Abstract
A 50-year-old male presented to our hospital complaining of dry cough and slight fever. A chest CT scan showed a mass in the right upper lung lobe, pleural effusion on both sides, and multiple liver tumors. He was diagnosed with small cell lung cancer (SCLC), and then antitumor chemotherapy was started. Thereafter, his condition deteriorated rapidly, and died 2 days later. An autopsy revealed that the cause of death was ruptured liver metastases. SCLC is a highly invasive disease and often metastasizes to the liver, but the rupture of liver metastases is rare. Clinical features and imaging findings were of a great help in diagnosing ruptured hepatic metastasis. Physicians need to pay attention to this condition, especially after chemotherapy has initiated.
Keywords: Small cell lung cancer, Liver metastases, Hemoperitoneum
1. Introduction
SCLC is highly invasive and the liver is the most common site of metastasis. However, the incidence of ruptured liver metastasis is rare in comparison to hepatocellular carcinoma. We herein describe an autopsy case of SCLC involving a patient who died of this condition, in order to make clinicians aware of patients with associated risk factors (see Fig. 3).
Fig. 3.
A: The liver was enlarged, weighing 3120 g. B: More than 60% of the liver was replaced by metastasis. C and D: Immunohistochemical staining of the metastatic liver tumor cells was diffusely positive for CD34 (C) and MIB1 (D).
1.1. Case presentation
A 50-year-old man was referred to our department for further investigation of a chest X-ray abnormality (Fig. 1A). He complained of dry cough and slight fever, which had persisted for three weeks. Although he had bronchial asthma, he was not taking any medications. He had no history of blood transfusion, hepatitis, or alcohol abuse, but had a 37 pack-year history of cigarette smoking He had no known allergies. His consciousness level was clear. His heart rate was 128 beats/min and his blood pressure was 195/120 mmHg; his other vital signs were normal. He was sweaty and had bulbar conjunctiva and had many petechiae on his lower extremities. The results of cardiopulmonary and abdominal examinations were normal (see Fig. 2).
Fig. 1.
A chest radiograph showed a huge mass in the right upper lung field and enlargement of the right hilum.
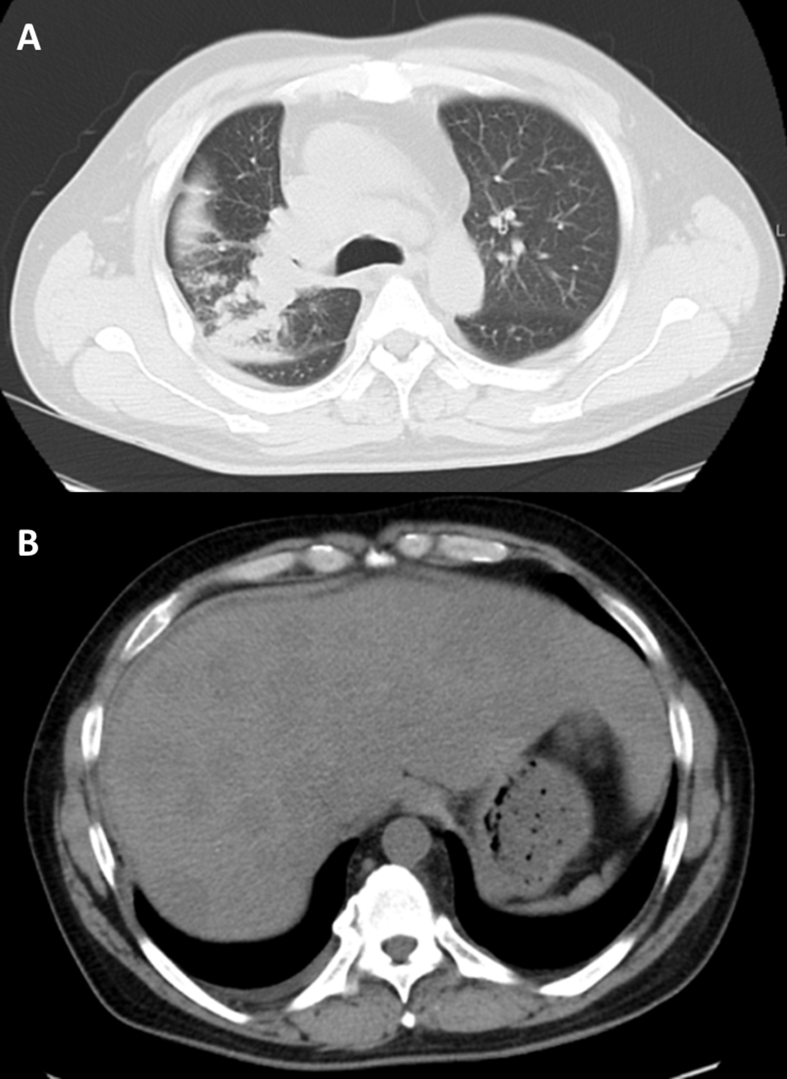
Fig. 2.
