Key Points
The increasing number of reports of direct transmission of avian influenza viruses to humans in the past few years and the ongoing outbreak of H5N1 influenza virus infections in birds and humans highlight the pandemic threat posed by avian influenza viruses.
Although vaccination is the key strategy for the prevention of severe illness and death from pandemic influenza viruses and despite the long-term experience with vaccines against human influenza viruses, researchers face several obstacles in developing successful vaccines against avian influenza viruses.
The haemagglutinin (HA) and neuraminidase (NA) glycoproteins of influenza viruses are the main targets of the protective immune response. Licensed influenza virus vaccines are designed to induce HA-specific antibody responses to protect the host from infection. However, the presence of 16 subtypes of HA and 9 subtypes of NA glycoproteins among avian influenza viruses and the genetic and antigenic diversity among each subtype in nature present several unique challenges for the generation of broadly cross-protective vaccines.
Inactivated virus and live attenuated virus vaccines against pandemic influenza are being developed on the basis of plasmid-based reverse-genetics technology. Vaccines based on various other platforms, including live virus vectors and DNA vaccines, are also being developed and show promise in preclinical studies.
The available data indicate that inactivated avian influenza virus vaccines are poorly immunogenic and require a high concentration of HA glycoprotein or co-administration with an adjuvant to achieve the desired antibody response in humans. The biological basis for the poor immunogenicity of avian HA glycoproteins is not well understood.
Assays to measure the immune response to avian influenza viruses, in particular cell-mediated immune responses, are not available and the immune correlates of protection are not well understood. The choice of assay(s) for assessment of the immune response to pandemic influenza vaccines is a practical challenge in the evaluation of candidate vaccines.
As it is difficult to predict which avian influenza virus will cross the species barrier and cause a future pandemic, a library of candidate vaccines of different subtypes must be generated and evaluated in animal models and humans.
Although an ideal vaccine would prevent infection, a more realistic goal for a pandemic influenza vaccine might be to prevent severe illness and death.
The pandemic threat posed by avian influenza viruses highlights the need for new safe and efficient vaccines. However, several unique obstacles are faced by researchers in the development of these vaccines against avian influenza viruses. What are these obstacles and how can we overcome them?
Abstract
The increasing number of reports of direct transmission of avian influenza viruses to humans underscores the need for control strategies to prevent an influenza pandemic. Vaccination is the key strategy to prevent severe illness and death from pandemic influenza. Despite long-term experience with vaccines against human influenza viruses, researchers face several additional challenges in developing human vaccines against avian influenza viruses. In this Review, we discuss the features of avian influenza viruses, the gaps in our understanding of infections caused by these viruses in humans and of the immune response to them that distinguishes them from human influenza viruses, and the current status of vaccine development.
Main
Emerging and re-emerging infectious diseases in humans and animals have been reported with increased frequency in recent years1. The growing demands on land use, intensified farming practices to feed a larger population and the increase in travel and transportation allow the emergence, re-emergence and rapid spread of infectious agents around the globe. The emergence of high-pathogenicity avian influenza (HPAI) viruses in domestic poultry and the increasing number of cases of direct transmission of avian influenza viruses of different subtypes to humans are a significant threat to public health because of the potential for pandemic spread of these viruses. The ongoing outbreak of HPAI H5N1 viruses in the bird population and the nearly 50% case-fatality rate among people who become infected with H5N1 viruses underscore the need for control strategies to prevent a potential influenza pandemic.
Research efforts to control emerging viral diseases are focused on improving surveillance and diagnostic methods, and on the development of antiviral drugs and effective vaccines. Vaccination is the cornerstone of prevention. Interest in the development of pandemic influenza vaccines intensified with the outbreak of H5N1 influenza virus infections of humans in Hong Kong in 1997 and has increased further as H5N1 viruses have spread in birds and humans since 2003. Despite extensive experience with vaccines against human influenza viruses, researchers face several additional obstacles in developing successful vaccines against avian influenza viruses with pandemic potential. In this Review, we discuss the challenges associated with generating and evaluating vaccines against avian influenza viruses and the current status of pandemic vaccine development.
Avian influenza viruses
Influenza viruses belong to the Influenza A genus of the family Orthomyxoviridae. Although the natural reservoir for all influenza A viruses is aquatic birds, these viruses also infect and cause disease of varying severity in domestic poultry and several species of mammal, including humans2,3. The genome of influenza A viruses consists of 8 single-stranded RNA segments that encode 11 proteins (Fig. 1a). Influenza A viruses can be divided into subtypes on the basis of genetic and antigenic differences in their main surface glycoproteins, haemagglutinin (HA) and neuraminidase (NA)3. So far, 16 HA (H1–H16) and 9 NA (N1–N9) glycoprotein subtypes have been identified in influenza A viruses3,4. The HA and NA glycoproteins are the targets of the protective immune response and can vary as a result of antigenic drift and antigenic shift. The genes encoding the internal virus proteins are highly conserved between influenza A viruses. The matrix 2 (M2) protein is an integral membrane protein that functions as an ion channel that is important in the uncoating of the virus. Antibodies specific for the M2 protein cannot neutralize virus infectivity but are protective in vivo5,6. The cellular immune response is directed against the nucleoprotein (NP) and the RNA polymerase proteins PB2 (polymerase basic protein 2) and PA (polymerase acidic protein).
Figure 1. Schematic of an influenza A virus.
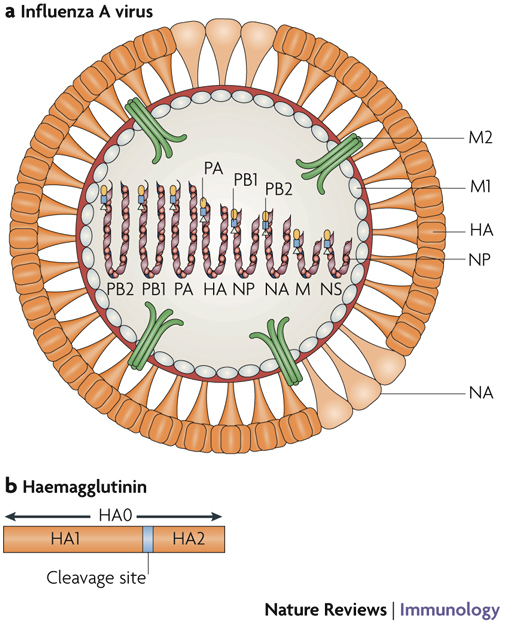
a | The influenza A virus particle has a lipid envelope that is derived from the host cell membrane. Three envelope proteins — haemagglutinin (HA), neuraminidase (NA) and an ion channel protein (matrix protein 2, M2) — are embedded in the lipid bilayer of the viral envelope. HA (rod shaped) and NA (mushroom shaped) are the main surface glycoproteins of influenza A viruses. The ratio of HA to NA molecules in the viral envelope usually ranges from 4:1 to 5:1. b | The HA glycoprotein is synthesized as an HA0 molecule that is post-translationally cleaved into HA1 and HA2 subunits; this cleavage is essential for virus infectivity. The HA glycoprotein is responsible for binding of the virus to sialic-acid residues on the host cell surface and for fusion of the viral envelope with the endosomal membrane during virus uncoating. The NA glycoprotein cleaves sialic-acid receptors from the cell membrane and thereby releases new virions from the cell surface. M2 functions as a pH-activated ion channel that enables acidification of the interior of the virion, leading to uncoating of the virion. Matrix protein 1 (M1), which is the most abundant protein in the virion, underlies the viral envelope and associates with the ribonucleoprotein (RNP) complex. Inside the M1 inner layer are eight single-stranded RNA molecules of negative sense that are encapsidated with nucleoprotein (NP) and associated with three RNA polymerase proteins — polymerase basic protein 1 (PB1), PB2 and polymerase acidic protein (PA) — to form the RNP complex. The PB1, PB2 and PA proteins are responsible for the transcription and replication of viral RNA. The virus also encodes a non-structural protein (NS) that is expressed in infected cells and a nuclear export protein (NEP). The location of NEP in the virion is not known.
Aquatic birds do not usually show signs of disease when infected with avian influenza viruses7, but infections of domestic poultry can be associated with disease. In addition to their division into subtypes on the basis of genetic and antigenic differences, avian influenza viruses can also be divided into two groups on the basis of their ability to cause severe disease in chickens — namely, HPAI viruses and low-pathogenicity avian influenza (LPAI) viruses. HPAI viruses are restricted to the HA glycoprotein subtypes H5 and H7 and cause systemic infection, which can result in 100% mortality within a flock. Specific NA subtypes have not been associated with the pathogenicity of HPAI viruses. LPAI viruses, which include viruses of all HA subtypes, cause milder infection and do not usually cause mortality7.
