Key Points
The interplay between tumour cells and the extracellular microenvironment has been recognized as one of the key determinants of cancer development and progression
Interaction between extracellular matrix components and bacterial products controls tissue homeostasis; its dysregulation might prepare protumorigenic environmental niches, which could also favour disease relapse
Bladder colonization with specific bacterial genera throughout an individual's lifetime might influence the propensity for bladder pathology and partly explain the gender differences in the rate of urinary diseases
Extracellular-matrix-based and microbiota-based biomarkers might be new prognostic factors in bladder cancer, exploitable for risk stratification, tumour staging and for predicting relapse and outcome
Targeting the bladder extracellular matrix or the associated microbiota might bypass treatment resistance mechanisms of tumour cells
Supplementary information
The online version of this article (doi:10.1038/nrurol.2015.292) contains supplementary material, which is available to authorized users.
Subject terms: Bladder cancer, Cancer microenvironment, Microbiota
The interaction between tumour cells and their microenvironment has an important role in cancer pathogenesis. Alfano et al. review how dysregulation of the extracellular matrix and microbiota associated with the human epithelium might influence the development and progression of urothelial carcinomas.
Supplementary information
The online version of this article (doi:10.1038/nrurol.2015.292) contains supplementary material, which is available to authorized users.
Abstract
Many pathological changes in solid tumours are caused by the accumulation of genetic mutations and epigenetic molecular alterations. In addition, tumour progression is profoundly influenced by the environment surrounding the transformed cells. The interplay between tumour cells and their microenvironment has been recognized as one of the key determinants of cancer development and is being extensively investigated. Data suggest that both the extracellular matrix and the microbiota represent microenvironments that contribute to the onset and progression of tumours. Through the introduction of omics technologies and pyrosequencing analyses, a detailed investigation of these two microenvironments is now possible. In urological research, assessment of their dysregulation has become increasingly important to provide diagnostic, prognostic and predictive biomarkers for urothelial bladder cancer. Understanding the roles of the extracellular matrix and microbiota, two key components of the urothelial mucosa, in the sequelae of pathogenic events that occur in the development and progression of urothelial carcinomas will be important to overcome the shortcomings in current bladder cancer treatment strategies.
Supplementary information
The online version of this article (doi:10.1038/nrurol.2015.292) contains supplementary material, which is available to authorized users.
Introduction
Urothelial bladder cancer (UBC) is the most common malignancy of the urinary tract, causing 145,000 patient deaths per year1. Based on tumour stage classification, UBC is grouped into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC)2,3. NMIBC includes carcinoma in situ (Cis or Tis), pTa and pT1 tumours, with cancer cells located in the mucosa and submucosa of the bladder, and MIBC includes pT2–pT4 tumour stages, indicating invasion of cancer cells into the muscle layer of the bladder and beyond2,3. Most low-grade NMIBCs are prone to recurrence after treatment; flat Cis noninvasive lesions and papillary tumours are always characterized by high-grade cancer cells, with the risk of progression to MIBC and subsequent occurrence of metastases in 40–80% of patients, depending on the extent of the disease2,4,5. Patients with MIBC are treated with radical cystectomy, lymph node dissection and urinary diversion, and these patients are at high risk of metastatic tumour progression and cancer-related death3,6.
Modifications of the extracellular environments are mandatory for tumour progression, both at the primary site and in the metastatic niches7,8. Data published in the past decade suggest that the tumour extracellular matrix (ECM) might have a principal rather than a supporting role in the onset of carcinoma. Indeed, the dysregulation of the composition and stiffness of the ECM are associated with a lack of asymmetric division and differentiation of stem cells9,10, as well as an epithelial–mesenchymal transition of cancer stem cells; thus, the ECM regulates tissue homeostasis and sustains the onset and progression of cancer, including bladder cancer7,9,11,12.
The bacterial flora — collectively known as microbiota — is another extracellular microenvironment that is in contact with epithelium. Bacteria produce proteases, which can act intracellularly and/or extracellularly13. These enzymes function as extracellular virulence factors with important roles in host tissue degradation, as well as evasion and destruction of host physical barriers. Among these virulence factors, many bacterial enzymes that can degrade the ECM have been extensively characterized, including collagenase, elastase and hyaluronidase14,15. In addition, the bacterial invasion of tissues induces inflammation, a reaction which further sustains ECM remodelling, as well as the generation of oxygen radicals, which induce DNA damage and mutations that drive cancer and cancer recurrence16.
Current methods of oncological outcome evaluation and imaging assessment still have a limited ability to provide reliable risk stratification and predict UBC aggressiveness, disease recurrence and survival probability. Hence, an urgent need exists to better understand processes underlying bladder carcinogenesis, progression and metastasis, and to identify new prognostic and predictive markers, as well as novel potential therapeutic targets17,18. The ECM and microbiota represent two microenvironments that contribute to the onset and progression of tumours, which has been extensively studied in colorectal cancer19. In this Review, we provide an overview of the ECM and microbiota of the human epithelium, highlighting how dysregulation of either environment might influence the development and progression of UBC. An in-depth investigation of these two microenvironments and the role of their dysregulation in UBC might eventually provide novel prognostic, diagnostic and predictive biomarkers and new treatment strategies for this disease.
ECM
Molecular genetic evidence supports the existence of distinct pathogenetic pathways for the development of NMIBC and MIBC20. Indeed, changes in the composition and biomechanical properties of the extracellular environment are required for disease progression, both at the primary site and in metastatic niches7,8. Over the past years, it has become evident that tumour progression requires a microenvironment that is conducive to tumour growth, spread of tumour cells via the vasculature and lymphatic system and the formation of metastases in distant organs21. These steps in tumour progression are influenced by tumour cells as well as host factors22. Tumour-associated cells, such as fibroblast and macrophages, although they could be classified as nonmalignant based on the absence of specific genetic mutations, harbour epigenetic changes that modify their protein expression, resulting in modulation of the composition of the ECM itself and of ECM-embedded growth factors surrounding the neoplastic area22,23,24.
The ECM is a complex environment made up of a network of proteins and proteoglycans that interact with each other. In addition, these components form supramolecular structures in which their biological properties are modified. This system also incorporates cytokines and growth factors that are bound to glycosylated components of the ECM, thus, locating their activity to a specific compartment. Components of the ECM have been categorized as structural molecules (the core matrisome, composed of 200 glycoproteins, 43 collagen subunits and 35 proteoglycans) and matrisome-associated molecules (176 ECM-affiliated proteins, 250 ECM regulators, 352 secreted factors bound to the ECM, such as matrix metalloproteinases (MMPs), mucins, MMP inhibitors and TGF-β)25. In this context, not only ECM deposition but also its breakdown into smaller fragments and the crosslinking among ECM components contribute to the supramolecular assemblies found in the extracellular space. Intrinsic domains of the stromal proteins have growth-factor-like structures, which act as ligands for canonical growth factor receptors. Proteases and MMPs participate in ECM remodelling and turnover, thereby regulating cell–cell and cell–ECM adhesion, releasing ECM-bound cytokines and growth factors, as well as fragments of stromal components that function as growth factors, or proangiogenic or antiangiogenic molecules26,27,28. Thus, the ECM has been described as a solid-phase organized assembly of ligands29. Oxygen-dependent crosslinking has been reported to be mediated by lysyl oxidase30,31 and glycation end products32, which are increased in case of enhanced neovascularization.
In addition to the biochemical signals provided to cells by ECM proteins, the stiffness, dimensionality and the geometry of the extracellular space also modulate the cell behaviour, cell phenotype and function of any organ33,34,35. As a result, the extracellular environment of different tissues and organs also regulates recruitment of cells from the bloodstream and the fate of these cells, with stem cells or monocytes differentiating into a variety of cellular populations33,34,35.
Bladder ECM
In the past few years, the function of the ECM in the bladder has been examined and a number of genes regulating ECM remodelling and composition have been found to be associated with UBC progression and poor prognosis. The ECM composition of the bladder mucosa, in particular the basal membrane (Box 1) and the submucosa (Boxes 2,3), have been assessed for healthy tissue and tumours of epithelial origin.
Urothelium. The urothelium is covered by glycosaminoglycans (such as hyaluronic acid, heparan sulfate, heparin, chondroitin sulfate, dermatan sulfate and keratan sulfate), which form a gel-like barrier against urine and bacteria36,37. Other proteoglycans (such as decorin, nidogen-1, biglycan, fibulin-1 and tenascins) are also present in the urothelium, but their role in the modulation of tissue functionality has not been clarified38.
Hyaluronic acid is the main ECM-related glycosaminoglycan and it regulates biomechanical activity of tissues and cell functionality. Hyaluronic acid does not induce cell transformation, but it supports many important aspects of the malignant cell phenotype, such as proliferation, migration, resistance to apoptosis and epithelial–mesenchymal transition39,40. Formation of supramolecular hyaluronic acid structures (termed hyaluronic acid cables) can even promote inflammation through the binding of monocytes and lymphocytes41.
Lamina propria and muscularis propria. Collagen is the main structural protein of the ECM of various connective, fibrous and muscle tissues. Upon interaction with other ECM components, collagen provides the proper structure and function to tissues and organs. In the lamina propria and muscularis propria, the ECM is composed of collagens, and studies in female Sprague-Dawley rats and in women have revealed that a specific ratio among different collagens (mainly collagen I, III, IV, VI and XII)36 is required for adequate bladder functioning42,43,44,45. Collagenases (MMP-1, MMP-2, MMP-9 and MMP-13) break peptide bonds in collagen, enabling tissue ontogenesis and turnover, and participate in the normal immune response (that is, wound healing). Collagenases generate a variety of collagen-derived fragments with anti-angiogenic activity, such as endostatin46, tumstatin47, arresten48, canstatin49 and restin50. In the bladder, MMPs (such as MMP-1, MMP-2, MMP-7, MMP-9, MMP-11, MMP-14 and MMP-28), serine proteases (plasmin and kallikreins) and urokinase plasminogen activator have been identified36,51,52,53,54,55,56.
Osteopontin and fibronectin are also expressed in both the lamina propria and the muscularis propria of the bladder36. Fibronectin is present in the ECM in its insoluble form and is critical to the assembly of collagen fibrils in the extracellular space during development in vivo57. In the bladder, several fibronectin variants generated by alternative splicing are present58,59. Many studies have used rats or in vitro models to assess the role of plasma-purified fibronectin and fibronectin variants in tissue homeostasis and remodelling throughout disease processes60,61,62,63; however, a study focusing on the human bladder has not yet been reported.
Laminins are also present in the lamina propria and, together with elastic fibres, contribute to bladder functionality64. Elastin is also an ECM protein, which, along with collagen, determines the structural and mechanical properties of connective tissues. Elastic fibres (microfibrils) are formed by elastin covalently linking to fibrillin, fibulin and microfibril-associated glycoprotein53,65,66,67, by the action of lysyl oxidase65. Increased levels of lysyl oxidase have been reported in a variety of solid tumours as a responsible factor for the increased stiffness of neoplasia compared with normal tissues and they have been associated with poor prognosis68,69,70,71. In a murine model, lysyl oxidase was shown to regulate bladder tissue elasticity, and a deficiency in that enzyme has been associated with pelvic prolapse72. Indeed, elastic fibres are present in all bladder layers and are long-lasting molecules with only 1% turnover per year66, suggesting that any damage in the microfibrils is also long-lasting. Chymotrypsin, cathepsin G, neutrophil elastase and macrophage metalloelastase are enzymes that can cleave elastin73.
Apart from the crosslinking enzyme lysyl oxidase, advanced glycation end products, such as carboxymethyllysine and pentosidine, have been observed in connective tissues between muscle bundles and in the connective tissue between muscle fibres in non-neoplastic areas of human bladder specimens obtained from patients who underwent radical cystectomy for cancer32.
