Abstract
Polycystic liver disease (PLD) is a rare hereditary disease that independently exists in isolated PLD, or as an accompanying symptom of autosomal dominant polycystic kidney disease and autosomal recessive polycystic kidney disease with complicated mechanisms. PLD currently lacks a unified diagnostic standard. The diagnosis of PLD is usually made when the number of hepatic cysts is more than 20. Gigot classification and Schnelldorfer classification are now commonly used to define severity in PLD. Most PLD patients have no clinical symptoms, and minority with severe complications need treatments. Somatostatin analogues, mammalian target of rapamycin inhibitor, ursodeoxycholic acid and vasopressin-2 receptor antagonist are the potentially effective medical therapies, while cyst aspiration and sclerosis, transcatheter arterial embolization, fenestration, hepatic resection and liver transplantation are the options of invasion therapies. However, the effectiveness of these therapies except liver transplantation are still uncertain. Furthermore, there is no unified strategy to treat PLD between medical centers at present. In order to better understand recent study progresses on PLD for clinical practice and obtain potential directions for future researches, this review mainly focuses on the recent progress in PLD classification, clinical manifestation, diagnosis and treatment. For information, we also provided medical treatment processes of PLD in our medical center.
Keywords: Polycystic liver disease, Autosomal dominant polycystic kidney disease, Autosomal recessive polycystic kidney disease, Isolated polycystic liver disease, Diagnosis, Treatment
Core tip: Polycystic liver disease (PLD) is a rare hereditary disease. However, there is no unified strategy in the treatment of PLD so far. In order to better understand recent progresses on clinical practice of PLD and contribute to potential directions for future researches, we conducted this review mainly focusing on recent progresses of PLD classification, clinical manifestation, diagnosis and treatments. For information, we also provided medical treatment process of PLD that is being used in our medical center.
INTRODUCTION
Polycystic liver disease (PLD) is a rare hereditary disease and often defined as multiple diffuse cysts of the liver[1]. It can independently exist in isolated PLD (PCLD), or as an accompanying symptom of autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD), while the mechanisms of cysts in PLD and PKD are complicated. In the one hand, they are both related to the primary cilia of biliary epithelial cells and the key proteins associated with cilia function, thus classified as fibrocystic diseases or ciliary diseases[2]. In the other hand, some scholars have classified them as cholangiopathic disease due to the source of PLD cysts which is from congenital bile duct dysplasia through multiple mechanisms[3]. Meanwhile, there is an opinion that the two mentioned above are actually associated with each other for there is a causality between fibrocystic malformation and dysgenesis of the biliary structures. In the limited treatments of PLD, drug therapy has gradually become a hot spot along with the deepening understanding on the pathogenesis of PLD, while there are still controversies in surgical treatments. In order to better understand recent progresses on PLD for clinical practice and contribute to potential directions for future researches, we conducted this non-systematic review mainly focusing on the recent progresses on PLD classification, clinical manifestation, diagnosis and treatments.
BRIEF SUMMARY OF EPIDEMIOLOGY AND MECHANISMS
Despite the genes associated with pathogenesis, the natural courses of various PLD in the liver are basically the same, showing as a continuous increase in the number and volume of cysts in liver[4]. However, it is essential to clarify the various forms of PLD (Table 1).
Table 1.
Brief summary of various polycystic liver disease
| Disease | Genes mutation | Kidney involvement |
| ADPKD | PKD1, PKD2, GANAB | Yes |
| ARPKD | PKHD1 | Yes |
| PCLD | PRKCSH, SEC63, LRP5, GANAB, ALG8, SEC61B, PKHD1 | Usually not |
ADPKD: Autosomal dominant polycystic kidney disease; ARPKD: Autosomal recessive polycystic kidney disease; PCLD: Isolated polycystic liver disease.
PLD in ADPKD
ADPKD is the most common monogenic genetic disease in the kidneys, with a global incidence of about 0.25% to 1%[5]. PLD is the most common extrarenal symptom of ADPKD, which involved with 94% ADPKD patients[6]. Mutations in two genes (PKD1 and PKD2) cause the development of ADPKD. PKD1 is located at chromosome 16p13.3 with 80% of cases related to it, while PKD2 is located at chromosome 4q21-22, which is responsible for the remaining 5-10% of cases[5]. PKD3 was once thought to be associated with ADPKD but excluded according to the recent family reanalysis[7]. Additionally, GANAB, which is involved in protein folding, was also reported to be responsible for ADPKD[5,8].
PLD in ARPKD
ARPKD is pretty rare with the incidence about 1:20000. It often occurs in children, of which 30% die from severe lung dysplasia and secondary respiratory failure, with renal collecting duct dilatation, bile duct dysplasia and portal fibrosis as the mainly clinical manifestations[9]. At present, a mutation of PKHD1 gene on the short arm of chromosome 6 encoding a fibrocystic protein, of which function is still not well-known, is found to be responsible for ARPKD. As well as PKD1 and PKD2, PKHD1 is also involved in the processes of forming the original cilia of liver and kidney, eventually causing cyst formation[2].
PLD in PCLD
Unlike ADPKD and ARPKD, PCLD often does not involve the kidneys[10]. In the previous studies of variant genes in PCLD, PRKCSH gene mutation accounted for the highest proportion of 15%, followed by SEC63 and LRP5. Meanwhile, GANAB is the first gene found to be associated with PCLD with a small proportion (approximately 1%). However, there are still a big amount of cases where a pathogenic gene cannot be found. The products of PRKCSH, SEC63 and GANAB genes are important proteins involved in the process of co-translational transport and maturation of glycoproteins in the endoplasmic reticulum[11], while the unidirectional transmembrane molecules encoded by LRP5 gene, with Frizzled receptors together, can bind to Wnt proteins, thereby initiating the Wnt signaling pathway and participating in the pathophysiological changes of PCLD[12]. Furthermore, in the recent PCLD pathogenic gene research, mutations in three genes, ALG8, SEC61B and PKHD1, are also found to be involved in the development of PCLD, which together with the above-mentioned PRKCSH, SEC63, LRP5, and GANAB genes can explain nearly 50% PLD cases[13]. The α-1,3-glycosyltransferase encoded by the ALG8 gene is an endoplasmic reticulum integral membrane protein[14], and the SEC61B gene-encoded product is an important component of the SEC63 protein complex on the endoplasmic reticulum. Both the two genes play important roles in protein quality regulation[15]. In addition, recent study[16] showed cholangiocyte autophagy contributed to hepatic cystogenesis in PLD and represented as a potential therapeutic target.