The molecular basis of the difference in pathogenicity between HPAI and LPAI viruses is mainly attributed to the cleavability of the precursor HA glycoprotein HA0 into HA1 and HA2 subunits. Cleavage of HA0 generates the carboxyl terminus of HA1 and the amino terminus of HA2, the latter being necessary for membrane fusion and for virus infectivity8 (Fig. 1b). The HA0 glycoproteins of LPAI viruses and human influenza viruses are cleaved at a conserved arginine residue by trypsin-like proteases9. Therefore, infection with these viruses is restricted to tissues in which trypsin and trypsin-like proteases are present: the respiratory tract of humans and the intestinal tract of birds. By contrast, the HA0 glycoproteins of HPAI viruses are highly cleavable; they can have multiple basic amino acids at the cleavage site9 that can be cleaved by ubiquitous intracellular proteases, such as furins10, or by non-trypsin-like extracellular proteases. The HA0 glycoprotein can also have additional glycosylation sites11 that are associated with virulence. Because the HA0 glycoproteins of HPAI viruses are easily cleaved in extrapulmonary sites, these viruses can replicate in extrapulmonary organs, including the brain, causing fatal disease and death of the infected birds7. However, the significance of the highly cleavable HA0 glycoprotein as a virulence determinant in human infections with HPAI viruses is not known. HPAI viruses are believed to emerge as a result of mutations that occur after LPAI viruses move into domestic poultry12. This has prompted the establishment of agricultural controls for LPAI virus H5 and H7 subtype infections in poultry in an effort to prevent the emergence of HPAI viruses13,14.
HA glycoproteins bind to sialic-acid residues with terminal oligosaccharides on the host cell surface. An important difference between avian influenza viruses and human influenza viruses is their preference for specific sialic-acid linkages. Most avian influenza viruses preferentially bind to sialic acids that are linked to galactose with α-2,3 linkages, whereas human influenza viruses preferentially bind to sialic acids with α-2,6 linkages15,16,17. NA glycoproteins cleave sialic-acid residues from the surface of the infected cell to release progeny virions from that cell, thereby facilitating virus dissemination3.
The transmission of avian influenza viruses to humans was thought to occur rarely because of the host-range restriction of the viruses. The observation that the 1957 H2N2 and 1968 H3N2 viruses that caused human pandemics were reassortant viruses, with gene segments derived from both avian and human influenza A viruses, indicated that the transmission of avian influenza viruses to humans might require reassortment between a human and avian influenza virus. Reassortment was proposed to occur in an intermediate host, such as the pig, which has both α-2,3-linked and α-2,6-linked sialic-acid residues in the respiratory tract. However, the outbreak of human infections with H5N1 viruses in 1997 in Hong Kong showed that avian influenza viruses could be transmitted directly from domestic poultry to humans18,19. Since 1997, LPAI and HPAI viruses of several subtypes, including H9N2, H5N1, H7N7, H7N3 and H10N7, have been implicated in human infections by direct transmission from birds (Table 1). In each case, poultry in the region were infected with viruses that were genetically related to the viruses isolated from humans, which indicates that the human infections occurred as a result of direct transmission from birds to humans. Severe illness and death have not occurred following infection with LPAI viruses, whereas human infections with HPAI viruses have been more severe and fatal in many cases. However, human infections with H5 and H7 subtype viruses are not uniformly severe or fatal.
Table 1.
Laboratory-confirmed cases of human infection with avian influenza viruses
| Virus subtype | Year; country | Number of cases (deaths) | Clinical illness | Characteristics of the virus isolated from human case(s) | Refs | |
|---|---|---|---|---|---|---|
| Genetically related avian influenza virus isolate(s)* | Sialic-acid linkage specificity‡ | |||||
| H5N1 | 1997; Hong Kong | 18 (6) | Fever, respiratory symptoms, pneumonia, ARDS, multi-organ dysfunction in fatal cases |
HA: A/goose/Guangdong/1/96 (H5N1) NA: A/teal/Hong Kong/W312/97 (H6N1) Internal protein genes: A/chicken/Hong Kong/G1/97 (H9N2) or A/teal/Hong Kong/W312/97 (H6N1) |
α-2,3 | 18,19, 114–117 |
| 2003–present; Cambodia, China, Egypt, Indonesia, Iraq, Thailand, Turkey, Vietnam | 256 (151) | Respiratory symptoms, ARDS, multi-organ dysfunction | ND | α-2,3 | 16,17, 118–121 | |
| H7N7 | 2003; Netherlands | 89 (1) | Conjunctivitis, mild influenza-like illness, pneumonia followed by respiratory-distress syndrome in the fatal case | A/chicken/Netherlands/1/03 (H7N7) | ND | 31,122 |
| H7N3 | 2004; Canada | 2 | Conjunctivitis |
A/chicken/Canada/AVFV1/04 (H7N3) A/chicken/Canada/AVFV2/04 (H7N3) |
ND | 32,123 |
| H9N2 | 1999; Hong Kong | 2 | Mild, self-limiting febrile pharyngitis | A/quail/Hong Kong/G1/97 (H9N2) | α-2,6 | 116,124 |
| 1999; China | 5 | Mild | A/chicken/Hong Kong/G9/97 (H9N2) | α-2,6 | 124,125 | |
| 2003; Hong Kong | 1 | Mild | A/chicken/Hong Kong/G9/97 (H9N2) | α-2,6 | 126 | |
| H10N7 | 2004; Egypt | 2 | Fever and cough | ND | ND | 127 |
| *Genetically related avian influenza viruses that were identified as the probable source of the indicated gene segment of the virus that caused human infection. | ||||||
| ‡Denotes the binding preference of the viral HA glycoprotein for avian (α-2,3-linked) or human (α-2,6-linked) sialic-acid residues. ARDS, adult respiratory distress syndrome; HA, haemagglutinin; NA, neuraminidase; ND, not determined. | ||||||
The goal of a pandemic influenza vaccine
The increasing number of reports of direct transmission of avian influenza viruses to humans in the past few years (reviewed in Refs 20–22) and the ongoing outbreak of H5N1 influenza virus infections in avian species and humans in several countries (reviewed in Refs 20, 23, 24) highlight the significant threat posed by HPAI and LPAI viruses to human health. Because it is not possible to predict which subtype of avian influenza virus will cause the next human pandemic, an ideal vaccine would elicit an immune response that protects the host from infection with a broad range of influenza viruses from the same or different subtypes. Lessons from the previous influenza pandemics guide our efforts to prepare for a future pandemic (Box 1).
The HA and NA glycoproteins of influenza viruses (Fig. 1) undergo genetic and antigenic variation to escape the immune response25,26. The presence of neutralizing antibodies specific for the HA glycoprotein at systemic or mucosal sites of infection provides immediate protection against infection with influenza viruses, whereas the clearance of human influenza viruses depends mainly on cell-mediated immunity27 (Fig. 2). Although antibodies specific for the NA glycoprotein do not neutralize infectivity, they restrict virus replication by preventing the release of new virus particles, a process that requires viral NA proteins. Therefore, antibodies specific for NA can decrease the severity of the disease28,29. Epitopes recognized by cytotoxic T lymphocytes (CTLs) are present on NP, PB2 and PA proteins of human influenza viruses. Therefore, if a virus with a new HA and/or NA glycoprotein emerges in the human population, cell-mediated immunity directed against the highly conserved internal proteins could have a role in protection at the time of a pandemic. The principle underlying the currently licensed vaccines against human influenza viruses is the induction of protective antibodies specific for the HA glycoprotein of the predicted epidemic strain. The concentration of HA glycoprotein in licensed, inactivated virus vaccines for seasonal influenza is standardized, but the concentration of NA glycoprotein is not standardized.
Figure 2. The adaptive immune response during infection with influenza virus.
