Key Points
Translating knowledge of genetic disease mechanisms into gene therapies has been slow with limited clinical success. One major reason is that the transfer vectors, which are most often of viral origin, are not targeted sufficiently towards the cells of interest.
To achieve successful delivery of genetic material, transductional targeting is often essential to enter the target cell and to avoid side effects from the transduction of non-target cells.
Many techniques to target viral vectors to specific cells have been developed. They can be divided into three types: systems that use adaptor proteins from other viruses (pseudotyping); systems that use adaptors to couple the targeting ligand to the vector; and systems that genetically incorporate the targeting moiety into the viral genome.
Whereas systems involving adaptor proteins are highly useful in preclinical evaluations, systems that make use of genetically incorporated targeting ligands are advantageous for clinical applications.
Combinations of several targeting principles (including ablation of natural tropism, pseudotyping and adaptors) and novel combinations (such as the adeno-associated virus (AAV) genome in a phage vector) allow systemic vector application.
An initial clinical study with a targeted retrovirus showed feasibility to transfer laboratory success to patient application, underlining that there are no principal regulatory barriers for targeted vectors.
Systemic vector applications will be facilitated by enabling the vector to move beyond the vascular endothelium at specific sites, using transcytosis or cellular vehicles. The application of existing targeting techniques to new viral vector serotypes and new vector classes is extending the therapeutic capabilities further.
Obstacles to systemic application of vectors are found in the blood as immune reactions against the vector and as binding of blood proteins to the vector. Some targeting approaches might have the potential to circumvent these obstacles.
To preclinically evaluate new targeting strategies, several models that reflect the human situation to varying degrees are available. The use of primary cells, tissue-slice systems and transgenic animals seems to be especially promising.
Imaging technologies provide the ability to monitor the vector in vivo in real time without sacrificing the animal model. These techniques facilitate vector targeting and biodistribution studies.
A key challenge in gene therapy is vector targeting to specific cells, while avoiding effects on other tissues. Several strategies have been developed recently to enable targeting of the main viral vectors, moving them a step closer to clinical use.
Abstract
To achieve therapeutic success, transfer vehicles for gene therapy must be capable of transducing target cells while avoiding impact on non-target cells. Despite the high transduction efficiency of viral vectors, their tropism frequently does not match the therapeutic need. In the past, this lack of appropriate targeting allowed only partial exploitation of the great potential of gene therapy. Substantial progress in modifying viral vectors using diverse techniques now allows targeting to many cell types in vitro. Although important challenges remain for in vivo applications, the first clinical trials with targeted vectors have already begun to take place.
Main
Despite the remarkable preclinical success of gene therapy, its clinical applications remain limited1,2,3. Clinical trials have been crucial for highlighting the main challenges, one of which is the high cost of vector production. Another challenge relates to vector targeting: to achieve successful gene therapy, the appropriate genes must be delivered to and expressed in target cells, without harming non-target cells. One approach is to use promoters that are active only in the target cell (transcriptional targeting; for a review, see Ref. 4). Although this strategy can reduce or even eliminate potential toxic side effects of the transgene, it does not address the need to avoid those that result from the mislocalization of vector particles. Furthermore, transcriptional targeting alone is not sufficient to ensure gene expression in the target cell, which also requires efficient introduction of the therapeutic nucleic acid into the correct cells.
The development of technologies that allow targeting of specific cells has progressed substantially in recent years for several types of vectors, particularly viral vectors, which have been used in 70% of gene therapy clinical trials as of January 2007 (Ref. 5). Non-viral gene therapy, although promising, presents greater challenges with regard to gene-transfer efficiency (these approaches are discussed in Refs 6,7). This reflects the vast time that viruses have had to evolve naturally into efficient gene-transfer vehicles (Fig. 1). Building on this advantage, many groups are currently working towards improving the features of viral vectors for gene therapy purposes.
Figure 1. Native entry mechanisms of unmodified viral vectors.