CLINICAL MANIFESTATION
Although the volume of PLD liver increases by 1.8% per 6 to 12 mo[17,18], most patients have no clinical symptoms regardless of the type of PLD. About 20% of patients develop obvious clinical symptoms including dyspnea, early satiety, abdominal distension, malnutrition, gastroesophageal reflux, back pain due to hepatomegaly pressing surrounding organs or cyst complications, which will seriously affect the quality of life[19-21]. Moreover, patients suffering from PLD may develop hepatic venous outflow obstruction because of cystic mass effect, resulting in portal hypertension, ascites, variceal haemorrhage or splenomegaly[22,23]. Gabow et al[24] found that the risk factors for hepatic cyst symptoms in ADPKD patients were older age, female gender and multiple pregnancy history. Studies[25,26] have also shown that in female, hepatic cysts grow rapidly under the influence of hormones, which may be related to the expression of estrogen receptors α and β[27]. Moreover, lower age was reported to be independently associated with larger liver volume in ADPKD females patients, whereas the higher age in male patients[28]. The gender differences and related mechanisms should be investigated in future.
In most patients with PLD, liver function tests are usually normal because liver parenchyma is not completely destroyed[29], however elevated γ-glutamyltransferase, alkaline phosphatase, aspartate aminotransferase and total bilirubin are reported in some serious cases[30,31]. Elevation of γ-glutamyltransferase and alkaline phosphatase may be the result of biliary cell activation[24,32], while the increase in total bilirubin can be seen in some cases of cystic compressing the bile duct. Furthermore, a study by Waanders et al[33] found that 45% PLD patients showed an increase in CA19-9 with a degree of elevation positively correlated with polycystic liver volume. Besides, the possibility of cysts infection is needed to consider when detecting a significant increase of CA19-9, and decrease of CA19-9 can be seen following effective anti-infective treatments.
DIAGNOSIS
The diagnosis of PLD is usually made when the number of hepatic cysts is more than 20[31]. However, patient with a family history of PCLD can be diagnosed when number of cysts more than 4[10]. However, the type of PLD can be hard to distinguish. Because PCLD patients may have renal cysts while ADPKD or ARPKD patients may have hepatic cysts as the main clinical manifestations, the identification between them without family history may be difficult and requires genetic analysis.
Currently there are mainly two clinical classifications on PLD: Gigot classification[34] (Table 2) and Schnelldorfer classification[35] (Table 3). Both of them include the number and size of cysts and the remaining liver parenchyma volume as the criteria for typing, while the latter also considers the inflow and outflow of pre-retained liver segments, which is more conducive to the choice of treatment. There was a Qian classification[19] relying on the number of cysts and the presence of symptomatic hepatomegaly (Table 4), however it is seldom used now because of oversimplification and, more importantly, having no contribution to selection of treatments.
Table 2.
Gigot classification
| Number of cysts | Cyst size | Remaining areas of noncystic liver parenchyma | |
| Gigot type I | < 10 | Large (> 10 cm) | Large |
| Gigot type II | Multiple | Small, medium | Large |
| Gigot type III | Multiple | Small, medium | Few |
Table 3.
Schnelldorfer classification
| Symptoms | Cyst characteristics | Areas of relative normal liver parenchyma | Isosectoral portal vein or hepatic vein occlusion of preserved sector | |
| Type A | Absent or mild | Any | Any | Any |
| Type B | Moderate or severe | Limited Number with large cysts | > 2 sectors | Absent |
| Type C | Severe (or moderate) | Any | > 1 sector | Absent |
| Type D | Severe (or moderate) | Any | < 1 sector | Present |
Table 4.
Qian classification
| Number of cysts | Symptomatic hepatomegaly | |
| Grade 0 | 0 | No |
| Grade 1 | 1-10 | No |
| Grade 2 | 11-20 | No |
| Grade 3 | > 20 | No |
| Grade 4 | > 20 | Yes |
TREATMENTS
Most patients with asymptomatic PLD do not need any treatment, while minority need only when incapacitating symptoms and a lower quality of life caused by hepatomegaly or complications such as cyst rupture, infection, bleeding, or hepatic venous outflow obstruction[21,36]. At present, the treatments of PLD are divided into three main categories: drug therapy, percutaneous therapy and surgical therapy.
Drug therapy
Somatostatin analogues: Although there is currently no approved effective treatment for PLD, recent progresses in somatostatin analogues have been achieved with positive results[37]. Somatostatin is a neurohormone with a wide range of effects through combining with somatostatin receptor (SSTR). There are five subtypes of SSTR (SSTR-1 to SSTR-5), which are expressed in varied tissues of human body. Somatostatin analogues such as octreotide, lanreotide and etc. are able to interact with the SSTR on the surface of the cyst wall to reduce the cAMP level of the bile duct epitheliums, inhibit the secretion of cyst fluid and hyperplasia of the bile duct cells, therefore inhibiting the growth of hepatic cysts[38,39].
Multiple controlled trials[17,18,40,41] showed that the liver volume of the group using somatostatin analogues was significantly reduced comparing to the control group. For octreotide, Caroli et al[40] administered 40 mg of octreotide per month and found that the experimental group had a significant reduced liver volume 71 ± 57 mL within 6 mo. Meanwhile, Hogan et al[17] gave the same dosage of octreotide to patients with severe ADPKD and PLD and the liver volume decreased by 4.95% ± 6.77% within a year (P = 0.048). In some patients with symptomatic PLD, octreotide were reported for significantly slowing disease progression, reducing symptoms and improving quality of life for 4 years[42]. For lanreotide, the liver volume of PLD patients using lanreotide decreased by 2.9% on average in 6 mo, and 4.0% in a year (P = 0.01)[18]. And more recently, lanreotide also showed positive effects on decreasing liver volume in patients with ADPKD and PLD, compared with control arm[43]. For the pattern of effectiveness, the 120 mg lanreotide group benefited more than the 90 mg lanreotide group and the control group[41]. In another study, Temmerman et al[44] increased the therapeutic dose of lanreotide non-responder from 90 mg to 120 mg, which led to stopping liver volume growing. These studies have shown that the efficacy of lanreotide may be dose-dependent.
Pasireotide is a more stable somatostatin analogue than octreotide, with a half-life of 12 h and it is currently used to treat Cushing's syndrome. Unlike octreotide and lanreotide, pasireotide can combine with all the SSTR subtypes to function, except SSTR-4[45]. Studies[46] have shown that pasireotide is more effective in relieving hepatorenal cyst formation than octreotide in the PKD mouse model. Further clinical data are required to confirm its efficacy.
