Key Points
Measles remains a leading vaccine-preventable cause of child mortality in Africa and Asia, and continues to cause outbreaks in industrialized countries.
Remarkable progress in reducing measles incidence and mortality has been made in resource-poor countries, particularly in sub-Saharan Africa, as a consequence of increasing measles vaccine coverage and provision of a second opportunity for measles vaccination through supplementary immunization activities.
Measles virus (MV) is highly infectious, requiring a high level of population immunity to interrupt transmission, and might be more difficult to eliminate in regions of high population density and high prevalence of human immunodeficiency virus type 1 (HIV-1) infection.
The global elimination of measles has been debated since the 1960's, shortly after measles vaccines were first licensed.
Criteria necessary for disease eradication include: first, humans must be required for virus transmission; second, sensitive and specific diagnostic tools must exist; and finally an effective intervention must be available. Measles is thought by many experts to meet all of these criteria
Measles vaccines are safe, effective and have interrupted MV transmission in large geographic areas, providing a suitable tool for global measles elimination.
The ideal measles vaccine would be inexpensive, safe, heat-stable, immunogenic in neonates or very young infants, administered as a single dose without needle or syringe, and would not prime individuals for atypical measles or be associated with prolonged immunosuppression. Several vaccine candidates with some of these characteristics are undergoing development.
A significant challenge to global measles elimination efforts will be to maintain the resources, political will and public confidence to implement measles vaccination and surveillance programmes.
Safe and effective vaccines are available that could be used to eradicate measles, which is a primary cause of childhood vaccine-preventable deaths worldwide. This article reviews the pathogenesis of this deadly disease and the prospects for its elimination.
Abstract
Measles remains a leading vaccine-preventable cause of child mortality worldwide, particularly in sub-Saharan Africa where almost half of the estimated 454,000 measles deaths in 2004 occurred. However, great progress in measles control has been made in resource-poor countries through accelerated measles-control efforts. The global elimination of measles has been debated since measles vaccines were first licensed in the 1960's, and this debate is likely to be renewed if polio virus is eradicated. This review discusses the pathogenesis of measles and the likelihood of the worldwide elimination of this disease.
Main
Measles, which is caused by the measles virus (MV) (Box 1), was estimated to cause 454,000 deaths in 2004, almost half of which were in sub-Saharan Africa, and continues to cause outbreaks in communities with low vaccination coverage in industrialized nations1 (Fig. 1). It is one of the most important infectious diseases of humans and has caused millions of deaths since its emergence thousands of years ago. The disease is characterized by a prodromal illness of fever, cough, coryza and conjunctivitis followed by the appearance of a generalized maculopapular rash. Deaths from measles are mainly due to an increased susceptibility to secondary bacterial and viral infections, which is attributed to a prolonged state of MV-induced immune suppression (Box 2).
Figure 1. Immunization coverage with measles-containing vaccines in infants (2004).
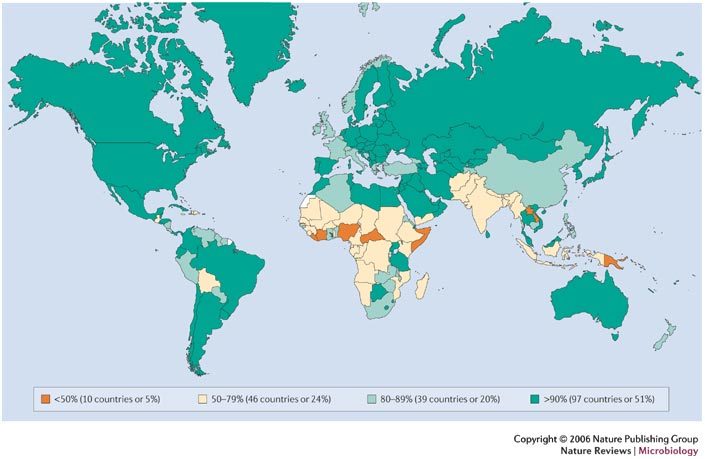
The figure was compiled using data provided by the WHO (2005), see Further information for details.
MV most closely resembles rinderpest virus, a pathogen of cattle, and might have evolved as a zoonotic infection from an ancestral virus that was present in communities in which cattle and humans lived in close proximity. MV is thought to have become established in human populations approximately 5,000 years ago, when human populations achieved sufficient size in the Middle Eastern river valley civilizations to maintain virus transmission. The virus was introduced into the Americas in the sixteenth century as a result of European exploration of the New World, resulting in thousands of deaths in native Amerindian populations. Deaths in this susceptible native American population due to measles and smallpox facilitated the European conquest of the Americas2. MV was first isolated from the blood of David Edmonston in 1954 by John Enders and Thomas Peebles3. The development of vaccines against measles soon followed4.
Pathogenesis of MV infection
Respiratory droplets from infected persons function as vehicles of transmission by delivering infectious virus to epithelial cells of the respiratory tract of susceptible hosts. During the 10–14 day incubation period between infection and the onset of clinical signs and symptoms, MV replicates and spreads in the infected host (Fig. 2). Initial viral replication occurs in epithelial cells at the entry site in the upper respiratory tract, and the virus then spreads to local lymphatic tissue. Replication in local lymph nodes is followed by viraemia and the dissemination of MV to many organs, including lymph nodes, skin, kidney, gastrointestinal tract and liver, in which the virus replicates in the epithelial and endothelial cells, and in lymphocytes, monocytes and macrophages.
Figure 2. Basic pathogenesis of measles-virus infection.
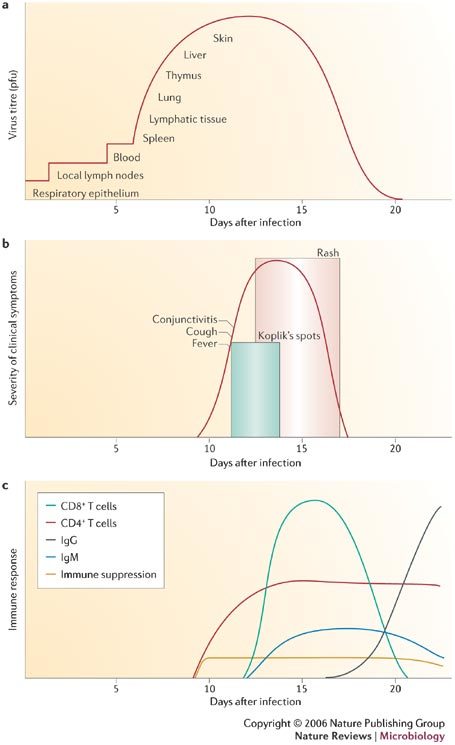
Panels summarize features of the basic pathogenesis of measles-virus infection. a | Panel shows the spread of the virus through the body from the initial site of infection in respiratory epithelia to the skin, in which the diagnostic rash is formed. Sites of infection are overlaid with virus titre (pfu). b | Illustrates the appearance of clinical symptoms over time, including the diagnostic Koplik's spots and rash. c | Panel summarizes immune responses over time, including both B- and T-cell responses. Clinical symptoms arise coincident with the onset of the immune response. Pfu; plaque-forming unit. Modified with permission from Ref. 87 © (2001) Lippincott Williams & Wilkins.
