Vaccine strategies are focused on developing protective responses to immunogenic peptide epitopes of pathogens that are normally recognized by T and B cells. However, some epitopes stimulate crossreactive T-cell responses between pathogens and can prime a host to damaging pathology on infection with the crossreactive pathogen. The removal of potentially pathogenic epitopes from vaccines might enhance prophylaxis and reduce the risk of side effects of vaccine-associated disease.
Abstract
Substantial research has been directed towards the development of a new generation of vaccines that are based on the inclusion of immunogenic epitopes in recombinant vectors. Here we examine the evidence that under certain conditions immunogenic epitopes can do more harm than good and might therefore be considered pathogenic. We suggest that the specific removal of such pathogenic epitopes from vaccines might increase their prophylactic potential, while minimizing the risk of side-effects from vaccine use.
Main
The goal of vaccination is to provide lasting immunity to a pathogen. Most vaccination strategies for viral infections aim to generate high titres of high-affinity neutralizing antibodies that bind to virions and prevent productive virus infections1. Variations in the virus-encoded epitopes that are the targets of these neutralizing antibodies complicate vaccination strategies. For example, there are three poliovirus serotypes, each of which has distinct neutralizing epitopes, so that poliovirus vaccines, which have high efficacy, include three strains of inactivated or attenuated polioviruses2. Influenza A viruses continually evolve strain variations due to mutations in the neutralizing epitopes encoded by their surface glycoproteins. These variations can either be due to point mutations (antigenic drift) that are driven in part by immune selection, or be attributable to the complete substitution of glycoprotein genes owing to reassortment with other influenza viruses (antigenic shift)3.
New influenza A virus strains frequently emerge and require novel influenza vaccines that can induce the appropriate neutralizing antibody responses. Influenza poses some interesting additional problems, as immunizations often boost the production of non-protective antibodies that crossreact with previously encountered strains, sometimes at the expense of inducing high quality, protective antibody responses. This phenomenon has been referred to as 'original antigenic sin', whereby a history of a response to crossreactive antigens can bias the response toward those antigens and inhibit the response to a new infection or vaccine4. Dengue viruses also pose problems for vaccine design, with no effective vaccine and millions of human infections every year. There are four dengue virus serotypes, as defined by neutralization, but these serotypes are otherwise antigenically similar. Non-neutralizing antibodies that are specific for one dengue serotype can bind to a second dengue serotype and facilitate the infection of cells that express antibody (Fc) receptors, in a process known as 'immune enhancement'5. Immune enhancement has been linked to severe cases of dengue haemorrhagic fever and dengue shock syndrome, so immunization against one dengue virus serotype has the potential to prime an individual for a more severe infection with a second serotype.
Viral vaccines that are currently licensed for use in humans include inactivated virus preparations (hepatitis A, influenza, Japanese encephalitis, polio and rabies), recombinant proteins (hepatitis B and papilloma) and attenuated viruses that have been passaged in vitro or in eggs to reduce their virulence towards humans (adenovirus, measles, mumps, polio, rubella, varicella-zoster, yellow fever and the modified vaccinia virus (VV) vaccine for smallpox)1,6. Although studies using the passive transfer of serum have clearly shown that antibodies alone can provide protective immunity to some viruses, it is generally thought that vaccines that induce T-cell responses in addition to B-cell responses should produce better and longer lasting protective immunity. Inactivated and recombinant vaccines induce responses by helper CD4+T cells, whose T-cell receptors (TCR) recognize viral peptides that are captured by antigen-presenting cells, processed and presented by their class II major histocompatibility complex (MHC) molecules. Attenuated vaccines are better than inactivated or recombinant vaccines at stimulating good CD8+ T-cell responses, because cells infected with live replicating virus load viral peptide epitopes onto their class I MHC molecules and these epitope-charged MHC molecules are the targets of CD8+ T cells. Many papers have indicated that CD8+ T cells are effective at clearing acute viral infections and at controlling low-level persistent infections, such as those caused by cytomegalovirus (CMV), Epstein-Barr virus (EBV), and herpes simplex viruses (HSV)7. CD8+ T cells are thought to be especially important for the control of HIV-1 and hepatitis C virus (HCV) infections and are being targeted in experimental vaccination studies for both8,9,10.
Three general conclusions can be reached regarding T-cell responses to viral infections. First, although many viral peptides can be processed and presented by MHC molecules, the T-cell response is mostly directed against a relatively small number of discrete 'immunodominant' epitopes. What causes immunodominance is not completely understood, but it requires the ability of the cell to enzymatically digest a protein into a particular peptide, and requires that peptide to have the appropriate amino-acid sequence to bind with good affinity to the antigen-presenting groove of the MHC molecule11. The amount of protein synthesized, and when it is synthesized, during the infection cycle are also important factors. Many dominant epitopes are derived from proteins that are expressed early during infection, before the cellular antigen-presenting system is shut down, an event which occurs in many, but not all, viral infections. Of great importance is the availability of a repertoire of T cells that can recognize the epitope12,13,14. Some studies have indicated that one in 50–100,000 naive T cells recognizes a specific epitope, though this average number is likely to vary with the epitope15,16. Immunodominance patterns of antigenic epitopes alter if a host has been previously infected with a pathogen that has T-cell crossreactive epitopes17,18. T-cell recognition of peptide–MHC complexes can be quite degenerate, with estimates that a single TCR can recognize up to 105 to 106 peptide–MHC combinations19,20. This might be partly due to the ability of a TCR to adjust its conformation to bind to the part of the peptide presented by the MHC21. If the T-cell memory pool contains T cells that crossreact with an epitope of a newly encountered virus, those T cells could proliferate and dominate the subsequent response. In humans it can be difficult to determine whether an epitope is intrinsically immunodominant, or whether its immunodominance is attributed to crossreactivity with a previously encountered pathogen.
Second, broad-based T-cell responses that are directed against several epitopes are desirable22,23,24. It was noted, for example, that HIV-1-infected individuals with CD8+ T-cell responses directed mainly against a single epitope developed full-blown AIDS more rapidly than individuals with CD8+ T-cell responses directed against several epitopes24. One advantage of a broad-based immune response is that the virus is less likely to accumulate mutations that enable escape from the immune response. Studies of HCV in chimpanzees and lymphocytic choriomeningitis virus (LCMV) in mice have correlated narrow T-cell repertoires with the generation of escape mutants23,25. A second advantage of a broad-based T-cell response under conditions of high viral load is that it might be more difficult for the virus to exhaust the immune response to several epitopes than it is to exhaust the response to a single epitope26,27,28. Repeated stimulation of T cells can 'stun' them and render them dysfunctional (anergic), or drive them into apoptosis through the process known as activation-induced cell death27,29. This impairment or elimination of specific T cells is known as clonal exhaustion. Studies of persistent LCMV infections in mice revealed different degrees of T-cell exhaustion that varied with the epitope26,27,28.