On the contrary, a research[47] systematically reviewed seven PLD drug treatment studies from January 1966 to August 2014 and found that though the use of somatostatin analogues significantly reduced liver volume in 6 mo, however the improvement of patient quality of life and relief of clinical symptoms were very limited. In addition, there are some controversies about duration of efficacy and effect of cessation of treatment (also called drug holiday). Some studies[48] have also shown that the efficacy of somatostatin analogue therapy can only last for 2 years, and cessation of treatment would lead to disappearance of effect or even a rebound effect[42,49,50]. However, a study[43] showed the benefit to reduce liver volume from lanreotide still persisted 4 mo after cessation of the drug. Meanwhile, second cycle of somatostatin analogues after a drug holiday would still be as effective as the first in reducing liver volume[50]. This issue should be investigated in future clinical practicing.
Mammalian target of rapamycin (mTOR): mTOR is a serine/threonine protein kinase belonging to the phosphatidylinositol 3-kinase-associated kinase (PI3K) family and plays an important role in regulating signaling in many pathways. It mainly presents in two different complexes: Rapamycin target protein complex 1 (mTORC1) and rapamycin target protein complex 2 (mTORC2). mTORC1 is a growth regulator that can sense and aggregate trophic and environmental factors, while mTORC2 can promote cell survival, regulate cytoskeletal remodeling, ion transport and growth[51]. mTOR inhibitor is a targeted drug currently used in the treatment of cancer, including sirolimus, everolimus, and etc. In the PKD animal model, they showed significant inhibition of cyst growth and delaying the progression of the disease[52-55]. Qian et al[56] showed that sirolimus significantly reduced liver volume (11.9%) in ADPKD patients after renal transplantation (P = 0.009). However, in the clinical randomized controlled trials by Serra et al[57] and Walz et al[58] 18 mo of sirolimus and 2 years of everolimus were used, respectively, and had no significant effect on progression of renal cysts (P = 0.26, P = 0.06). Chrispijn et al[59] used different drug regimens for PCLD and ADPKD patients. The efficacy of everolimus-octreotide combination therapy was not significantly different in reducing liver volume compared with octreotide monotherapy (P = 0.73). On the other hand, as an immunosuppressive agent, mTOR inhibitors could increase the incidence of infection and malignant tumors as well as other side effects including dyslipidemia, thrombosis and lung diseases. Although most of them are moderate and may regress with lower doses, these side effects are unpredictable and idiosyncratic, which medics need to pay highly cautions to in clinical practice[60].
In summary, though with acceptable safety profile, there is not enough evidence to prove that mTOR inhibitors can benefit PLD patients. Thus, the use of mTOR inhibitors for the treatment of PLD is not recommended until more systematic and comprehensive results are obtained.
Ursodeoxycholic acid: Ursodeoxycholic acid (UDCA), which is a Ca2+ agonist in hepatocytes and biliary epithelial cells, has been shown to delay the growth of hepatic cysts in PLD animal model experiments. The mechanism is to inhibit cystic hyperplasia of biliary epithelial cells by inhibiting the proliferation of cystic bile duct epithelium and decreasing cytotoxic bile acid levels in the liver without affecting apoptosis by the PI3K/AKT/MEK/ERK1/2 pathway[61]. However, a multicenter randomized controlled trial[62] showed that liver volume insignificantly increased by 4.6% ± 7.7% in advanced PLD patients after 24 wk of UDCA treatment, with a liver volume increase of 3.1% ± 3.8% in the control group (P = 0.493), but subgroup analysis showed significant delay on the growth of hepatic cysts in ADPKD patients (P = 0.049).
Vasopressin-2 receptor antagonist: Vasopressin-2 receptor (V2R) is localized in the renal tubular epithelium, which promotes vesicle secretion and cell proliferation by up-regulating cAMP level[63]. Studies[64] have shown that antagonizing V2R in the kidney can delay the growth of renal cysts and improve renal function in the PCK mouse model. In a randomized controlled trial[65], the growth rate of renal cysts in the treatment group treated with tolvaptan was slower than the control group (P < 0.001). Meanwhile, even in advanced ADPKD patients, tolvaptan also showed protective effect on kidney function[66]. Although V2R is theoretically not expressed in biliary epithelial cells, meaning V2R antagonists are not effective against hepatic cysts, successful V2R treatment in reducing liver volume in PLD patients have recently been reported[67].
Percutaneous therapy
Cyst aspiration and sclerosis: This treatment is often used for patients with a single giant cyst, as the Gigot type I[68]. Besides completely suction of fluid, the sclerosing agent will be injected into the cyst to destroy the epitheliums of the cyst wall. The most commonly used agent is ethanol, followed by ethanolamine oleate, minocycline, tetracycline, etc.[68,69]. A retrospective study by Benzimra et al[70] collected 58 cases of hepatic cysts treated with puncture and ethanol sclerotherapy, and the cyst volume was reduced by an average of 94% and the symptom relief rate was 95%. In meta-analysis review[71] of cystic puncture and sclerotherapy including a total of 526 patients in 16 studies, 76%-100% of cases had partial cyst volume remission, while 72%-100% of cases had partial symptom remission, and 56%-100% of cases reported disappearance of symptoms. However, some researchers reported that the recurrence rate of cysts was as high as 80% undergone cyst aspiration and sclerosis, and the recurrence rate of symptoms was as high as 50%[72]. Nevertheless, PLD patients are often diagnosed with multiple cysts, thus this procedure actually is seldom used in PLD patients.
Transcatheter arterial embolization: Transcatheter arterial embolization (TAE) is using embolic agents to selectively embolize the branches of the arteries that supply blood to the cysts, thereby destroying the cells of the cystic wall, cutting off the source of the cystic fluid, and controlling the disease progression[73]. The application of this treatment is mainly due to the recent study showed that the cysts in PLD were mainly supplied by the hepatic artery[74]. A retrospective study with a small sample by Zhang et al[75] found that liver volume of PLD patients after TAE decreased by 32%, 31%, and 33% at 1 year, 2 years, and 3 years, respectively, while liver cyst volume reduced by 36%, 37%, 38%. Hoshino et al[76] collected 244 PLD cases undergone liver TAE, and the liver volume decreased by 94.7% (95%CI: 93.5%-95.8%) at 6 mo and 90.8% (95%CI: 88.7%-92.9%) at 1 year after TAE, respectively. A recent preliminary study[77] also showed positive effects on improvement of symptoms and shrinkage of cyst volume in PLD patients. Meanwhile, a study[78] showed its failure rate is as high as 69.6%, including uncontrolled symptoms, postoperative liver failure and death. Nevertheless, there is still a need for more well-designed large-scale studies to investigate their safety and efficacy before widespread adoption. And efforts should be made to investigate its potential as an adjuvant therapy.