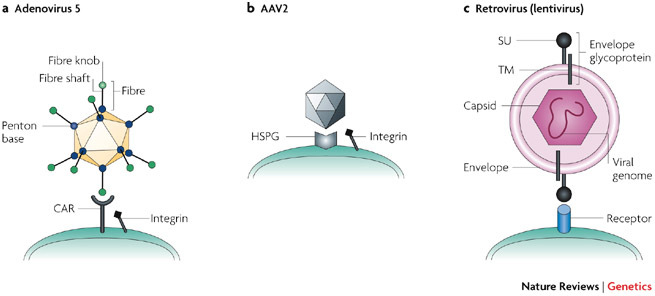
a | Adenovirus (Ad). Ad serotype 5 binds to its receptor CAR (coxsackie and adenovirus receptor) through its fibre knob. Subsequently, integrins interact with the RGD peptide motif in the penton base (the capsid protein at the base of the fibre) and facilitate cell entry by endocytosis138. b | Adeno-associated virus (AAV). Several basic residues of the AAV2 (adeno-associated virus serotype 2) capsid protein VP3 (especially positions R585 and R588) are involved in heparin binding. AAV2 first binds to heparan-sulphate proteoglycan (HSPG)139 and then to the co-receptor, which can be either an integrin (shown here), human fibroblast growth factor receptor or hepatocyte growth factor receptor. The virus is internalized by endocytosis. Other AAV serotypes either resemble AAV2 in its heparin binding (such as AAV3 and AAV6), or use different primary receptors (for example, sialic acid for AAV4 and AAV5)140. c | Retrovirus (lentivirus): Membrane fusion is the main mechanism whereby enveloped viruses deliver their genomes into target cells141. After initial nonspecific adhesion of the virus to the cell surface142, viral attachment glycoproteins bind specifically to their cognate receptors, whereupon binding becomes irreversible. The host range of retroviral vectors is determined by the interaction of the viral envelope protein (Env) and the cellular receptor143. Subsequent steps in the viral entry process vary between different viruses but always result in fusion between the lipid membranes of the virus and the host cell, following which the viral nucleocapsid is released into the cytoplasm144. In some cases, receptor binding triggers conformational changes in the viral proteins that mediate membrane fusion. In others, the cell-bound virus is transported by its receptor into an endosomal compartment where a reduction in pH triggers a conformational rearrangement of the viral fusion machinery. SU, surface subunit; TM, transmembrane subunit.
One major technical challenge in facilitating the efficient infection of the correct cells by viral vectors — known as transductional targeting — is that the native tropism of the virus often does not meet the therapeutic need. To avoid toxic side effects, the natural tropism of the vector must often be ablated or diminished. The vector might also need to be engineered to infect target cells that it does not infect naturally. If target cells are easily isolated from the patient (for example, from the blood or bone marrow) and re-transferred, ex vivo gene transfer might be ideal, and broadening of the vector tropism might be advantageous. Alternatively, a local application might be sufficient (for example, for a locally restricted tumour or a small organ such as the eye) and, depending on the possible side effects of gene transfer to local non-target cells, broadening or narrowing the tropism might be required. Finally, systemic treatment might be necessary, for example, to reach disseminated metastases or a large number of somatic cells to correct a genetic defect. In this situation, the tropism must be narrowed down to the target cells only.
Here we discuss new approaches aimed at improving viral vector targeting, focusing mainly on systemic targeting, which promises ease of application and great therapeutic returns. There are several obstacles that need to be overcome in order for a systematically applied vector to reach its target cells (Box 1). The challenge that has been most widely studied, and on which we focus here, is the final step of infecting the target cell. To accomplish this, the vector must display a suitable ligand to bind a target-cell receptor. The natural tropism of some viruses matches their vector utility, as is the case for the herpes virus, which can be used for neuronal gene delivery8, but in many cases the vector must be engineered to have a new tropism. This final obstacle to targeting has received the most attention from researchers, mainly owing to the fact that its study has been easier because of the availablity of in vitro models (Box 2), unlike the other obstacles that are discussed in Box 1.
Most progress in vector development has been achieved using adenovirus (Ad), adeno-associated virus (AAV) and vectors that are derived from retroviruses, particularly lentiviruses (Table 1). The technical hurdles that must be overcome in developing effective therapeutic systems are similar for most viral vector systems; so, methods to enhance one vector system often have general relevance. Here we discuss the main approaches that have been applied to vector targeting, and outline the important challenges that need to be addressed in order for gene therapy using viral vectors to reach widespread clinical application.
Table 1.