Box 1: Composition of bladder basal membrane extracellular matrix.
Collagen IV
• One of the main components of the basal membrane
• Composed of six distinct α chains
Laminins
• Some of the main components of the basal membrane
• A large family of heterotrimeric glycoproteins, consisting of α, β and γ chains, which are encoded by several different genes
• Involved in cell differentiation, migration and adhesion
• Support attachment of epithelial cells by participating in the formation of hemidesmosomes203
• In particular, laminin-5 is the major component of the anchoring filament that attaches hemidesmosomes to the basal membrane
• Cells interact with laminins through several cell surface receptors, such as integrins, membrane-bound proteoglycans and glycoproteins
Nidogen-1 (Ref. 204) and nidogen-2 (Ref. 205)
• Also known as entactin-1 and entactin-2
• Glycoproteins mainly expressed by mesenchymal cells and deposited into the epithelial and endothelial basal membranes during development206
• Structures and abilities to bind to extracellular matrix proteins are similar for both proteins
• Bind and form a ternary complex with collagen IV and laminins, connecting the two networks and stabilizing the 3D structure of the basal membrane207
• Can compensate for each other, owing to their similar structures and binding affinities208,209, but have different tissue distributions210,211,212, interact with different receptors212,213,214 and have diverse functions215,216
• Highly sensitive to proteolytic cleavage, although the binding of nidogen-1 to laminin-γ1 decreases susceptibility to proteolysis217, protecting laminins from proteolysis and contributing to basal membrane stability
• Removal of nidogens contributes to basal membrane disintegration, favouring epithelial–mesenchymal transition and metastasis206
Perlecan
• A ubiquitous proteoglycan supporting the basal membrane structure
• Binds to a variety of growth factors, such as FGF, VEGF, PDGF and TGF-β, through both a domain of the core protein and the carbohydrate chain218,219,220, highlighting a function in cell differentiation, cell proliferation and, in particular, in angiogenesis218
Box 2: Composition of bladder submucosa extracellular matrix.
Collagen
• Main structural protein of the extracellular matrix (ECM) of various connective, fibrous and muscle tissues
• 28 forms have been identified
• Interaction with other ECM components provides the structure and function of tissues and organs
• Together with elastin, forms elastic fibres (microfibrils) through covalent links with fibrillin, fibulin and microfibril-associated glycoprotein53,65,66,67, determining structural and mechanical properties of connective tissues
Proteoglycans
• Consist of a core protein with one or more covalently attached glycosaminoglycan chains
• Divided into small proteoglycans (decorin, biglycan, fibromodulin, lumican and testican) and large proteoglycans (versican, perlecan, neurocan and aggrecan)
• The extracellular proteoglycan decorin acts as an important regulator of collagen fibrillogenesis and inhibitor of cellular proliferation via sequestration of TGF-β and other growth factors221,222
• The decorin-related proteoglycan biglycan does not participate in fibrotic processes223, but mainly sustains pro-inflammatory signalling via the binding of TLR-2 and TLR-4 (Refs 224,225)
Fibromodulin
• Controls collagen assembly and the maintenance of the matrix structure in tendons and ligaments226,227,228
• Has also been detected in the stroma of a variety of solid malignancies, such as lung, breast and prostate carcinomas229,230,231
Hyaluronic acid
• Main glycosaminoglycan of the ECM, regulating biomechanical activity of tissues and cell functionality
• Does not induce cell transformation but supports aspects of the malignant cell phenotype, such as proliferation, migration, resistance to apoptosis and epithelial–mesenchymal transition39,40
• Can promote inflammation by forming supramolecular structures (hyaluronic acid cables) that bind monocytes and lymphocytes41
Box 3: Glycoproteins of bladder submucosa extracellular matrix.
Tenascin
• Polymorphic with a high molecular mass, mainly expressed during embryonic development
• In adults, normally absent or expressed at greatly reduced levels, but again expressed in association with cell migration, for example during inflammation, wound healing and in tumours232
• Structure and size vary owing to alternative splicing, with some forms appearing to be expressed in a tumour-specific manner233
Fibronectin
• Multifunctional and adhesive glycoprotein widely distributed in connective tissues and subendothelial matrices, as well as in many cell types
• Present in a soluble form in body fluids and in an insoluble form in the extracellular matrix where it interacts with many other matrix components, such as collagen, fibrin, several integrins and syndecans234
• Originates from a primary transcript, which can be alternatively spliced generating at least 20 different variants
Osteopontin
• Multifunctional glycophosphoprotein highly expressed in bone, but also by various other cell types235,236
• Participates in the regulation of both physiological and pathological mineralization237, but also in acute and chronic inflammation in which it can be expressed by resident epithelial, endothelial and smooth muscle cells, and infiltrating macrophages and T cells
• Might have both proinflammatory and anti-inflammatory effects, depending on the biological scenario238
ECM and UBCs
Expression of extracellular matrix metalloproteinase inducer (EMMPRIN, also known as CD147) on malignant cells has been associated with an invasive phenotype, as well as advanced stage and grade of both transitional cell carcinoma and squamous cell carcinoma. Its expression has also been associated with disease progression74, because of the induced expression of MMPs75. Indeed, increased expression levels of MMP-10 in the bladder have been associated with an invasive phenotype of UBC76, increased expression levels of MMP-7 (both in tissue and serum samples) have been associated with the occurrence of metastatic disease in patients with UBC77, and MMP-14 expression levels correlated with tumour stage and grade, as well as poor prognosis78,79. MMP-2 and MMP-9 have also been extensively evaluated in UBC, owing to their collagenolytic activity against collagen IV, which is distributed in the basal membrane, but the results were conflicting80. In agreement with the increased expression levels of MMP-7 in UBC, MMP-7-mediated shedding of the transmembrane proteoglycan syndecan-1 was found to be independently associated with UBC progression and poor survival81, and the same process has been reported to be involved in chemotherapy resistance of colorectal cancer82.
Clearly, MMPs have a fundamental role in tumorigenesis and disease progression, but their application as diagnostic and prognostic markers or as therapeutic targets was not very successful80, probably because MMPs represent only few of the many extracellular factors (both stromal and microbial) contributing to UBC pathogenesis. Mapping the upstream regulatory pathways or establishing combination therapies that include MMP-targeted agents might offer new approaches for more effective treatments against UBC progression and relapse in comparison with agents targeting MMPs alone.
Regarding matrix-associated enzymes that degrade and remodel the ECM, the tissue level of hyaluronidase-1 has been reported as a potential prognostic marker predicting progression to muscle invasion and tumour recurrence83. The turnover of hyaluronic acid is profoundly altered during UBC progression, owing to increased expression of hyaluronan synthase 1 (Ref. 84) and hyaluronidase-1 (Ref. 83), resulting in increased levels of hyaluronic acid fragments, and hyaluronic acid receptors (CD44 and the receptor for hyaluronan-mediated motility, also known as RHAMM)84. These factors contribute to the formation of a protumoural environment. Hyaluronic acid fragments boost the inflammatory response by releasing hyaluronic-acid-bound proinflammatory mediators85, and by inhibiting type 2 immune responses, thus, favouring angiogenesis and cell motility86, which are likely to contribute to the tumour-supporting effect of hyaluronic acid87,88. In addition, RHAMM expression in the urothelium has been associated with MIBC progression84, holding promise for further evaluation as a prognostic marker or therapeutic target in UBC treatment. In 2014, the determination of urinary levels of hyaluronic acid and hyaluronidase was reported to be a highly accurate and noninvasive method for detecting bladder transitional cell carcinoma regardless of tumour grade89,90.
The small leucine-rich proteoglycan (SLRP) decorin has been reported to inhibit cell motility and its loss has been associated with tumour aggressiveness and unfavourable prognosis in both NMIBC and MIBC91,92,93. The SLRP biglycan has been reported to inhibit UBC cell proliferation, with an increased tissue level being associated with a favourable prognosis91. However, in colorectal, gastric and pancreatic adenocarcinoma, biglycan expression has been associated with disease progression94,95,96.
Matrix-associated galectin-3 has been identified in the healthy urothelium97. Several authors have reported overexpression of galectin-3 in UBCs, but contrasting findings were reported when comparing different tumour grades of MIBC and NMIBC96,97,98. Tissue overexpression and increased urinary levels of galectin-3 have been suggested as potential biomarkers for UBC diagnosis, staging and outcome prognosis98. Considering all discussed ECM components, the contribution of galectin-3 expression to tumour outcome seems to be tumour-type-specific and, correspondingly, microenvironment-specific: a positive correlation between the expression of galectin-3 and tumour progression has been observed in both UBC and colon cancer98,99, but not in breast and gastric cancers100,101.
Taken together, these findings highlight the important role of the ECM in UBC outcome, while outlining the relevance of the tissue-specific composition and complexity of the different 3D microenvironments. Indeed, ECM derived from healthy small-intestine submucosa, but not ECM from mouse sarcoma (Matrigel®, Corning, New York, USA) or composed of collagen I, suppressed the malignant phenotype of highly invasive J82 bladder cancer cells102. In agreement, the ECM from healthy human colon allowed binding but not invasion of metastatic LoVo cells, whereas the same cells were able to infiltrate peritumoural ECM and ECM derived from colorectal carcinoma103. In 2015, IL-1α from UBC cell lines was shown to induce the expression of ECM-associated chemokine MCP-1 (encoded by CCL2) from fibroblasts, and the level of MCP-1 expressed from normal fibroblasts was lower than that of tumour-associated fibroblasts104.
Overall, these findings indicate that features of the healthy ECM suppress malignancy. Understanding the ECM features that account for the suppression mechanism might help identify new therapeutic targets to control UBC outcomes. Numerous findings support the notion that oncogenesis recapitulates embryogenesis105,106,107,108,109, as cells in both processes are plastic, hypoxia-dependent and perform epithelial–mesenchymal transitions (in the primary tumour) and mesenchymal–epithelial transitions (in metastases)9. Thus, pathways associated with embryogenesis are now being investigated as therapeutic targets in cancer110.
Recapitulation of fetal gene expression also occurs during wound healing and organ regeneration, with generation of differentiated cells from stem cells and deposition of ECM components. Studies in murine models have shown activation and deactivation of ECM-related genes during bladder development. Of particular interest might be genes that are switched off after birth, such as those coding for tenascin-C111, decorin112, elastin and collagen IV113. In the normal human bladder, tenascin-C is located in the basal membrane; however, in UBC, increased levels of tenascin-C and diffuse tenascin-C staining in the stroma of invasive tumours have been associated with significantly worse prognosis114, which had also been observed in other solid tumours115,116. Loss of basal membrane components, such as laminin-5, collagen IV and collagen VII, has also been investigated in UBC, without univocal findings114. Conversely, in pancreatic cancer, an increase in collagen IV expression has been reported117.
Microbiota
A vast number of ecological niches exist in the human body, and the continuous process of tissue morphogenesis and structural regeneration — during both physiological and pathological conditions — can be directly or indirectly influenced by the resident normal microbiota or by pathogenic microorganisms. The organisms involved in this process can include bacteria, viruses and fungi. For instance, in physiological conditions, the mucosal barriers (such as the intestinal or bladder mucosa together with the gut mucus or the glycosaminoglycans secreted by the urothelium, respectively)37 might limit the direct interaction of the majority of bacteria that are present in the intestinal or in the bladder lumen with the ECM environment. However, as observed in the gut118, continuous transitory bacterial translocations or tissue invasions usually occur by members of the microbiota or by pathogenic bacteria, which can adhere to the mucosal surfaces. Moreover, bacteria present in the bladder can form biofilms that enable a continuous direct and prolonged contact with the urothelium. These events can bring bacteria to a location where they can directly alter the composition and structure of ECMs.