Surgical therapy
Fenestration: Being different with cyst puncture and sclerotherapy, fenestration is often used for the treatment of multiple cysts, as Gigot type I-II or Schnelldorfer type B patients. In addition, the procedure can also be applied to cases when cyst puncture and sclerotherapy failed[79]. With the development of laparoscopic techniques, fenestration is now usually performed using laparoscope, but sometimes it has to be completed under the ordinary surgery due to uncontrollable bleeding, blind spots or technique, etc[1]. Symptoms are greatly relieved in 92% of cases undergone fenestration[80], however 33.7% of patients suffer symptomatic recurrence and 26.4% need reintervention[81]. Patients with multiple cysts larger than 5 cm in diameter have a higher recurrence rate than patients with smaller volume cysts[82]. Common complications of this procedure include ascites, pleural effusion, hemorrhage, and bile leakage. A meta-analysis[83] showed that the recurrence rate through open surgery was slightly lower, however without statistical significance, than through laparoscopic approach (5% vs 6%), and most recurrent cysts do not require second surgery. However, we believe the incidence of complication of laparoscopic fenestration will reduce, which is related to the continuous updating of surgical instruments and the increasing experience of surgeons. Besides, considering the convenience and small trauma, we still recommend laparoscope as first priority.
Hepatic resection: Hepatectomy is often used in severe Gigot II PLD patients with at least one liver segment that is not affected by the cysts[35]. The extent of resection depends on the size and distribution of the cysts. However, due to the compression of the cyst, the distorted Glison system and hepatic venous outflow obstruction increase the difficulty of resection. Thus, for cysts that cannot be removed, fenestration is often performed with hepatic resection[3]. Meanwhile, hepatic venous outflow obstruction is frequent in PLD and has major consequences on intraoperative bleeding and postoperative ascites and liver failure[84]. In a retrospective study by Chebib et al[85] including 186 PLD patients undergone hepatectomy, postoperative liver volume is reduced by an average of 61% comparing to preoperative, with a high complication rate of 21% and a mortality rate of 2.7%. On the other hand, some studies[83,86] claimed that hepatectomy could greatly alleviate symptoms and prolong the needs of liver transplantation with an acceptable safety profile. In addition, application of somatostatin analogues after hepatectomy can inhibit the growth of residual cysts and prevent the occurrence of new cysts[86]. Despite the compromising relief of symptom and reduction of liver volume, it is presently not recommended as a first-line treatment plan, because of the high complication and mortality rate and the potential difficulties for the future liver transplantation due to abdominal adhesion[1,87]. Nevertheless, we consider the most important issues may be when and how to perform this procedure, or in other words, which group of patients benefit from hepatotectmy, which would maximize the value of the surgery.
Liver transplantation: Liver transplantation is currently the only cure for PLD, which is mainly applied in Gigot type III patients with severe symptoms that seriously affect the quality of life of patients, as well as untreated complications such as portal hypertension and malnutrition[1]. In the PLD classification designed by Schnelldorfer et al[35], liver transplantation is suitable for patients with type D. Compared with hepatocellular carcinoma and chronic liver failure, the survival rate of PLD is significantly higher than that of the former two, respectively[88,89]. It has been reported that the 5-year survival rate was 92.3%[90], while a more recent research found it as 85.1%[88]. Meanwhile, most of patients have a significant improvement on health-related quality of life assessment after transplantation[91]. Even liver transplantation performs comparatively well in PLD, however, due to the lack of liver donors, relatively low urgency and low mortality rate, it is difficult to extensive perform and necessary to carefully evaluate the indications of liver transplantation.
MEDICAL TREATMENT PROCESS
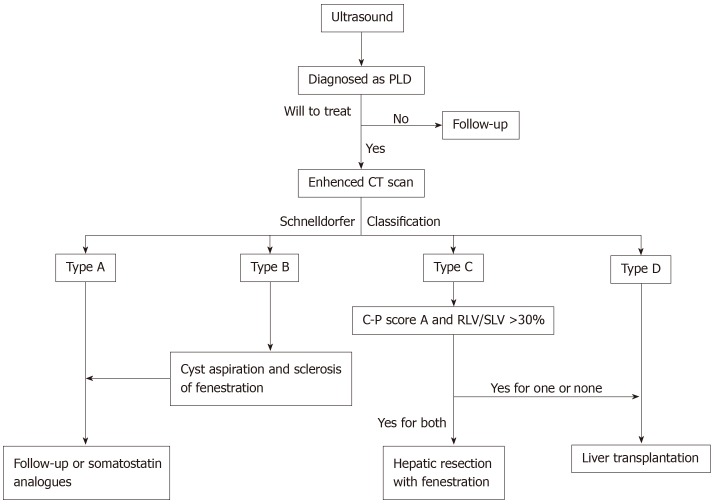
Although some institutions have attempted to make guidance in treating PLD[92], there is currently no widely accepted international guideline for treatment of PLD. Various therapies were used to treat different types of PLD in different medical centers. Schnelldorfer et al[35] gave the corresponding preferred treatment based on Schnelldorfer classification. Base on this, we modified the process according to the clinical experience of our medical center in treating PLD, which is being used in our medical center, and here we summarize it as Figure 1.
Figure 1.
Polycystic liver disease diagnosis and treatment process in our medical center based on Schnelldorfer classification. PLD: Polycystic liver disease; LV/SLV: Remaining liver volume / standard liver volume; CT: Computer tomography.
Ultrasound should be the first choice for first visit of suspicious PLD patients. Enhanced computer tomography is used for patients diagnosed with PLD to determine Schnelldorfer classification. The treatment plan is determined based on the classification, liver function and computerized three-dimensional imaging which is used to calculate liver volume and estimate resident liver volume after resection for ensuring safety of hepatectomy. For patients with asymptomatic or mild symptoms, Schnelldorfer type A, simple observation or long-acting somatostatin analogue can be applied without any surgical interventions. Schnelldorfer B patients with large cysts but limited number should be treated with cyst aspiration and sclerosis or fenestration depending on situation of cysts, to reduce cyst volume and relieve symptoms. For Schnelldorfer type C patients with excessive cysts, normal liver function and sufficient liver parenchyma volume, only fenestration cannot achieve long-term relief of symptoms. Under the premise of ensuring the pre-retained inflow of the hepatic lobe and the safety of the outflow tract, it is more appropriate to choose hepatectomy with fenestration to remove the liver segment occupied by the cyst, which would minimize the liver volume for controlling symptoms for a longer time. However, if Schnelldorfer is classified as type C with impaired liver function (child-pugh score B or C) or insufficient residual liver volume (remaining liver volume / standard liver volume < 30%), liver transplantation is recommended. In addition, somatostatin analogue can be considered after fenestration or hepatectomy. Hepatectomy is no longer suit for Schnelldorfer type D patients, and liver transplantation is most appropriate regardless of the condition of liver function.