Third, there can be a delicate balance between the ability of T cells to provide protective immunity and clear an infection, and their propensity to mediate damaging immunopathology. The balance between protective immunity and immunopathology might be a consequence of the number of responding T cells compared with their efficacy in clearing the virus. DNA immunization of mice with the LCMV nucleoprotein gene, which encodes a potent CD8+ T-cell epitope, results in a rapid immunopathology when mice are subsequently infected intracerebrally with LCMV. In contrast, DNA immunization with the glycoprotein gene, which encodes neutralizing antibody epitopes as well as CD8+ and CD4+ T-cell epitopes, results in protective immunity30.
Over the past few years, concerted efforts have been made to identify the immunodominant T-cell epitopes of several human viruses and to incorporate these epitopes into vaccines. Specific sequences coding for epitopes and cytokine enhancing factors can be incorporated into viral vectors or DNA vaccines. The same approach offers promise in tumour immunotherapy, through the design of vaccines that incorporate immunogenic epitopes presented by human tumour cells31. In this Opinion, however, we question whether designing vaccines against a small number of epitopes is a good strategy for virus infections, in which there are many potential epitopes that are presented by various MHC molecules, with the attendant possibility of viral escape variants and different crossreactivities. In particular, we question this because viral epitopes can sometimes be pathogenic and induce T-cell responses that cause immunopathology rather than protective immunity. Alternatively, they can induce poorly protective T-cell responses, which inhibit the induction of responses against more protective epitopes.
T cells and heterologous immunity
Heterologous immunity is a term used to describe the partial immunity (or altered immunopathology) that occurs in response to a pathogen if the host has been previously infected or immunized with an unrelated pathogen. In some cases, heterologous immunity is attributed to T cells that crossreact with both pathogens. CD8+ T-cell crossreactivity has been documented between two epitopes on the same viral protein32, between epitopes of different proteins from the same virus33 and, more importantly, between epitopes of proteins from different viruses34. Many examples of T-cell crossreactivity among viruses have been documented, and this crossreactivity can, perhaps unsurprisingly, occur between genetically related viruses within virus groups, such as arenaviruses18, flaviviruses (dengue)35, polyomaviruses36, hantaviruses37 and orthomyxoviruses (influenza A)38.
More surprisingly, T-cell crossreactivity has been found between unrelated viruses. For example, T cells that are specific for distinct influenza A virus antigens crossreact with HCV39, HIV-140 and EBV41 antigens. Some T cells that are specific for the arenaviruses LCMV and Pichinde virus (PV) crossreact with antigens of the poxvirus vaccinia42. Some human T cells that are specific for a coronavirus epitope crossreact with a papillomavirus epitope43. These crossreactivities might be due to a high level of amino-acid sequence similarity between epitopes, although this is not always the case. Crossreactivity occurs between the immunodominant HLA-A2.1-restricted influenza A virus epitope M158–66 and the major EBV-specific epitope BMLF1280–288 and these epitopes share only three out of nine amino acids. When a host that is immune to one virus is infected with another virus that encodes a crossreactive epitope, there can be a shift in the immunodominance hierarchy18, because crossreactive memory-T-cell populations might be present at much higher frequencies than antigen-specific naive T cells and could therefore dominate the response.
Narrowing of TCR repertoires. An extensive analysis of the T cell repertoire that is produced in response to a crossreactive class I MHC Kb-restricted epitope (NP205–212) between LCMV and PV has been carried out44. These two epitopes share six out of eight amino acids and are highly crossreactive at the effector level18. TCR Vβ clonal analyses showed that mice infected with either LCMV or PV generated broad-based T-cell responses, in which for every 14 clones sequenced there were at least 10 distinct clonotypes. Homologous challenge of LCMV-immune mice with LCMV slightly skewed the response toward a predictable Vβ clonotype with a typical amino-acid pattern (or 'motif'), in the antigen-binding complementarity-determining region 3 (CDR3) of the TCR. However, following heterologous viral challenge, a dramatic narrowing of the T-cell repertoire was observed. This is probably because only a subset of the memory CD8+ T cells reacted to the crossreactive peptide with sufficient affinity to induce proliferation. Thus, not only was the T-cell response skewed toward a normally subdominant epitope (Fig. 1), but the normally diverse TCR usage was reduced to a narrow oligoclonal response. Predictably, PV-immune mice that were infected with a dose of LCMV sufficient to cause a persistent infection generated an NP205–212 epitope escape mutant of LCMV in the presence of such a narrow T-cell response44.
Figure 1. Narrowing of a T-cell response by crossreactivity and heterologous immunity.
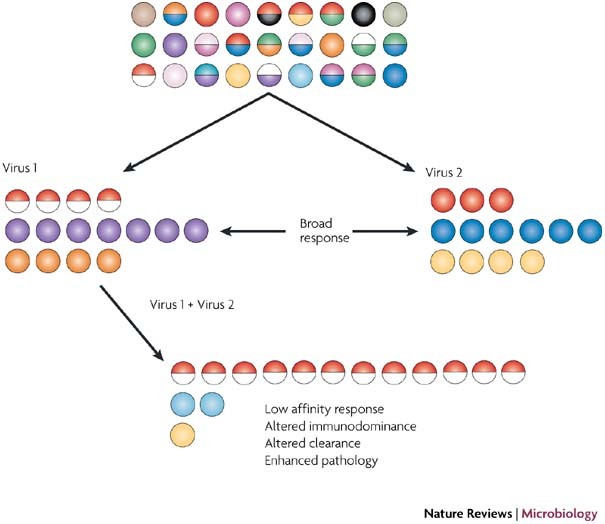
This figure shows a diverse naive repertoire of T cells. Two different, but crossreactive, viruses stimulate T cells with different specificities to different epitopes. The red and half-red circles indicate crossreactive responses. Infection of a virus immune host with the crossreactive virus (virus 2) causes a change in immunodominance and a focusing of the immune response by expanding crossreactive T cells.
One caveat is that although repertoire narrowing might be expected to occur in most cases of crossreactivity44,45, it does not always occur if the repertoire is narrow to start with. In such cases heterologous immunity might actually broaden the response by recruiting T cells that would not normally be dominant clones46. Why TCR repertoires for specific epitopes are diverse in the first place is unclear, although one report has correlated this diversity with the complexity of the amino-acid epitope side chains that are presented by the MHC47.
Impact of TCR private specificity. Genetically identical individuals, such as monozygotic twins, have genetically different immune systems because T- and B-cell repertoires are generated from independent, random DNA recombination events22. A naive T-cell repertoire has millions of T cells present at low frequencies, so that immune responses to viral infections are similar in genetically identical naive mice, despite differences in TCR usage46. This is shown in Fig. 2, in which genetically identical individuals mounted quantitatively similar acute responses that used different T-cell repertoires. However, when an immune repertoire is altered by the deposition of highly expanded clones of memory T cells that vary in sequences between individuals, the impacts of these private TCR repertoires might subsequently be observed following infection with a heterologous pathogen.