Host immune responses (Fig. 2) to MV are essential for viral clearance, clinical recovery and the establishment of long-term immunity. Early innate immune responses occur during the prodromal phase and include activation of natural killer (NK) cells and increased production of interferons (IFN)-α and β. The adaptive immune responses consist of MV-specific humoral and cellular responses. The protective efficacy of antibodies to MV is shown by the immunity conferred to infants from passively acquired maternal antibodies and the protection of exposed, susceptible individuals following post-exposure administration of anti-MV immune globulin5. The most abundant and rapidly produced antibodies are against the nucleoprotein (N). Antibodies to the haemagglutinin (H) and fusion (F) proteins contribute to virus neutralization and are sufficient to provide protection.
Evidence for the importance of cellular immunity to MV is demonstrated by the ability of children with agammaglobulinaemia to fully recover from measles, whereas children with severe defects in T-lymphocyte function often develop severe or fatal disease6. Monkeys depleted of CD8+ T-lymphocytes and challenged with wild-type MV had a more extensive rash, higher levels of MV in the blood and a longer duration of viraemia than control animals7. CD4+ T-lymphocytes are activated in response to MV infection and secrete cytokines capable of directing humoral and cellular immune responses. Plasma cytokine profiles show increased levels of IFN-γ during the acute phase, followed by a shift to high levels of interleukin (IL)-4 and IL-10 during convalescence8. The initial predominant T-helper-1 (Th1)-response is essential for viral clearance, and the later Th2 response promotes the development of protective MV-specific antibodies.
The immune responses induced by MV infection are paradoxically associated with depressed responses to non-MV antigens, and this effect continues for several weeks to months after resolution of the acute illness. Following MV infection, delayed-type hypersensitivity (DTH) responses to recall antigens, such as tuberculin, are suppressed9 and cellular and humoral responses to new antigens are impaired10. This MV-induced immune suppression renders individuals more susceptible to secondary bacterial and viral infections that can cause pneumonia and diarrhoea, and is responsible for much of the measles-related morbidity and mortality11,12. Pneumonia, the most common fatal complication of measles, occurs in 56–86% of measles-related deaths13.
Abnormalities of both the innate and adaptive immune responses have been described following MV infection. Transient lymphopaenia with a reduction in CD4+ and CD8+ T-lymphocytes occurs in children with measles. Functional abnormalities of immune cells have also been detected, including decreased lymphocyte proliferative responses14. Dendritic cells that are infected with MV in vitro mature poorly, lose the ability to stimulate responses in lymphocytes and undergo cell death15. The dominant Th2-response in children recovering from measles can inhibit Th1 responses and increase susceptibility to intracellular pathogens16,17. The production of IL-12, which is important for the generation of the Th1-type immune response, is decreased in vitro following binding of CD46 (Ref. 18), an MV receptor, and is reduced for several weeks in children with measles19. Conversely, IL-10 production is elevated for several weeks in the plasma of children with measles8. IL-10 downregulates the synthesis of cytokines, suppresses macrophage activation and T-cell proliferation and inhibits DTH responses.
Progress in measles control
Prior to the development and widespread use of measles vaccines, measles was estimated to result in 5–8 million deaths annually. The decline in mortality from measles in developed countries was associated with economic development, improved nutritional status and supportive care, and antibiotic therapy for secondary bacterial pneumonia. Remarkable progress in reducing measles incidence and mortality has been made in resource-poor countries, particularly in sub-Saharan Africa20, as a consequence of increasing measles vaccine coverage, provision of a second opportunity for measles vaccination through supplementary immunization activities, and efforts by the WHO, the United Nations Children's Fund (UNICEF) and their partners to target 45 countries for accelerated and sustained measles mortality reduction21. Provision of vitamin A through polio and measles vaccination campaigns has contributed further to the reduction in measles mortality1. In 2003, the World Health Assembly endorsed a resolution urging member countries to reduce the number of deaths attributed to measles by 50% by the end of 2005 compared with the estimated number of measles-related deaths in 1999, a target that is likely to have been met. Overall global measles mortality in 2004 was estimated to be 454,000 deaths (uncertainty over exact numbers means that this estimate could range from 329,000 to 596,000 deaths), a 48% decrease from the measles-related deaths recorded in 1999 (Ref. 22).
Measles eradication
The global elimination of measles has been debated since the 1960's, shortly after measles vaccines were first licensed23. The Dahlem Conference on Disease Eradication (1997) defined eradication as the permanent reduction to zero of the global incidence of infection caused by a specific pathogen as a result of deliberate efforts, with the consequence that interventions would no longer be necessary. Although modifications to this definition were subsequently proposed, three criteria were deemed necessary for a disease to be considered eradicable: first, humans must be crucial for transmission; second, sensitive and specific diagnostic tools must exist; and finally, an effective intervention must be available. Demonstration of the interruption of transmission in a large geographic area for a prolonged period further supports the feasibility of eradication. Measles is thought by many experts to meet these criteria for eradication24,25.
Biological feasibility of measles elimination
MV has no non-human reservoirs, can be readily diagnosed after the onset of a rash and has not mutated or evolved to significantly alter immunogenic epitopes. However, MV is highly infectious, requiring a high level of population immunity to interrupt transmission, is contagious for several days prior to the onset of the rash (the first easily diagnosed symptom), and might be more difficult to eliminate in regions that have a high prevalence of human immunodeficiency virus type 1 (HIV-1).
Non-human reservoirs. Humans are the only reservoir for MV. Non-human primates can be infected with MV and develop an illness similar to measles in humans. However, populations of wild primates are not of a sufficient size to maintain MV transmission. To provide a sufficient number of new susceptible individuals through births to maintain MV transmission, a population size of several hundred thousand individuals with 5,000 to 10,000 births per year is required26.
Case detection. The characteristic clinical features of measles are of sufficient sensitivity and specificity to have a high predictive value in regions where measles is endemic. However, laboratory diagnosis is necessary if MV transmission rates are low or in immunocompromised persons who might not have the characteristic clinical manifestations (Box 2). Infection with rubella virus, parvovirus B19, human herpes virus 6 and dengue viruses all cause symptoms that can mimic those of measles. Detection of IgM antibodies to MV by enzyme immunoassay is the standard laboratory method for diagnosing acute measles27.
Subclinical measles is defined as a fourfold rise in MV-specific IgG antibodies following exposure to wild-type MV in an asymptomatic individual with some prior measles immunity. Subclinical infection might be important in boosting protective antibody levels in children with decreasing immunity28, but raises the concern that persons with incomplete immunity and subclinical infection might be capable of transmitting MV29. MV has been isolated from a naturally immune, asymptomatically re-infected individual30, and extensive epidemiological investigation of a person with measles in the Netherlands failed to identify a contact with clinically apparent measles, implying that transmission might have occurred from a person with subclinical infection31.