CONCLUSION
PLD is a hereditary genetic disease. Although PLD progresses with age, only a small number of patients have symptoms that require treatment. At present, the treatment of PLD is mainly drugs intervention, cyst puncture and sclerotherapy, fenestration, transcatheter arterial embolization, liver resection, liver transplantation. Clinical drug therapy for PLD is currently focused on somatostatin analogues, while many other drug targets are being developed as more and more clinical trials validating their effectiveness. Liver transplantation is now the only cure for PLD, but it cannot be carried out in large quantities due to complicated reasons. Except liver transplantation, the other four surgical and interventional treatments can be widely used for PLD patients with different conditions. But considering the high recurrence rate, serious complications and mortality, it is necessary to carefully consider the indications. Besides, various combination therapies should be investigated in future researches for better effectiveness. In addition, for reference, we provide the diagnosis and treatment process being applied in our medical center, which is based on Schnelldorfer classification and the experience of the medical center.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: November 28, 2019
First decision: January 7, 2020
Article in press: March 1, 2020
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Drenth JPH, Martínez-Pérez A, Mochizuki T, Souftas VD S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
Contributor Information
Ze-Yu Zhang, Department of Hepatobiliary Surgery, Xiangya Hospital, Central South University, Changsha 410000, Hunan Province, China.
Zhi-Ming Wang, Department of Hepatobiliary Surgery, Xiangya Hospital, Central South University, Changsha 410000, Hunan Province, China.
Yun Huang, Department of Hepatobiliary Surgery, Xiangya Hospital, Central South University, Changsha 410000, Hunan Province, China. huangyun-1002@163.com.
References
- 1.Drenth JP, Chrispijn M, Nagorney DM, Kamath PS, Torres VE. Medical and surgical treatment options for polycystic liver disease. Hepatology. 2010;52:2223–2230. doi: 10.1002/hep.24036. [DOI] [PubMed] [Google Scholar]
- 2.Chandok N. Polycystic liver disease: a clinical review. Ann Hepatol. 2012;11:819–826. [PubMed] [Google Scholar]
- 3.Wong MY, McCaughan GW, Strasser SI. An update on the pathophysiology and management of polycystic liver disease. Expert Rev Gastroenterol Hepatol. 2017;11:569–581. doi: 10.1080/17474124.2017.1309280. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Wasel B, Walsh C, Keough V, Molinari M. Pathophysiology, epidemiology, classification and treatment options for polycystic liver diseases. World J Gastroenterol. 2013;19:5775–5786. doi: 10.3748/wjg.v19.i35.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nat Rev Dis Primers. 2018;4:50. doi: 10.1038/s41572-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:173–180. doi: 10.1053/j.ackd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Xue C, Zhou CC, Wu M, Mei CL. The Clinical Manifestation and Management of Autosomal Dominant Polycystic Kidney Disease in China. Kidney Dis (Basel) 2016;2:111–119. doi: 10.1159/000449030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornec-Le Gall E, Torres VE, Harris PC. Genetic Complexity of Autosomal Dominant Polycystic Kidney and Liver Diseases. J Am Soc Nephrol. 2018;29:13–23. doi: 10.1681/ASN.2017050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp AM, Messiaen LM, Page G, Antignac C, Gubler MC, Onuchic LF, Somlo S, Germino GG, Guay-Woodford LM. Comprehensive genomic analysis of PKHD1 mutations in ARPKD cohorts. J Med Genet. 2005;42:336–349. doi: 10.1136/jmg.2004.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian Q. Isolated polycystic liver disease. Adv Chronic Kidney Dis. 2010;17:181–189. doi: 10.1053/j.ackd.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besse W, Choi J, Ahram D, Mane S, Sanna-Cherchi S, Torres V, Somlo S. A noncoding variant in GANAB explains isolated polycystic liver disease (PCLD) in a large family. Hum Mutat. 2018;39:378–382. doi: 10.1002/humu.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Laarschot LFM, Drenth JPH. Genetics and mechanisms of hepatic cystogenesis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1491–1497. doi: 10.1016/j.bbadis.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Besse W, Dong K, Choi J, Punia S, Fedeles SV, Choi M, Gallagher AR, Huang EB, Gulati A, Knight J, Mane S, Tahvanainen E, Tahvanainen P, Sanna-Cherchi S, Lifton RP, Watnick T, Pei YP, Torres VE, Somlo S. Isolated polycystic liver disease genes define effectors of polycystin-1 function. J Clin Invest. 2017;127:1772–1785. doi: 10.1172/JCI90129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breitling J, Aebi M. N-linked protein glycosylation in the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5:a013359. doi: 10.1101/cshperspect.a013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park E, Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys. 2012;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- 16.Masyuk AI, Masyuk TV, Lorenzo Pisarello MJ, Ding JF, Loarca L, Huang BQ, LaRusso NF. Cholangiocyte autophagy contributes to hepatic cystogenesis in polycystic liver disease and represents a potential therapeutic target. Hepatology. 2018;67:1088–1108. doi: 10.1002/hep.29577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, King BF, Glockner J, Holmes DR, 3rd, Rossetti S, Harris PC, LaRusso NF, Torres VE. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, de Man RA, Drenth JP. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661–8.e1-2. doi: 10.1053/j.gastro.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 19.Qian Q, Li A, King BF, Kamath PS, Lager DJ, Huston J, 3rd, Shub C, Davila S, Somlo S, Torres VE. Clinical profile of autosomal dominant polycystic liver disease. Hepatology. 2003;37:164–171. doi: 10.1053/jhep.2003.50006. [DOI] [PubMed] [Google Scholar]