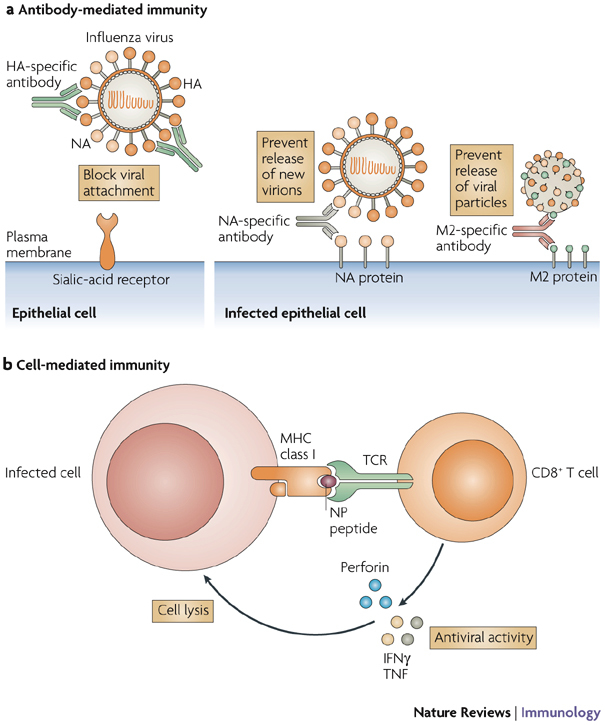
Influenza viruses attach to the epithelial cell surface of host cells through binding of the viral haemagglutinin (HA) glycoprotein to cell-surface sialic-acid residues. The virion is internalized through endocytosis and fusion between host and viral membranes occurs in acidic vacuoles. Opening of the ion channel formed by matrix protein 2 (M2) triggers this fusion and the release of viral genes into the cytoplasm, through which they travel to the nucleus. Viral mRNAs are transported from the nucleus to the cytoplasm, where viral proteins are translated and progeny virions assemble and bud from the cell membrane. The release of progeny virions requires the action of the viral neuraminidase (NA) glycoprotein, which cleaves sialic-acid receptors from the host cell membrane. a | Antibodies specific for HA block virus attachment, thereby preventing infection of cells, or they can prevent fusion. Antibodies specific for NA bind virus to the cell, thereby preventing the release of virions. Antibodies specific for M2 bind virus to the cell and prevent the release of viral particles into the extracellular fluid. b | Cell-mediated immunity contributes to host resistance when CD8+T cells specific for viral proteins such as nucleoprotein (NP) or the RNA polymerase proteins polymerase basic protein 2 (PB2) and polymerase acidic protein (PA) recognize viral peptides presented by MHC class I molecules, resulting in the release of cytokines with antiviral activity — such as interferon-γ (IFNγ) and tumour-necrosis factor (TNF) — and perforins that mediate cytolysis of the infected cell. Lysis of the infected cell decreases the amount of virus released by the cell. The latter three mechanisms, NA-specific antibodies, M2-specific antibodies and CD8+ T cells, operate after a cell becomes infected. Only antibodies specific for HA can prevent infection; this is probably why they are the most effective mediators of immunity in vivo. TCR, T-cell receptor.
The role of cell-mediated immunity in the control of avian influenza virus infections is not known. A potential advantage of a vaccine that induces a cell-mediated immune response, over one that elicits a protective antibody response, is that the internal proteins of the virus that are targets of the cell-mediated immune response tend to be conserved and are less prone to genetic drift (see later) than are the antibody-binding sites on the HA and NA glycoproteins.
There are at least two scenarios in which a vaccine that elicits a cell-mediated immune response might be useful in the event of a pandemic. First, although a cell-mediated immune response might not prevent infection with the virus, it may prevent severe illness and death from influenza. Although this might not be an acceptable outcome for a seasonal influenza vaccine, it might be reasonable for a pandemic influenza vaccine. Second, if the incubation period and course of infection with avian influenza viruses are longer than those with human influenza viruses, as has been reported23, vaccines that elicit protection through a cell-mediated immune response might be effective.
Diversity of avian viruses and vaccine design
Although most influenza vaccines are designed to induce HA-specific antibody responses to protect the host from infection, the biology of avian influenza viruses presents several unique challenges, compared with human influenza viruses. These challenges include the presence of different subtypes of HA and NA glycoproteins and the genetic and antigenic diversity within each subtype. The antigenic diversity has consequences for pandemic vaccines that must be considered in the design of a protective vaccine.
One of the first questions that must be considered is whether vaccines should be developed against all of the subtypes of HA and NA glycoproteins. The 16 HA and 9 NA subtypes of avian influenza viruses might not have similar pandemic potential (Box 2). Although HPAI H5N1 viruses are the main focus of global attention, LPAI H9N2 viruses are also widespread in poultry in Asia30 and HPAI H7 viruses have caused large outbreaks in poultry in Europe31, North America123 and South America33. Although HPAI viruses cause morbidity and mortality in poultry, HPAI viruses might not be intrinsically more likely to cause a human pandemic than LPAI viruses; interestingly, there are no known examples of a pandemic caused by an H5 or H7 HPAI virus, although virological data are limited to those from the three influenza pandemics that occurred in the last century.
Because of this uncertainty, it will be prudent to develop vaccines against each of the subtypes of avian influenza virus, although the order of development can be prioritized on the basis of epidemiological data. It is probable that avian influenza virus subtypes that are widely prevalent in nature and those that have been shown to infect humans will be of greater concern than subtypes that are not circulating widely in nature. A comparison of the predicted protein structures of HA glycoprotein subtypes 1 to 15 has led to the classification of these subtypes into four different clades — namely, clade 1 (H1, H2, H5, H6, H11 and H13), clade 2 (H8, H9 and H12), clade 3 (H3, H4 and H14) and clade 4 (H7, H10 and H15)34. The implications for vaccine development of the phylogenetic relationships between the HA glycoproteins of different subtypes are not yet known. Can HA glycoproteins that are grouped together in the same clade elicit crossreactive immunity against other subtypes in the same clade?
Phylogenetic analysis of the genes encoding certain subtypes of HA glycoprotein reveals a separation into lineages that correspond to the geographical separation of the birds that they infect. These genetic lineages are referred to as the Eurasian and North American lineages and they generally correspond to the flight paths of migratory birds7,12,35. Viruses from these two lineages might also be antigenically distinguishable, but the consequences of these genetic and antigenic differences for vaccine development are not known. Are the antigenic differences that have been identified between avian influenza viruses of significance for human infections? Do viruses of the two lineages have similar pandemic potential? If the genetic differences between viruses are the result of co-evolution of an avian influenza virus with its avian host, and if the antigenic differences are driven by evolution in an avian host rather than by positive selection by an immune response to infection, then a vaccine generated against a virus of the North American lineage might protect against a virus of the Eurasian lineage. Unfortunately, this information is not known yet.
Circulating human influenza viruses undergo rapid mutation owing to the low fidelity of the viral RNA-dependent RNA polymerase36. Antigenic drift occurs when the genes encoding the HA and/or NA glycoproteins undergo stepwise mutations, resulting in variant viruses with amino-acid changes at one or more antibody-binding sites of HA and/or NA37 that allow the viruses to evade neutralization by antibodies generated as a result of previous natural infection or vaccination. The internal protein genes of avian influenza viruses are not under positive immune selection in waterfowl and shorebirds. However, the use of veterinary vaccines to protect poultry from infection with avian influenza viruses might drive evolution of the HA glycoprotein if such vaccines do not induce sterilizing immunity. This was particularly evident when a widespread vaccination programme was launched in 1995 in Mexico to control the outbreak of a LPAI H5N2 virus infection that started in 1993. Phylogenetic and serological analyses of viruses that were isolated a few years after the introduction of the vaccine indicated the presence of multiple sublineages of HA glycoproteins with marked antigenic differences from the HA glycoproteins used in the vaccine38. Several countries in Asia are using veterinary vaccines to control H5N1 virus infections in poultry. If avian influenza viruses undergo antigenic drift in poultry as a consequence of vaccine use, then the pandemic influenza vaccines that are stockpiled for human use might need to be updated. However, an effective animal vaccination programme would reduce the burden of disease in poultry substantially, thereby reducing the risk of a human pandemic. Also, changes in the virus that are driven by positive immune selection in poultry might not be significant in humans. It will be important to assess the potential advantages and disadvantages of animal vaccination and to determine the significance of antigenic drift in avian influenza viruses for humans.
The viral determinants of pathogenicity of avian influenza viruses in humans are multigenic. Further studies are required to understand how the pathogenicity of avian influenza viruses affects the infectivity and transmissibility of these viruses in humans and to establish whether these factors have implications for vaccine design.
Types of vaccine
Inactivated virus vaccines and live attenuated virus vaccines that are being developed for pandemic influenza are based on technologies that are licensed for the existing seasonal human influenza vaccines. Vaccines based on various other platforms, such as live virus vectors expressing influenza virus proteins and DNA vaccines, are also being developed and have shown promise in preclinical studies (Table 2).
Table 2.