A: Chest CT revealed a mass in the right upper lung lobe, right hilar lymphadenopathy, and pleural effusion on both sides. B: Abdominal CT revealed multiple liver tumors, and some lesions were located at the subcapsular region.
A laboratory test revealed the following results: hemoglobin, 13.5 g/dL; leukocyte count, 11,150/mm3; platelet count, 77,000/mm3; prothrombin time, 92%; serum albumin, 3.0 g/dL; total bilirubin (T.Bil), 1.7 mg/dL; serum aspartate transaminase (AST), 115 IU/L; serum alanine transferase (ALT), 215 IU/L; alkaline phosphatase (ALP), 1209 IU/L; lactate dehydrogenase (LDH), 1277 IU/L; γGTP, 936 IU/L; and serum C-reactive protein, 2.84 mg/dL. The renal function and ionogram results were in the normal ranges. Tests for hepatitis B surface (HBs) antigen and HCV antibodies were negative. Tumor marker tests revealed the following results: carcino-embryonal antigen (CEA), 19.1 ng/ml; soluble cytokeratin 19 fragment (CYFRA), 4.3 ng/ml; and pro-gastrin releasing-peptide (proGRP), 72,098 pg/ml.
A computed tomography (CT) scan revealed a mass in the right upper lung lobe (7.0 cm in diameter) that subsequently expanded to the right hilum, and pleural effusion on both sides (Fig. 1B). Multiple liver tumors were also detected and some were located at the subcapsular region (Fig. 1C). A histological examination of the tumor specimen obtained by bronchoscopy from the right upper lung lobe confirmed the diagnosis of small cell lung cancer (SCLC), and antitumor chemotherapy (carboplatin and etoposide) was started. Several hours after the completion of the chemotherapy, the patient suddenly complained of nausea and general malaise. Laboratory tests showed anemia (hemoglobin, 9.8 g/dL), coagulation abnormality (PT, 76%; APTT, 21.4 sec), elevation of hepatic and biliary enzymes (T.Bil, 5.2 mg/dL; AST, 562 IU/L; ALT, 723 IU/L; ALP 1802 IU/L; LDH 2013 IU/L; γGTP, 1517 IU/L), acute renal insufficiency (BUN, 46 mg/dL; Cre 1.48 mg/dL), and hyperpotassemia (5.0 mEq/L). After that, his condition deteriorated rapidly and he died the next morning.
An autopsy revealed a mass (6.0 cm by 7.0 cm) in the right upper lobe. A histological examination revealed small cell lung cancer. His liver was enlarged, weighing 3120 g. A subcapsular hematoma (6 cm) and 1600 ml of ascites were also detected. The cut surface of the liver showed widely distributed tumor nodules (SCLC) of varying sizes and necrotic tissue. More than 60% of the liver parenchyma was replaced with metastatic tumors (Fig. 1D). Immunohistochemical staining of his metastatic liver tumor cells was diffusely positive for CD34 and MIB1, suggesting hypervascularity and high proliferation (Fig. 1E). From these findings, hemoperitoneum was thought to have occurred due to the rupture of subcapsular liver metastasis from lung cancer. Cancer had also spread to the distant lymph nodes, spleen and bone marrow.
2. Discussion
We described a case of hemoperitoneum due to the rupture of liver metastasis from SCLC. SCLC is a highly invasive disease and often metastasizes to the liver; however, the rupture of liver metastasis is rare. Hepatocellular carcinoma (HCC) is reported to be the most common cause of spontaneous rupture of liver tumors. HCC rupture is thought to occur due to its hypervascularity, high invasiveness, tendency to penetrate the liver capsule, and decreased coagulation factors due to underlying liver cirrhosis [1,2]. Other contributing factors have been proposed, including necrotic tendency, subcapsular location, congestion of the local hepatic vein, increased intra-abdominal pressure, and rapid tumor growth [3]. Our patient had some of these risk factors, and based on his clinical course, chemotherapy was also considered to be one of the triggers of this condition due to tumor necrosis. Rupture of liver metastasis of various tumors, including lung cancer, pancreatic cancer, skin cancer, prostate cancer, kidney tumor, testicular cancer, and gastric cancer, has been reported [4,5]. To the best of our knowledge, more than 50 cases of hemoperitoneum due to rupture of liver metastasis have been reported [2,4]. We comprehensively reviewed the relevant literature, and finally found 11 similar cases with rupture of liver metastasis from lung cancer (Table 1) [4,[6], [7], [8], [9], [10], [11], [12], [13], [14]].
Table 1.