- 20.Bistritz L, Tamboli C, Bigam D, Bain VG. Polycystic liver disease: experience at a teaching hospital. Am J Gastroenterol. 2005;100:2212–2217. doi: 10.1111/j.1572-0241.2005.50258.x. [DOI] [PubMed] [Google Scholar]
- 21.Neijenhuis MK, Kievit W, Verheesen SM, D'Agnolo HM, Gevers TJ, Drenth JP. Impact of liver volume on polycystic liver disease-related symptoms and quality of life. United European Gastroenterol J. 2018;6:81–88. doi: 10.1177/2050640617705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernts LHP, Drenth JPH, Tjwa ETTL. Management of portal hypertension and ascites in polycystic liver disease. Liver Int. 2019;39:2024–2033. doi: 10.1111/liv.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernts LHP, Tjwa ETTL, D'Agnolo HMA, Jenniskens SFM, Drenth JPH. Venous Stent Placement for Refractory Ascites due to Hepatic Venous Outflow Obstruction in Polycystic Liver Disease. J Vasc Interv Radiol. 2019;30:1617–1619. doi: 10.1016/j.jvir.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology. 1990;11:1033–1037. doi: 10.1002/hep.1840110619. [DOI] [PubMed] [Google Scholar]
- 25.Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser SS, Alpini G, Francis H, Marucci L, Sterpetti P, Ginanni-Corradini S, Onetti Muda A, Dostal DE, De Santis A, Attili AF, Benedetti A, Gaudio E. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169:877–888. doi: 10.2353/ajpath.2006.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Aerts RMM, Bernts LHP, Gevers TJG, Kievit W, Koopmans L, Nieboer TE, Nevens F, Drenth JPH. Estrogen-Containing Oral Contraceptives Are Associated With Polycystic Liver Disease Severity in Premenopausal Patients. Clin Pharmacol Ther. 2019;106:1338–1345. doi: 10.1002/cpt.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvaro D, Alpini G, Onori P, Franchitto A, Glaser SS, Le Sage G, Folli F, Attili AF, Gaudio E. Alfa and beta estrogen receptors and the biliary tree. Mol Cell Endocrinol. 2002;193:105–108. doi: 10.1016/s0303-7207(02)00103-x. [DOI] [PubMed] [Google Scholar]
- 28.van Aerts RMM, Kievit W, de Jong ME, Ahn C, Bañales JM, Reiterová J, Nevens F, Drenth JPH. Severity in polycystic liver disease is associated with aetiology and female gender: Results of the International PLD Registry. Liver Int. 2019;39:575–582. doi: 10.1111/liv.13965. [DOI] [PubMed] [Google Scholar]
- 29.Everson GT, Scherzinger A, Berger-Leff N, Reichen J, Lezotte D, Manco-Johnson M, Gabow P. Polycystic liver disease: quantitation of parenchymal and cyst volumes from computed tomography images and clinical correlates of hepatic cysts. Hepatology. 1988;8:1627–1634. doi: 10.1002/hep.1840080626. [DOI] [PubMed] [Google Scholar]
- 30.Arnold HL, Harrison SA. New advances in evaluation and management of patients with polycystic liver disease. Am J Gastroenterol. 2005;100:2569–2582. doi: 10.1111/j.1572-0241.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Keimpema L, De Koning DB, Van Hoek B, Van Den Berg AP, Van Oijen MG, De Man RA, Nevens F, Drenth JP. Patients with isolated polycystic liver disease referred to liver centres: clinical characterization of 137 cases. Liver Int. 2011;31:92–98. doi: 10.1111/j.1478-3231.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- 32.Hoevenaren IA, Wester R, Schrier RW, McFann K, Doctor RB, Drenth JP, Everson GT. Polycystic liver: clinical characteristics of patients with isolated polycystic liver disease compared with patients with polycystic liver and autosomal dominant polycystic kidney disease. Liver Int. 2008;28:264–270. doi: 10.1111/j.1478-3231.2007.01595.x. [DOI] [PubMed] [Google Scholar]
- 33.Waanders E, van Keimpema L, Brouwer JT, van Oijen MG, Aerts R, Sweep FC, Nevens F, Drenth JP. Carbohydrate antigen 19-9 is extremely elevated in polycystic liver disease. Liver Int. 2009;29:1389–1395. doi: 10.1111/j.1478-3231.2009.02055.x. [DOI] [PubMed] [Google Scholar]
- 34.Gigot JF, Jadoul P, Que F, Van Beers BE, Etienne J, Horsmans Y, Collard A, Geubel A, Pringot J, Kestens PJ. Adult polycystic liver disease: is fenestration the most adequate operation for long-term management? Ann Surg. 1997;225:286–294. doi: 10.1097/00000658-199703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnelldorfer T, Torres VE, Zakaria S, Rosen CB, Nagorney DM. Polycystic liver disease: a critical appraisal of hepatic resection, cyst fenestration, and liver transplantation. Ann Surg. 2009;250:112–118. doi: 10.1097/SLA.0b013e3181ad83dc. [DOI] [PubMed] [Google Scholar]
- 36.van Keimpema L, Höckerstedt K. Treatment of polycystic liver disease. Br J Surg. 2009;96:1379–1380. doi: 10.1002/bjs.6738. [DOI] [PubMed] [Google Scholar]
- 37.Gevers TJ, Drenth JP. Somatostatin analogues for treatment of polycystic liver disease. Curr Opin Gastroenterol. 2011;27:294–300. doi: 10.1097/MOG.0b013e328343433f. [DOI] [PubMed] [Google Scholar]
- 38.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 39.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 40.Caroli A, Antiga L, Cafaro M, Fasolini G, Remuzzi A, Remuzzi G, Ruggenenti P. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol. 2010;5:783–789. doi: 10.2215/CJN.05380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Temmerman F, Vanslembrouck R, Coudyzer W, Bammens B, Laleman W, Cassiman D, van der Merwe S, Verslype C, Van Steenbergen W, van Pelt J, Pirson Y, Drenth J, Nevens F. The reduction in liver volume in polycystic liver disease with lanreotide is dose dependent and is most pronounced in patients with the highest liver volume. J Hepatol. 2012;56:S547. [Google Scholar]