Current status of the development of vaccines against avian influenza viruses
| Virus subtype | Type of vaccine | Vaccines evaluated in preclinical studies based on indicated virus | Vaccines evaluated in clinical studies based on indicated virus |
|---|---|---|---|
| H5N1 | Inactivated virus |
A/Hong Kong/156/97 (H5N1) virus43 A/duck/Singapore/97 (H5N3) virus42 A/duck/Hokkaido/67/96 (H5N4) virus54 Recombinant H5N1–PR8 virus45 Recombinant A/Hong Kong/213/03 (H5N1)–PR8 virus55,56,57,58 Recombinant A/duck/Singapore/97 (H5N3)–PR8 virus56 |
A/duck/Singapore/97 (H5N3) virus59,60,128 Recombinant A/Vietnam/1203/04 (H5N1)–PR8 virus70 |
| Live attenuated virus | H5N1–AA cold-adapted reassortant virus (H5N1 genes from A/Vietnam/1203/04, A/Hong Kong/213/2003 and A/Hong Kong/491/97 viruses)75 | In progress | |
| Subunit |
HA and NA proteins of A/Hong Kong/156/97 (H5N1) virus80 M2 protein of A/Hong Kong/483/97 (H5N1) virus79 |
Baculovirus-expressed HA proteins of A/Hong Kong/156/97 and A/Hong Kong/483/97 (H5N1) viruses69 | |
| DNA |
HA gene of A/Hong Kong/156/97 and A/Hong Kong/483/97 (H5N1) viruses81 |
– | |
| Vectored | Adenovirus expressing HA protein of A/Hong Kong/156/97 and A/Vietnam/1203/04 (H5N1) viruses86,87 | – | |
| H7 | Subunit | HA protein of A/mallard/Netherlands/12/00 (H7N3) virus and NA protein of A/Netherlands/33/03 (H7N7) virus78 | – |
| H9 | Inactivated virus |
A/Hong Kong/1073/99 (H9N2) G1-like virus53 Recombinant A/chicken/Hong Kong/G9/97 (H9N2)–PR8 virus52 |
A/Hong Kong/1073/99 (H9N2) G1-like virus63,66 A/chicken/Hong Kong/G9/97 (H9N2) virus61 |
| Live attenuated virus | H9N2–AA cold-adapted reassortant virus (H9N2 genes from A/chicken/Hong Kong/G9/97 virus)74 | In progress | |
| Subunit | M2 protein of H9N2 virus79 | – | |
| Virus-like particles | Expressing HA, NA and matrix proteins of A/Hong Kong/1073/99 (H9N2) virus129 | – | |
| DNA | HA and NA genes of A/chicken/Jiangsu/7/02 (H9N2) virus84 | – | |
| AA, A/Ann Arbor/6/60 influenza virus; HA, haemagglutinin; M2, matrix protein 2; NA, neuraminidase; NP, nucleoprotein; PR8, A/Puerto Rico/8/34 (H1N1) influenza virus. | |||
The currently licensed vaccines against human influenza viruses are produced in embryonated chicken eggs and the manufacturing process can take 6–9 months. Therefore, for vaccines that are based on the currently licensed technologies, the availability of embryonated eggs is a crucial factor and if the pandemic virus causes widespread morbidity and mortality in poultry, the supply of embryonated eggs might be compromised. Therefore, alternative substrates, including mammalian cell lines such as Madin–Darby canine kidney (MDCK) cells and Vero cells, must be developed for the production of influenza viruses for use in vaccines. Considerable progress has been made in the development of vaccines based on inactivated influenza viruses and live cold-adapted influenza viruses grown in these cell lines in microcarrier fermentors39,40,41.
The virulence of HPAI viruses for chickens, embryonated eggs and humans, as well as safety concerns for agriculture and humans, has limited the use of conventional methods for the production of vaccines from wild-type HPAI viruses. Two strategies have been used to address this issue. One strategy is the use of antigenically related surrogate avian influenza viruses that are not pathogenic for poultry or humans. Such viruses can be handled safely in the laboratory or a vaccine-manufacturing plant, and a vaccine manufactured from the surrogate virus should elicit an immune response that crossreacts well with the antigenically related HPAI virus. An example of this approach is the use of a LPAI H5N3 virus (A/duck/Singapore/97) to generate a vaccine to protect against HPAI H5N1 viruses42,43.
The other strategy is to modify the virulence of the HPAI viruses by genetic engineering44,45. This involves the use of plasmid-based reverse genetics, whereby infectious influenza viruses can be recovered from cells transfected with plasmids encoding each of the eight gene segments of the virus46,47,48 (Fig. 3). The cells that are transfected with plasmids for the generation of a vaccine seed virus must be approved for use in humans. However, another major challenge in the field of vaccine development is the choice of appropriate animal models for preclinical studies.
Figure 3. The eight-plasmid reverse-genetics system.
Generation of recombinant vaccines for pandemic influenza. a | Six plasmids encoding the internal proteins of the high-growth influenza A/Puerto Rico/8/34 (PR8) donor virus or the attenuated, cold-adapted (ca) H2N2 A/Ann Arbor/6/60 (AA) donor virus are co-transfected with two plasmids encoding the avian influenza virus haemagglutinin (HA; modified to remove virulence motifs, if necessary) and neuraminidase (NA) glycoproteins into qualified mammalian cells and the recombinant virus is then isolated. Recombinant viruses containing internal protein genes from the PR8 virus are used to prepare inactivated influenza virus vaccines. Recombinant viruses containing internal protein genes from the attenuated, cold-adapted AA virus are used to prepare live attenuated influenza virus vaccines. b | The generation of pandemic influenza vaccine viruses by classical reassortment. The reassortant viruses derive six internal protein genes from the vaccine donor virus and the HA and NA genes from the circulating avian influenza virus. The reassortant virus is selected using antisera specific for the HA and NA glycoproteins of the donor virus. M, matrix protein; NP, nucleoprotein; NS, non-structural protein; PA, polymerase acidic protein; PB, polymerase basic protein.
Influenza A viruses replicate in several experimental animals, including chickens, mice, cotton rats, ferrets, hamsters, guinea pigs and non-human primates. The use of mouse models for the study of influenza is limited because intranasally administered influenza A viruses do not cause symptoms of respiratory-tract disease in mice, although some influenza A viruses are lethal in some other animal models. In addition, in general, human influenza viruses require an adaptation to replicate to high titres in mice. However, it is probable that mice will continue to be used for research into influenza viruses because reagents for immunological studies in mice are widely available.
Ferrets are generally thought to be the best available model for influenza research. Unlike mice, ferrets develop fever, rhinorrhea and sneezing after infection with intranasally administered human influenza viruses and the virus replicates in the respiratory tract of these animals. Seronegative ferrets develop a strain-specific immune response to human influenza viruses. However, less is known about the replication of avian influenza viruses in mice and ferrets or the relevance of observed morbidity and mortality to humans, so the choice of either animal as an ideal model for the development of pandemic influenza vaccines requires further research. Golden Syrian hamsters, cotton rats49,50, guinea pigs51 and non-human primates support the replication of influenza A viruses, but experience with these models is not as extensive as with mice and ferrets for human or avian influenza viruses. Currently, preclinical studies of pandemic influenza vaccines are carried out in mice and ferrets.
Inactivated virus vaccines. In preclinical studies, parenterally administered, inactivated whole-virus H9 and H5 subtype vaccines have been shown to be effective in mice against challenge with homologous and heterologous viruses42,43,52,53,54. Recombinant H5 influenza viruses — which contain a modified HA glycoprotein, a wild-type NA glycoprotein from the 1997 or 2003 H5N1 viruses or from an LPAI H5N3 virus, and internal protein genes from the PR8 H1N1 influenza virus (A/Puerto Rico/8/34) that confer high yield in eggs — have been generated by reverse genetics45,55,56,57,58. The removal of the multibasic amino-acid motif in HA that makes the HA0 precursor of HPAI viruses highly cleavable attenuated the virus for infection of chickens, mice and ferrets without altering the antigenicity of the HA glycoprotein. Two doses of these inactivated virus vaccines provided complete protection from lethal challenge with homologous and heterologous H5N1 viruses in mice and ferrets45,55,56,57,58.
Data from phase I clinical trials of inactivated virus vaccines against H9N2, H5N3, H5N1 and H2N2 viruses have been reported and other vaccines are still under evaluation (Table 2). Studies that have been carried out so far indicate that inactivated split-virion vaccines against avian influenza viruses — in which the virions are disrupted or split by detergent treatment and the surface glycoproteins are then partially purified — are not optimally immunogenic59 and require multiple doses60 or the inclusion of an adjuvant61,62,63,64 to induce a protective immune response. Whole-virus vaccines are more immunogenic than split-virion vaccines, but they are likely to be more reactogenic65. Adjuvants are required to increase the immunogenicity of inactivated virus vaccines and to decrease the concentration of viral proteins that is required to induce protective immunity, and several adjuvants for this purpose are under investigation, including aluminium salts, the squalene–oil–water emulsion (MF59) and other proprietary compounds that cannot be discussed in detail.
An inactivated whole-virus H9N2 vaccine was shown to be immunogenic in individuals who had circulating antibodies induced by prior exposure to H2N2 viruses that crossreacted with H9N2 viruses, but the vaccine was not immunogenic in individuals who were born after 1968, when H2N2 viruses stopped circulating in humans66. This observation is consistent with findings from studies of an H1N1 vaccine in 1976–1977, when prior exposure to H1N1 viruses that had circulated in the population earlier ('priming') was found to be a determinant of the response to vaccination65,67. These studies also showed the need for two doses of vaccine in 'unprimed' individuals. In other studies of vaccines against H9N2 viruses, aluminium hydroxide and MF59 adjuvants improved immunogenicity61,63.