Viral evolution and diversity. Although RNA viruses have high mutation rates32, MV is an antigenically monotypic virus: the surface proteins that are responsible for inducing protective immunity have retained their antigenic structure across time and space. The public health significance of this is that measles vaccines developed decades ago from a single MV strain remain protective worldwide. However, variability in the genome is sufficient to allow for molecular epidemiologic investigation. One of the most variable regions of the MV genome is the 450-nucleotide sequence at the carboxyl terminus of the N protein, with up to 12% variability between wild-type viruses. The WHO recognizes 8 clades of MV (designated A to H) and 23 genotypes33,34. New genotypes will probably be identified with improved surveillance and molecular characterization. As measles-control efforts intensify, molecular surveillance of circulating MV strains could be used to document the interruption of MV transmission and to identify the source and transmission pathways of MV outbreaks35,36.
Infectiousness. MV is one of the most highly contagious infectious agents and outbreaks can occur in populations in which less than 10% of individuals are susceptible. The contagiousness of MV is best expressed by the basic reproductive number (Ro), which is the mean number of secondary cases that would arise if an infectious agent were introduced into a completely susceptible population. Ro is a function not only of the infectious agent but also of the host population. The estimated Ro for MV is generally assumed to be 12–18, in contrast to only 5–7 for smallpox virus and 2–3 for SARS coronavirus. In the 1951 measles epidemic in Greenland, the index case attended a community dance during the infectious period resulting in an Ro of 200 (Ref. 37). The high infectivity of MV implies that a high level of population immunity (approximately 95%) is required to interrupt MV transmission.
Further hindering elimination efforts is the fact that persons with measles are infectious during the prodromal phase, several days prior to the onset of rash, and therefore transmit the virus prior to clinical case detection. MV can be isolated in tissue culture from urine as late as one week after the onset of rash. Detection of MV in body fluids by various means, including identification of multinucleated giant cells in nasal secretions and the use of PCR after reverse transcription of RNA (RT-PCR), indicates the potential for prolonged infectious periods in persons immunocompromised by severe malnutrition or HIV-1 infection38,39. However, whether detection of MV by these methods indicates prolonged contagiousness is unclear, and prolonged transmission of MV is not likely to be a significant obstacle to eradication. In contrast to polioviruses40, transmission of the live attenuated measles vaccine virus has not been reported.
HIV-1 epidemic. In regions of high HIV-1 prevalence and crowding, such as urban centres in sub-Saharan Africa, HIV-1-infected children could have a role in the sustained transmission of MV41. HIV-1-infected mothers have defective transfer of IgG antibodies across the placenta, resulting in lower titres of protective antibodies in the infant, which increases the period of susceptibility to MV infection prior to routine immunization. HIV-1-infected children that are vaccinated against MV might become susceptible to MV owing to progressive HIV-1-induced immunosuppression. Children with defective cell-mediated immunity might not develop the characteristic measles rash, and infection might therefore go unrecognized, with the potential for widespread transmission of MV, particularly in healthcare settings. Finally, HIV-1-infected children might have impaired cell-mediated immune clearance and prolonged shedding of MV39, increasing the period of infectivity and the spread of MV to secondary contacts. However, even if HIV-1-infected children lose protective immunity over time, the high mortality rate of HIV-infected children, particularly in sub-Saharan Africa where approximately one third of untreated HIV-1-infected children die by one year of age and half are dead by two years of age, is such that they do not live long enough for a sizeable pool of MV-susceptible children to accumulate42. This might change with increased access to antiretroviral drugs. Successful control of measles in the countries of southern Africa suggests that the HIV-1 epidemic is not a significant barrier to measles control20,43.
Technical feasibility of measles elimination
Measles vaccines are safe, effective and have interrupted MV transmission in large geographic areas, providing a crucial tool for global measles elimination.
Measles vaccines. Several attenuated measles vaccines are available worldwide, either as single-virus vaccines or in combination with other vaccine viruses (commonly rubella and mumps). Most of the currently used measles vaccines were derived from the Edmonston strain of MV isolated by Enders and Peebles in 1954. These vaccines have undergone different passage histories in cell culture, but nucleotide sequence analyses show genetic differences of less than 0.6% between most vaccine strains (Fig. 3).
Figure 3. Measles virus vaccines.
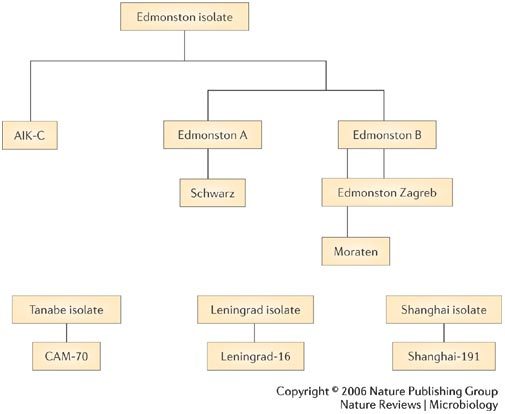
Most attenuated measles vaccines were developed from the Edmonston strain of measles virus. The Edmonston B vaccine was the first licensed measles vaccine but was associated with a high frequency of fever and rash. The further attenuated Schwarz and Edmonston-Zagreb vaccines are widely used throughout the world, although the Moraten vaccine is the only measles vaccine used in the United States. Modified with permission from Ref. 88 (1993) WHO.
The measles vaccine induces both humoral and cellular immune responses. Antibodies first appear between 12 and 15 days after vaccination and peak at 21 to 28 days. IgG antibodies persist in the blood for years (Box 2). Vaccination also induces MV-specific cellular immune responses44. The proportion of children who develop protective antibody titres following measles vaccination depends on the presence of inhibitory maternal antibodies and the immunological maturity of the vaccine recipient, as well as the dose and strain of vaccine virus. Polymorphisms in human immune-response genes (for example, TAP2 and HLA-DQA1) also influence immune responses to measles vaccine45. Frequently cited statistics are that approximately 85% of children develop protective antibody titres when the measles vaccine is administered at 9 months of age, and 90–95% have a protective antibody response after vaccination at 12 months of age46. The duration of protective antibody titres following measles vaccination is more variable and shorter than that acquired through infection with wild-type MV, with an estimated 5% of children losing protective antibody titres 10–15 years after vaccination47. However, decreasing antibody titres do not necessarily imply a loss of protective immunity, as a secondary immune response usually develops after re-exposure to MV, with a rapid rise in IgG antibodies.
Limitations of licensed measles vaccines. Despite the public health benefits of measles vaccines, there are several limitations of the licensed vaccines that might be important for global measles elimination. First, attenuated measles vaccines are inactivated by light and heat, and lose about half of their potency after reconstitution if stored at 20°C for one hour and almost all potency if stored at 37°C for one hour. A cold chain must be maintained to support measles immunization activities. Second, measles vaccines must be injected subcutaneously or intramuscularly, necessitating trained healthcare workers, needles, syringes and the proper disposal of hazardous waste. Third, both maternally acquired antibodies and immunological immaturity reduce the protective efficacy of measles vaccination in early infancy, hindering effective immunization of young infants48. Fourth, the attenuated measles vaccine has the potential to cause serious outcomes, such as lung or brain infection, in severely immunocompromised persons49. Finally, as discussed below, a second opportunity for measles vaccination, in addition to the first dose through routine immunization services, must be provided to achieve high enough levels of population immunity to interrupt MV transmission.