Bacterial proteases and the ECM
A number of bacteria produce proteases (Box 4), which can act in an intracellular and/or extracellular manner13. These enzymes serve as extracellular virulence factors, as they have an important role in host tissue degradation and immune system evasion and/or destruction of host physical barriers. Among them, alkaline protease, elastase and phospholipase C have been extensively characterized14,15. In addition, most Gram-positive bacteria produce hyaluronidase as a means of using hyaluronic acid as a carbon source, and probably to facilitate the spread of pathogens through the mucosa of the host organism and the onset of productive infection119,120.
Many bacteria also produce and release collagenases that promote bacterial spread121,122,123. Collagenases are endopeptidases that digest native collagen in its triple helix region124,125. Bacterial collagenases exhibit broader substrate specificity than vertebrate collagenases and, thus, digest collagens regardless of their type or size126,127; they can nonspecifically bind to and degrade various types of fibrils and sheets formed by collagens128,129. Interestingly, abnormal collagen degradation can be observed in many human diseases in which a role for microorganisms has been hypothesized, such as cancer, arthritis and atherosclerosis130,131. Collagenases enable tissue degradation, the acquisition of nutrients for growth and proliferation, colonization, evasion of host defences and the dissemination of biofilm-forming bacteria. In particular, these extracellular proteases are essential in hydrolysis of proteins in cell-free environments, enabling bacteria to absorb the hydrolytic products132.
Exoprotease production by bacteria is also usually regulated by the environment and by the bacterial growth modality, for example in biofilm formation or gaining access to specific environments, such as the suburothelial space and the bladder ECM. For example, the proteases secreted by Pseudomonas aeruginosa via the type II or general secretory pathway differ under aerobic and anaerobic growth conditions: under aerobic growth, this microorganism primarily secretes elastase, whereas in anaerobic conditions in vitro (and, hence, probably within tumour tissues in vivo) alkaline protease represents the predominantly secreted protease133. Importantly, during biofilm formation, bacteria such as Aeromonas hydrophila and P. aeruginosa can increase the production of extracellular proteases, such as serine proteases, metalloproteinases and elastases134. Some pathogens, for example Streptococcus pyogenes, can cause invasive diseases through the degradation of intercellular junctions together with the host cysteine protease calpain135. During infection of human tissues, S. pyogenes produces numerous secreted and cell-associated proteins, including a number of known proteases. Among the secreted cysteine proteases, streptococcal pyrogenic exotoxin B (SpeB) effectively cleaves transmembrane proteins associated with the epithelial barrier to enable bacterial penetration and direct access to the ECM135. Moreover, if a lesion of the mucocutaneous barrier (such as a wound) occurs, many bacteria can directly and specifically bind to ECM components, such as collagen fibrils, and start the formation of microcolonies or biofilms136.
Post-translational modifications of host proteins can also be induced by bacterial enzymes, enabling diversification of activities of the host protein137. Regarding ECM proteins, bacterial peptidyl-arginine deiminase expressed from Porphyromonas gingivalis has been suggested to citrullinate collagen I, thus, affecting interaction of fibroblasts expressing integrin α11β1 with collagen I, and has been associated with the development and progression of destructive arthritis138,139. However, whether bacterially induced post-translational modifications of ECM proteins have a role in the onset and progression of bladder cancer, or other solid tumours, has not yet been clarified. Future studies should evaluate the relevance of this hypothesis.
Box 4: Bacterial enzymes acting on the ECM and affecting immune responses.
Alkaline protease
• Has optimal enzymatic activity at neutral and alkaline pH
• Degrades extracellular matrix (ECM) components and interferes with lymphocyte proliferation through degradation and inactivation of IFN-γ
• Expressed by Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Listeria monocytogenes, Bacillus subtilis, Clostridium perfringens and others13,14,146,239
Elastase
• Breaks down elastin and other molecules
• Disrupts tight junctions, damages tissue, degrades cytokines and α protease inhibitors
• Cleavage of IgA, IgG, complement factor C3b and complement receptor type 1 decreases phagocytosis by neutrophils
• Expressed by S. aureus, P. aeruginosa, S. marcescens, L. monocytogenes, B. subtilis, C. perfringens and others13,14,146,239
Phospholipase C
• Highly heterogeneous class of enzymes
• Cleaves various bonds in phospholipids
• Lipase activity releases the secondary messengers inositol triphosphate and diacylglycerol in host cells, ultimately resulting in degradation of host cell membranes
• Expressed by S. aureus, P. aeruginosa, S. marcescens, L. monocytogenes, B. subtilis, C. perfringens and others13,14,146,239
Hyaluronidases
• Degrade hyaluronic acid, resulting in a carbon source and destabilization of eukaryotic cells
• Expressed by most Gram-positive bacteria119,120
Collagenases
• Endopeptidases that digest collagen in its triple helix region
• Bacterial collagenases have broader substrate specificity than vertebrate collagenases and can digest collagen regardless of type or size
• Expressed by S. aureus, Streptococcus agalactiae, P. aeruginosa, Aeromonas hydrophila, Streptococcus bovis, Bacteroides fragilis, S. marcescens and C. perfringens121,122,123,124,125,132,133
Serine proteases
• Ubiquitously present in nature, classified into >50 families
• Differently distributed in bacteria, owing to environmental adaptation
• Cleave peptide bonds in which serine serves as the nucleophilic amino acid at the enzyme's active site
• Expressed by S. aureus, S. agalactiae, P. aeruginosa, A. hydrophila, S. bovis, B. fragilis, S. marcescens and C. perfringens121,122,123,124,125,132,133
Metalloproteinases
• Metal-containing proteases classified into nine families on the basis of differing primary sequences and structural characteristics
• Degrade environmental proteins and peptides for bacterial heterotrophic nutrition
• Expressed by S. aureus, S. agalactiae, P. aeruginosa, A. hydrophila, S. bovis, B. fragilis, S. marcescens and C. perfringens121,122,123,124,125,132,133
Streptococcal pyrogenic exotoxin B cysteine protease
• Secreted protease
• Degrades host serum proteins, such as immunoglobulins, ECM and complement components
• Cleaves transmembrane proteins associated with the epithelial barrier, enabling bacterial penetration and direct access to the ECM
• Expressed by Group A Streptococcus pyogenes133
These enzymes were characterized in the gut microbiota.
Bacterial proteases and immune responses
Bacterial proteases are able to degrade growth factors and their receptors and can perturb host cytokine networks140,141. Bacteria also release elastases that cleave and release matrix-associated components121, such as cytokines and protease inhibitors, factors of the complement system and complement receptors from neutrophils, thus, reducing immune responses (that is, proinflammatory activity and phagocytosis) against the invading bacteria. Furthermore, bacterial elastase has been shown to disrupt epithelial tight junctions142,143,144, causing proteolytic damage to tissues. In this context, bacterial elastases have been shown to be involved in the formation of leg ulcers121, as well as in a variety of chronic diseases, such as cystic fibrosis and chronic wounds136,145.
As shown in vitro, both alkaline proteases and elastases from P. aeruginosa are able to modulate the local host immune response and the machinery that produces and degrades the ECM by inhibiting IL-2-induced proliferation of lymphocytes and through degradation and inactivation of IFN-γ146. Proteases expressed by several microorganisms can cleave the IL-6 receptor from human monocytes13. Bacterial proteases have been shown to mimic endogenous host-membrane-bound metalloproteinases and enable the shedding of various host signalling factors, including the IL-6 receptor, the FAS ligand, as well as TNF and its receptors147,148. Hence, these mechanisms enable bacterial proteases to exert not only direct enzymatic activity on the ECM but also an indirect activation or increased production of host MMPs by inflammatory cells, which in turn can enhance the perturbation of the physiological process of ECM regeneration, thus, promoting an altered and potentially cancer-promoting extracellular environment.
Many members of this vast array of proteases, which is produced by both Gram-positive and Gram-negative bacteria and fungi (viruses do not produce metalloproteinases but usually endopeptidases), can directly digest ECM components and are also becoming commercially important, particularly in terms of protein degradation in various pharmaceutical, clinical or industrial applications149,150.
Although many mechanisms involve direct enzymatic action on ECM components, other immune-system-related processes can influence the ECM indirectly. Endotoxins, for example lipopolysaccharide, can recruit and activate inflammatory cells, such as neutrophils, macrophages and even T cells and B cells, owing to their superantigenic nature. The release of pathogen-associated molecular patterns occurs during both physiological and pathological bacterial replication and induces the migration and activation of inflammatory cells. This continuous stimulation has been shown to be essential in the development of a mature and effective immune system, although perturbations of this equilibrium are often observed during pathogenic processes.
Direct evidence is still lacking for bladder diseases, but, according to what has been observed in inflammatory bowel diseases, extensive alterations in the gut mucosal structure are always associated with modifications in the microbiota composition151. Indeed, in the gut, the microbiota has been shown to induce the epithelial expression of genes involved in ECM formation152, and microbiota-derived proteases degrade collagens of the intestinal mucosa ECM153. Hence, these phenomena might also have an important role in the bladder, which is constantly colonized by microorganisms that are predominantly of gastrointestinal origin. The microbiota could affect both the integrity of the bladder urothelial barrier and ECM formation in physiological as well as in disease conditions154.
Indeed, in several animal models, microorganisms either initiate or perpetuate organ inflammation and fibrosis155,156. Although almost all available data come from observations made in the gut, they clearly demonstrate that microbial products can be directly profibrogenic. For example, progressive fibrosis was observed in the intestine of rats 17–26 days after injection of a bacterial cell wall component into the rat bowel155,156. Similarly, the same consequences were observed 7 days after autologous injection of faecal material or anaerobic bacteria into rats157. In both experiments, fibrogenesis was associated with an increase in classical profibrotic mediators, such as TGF-β in the mucosa, and could be inhibited by antibiotic treatment. These findings suggest that if microbial components can cross through urothelial barriers, similar to intestinal ones, they could also trigger inflammation, as well as fibrosis and bladder stiffness at the same sites. Moreover, underscoring the complexity of this interaction, the microorganisms found in a patient with established fibrosis might not be the same as the organisms that triggered the initial events. Indeed, as observed in intestinal fibrosis in mice, once fibrogenesis is initiated, it might be self-perpetuating158. In addition, ECM accumulation leads to increased tissue stiffness that can in itself drive fibrogenesis through an integrin-mediated fibroblast activation159.
The many interactions of bacteria with the host ECM metabolism have also evolved in a way that, in physiological conditions, resulted in the microbiota having a key role in the regeneration of a disrupted host epithelial layer160. The microbiota has the capacity to contribute to host mucosal homeostasis and is likely to participate in the pathogenesis of a variety of diseases including carcinoma. Recent observations have shown that in patients with colorectal carcinoma, the tumour-associated microbiome differs in composition compared with the microbiome of healthy individuals, with either increased or reduced representation of specific bacterial genera or species161,162. Unfortunately, no data exist for the microbiome associated with bladder cancer.
Urinary and bladder-associated microbiota
Urinary microbiota
Historically, the bladder and urine — until reaching the urethra — have been considered sterile in healthy individuals. Conventional microbiological methods could neither isolate nor characterize the full spectrum of urinary bacterial species, which can now be identified by 16S ribosomal RNA sequencing and have been shown to be present in the urinary tract163. Ultra–deep pyrosequencing revealed the (relatively) most abundant bacterial taxa in the urine of healthy individuals: Lactobacillus, Corynebacterium, Staphylococcus, Prevotella, Gardnerella and Streptococcus, with a preponderance of Lactobacillus, Prevotella and Gardnerella in women and Corynebacterium in men163,164. The urinary tract has its own microbiome because urine passing through the urethra is contiguous to the external environment and is exposed to the skin and the openings of the gastrointestinal tract and vaginal mucosae, which host their own microbiota.