Inactivated virus vaccines prepared from recombinant PR8 viruses that contain a modified HA glycoprotein and wild-type NA glycoproteins from H5N1 viruses isolated in 2004 have been evaluated as subvirion vaccines or whole-virus vaccines, with or without adjuvants58,62,64,68,70. The subvirion vaccines were safe and well-tolerated in healthy adults, and the antibody response that was induced could be enhanced by increasing the dose of antigen used or by the addition of an adjuvant62,70. A whole-virus vaccine was also well-tolerated by humans, and when administered with an adjuvant this vaccine was immunogenic at a lower dose than the subvirion vaccines64. However, the available data indicate that inactivated H5 influenza virus vaccines are poorly immunogenic and require a large concentration of HA glycoprotein or co-administration with an adjuvant to achieve the desired antibody response.
Live attenuated virus vaccines. Live attenuated, cold-adapted influenza virus vaccines against human influenza viruses elicit both systemic immunity and mucosal immunity at the primary portal of infection. The vaccine strains are generated by the reassortment of a wild-type influenza virus carrying the HA and NA genes of interest with a cold-adapted donor AA (H2N2) influenza virus (A/Ann Arbor/6/60), which was generated by serial passage of the wild-type AA virus at successively lower temperatures71. The temperature-sensitive, attenuated, cold-adapted donor AA virus has five mutations in three gene segments that contribute to the temperature-sensitive or attenuation phenotype72 and the virus has a high degree of phenotypic and genotypic stability73. Candidate live attenuated virus vaccines against H9N2 and H5N1 avian influenza viruses generated on this cold-adapted donor backbone using reassortment and plasmid-based reverse genetics, respectively (Fig. 3), were safe and effective in mice and ferrets44,74,75. Phase I clinical evaluation of these vaccines is currently in progress.
Generally, live attenuated virus vaccines must retain some infectivity to be immunogenic. Therefore, virus shedding during clinical testing of these vaccines must be closely monitored. The potential challenges in the development of live attenuated virus vaccines for pandemic influenza are: first, to generate reassortant viruses that are sufficiently infectious when the HA glycoprotein is derived from an avian influenza virus, in particular if the HA used has a preference for α-2,3-linked oligosaccharides; second, to reproducibly achieve the desired level of viral attenuation with different combinations of HA and NA genes; and third, to minimize the risk of reassortment with circulating human influenza viruses. The evaluation of live attenuated virus vaccines of different subtypes in preclinical studies in appropriate animal models and in clinical studies will address the first two challenges. The standard approach of preclinical evaluation that is applied to vaccines against human influenza viruses might not be uniformly applicable to avian influenza viruses, because the infectivity, immunogenicity and protective efficacy of avian influenza viruses of different subtypes have not been studied extensively76. The risk of reassortment of the live attenuated vaccine virus with human influenza viruses during clinical trials can be minimized by conducting vaccine studies in isolation units when human influenza viruses are not circulating in the community. In the event of an influenza pandemic, the potential benefits of a live attenuated virus vaccine will have to balanced against the risks associated with it, and this type of vaccine will only be introduced judiciously when a pandemic is imminent.
Recombinant subunit, DNA and vectored vaccines. The use of recombinant or expressed proteins of the influenza virus in a vaccine is an attractive option for vaccine development because these approaches do not require handling of HPAI or infectious viruses for vaccine production.
Preclinical studies of recombinant HA, NA and M2 proteins as vaccine antigens (Table 2) showed that the proteins were poorly immunogenic and required multiple doses77 or the inclusion of adjuvants78,80 for improved immunogenicity and efficacy. DNA vaccines encoding the HA and NA glycoproteins of avian influenza viruses or conserved internal virus proteins, such as matrix proteins and nucleoproteins, induced protective immunity in mice and chickens81,82,83,84. The protective efficacy of a nucleoprotein-encoding DNA vaccine was increased by a booster vaccination in the form of a recombinant replication-defective adenovirus (rADV) expressing the nucleoprotein85. In two recent studies, intramuscular or intranasal immunization of mice with a human rADV vaccine expressing the influenza virus HA glycoprotein induced both humoral and cell-mediated immune responses and conferred protection against challenge with the wild-type virus in mice and chickens86,87. A recombinant baculovirus-expressed H5 glycoprotein subunit vaccine was well-tolerated but was poorly immunogenic in humans, indicating the need for an adjuvant69. The production of recombinant proteins and DNA vaccines is safe and economical, but clinical studies of their safety and immunogenicity in humans are awaited.
Universal influenza virus vaccines. An ideal influenza vaccine would be effective against a range of virus subtypes and could be useful during pandemic and inter-pandemic periods. One approach to creating a universal vaccine would be to target an antigenically stable protein or an antigenically stable part of a variable protein that is essential for virus replication. The high degree of conservation of the M2 protein makes it a prime candidate for a universal influenza vaccine. The M2 protein induced crossreactive immunity that decreased the severity of disease in animal models after challenge with wild-type virus79,88. However, the emergence of immune-escape mutants of the M2 protein in mice in the presence of specific antibodies raises concerns regarding the usefulness of the M2 protein as a target for a universal vaccine89. Clinical studies are required to evaluate the immunogenicity of the M2 protein in humans.
It has been suggested that the use of the NA glycoprotein, which is less variable than the HA glycoprotein, to induce cross-protective immunity might be worth exploring90. NA-specific immunity in mice provides significant cross-protection against antigenically distinct viruses of the same subtype91,92. Although NA-specific antibodies do not prevent infection with influenza viruses, they decrease the severity and duration of illness in humans by limiting the release and spread of the virus28,29.
Also, if common immunogenic epitopes are identified within the four clades of HA glycoprotein subtypes, HA-based immunogens could induce widely crossreactive immunity93. Alternatively, genetically engineered viruses that have several conserved immunogenic epitopes on the viral envelope could be developed and evaluated for use as a universal influenza vaccine90. Recombinant viruses expressing chimeric HA glycoproteins have been described recently94. Although universal influenza vaccines are still in preclinical development, the potential benefits of such vaccines are so great that strategies to develop them must be encouraged.
Immunogenicity of pandemic influenza vaccines
Although principles derived from decades of experience with seasonal vaccines against human influenza viruses form the basis for the development of pandemic influenza vaccines, our knowledge of the human immune response to avian influenza viruses is incomplete. Investigators rely on extrapolation from experience with vaccines against human influenza viruses, where the immune correlates of protection were elucidated in challenge studies in humans. However, the evaluation of vaccines against potential pandemic strains of avian influenza viruses presents a unique challenge: vaccines developed against these viruses can only be evaluated for safety and immunogenicity, and not for protection, in clinical trials, because challenge studies to assess the efficacy of the vaccines cannot be undertaken in humans. When the immunogenicity of candidate pandemic vaccines is assessed, the data can be difficult to interpret because specific information on the nature and magnitude of the antibody response that correlates with protection is lacking. If a vaccine is immunogenic, it might be possible to assess its efficacy by testing the vaccine in a large group of people who are at high risk from infection with avian influenza virus, such as poultry farmers in areas with severe epizootics.
Serum and mucosal antibodies can independently mediate immunity to influenza viruses. Live viruses and inactivated virus vaccines differ in the induction of protective antibodies, but there are no standardized methods for evaluating the mucosal antibody response. The conventional assay for assessing the immunogenicity of a human influenza vaccine is the haemagglutination-inhibition assay. The standard haemagglutination-inhibition assay, which uses chicken or turkey erythrocytes, is relatively insensitive for the detection of antibodies specific for H5N1 viruses, but a modified assay using horse erythrocytes was found to be more sensitive95,96 because horse erythrocytes exclusively express the α-2,3-linked oligosaccharide side chains that are preferred for binding by avian influenza viruses. Most studies have shown that serum haemagglutination-inhibition antibody titres of approximately 1:32 to 1:40 protected 50% of the study subjects from infection after immunization with inactivated human influenza virus vaccines25,26,97. However, in other studies of experimental infection in adult humans, serum haemagglutination-inhibition antibody titres as low as 1:8 provided resistance to infection with human influenza viruses, which indicates that the levels of antibody required for protection are fairly low98. The horse erythrocyte haemagglutination-inhibition assay has not been well standardized and the antibody titres determined by this assay that correlate with protection are not known. Therefore, the choice of assays by which the immune response is assessed poses a practical challenge for the evaluation of pandemic influenza vaccines.