Logistical feasibility of measles elimination
High levels of population immunity against measles, achieved through high measles-vaccine coverage, can interrupt MV transmission. Perhaps the main challenge to global measles-elimination efforts will be to maintain the resources, political will and public confidence to implement intensive measles vaccination and surveillance activities.
Measles elimination strategies. Measles elimination is the interruption of MV transmission in a defined geographical area. Small outbreaks of primary and secondary cases might still occur following importation from outside the region, but sustained transmission does not occur. Because of the high infectivity of MV and the fact that not all individuals develop protective immunity following vaccination, a single dose of measles vaccine does not achieve a sufficient level of population immunity to eliminate measles. A second opportunity for measles immunization is necessary to eliminate measles by providing protective immunity to children who failed to respond to the first dose and to those who were not previously vaccinated. Two broad strategies to administer the second dose have been used. In countries with sufficient infrastructure, the second dose of measles vaccine is administered through routine immunization services, typically prior to the start of school. High coverage levels are ensured by school entry requirements. A second approach, first developed by the Pan American Health Organization (PAHO) for South and Central America50, involves mass-immunization campaigns (called supplementary immunization activities, or SIAs) to deliver the second dose of measles vaccine. This strategy was successful in eliminating measles in Latin America, and has resulted in a marked reduction in measles incidence and mortality in parts of sub-Saharan Africa43.
The PAHO strategy consists of four sub-programmes: Catch-Up, Keep-Up, Follow-Up and Mop-Up50. The Catch-Up phase is a one-time, mass-immunization campaign that targets all children in a broad age group regardless of whether they previously had wild-type MV infection or measles vaccination. The goal is to rapidly achieve a high level of population immunity and interrupt MV transmission. If successful, SIA activities are cost effective51,52 and can abruptly interrupt MV transmission with dramatic declines in incidence and mortality. Keep-Up refers to the need to maintain >90% routine measles vaccine coverage through improved access to measles vaccination and a reduction in missed opportunities. Follow-Up refers to periodic mass campaigns to prevent the accumulation of susceptible children that typically target children 1–4 years of age, a narrower age group than targeted in Catch-Up campaigns. Mop-Up campaigns target children that are difficult to reach in sites of measles outbreaks or low vaccine coverage.
War, population movement and demographics. Polio vaccination campaigns have been successfully conducted during scheduled ceasefires in regions of conflict53. Similar results should be achievable for measles vaccination campaigns, although additional highly skilled healthcare workers are needed to administer parenteral measles vaccine compared with oral poliovirus vaccine. However, maintaining high levels of routine measles vaccine coverage in areas of conflict is extremely difficult, and devastating measles outbreaks frequently occur in refugee populations54. The ease of global travel facilitates the importation of MV into regions where measles has been successfully controlled (as exemplified by the 2006 measles outbreak in Boston, USA) and cross-border population movements necessitate regional rather than country level control strategies. Finally, measles control might be particularly difficult in the high-density slum areas of large cities in Africa and Asia, where several factors converge to facilitate MV transmission among susceptible persons, including high population density and difficulties in achieving high vaccination coverage.
Loss of public confidence. A loss of public confidence in vaccines can significantly impair elimination efforts, as demonstrated by the poliovirus outbreaks in northern Nigeria that spread across several continents after a loss of public confidence in polio vaccines55. Measles outbreaks have occurred in communities that are opposed to vaccination on religious or philosophical grounds56. Much public attention has focused on a purported association between the measles-mumps-rubella (MMR) vaccine and autism following the publication of a report in 1998 hypothesizing that the MMR vaccine might cause a syndrome of autism and intestinal inflammation57. The events that followed, and the public concern over the safety of the MMR vaccine, led to diminished vaccine coverage in the United Kingdom and provide important lessons in the misinterpretation of epidemiological evidence and the communication of scientific results to the public58. As a consequence, measles outbreaks became more frequent and larger in size59. Subsequently, several comprehensive reviews and epidemiological studies rejected evidence for a causal relationship between MMR vaccination and autism60,61.
New tools for measles eradication
Aerosol administration of measles vaccine was first evaluated in the early 1960s in several countries, including the former Soviet Union and the United States. More recent studies in South Africa62 and Mexico63 have shown that aerosol administration of measles vaccine is highly effective in boosting antibody titres, although the primary immune response to aerosolized measles vaccine is reduced compared with subcutaneous administration64. Administration of measles vaccine by aerosol has the potential to facilitate measles vaccination during mass campaigns, and the WHO plans to test and bring to licensure an aerosol measles vaccine by 2009.
The ideal measles vaccine would be inexpensive, safe, heat-stable, immunogenic in neonates or very young infants, and administered as a single dose without the need to use a needle or syringe. The age at vaccination should coincide with the Expanded Programme on Immunization (EPI) schedule to maximize compliance and share resources. Finally, a new vaccine should not prime individuals for atypical measles on exposure of immunized individuals to wild-type MV (a complication of formalin-inactivated measles vaccines) and should not be associated with prolonged immunosuppression adversely affecting immune responses to subsequent infections (a complication of high-titre measles vaccines).
Several vaccine candidates with some of these characteristics are undergoing development and testing. Naked cDNA vaccines are thermostable, inexpensive and could theoretically elicit antibody responses in the presence of passively acquired maternal antibody. DNA vaccines encoding either or both of the measles H and F proteins are safe, immunogenic and protective against measles challenge in naive, juvenile rhesus macaque monkeys65. A different DNA vaccine, containing H, F and N genes and an IL-2 molecular adjuvant, provided protection to infant macaques in the presence of neutralizing antibody66,67. Alternative vectors for administering MV genes, such as alphavirus68, parainfluenza virus69 or enteric bacteria70 are also under investigation. Immune responses to intranasal administration of MV vaccines are enhanced by the use of adjuvants71. Novel oral immunization strategies have been developed using plant-based expression of the H protein from MV in tobacco72.
Prospects for global measles elimination
The elimination of measles in large areas, such as the Americas, suggests that global measles elimination is feasible with current vaccination strategies25,73,74. The American Red Cross, along with other partners, has played a significant role in establishing the Measles Initiative, which has been responsible for much of the success in measles control in Africa and is committed to reducing global measles deaths by providing measles vaccine to children. The key questions regarding global measles elimination are whether epidemiological conditions are sufficiently different in parts of Africa and Asia to hinder measles-elimination efforts, and whether there will be the political and public support necessary for elimination activities. Interruption of MV transmission could be difficult in densely populated urban environments of Africa and Asia and in regions of high HIV-1 prevalence24. Other potential obstacles to global measles elimination have been identified, including immunity that decreases over time, and the possibility of transmission from subclinical cases, although these have not hindered measles elimination in the Americas24,75. Measles outbreaks have occurred in susceptible adults, most notably the large outbreak in Sâo Paulo, Brazil, in 1997, but have not led to sustained endemic transmission24. Garnering the political will and public support is likely to be more difficult in resource-rich countries where the burden of disease due to measles is not recognized and unfounded fears of serious adverse events from vaccination are more prevalent. Whether the threat from bioterrorism precludes stopping measles vaccination after eradication is a topic of debate, but, at the least, a single-dose rather than a two-dose measles vaccination strategy could be adopted73.