Reported cases of liver rupture due to metastases from lung cancer.
| Ref. | Pathology | Age | Sex | Latest treatment | Initial symptoms | Treatment | Time to death |
|---|---|---|---|---|---|---|---|
| Sakai M et al. [6] | Ad | 64 | M | none | Abdominal discomfort hypotension | TAE | survival |
| Schoedel KE et al. [7] | Ad | 57 | M | none | Confusion tachycardia hypotension | conservative | 6 days |
| Mittleman RE et al. [8] | Sm | 62 | M | none | abdominal pain back pain tachycardia | operation | <1 day |
| Kadowaki T et al. [9] | Sq | 72 | M | none | abdominal pain tachycardia hypotension | conservative | 2 months |
| Fujikawa T et al. [10] | Sm | 69 | M | amrubicin (8 weeks prior) | dizziness nausea abdominal pain |
conservative | 3 days |
| Nishikawa S et al. [11] | Ad | 65 | F | erlotinib (current) | abdominal pain | conservative | 3 months |
| Nishikawa S et al. [11] | Sm | 79 | M | amrubicin (current) | (no data) | conservative | 2 months |
| Umemoto K et al. [12] | Ad | 60 | M | enterectomy (6 days prior) | shock | operation | 7 days |
| Kadowaki T et al. [13] | Sq | 72 | M | none | abdominal pain hypotension | conservative | 2 months |
| Odagiri H et al. [14] | La | 74 | M | lung lobectomy | (no data) | conservative | 2–3 weeks |
| Mochimaru T et al. [4] | Sm | 65 | M | CDDP + ETP (current) | abdominal discomfort hypotension | TAE | survival |
Liver metastasis is found in 30–45% of cases of non-small cell lung cancer, and in 17–34% cases of small cell lung cancer on autopsy [15]. In our patient, >60% of the liver parenchyma had been replaced by metastasis, resulting in acute liver failure (ALF). ALF is defined as a rapid loss of the liver function in a patient with no preexisting liver disease. The most common cause of ALF is viral infection, followed by drugs and autoimmune hepatitis. SCLC often metastasizes to the liver, but metastatic liver lesions remain asymptomatic in most patients. Thus, ALF due to liver metastasis of SCLC is extremely rare. In a previous study, diffusely spread carcinoma cells were reported to destroy liver cells and cause hepatic ischemia, necrosis, and potal vein thrombosis [3].
The initial symptoms of spontaneous rupture of liver metastasis depend on the extent of bleeding and range from subtle abdominal discomfort to acute abdomen and hemorrhagic shock (Table 1). Although there have been no published reviews of the CT features of ruptured liver metastasis, Choi et al. reviewed the CT findings of 12 patients with ruptured hepatocellular carcinoma and reported that peripheral location, protruding contour, discontinuity of the hepatic surface, and surrounding hemoperitoneum are helpful diagnostic indicators of ruptured hepatocellular carcinoma [16]. In this case, CT revealed multiple liver metastases, and some of the metastatic lesions of the right lobe were located at the subcapsular region with discontinuity of the hepatic surface. Abdominal ultrasound is usually the first examination performed and shows free intraperitoneal fluid. In the case of pre-existing ascites, there are reports that contrast-enhanced ultrasonography can be used to confirm active bleeding and determine the location of a bleeding lesion if contrast media is present in the ascites [5]. CT angiography is the diagnostic modality of choice and may show signs of ongoing bleeding with extravasation of contrast media. It also offers useful information regarding the extent of the metastatic disease in the liver and treatment by transarterial embolization (TAE).
The short-term prognosis of spontaneous rupture of liver metastasis is determined by bleeding severity. In many of the reported cases, the patients received conservative therapy and died within three months. While the long-term prognosis depends on the cancer stage and the patient's performance status, treatment of hemoperitoneum secondary to rupture of liver metastasis depends on the tumor size, tumor location, and severity of bleeding, with control of the hemorrhage being the major objective [1]. Most patients are in shock or unstable, and therapeutic options are limited. Especially in cases of advanced cancer, therapy tends to be palliative rather than curative. The goal of treatment should be to control the hemorrhage quickly and effectively. The role of TAE is well established in HCC hemorrhage [17] and case reports describe its successful application in the treatment of ruptured liver metastasis [4,6]. Surgical resection of liver metastasis or ligation of the hepatic artery is ineffective in unstable patients but may be considered after haemostasis has been achieved, especially in cases of solitary liver metastasis [17,18].
We presented a rare case of hemoperitoneum secondary to the spontaneous rupture of hepatic metastasis from lung cancer. The clinical features and imaging findings were very useful in the diagnosis of ruptured hepatic metastasis. Physicians should pay attention to this condition, especially after the initiation of chemotherapy.