An alternative to the haemagglutination-inhibition assay that might be more biologically relevant is a neutralization assay, in which the ability of antibodies to neutralize the infectivity of the avian influenza virus is assessed. Using paired sera from individuals infected with H5N1 influenza virus in 1997 in Hong Kong collected at the acute and convalescent stages of infection, a neutralizing antibody titre of 1:80 was shown to be indicative of infection with an H5N1 virus99. However, it is not known whether this antibody titre correlates with protection from re-infection. Other barriers to the use of the neutralization assay are the requirement for appropriate biosafety containment measures, as the assay requires handling of the infectious virus, and the fact that the test has not yet been standardized100.
Another consideration in the assessment of the immune response to pandemic influenza vaccines is the lack of reagents for quality control. Post-infection or hyperimmune post-vaccination animal sera are used as controls at present because human sera containing antibodies specific for avian influenza viruses are not available.
Careful analysis of the immune responses in individuals who have recovered from natural infections with avian influenza viruses and in volunteers who participate in clinical trials of pandemic influenza vaccine candidates using standardized assays will increase our understanding of the human immune response to infection with avian influenza viruses. The efficacy of pandemic influenza vaccine candidates cannot be assessed directly in humans; therefore, this information will have to be extrapolated from studies in experimental animals.
Future prospects
The circulation of influenza A viruses of all subtypes in aquatic and wild birds, and the apparent expansion of the host range of avian influenza viruses increase the probability of a pandemic in humans, either by the direct introduction of an avian influenza virus into humans or by the generation of an avian–human reassortant virus that has an increased ability to replicate and spread in the human population. Because it is difficult to predict which avian influenza virus will cross the species barrier and cause a future pandemic, it is crucial that a library of candidate vaccines of different influenza virus subtypes is generated and evaluated in animal models and humans. Animal models that allow comparison with the corresponding wild-type avian influenza virus should be used.
The immune correlates of protection against influenza are not well understood and assays for different aspects of the immune response to avian influenza viruses, in particular cell-mediated immune responses, are not available. The contribution of viral determinants of infectivity, transmissibility and virulence of avian influenza viruses to infection of humans and their implications for vaccine design require further study. The relevance of adaptive genetic changes in avian influenza viruses as they replicate in poultry and other avian species to their infectivity for humans, and the effect of veterinary vaccine use on the evolution of avian influenza viruses are both areas of research that could have a direct impact on vaccines developed for human use. The biological basis for the poor immunogenicity of avian HA glycoproteins should be explored. Until this is understood, there is a need for adjuvants to increase the immunogenicity of avian influenza vaccines. Although an ideal vaccine would prevent infection, a more realistic goal for a pandemic influenza vaccine might be to prevent severe illness and death. Efforts to develop cell-culture-based vaccines are under way. The development of a safe, immunogenic universal vaccine that could be used to control both seasonal and pandemic influenza would be a great achievement.
Box 1 | Influenza pandemics of the twentieth century.
The 1918 Spanish influenza pandemic
Of the three influenza pandemics that occurred in the twentieth century, the Spanish influenza (H1N1 virus) pandemic of 1918–1919 was the most notable. More than 40 million people around the world died from influenza101. An important feature of this pandemic was the high mortality in the unusually young age group of 20–40-year olds102. Studies in a mouse model using viruses containing genes from a reconstructed 1918 virus generated by reverse genetics indicate that the haemagglutinin (HA) glycoprotein has an important role in the pathogenicity of this virus. The introduction of the HA glycoprotein of the 1918 virus into a non-pathogenic human influenza virus made it highly pathogenic in mice103,104. However, the fully reconstructed 1918 virus was even more virulent104,105. Structural analysis showed that the HA glycoprotein of the 1918 virus could bind to α-2,6-linked sialic-acid residues, despite the presence of amino-acid residues in the receptor-binding site that are characteristic of the HA glycoprotein of an avian influenza virus106. Further studies of host, as well as viral, factors that contributed to the virulence of the 1918 pandemic H1N1 virus might help in the development of strategies to combat future pandemics.
The 1957 and 1968 Asian influenza pandemics
The 1957 Asian influenza (H2N2 virus) pandemic and the 1968 Hong Kong influenza (H3N2 virus) pandemic were milder than the 1918 pandemic, but both still caused significant morbidity and mortality around the world. The 1957 pandemic was caused by a reassortant virus that was derived from the HA (H2), neuraminidase (N2) and PB1 (polymerase basic protein 1) genes from an avian influenza virus infecting ducks and the remaining gene segments from the previously circulating human H1N1 virus107,108. The H3N2 virus that caused the 1968 pandemic consisted of avian HA (H3) and PB1 genes in a background of other internal protein genes of the human H2N2 virus that was circulating at the time107,108. The presence of an avian HA H3 glycoprotein made the reassortant virus antigenically novel to humans and it spread in the susceptible human population causing a pandemic.
Box 2 | Factors contributing to the emergence of pandemic influenza.
Factors of probable relevance
The prevalence of an avian influenza virus subtype in domestic poultry
Documented human infection and human-to-human transmission of the virus
Factors of unknown relevance
High pathogenicity of the avian influenza virus109
The pathogenicity of the virus in mammals other than humans110
The ability of the viral haemagglutinin glycoprotein to bind to sialic-acid residues with an α-2,3-linkage (avian) or with an α-2,6-linkage (human)16,17
The stalk length of the viral neuraminidase glycoprotein111,112
The presence of a glutamic acid to lysine mutation at position 627 of the viral RNA polymerase protein PB2 (polymerase basic protein 2)110,113
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Glossary
- Pandemic influenza virus
An influenza virus of a new subtype to which the general population has little or no immunity that causes disease in humans and spreads efficiently from person to person, causing community-wide outbreaks and resulting in a global outbreak of influenza.
- Haemagglutinin
A type I integral membrane glycoprotein that binds to cell-surface receptors and facilitates fusion between the viral envelope and endosomal membrane. It is the main target antigen of the humoral immune response to influenza viruses.
- Neuraminidase
A type II integral membrane glycoprotein that facilitates virus release from cells by removing sialic acid from sialyloligosaccharides on the cell and viral surfaces. It is also a target of the protective immune response.
- Antigenic drift
A process by which circulating influenza viruses are constantly changing, which allows the virus to cause annual epidemics of illness. Antigenic drift occurs when mutations accumulate in the haemagglutinin and neuraminidase genes that alter the antigenicity of these proteins such that the 'drifted' strains are no longer neutralized by antibodies that were specific for previously circulating strains.
- Antigenic shift
A process by which a new influenza A virus haemagglutinin subtype (with or without an accompanying new neuraminidase subtype) is introduced into the human population, which lacks prior experience of and immunity to the subtype. Antigenic shift can occur as a result of the direct introduction of an influenza virus from an animal or avian host into humans or by the exchange or reassortment of gene segments between human and non-human influenza viruses when they co-infect animals or humans.
- Matrix protein
The most abundant structural protein of influenza virus, which lies beneath the virus envelope.
- Nucleoprotein
Encapsidates viral genomic RNA and forms a ribonucleoprotein complex in association with viral polymerase proteins.
- Positive immune selection
This is usually defined as a significant excess of non-silent over silent nucleotide substitutions in a gene, and occurs when natural selection favours a particular genetic variation and therefore the frequency of the genetic variation shifts.
- Vaccine seed virus
A virus that is used for the large-scale production of vaccines.
- PR8 H1N1 influenza virus (A/Puerto Rico/8/34)
A well-characterized laboratory strain of influenza virus that confers high growth in eggs and is used as the genetic backbone for viruses from which inactivated influenza virus vaccines are generated.
- Adjuvant
An agent mixed with an antigen that increases the immune response to that antigen after immunization.
- Subvirion vaccine
In a subvirion vaccine, the virions are disrupted or split by detergent treatment and the surface glycoproteins are then partially purified.
- Cold-adapted virus
A virus that replicates efficiently at low temperatures, which can be generated by serial passage of a wild-type virus at successively lower temperatures.
- Haemagglutination-inhibition assay
An assay used to measure the concentration of antibodies that inhibit the agglutination of erythrocytes by a standard amount of influenza virus. Serum haemagglutination-inhibition antibody titres correlate with protection from infection with human influenza viruses.
Biographies
Kanta Subbarao is a senior investigator in the Laboratory of Infectious Diseases of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), USA. Dr Subbarao received her medical training (M.B., B.S.) from the Christian Medical College, Vellore, University of Madras, India, and completed a residency in paediatrics, a fellowship in paediatric infectious diseases and a Master of Public Health degree in epidemiology in the United States. She received postdoctoral training in virology and vaccine development in the Laboratory of Infectious Diseases, NIAID. Dr Subbarao's research is focused on the development of vaccines against pandemic strains of influenza virus and the development of animal models for and evaluation of vaccines against the coronavirus that causes severe acute respiratory syndrome (SARS).