The measles eradication end game is likely to be different from that for smallpox and polioviruses76,77. Higher levels of population immunity are necessary to interrupt MV transmission. Linking surveillance activities with rapid outbreak response and vaccination efforts will be crucial for measles elimination, as it is for smallpox and polio. However, the rapid spread of MV and the need for trained healthcare workers to administer measles vaccine will be additional obstacles. Ensuring that the necessary supply of measles vaccine is maintained as elimination efforts progress will also be crucial, requiring close collaboration with vaccine manufacturers.
Critics of eradication programmes claim that they divert resources from primary healthcare and are imposed on countries or communities from outside. Enormous resources and efforts might be required to eradicate the few remaining cases of disease, and the economic and social costs of eradication need to be considered78. The polio eradication campaign in Nigeria was seen to trigger a massive outbreak response for three confirmed cases in Adamawa State in 2005, whereas hundreds of deaths due to measles did not result in a comparable response79. But delays in achieving polio eradication will make many people hesitant to move forward with measles eradication. Serious discussion of measles eradication will probably take place after polio eradication is achieved, and will be a focus of intense debate in the years to come.
Box 1 | Measles virus – the basics.
The measles virus (MV) is a spherical, non-segmented, single-stranded, negative sense RNA virus (see the figure part a) and is a member of the Morbillivirus genus in the family of Paramyxoviridae. Other members of the Morbillivirus genus that are not pathogenic to humans include rinderpest virus and canine distemper virus. MV is killed by ultraviolet light and heat. Attenuated measles vaccine viruses retain these characteristics, necessitating a cold chain for the transport and storage of measles vaccines.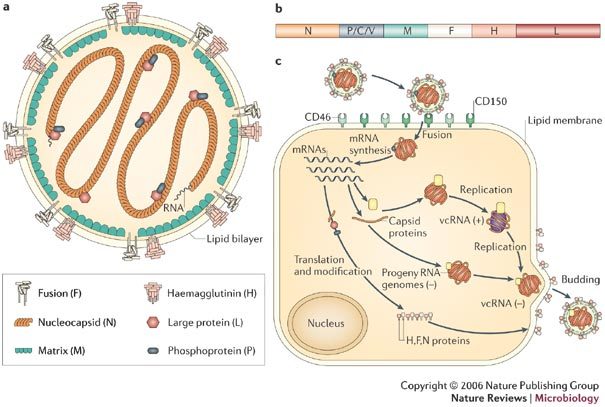
The MV RNA genome comprises approximately 16,000 nucleotides (see the figure part b) and is enclosed in a lipid-containing envelope that is derived from the host cell. The genome encodes eight proteins, two of which (V and C) are non-structural proteins and are alternatively translated from the RNA, or an edited RNA, coding for the phosphoprotein (P). Of the six structural proteins, P, large protein (L) and nucleoprotein (N) form the nucleocapsid that encloses the viral RNA. The haemagglutinin protein (H), fusion protein (F) and matrix protein (M), together with lipids from the host cell membrane, form the viral envelope.
The H protein interacts with F to mediate attachment and fusion of the viral envelope with the host cell membrane, enabling viral entry into the cell80 (see the figure part c). The primary function of the H protein is to bind to the host cellular receptors for MV. The two identified receptors are CD46 and CD150 (also known as SLAM). CD46 is a complement regulatory molecule expressed on all nucleated cells in humans. SLAM (signalling lymphocyte activation molecule) is expressed on activated T and B lymphocytes and antigen-presenting cells. The binding sites on H for these receptors overlap and strains of MV differ in the efficiency with which each receptor is used. Wild-type MV binds to cells primarily through the cellular receptor SLAM, whereas most vaccine strains bind to CD46, as well as to SLAM81. Other unidentified receptors for MV probably exist on human endothelial and epithelial cells82.
Remaining MV proteins are involved in viral replication. The P protein regulates transcription, replication and the efficiency with which the nucleoprotein assembles into nucleocapsids83. The M protein links ribonucleoproteins with envelope proteins during virion assembly. The functions of the V and C proteins have not been clearly defined, but both proteins seem to contribute to the virulence of MV by regulating transcription and sensitivity to the antiviral effects of IFNα/β84,85. Part a of the figure is modified with permission from Ref. 86 © (2002) Cambridge press.
Box 2 | Measles – the disease.
Clinically apparent measles begins with a prodrome characterized by fever, cough, coryza (runny nose) and conjunctivitis (Fig. 2). Koplik's spots, which are small white lesions on the buccal mucosa inside the mouth, might be visible during the prodrome. The prodromal symptoms intensify several days before the onset of rash. The characteristic erythematous and maculopapular rash appears first on the face and behind the ears, and then spreads to the trunk and the extremities. The rash lasts for 3–4 days after which it fades, disappearing from the face first.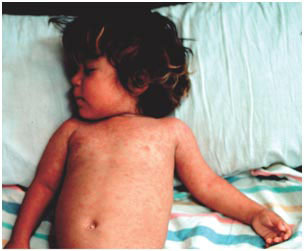
In uncomplicated measles, clinical recovery begins soon after the appearance of the rash. The risk of complication is increased by extremes of age, malnutrition and vitamin A deficiency. Complications of measles have been described in almost every organ system. Pneumonia accounts for most of the approximately 500,000 measles-associated deaths annually, and is caused by secondary viral or bacterial infections or by MV itself. Other respiratory complications include laryngotracheobronchitis (croup) and otitis media (ear infection). Mouth ulcers, or stomatitis, might hinder children from eating or drinking. Many children with measles develop diarrhoea, further contributing to malnutrition. Eye disease (keratoconjunctivitis) is common after measles, particularly in children with vitamin A deficiency, and can cause blindness.
Rare but serious complications of measles involve the central nervous system (CNS). Post-measles encephalomyelitis, an autoimmune disorder triggered by MV infection, occurs in approximately 1 in 1000 cases, and is mainly confined to older children and adults. Encephalomyelitis occurs within two weeks of the onset of rash and is characterized by fever, seizures and various neurological abnormalities. Other CNS complications that occur months to years after acute infection are measles inclusion body encephalitis (MIBE) and subacute sclerosing panencephalitis (SSPE). MIBE and SSPE are caused by persistent MV infection. MIBE is a rare but fatal complication that affects individuals with defective cellular immunity and typically occurs months after infection. SSPE is a slow progressive disease characterized by seizures, progressive deterioration of cognitive and motor functions and by death, which occurs 5–15 years after MV infection, most often in persons infected with MV before 2, which years of age.