Key features of viral vectors
| Feature | Adenoviral vector | Helper-dependent adenoviral vector | AAV vector | Retroviral vector | Lentiviral vector |
|---|---|---|---|---|---|
| Particle size (nm) | 70–100 | 70–100 | 20–25 | 100 | 100 |
| Cloning capacity (kb) | 8–10 | ∼30 | 4.9 (10 after heterodimerization of two AAV virions) | 8 | 9 |
| Chromosomal integration | No | No | No (yes if rep gene is included) | Yes | Yes |
| Vector yield (transducing units/ml) | High (1012) | High (1012) | High (1012) | Moderate (1010) | Moderate (1010) |
| Entry mechanism | Receptor (CAR)-mediated endocytosis, endosomal escape and microtubule transport to the nucleus | Receptor-mediated endocytosis, endosomal escape and transport to the nucleus | Receptor binding, conformational change of Env, membrane fusion, internalization, uncoating, nuclear entry of reverse-transcribed DNA | ||
| Transgene expression and practical application | Weeks to months; highly efficient short-term expression (e.g. for cancer or in acute cardiovascular diseases) | >1 year; highly efficient medium- to long-term expression | >1 year; medium- to long-term gene expression for non-acute diseases (onset of transgene expression after ∼3 weeks) | Long-term correction of genetic defects | |
| Oncolytic potential? | Yes | No | No | No (but has potential to spread through the tumour without lysis, thereby spreading a suicide gene that encodes a pro-drug-converting enzyme) | |
| Emergence of replication-competent vector in vivo? | Possible but not a major concern | Negligible, low risk | Possible but not a major concern | Risk is a concern | Risk is a concern |
| Infects quiescent cells? | Yes | Yes | Yes | No | Yes |
| Transcriptional targeting affected by chromosomal integration site? | No | No | No | Yes | Yes |
| Risk of oncogene activation by the vector? | No | No | No | Yes | Yes |
| AAV, adeno-associated virus; CAR, coxsackie and adenovirus receptor; Env, viral envelope protein. | |||||
Vector targeting by pseudotyping
Pseudotyping, which was the first method used to alter viral vector tropism, involves transferring viral attachment proteins either between strains within a family of viruses or between virus families (Fig. 2; Table 2). Pseudotyping can be achieved by co-transfection of plasmids, with one encoding the attachment protein to be pseudotyped and separate plasmids encoding all other vector components. This approach is used routinely to pseudotype AAV and retroviral or lentiviral vectors. Alternatively, the viral attachment protein can be expressed in trans from the production cell line, or genetically incorporated into the viral genome, an approach that is particularly well suited to the generation of adenovirus pseudotypes.
Figure 2. Targeting options for viral vectors.
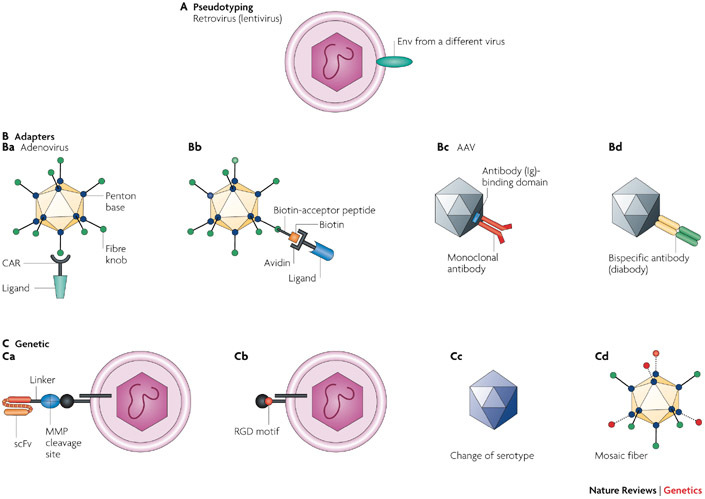
Many targeting modalities have been implemented for all three vector types discussed in this Review. The targeting techniques are illustrated for only one viral attachment protein in most of the panels. A |Pseudotyping. A retroviral (lentiviral) vector is pseudotyped with an envelope protein (Env) from a different virus9. B |Adaptors. In part Ba, an adenoviral vector is coupled with a receptor–ligand fusion; in this example, the ectodomain of the adenoviral receptor is fused to a ligand that is expressed on a target cell type (for example, CD40L, the ligand for the CD40 receptor on dendritic cells)22. In part Bb, a biotin-acceptor peptide is integrated into the fibre knob, biotinylated and coupled to an avidin-containing ligand21. In part Bc, an antibody-binding domain is genetically incorporated into the adeno-associated virus (AAV) capsid to couple a monoclonal antibody to the vector43. In part Bd, a bispecific antibody is attached to the AAV capsid145. C | Genetic incorporation of a targeting ligand. In part Ca, a single-chain antibody (single-chain variable fragment (scFv) against human carcinoembryonic antigen (CEA)) and a matrix metalloprotease (MMP) cleavage site are coupled to the viral envelope protein (Env). This allows binding to tumour cells that express CEA, followed by cleavage of the MMP cleavage site by tumour-secreted MMP48. The vector can also be targeted to tumour cells by incorporating a tumour-specific scFv directly into Env. However, these insertions can perturb infection if the targeted receptor does not support the required post-binding steps towards viral entry. The MMP cleavage site allows release of the scFv before fusion with the target cell. In part Cb, incorporation of a small targeting ligand (for example, an RGD peptide) can be used to target a vector to integrin receptors73. In part Cc, the serotype is changed to achieve desired targeting140. In part Cd, the use of different fibres in the same vector allows multifunctionality in a mosaic fibre virus93. CAR, coxsackie and adenovirus receptor.