Tomy Joseph received his veterinary medical degree (DVM) from the Kerala Veterinary College in India. He obtained an M.Sc. and Ph.D in Molecular Virology from the Atlantic Veterinary College, University of Prince Edward Island, Canada. Dr Joseph is currently a postdoctoral visiting fellow in the Laboratory of Infectious Diseases at the NIAID, NIH, USA. His research focuses on the generation and preclinical evaluation of live attenuated virus vaccines against avian influenza A H7 subtype viruses. He is also studying the pathogenesis of and immune response to avian influenza viruses in animal models.
Competing interests
Kanta Subbarao and Tomy Joseph
Scientific barriers to developing vaccines against avian influenza viruses. Nature Reviews Immunology, published online 16 March 2007; doi: 10.1038/nri2054.
The laboratory of Dr Subbarao has a cooperative research and development agreement with MedImmune Vaccines to develop vaccines against potential pandemic strains of influenza.
References
- 1.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford PC, et al. Transmission of equine influenza virus to dogs. Science. 2005;310:482–485. doi: 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- 3.Wright PF, Webster RG. Fields Virology. 2001. pp. 1533–1579. [Google Scholar]
- 4.Fouchier RA, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005;79:2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treanor JJ, Tierney EL, Zebedee SL, Lamb RA, Murphy BR. Passively transferred monoclonal antibody to the M2 protein inhibits influenza A virus replication in mice. J. Virol. 1990;64:1375–1377. doi: 10.1128/jvi.64.3.1375-1377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J. Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 9.Perdue ML, Garcia M, Senne D, Fraire M. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res. 1997;49:173–186. doi: 10.1016/S0168-1702(97)01468-8. [DOI] [PubMed] [Google Scholar]
- 10.Klenk HD, Garten W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842X(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 11.Kawaoka Y, Naeve CW, Webster RG. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology. 1984;139:303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- 12.Banks J, Speidel EC, McCauley JW, Alexander DJ. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch. Virol. 2000;145:1047–1058. doi: 10.1007/s007050050695. [DOI] [PubMed] [Google Scholar]
- 13.Akey BL. Low-pathogenicity H7N2 avian influenza outbreak in Virginia during 2002. Avian Dis. 2003;47:1099–1103. doi: 10.1637/0005-2086-47.s3.1099. [DOI] [PubMed] [Google Scholar]
- 14.Marangon S, Capua I. Control of avian influenza in Italy: from stamping out to emergency and prophylactic vaccination. Dev. Biol. (Basel) 2006;124:109–115. [PubMed] [Google Scholar]
- 15.Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 16.Shinya K, et al. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 17.van Riel D, et al. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 18.Claas EC, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 19.Subbarao K, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 20.Gillim-Ross L, Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 2006;19:614–636. doi: 10.1128/CMR.00005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis DB. Avian flu to human influenza. Annu. Rev. Med. 2006;57:139–154. doi: 10.1146/annurev.med.57.121304.131333. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect. Dis. 2004;4:499–509. doi: 10.1016/S1473-3099(04)01105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigel JH, et al. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 24.de Jong MD, Hien TT. Avian influenza A (H5N1) J. Clin. Virol. 2006;35:2–13. doi: 10.1016/j.jcv.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couch RB, Kasel JA. Immunity to influenza in man. Annu. Rev. Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 26.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br. Med. Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 27.Gerhard W. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 28.Kilbourne ED, Laver WG, Schulman JL, Webster RG. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J. Virol. 1968;2:281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy BR, Kasel JA, Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N. Engl. J. Med. 1972;286:1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 30.Choi YK, et al. Continuing evolution of H9N2 influenza viruses in Southeastern China. J. Virol. 2004;78:8609–8614. doi: 10.1128/JVI.78.16.8609-8614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fouchier RA, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl Acad. Sci. USA. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tweed SA, et al. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 2004;10:2196–2199. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suarez DL, et al. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg. Infect. Dis. 2004;10:693–699. doi: 10.3201/eid1004.030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell RJ, et al. H1 and H7 influenza haemagglutinin structures extend a structural classification of haemagglutinin subtypes. Virology. 2004;325:287–296. doi: 10.1016/j.virol.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 35.Makarova NV, Kaverin NV, Krauss S, Senne D, Webster RG. Transmission of Eurasian avian H2 influenza virus to shorebirds in North America. J. Gen. Virol. 1999;80:3167–3171. doi: 10.1099/0022-1317-80-12-3167. [DOI] [PubMed] [Google Scholar]
- 36.Cox NJ, Bender CA. The molecular epidemiology of influenza virus. Semin. Virol. 1995;6:359–370. doi: 10.1016/S1044-5773(05)80013-7. [DOI] [Google Scholar]
- 37.Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu. Rev. Immunol. 1990;8:737–771. doi: 10.1146/annurev.iy.08.040190.003513. [DOI] [PubMed] [Google Scholar]
- 38.Lee CW, Senne DA, Suarez DL. Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J. Virol. 2004;78:8372–8381. doi: 10.1128/JVI.78.15.8372-8381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghendon YZ, et al. Development of cell culture (MDCK) live cold-adapted (CA) attenuated influenza vaccine. Vaccine. 2005;23:4678–4684. doi: 10.1016/j.vaccine.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 40.Oxford JS, et al. A new European perspective of influenza pandemic planning with a particular focus on the role of mammalian cell culture vaccines. Vaccine. 2005;23:5440–5449. doi: 10.1016/j.vaccine.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 41.Palker T, et al. Protective efficacy of intranasal cold-adapted influenza A/New Caledonia/20/99 (H1N1) vaccines comprised of egg- or cell culture-derived reassortants. Virus Res. 2004;105:183–194. doi: 10.1016/j.virusres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Lu X, et al. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood JM, et al. Vaccines against H5N1 influenza. Vaccine. 1999;18:579–580. doi: 10.1016/S0264-410X(99)00268-6. [DOI] [PubMed] [Google Scholar]
- 44.Li S, et al. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J. Infect. Dis. 1999;179:1132–1138. doi: 10.1086/314713. [DOI] [PubMed] [Google Scholar]
- 45.Subbarao K, et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology. 2003;305:192–200. doi: 10.1006/viro.2002.1742. [DOI] [PubMed] [Google Scholar]
- 46.Fodor E, et al. Rescue of influenza A virus from recombinant DNA. J. Virol. 1999;73:9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–3170. doi: 10.1016/S0264-410X(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 48.Neumann G, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl Acad. Sci. USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ottolini MG, et al. The cotton rat provides a useful small-animal model for the study of influenza virus pathogenesis. J. Gen. Virol. 2005;86:2823–2830. doi: 10.1099/vir.0.81145-0. [DOI] [PubMed] [Google Scholar]
- 50.Straight TM, Ottolini MG, Prince GA, Eichelberger MC. Evidence of a cross-protective immune response to influenza A in the cotton rat model. Vaccine. 2006;24:6264–6271. doi: 10.1016/j.vaccine.2006.05.092. [DOI] [PubMed] [Google Scholar]
- 51.Lowen AC, Mubareka S, Tumpey TM, Garcia-Sastre A, Palese P. The guinea pig as a transmission model for human influenza viruses. Proc. Natl Acad. Sci. USA. 2006;103:9988–9992. doi: 10.1073/pnas.0604157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H, et al. Generation and evaluation of a high-growth reassortant H9N2 influenza A virus as a pandemic vaccine candidate. Vaccine. 2003;21:1974–1979. doi: 10.1016/S0264-410X(02)00809-5. [DOI] [PubMed] [Google Scholar]
- 53.Lu X, et al. Immunity to influenza A H9N2 viruses induced by infection and vaccination. J. Virol. 2001;75:4896–4901. doi: 10.1128/JVI.75.10.4896-4901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takada A, et al. Avirulent avian influenza virus as a vaccine strain against a potential human pandemic. J. Virol. 1999;73:8303–8307. doi: 10.1128/jvi.73.10.8303-8307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J. Infect. Dis. 2006;194:159–167. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- 56.Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97 (H5) vaccines against infection with A/Vietnam/1203/04 (H5N1) virus in ferrets. J. Infect. Dis. 2006;194:1040–1043. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- 57.Nicolson C, Major D, Wood JM, Robertson JS. Generation of influenza vaccine viruses on Vero cells by reverse genetics: an H5N1 candidate vaccine strain produced under a quality system. Vaccine. 2005;23:2943–2952. doi: 10.1016/j.vaccine.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 58.Webby RJ, et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363:1099–1103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicholson KG, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 60.Stephenson I, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21:1687–1693. doi: 10.1016/S0264-410X(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 61.Atmar RL, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin. Infect. Dis. 2006;43:1135–1142. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 62.Bresson JL, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 63.Hehme N, Engelmann H, Kunzel W, Neumeier E, Sanger R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med. Microbiol. Immunol. (Berlin) 2002;191:203–208. doi: 10.1007/s00430-002-0147-9. [DOI] [PubMed] [Google Scholar]