Similar to the intestinal microbiota, urinary microbiota is age-dependent165, with significant differences among age groups. For instance, Jonquetella, Parvimonas, Proteiniphilum and Saccharofermentans mostly occur in adults over the age of 70.165 Data from investigations of midstream urine (used as a proxy of the bladder microbiome) showed that a more heterogeneous mix of bacterial genera is present in samples from women (6–36 genera) than in samples from men (1–8 genera, but also one sample with 51 genera)165. Moreover, regardless of sex, in 75% of samples, more than 50% of bacteria belonged to the phylum Firmicutes. Samples from women also had members of the phyla Actinobacteria and Bacteroidetes, which were generally absent from the samples collected from men. In addition, midstream urinary analysis confirmed the existence of a core microbiome — also in the bladder — but with variability in the amount of the core bacteria, along with a variable prevalence of other bacteria, across age groups. This observation was even more pronounced in the urinary tract than in the gut microbiome166,167, supporting the hypothesis that bladder colonization with specific genera over the course of a lifetime might ultimately influence the propensity for bladder pathology in later life. These findings might also explain the difference in the frequency of urinary diseases observed in men and women.
Bladder-associated microbiota
Obtaining bladder biopsies or suprapubic aspirates in healthy individuals, which would provide the best samples to characterize the bladder microbiome without sample contamination with microorganisms present in the urethra, is unethical. Indeed, this was one of the reasons why the bladder microbiome was not originally included in the Human Microbiome Project. However, sampling of the midstream bacterial population only might not enable detection of bacterial communities in biofilms in the bladder that adhere to the mucosa for long periods of time in direct interaction with the urothelium. For example, a different composition of mucosa-associated and luminal microbiota has been reported in the process of characterizing the intestinal microbiota168,169,170,171. Thus, similar to the intestinal microbiota, further studies are needed to help understand the modulation of the composition and variety of bladder-associated bacterial strains, which are likely to be dependent on age, gender, breast feeding, dietary and socioeconomic status.
Indeed, epidemiological studies have revealed that UBC incidence is age-dependent, with men having a higher risk than women with a rate ratio of at least 3:1 (Refs 2,3). The association between bladder-associated microbiota and the incidence of cancer in men and women has not yet been comprehensively assessed. For example, whether the preponderant bacterial strain Lactobacillus in the bladder of women might provide protection from UBC is not known, although many reports have shown that Lactobacillus might reduce chronic inflammation and potentiate a number of immune responses172,173,174,175,176. A multicentre, double-blind, placebo-controlled, randomized trial in 138 patients with primary bladder tumours reported that daily oral administration of freeze-dried Lactobacillus casei sp. Shirota for 1 year prevented the recurrence of UBC after transurethral resection of the tumours177. Another multicentre, prospective, randomized, controlled trial that enrolled 207 patients demonstrated that patients treated orally for 1 year with L. casei sp. Shirota in addition to transurethral epirubicin (for 3 months) had a significantly lower UBC recurrence rate at 3 years compared with the epirubicin only group, although the overall survival did not differ between the groups178. In addition, a case-control study in 180 patients and 445 population-based controls showed that regular (1–2 times per week) probiotic intake reduced UBC risk in the healthy population179. Taken together, these results strongly support the protective role of L. casei Shirota against bladder cancer.
The bladder epithelium can act as a persistent reservoir for viable but nonculturable uropathogenic bacterial strains, which can ultimately lead to bladder or kidney infection163,180,181. In these cases, Escherichia coli, Klebsiella pneumoniae and Staphylococcus saprophyticus strains are the most often isolated species, but many other bacteria can also be found163,180,181,182, suggesting that bladder commensal populations are polymicrobial and variable162,164,175,176,177.
In a murine model, researchers found that 2 weeks after acute E. coli infection the bladder can tolerate the colonization of bacterial strains that are in a resting state, which, thus, do not induce an immune response and are unresponsive to many antibiotics183,184. Whether a chronic state of bacterial colonization is associated with a chronic state of low-grade bladder inflammation still requires investigation. In healthy individuals, intentional instillation into the bladder of E. coli sp. 83972 (the prototype strain of asymptomatic bacteriuria)185 resulted in a normal acute neutrophil response but only a modest inflammatory response186. This finding suggests that, indeed, chronic low-grade bladder inflammation can be present even in the absence of symptomatic infection and that this inflammation can be associated with low pathogenic or nonpathogenic bacterial strains that colonize the urothelium. The association between chronic inflammation, mucosa-associated microbiota and the development and outcome of solid tumours has been validated for a variety of neoplasia, in particular colorectal cancer187,188. In bladder cancer, a preliminary study found an association between urinary dysbiosis (specifically, an altered ratio among Pseudomonas and Anaerococcus versus Streptococcus) and urothelial carcinoma189.
Overall, the urinary microbiota differs between men and women and urinary dysbiosis might be associated with UBC. Indeed, the urinary microbiome might be different from the bacteria strictly associated with the urothelium, and a clear association between mucosa-associated microbiota and the incidence and outcome of UBC is lacking. Identification of bacterial strains associated with UBC and clarification of their interaction with the ECM might lead to new therapeutic options for patients with NMIBC experiencing tumour recurrence after BCG treatment190,191, and those with MIBC for whom immunotherapy is not indicated2,3,192.
Conclusions
In UBC, a patient's most important prognostic factors are still based on morphology, including tumour size, multiplicity, associated Cis, grade and stage2,3. Further thorough studies are needed to establish the detailed ECM composition of all layers of the human bladder and UBC. Despite many authors reporting expression and localization of a single or some ECM components in healthy and neoplastic bladders, a thorough examination of composition, 3D structure and biomechanical properties of the ECMs of the human bladder and human bladder carcinoma has not yet been undertaken. Assessing the entire complexity of ECM composition, for example via a proteomic approach, and biomechanical features (that is, ultrastructure and stiffness) will reveal new or dysregulated pathways that are associated with UBCs.
Many attempts to develop bladder scaffolds or substitutes have failed because of mechanical, structural or functional problems193. Given the critical role of the ECM in determining cell behaviour, a thorough examination of the features of the ECM of the healthy bladder will pave the way for bladder regenerative medicine in patients needing bladder augmentation (that is, patients with congenital bladder exstrophy, posterior urethral valves and benign prostatic hyperplasia)193,194 or undergoing nonradical surgery. As an example, ECM stiffness modulates cellular functions as diverse as migration, proliferation, differentiation and apoptosis, and is crucial for organ development and homeostasis10. Establishing the stiffness of bladder ECM and the correct ratio of ECM components, in particular the total amount of collagens and elastin, will probably contribute to the design of scaffolds resembling native features of the bladder. Moreover, elastin content in the bladder is continuously decreasing after birth, suggesting that features of bladder scaffolds should differ according to the decade of life.
The human body coexists with a variety of commensal microorganisms, referred to as the normal microbiota. The variety of bacterial strains has been described for most but not all human tissues195, and the accurate amount and diversity of microbial species of any healthy tissue, sometimes termed eubiosis, was associated with the function of the organ (Fig. 1a). The microbial translocation of commensal bacteria through the epithelium with altered permeability, dysbiosis and infection by pathogenic bacteria contribute to a variety of diseases. Tissue invasion by resident bacteria, biofilm formation in the mucosa or colonization by pathogenic bacteria might support the onset, progression and relapse of carcinoma. Malignant tumours often develop at sites of chronic injury, with chronic inflammation being the most important risk factor, as it increases the risk that a mutated cell accumulates additional deleterious mutations16.
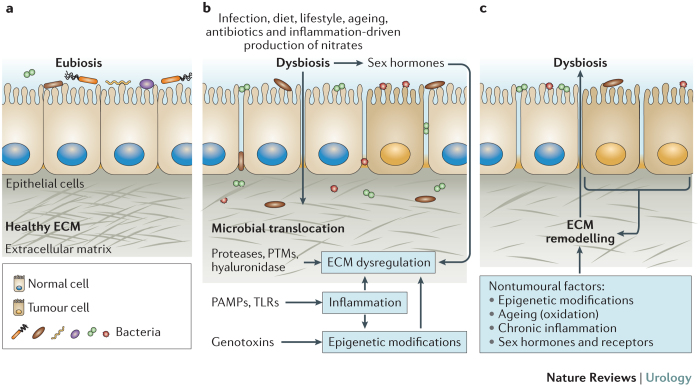
Figure 1. Dysbiosis and ECM modifications.
a | In many tissues, homeostasis depends on ecological community with microbial organisms (eubiosis). Disruption of this balance (dysbiosis) and extracellular matrix (ECM) remodelling are associated with a variety of diseases, for example, colorectal carcinoma. Future studies are likely to also establish associations between the functions of the microbiota and the urothelium, connecting dysbiosis with the onset, progression and relapse of urothelial bladder cancer. b | These associations have been mainly viewed as dysbiosis inducing ECM remodelling, for example, through the release of bacterial enzymes that degrade ECM components or introduce post-translational modifications (PTMs)137, activation of inflammatory pathways and epigenetic modifications of fibroblasts. Microbial translocation can occur during this process and exacerbate ECM dysregulation. Interaction between the microbiome and sex hormones can also regulate ECM features240,241. c | However, dysbiosis can also follow ECM remodelling at the onset of disease; for example, a solid tumour can establish a new niche for the growth of bacterial strains by creating hypoxic and/or acidic conditions or changing the ECM composition242,243. Dysregulation of the ECM composition can also follow nontumoural conditions, for example, through epigenetic modifications of fibroblasts induced by toxins, irradiation and chronic inflammatory responses, an imbalance between sex hormones and/or their receptors, and ageing. Changes in the ECM composition provide conditions for altered binding of bacterial strains, thus, favouring dysbiosis and microbial translocation. PAMPs, pathogen-associated molecular patterns; TLRs, Toll-like receptors.
Some bacterial strains release exoproteases that degrade the ECM to enable tissue colonization and the evasion of host defences, acquisition of nutrients for growth and proliferation and dissemination of biofilm-forming bacteria to different areas of the organism. Among these bacterial virulence factors, enzymes degrading ECM components have been extensively characterized and include bacterial hyaluronidase, collagenase and elastase14,15 (Box 4). In terms of cancer outcome, different mechanisms have been proposed, such as the release of antiapoptotic and proliferative cytokines by TLR+ cells and the induction of mutations in epithelial cells (both induction of neoplastic mutations and accumulation in addition to pre-existing mutations). These mutations can be caused by oxygen species and reactive nitrogen species released from inflammatory cells, genotoxins or cancerogenic metabolites occurring during bacterial metabolism196 (Fig. 1b).
Indeed, in order to spread, bacteria invading tissues need to break the epithelial barrier and create an ecological niche within the lamina propria. Bacteria release a variety of enzymes that degrade ECM components, such as collagenase, hyaluronidase and elastase, which degrade the tight junctions between epithelial cells and ECM components in the lamina propria197. Thus, bacterial enzymes that degrade ECM components might create an environmental niche that supports the productive seeding of transformed cells. Abnormal ECM composition and structure affect cancer progression by either promoting cellular transformation and metastasis or deregulating behaviour of stromal cells, eventually facilitating tumour-associated angiogenesis and inflammation9. Furthermore, an inflammatory response against bacterial antigens and as a consequence of the presence of bacterial elastase would contribute to the acquisition of new mutations.
In addition, bacteria-induced post-translational modifications of ECM components are likely to represent a new field of research. As post-translational modifications of host proteins have a fundamental role in the diversification of protein function, it is not surprising that bacteria evolved strategies to interfere with the functionality of host proteins to create their own niche and to evade the immune system. Bacteria-induced posttranslational modifications of host proteins might also alter functions that are regulated by ECM components, such as cell localization, proliferation and adhesion. Identification of pathogenic bacteria associated with specific diseases will open the way for searching post-translational modifications associated with the onset and progression of these diseases. Post-translational modifications of ECM components will probably also be discovered for solid tumours and other solid diseases. Finally, ECM modifications that occur in ageing tissues, induced by chronic immune responses or fibrosis, as well as by tumour-associated microenvironments (for example, acidosis), probably selectively promote the growth of specific bacterial species (Fig. 1c).