Acknowledgements
Work from the authors' laboratory was supported by grants from the National Institutes of Health, the Wellcome Trust–Burroughs Wellcome Fund Infectious Disease Initiative, the Thrasher Research Fund and the Bill and Melinda Gates Foundation.
Glossary
- Agammaglobulinaemia
A disease state in which B lymphocytes fail to produce antibodies.
- Recall antigen
An antigen to which a host has previously been exposed.
- Lymphopaenia
A decrease in the number of circulating lymphocytes in the blood.
- Reproductive number
(Ro). The average number of secondary cases that occur when an infected person enters a completely susceptible population.
- Index case
The infected person who first introduces an infection into a population.
- Immunological immaturity
Developmental deficiencies in the immune responses of newborns and infants.
- Parenteral
The administration of a drug or vaccine other than through the intestine, usually by injection.
Biographies
William Moss is a paediatrician with subspecialty training in infectious diseases who has worked extensively in sub-Saharan Africa. His primary research interest is in understanding the impact of the HIV-1 epidemic on measles control and eradication. With Diane Griffin, he has collaborated with investigators in Zambia and the London School of Hygiene and Tropical Medicine to study the clinical, epidemiological and immunological interactions between measles virus and HIV-1, and the impact of HIV-1 infection on immune responses to measles vaccination. His work is now focused on understanding the impact of antiretroviral therapy on immune responses to measles vaccine.
Diane Griffin is a virologist and immunologist who trained in infectious diseases and whose primary research interest is in the pathogenesis of viral diseases. She earned her M.D. and Ph.D. at Stanford University and was a postdoctoral fellow at Johns Hopkins before joining the faculty. Her laboratory studies the pathogenesis of alphavirus encephalomyelitis in mice and measles in humans and monkeys. A rhesus macaque model of measles is being used to study immunosuppression, virus clearance and correlates of protective immunity with the goal of developing a new measles vaccine that can be administered to infants under the age of 6 months.
Related links
DATABASES
Entrez Genome
FURTHER INFORMATION
Competing interests
The authors declare no competing financial interests.
References
- 1.WHO. Progress in reducing measles mortality — worldwide 1999–2003. Wkly Epidemiol. Rec. 78–81 (2005).
- 2.McNeill WH. Plagues and Peoples. 1976. [PubMed] [Google Scholar]
- 3.Enders JF, Peebles TC. Propagation in tissue cultures of cytopathic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 1954;86:277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- 4.Enders JF, Katz SL, Milovanovic MV, Holloway A. Studies on an attenuated measles-virus vaccine. I. Development and preparations of the vaccine: technics for assay of effects of vaccination. N. Engl. J. Med. 1960;263:153–159. doi: 10.1056/NEJM196007282630401. [DOI] [PubMed] [Google Scholar]
- 5.Black FL, Yannet H. Inapparent measles after γ globulin administration. JAMA. 1960;173:1183–1188. doi: 10.1001/jama.1960.03020290009002. [DOI] [PubMed] [Google Scholar]
- 6.Good RA, Zak SJ. Disturbances in γ globulin synthesis as 'experiments of nature'. Pediatrics. 1956;18:109–149. [PubMed] [Google Scholar]
- 7.Permar SR, et al. Role of CD8+ lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J. Virol. 2003;77:4396–4400. doi: 10.1128/JVI.77.7.4396-4400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moss WJ, Ryon JJ, Monze M, Griffin DE. Differential regulation of interleukin (IL)-4, IL-5, and IL-10 during measles in Zambian children. J. Infect. Dis. 2002;186:879–887. doi: 10.1086/344230. [DOI] [PubMed] [Google Scholar]
- 9.Tamashiro VG, Perez HH, Griffin DE. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr. Infect. Dis. J. 1987;6:451–454. doi: 10.1097/00006454-198705000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Coovadia HM, et al. Alterations in immune responsiveness in acute measles and chronic post-measles chest disease. Int. Arch. Allergy Appl. Immunol. 1978;56:14–23. doi: 10.1159/000231998. [DOI] [PubMed] [Google Scholar]
- 11.Beckford AP, Kaschula RO, Stephen C. Factors associated with fatal cases of measles. A retrospective autopsy study. S. Afr. Med. J. 1985;68:858–863. [PubMed] [Google Scholar]
- 12.Greenberg BL, et al. Measles-associated diarrhea in hospitalized children in Lima, Peru: pathogenic agents and impact on growth. J. Infect. Dis. 1991;163:495–502. doi: 10.1093/infdis/163.3.495. [DOI] [PubMed] [Google Scholar]
- 13.Duke T, Mgone CS. Measles: not just another viral exanthem. Lancet. 2003;361:763–773. doi: 10.1016/S0140-6736(03)12661-X. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch RL, et al. Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin. Immunol. Immunopathol. 1984;31:1–12. doi: 10.1016/0090-1229(84)90184-3. [DOI] [PubMed] [Google Scholar]
- 15.Servet-Delprat C, et al. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J. Virol. 2000;74:4387–4393. doi: 10.1128/JVI.74.9.4387-4393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin DE, et al. Changes in plasma IgE levels during complicated and uncomplicated measles virus infections. J. Allergy Clin. Immunol. 1985;76:206–213. doi: 10.1016/0091-6749(85)90703-1. [DOI] [PubMed] [Google Scholar]
- 17.Griffin DE, Ward BJ. Differential CD4 T cell activation in measles. J. Infect. Dis. 1993;168:275–281. doi: 10.1093/infdis/168.2.275. [DOI] [PubMed] [Google Scholar]
- 18.Karp CL, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 19.Atabani SF, et al. Natural measles causes prolonged suppression of interleukin-12 production. J. Infect. Dis. 2001;184:1–9. doi: 10.1086/321009. [DOI] [PubMed] [Google Scholar]
- 20.Otten M, et al. Public-health impact of accelerated measles control in the WHO African Region 2000–03. Lancet. 2005;366:832–839. doi: 10.1016/S0140-6736(05)67216-9. [DOI] [PubMed] [Google Scholar]
- 21.WHO & United Nations Children's Fund. Measles Mortality Reduction and Regional Elimination Strategic Plan 2001–2005. (WHO, Geneva, 2001).