Figure 2. Impact of TCR private specificities on heterologous viral challenge.
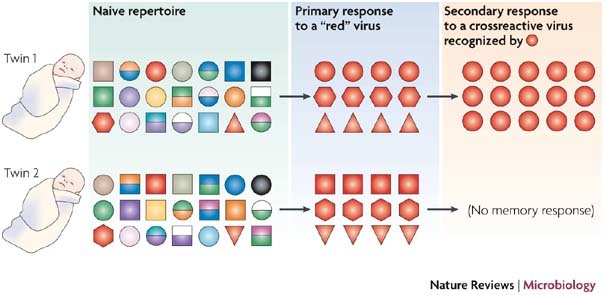
This figure shows genetically different naive T-cell repertoires from genetically identical individuals, such as identical twins. Although using different TCRs, the twins' naive immune systems make quantitatively similar responses to a primary virus challenge that stimulates the T cells that have a 'red' TCR. However, they now have expanded clones of memory T cells, which might (red circles, top) or might not (no red circles, bottom) respond to a non-identical but crossreactive heterologous pathogen. These private TCR specificities cause an alteration in memory responses to a second pathogen. TCR, T-cell receptor.
For example, the sequential infection studies using LCMV and PV (discussed previously) revealed dramatic differences in the magnitude of the crossreactive responses and in the crossreactive epitope-specific TCR usage between mice44. These variations in the crossreactive responses reflected the private specificities of the immune repertoire of individual immune mice, because transfer of splenocytes from one immune donor into three naive recipient mice resulted in quantitatively and qualitatively similar T-cell responses after challenge with the heterologous virus. These studies indicate that the private specificity of the TCR repertoire (which is unique to the individual) might have a central role in immune responses to viruses during infection if crossreactive responses and heterologous immunity are present. It also implies that individuals might experience different types of pathologies when heterologous immunity is a factor.
Altered immunopathology and patterns of crossreactivity. Experimental models have suggested that changes in T-cell hierarchies are accompanied by changes in viral load and alterations in immunopathology34. Changes in pathology as a consequence of heterologous immunity have been studied by challenging mice that are immune to LCMV with VV. VV grows to 10–100-fold higher titres in naive mice compared with LCMV-immune mice, and adoptive transfer studies have implicated LCMV-specific memory CD4+ and CD8+ T cells in this protective immunity48.
Despite these changes in protective immunity there are considerable differences in immunopathology. Naive mice that were intranasally infected with VV developed severe airway-obstructing oedema in the lungs, whereas LCMV-immune mice that were infected with VV have little oedema, but instead have large defined cellular infiltrates (bronchus-associated lymphoid tissue (BALT)) that contain LCMV-specific T cells49. In addition, these mice present with bronchiolitis obliterans, a pathology of unknown aetiology in humans that occurs after infection or lung transplantation. Whereas naive mice infected intraperitoneally with VV have little pathology in the visceral fat of the peritoneal cavity, LCMV-immune mice that are challenged with VV experience a severe fatty necrosis and panniculitis that is associated with the infiltration of fat with LCMV-specific T cells48. This resembles the pathology of erythema nodosum, a human disease of unknown origin that sometimes occurs after vaccination or viral infection.
T-cell crossreactivity between LCMV and VV is complex and involves several epitopes, but the epitope specificity of LCMV-specific T cells that proliferate in response to VV infection varies among LCMV-immune mice42. This variation in T-cell crossreactivity between individuals is also a function of the private specificity of the T-cell repertoire, rather than random events, because adoptive transfers of splenocytes from LCMV-immune donor mice into three naive recipients resulted in similar specificities of the LCMV-specific recall responses following VV challenge. This indicates that even though two individuals with a similar MHC composition can experience infections with the same two viruses, these individuals will not necessarily generate strong crossreactive T-cell responses to the same epitopes, as shown in Fig. 2. This might be reflected by variations in the resultant immunopathology suffered by individuals42.
Viral load and heterologous immunity. Most studies on heterologous immunity between pathogens in C57BL/6 mice have shown reduced levels of replication of the challenge virus, as quantified by viral plaque assays as early as 2–3 days post-infection. Associated with this inhibition is the activation of the pre-existing memory T cells and their synthesis of high levels of interferon (IFN)-γ48,49,50. In some pathogen combinations there is increased replication of the challenge pathogen, much like that observed for antibody-mediated immune enhancement with dengue virus. For example, although prior infection with influenza virus might render a mouse more resistant to a subsequent VV infection, it increases the susceptibility of the mouse to infection with LCMV or murine CMV50. The mechanism underlying this phenomenon has not been elucidated, but might relate to altered T-cell responses, owing to heterologous immunity, that are less efficient at controlling viral titres compared with newly generated T cells in a naive repertoire.
Human disease
Heterotypic immunity to influenza A virus. The term heterologous immunity is currently used to describe the potential impact of T-cell-dependent crossreactive immunity between unrelated viruses34. The term 'heterotypic immunity' has long been used to describe T-cell responses to preserved determinants on different variants of influenza virus, but the systems are conceptually similar51. Through original antigenic sin, antibodies and T cells can crossreact to different influenza strains and dominate immune responses4, and when viral strains have mutations in T-cell epitopes, more narrow T-cell repertoires are generated52. It is noteworthy that all influenza A viruses that have infected humans in the past 80 years have expressed a conserved HLA-A2.1-restricted M158–66 immunodominant epitope53. As individuals are infected by a series of influenza A strains during their lifetimes, a TCR repertoire that is specific for this epitope evolves from the diverse use of several Vβ families to a virtually exclusive Vβ 17 response using a discrete amino-acid motif in the CDR3 region of the TCR54. Whether these responses are helpful in controlling influenza A viral infections is unclear, but it seems unlikely that they are particularly beneficial, because immune individuals remain susceptible to infection with crossreactive influenza virus strains.
Crossreactivity between influenza A virus and EBV. Many viral diseases result in more severe symptoms in teenagers and young adults than in young children55,56. Such diseases include chicken pox, measles, mumps, polio and, perhaps most characteristically, infectious mononucleosis. The difference between a clinical and a subclinical case of infectious mononucleosis seems to be related to the magnitude of the CD8+ T-cell response rather than the virus load57. It is proposed that diseases that have more severe pathologies in this older age group might involve the activation of crossreactive memory T cells that were generated in response to a previously encountered virus or other microorganism34. Such immune responses may be robust, owing to their generation from high frequencies of memory cells, but they may not have sufficient affinity, or be broadly based enough, to rapidly clear the pathogen. Consistent with this hypothesis, T cells that are crossreactive between the influenza M1 epitope and the main immunodominant BMLF1 epitope of EBV are present at a high frequency during infectious mononucleosis41.