New prognostic factors, such as ECM-based and microbiota-based biomarkers, might be exploitable for risk stratification, tumour staging and to predict relapse and outcome of UBCs. Similarly, ECM-based biomarkers might also be highlighting targets for antineoplastic drugs and the design of new therapeutic strategies. In addition, once ECM modifications and the diversity of the bacterial flora associated with UBC have been established, urine samples should be investigated for the same biomarkers, as urine samples ultimately represent the most useful specimen to be assessed in the real-life clinical setting. Extending the search to other biomarkers in addition to hyaluronic acid and hyaluronidase will improve the chance to identify rapid, cheap, noninvasive and easily repeatable assays that combine multiple parameters for diagnosis, as well as prognostic indicators for UBC relapse. Integration of biomarkers indicating ECM modifications induced by the bacterial flora associated with UBC into current diagnostic and prognostic assays is likely to strengthen their power.
Since the 1950s, faecal microbiota transplantation has been used to 're-establish the balance of nature' within the intestinal environment, by correcting the dysbiosis caused by antibiotic treatment198. Now, the modulation of gut microbiota composition is regarded as an emerging treatment for several gastrointestinal and metabolic disorders, such as refractory Clostridium difficile infection and ulcerative colitis199,200. Animal models have been used to establish evidence (for example, lower incidence of certain cancers in germ-free mice compared to conventionally raised animals) and mechanisms underlying the observation that bacterial microbiota promote colorectal, gastric, liver, lung and breast cancer196, but such studies have not been performed for UBC. Indeed, strategies to restore normal bladder-associated microbiota might be a potential option to reduce UBC incidence or relapse. Future studies assessing efficacy of intravesical instillation of prebiotics and probiotics are likely to prove the beneficial effects of targeting the bladder-associated microbiome in patients with UBC.
Finally, targeting the bladder ECM or the associated microbiota might bypass treatment resistance mechanisms of tumour cells. Bladder-associated microbiota should also be investigated in UBC responses to antineoplastic therapy, as has previously been reported for colorectal carcinoma201,202. Strategies to improve UBC responses to antineoplastic therapies might come from studies assessing the difference of bladder-associated microbiota between men and women according to different decades of patient age, ultimately leading to a more patient-tailored approach.
Review criteria
A systematic literature search for English-language original and review articles was performed using Google and PubMed. Key words used were “bladder”, “urine”, “extracellular matrix”, “bacteria”, “microbioma”, “microbiota”. All available full-text original articles and reviews published since 1970 were used. Original articles referenced in the identified reviews were also searched and discussed.
Acknowledgements
M.A. and F.C. contributed equally to this article. The authors thank Dana Kuefner PhD for reviewing the language in this manuscript.
Biographies
Dr Massimo Alfano, PhD, is group leader at IRCCS Ospedale San Raffaele in Milan, Italy, and is author of 66 peer-reviewed publications and two patents. In the past years, he has developed an interest for and expertise in tissue extracellular microenvironments, such as the extracellular matrix (ECM) and tissue-associated microbiota. His research group is working on the role of the ECM in several malignancies to assess which ECM changes have causative effects on disease progression and how these changes, alone or in combination with others, might affect cancer cells and cells in the stromal compartment.
Dr Filippo Canducci, MD PhD, is Assistant Professor of Microbiology at the University of Insubria in Varese, Italy, and a scientist at the San Raffaele Hospital in Milan, Italy. At the beginning of his career, he was a pioneer in the therapeutic use of probiotics and has now focused his interests on microbiome analysis in human diseases, such as cancer and inflammatory diseases. Dr Canducci has also studied the prevalence of emerging respiratory viruses and, in 2003, he contributed to the isolation and characterization of the SARS coronavirus strain HSR1. Dr Canducci is author of more than 60 papers in international peer-reviewed journals.
Prof. Manuela Nebuloni, MD, is Associate Professor of Pathology at the University of Milan, Head of the pathology unit at Luigi Sacco Hospital in Milan, Italy, and author of 106 original manuscripts in peer-reviewed journals. Her main research fields of interest and expertise are infectious and neoplastic human and murine diseases, intestinal inflammatory diseases, renal pathology in HIV infection and in transplant patients, as well as distribution, pathology and clinical significance of polyomavirus infection in the urinary tract. In the past years, Prof. Nebuloni has also developed an interest and expertise in the isolation, characterization and relevance of the extracellular matrix.
Prof. Massimo Clementi, MD, is Professor of Microbiology and Virology at the Vita-Salute San Raffaele University, Dean of the Faculty of Medicine, and Chief of the Laboratory of Microbiology and Virology of the San Raffaele Hospital and Research Institute in Milan, Italy. His research interests lie in the molecular biology of hepatitis C virus and HIV infections, virus–host relationships, microbiology of emerging infectious diseases, infections in immunocompromised patients, as well as generation and characterization of virus-neutralizing human monoclonal antibodies. He is the author of more than 300 papers in all microbiological research areas and of 15 international patents in the field; he is Editor of New Microbiologica and Clinical Microbiology and Infection, founder and former President of the Italian Society of Medical Virology.
Prof. Francesco Montorsi, MD, is Professor and Chairman of the Department of Urology and Director of the Urology Residency programme at Vita-Salute San Raffaele University and the current Scientific Director of the San Raffaele Scientific Institute in Milan, Italy. In 1998, he received the Matula Award, assigned yearly by the European Association of Urology to the most promising academic urologist younger than 40 years of age. His main areas of scientific interest include the pathophysiology and management of prostate cancer and benign prostatic hyperplasia, sexual dysfunctions and genitourinary oncology. Prof. Montorsi is author and co-author of more than 1,000 peer-reviewed manuscripts.
Prof. Andrea Salonia, MD PhD, is Associate Professor of Urology at Vita-Salute San Raffaele University and the current Director of the Urological Research Institute at IRCCS Ospedale San Raffaele in Milan, Italy. He is author and co-author of more than 270 peer-reviewed publications in the area of sexual medicine, reproductive medicine and uro-oncology, predominantly focusing on clinical experimental and surgical treatment of non-muscle-invasive and muscle-invasive bladder cancer, sexual and reproductive outcomes of testicular cancer survivors, hormonal biology, physiopathology and surgery of organ-confined and locally advanced prostate cancer.
Related links
FURTHER INFORMATION
PowerPoint slides
Author Contributions
M.A., F.C. and A.S. researched data for the article, wrote and reviewed and/or edited the manuscript before submission. All authors substantially contributed to discussion of the article content.
Competing interests
The authors declare no competing financial interests.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M. Guidelines on non-muscle-invasive (TA, T1, CIS) bladder cancer. Eur. Urol. 2013;59:584–594. [Google Scholar]
- 3.Witjes JA. European Association of Urology. 2015. Guidelines on muscle-invasive and metastatic bladder cancer. [DOI] [PubMed] [Google Scholar]
- 4.Zargar H, Aning J, Ischia J, So A, Black P. Optimizing intravesical mitomycin C therapy in non-muscle-invasive bladder cancer. Nat. Rev. Urol. 2014;11:220–230. doi: 10.1038/nrurol.2014.52. [DOI] [PubMed] [Google Scholar]
- 5.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat. Rev. Urol. 2014;11:153–162. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 6.Stein JP. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 7.Iozzo RV. Tumor stroma as a regulator of neoplastic behavior. Agonistic and antagonistic elements embedded in the same connective tissue. Lab. Invest. 1995;73:157–160. [PubMed] [Google Scholar]
- 8.Vessella RL, Pantel K, Mohla S. Tumor cell dormancy: an NCI workshop report. Cancer Biol. Ther. 2007;6:1496–1504. doi: 10.4161/cbt.6.9.4828. [DOI] [PubMed] [Google Scholar]
- 9.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Migrating cancer stem cells — an integrated concept of malignant tumour progression. Nat. Rev. Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 12.Berndt A, Richter P, Kosmehl H, Franz M. Tenascin-C and carcinoma cell invasion in oral and urinary bladder cancer. Cell Adh. Migr. 2015;9:105–111. doi: 10.1080/19336918.2015.1005463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vollmer P, Walev I, Rose-John S, Bhakdi S. Novel pathogenic mechanism of microbial metalloproteinases: liberation of membrane-anchored molecules in biologically active form exemplified by studies with the human interleukin-6 receptor. Infect. Immun. 1996;64:3646–3651. doi: 10.1128/iai.64.9.3646-3651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berka RM, Gray GL, Vasil ML. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect. Immun. 1981;34:1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 16.Kiraly O, Gong G, Olipitz W, Muthupalani S, Engelward BP. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet. 2015;11:e1004901. doi: 10.1371/journal.pgen.1004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rhijn BW. Molecular markers for urothelial bladder cancer prognosis: toward implementation in clinical practice. Urol. Oncol. 2014;32:1078–1087. doi: 10.1016/j.urolonc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Kamat AM. Considerations on the use of urine markers in the management of patients with high-grade non-muscle-invasive bladder cancer. Urol. Oncol. 2014;32:1069–1077. doi: 10.1016/j.urolonc.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 20.Netto GJ. Molecular biomarkers in urothelial carcinoma of the bladder: are we there yet? Nat. Rev. Urol. 2012;9:41–51. doi: 10.1038/nrurol.2011.193. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 22.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 23.Hu M. Distinct epigenetic changes in the stromal cells of breast cancers. Nat. Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 24.Polyak K. Breast cancer: origins and evolution. J. Clin. Invest. 2007;117:3155–3163. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hynes RO, Naba A. Overview of the matrisome — an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br. J. Cancer. 2003;89:1817–1821. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 28.Klingberg F. Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J. Cell Biol. 2014;207:283–297. doi: 10.1083/jcb.201402006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell. Mol. Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F. The biomechanical effect of corneal collagen cross-linking (CXL) with riboflavin and UV-A is oxygen dependent. Transl. Vis. Sci. Technol. 2013;2:6. doi: 10.1167/tvst.2.7.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto K, Fujiwara Y, Nagai R, Yoshida M. Immunohistochemical detection of advanced glycation end products in human bladder with specific monoclonal antibody. Int. J. Urol. 2009;16:402–405. doi: 10.1111/j.1442-2042.2009.02259.x. [DOI] [PubMed] [Google Scholar]
- 33.Charras G, Sahai E. Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 2014;15:813–824. doi: 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- 34.Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape — the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014;15:825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aitken KJ. Mechanotransduction of extracellular signal-regulated kinases 1 and 2 mitogen-activated protein kinase activity in smooth muscle is dependent on the extracellular matrix and regulated by matrix metalloproteinases. Am. J. Pathol. 2006;169:459–470. doi: 10.2353/ajpath.2006.050969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilly JD, Parsons CL. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surg. Gynecol. Obstet. 1990;171:493–496. [PubMed] [Google Scholar]