- 22.Centers for Disease Control and Prevention. Progress in reducing global measles deaths, 1999–2004. Morb. Mortal. Wkly Rep.55, 247–249 (2006). [PubMed]
- 23.Sencer DJ, Dull HB, Langmuir AD. Epidemiologic basis for eradication of measles in 1967. Public Health Rep. 1967;82:253–256. doi: 10.2307/4592985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orenstein WA, et al. Measles eradication: is it in our future? Am. J. Public Health. 2000;90:1521–1525. doi: 10.2105/AJPH.90.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Quadros CA. Can measles be eradicated globally? Bull. World Health Organ. 2004;82:134–138. [PMC free article] [PubMed] [Google Scholar]
- 26.Black FL. Measles endemicity in insular populations: critical community size and its evolutionary implication. J. Theor. Biol. 1966;11:207–211. doi: 10.1016/0022-5193(66)90161-5. [DOI] [PubMed] [Google Scholar]
- 27.Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J. Infect. Dis. 2003;187(Suppl. 1):S283–S290. doi: 10.1086/368040. [DOI] [PubMed] [Google Scholar]
- 28.Whittle HC, et al. Effect of subclinical infection on maintaining immunity against measles in vaccinated children in West Africa. Lancet. 1999;353:98–101. doi: 10.1016/S0140-6736(98)02364-2. [DOI] [PubMed] [Google Scholar]
- 29.Mossong J, et al. Modeling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am. J. Epidemiol. 1999;150:1238–1249. doi: 10.1093/oxfordjournals.aje.a009951. [DOI] [PubMed] [Google Scholar]
- 30.Vardas E, Kreis S. Isolation of measles virus from a naturally-immune asymptomatically re-infected individual. J. Clin. Virol. 1999;13:173–179. doi: 10.1016/S1386-6532(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 31.WHO. Strategies for reducing global measles mortality. Wkly Epidem. Rec.75, 411–416 (2000). [PubMed]
- 32.Kuhne M, Brown DW, Jin L. Genetic variability of measles virus in acute and persistent infections. Infect. Genet. Evol. 2006;6:269–276. doi: 10.1016/j.meegid.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 33.WHO. Update of the nomenclature for describing the genetic characteristics of wild-type measles viruses: new genotypes and reference strains. Wkly Epidem. Rec.78, 229–232 (2003). [PubMed]
- 34.Muwonge A, et al. New measles genotype, Uganda. Emerg. Infect. Dis. 2005;11:1522–1526. doi: 10.3201/eid1110.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rota PA, Bellini WJ. Update on the global distribution of genotypes of wild type measles viruses. J. Infect. Dis. 2003;187(Suppl. 1):S270–S276. doi: 10.1086/368042. [DOI] [PubMed] [Google Scholar]
- 36.Rota PA, Rota JS, Redd SB, Papania MJ, Bellini WJ. Genetic analysis of measles viruses isolated in the united states between 1989 and 2001: absence of an endemic genotype since 1994. J. Infect. Dis. 2004;189(Suppl. 1):S160–S164. doi: 10.1086/374607. [DOI] [PubMed] [Google Scholar]
- 37.Christensen PE, et al. An epidemic of measles in southern Greenland, 1951. Measles in virgin soil. II. The epidemic proper. Acta. Med. Scand. 1953;144:430–449. doi: 10.1111/j.0954-6820.1953.tb15717.x. [DOI] [PubMed] [Google Scholar]
- 38.Dossetor J, Whittle HC, Greenwood BM. Persistent measles infection in malnourished children. BMJ. 1977;1:1633–1635. doi: 10.1136/bmj.1.6077.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Permar SR, et al. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J. Infect. Dis. 2001;183:532–538. doi: 10.1086/318533. [DOI] [PubMed] [Google Scholar]
- 40.Kew OM, et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. World Health Organ. 2004;82:16–23. [PMC free article] [PubMed] [Google Scholar]
- 41.Moss WJ, Cutts F, Griffin DE. Implications of the human immunodeficiency virus epidemic for control and eradication of measles. Clin. Infect. Dis. 1999;29:106–112. doi: 10.1086/520136. [DOI] [PubMed] [Google Scholar]
- 42.Helfand RF, Moss WJ, Harpaz R, Scott S, Cutts F. Evaluating the impact of the HIV pandemic on measles control and elimination. Bull. World Health Organ. 2005;83:329–337. [PMC free article] [PubMed] [Google Scholar]
- 43.Biellik R, et al. First 5 years of measles elimination in southern Africa: 1996–2000. Lancet. 2002;359:1564–1568. doi: 10.1016/S0140-6736(02)08517-3. [DOI] [PubMed] [Google Scholar]
- 44.Ovsyannikova IG, Dhiman N, Jacobson RM, Vierkant RA, Poland GA. Frequency of measles virus-specific CD4+ and CD8+ T cells in subjects seronegative or highly seropositive for measles vaccine. Clin. Diagn. Lab. Immunol. 2003;10:411–416. doi: 10.1128/CDLI.10.3.411-416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ovsyannikova IG, Jacobson RM, Poland GA. Variation in vaccine response in normal populations. Pharmacogenomics. 2004;5:417–427. doi: 10.1517/14622416.5.4.417. [DOI] [PubMed] [Google Scholar]
- 46.Cutts F, Grabowsky M, Markowitz LE. The effect of dose and strain of live attenuated measles vaccines on serological responses in young infants. Biologicals. 1995;23:95–106. doi: 10.1016/1045-1056(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 47.Anders JF, Jacobson RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studies. Pediatr. Infect. Dis. J. 1996;15:62–66. doi: 10.1097/00006454-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Gans HA, et al. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA. 1998;280:527–532. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 49.Angel JB, et al. Vaccine-associated measles pneumonitis in an adult with AIDS. Ann. Intern. Med. 1998;129:104–106. doi: 10.7326/0003-4819-129-2-199807150-00007. [DOI] [PubMed] [Google Scholar]
- 50.Pan American Health Organization. Measles Eradication. Field Guide. (Pan American Health Organization, Washington DC, 1999).