- 42.Hogan MC, Masyuk T, Bergstralh E, Li B, Kremers WK, Vaughan LE, Ihrke A, Severson AL, Irazabal MV, Glockner J, LaRusso NF, Torres VE. Efficacy of 4 Years of Octreotide Long-Acting Release Therapy in Patients With Severe Polycystic Liver Disease. Mayo Clin Proc. 2015;90:1030–1037. doi: 10.1016/j.mayocp.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Aerts RMM, Kievit W, D'Agnolo HMA, Blijdorp CJ, Casteleijn NF, Dekker SEI, de Fijter JW, van Gastel M, Gevers TJ, van de Laarschot LFM, Lantinga MA, Losekoot M, Meijer E, Messchendorp AL, Neijenhuis MK, Pena MJ, Peters DJM, Salih M, Soonawala D, Spithoven EM, Visser FW, Wetzels JF, Zietse R, Gansevoort RT, Drenth JPH DIPAK-1 Investigators. Lanreotide Reduces Liver Growth In Patients With Autosomal Dominant Polycystic Liver and Kidney Disease. Gastroenterology. 2019;157:481–491.e7. doi: 10.1053/j.gastro.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Temmerman F, Ho TA, Vanslembrouck R, Coudyzer W, Billen J, Dobbels F, van Pelt J, Bammens B, Pirson Y, Nevens F. Lanreotide Reduces Liver Volume, But Might Not Improve Muscle Wasting or Weight Loss, in Patients With Symptomatic Polycystic Liver Disease. Clin Gastroenterol Hepatol. 2015;13:2353–9.e1. doi: 10.1016/j.cgh.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 45.Lesche S, Lehmann D, Nagel F, Schmid HA, Schulz S. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J Clin Endocrinol Metab. 2009;94:654–661. doi: 10.1210/jc.2008-1919. [DOI] [PubMed] [Google Scholar]
- 46.Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Gradilone SA, Huang B, Masyuk AI, Hogan MC, Torres VE, Larusso NF. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013;58:409–421. doi: 10.1002/hep.26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan S, Dennison A, Garcea G. Medical therapy for polycystic liver disease. Ann R Coll Surg Engl. 2016;98:18–23. doi: 10.1308/rcsann.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogan MC, Masyuk TV, Page L, Holmes DR, 3rd, Li X, Bergstralh EJ, Irazabal MV, Kim B, King BF, Glockner JF, Larusso NF, Torres VE. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27:3532–3539. doi: 10.1093/ndt/gfs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chrispijn M, Nevens F, Gevers TJ, Vanslembrouck R, van Oijen MG, Coudyzer W, Hoffmann AL, Dekker HM, de Man RA, van Keimpema L, Drenth JP. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment Pharmacol Ther. 2012;35:266–274. doi: 10.1111/j.1365-2036.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- 50.van Aerts RMM, Kolkman M, Kievit W, Gevers TJG, Nevens F, Drenth JPH. Drug holiday in patients with polycystic liver disease treated with somatostatin analogues. Therap Adv Gastroenterol. 2018;11:1756284818804784. doi: 10.1177/1756284818804784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren XS, Sato Y, Harada K, Sasaki M, Furubo S, Song JY, Nakanuma Y. Activation of the PI3K/mTOR pathway is involved in cystic proliferation of cholangiocytes of the PCK rat. PLoS One. 2014;9:e87660. doi: 10.1371/journal.pone.0087660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Temmerman F, Chen F, Libbrecht L, Vander Elst I, Windmolders P, Feng Y, Ni Y, De Smedt H, Nevens F, van Pelt J. Everolimus halts hepatic cystogenesis in a rodent model of polycystic-liver-disease. World J Gastroenterol. 2017;23:5499–5507. doi: 10.3748/wjg.v23.i30.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wüthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 54.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 56.Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, Torres VE. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631–638. doi: 10.1681/ASN.2007050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 58.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 59.Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: results from a randomized controlled trial. J Hepatol. 2013;59:153–159. doi: 10.1016/j.jhep.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Pallet N, Legendre C. Adverse events associated with mTOR inhibitors. Expert Opin Drug Saf. 2013;12:177–186. doi: 10.1517/14740338.2013.752814. [DOI] [PubMed] [Google Scholar]
- 61.Munoz-Garrido P, Marin JJ, Perugorria MJ, Urribarri AD, Erice O, Sáez E, Úriz M, Sarvide S, Portu A, Concepcion AR, Romero MR. Monte MJ, Santos-Laso Á, Hijona E, Jimenez-Agüero R, Marzioni M, Beuers U, Masyuk TV, LaRusso NF, Prieto J, Bujanda L, Drenth JP, Banales JM. Ursodeoxycholic acid inhibits hepatic cystogenesis in experimental models of polycystic liver disease. J Hepatol. 2015;63:952–961. doi: 10.1016/j.jhep.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Agnolo HM, Kievit W, Takkenberg RB, Riaño I, Bujanda L, Neijenhuis MK, Brunenberg EJ, Beuers U, Banales JM, Drenth JP. Ursodeoxycholic acid in advanced polycystic liver disease: A phase 2 multicenter randomized controlled trial. J Hepatol. 2016;65:601–607. doi: 10.1016/j.jhep.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Gattone VH 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Gattone VH 2nd, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16:846–851. doi: 10.1681/ASN.2004121090. [DOI] [PubMed] [Google Scholar]
- 65.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O REPRISE Trial Investigators. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2017;377:1930–1942. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 67.Mizuno H, Hoshino J, Suwabe T, Sumida K, Sekine A, Oshima Y, Oguro M, Kunizawa K, Kawada M, Hiramatsu R, Hayami N, Hasegawa E, Yamanouchi M, Sawa N, Takaichi K, Ubara Y. Tolvaptan for the Treatment of Enlarged Polycystic Liver Disease. Case Rep Nephrol Dial. 2017;7:108–111. doi: 10.1159/000477664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamada N, Shinzawa H, Ukai K, Makino N, Matsuhashi T, Wakabayashi H, Togashi H, Takahashi T. Treatment of symptomatic hepatic cysts by percutaneous instillation of minocycline hydrochloride. Dig Dis Sci. 1994;39:2503–2509. doi: 10.1007/BF02087673. [DOI] [PubMed] [Google Scholar]
- 69.Nakaoka R, Das K, Kudo M, Chung H, Innoue T. Percutaneous aspiration and ethanolamine oleate sclerotherapy for sustained resolution of symptomatic polycystic liver disease: an initial experience. AJR Am J Roentgenol. 2009;193:1540–1545. doi: 10.2214/AJR.08.1681. [DOI] [PubMed] [Google Scholar]
- 70.Benzimra J, Ronot M, Fuks D, Abdel-Rehim M, Sibert A, Farges O, Vilgrain V. Hepatic cysts treated with percutaneous ethanol sclerotherapy: time to extend the indications to haemorrhagic cysts and polycystic liver disease. Eur Radiol. 2014;24:1030–1038. doi: 10.1007/s00330-014-3117-x. [DOI] [PubMed] [Google Scholar]
- 71.Wijnands TF, Görtjes AP, Gevers TJ, Jenniskens SF, Kool LJ, Potthoff A, Ronot M, Drenth JP. Efficacy and Safety of Aspiration Sclerotherapy of Simple Hepatic Cysts: A Systematic Review. AJR Am J Roentgenol. 2017;208:201–207. doi: 10.2214/AJR.16.16130. [DOI] [PubMed] [Google Scholar]