Table 2.
Targeting systems
| Approach | Principle | Advantages | Disadvantages | Examples |
|---|---|---|---|---|
| Pseudotyping | ||||
| Approach overview | Use of a viral attachment protein from a different virus strain or family | Technically easy when the biology is supportive or compatible | Limited availability of pseudotypes that fit the desired target cell; possible reduction of transfection efficiency (retrovirus) | Ad (in vitro)18 (in vivo)120; AAV (in vivo)138; Lentivirus (in vivo)30 |
| Adaptor systems | ||||
| Approach overview | Use of a molecule that binds both the vector and target-cell receptor to facilitate transduction | Limited knowledge of capsid structure is sufficient; flexibility; no/minimal change in vector structure; easy preclinical testing of different targeting ligands | Two-component system; stoichiometry of adaptor to vector might vary between batches; two molecules must be produced separately; issues with regulatory agencies; adaptor might dissociate in vivo; clinical applicability can be limited | |
| Receptor–ligand | A native viral receptor is fused to the targeting ligand | Easy preclinical testing | Correct folding of each new receptor–ligand pair must be determined | Ad (in vivo)22; retrovirus (in vitro)24 |
| Bispecific antibody | Two antibodies are coupled, with the resulting molecule having specificity for the vector and the target | Using existing reagents, the antibody is easy to make; screening for different targets is readily possible | Binding affinity of the targeting complex to the vector can vary | Ad (in vivo)139; AAV (in vitro)145; coronavirus (in vitro)148 |
| Chemical linkage | Targeting moiety is bound to the vector by chemical means | A covalent bond is formed with the targeting complex, thus no adaptor dissociation from the vector | Technically more demanding than other adaptor systems (but nevertheless scaleable for clinical applications) | Ad (in vitro)29,31 |
| Avidin–biotin | Biotin is coupled to the vector and then bound to the avidin–ligand complex | High-affinity binding of the targeting complex to the vector; allows easy vector purification | Some risk for toxicity in clinical applications (biotin from the circulation could be complexed) | Ad (in vitro)38; AAV (in vitro)39; retrovirus (in vitro)34,35 |
| Antibody | Antibody binds to a genetically incorporated Ig-binding domain of the vector | Vast pool of available antibodies for targeting; easy coupling | Antibodies from the circulation could interfere with targeting | Ad (in vitro)44; AAV (in vitro)43; retrovirus (in vitro)45 |
| Genetic systems | ||||
| Approach overview | A polypeptide is incorporated into the vector by genetic means to facilitate transduction | Single-component system; favoured for clinical application; ease of high-titre vector production | Technically more challenging than adaptor approaches; can be detrimental to vector or ligand structure | |
| Serotype switching | Use of a different serotype from within the same virus family | Biological compatibility makes it feasible | Limited availability of serotypes; the precise cellular receptor is frequently unknown | AAV (in vivo)140; Ad (in vivo)149 |
| Small targeting motifs | Small peptides are inserted into the capsid or viral attachment protein | Minimal disturbance of vector structure | Broadens tropism without ablating native tropism; limited number of available motifs, thus not applicable for all cell types | Ad (in vivo)76; AAV (in vitro)70; retrovirus (in vitro)73; phage–AAV (in vivo)20 |
| Single-chain antibody | A single-chain antibody is incorporated into the viral attachment protein | Vast pool of tested antibodies available for targeting | Antibody might need adaptation to a biosynthetic pathway of virus protein production (Ad) | Ad (in vivo)19; AAV (in vitro)47; retrovirus (in vivo)48 |
| Mosaic viral attachment proteins | Two viral attachment proteins with different properties are combined, allowing targeting, production or imaging in parallel | True multifunctionality in a virion can be achieved | Desired stoichiometry can be difficult to achieve | Ad (in vitro)93; AAV (in vitro)70 |