CRediT authorship contribution statement
Yuki Goto: Data curation, Writing - original draft. Kazunori Tobino: Data curation. Kohei Yoshimine: Data curation. Takuto Sueyasu: Data curation. Masanobu Okahisa: Data curation. Mitsukuni Sakabe: Writing - original draft, Data curation.
Declaration of competing interest
All the authors have no conflict of interest about this case report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101039.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Urdaneta L.F., Nielsen J.V. Massive hemoperitoneum secondary to spontaneous rupture of hepatic metastases: report of two cases and review of the literature. J. Surg. Oncol. 1986;31:104–107. doi: 10.1002/jso.2930310206. [DOI] [PubMed] [Google Scholar]
- 2.Akriviadis E.A. Hemoperitoneum in patients with ascites. Am. J. Gastroenterol. 1997;92:567–575. [PubMed] [Google Scholar]
- 3.van Marcke C., Coulier B., Gielen I., Maldague P. Acute liver failure secondary to metastatic liver infiltration: case report and review of the literature. Acta Gastro-Enterol. Belg. 2013;76(4):436–438. [PubMed] [Google Scholar]
- 4.Mochimaru T., Minematsu N., Ohsawa K. Hemoperitoneum secondary to rupture of a hepatic metastasis from small cell lung cancer during chemotherapy: a case with a literature review. Intern. Med. 2017;56:695–699. doi: 10.2169/internalmedicine.56.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naganuma H., Funaoka M., Fujimori S. Rupture of liver metastasis: report of a case with an emphasis on contrast-enhanced US. J. Med. Ultrason. 2007;34:113–116. doi: 10.1007/s10396-007-0140-4. [DOI] [PubMed] [Google Scholar]
- 6.Sakai M., Oguri T., Sato S. Spontaneous hepatic rupture due to metastatic tumor of lung adenocarcinoma. Intern. Med. 2005;44:50–54. doi: 10.2169/internalmedicine.44.50. [DOI] [PubMed] [Google Scholar]
- 7.Schoedel K.E., Dekker A. Hemoperitoneum in the setting of metastatic cancer to the liver. A report of two cases with review of the literature. Dig. Dis. Sci. 1992;37:153–154. doi: 10.1007/BF01308360. [DOI] [PubMed] [Google Scholar]
- 8.Mittleman R.E. Hepatic rupture due to metastatic lung carcinoma. Am. J. Clin. Pathol. 1987;88:506–509. doi: 10.1093/ajcp/88.4.506. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki T., Hamada H., Yokoyama A. Hemoperitoneum secondary to spontaneous rupture of hepatic metastasis from lung cancer. Intern. Med. 2005;44:290–293. doi: 10.2169/internalmedicine.44.290. [DOI] [PubMed] [Google Scholar]
- 10.Fujikawa T., Nagahama K., Minakata K. A case of intraperito neal bleeding due to spontaneous rupture of liver metastases of small-cell lung carcinoma. Jpn. J. Chest Dis. 2014;57:59–66. (in Japanese) [Google Scholar]
- 11.Nishikawa S., Kiba H., Watanabe K. Two cases of adenocarcinoma with hepatic metastasis causing hemoperitoneum. Jpn. J. Lung Canc. 2014;54:593. (in Japanese) [Google Scholar]
- 12.Umemoto K., Oura T., Nakayama M. A case of ruptured hepatic metastasis from lung cancer. Hokkaido J. Surg. 2011;56:60. (in Japanese) [Google Scholar]
- 13.Kadowaki T., Hamada Y., Itoh R. A case of hemoperitoneum in the elderly secondary to rupture of a hepatic metastasis from lung cancer. Nihon Ronen Igakkai Zasshi. 2004;41:566. (in Japanese) [Google Scholar]
- 14.Odagiri H., Sugimoto H., Hanada S. An autopsy case of large cell carcinoma of the lung with rhabdoid phenotype, causing rupture of a metastatic hepatoma in an early time after operation. Jpn. J. Lung Canc. 2010;50:387. (in Japanese) [Google Scholar]
- 15.Fishman A.P., Elias J.A., Fishman J.A. third ed. McGrow Hill; New York: 1997. Fishman's Pulmonary Diseases and Disorders; pp. 1770–1821. [Google Scholar]
- 16.Choi B.G., Park S.H., Byun J.Y. The findings of ruptured hepatocellular carcinoma on helical CT. Br. J. Radiol. 2001;74:142–146. doi: 10.1259/bjr.74.878.740142. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasa S., Lee W.G., Aldameh A. Spontaneous hepatic haemorrhage: a review of pathogenesis, aetiology and treatment. HPB. 2015;17:872–880. doi: 10.1111/hpb.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.J., Park Y.E., Ki M.S. Spontaneous rupture of hepatic metastasis from a thymoma: a case report. World J. Gastroenterol. 2016;22:9860–9864. doi: 10.3748/wjg.v22.i44.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.