- 72.Russell RT, Pinson CW. Surgical management of polycystic liver disease. World J Gastroenterol. 2007;13:5052–5059. doi: 10.3748/wjg.v13.i38.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ubara Y, Takei R, Hoshino J, Tagami T, Sawa N, Yokota M, Katori H, Takemoto F, Hara S, Takaichi K. Intravascular embolization therapy in a patient with an enlarged polycystic liver. Am J Kidney Dis. 2004;43:733–738. doi: 10.1053/j.ajkd.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 74.Ubara Y. New therapeutic option for autosomal dominant polycystic kidney disease patients with enlarged kidney and liver. Ther Apher Dial. 2006;10:333–341. doi: 10.1111/j.1744-9987.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhang JL, Yuan K, Wang MQ, Yan JY, Xin HN, Wang Y, Liu FY, Bai YH, Wang ZJ, Duan F, Fu JX. Transarterial Embolization for Treatment of Symptomatic Polycystic Liver Disease: More than 2-year Follow-up. Chin Med J (Engl) 2017;130:1938–1944. doi: 10.4103/0366-6999.211882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoshino J, Ubara Y, Suwabe T, Sumida K, Hayami N, Mise K, Hiramatsu R, Hasegawa E, Yamanouchi M, Sawa N, Takei R, Takaichi K. Intravascular embolization therapy in patients with enlarged polycystic liver. Am J Kidney Dis. 2014;63:937–944. doi: 10.1053/j.ajkd.2014.01.422. [DOI] [PubMed] [Google Scholar]
- 77.Yan JY, Zhang JL, Yuan K, Fu JX, Wang Y, Yuan B, Wang MQ. Transarterial embolisation with bleomycin and N-butyl-2-cyanoacrylate -Lipiodol mixture for symptomatic polycystic liver disease: preliminary experience. Clin Radiol. 2019;74:975.e11–975.e16. doi: 10.1016/j.crad.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Yang J, Ryu H, Han M, Kim H, Hwang YH, Chung JW, Yi NJ, Lee KW, Suh KS, Ahn C. Comparison of volume-reductive therapies for massive polycystic liver disease in autosomal dominant polycystic kidney disease. Hepatol Res. 2016;46:183–191. doi: 10.1111/hepr.12560. [DOI] [PubMed] [Google Scholar]
- 79.van Keimpema L, Ruurda JP, Ernst MF, van Geffen HJ, Drenth JP. Laparoscopic fenestration of liver cysts in polycystic liver disease results in a median volume reduction of 12.5% J Gastrointest Surg. 2008;12:477–482. doi: 10.1007/s11605-007-0376-8. [DOI] [PubMed] [Google Scholar]
- 80.Martin IJ, McKinley AJ, Currie EJ, Holmes P, Garden OJ. Tailoring the management of nonparasitic liver cysts. Ann Surg. 1998;228:167–172. doi: 10.1097/00000658-199808000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernts LHP, Echternach SG, Kievit W, Rosman C, Drenth JPH. Clinical response after laparoscopic fenestration of symptomatic hepatic cysts: a systematic review and meta-analysis. Surg Endosc. 2019;33:691–704. doi: 10.1007/s00464-018-6490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Keimpema L, de Koning DB, Strijk SP, Drenth JP. Aspiration-sclerotherapy results in effective control of liver volume in patients with liver cysts. Dig Dis Sci. 2008;53:2251–2257. doi: 10.1007/s10620-007-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pantè S, Di Dio V, Putorti A, Salvo A, Barbera N, Catalfamo G, Leonello G, Mastrojeni C. Laparoscopic cyst fenestration in the treatment of polycystic liver disease. Ann Ital Chir. 2014;85:298–303. [PubMed] [Google Scholar]
- 84.Barbier L, Ronot M, Aussilhou B, Cauchy F, Francoz C, Vilgrain V, Soubrane O, Paradis V, Belghiti J. Polycystic liver disease: Hepatic venous outflow obstruction lesions of the noncystic parenchyma have major consequences. Hepatology. 2018;68:652–662. doi: 10.1002/hep.29582. [DOI] [PubMed] [Google Scholar]
- 85.Chebib FT, Harmon A, Irazabal Mira MV, Jung YS, Edwards ME, Hogan MC, Kamath PS, Torres VE, Nagorney DM. Outcomes and Durability of Hepatic Reduction after Combined Partial Hepatectomy and Cyst Fenestration for Massive Polycystic Liver Disease. J Am Coll Surg. 2016;223:118–126.e1. doi: 10.1016/j.jamcollsurg.2015.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tseng J, Orloff SL. Management of symptomatic polycystic liver disease with hepatic resection. JAMA Surg. 2015;150:81–82. doi: 10.1001/jamasurg.2014.307. [DOI] [PubMed] [Google Scholar]
- 87.Aussilhou B, Douflé G, Hubert C, Francoz C, Paugam C, Paradis V, Farges O, Vilgrain V, Durand F, Belghiti J. Extended liver resection for polycystic liver disease can challenge liver transplantation. Ann Surg. 2010;252:735–743. doi: 10.1097/SLA.0b013e3181fb8dc4. [DOI] [PubMed] [Google Scholar]
- 88.Doshi SD, Bittermann T, Schiano TD, Goldberg DS. Waitlisted Candidates With Polycystic Liver Disease Are More Likely to be Transplanted Than Those With Chronic Liver Failure. Transplantation. 2017;101:1838–1844. doi: 10.1097/TP.0000000000001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coquillard C, Berger J, Daily M, Shah M, Mei X, Marti F, Gedaly R. Combined liver-kidney transplantation for polycystic liver and kidney disease: analysis from the United Network for Organ Sharing dataset. Liver Int. 2016;36:1018–1025. doi: 10.1111/liv.13041. [DOI] [PubMed] [Google Scholar]
- 90.van Keimpema L, Nevens F, Adam R, Porte RJ, Fikatas P, Becker T, Kirkegaard P, Metselaar HJ, Drenth JP European Liver and Intestine Transplant Association. Excellent survival after liver transplantation for isolated polycystic liver disease: an European Liver Transplant Registry study. Transpl Int. 2011;24:1239–1245. doi: 10.1111/j.1432-2277.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- 91.Kirchner GI, Rifai K, Cantz T, Nashan B, Terkamp C, Becker T, Strassburg C, Barg-Hock H, Wagner S, Lück R, Klempnauer J, Manns MP. Outcome and quality of life in patients with polycystic liver disease after liver or combined liver-kidney transplantation. Liver Transpl. 2006;12:1268–1277. doi: 10.1002/lt.20780. [DOI] [PubMed] [Google Scholar]
- 92.Savige J, Mallett A, Tunnicliffe DJ, Rangan GK. KHA-CARI Autosomal Dominant Polycystic Kidney Disease Guideline: Management of Polycystic Liver Disease. Semin Nephrol. 2015;35:618–622.e5. doi: 10.1016/j.semnephrol.2015.10.015. [DOI] [PubMed] [Google Scholar]