| Ablation of native tropism | Mutation of the amino acids responsible for native tropism | Can be combined with other techniques | Can confound production in packaging cell line | Ad (in vivo)88; AAV (in vivo)87; Lentivirus (in vivo)13 |
| AAV, adeno-associated virus; Ad, adenovirus. | ||||
Pseudotyping of enveloped vectors. Pseudotyping has been used most extensively to modulate the host-cell tropisms of retroviral (including lentiviral) vectors because they are highly permissive for incorporation of heterologous attachment glycoproteins9,10. The most widely used retroviral vector pseudotypes are those that incorporate the attachment glycoprotein of the vesicular stomatitis virus (VSV-G)11, which both allows the production of high-titre vector stocks and confers a broad host range. The list of other foreign envelope glycoproteins that have been incorporated into lentiviral vectors is long, including representatives from several virus families. These pseudotypes show large differences in their relative transduction efficiencies for different tissues. Notable in vivo findings include the high efficiencies of neural tissue transduction by lyssavirus pseudotypes (the rabies and Mokola viruses)9 and efficient transduction of airway epithelium by filovirus (Ebola Zaire)9 or paramyxovirus (Sendai) pseudotypes12. If lentiviral gene transfer gains broader acceptance as a clinically viable vector system, these vectors could be used to explore gene therapies for Parkinson disease and cystic fibrosis, respectively.
Unfortunately, the specificities of naturally occurring viral attachment proteins frequently do not coincide with those that are required for targeted gene delivery. In response to this, several encouraging studies have demonstrated the feasibility of pseudotyping retroviral vectors with chimeric envelope glycoproteins from Sindbis viruses and gammaretroviruses that have been genetically engineered to incorporate polypeptide ligands that direct targeting to specific cell types9,13,14 (see later for a detailed discussion of the genetic incorporation of targeting ligands into viruses). As for many retroviral targeting approaches, however, accuracy often comes at the price of low gene-transfer efficiency via the targeted receptors, and optimizing the efficiency of targeted gene transfer will be needed to justify clinical testing.
The concept of pseudotyping has recently been extended to the incorporation of host-cell viral receptors (CD4 and CXCR4 or CCR5) into viral envelopes for targeted entry into HIV-infected cells. Feasibility was demonstrated using a replication-competent rhabdovirus (VSV) and non-replicating lentiviral or murine leukaemia virus (MLV) vectors to mediate the targeted destruction of HIV-infected cells by redirecting them to use the HIV-derived glycoprotein HIVgp120 as a receptor15,16,17. However, the efficiency of targeted entry into HIV-infected cells was low, for reasons that are not understood.
Pseudotyping of non-enveloped vectors. Pseudotyping has also been used for non-enveloped vectors, including AAV and adenovirus. An important challenge here is that the viral attachment protein must be incorporated into a protein capsid instead of a lipid bilayer. This has mainly been achieved by substituting coat proteins with homologous proteins of other related serotypes, giving rise to a new tropism without changing the rest of the genome and thus enabling the use of established cloning systems that have been developed for the previous serotype. It is also possible to incorporate the coat proteins of unrelated viruses, although structural incompatibility can preclude this. For example, on the basis of the structural similarities of the trimeric Ad fibre and the trimeric reovirus attachment protein σ1, a chimeric fibre–σ1 protein was introduced into the Ad capsid by modifying the Ad genome, enabling efficient infection of primary dendritic cells and intestinal epithelial cells18. In cases in which structural incompatibilities hinder incorporation of the desired part of the foreign viral attachment protein, artificial fibre molecules can be used. For example, these have been exploited for fusing single-chain variable fragment (scFv) antibodies to the Ad vector19.