There can be wide variations in the severity of infectious mononucleosis, but crossreactivity is not found in all patients, perhaps reflecting private specificities in the patients' immune T-cell repertoires. Although it is plausible that the EBV infection, as well as infections with other potentially crossreactive viruses, serve to modify the influenza-specific T-cell repertoire as individuals age, this possibility has not been studied.
Crossreactivity between influenza A virus and HCV. One viral disease that presents with extreme differences in symptoms between individuals is hepatitis C. HCV can cause either clinical or subclinical infections and can either be cleared from the host or cause a lifelong persistent infection. The reasons for this variation are unknown. T-cell crossreactivity, however, has been found between a major immunodominant HLA-A2.1-restricted epitope of HCV (NS31073–1081) and the NA231–239 epitope of influenza A virus39. A study carried out on the breadth of the T-cell response to a library of peptides that encompass the entire HCV genome revealed that many acute HCV patients generated T cells that recognized a wide array of peptides. However, two patients with persistent severe fulminant HCV were found to have narrow T-cell responses that were almost exclusively directed against a peptide that spans the crossreactive influenza virus and HCV epitopes58. This important finding, in common with studies carried out using animal models, links severe pathology to T-cell crossreactivity in humans. As all of the patients had presumably previously encountered influenza A viral infections, their private specificities probably dictated the dominance of the crossreactive T-cell responses.
Crossreactivity between different serotypes of dengue virus. Dengue haemorrhagic fever and dengue shock syndrome are severe diseases which occur most frequently in individuals that are immune to one dengue virus serotype and become infected with a second serotype (there are four serotypes of dengue virus). These syndromes might be partly due to the presence of immune-enhancing antibodies that facilitate the infection of cells by dengue virus, but crossreactive T cells might also be involved. One study showed that T-cell responses to a crossreactive epitope during dengue virus serotype 3 infection had a high affinity to a previously encountered dengue virus serotype 2, but relatively low affinity to the newly encountered dengue virus serotype 3 (Ref. 59). This could have been due to the elicitation of crossreactive memory T cells that dominated the immune response but were inefficient at clearing virus. The dramatic variations in disease between individuals infected with dengue viruses could indeed reflect private specificities of immune responses.
Immune deviation
Differentiation of T cells produces T cell subtypes that are characterized by distinct patterns of cytokine production, and one consequence of reactivating memory T cells is the synthesis and secretion of large concentrations of the cytokines that they have been programmed to synthesize49. Reactivation of memory T cells that produce type 1 cytokines (for example, IFN-γ) might therefore help to prime a new response into a type 1 direction, whereas reactivation of memory cells that express type 2 cytokines (for example, interleukin-4 (IL-4)) might drive immune responses in a type 2 direction.
The classic example of a 'vaccine gone wrong' is that of the formalin-inactivated respiratory syncytial virus (RSV) vaccine that was administered to children in the 1960s60. Following exposure of children to wild-type RSV several months after vaccination, many children were not protected from infection and instead developed severe infections, some of which were fatal. These patients presented at the clinic with an unusual lung pathology that included eosinophilia. Experimental mouse models of RSV have reproduced some of the features of this disease. Balb/c mice that were immunized with the RSV G protein, either provided in adjuvant, or in the form of a VV recombinant vaccine (VV-G), developed a severe eosinophilia and a TH2 CD4+ T-cell response after subsequent challenge with RSV. This contrasted with the TH1 response that was mounted by non-vaccinated RSV-challenged control mice, which easily controlled the infection61,62,63. The reason for the severe immune deviation resulted from a combination of the expression of a specific class II pathogenic epitope encoded by the RSV G protein and a rather limited repertoire of CD4+ T cells expressing Vβ14 TCR61. However, mice that were infected with influenza virus prior to VV-G immunization did not develop eosinophilia when subsequently challenged with RSV62. Influenza A virus-immune mice challenged with VV produce much higher levels of type 1 cytokines (IFN-γ) than VV-challenged naive mice49,50, so the IFN-γ induced by heterologous immunity between influenza and VV might have changed a type 2 cytokine response into a type 1 cytokine response following immunization with VV-G.
Molecular mimicry and self antigens
Pathogenic epitopes in autoimmunity. The term 'pathogenic epitope' was originally coined to describe epitopes that are the targets of autoimmune reactions. Experimental autoimmune encephalitis (EAE) is a central nervous system (CNS) disease of animal models for human multiple sclerosis (MS). In the mouse model of MS, pathogenic class I and II MHC-restricted epitopes encoded by CNS proteins have been identified. These include epitopes that are derived from myelin basic protein (MBP), myelin proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein and these proteins, and the encephalitogenic peptides that are derived from these proteins, can induce EAE when injected with adjuvant into mice. The pathogenic epitopes are highly specific. The N-terminal 9 amino-acid sequence from MBP comprises a pathogenic epitope for the PL/J strain of mouse, with the MHC of H2u. Its N-terminal amino acid is acetylated64, but if a non-acetylated peptide is used EAE is not induced. Development of EAE is a highly regulated process and epitopes administered under different immunization schemes can sometimes result in T cell anergy or tolerance instead of autoimmunity65.
Autoimmune T-cell responses share commonality with anti-viral T-cell responses, in that they can comprise T cells that react against several different epitopes. Injection of SJL/J mice (H2s) with the intact PLP molecule results in activation of CD4+ T cells that are specific for co-dominant PLP139–151 and PLP178–191 epitopes, and immunization of mice with either of these epitopes induces EAE. Mice immunized with a subdominant PLP104–117 epitope develop EAE but far more slowly65. Similarly, adoptive transfer of CD4+ T cells from mice sensitized with PLP104–117 into naive mice results in slower EAE development compared with that induced in PLP139–151- or PLP178–191- immunized mice66. Interestingly, little reactivity is observed to PLP104–117 when mice are immunized with intact PLP. Therefore, PLP104–117 is referred to as a non-dominant cryptic T-cell determinant.
Although autoimmunity is often initially associated with just a few pathogenic epitopes, these immune responses can sometimes spread to epitopes that are encoded by other proteins. For example, immunization of SJL/J mice with PLP139–151 will subsequently generate a response to MBP89–101. This has led to the concept of 'determinant spreading'67. In this case, activated antigen-presenting cells within the inflammatory milieu engulf myelin, degrade it and present other myelin epitopes to T cells. This might be the opposite of what is observed in sequential viral infections, in which crossreactivity could drive a narrowing of the T-cell response44.
Precipitation of autoimmunity by viral infection. Some viral and self epitopes can crossreact at the level of T cells or B cells. Infection of animals with viruses that encode epitopes that are crossreactive with self antigens, or infection with recombinant viruses encoding engineered self-epitopes, can result in activation of self reactive T cells that initiate autoimmune disease. An epitope expressed by the UL6 protein of HSV-1 crossreacts with a corneal antigen in an HSV-1-induced murine model of autoimmune keratitis68,69. Theiler's murine encephalomyelitis virus (TMEV) infection of mice induces TMEV viral protein (VP)-1-specific crossreactive T cells that can produce IFN-γ and kill uninfected host cells. Transfer of these T cells into naive uninfected mice induces CNS inflammation70,71. Insertion of a bacterial epitope from protease IV of Haemophilus influenzae into TMEV increases the rate of TMEV-induced inflammation in the CNS72. Incorporation of self CNS proteins into recombinant VV also primes mice for autoimmune disease73,74,75.
