.ejcb.2009.10.016 [DOI] [PubMed] [Google Scholar]
- Wagner O. I., Rammensee S., Korde N., Wen Q., Leterrier J. F. and Janmey P. A. (2007). Softness, strength and self-repair in intermediate filament networks. Exp. Cell Res. 313, 2228-2235. 10.1016/j.yexcr.2007.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ip W., Boissy R. and Grood E. S. (1995). Cell orientation response to cyclically deformed substrates: experimental validation of a cell model. J. Biomech. 28, 1543-1552. 10.1016/0021-9290(95)00101-8 [DOI] [PubMed] [Google Scholar]
- Wang J. H., Goldschmidt-Clermont P., Wille J. and Yin F. C. (2001). Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 34, 1563-1572. 10.1016/S0021-9290(01)00150-6 [DOI] [PubMed] [Google Scholar]
- Weber K. L. and Bement W. M. (2002). F-actin serves as a template for cytokeratin organization in cell free extracts. J. Cell Sci. 115, 1373-1382. [DOI] [PubMed] [Google Scholar]
- Weber G. F., Bjerke M. A. and Desimone D. W. (2012). A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell 22, 104-115. 10.1016/j.devcel.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstead B. and Gull K (2011). The evolution of the cytoskeleton. J. Cell Biol. 194, 513-525. 10.1083/jcb.201102065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windoffer R., Kolsch A., Wöll S. and Leube R. E. (2006). Focal adhesions are hotspots for keratin filament precursor formation. J. Cell Biol. 173, 341-348. 10.1083/jcb.200511124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. K., Lagendijk A. K., Hogan B. M., Gomez G. A. and Yap A. S. (2015). Active contractility at E-cadherin junctions and its implications for cell extrusion in cancer. Cell Cycle 14, 315-322. 10.4161/15384101.2014.989127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Qiu W., Liu R., Efremov A. K., Cong P., Seddiki R., Payre M., Lim C. T., Ladoux B., Mège R.-M. and et al. (2014). Force-dependent conformational switch of α-catenin controls vinculin binding. Nat. Commun. 5, 4525 10.1038/ncomms5525 [DOI] [PubMed] [Google Scholar]
- Yonemura S., Wada Y., Watanabe T., Nagafuchi A. and Shibata M (2010). alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12, 533-542. 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- Zhang H., Landmann F., Zahreddine H., Rodriguez D., Koch M. and Labouesse M (2011). A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 471, 99-103. 10.1038/nature09765 [DOI] [PubMed] [Google Scholar]
- Zhang J., Yue J. and Wu X (2017). Spectraplakin family proteins - cytoskeletal crosslinkers with versatile roles. J. Cell Sci. 130, 2447-2457. 10.1242/jcs.196154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Muroyama A., Underwood J., Leylek R., Ray S., Soderling S. H. and Lechler T (2013). Actin-related protein2/3 complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation. Proc. Natl Acad. Sci. USA 110, E3820-E3829. 10.1073/pnas.1308419110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski A., Linnartz C., Pleschka C., Dreissen G., Springer R., Merkel R. and Hoffmann B (2018). Reorientation dynamics and structural interdependencies of actin, microtubules and intermediate filaments upon cyclic stretch application. Cytoskeleton (Hoboken) 75, 385-394. 10.1002/cm.21470 [DOI] [PubMed] [Google Scholar]