- 51.Dayan GH, et al. Cost-effectiveness of three different vaccination strategies against measles in Zambian children. Vaccine. 2004;22:475–484. doi: 10.1016/j.vaccine.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Uzicanin A, Zhou F, Eggers R, Webb E, Strebel P. Economic analysis of the 1996–1997 mass measles immunization campaigns in South Africa. Vaccine. 2004;22:3419–3426. doi: 10.1016/j.vaccine.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 53.Tangermann RH, et al. Eradication of poliomyelitis in countries affected by conflict. Bull. World Health Organ. 2000;78:330–338. [PMC free article] [PubMed] [Google Scholar]
- 54.Connolly MA, et al. Communicable diseases in complex emergencies: impact and challenges. Lancet. 2004;364:1974–1983. doi: 10.1016/S0140-6736(04)17481-3. [DOI] [PubMed] [Google Scholar]
- 55.Katz SL. Polio — new challenges in 2006. J. Clin. Virol. 2006;36:163–165. doi: 10.1016/j.jcv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Feikin DR, et al. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA. 2000;284:3145–3150. doi: 10.1001/jama.284.24.3145. [DOI] [PubMed] [Google Scholar]
- 57.Wakefield AJ, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637–641. doi: 10.1016/S0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 58.Offit PA, Coffin SE. Communicating science to the public: MMR vaccine and autism. Vaccine. 2003;22:1–6. doi: 10.1016/S0264-410X(03)00532-2. [DOI] [PubMed] [Google Scholar]
- 59.Jansen VA, et al. Measles outbreaks in a population with declining vaccine uptake. Science. 2003;301:804. doi: 10.1126/science.1086726. [DOI] [PubMed] [Google Scholar]
- 60.DeStefano F, Thompson WW. MMR vaccine and autism: an update of the scientific evidence. Expert. Rev. Vaccines. 2004;3:19–22. doi: 10.1586/14760584.3.1.19. [DOI] [PubMed] [Google Scholar]
- 61.Madsen KM, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N. Engl. J. Med. 2002;347:1477–1482. doi: 10.1056/NEJMoa021134. [DOI] [PubMed] [Google Scholar]
- 62.Dilraj A, et al. Response to different measles vaccine strains given by aerosol and subcutaneous routes to schoolchildren: a randomized trial. Lancet. 2000;355:798–803. doi: 10.1016/S0140-6736(99)95140-1. [DOI] [PubMed] [Google Scholar]
- 63.Bennett JV, et al. Aerosolized measles and measles-rubella vaccines induce better measles antibody booster responses than injected vaccines: randomized trials in Mexican schoolchildren. Bull. World Health Organ. 2002;80:806–812. [PMC free article] [PubMed] [Google Scholar]
- 64.Wong-Chew RM, et al. Induction of cellular and humoral immunity after aerosol or subcutaneous administration of Edmonston-Zagreb measles vaccine as a primary dose to 12-month-old children. J. Infect. Dis. 2004;189:254–257. doi: 10.1086/380565. [DOI] [PubMed] [Google Scholar]
- 65.Polack FP, et al. Successful DNA immunization against measles: Neutralizing antibody against either the hemagglutinin or fusion glycoprotein protects rhesus macaques without evidence of atypical measles. Nature Med. 2000;6:776–781. doi: 10.1038/77506. [DOI] [PubMed] [Google Scholar]
- 66.Premenko-Lanier M, et al. DNA vaccination of infants in the presence of maternal antibody: a measles model in the primate. Virology. 2003;307:67–75. doi: 10.1016/S0042-6822(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 67.Premenko-Lanier M, Rota PA, Rhodes GH, Bellini WJ, McChesney MB. Protection against challenge with measles virus (MV) in infant macaques by an MV DNA vaccine administered in the presence of neutralizing antibody. J. Infect. Dis. 2004;189:2064–2071. doi: 10.1086/420792. [DOI] [PubMed] [Google Scholar]
- 68.Pan CH, et al. Inaugural article: modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles. Proc. Natl Acad. Sci. USA. 2005;102:11581–11588. doi: 10.1073/pnas.0504592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skiadopoulos MH, Surman SR, Riggs JM, Collins PL, Murphy BR. A chimeric human-bovine parainfluenza virus type 3 expressing measles virus hemagglutinin is attenuated for replication but is still immunogenic in rhesus monkeys. J. Virol. 2001;75:10498–10504. doi: 10.1128/JVI.75.21.10498-10504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pasetti MF, et al. Attenuated Salmonella enterica serovar Typhi and Shigella flexneri 2a strains mucosally deliver DNA vaccines encoding measles virus hemagglutinin, inducing specific immune responses and protection in cotton rats. J. Virol. 2003;77:5209–5217. doi: 10.1128/JVI.77.9.5209-5217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chabot S, et al. A novel intranasal Protollin-based measles vaccine induces mucosal and systemic neutralizing antibody responses and cell-mediated immunity in mice. Vaccine. 2005;23:1374–1383. doi: 10.1016/j.vaccine.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 72.Webster DE, Thomas MC, Huang Z, Wesselingh SL. The development of a plant-based vaccine for measles. Vaccine. 2005;23:1859–1865. doi: 10.1016/j.vaccine.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 73.Meissner H, Strebel PM, Orenstein WA. Measles vaccines and the potential for worldwide eradication of measles. Pediatrics. 2004;114:1065–1069. doi: 10.1542/peds.2004-0440. [DOI] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention. Measles eradication: Recommendations from a meeting cosponsored by the World Health Organization, the Pan American Health Organization, and the CDC. Morb. Mortal. Wkly Rep.46, 1–20 (1997). [PubMed]
- 75.Cutts FT, Henao-Restrepo AM, Olive JM. Measles elimination: progress and challenges. Vaccine. 1999;17:S47–S52. doi: 10.1016/S0264-410X(99)00309-6. [DOI] [PubMed] [Google Scholar]
- 76.Morgan OW. Following in the footsteps of smallpox: can we achieve the global eradication of measles? BMC Int. Health. Hum. Rights. 2004;4:1. doi: 10.1186/1472-698X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gounder C. The progress of the Polio Eradication Initiative: what prospects for eradicating measles? Health Policy Plan. 1998;13:212–33. doi: 10.1093/heapol/13.3.212. [DOI] [PubMed] [Google Scholar]
- 78.Cutts FT, Steinglass R. Should measles be eradicated? BMJ. 1998;316:765–767. doi: 10.1136/bmj.316.7133.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schimmer B, Ihekweazu C. Polio eradication and measles immunisation in Nigeria. Lancet Infect. Dis. 2006;6:63–65. doi: 10.1016/S1473-3099(06)70358-9. [DOI] [PubMed] [Google Scholar]
- 80.Malvoisin E, Wild TF. Measles virus glycoproteins: studies on the structure and interaction of the haemagglutinin and fusion proteins. J. Gen. Virol. 1993;74:2365–2372. doi: 10.1099/0022-1317-74-11-2365. [DOI] [PubMed] [Google Scholar]
- 81.Schneider U, von Messling V, Devaux P, Cattaneo R. Efficiency of measles virus entry and dissemination through different receptors. J. Virol. 2002;76:7460–7467. doi: 10.1128/JVI.76.15.7460-7467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andres O, Obojes K, Kim KS, ter Meulen V, Schneider-Schaulies J. CD46- and CD150-independent endothelial cell infection with wild-type measles viruses. J. Gen. Virol. 2003;84:1189–1197. doi: 10.1099/vir.0.18877-0. [DOI] [PubMed] [Google Scholar]
- 83.Spehner D, Drillien R, Howley PM. The assembly of the measles virus nucleoprotein into nucleocapsid-like particles is modulated by the phosphoprotein. Virology. 1997;232:260–268. doi: 10.1006/viro.1997.8568. [DOI] [PubMed] [Google Scholar]
- 84.Valsamakis A, et al. Recombinant measles viruses with mutations in the C, V, or F gene have altered growth phenotypes in vivo. J. Virol. 1998;72:7754–7761. doi: 10.1128/jvi.72.10.7754-7761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patterson JB, Thomas D, Lewicki H, Billeter MA, Oldstone MB. V and C proteins of measles virus function as virulence factors in vivo. Virology. 2000;267:80–89. doi: 10.1006/viro.1999.0118. [DOI] [PubMed] [Google Scholar]
- 86.Schneider-Schaulies S, ter Meulen V. Measles virus and immunomodulation: molecular bases and perspectives. Exp. Rev. Mol. Med. 2002;30:1–18. doi: 10.1017/S1462399402004696. [DOI] [PubMed] [Google Scholar]
- 87.Griffin DE, et al. Fields Virology. 2001. pp. 1401–1441. [Google Scholar]
- 88.Cutts FT. World Health Organization. 1993. Measles Module 7: The Immunological Basis for Immunization Series. [Google Scholar]