Some studies have indicated that autoimmunity might be induced by viral infection in humans as well as in mice. HLA-DR3 predisposes humans to autoimmune diabetes, with glutamic acid decarboxylase (GAD65) the targeted autoantigen in this disease. Studies have defined a CD4+ T-cell crossreactive HLA-DR3-restricted response against epitopes with sequence similarity between the autoantigen and a CMV-encoded antigen76. MBP-reactive T cells that were isolated from MS patients react with various viral and bacterial peptides. The crossreactive viral or microbial peptide epitopes do not always share amino-acid similarity but can still activate the MBP-specific T-cell clones. Recently, however, CD4+ T cells that were isolated from MS cerebrospinal fluid were shown to react with polyarginine sequences that are similar to those encoded by the CNS α-1B adrenergic receptor and several viruses. One virus that encodes an epitope homologous to the CNS α-1B adrenergic receptor is the ubiquitous Torque Teno 'orphan' virus, a small DNA virus that replicates in the CNS but has not been associated with any disease77. Arginine-rich domains are found in the DNA-binding proteins of many viruses, leading to the possibility that exacerbation of MS could be related to infections with different viruses.
On occasion, individuals develop post-infectious encephalomyelitis following acute measles virus infection78. This is an inflammatory response within the CNS that mirrors EAE in the Lewis rat. Post infectious encephalomyelitis following infection with measles virus might be due to a crossreactive immune response between measles virus and a CNS protein, but this has yet to be proven.
Autoimmunity and heterologous immunity. Experimental model systems for studying the ability of viral infections to break tolerance and induce autoimmunity have included transgenic mice that express viral proteins as 'self' antigens79,80,81,82. LCMV infection induces a transient encephalitis (or limited insulitis) in transgenic mice that express an LCMV nucleoprotein in the brain or pancreatic islets. After the LCMV infection is cleared, a heterologous infection with either PV or VV can induce a second wave of CNS inflammation80 (Fig. 3). This model might mimic the viral infection-associated exacerbation of human MS77. After LCMV infection in the insulitis model, it was noted that PV challenge induced pancreatic inflammation and diabetes associated with the infiltration and proliferation of crossreactive NP205–212-specific T cells. The LCMV infection (but not the PV infection) could break tolerance to NP205–212, but the PV infection of LCMV-immune mice further stimulated crossreactive cells and pushed the host into diabetic disease82.
Figure 3. Progression of virus-induced autoimmunity.
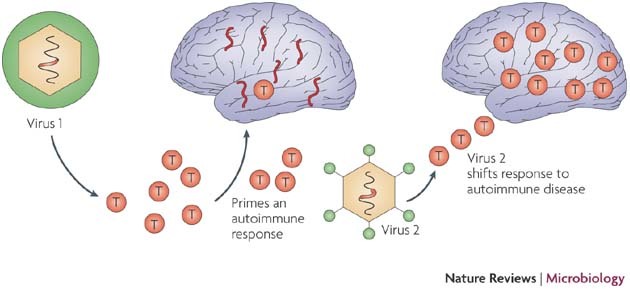
This figure shows how infection with a virus (virus 1) that encodes a self-like epitope might initiate an autoimmune process and how infection with a second virus (virus 2) might shift a controlled response into one that causes an autoimmune disease.
In a different experimental model for MS, mice that were immune to one virus that encodes epitopes that mimic myelin developed CNS inflammatory lesions and associated demyelination, after infection with a different virus or Mycobacterium tuberculosis in adjuvant. Mice infected with a recombinant VV encoding the CNS protein PLP cleared the virus after two weeks. If these VV-immune mice were then challenged with M. tuberculosis in adjuvant or were infected with murine CMV, they developed CNS white matter lesions73,74. One explanation for this observation is that the first virus infection primes autoreactive T cells that are stimulated by the second pathogen, possibly owing to crossreactivity. However, because the autoantigen is present, the second pathogen might be stimulating the disease through a bystander activation event. In these examples the first pathogen does not expand the autoreactive T cells to sufficient numbers to initiate CNS lesions, but the second challenge expands crossreactive T cells, thereby causing the CNS lesions (Fig. 3). It is known that a threshold number of autoreactive T cells must be generated during a sensitization regimen, or provided by adoptive transfers of T cells, for an autoimmune disease to occur. Heterologous immunity between viruses provides a potential mechanism for amplifying an autoimmune response that is initiated by the first virus. One natural situation in which this is posited to occur is human MS, in which several viruses encode polyarginine sequences that can be recognized by CD4+ T cells that are isolated from the CNS77.
Pathogenic epitopes and vaccine design
It is clear that specific virus-encoded T-cell epitopes could contribute to aberrant immunopathologies during viral infections. This might be achieved through: stimulating immunodominant low-affinity oligoclonal responses that inhibit broad-based high-affinity responses to other well-presented epitopes; deviating protective cytokine responses to damaging cytokine responses; or eliciting responses against autoantigens. In this Opinion we have described how the LCMV NP205–212 epitope might function as a pathogenic epitope in models of heterologous immunity and immunopathology18,44,82, how the influenza M158–66 epitope could be a pathogenic epitope in infectious mononucleosis patients infected with EBV41, and how crossreactive dengue virus epitopes might contribute to low-affinity T-cell responses and poor clearance of virus during dengue haemorrhagic fever59. We have also discussed how an RSV G protein epitope might produce a deviated type 2 cytokine-induced pathogenic immune response61,62,63 and how an influenza virus NA231–239 epitope could contribute to narrowly focused immunodominant T-cell responses that are associated with fulminant HCV. In all of these examples the pathogenic epitope seems to direct immune responses away from those that are best suited to clear the viral infection with minimal pathology.
Research is now being directed at defining immunodominant viral epitopes, in order to include them in vaccines that are designed to strongly focus an immune response against protective epitopes, to improve the efficacy of immunization. We caution against emphasis on directing vaccine strategies towards a small number of immunodominant epitopes, because broad-based responses using diverse TCR repertoires that are directed against several epitopes might provide better protective immunity and could reduce the possibility of selecting disease-producing virus escape variants, as has been suggested by clinical and experimental studies8,23,24,25,44. In addition, the large variation in human MHC molecules that present different peptide epitopes and in virus-encoded epitope sequences, which sometimes occur through the generation of escape mutations, are problematic for epitope-based vaccine designs.