- 64.Lin J, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 65.Wright PF, et al. Trials of influenza A/New Jersey/76 virus vaccine in normal children: an overview of age-related antigenicity and reactogenicity. J. Infect. Dis. 1977;136:S731–S741. doi: 10.1093/infdis/136.Supplement_3.S731. [DOI] [PubMed] [Google Scholar]
- 66.Stephenson I, et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003;362:1959–1966. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- 67.Parkman PD, Hopps HE, Rastogi SC, Meyer HM., Jr. Summary of clinical trials of influenza virus vaccines in adults. J. Infect. Dis. 1977;136:S722–S730. doi: 10.1093/infdis/136.Supplement_3.S722. [DOI] [PubMed] [Google Scholar]
- 68.Ozaki H, et al. Generation of high-yielding influenza A viruses in African green monkey kidney (Vero) cells by reverse genetics. J. Virol. 2004;78:1851–1857. doi: 10.1128/JVI.78.4.1851-1857.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Treanor JJ, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/S0264-410X(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 70.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 71.Maassab HF, Bryant ML. The development of live attenuated cold-adapted influenza virus vaccine for humans. Rev. Med. Virol. 1999;9:237–244. doi: 10.1002/(SICI)1099-1654(199910/12)9:4<237::AID-RMV252>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 72.Jin H, et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology. 2003;306:18–24. doi: 10.1016/S0042-6822(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 73.Cha TA, et al. Genotypic stability of cold-adapted influenza virus vaccine in an efficacy clinical trial. J. Clin. Microbiol. 2000;38:839–845. doi: 10.1128/jcm.38.2.839-845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen H, et al. Generation and characterization of a cold-adapted influenza A H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine. 2003;21:4430–4436. doi: 10.1016/S0264-410X(03)00430-4. [DOI] [PubMed] [Google Scholar]
- 75.Suguitan AL, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3:e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luke CJ, Subbarao K. Vaccines for pandemic influenza. Emerg. Infect. Dis. 2006;12:66–72. doi: 10.3201/eid1201.051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nwe N, et al. Expression of hemagglutinin protein from the avian influenza virus H5N1 in a baculovirus/insect cell system significantly enhanced by suspension culture. BMC Microbiol. 2006;6:16. doi: 10.1186/1471-2180-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Wit E, et al. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J. Virol. 2005;79:12401–12407. doi: 10.1128/JVI.79.19.12401-12407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ernst WA, et al. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24:5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Rimmelzwaan GF, Claas EC, van Amerongen G, de Jong JC, Osterhaus AD. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine. 1999;17:1355–1358. doi: 10.1016/S0264-410X(98)00390-9. [DOI] [PubMed] [Google Scholar]
- 81.Bright RA, Ross TM, Subbarao K, Robinson HL, Katz JM. Impact of glycosylation on the immunogenicity of a DNA-based influenza H5 HA glycoprotein vaccine. Virology. 2003;308:270–278. doi: 10.1016/S0042-6822(03)00008-4. [DOI] [PubMed] [Google Scholar]
- 82.Epstein SL, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg. Infect. Dis. 2002;8:796–801. doi: 10.3201/eid0805.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kodihalli S, Kobasa DL, Webster RG. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine. 2000;18:2592–2599. doi: 10.1016/S0264-410X(99)00485-5. [DOI] [PubMed] [Google Scholar]
- 84.Qiu M, et al. Protection against avian influenza H9N2 virus challenge by immunization with hemagglutinin- or neuraminidase-expressing DNA in BALB/c mice. Biochem. Biophys. Res. Commun. 2006;343:1124–1131. doi: 10.1016/j.bbrc.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 85.Epstein SL, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 86.Gao W, et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 2006;80:1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoelscher MA, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–481. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerhard W, Mozdzanowska K, Zharikova D. Prospects for universal influenza virus vaccine. Emerg. Infect. Dis. 2006;12:569–574. doi: 10.3201/eid1204.051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zharikova D, Mozdzanowska K, Feng J, Zhang M, Gerhard W. Influenza type A virus escape mutants emerge in vivo in the presence of antibodies to the ectodomain of matrix protein 2. J. Virol. 2005;79:6644–6654. doi: 10.1128/JVI.79.11.6644-6654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palese P. Making better influenza virus vaccines? Emerg. Infect. Dis. 2006;12:61–65. doi: 10.3201/eid1201.051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen J, Fang F, Li X, Chang H, Chen Z. Protection against influenza virus infection in BALB/c mice immunized with a single dose of neuraminidase-expressing DNAs by electroporation. Vaccine. 2005;23:4322–4328. doi: 10.1016/j.vaccine.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 92.Kilbourne ED, et al. Protection of mice with recombinant influenza virus neuraminidase. J. Infect. Dis. 2004;189:459–461. doi: 10.1086/381123. [DOI] [PubMed] [Google Scholar]
- 93.Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Li ZN, et al. Chimeric influenza virus hemagglutinin proteins containing large domains of the Bacillus anthracis protective antigen: protein characterization, incorporation into infectious influenza viruses, and antigenicity. J. Virol. 2005;79:10003–10012. doi: 10.1128/JVI.79.15.10003-10012.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–95. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 96.Stephenson I, Wood JM, Nicholson KG, Zambon MC. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J. Med. Virol. 2003;70:391–398. doi: 10.1002/jmv.10408. [DOI] [PubMed] [Google Scholar]
- 97.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (London) 1972;70:767–777. doi: 10.1017/S0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev. Biol. (Basel) 2003;115:97–104. [PubMed] [Google Scholar]
- 99.Rowe T, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stephenson I. H5N1 vaccines: how prepared are we for a pandemic? Lancet. 2006;368:965–966. doi: 10.1016/S0140-6736(06)69340-9. [DOI] [PubMed] [Google Scholar]
- 101.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 'Spanish' influenza pandemic. Bull. Hist. Med. 2002;76:105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 102.Reid AH, Taubenberger JK. The origin of the 1918 pandemic influenza virus: a continuing enigma. J. Gen. Virol. 2003;84:2285–2292. doi: 10.1099/vir.0.19302-0. [DOI] [PubMed] [Google Scholar]
- 103.Kobasa D, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 104.Tumpey TM, et al. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc. Natl Acad. Sci. USA. 2004;101:3166–3171. doi: 10.1073/pnas.0308391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tumpey TM, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 106.Gamblin SJ, et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004;303:1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- 107.Kawaoka Y, Krauss S, Webster RG. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 1989;63:4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scholtissek C, Rohde W, Von Hoyningen V, Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978;87:13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- 109.Proceedings of the 98th Annual Meeting of the United States Animal Health Association, Grand Rapids, Michigan (1994).
- 110.Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science. 2001;293:1840–1842. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- 111.Castrucci MR, Kawaoka Y. Biologic importance of neuraminidase stalk length in influenza A virus. J. Virol. 1993;67:759–764. doi: 10.1128/jvi.67.2.759-764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Subbarao EK, London W, Murphy BR. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guan Y, Shortridge KF, Krauss S, Webster RG. Molecular characterization of H9N2 influenza viruses: were they the donors of the 'internal' genes of H5N1 viruses in Hong Kong? Proc. Natl Acad. Sci. USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoffmann E, et al. Characterization of the influenza A virus gene pool in avian species in southern China: was H6N1 a derivative or a precursor of H5N1? J. Virol. 2000;74:6309–6315. doi: 10.1128/JVI.74.14.6309-6315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin YP, et al. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc. Natl Acad. Sci. USA. 2000;97:9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu X, Subbarao K, Cox NJ, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreak in Hong Kong. Virology. 1999;261:15–19. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- 118.Apisarnthanarak A, et al. Atypical avian influenza (H5N1) Emerg. Infect. Dis. 2004;10:1321–1324. doi: 10.3201/eid1007.040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Jong MD, et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 120.Tran TH, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 121.Epidemic and Pandemic Alert and Response (EPR): Avian influenza. World Health Organisation[online]
- 122.Koopmans M, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 123.Hirst M, et al. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg. Infect. Dis. 2004;10:2192–2195. doi: 10.3201/eid1012.040743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matrosovich MN, Krauss S, Webster RG. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology. 2001;281:156–162. doi: 10.1006/viro.2000.0799. [DOI] [PubMed] [Google Scholar]
- 125.Guo YJ, et al. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267:279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- 126.Butt KM, et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J. Clin. Microbiol. 2005;43:5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Avian influenza virus A (H10N7) circulating among humans in Egypt. EID Weekly Updates[online], (2004).
- 128.Stephenson I, et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J. Infect. Dis. 2005;191:1210–1215. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]
- 129.Pushko P, et al. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23:5751–5759. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]