Combining prokaryotic and eukaryotic vectors. Current eukaryotic viral vectors can infect cells with high efficiency, but they have the disadvantage that their native tropism must be ablated to achieve ligand-directed targeting upon systemic administration. Prokaryotic viruses infect mammalian cells with a low efficiency at best, but can be adapted to bind mammalian receptors by genetically engineering a eukaryotic ligand into their capsid. For example, one study reported the construction of a hybrid vector that comprises an AAV cassette inserted in the phage genome and that targets αv integrins through an Arg-Gly-Asp (RGD-4C) peptide motif that is displayed on the phage capsid20. Following systemic application in nude mice, this vector showed specific targeting to tumours derived from injection of human prostate cancer cells, and tumour shrinkage was achieved when a therapeutic transgene was introduced into the vector. Monitoring the biodistribution of the vectors in this study was facilitated using in vivo vector imaging (Box 3). Remarkably, the chimeric vector was also highly effective for anti-tumour therapy in the context of immunocompetent mice, even following prior phage vaccination and high anti-phage antibody titres. A similar strategy could be used for other vectors with double-stranded genomes, such as adenoviruses.
Vector targeting using adaptors
Pseudotyping is limited by the number of viral attachment proteins for which receptors are expressed exclusively and abundantly on target cells of interest. Genetic modifications that can overcome this limitation require structural knowledge to guide modification of the viral attachment protein — knowledge that is only just becoming available. The use of adaptor proteins, which can be applied even with a limited knowledge of the viral structure, has been explored as an alternative. Adaptors are molecules with dual specificities: one end binds the viral attachment protein and the other binds the receptor on the target cell. The advantages of this approach are its great flexibility, as different adaptors can readily be coupled to the same vector, and the fact that it does not require changes in vector structure that could be detrimental to vector production or gene transfer. Most adaptors can achieve the two main goals of targeted delivery: ablating native tropism and conferring a novel tropism towards the desired target. Adaptor systems have proved particularly useful for proof-of-principle preclinical studies, allowing easy testing of several target receptors21.
Receptor–ligand complexes. Receptor–ligand complexes are an important class of adaptors that exploit native viral tropisms and are widely used for retargeting vectors. The viral receptor is genetically fused to the ligand of a receptor that is expressed on the target cell. For example, fusing the ectodomain of the adenovirus receptor (coxsackie and adenovirus receptor; CAR) with CD40L (the ligand for the CD40 receptor on dendritic cells) through a trimerization motif successfully targeted Ad vectors to dendritic cells with more than four orders of magnitude higher efficiency than untargeted Ad vectors22 (Fig. 2Ba). Fusing the ectodomain of CAR to a single-chain antibody against human carcinoembryonic antigen (CEA) allowed vector targeting to subcutaneous tumours as well as hepatic metastases of colon cancer in nude mice, while simultaneously ablating liver tropism23.
The same principle has been applied for retroviral vectors. A fusion of the extracellular domain of the avian sarcoma and leukaemia virus (ASLV) retroviral receptor (tumour virus subgroup A receptor; TVA) to heregulin-β1 successfully targeted the vector to cells expressing heregulin receptors24, providing a potential therapeutic strategy for the treatment of various malignancies. Adaptors that incorporate the ASLV receptor as the virus-binding moiety have been explored in particular, as they can trigger conformational changes in the ASLV envelope glycoprotein that are required for membrane fusion and virus entry.
In another important example, this type of targeting has been achieved for one of the coronaviruses (a class of enveloped RNA viruses), which are potential oncolytic agents. The tropism of a replication-competent coronavirus, mouse hepatitis virus (MHV), was recently retargeted by engineering the viral genome to express an adaptor protein that consisted of part of the natural cellular receptor of MHV and a targeting peptide, allowing multiround infection and killing of target cells25.
Altogether, the receptor–ligand approach shows promise for use in a range of preclinical studies. However, for clinical applications, other targeting methods (such as genetic targeting, discussed below) might be preferable because of the potential risk that the adaptor could dissociate from the vector.