Perhaps equal attention should be directed toward the elimination of pathogenic epitopes from vaccines. When empirical studies link aberrant human immune T-cell responses and immunopathology to specific epitopes, eliminating them from vaccines might be appropriate. For example, would it be wise to develop an attenuated HCV vaccine that contained an epitope that is crossreactive with influenza A virus, when it is likely that almost all recipients of any HCV vaccine would have previously been infected with the influenza A virus? It is probable that some of the vaccinated individuals would develop a focused oligoclonal response to the crossreactive epitope that might promote, rather than inhibit, disease. Is it wise to include the M158–66 epitope in an attenuated live influenza A virus vaccine, when most HLA-A2.1-expressing adults have T-cell responses to that epitope already and when it might inhibit the induction of broad-based responses to other influenza epitopes (as shown in Fig. 4)? Would it make sense to mutate the BMLF1280–288 epitope from a putative EBV vaccine, because it might crossreact with that same ubiquitous influenza A virus M1 epitope? Is it advisable to immunize HLA-DR3+ individuals with an attenuated CMV containing the epitope that stimulates crossreactivity with the diabetes-associated autoimmune antigen GAD65?76 These types of issues have, for a long time, been considered problematic for developing dengue virus vaccines, given the correlation of immune-enhancing antibodies with dengue haemorrhagic fever.
Figure 4. Heterotypic immunity with influenza A virus.
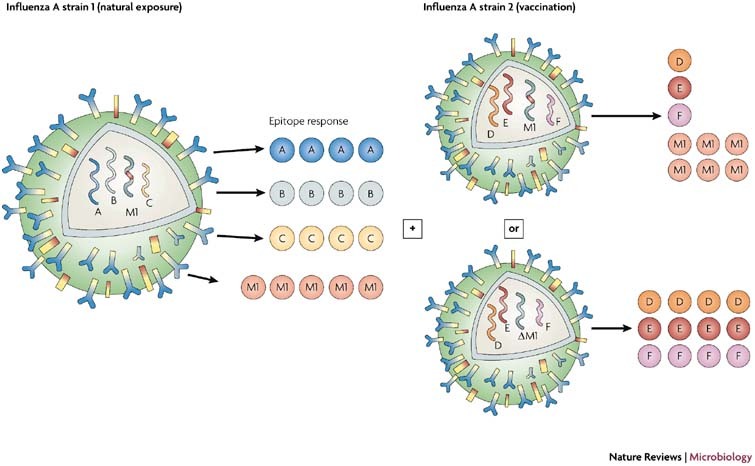
This figure illustrates a theoretical T-cell response to various influenza A virus epitopes (influenza virus A strain 1), including the highly conserved and immunodominant M158–66 epitope (coloured red). Vaccination with a different influenza A strain (strain 2) should boost the response to the M158–66 epitope at the expense of responses to other epitopes. We propose that if the M158–66 epitope was deleted (ΔM1) or mutated, a more effective response might be induced to a broader selection of epitopes (right-hand side, lower panel).
We argue that these questions should be considered as new vaccines are being designed, but we recognize that such considerations come with several caveats. Some epitopes, such as the highly conserved M158–66 influenza epitope, might be difficult to delete while preserving the viability of an attenuated virus. Importantly, many epitopes that could potentially be deleted might contribute to protective immune responses in most individuals, and a risk/benefit analysis might be needed to assess the protective benefits versus what could be rare incidences of damaging immune pathology. Heterologous immunity is often beneficial because crossreactive responses can augment clearance of the challenge virus34,48. Indeed, the private specificities of immune responses that are unique to the individual might ensure variability in the responses (and pathologies) experienced by vaccine recipients.
Acknowledgements
This work was supported by the National Institute of Health (NIH) research grants AI17672 and AR35506 to R.M.W. and NS040350 and AI581501 to R.S.F. The opinions expressed are solely those of the authors and do not necessarily reflect the official views of the NIH. We thank L. Selin for reviewing the manuscript and K. Daniels and M. Cornberg for artwork.
Glossary
- Bystander activation
The term as it is used here refers to the virus-induced activation of T cells whose TCRs are not being triggered by the antigens that are driving the immune response. The TCRs are triggered by third party self (auto) antigens and this activation might be mediated by cytokines.
- Complementarity-determining region
(CDR). The antigen-receptor region (in T- and B-cell receptors) that interacts with the antigen, in which hypervariable sequences are located. CDR3 is partly encoded by the germline variable (V), diversity (D) and joining (J) regions of each receptor chain. Extensive diversity is generated during gene rearrangement by nucleotide trimming and/or template-independent nucleotide additions by terminal deoxynucleotidyl transferase.
- Immune deviation
The channelling of T cells through discrete differentiation series associated with distinct production of and responses to cytokines. For example, TH1 CD4+ T cells that preferentially synthesize IFN-g and TH2 cells which preferentially synthesize IL-4.
- Memory CD8+ T cells
Class I MHC-restricted T cells that have previously been stimulated by antigen and are currently in a resting state. Memory T cells are present at high frequencies in immune hosts.
- Oligoclonal
This refers to a T-cell response that is composed of a small number of T-cell clones, in contrast to a polyclonal response, which involves many clones.
- Recall response
When the first encounter with the antigen has been cleared, a subsequent immunization with the same antigen induces a memory T- and B-cell response that is referred to as a recall response.
- Tolerance
A state of T-cell unresponsiveness to stimulation with antigen. It can be induced by either improper stimulation or stimulation with a large amount of specific antigen in the absence of the engagement of co-stimulatory molecules.
Biographies
Raymond M. Welsh, Ph.D., is Professor of Pathology, Molecular Genetics and Microbiology at the University of Massachusetts Medical School in Worcester, USA. He received his Ph.D. at the University of Massachusetts at Amherst and subsequently carried out postdoctoral training at the University of Kansas at Lawrence, USA, and at the Scripps Research Institute in La Jolla, USA, where he later became a junior faculty member. His research focuses on the regulation of NK cell and T-cell responses to viral infections and on the role of heterologous immunity in viral pathogenesis.
Robert S. Fujinami, Ph.D., is Professor of Neurology at the University of Utah School of Medicine in Salt Lake City, USA. He received his Ph.D. at Northwestern University in Chicago, USA, and carried out postdoctoral training at the Scripps Research Institute prior to appointment as a junior faculty member at the Scripps Research Institute in La Jolla, USA. He was subsequently appointed Associate Professor of Pathology at the University of California at San Diego before moving to his current position. His research focuses on autoimmunity, viral immunology, neurovirology and on the role of molecular mimicry in virus-induced immunopathology of the central nervous system.
Related links
DATABASES
Entrez Genome
FURTHER INFORMATION
Competing interests
The authors declare no competing financial interests.