- 38.Aitken KJ, Bagli DJ. The bladder extracellular matrix. Part I: architecture, development and disease. Nat. Rev. Urol. 2009;6:596–611. doi: 10.1038/nrurol.2009.201. [DOI] [PubMed] [Google Scholar]
- 39.Toole BP, Zoltan-Jones A, Misra S, Ghatak S. Hyaluronan: a critical component of epithelial–mesenchymal and epithelial–carcinoma transitions. Cells Tissues Organs. 2005;179:66–72. doi: 10.1159/000084510. [DOI] [PubMed] [Google Scholar]
- 40.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 41.de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-α-trypsin inhibitor is crucial to structure and function. Am. J. Pathol. 2003;163:121–133. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longhurst PA, Eika B, Leggett RE, Levin RM. Urinary bladder function in the tight-skin mouse. J. Urol. 1992;148:1611–1614. doi: 10.1016/s0022-5347(17)36980-x. [DOI] [PubMed] [Google Scholar]
- 43.Liapis A. Changes in the quantity of collagen type I in women with genuine stress incontinence. Urol. Res. 2000;28:323–326. doi: 10.1007/s002400000120. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl Acad. Sci. USA. 1997;94:1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevenson K, Kucich U, Whitbeck C, Levin RM, Howard PS. Functional changes in bladder tissue from type III collagen-deficient mice. Mol. Cell. Biochem. 2006;283:107–114. doi: 10.1007/s11010-006-2388-1. [DOI] [PubMed] [Google Scholar]
- 46.Folkman J. Antiangiogenesis in cancer therapy — endostatin and its mechanisms of action. Exp. Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Sudhakar A. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc. Natl Acad. Sci. USA. 2003;100:4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 48.Aikio M. Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma. PLoS ONE. 2012;7:e51044. doi: 10.1371/journal.pone.0051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang-Bo J, Yoo KH, Park JH, Jeong HS, Chung IS. Recombinant canstatin inhibits angiopoietin-1-induced angiogenesis and lymphangiogenesis. Int. J. Cancer. 2012;131:298–309. doi: 10.1002/ijc.26353. [DOI] [PubMed] [Google Scholar]
- 50.John H, Radtke K, Standker L, Forssmann WG. Identification and characterization of novel endogenous proteolytic forms of the human angiogenesis inhibitors restin and endostatin. Biochim. Biophys. Acta. 2005;1747:161–170. doi: 10.1016/j.bbapap.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Saban R. Mandatory role of proteinase-activated receptor 1 in experimental bladder inflammation. BMC Physiol. 2007;7:4. doi: 10.1186/1472-6793-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saban R. Regulatory network of inflammation downstream of proteinase-activated receptors. BMC Physiol. 2007;7:3. doi: 10.1186/1472-6793-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang R, Amir J, Liu H, Chaqour B. Mechanical strain activates a program of genes functionally involved in paracrine signaling of angiogenesis. Physiol. Genom. 2008;36:1–14. doi: 10.1152/physiolgenomics.90291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutherland RS, Baskin LS, Elfman F, Hayward SW, Cunha GR. The role of type IV collagenases in rat bladder development and obstruction. Pediatr. Res. 1997;41:430–434. doi: 10.1203/00006450-199703000-00021. [DOI] [PubMed] [Google Scholar]
- 55.Hipp JD. Using gene chips to identify organ-specific, smooth muscle responses to experimental diabetes: potential applications to urological diseases. BJU Int. 2007;99:418–430. doi: 10.1111/j.1464-410X.2007.06676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aitken KJ. Mammalian target of rapamycin (mTOR) induces proliferation and de-differentiation responses to three coordinate pathophysiologic stimuli (mechanical strain, hypoxia, and extracellular matrix remodeling) in rat bladder smooth muscle. Am. J. Pathol. 2010;176:304–319. doi: 10.2353/ajpath.2010.080834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 2011;3:a005041. doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glukhova MA, Frid MG, Shekhonin BV, Balabanov YV, Koteliansky VE. Expression of fibronectin variants in vascular and visceral smooth muscle cells in development. Dev. Biol. 1990;141:193–202. doi: 10.1016/0012-1606(90)90114-x. [DOI] [PubMed] [Google Scholar]
- 60.Serini G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J. Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Little WC, Smith ML, Ebneter U, Vogel V. Assay to mechanically tune and optically probe fibrillar fibronectin conformations from fully relaxed to breakage. Matrix Biol. 2008;27:451–461. doi: 10.1016/j.matbio.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gee EP, Ingber DE, Stultz CM. Fibronectin unfolding revisited: modeling cell traction-mediated unfolding of the tenth type-III repeat. PLoS ONE. 2008;3:e2373. doi: 10.1371/journal.pone.0002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krammer A, Craig D, Thomas WE, Schulten K, Vogel V. A structural model for force regulated integrin binding to fibronectin's RGD-synergy site. Matrix Biol. 2002;21:139–147. doi: 10.1016/s0945-053x(01)00197-4. [DOI] [PubMed] [Google Scholar]
- 64.Hubschmid U, Leong-Morgenthaler PM, Basset-Dardare A, Ruault S, Frey P. In vitro growth of human urinary tract smooth muscle cells on laminin and collagen type I-coated membranes under static and dynamic conditions. Tissue Eng. 2005;11:161–171. doi: 10.1089/ten.2005.11.161. [DOI] [PubMed] [Google Scholar]
- 65.Mithieux SM, Weiss AS. Elastin. Adv. Protein Chem. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- 66.Koo HP, Macarak EJ, Chang SL, Rosenbloom J, Howard PS. Temporal expression of elastic fiber components in bladder development. Connect. Tissue Res. 1998;37:1–11. doi: 10.3109/03008209809028896. [DOI] [PubMed] [Google Scholar]
- 67.Rosenbloom J. Expression of microfibrillar proteins by bovine bladder urothelium. Urology. 1997;49:287–292. doi: 10.1016/S0090-4295(96)00437-2. [DOI] [PubMed] [Google Scholar]
- 68.El-Haibi CP. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl Acad. Sci. USA. 2012;109:17460–17465. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirschmann DA. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- 70.Shi W. The effect of lysyl oxidase polymorphism on susceptibility and prognosis of nonsmall cell lung cancer. Tumour Biol. 2012;33:2379–2383. doi: 10.1007/s13277-012-0501-5. [DOI] [PubMed] [Google Scholar]
- 71.Baker AM. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J. Natl Cancer Inst. 2011;103:407–424. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 72.Lee UJ. Lower urogenital tract anatomical and functional phenotype in lysyl oxidase like-1 knockout mice resembles female pelvic floor dysfunction in humans. Am. J. Physiol. Renal Physiol. 2008;295:F545–F555. doi: 10.1152/ajprenal.00063.2008. [DOI] [PubMed] [Google Scholar]
- 73.Heutinck KM, ten Berge IJ, Hack CE, Hamann J, Rowshani AT. Serine proteases of the human immune system in health and disease. Mol. Immunol. 2010;47:1943–1955. doi: 10.1016/j.molimm.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 74.El-Rehim DM, El-Maqsoud NM, El-Hamid AM, El-Bab TK, Galal EM. Expression of extracellular matrix metalloproteinase inducer and fascin in urinary bladder cancer: correlation with clinicopathological characteristics. Mol. Clin. Oncol. 2013;1:297–304. doi: 10.3892/mco.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb. Haemost. 2005;93:199–204. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 76.Zhang G, Miyake M, Lawton A, Goodison S, Rosser CJ. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer. 2014;14:310. doi: 10.1186/1471-2407-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szarvas T. Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer Sci. 2010;101:1300–1308. doi: 10.1111/j.1349-7006.2010.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wallard MJ. Comprehensive profiling and localisation of the matrix metalloproteinases in urothelial carcinoma. Br. J. Cancer. 2006;94:569–577. doi: 10.1038/sj.bjc.6602931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanayama H. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer. 1998;82:1359–1366. [PubMed] [Google Scholar]
- 80.Szarvas T, vom Dorp F, Ergun S, Rubben H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat. Rev. Urol. 2011;8:241–254. doi: 10.1038/nrurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 81.Szarvas T. Enhanced stromal syndecan-1 expression is an independent risk factor for poor survival in bladder cancer. Hum. Pathol. 2014;45:674–682. doi: 10.1016/j.humpath.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 82.Wang X. Shed Syndecan-1 is involved in chemotherapy resistance via the EGFR pathway in colorectal cancer. Br. J. Cancer. 2014;111:1965–1976. doi: 10.1038/bjc.2014.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kramer MW. HYAL-1 hyaluronidase: a potential prognostic indicator for progression to muscle invasion and recurrence in bladder cancer. Eur. Urol. 2010;57:86–93. doi: 10.1016/j.eururo.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niedworok C. The impact of the receptor of hyaluronan-mediated motility (RHAMM) on human urothelial transitional cell cancer of the bladder. PLoS ONE. 2013;8:e75681. doi: 10.1371/journal.pone.0075681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 86.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matou-Nasri S, Gaffney J, Kumar S, Slevin M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and γ-adducin. Int. J. Oncol. 2009;35:761–773. doi: 10.3892/ijo_00000389. [DOI] [PubMed] [Google Scholar]
- 88.Slevin M, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J. Biol. Chem. 2002;277:41046–41059. doi: 10.1074/jbc.M109443200. [DOI] [PubMed] [Google Scholar]
- 89.Jamshidian H. Sensitivity and specificity of urinary hyaluronic acid and hyaluronidase in detection of bladder transitional cell carcinoma. Urol. J. 2014;11:1232–1237. [PubMed] [Google Scholar]
- 90.Nossier AI, Eissa S, Ismail MF, Hamdy MA, Azzazy HM. Direct detection of hyaluronidase in urine using cationic gold nanoparticles: a potential diagnostic test for bladder cancer. Biosens. Bioelectron. 2014;54:7–14. doi: 10.1016/j.bios.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 91.Niedworok C. Inhibitory role of the small leucine-rich proteoglycan biglycan in bladder cancer. PLoS ONE. 2013;8:e80084. doi: 10.1371/journal.pone.0080084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iozzo RV. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J. Biol. Chem. 2011;286:34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sainio A. Lack of decorin expression by human bladder cancer cells offers new tools in the therapy of urothelial malignancies. PLoS ONE. 2013;8:e76190. doi: 10.1371/journal.pone.0076190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang B. Biglycan expression correlates with aggressiveness and poor prognosis of gastric cancer. Exp. Biol. Med. 2011;236:1247–1253. doi: 10.1258/ebm.2011.011124. [DOI] [PubMed] [Google Scholar]
- 95.Aprile G. Biglycan expression and clinical outcome in patients with pancreatic adenocarcinoma. Tumour Biol. 2013;34:131–137. doi: 10.1007/s13277-012-0520-2. [DOI] [PubMed] [Google Scholar]
- 96.Langbein S. Gene-expression signature of adhesion/growth-regulatory tissue lectins (galectins) in transitional cell cancer and its prognostic relevance. Histopathology. 2007;51:681–690. doi: 10.1111/j.1365-2559.2007.02852.x. [DOI] [PubMed] [Google Scholar]
- 97.Cindolo L. Galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int. J. Cancer. 1999;84:39–43. doi: 10.1002/(sici)1097-0215(19990219)84:1<39::aid-ijc8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 98.Canesin G. Galectin-3 expression is associated with bladder cancer progression and clinical outcome. Tumour Biol. 2010;31:277–285. doi: 10.1007/s13277-010-0033-9. [DOI] [PubMed] [Google Scholar]
- 99.Nakamura M. Involvement of galectin-3 expression in colorectal cancer progression and metastasis. Int. J. Oncol. 1999;15:143–148. doi: 10.3892/ijo.15.1.143. [DOI] [PubMed] [Google Scholar]
- 100.Idikio H. Galectin-3 expression in human breast carcinoma: correlation with cancer histologic grade. Int. J. Oncol. 1998;12:1287–1290. doi: 10.3892/ijo.12.6.1287. [DOI] [PubMed] [Google Scholar]
- 101.Okada K, Shimura T, Suehiro T, Mochiki E, Kuwano H. Reduced galectin-3 expression is an indicator of unfavorable prognosis in gastric cancer. Anticancer Res. 2006;26:1369–1376. [PubMed] [Google Scholar]
- 102.Hurst RE. Suppression and activation of the malignant phenotype by extracellular matrix in xenograft models of bladder cancer: a model for tumor cell 'dormancy'. PLoS ONE. 2013;8:e64181. doi: 10.1371/journal.pone.0064181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Genovese L. Cellular localization, invasion, and turnover are differently influenced by healthy and tumor-derived extracellular matrix. Tissue Eng. Part A. 2014;20:2005–2018. doi: 10.1089/ten.tea.2013.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grimm S. Malignancy of bladder cancer cells is enhanced by tumor-associated fibroblasts through a multifaceted cytokine–chemokine loop. Exp. Cell Res. 2015;335:1–11. doi: 10.1016/j.yexcr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 105.Brown RE, McGuire MF. Oncogenesis recapitulates embryogenesis via the hypoxia pathway: morphoproteomics and biomedical analytics provide proof of concept and therapeutic options. Ann. Clin. Lab. Sci. 2012;42:243–257. [PubMed] [Google Scholar]
- 106.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cameron CM, Harding F, Hu WS, Kaufman DS. Activation of hypoxic response in human embryonic stem cell-derived embryoid bodies. Exp. Biol. Med. (Maywood) 2008;233:1044–1057. doi: 10.3181/0709-RM-263. [DOI] [PubMed] [Google Scholar]
- 108.Cannito S. Epithelial–mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid. Redox Signal. 2010;12:1383–1430. doi: 10.1089/ars.2009.2737. [DOI] [PubMed] [Google Scholar]
- 109.Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin. Radiat. Oncol. 2009;19:106–111. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Harris PJ, Speranza G, Dansky Ullmann C. Targeting embryonic signaling pathways in cancer therapy. Expert Opin. Ther. Targets. 2012;16:131–145. doi: 10.1517/14728222.2011.645808. [DOI] [PubMed] [Google Scholar]
- 111.Saga Y, Tsukamoto T, Jing N, Kusakabe M, Sakakura T. Murine tenascin: cDNA cloning, structure and temporal expression of isoforms. Gene. 1991;104:177–185. doi: 10.1016/0378-1119(91)90248-a. [DOI] [PubMed] [Google Scholar]
- 112.Scholzen T. The murine decorin. Complete cDNA cloning, genomic organization, chromosomal assignment, and expression during organogenesis and tissue differentiation. J. Biol. Chem. 1994;269:28270–28281. [PubMed] [Google Scholar]
- 113.Smeulders N, Woolf AS, Wilcox DT. Extracellular matrix protein expression during mouse detrusor development. J. Pediatr. Surg. 2003;38:1–12. doi: 10.1053/jpsu.2003.50000. [DOI] [PubMed] [Google Scholar]
- 114.Brunner A, Tzankov A. The role of structural extracellular matrix proteins in urothelial bladder cancer. Biomark. Insights. 2007;2:418–427. doi: 10.4137/bmi.s294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett. 2006;244:143–163. doi: 10.1016/j.canlet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 116.Lapis K, Timar J. Role of elastin–matrix interactions in tumor progression. Semin. Cancer Biol. 2002;12:209–217. doi: 10.1016/S1044-579X(02)00024-X. [DOI] [PubMed] [Google Scholar]
- 117.Ohlund D. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br. J. Cancer. 2009;101:91–97. doi: 10.1038/sj.bjc.6605107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kostyukova NN, Volkova MO, Ivanova VV, Kvetnaya AS. A study of pathogenic factors of Streptococcus pneumoniae strains causing meningitis. FEMS Immunol. Med. Microbiol. 1995;10:133–137. doi: 10.1111/j.1574-695X.1995.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 120.Hynes WL, Walton SL. Hyaluronidases of Gram-positive bacteria. FEMS Microbiol. Lett. 2000;183:201–207. doi: 10.1111/j.1574-6968.2000.tb08958.x. [DOI] [PubMed] [Google Scholar]
- 121.Wysocki AB. Proteolytic activity by multiple bacterial species isolated from chronic venous leg ulcers degrades matrix substrates. Biol. Res. Nurs. 2013;15:407–415. doi: 10.1177/1099800412464683. [DOI] [PubMed] [Google Scholar]
- 122.Langston JP, Carson CC. 3rd. Peyronie's disease: review and recent advances. Maturitas. 2014;78:341–343. doi: 10.1016/j.maturitas.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 123.Watanabe K. Collagenolytic proteases from bacteria. Appl. Microbiol. Biotechnol. 2004;63:520–526. doi: 10.1007/s00253-003-1442-0. [DOI] [PubMed] [Google Scholar]
- 124.Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116–126. [PubMed] [Google Scholar]
- 125.Matsushita O, Koide T, Kobayashi R, Nagata K, Okabe A. Substrate recognition by the collagen-binding domain of Clostridium histolyticum class I collagenase. J. Biol. Chem. 2001;276:8761–8770. doi: 10.1074/jbc.M003450200. [DOI] [PubMed] [Google Scholar]
- 126.Peterkofsky B, Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971;10:988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- 127.Birkedal-Hansen H. Catabolism and turnover of collagens: collagenases. Methods Enzymol. 1987;144:140–171. doi: 10.1016/0076-6879(87)44177-3. [DOI] [PubMed] [Google Scholar]
- 128.Gross J. Animal collagenases: specificity of action, and structures of the substrate cleavage site. Biochem. Biophys. Res. Commun. 1974;61:605–612. doi: 10.1016/0006-291x(74)91000-6. [DOI] [PubMed] [Google Scholar]
- 129.Woolley DE, Lindberg KA, Glanville RW, Evanson JM. Action of rheumatoid synovial collagenase on cartilage collagen. Different susceptibilities of cartilage and tendon collagen to collagenase attack. Eur. J. Biochem. 1975;50:437–444. doi: 10.1111/j.1432-1033.1975.tb09821.x. [DOI] [PubMed] [Google Scholar]
- 130.Woolley DE, Grafton CA. Collagenase immunolocalization studies of cutaneous secondary melanomas. Br. J. Cancer. 1980;42:260–265. doi: 10.1038/bjc.1980.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 132.Kalisz HM. Microbial proteinases. Adv. Biochem. Eng. Biotechnol. 1988;36:1–65. doi: 10.1007/BFb0047944. [DOI] [PubMed] [Google Scholar]
- 133.Braun P, de Groot A, Bitter W, Tommassen J. Secretion of elastinolytic enzymes and their propeptides by Pseudomonas aeruginosa. J. Bacteriol. 1998;180:3467–3469. doi: 10.1128/jb.180.13.3467-3469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swift S. Quorum sensing-dependent regulation and blockade of exoprotease production in Aeromonas hydrophila. Infect. Immun. 1999;67:5192–5199. doi: 10.1128/iai.67.10.5192-5199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sumitomo T, Nakata M, Higashino M, Terao Y, Kawabata S. Group A streptococcal cysteine protease cleaves epithelial junctions and contributes to bacterial translocation. J. Biol. Chem. 2013;288:13317–13324. doi: 10.1074/jbc.M113.459875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McCarty SM, Cochrane CA, Clegg PD, Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012;20:125–136. doi: 10.1111/j.1524-475X.2012.00763.x. [DOI] [PubMed] [Google Scholar]
- 137.Ribet D, Cossart P. Post-translational modifications in host cells during bacterial infection. FEBS Lett. 2010;584:2748–2758. doi: 10.1016/j.febslet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 138.Maresz KJ. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD) PLoS Pathog. 2013;9:e1003627. doi: 10.1371/journal.ppat.1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zeltz C, Gullberg D. Post-translational modifications of integrin ligands as pathogenic mechanisms in disease. Matrix Biol. 2014;40:5–9. doi: 10.1016/j.matbio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 140.Theander TG. Inhibition of human lymphocyte proliferation and cleavage of interleukin-2 by Pseudomonas aeruginosa proteases. Infect. Immun. 1988;56:1673–1677. doi: 10.1128/iai.56.7.1673-1677.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect. Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Azghani AO, Gray LD, Johnson AR. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect. Immun. 1993;61:2681–2686. doi: 10.1128/iai.61.6.2681-2686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Read RC. Interaction of nontypable Haemophilus influenzae with human respiratory mucosa in vitro. J. Infect. Dis. 1991;163:549–558. doi: 10.1093/infdis/163.3.549. [DOI] [PubMed] [Google Scholar]
- 144.Wilson R, Dowling RB, Jackson AD. The biology of bacterial colonization and invasion of the respiratory mucosa. Eur. Respir. J. 1996;9:1523–1530. doi: 10.1183/09031936.96.09071523. [DOI] [PubMed] [Google Scholar]
- 145.Bieth JG. The elastases [French] J. Soc. Biol. 2001;195:173–179. [PubMed] [Google Scholar]
- 146.Horvat RT, Parmely MJ. Pseudomonas aeruginosa alkaline protease degrades human γ interferon and inhibits its bioactivity. Infect. Immun. 1988;56:2925–2932. doi: 10.1128/iai.56.11.2925-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kayagaki N. Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mullberg J. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J. Immunol. 1995;155:5198–5205. [PubMed] [Google Scholar]
- 149.Kochut A, Dersch P. Bacterial invasion factors: tools for crossing biological barriers and drug delivery? European J. Pharm. Biopharm. 2013;84:242–250. doi: 10.1016/j.ejpb.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 150.Ha M, Bekhit Ael D, Carne A, Hopkins DL. Comparison of the proteolytic activities of new commercially available bacterial and fungal proteases toward meat proteins. J. Food Sci. 2013;78:C170–177. doi: 10.1111/1750-3841.12027. [DOI] [PubMed] [Google Scholar]
- 151.Bakhtiar SM. Implications of the human microbiome in inflammatory bowel diseases. FEMS Microbiol. Lett. 2013;342:10–17. doi: 10.1111/1574-6968.12111. [DOI] [PubMed] [Google Scholar]
- 152.Hooper LV. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 153.Pruteanu M, Hyland NP, Clarke DJ, Kiely B, Shanahan F. Degradation of the extracellular matrix components by bacterial-derived metalloproteases: implications for inflammatory bowel diseases. Inflamm. Bowel Dis. 2011;17:1189–1200. doi: 10.1002/ibd.21475. [DOI] [PubMed] [Google Scholar]
- 154.Steck N. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology. 2011;141:959–971. doi: 10.1053/j.gastro.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 155.Pucilowska JB, Williams KL, Lund PK. Fibrogenesis. IV. Fibrosis and inflammatory bowel disease: cellular mediators and animal models. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G653–G659. doi: 10.1152/ajpgi.2000.279.4.G653. [DOI] [PubMed] [Google Scholar]
- 156.van Tol EA. Bacterial cell wall polymers promote intestinal fibrosis by direct stimulation of myofibroblasts. Am. J. Physiol. 1999;277:G245–G255. doi: 10.1152/ajpgi.1999.277.1.G245. [DOI] [PubMed] [Google Scholar]
- 157.Mourelle M. Stimulation of transforming growth factor β1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology. 1998;114:519–526. doi: 10.1016/s0016-5085(98)70535-9. [DOI] [PubMed] [Google Scholar]
- 158.Johnson LA. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a 'Top-Down' approach to intestinal fibrosis in mice. Inflamm. Bowel Dis. 2012;18:460–471. doi: 10.1002/ibd.21812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 160.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl Acad. Sci. USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu N. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Ecol. 2013;66:462–470. doi: 10.1007/s00248-013-0245-9. [DOI] [PubMed] [Google Scholar]
- 162.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fouts DE. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012;10:174. doi: 10.1186/1479-5876-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL, Jakobsen KS. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011;11:244. doi: 10.1186/1471-2180-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lewis DA. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell. Infect. Microbiol. 2013;3:41. doi: 10.3389/fcimb.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arumugam M. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jalanka-Tuovinen J. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS ONE. 2011;6:e23035. doi: 10.1371/journal.pone.0023035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sanapareddy N. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012;6:1858–1868. doi: 10.1038/ismej.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sobhani I. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang T. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shida K, Nomoto K. Probiotics as efficient immunopotentiators: translational role in cancer prevention. Indian J. Med. Res. 2013;138:808–814. [PMC free article] [PubMed] [Google Scholar]