References
- 1.Plotkin S, Orestein W. Vaccines. 1999. [Google Scholar]
- 2.Zimmerman RK, Spann SJ. Poliovirus vaccine options. Am. Fam. Physician. 1999;59:113–116. [PubMed] [Google Scholar]
- 3.Treanor J. Influenza vaccine — outmanoeuvering antigenic shift and drift. N. Engl. J. Med. 2004;350:218–220. doi: 10.1056/NEJMp038238. [DOI] [PubMed] [Google Scholar]
- 4.Fazekas de St. Groth S, Webster RG. Disquisitions on original antigenic sin. II. Proof in lower creatures. J. Exp. Med. 1966;124:347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead SB. Antibody, macrophages, dengue virus infection, shock and hemorrhage: a pathogenetic cascade. Rev. Infect. Dis. 1989;11:S830–S839. doi: 10.1093/clinids/11.Supplement_4.S830. [DOI] [PubMed] [Google Scholar]
- 6.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J. Clin. Invest. 2006;116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu. Rev. Immunol. 2004;22:711–743. doi: 10.1146/annurev.immunol.22.012703.104527. [DOI] [PubMed] [Google Scholar]
- 8.Gaschen B, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 9.Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 10.Sette A, et al. Optimizing vaccine design for cellular processing, MHC binding and TCR recognition. Tissue Antigens. 2002;59:443–451. doi: 10.1034/j.1399-0039.2002.590601.x. [DOI] [PubMed] [Google Scholar]
- 11.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Bennink JR, Morton PA, Yewdell JW. Mice deficient in perforin, CD4+ T cells, or CD28-mediated signaling maintain the typical immunodominance hierarchies of CD8+ T-cell responses to influenza virus. J. Virol. 2002;76:10332–10337. doi: 10.1128/JVI.76.20.10332-10337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Gruta NL, et al. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc. Natl Acad. Sci. USA. 2006;103:994–999. doi: 10.1073/pnas.0510429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Anton LC, Bennink JR, Yewdell JW. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity. 2000;12:83–93. doi: 10.1016/S1074-7613(00)80161-2. [DOI] [PubMed] [Google Scholar]
- 15.Blattman JN, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selin LK, Vergilis K, Welsh RM, Nahill SR. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J. Exp. Med. 1996;183:2489–2499. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klenerman P, Zinkernagel RM. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:421–422. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- 18.Brehm MA, et al. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nature Immunol. 2002;3:627–634. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 19.Mason D. A very high level of crossreactivity is an essential feature of the T cell repertoire. Immunol. Today. 1998;19:395–404. doi: 10.1016/S0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DB, et al. Specificity and degeneracy of T cells. Mol. Immunol. 2004;40:1047–1055. doi: 10.1016/j.molimm.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Boniface JJ, Reich Z, Lyons DS, Davis MM. Thermodynamics of T cell receptor binding to peptide-MHC: evidence for a general mechanism of molecular scanning. Proc. Natl Acad. Sci. USA. 1999;96:11446–11451. doi: 10.1073/pnas.96.20.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nature Rev. Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Olson D, et al. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J. Exp. Med. 2004;200:307–319. doi: 10.1084/jem.20040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrow P, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nature Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 25.Pircher H, et al. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 26.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 27.Zhou S, Ou R, Huang L, Moskophidis D. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 2002;76:829–840. doi: 10.1128/JVI.76.2.829-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ, Blattman JN, Murali-Krishna K, van der MR, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razvi ES, Welsh RM. Programmed cell death of T lymphocytes during acute viral infection: a mechanism for virus-induced immune deficiency. J. Virol. 1993;67:5754–5765. doi: 10.1128/jvi.67.10.5754-5765.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarozinski CC, Fynan EF, Selin LK, Robinson HL, Welsh RM. Protective CTL-dependent immunity and enhanced immunopathology in mice immunized by particle bombardment with DNA encoding an internal virion protein. J. Immunol. 1995;154:4010–4017. [PubMed] [Google Scholar]
- 31.Ramakrishna V, et al. Naturally occurring peptides associated with HLA-A2 in ovarian cancer cell lines identified by mass spectrometry are targets of HLA-A2-restricted cytotoxic T cells. Int. Immunol. 2003;15:751–763. doi: 10.1093/intimm/dxg074. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RW, Bennick JR, Yewdell JW, Maloy WL, Coligan JE. Influenza basic polymerase 2 peptides are recognized by influenza nucleoprotein-specific cytotoxic T lymphocytes. Mol. Immunol. 1992;29:1089–1096. doi: 10.1016/0161-5890(92)90041-U. [DOI] [PubMed] [Google Scholar]
- 33.Kuwano K, Reyes RE, Humphreys RE, Ennis FA. Recognition of disparate HA and NS1 peptides by an H-2kd- restricted, influenza specific CTL clone. Mol. Immunol. 1991;28:1–7. doi: 10.1016/0161-5890(91)90080-4. [DOI] [PubMed] [Google Scholar]
- 34.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nature Rev. Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 35.Zivny J, et al. Partial agonist effect influences the CTL response to a heterologous dengue virus serotype. J. Immunol. 1999;163:2754–2760. [PubMed] [Google Scholar]
- 36.Li J, et al. T-cell responses to peptide fragments of the BK virus T antigen: implications for cross-reactivity of immune response to JC virus. J. Gen. Virol. 2006;87:2951–2960. doi: 10.1099/vir.0.82094-0. [DOI] [PubMed] [Google Scholar]
- 37.Maeda K, et al. Identification and analysis for cross-reactivity among hantaviruses of H-2b-restricted cytotoxic T-lymphocyte epitopes in Sin Nombre virus nucleocapsid protein. J. Gen. Virol. 2004;85:1909–1919. doi: 10.1099/vir.0.79945-0. [DOI] [PubMed] [Google Scholar]
- 38.Effros RB, Doherty PC, Gerhard W, Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J. Exp. Med. 1977;145:557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J. Virol. 2001;75:11392–11400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acierno PM, et al. Cross-reactivity between HLA-A2-restricted FLU-M1:58–66 and HIV-1 p17 GAG:77–85 epitopes in HIV-1-infected and uninfected individuals. J. Transl. Med. 2003;1:3. doi: 10.1186/1479-5876-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clute SC, et al. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J. Clin. Invest. 2005;115:3602–3612. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SK, et al. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J. Exp. Med. 2005;201:523–533. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilges K, et al. Human papillomavirus type 16 E7 peptide-directed CD8+ T cells from patients with cervical cancer are cross-reactive with the coronavirus NS2 protein. J. Virol. 2003;77:5464–5474. doi: 10.1128/JVI.77.9.5464-5474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornberg M, et al. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J. Clin. Invest. 2006;116:1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haanan JB, Wolkers MC, Kruisbeek AM, Schumacher TN. Selective expansion of cross-reactive CD8+ memory T cells by viral variants. J. Exp. Med. 1999;190:1319–1328. doi: 10.1084/jem.190.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh RM, et al. The privacy of T cell memory to viruses. Curr. Top. Microbiol. Immunol. 2006;311:117–153. doi: 10.1007/3-540-32636-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner SJ, et al. Lack of prominent peptide-major histocompatibility complex features limits repertoire diversity in virus-specific CD8+ T cell populations. Nature Immunol. 2005;6:382–389. doi: 10.1038/ni1175. [DOI] [PubMed] [Google Scholar]
- 48.Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J. Exp. Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen HD, et al. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nature Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 50.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines antiviral immunity and immunopathology in the lung. Am. J. Pathol. 2003;163:1341–1355. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson PG, Hawke S, Bangham CR. Protection against lethal influenza virus encephalitis by intranasally primed CD8+ memory T cells. J. Immunol. 1996;157:3065–3073. [PubMed] [Google Scholar]
- 52.Haanen JBAG, Wolkers MC, Kruisbeek AM, Schumacher TNM. Selective expansion of cross-reactive CD8+ memory T cells by viral variants. J. Exp. Med. 1999;190:1319–1328. doi: 10.1084/jem.190.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park KY, Lee MG, Ryu JC, Park YK. Evolutionary stasis of M1 gene of human influenza A viruses and the possibility of their subtyping by restriction analysis of M1 gene polymerase chain reaction product. Acta Virol. 1997;41:231–239. [PubMed] [Google Scholar]
- 54.Lawson TM, et al. Influenza A antigen exposure selects dominant Vβ17+ TCR in human CD8+ cytotoxic T cell responses. Int. Immunol. 2001;13:1373–1381. doi: 10.1093/intimm/13.11.1373. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein L, Meade RH. Respiratory manifestations of chickenpox. Arch. Intern. Med. 1956;98:91–99. doi: 10.1001/archinte.1956.00250250097013. [DOI] [PubMed] [Google Scholar]
- 56.Rickinson AB, Kieff E. Fields Virology. 1996. pp. 2397–2446. [Google Scholar]
- 57.Silins SL, et al. Asymptomatic primary Epstein-Barr virus infection occurs in the absence of blood T-cell repertoire perturbations despite high levels of systemic viral load. Blood. 2001;98:3739–3744. doi: 10.1182/blood.V98.13.3739. [DOI] [PubMed] [Google Scholar]
- 58.Urbani S, et al. Heterologous T cell immunity in severe hepatitis C virus infection. J. Exp. Med. 2005;201:675–680. doi: 10.1084/jem.20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mongkolsapaya J, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nature Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 60.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiological study of altered clinical reactivity to respiratory syncitial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 61.Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity. 2001;15:637–646. doi: 10.1016/S1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 62.Walzl G, Tafuro S, Moss P, Openshaw PJ, Hussell T. Influenza virus lung infection protects from respiratory syncitial virus-induced immunopathology. J. Exp. Med. 2000;192:1317–1326. doi: 10.1084/jem.192.9.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson TR, Graham BS. Secreted respiratory syncytial virus G glcoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-dependent mechanism. J. Virol. 1999;73:8485–8495. doi: 10.1128/jvi.73.10.8485-8495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zamvil SS, et al. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 65.Gaur A, Wiers B, Liu A, Rothbard J, Fathman CG. Amelioration of autoimmune encephalomyelitis by myelin basic protein synthetic peptide-induced anergy. Science. 1992;258:1491–1494. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- 66.Tuohy VK, Thomas DM, Haqqi T, Yu M, Johnson JM. Determinant-regulated onset of experimental autoimmune encephalomyelitis: distinct epitopes of myelin proteolipid protein mediate either acute or delayed disease in SJL/J mice. Autoimmunity. 1995;21:203–214. doi: 10.3109/08916939509008017. [DOI] [PubMed] [Google Scholar]
- 67.Kumar V. Determinant spreading during experimental autoimmune encephalomyelitis: is it potentiating, protecting or participating in the disease? Immunol. Rev. 1998;164:73–80. doi: 10.1111/j.1600-065X.1998.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 68.Panoutsakopoulou V, et al. Analysis of the relationship between viral infection and autoimmune disease. Immunity. 2001;15:137–147. doi: 10.1016/S1074-7613(01)00172-8. [DOI] [PubMed] [Google Scholar]
- 69.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 70.Tsunoda I, Libbey JE, Kobayashi-Warren M, Fujinami RS. IFN-γ production and astrocyte recognition by autoreactive T cells induced by Theiler's virus infection: role of viral strains and capsid proteins. J. Neuroimmunol. 2006;172:85–93. doi: 10.1016/j.jneuroim.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Tsunoda I, Kuang LQ, Kobayashi-Warren M, Fujinami RS. Central nervous system pathology caused by autoreactive CD8+ T-cell clones following virus infection. J. Virol. 2005;79:14640–14646. doi: 10.1128/JVI.79.23.14640-14646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Croxford JL, Anger HA, Miller SD. Viral delivery of an epitope from Haemophilus influenzae induces central nervous system autoimmune disease by molecular mimicry. J. Immunol. 2005;174:907–917. doi: 10.4049/jimmunol.174.2.907. [DOI] [PubMed] [Google Scholar]
- 73.McCoy L, Tsunoda I, Fujinami RS. Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation. Autoimmunity. 2006;39:9–19. doi: 10.1080/08916930500484799. [DOI] [PubMed] [Google Scholar]
- 74.Theil DJ, Tsunoda I, Rodriguez F, Whitton JL, Fujinami RS. Viruses can silently prime for and trigger central nervous system autoimmune disease. J. Neurovirol. 2001;7:220–227. doi: 10.1080/13550280152403263. [DOI] [PubMed] [Google Scholar]
- 75.Wang LY, Fujinami RS. Enhancement of EAE and induction of autoantibodies to T-cell epitopes in mice infected with a recombinant vaccinia virus encoding myelin proteolipid protein. J. Neuroimmunol. 1997;75:75–83. doi: 10.1016/S0165-5728(96)00235-4. [DOI] [PubMed] [Google Scholar]
- 76.Hiemstra HS, et al. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc. Natl Acad. Sci. USA. 2001;98:3988–3991. doi: 10.1073/pnas.071050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sospedra M, et al. Recognition of conserved amino acid motifs of common viruses and its role in autoimmunity. PLoS. Pathog. 2005;1:e41. doi: 10.1371/journal.ppat.0010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirsch RL, et al. Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin. Immunol. Immunopathol. 1984;31:1–12. doi: 10.1016/0090-1229(84)90184-3. [DOI] [PubMed] [Google Scholar]
- 79.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-U. [DOI] [PubMed] [Google Scholar]
- 80.Evans CF, Horwitz MS, Hobbs MV, Oldstone MBA. Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J. Exp. Med. 1996;184:2371–2384. doi: 10.1084/jem.184.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kagi D, Odermatt B, Ohashi PS, Zinkernagel RM, Hengartner H. Development of insulitis without diabetes in transgenic mice lacking perforin-dependent cytotoxicity. J. Exp. Med. 1996;183:2143–2152. doi: 10.1084/jem.183.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Christen U, et al. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J. Clin. Invest. 2004;114:1290–1298. doi: 10.1172/JCI200422557. [DOI] [PMC free article] [PubMed] [Google Scholar]