- 173.Kato I, Yokokura T, Mutai M. Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol. Immunol. 1984;28:209–217. doi: 10.1111/j.1348-0421.1984.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 174.Kato I, Yokokura T, Mutai M. Correlation between increase in Ia-bearing macrophages and induction of T cell-dependent antitumor activity by Lactobacillus casei in mice. Cancer Immunol. Immunother. 1988;26:215–221. doi: 10.1007/BF00199932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takagi A. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis. 2001;22:599–605. doi: 10.1093/carcin/22.4.599. [DOI] [PubMed] [Google Scholar]
- 176.Matsumoto S. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology. 2009;128:e170–e180. doi: 10.1111/j.1365-2567.2008.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aso Y. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur. Urol. 1995;27:104–109. doi: 10.1159/000475138. [DOI] [PubMed] [Google Scholar]
- 178.Naito S. Prevention of recurrence with epirubicin and Lactobacillus casei after transurethral resection of bladder cancer. J. Urol. 2008;179:485–490. doi: 10.1016/j.juro.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 179.Ohashi Y. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol. Int. 2002;68:273–280. doi: 10.1159/000058450. [DOI] [PubMed] [Google Scholar]
- 180.Anderson M. Viable but nonculturable bacteria are present in mouse and human urine specimens. J. Clin. Microbiol. 2004;42:753–758. doi: 10.1128/JCM.42.2.753-758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schilling JD, Hultgren SJ. Recent advances into the pathogenesis of recurrent urinary tract infections: the bladder as a reservoir for uropathogenic Escherichia coli. Int. J. Antimicrob. Agents. 2002;19:457–460. doi: 10.1016/s0924-8579(02)00098-5. [DOI] [PubMed] [Google Scholar]
- 182.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Dis. Mon. 2003;49:71–82. doi: 10.1067/mda.2003.8. [DOI] [PubMed] [Google Scholar]
- 183.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. USA. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 2002;70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat. Rev. Urol. 2012;9:85–93. doi: 10.1038/nrurol.2011.192. [DOI] [PubMed] [Google Scholar]
- 186.Gronberg-Hernandez J, Sunden F, Connolly J, Svanborg C, Wullt B. Genetic control of the variable innate immune response to asymptomatic bacteriuria. PLoS ONE. 2011;6:e28289. doi: 10.1371/journal.pone.0028289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014;20:9872–9881. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Irrazabal T, Belcheva A, Girardin SE, Martin A, Philpott DJ. The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell. 2014;54:309–320. doi: 10.1016/j.molcel.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 189.Xu W. Mini-review: perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am. J. Clin. Exp. Urol. 2014;2:57–61. [PMC free article] [PubMed] [Google Scholar]
- 190.Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed. Pharmacother. 2007;61:299–305. doi: 10.1016/j.biopha.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 191.Mungan NA, Witjes JA. Bacille Calmette–Guérin in superficial transitional cell carcinoma. Br. J. Urol. 1998;82:213–223. doi: 10.1046/j.1464-410x.1998.00720.x. [DOI] [PubMed] [Google Scholar]
- 192.Ingersoll MA, Albert ML. From infection to immunotherapy: host immune responses to bacteria at the bladder mucosa. Mucosal Immunol. 2013;6:1041–1053. doi: 10.1038/mi.2013.72. [DOI] [PubMed] [Google Scholar]
- 193.Song L. Bladder acellular matrix and its application in bladder augmentation. Tissue Eng. Part B Rev. 2014;20:163–172. doi: 10.1089/ten.TEB.2013.0103. [DOI] [PubMed] [Google Scholar]
- 194.Aitken KJ, Bagli DJ. The bladder extracellular matrix. Part II: regenerative applications. Nat. Rev. Urol. 2009;6:612–621. doi: 10.1038/nrurol.2009.202. [DOI] [PubMed] [Google Scholar]
- 195.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schwabe RF, Jobin C. The microbiome and cancer. Nat. Rev. Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Clark CA, Thomas LK, Azghani AO. Inhibition of protein kinase C attenuates Pseudomonas aeruginosa elastase-induced epithelial barrier disruption. Am. J. Respir. Cell Mol. Biol. 2011;45:1263–1271. doi: 10.1165/rcmb.2010-0459OC. [DOI] [PubMed] [Google Scholar]
- 198.Brandt LJ. American Journal of Gastroenterology lecture: intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am. J. Gastroenterol. 2013;108:177–185. doi: 10.1038/ajg.2012.450. [DOI] [PubMed] [Google Scholar]
- 199.Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am. J. Gastroenterol. 2012;107:1452–1459. doi: 10.1038/ajg.2012.93. [DOI] [PubMed] [Google Scholar]
- 200.Kelly CP. Fecal microbiota transplantation — an old therapy comes of age. N. Engl. J. Med. 2013;368:474–475. doi: 10.1056/NEJMe1214816. [DOI] [PubMed] [Google Scholar]
- 201.Iida N. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 204.Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G. Nidogen: a new, self-aggregating basement membrane protein. Eur. J. Biochem. 1983;137:455–465. doi: 10.1111/j.1432-1033.1983.tb07849.x. [DOI] [PubMed] [Google Scholar]
- 205.Kohfeldt E, Sasaki T, Gohring W, Timpl R. Nidogen-2: a new basement membrane protein with diverse binding properties. J. Mol. Biol. 1998;282:99–109. doi: 10.1006/jmbi.1998.2004. [DOI] [PubMed] [Google Scholar]
- 206.Dziadek M. Role of laminin–nidogen complexes in basement membrane formation during embryonic development. Experientia. 1995;51:901–913. doi: 10.1007/BF01921740. [DOI] [PubMed] [Google Scholar]
- 207.Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 208.Salmivirta K. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp. Cell Res. 2002;279:188–201. doi: 10.1006/excr.2002.5611. [DOI] [PubMed] [Google Scholar]
- 209.Schymeinsky J. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol. Cell. Biol. 2002;22:6820–6830. doi: 10.1128/MCB.22.19.6820-6830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fox MA, Ho MS, Smyth N, Sanes JR. A synaptic nidogen: developmental regulation and role of nidogen-2 at the neuromuscular junction. Neural Dev. 2008;3:24. doi: 10.1186/1749-8104-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ackley BD. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J. Neurosci. 2003;23:3577–3587. doi: 10.1523/JNEUROSCI.23-09-03577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kruegel J, Sadowski B, Miosge N. Nidogen-1 and nidogen-2 in healthy human cartilage and in late-stage osteoarthritis cartilage. Arthritis Rheum. 2008;58:1422–1432. doi: 10.1002/art.23480. [DOI] [PubMed] [Google Scholar]
- 213.Dedhar S, Jewell K, Rojiani M, Gray V. The receptor for the basement membrane glycoprotein entactin is the integrin α3/β1. J. Biol. Chem. 1992;267:18908–18914. [PubMed] [Google Scholar]
- 214.Dong LJ, Hsieh JC, Chung AE. Two distinct cell attachment sites in entactin are revealed by amino acid substitutions and deletion of the RGD sequence in the cysteine-rich epidermal growth factor repeat 2. J. Biol. Chem. 1995;270:15838–15843. doi: 10.1074/jbc.270.26.15838. [DOI] [PubMed] [Google Scholar]
- 215.Dong L. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab. Invest. 2002;82:1617–1630. doi: 10.1097/01.lab.0000042240.52093.0f. [DOI] [PubMed] [Google Scholar]
- 216.Lebel SP, Chen Y, Gingras D, Chung AE, Bendayan M. Morphofunctional studies of the glomerular wall in mice lacking entactin-1. J. Histochem. Cytochem. 2003;51:1467–1478. doi: 10.1177/002215540305101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mayer U. Binding properties and protease stability of recombinant human nidogen. Eur. J. Biochem. 1995;227:681–686. doi: 10.1111/j.1432-1033.1995.tb20188.x. [DOI] [PubMed] [Google Scholar]
- 218.Jiang X, Couchman JR. Perlecan and tumor angiogenesis. J. Histochem. Cytochem. 2003;51:1393–1410. doi: 10.1177/002215540305101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Iozzo RV. Structural and functional characterization of the human perlecan gene promoter. Transcriptional activation by transforming growth factor-β via a nuclear factor 1-binding element. J. Biol. Chem. 1997;272:5219–5228. doi: 10.1074/jbc.272.8.5219. [DOI] [PubMed] [Google Scholar]
- 220.Bix G, Iozzo RV. Novel interactions of perlecan: unraveling perlecan's role in angiogenesis. Microsc. Res. Tech. 2008;71:339–348. doi: 10.1002/jemt.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stander M, Naumann U, Wick W, Weller M. Transforming growth factor-β and p-21: multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999;296:221–227. doi: 10.1007/s004410051283. [DOI] [PubMed] [Google Scholar]
- 222.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am. J. Pathol. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-β-mediated fibrotic responses in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L1327–L1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 224.Schaefer L. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Moreth K. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J. Clin. Invest. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 227.Heinegard D. Two novel matrix proteins isolated from articular cartilage show wide distributions among connective tissues. J. Biol. Chem. 1986;261:13866–13872. [PubMed] [Google Scholar]
- 228.Saamanen AM, Salminen HJ, Rantakokko AJ, Heinegard D, Vuorio EI. Murine fibromodulin: cDNA and genomic structure, and age-related expression and distribution in the knee joint. Biochem. J. 2001;355:577–585. doi: 10.1042/bj3550577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.van 't Veer LJ. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 230.Welsh JB. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974–5978. [PubMed] [Google Scholar]
- 231.Garber ME. Diversity of gene expression in adenocarcinoma of the lung. Proc. Natl Acad. Sci. USA. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jones PL, Jones FS. Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol. 2000;19:581–596. doi: 10.1016/s0945-053x(00)00106-2. [DOI] [PubMed] [Google Scholar]
- 233.Chen J. Role of fibrillar Tenascin-C in metastatic pancreatic cancer. Int. J. Oncol. 2009;34:1029–1036. [PubMed] [Google Scholar]
- 234.Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell–fibronectin matrix interactions during tissue repair. J. Investig. Dermatol. Symp. Proc. 2006;11:73–78. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- 235.O'Brien ER. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler. Thromb. 1994;14:1648–1656. doi: 10.1161/01.atv.14.10.1648. [DOI] [PubMed] [Google Scholar]
- 236.Malyankar UM, Almeida M, Johnson RJ, Pichler RH, Giachelli CM. Osteopontin regulation in cultured rat renal epithelial cells. Kidney Int. 1997;51:1766–1773. doi: 10.1038/ki.1997.243. [DOI] [PubMed] [Google Scholar]
- 237.Hunter GK, Kyle CL, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994;300:723–728. doi: 10.1042/bj3000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mazzali M. Osteopontin — a molecule for all seasons. QJM. 2002;95:3–13. doi: 10.1093/qjmed/95.1.3. [DOI] [PubMed] [Google Scholar]
- 239.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 240.Menon R. Diet complexity and estrogen receptor β status affect the composition of the murine intestinal microbiota. Appl. Environ. Microbiol. 2013;79:5763–5773. doi: 10.1128/AEM.01182-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Novotny M. ER-α agonist induces conversion of fibroblasts into myofibroblasts, while ER-β agonist increases ECM production and wound tensile strength of healing skin wounds in ovariectomised rats. Exp. Dermatol. 2011;20:703–708. doi: 10.1111/j.1600-0625.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- 242.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.van Kempen LC, de Visser KE, Coussens LM. Inflammation, proteases and cancer. Eur. J. Cancer. 2006;42:728–734. doi: 10.1016/j.ejca.2006.01.004. [DOI] [PubMed] [Google Scholar]