Chemical conjugation. Chemical conjugation is a method for coupling adaptors to vectors in which the targeting ligand is covalently linked to the vector. Polyethylene glycol (PEG) and PEG-derived polymers have been used to couple Ad vectors to ligands such as fibroblast growth factor 2, which was used to target ovarian cancer cells26. Endothelial cells have also been targeted using this approach, by coupling the Ad vector to PEG and then coupling the Ad–PEG complex to an RGD peptide or E-selectin-antibody27. Importantly, PEGylation has the potential to shield the vector from the innate immune system in vivo28, and it allows infection in the presence of Ad antibodies29, which might enable repeated vector application. Whereas PEGylation can impede cell transduction in vitro, this effect does not seem to occur in vivo, possibly owing to different hydrodynamic conditions28. So far, targeting by PEGylation has been used for AAV and Ad vectors, but could potentially be extended to enveloped vectors, for which a PEGylation strategy has been recently developed30.
A promising extension of the chemical conjugation approach was recently introduced in a study that combined the flexibility of adaptor systems with the advantage of the stable covalent bonds that are provided by genetic targeting (see below)31. Highly reactive thiol groups were introduced into the Ad capsid by genetically inserting cysteines at solvent-exposed positions. The thiol groups were then coupled to transferrin, which mediated the successful targeting of the vector to cells expressing the receptor for this protein. Because the thiol groups are at the tip of the fibre, and thus distant from the surface amino groups that are used for PEGylation, both systems can be used simultaneously. This novel thiol-group coupling system allows not only the convenient introduction of full-length proteins such as transferrin, but also receptor ligands that cannot be introduced by genetic methods, such as sugars, fatty acids and small molecules. This approach has the potential to be extended to other vector systems, possibly with the exception of enveloped vectors: these vectors lack the necessary capsid sites for modification and, in addition, coupling to the Env protein (retroviral or lentiviral viral envelope protein) might be deleterious to its function and the chemical steps involved might disrupt the viral envelope.
Adaptor systems using avidin and biotin. The high-affinity binding between avidin and biotin has been used in many biotechnological applications32,33, and gene therapists have taken advantage of this to provide an adaptor strategy that has been exploited for several viral vectors and forms a valuable basis for targeting studies.
One of the first studies to demonstrate the feasibility of this approach for an enveloped virus was reported as long ago as 1989 (Ref. 34). An ecotropic retrovirus was crosslinked to human major histocompatibility complex class I (MHCI) in an adaptor strategy in which the virus was coated with a biotinylated anti-envelope antibody, then with streptavidin, and finally with a biotinylated anti-MHC antibody to redirect its attachment. Although entry via class I MHC was convincingly demonstrated in vitro, the efficiency of this approach was low, for reasons that were not fully determined35.
Biotinylation strategies have also been developed for Ad vectors. A biotin-acceptor peptide (BAP) has been cloned into the fibre capsid protein to produce the Ad–BAP fusion. The BAP is biotinylated during vector production in mammalian cells and can therefore be coupled to a biotinylated targeting ligand via tetrameric avidin (Fig. 2Bb). Compared with coating the vector with biotinylated antibody, this strategy makes use of a more robust covalent attachment of biotin to the vector. This approach has been used to screen for potential targeting ligands21, to purify adenoviral vectors36 and to evaluate Ad capsid proteins for targeting37. In terms of potential clinical applications, the avidin–biotin system has been used to target Ad vectors to dendritic cells in vitro using monoclonal antibodies or high-affinity binding peptides as ligands for the targeted cell receptor38. The high-affinity binding of avidin to biotin (10−15) qualifies this system for in vivo applications, including therapeutic ones. Possible toxicity from high levels of avidin — which can complex biotin in the circulation — could be a concern; however, we consider this risk to be low, because only vector-bound avidin is expected to be introduced into the patient.
AAV vectors can be biotinylated in a similar way to Ad vectors, and this system is now being used as a platform for purification and targeting of this vector type39. A different biotinylation approach has recently been taken with vaccinia viral vectors and has proved successful for in vitro targeting. The virus was chemically biotinylated, followed by the addition of avidin and subsequent incubation with a biotinylated antibody, which allowed targeting to MHCI- and B7.2-transfected tumour cells40. The avidin–biotin system seems to be suitable for any vector type that allows incorporation of a BAP, or that can be chemically biotinylated — techniques that should be universally applicable — and is expected to be utilized in many more applications in the future.
Monoclonal antibodies as adaptors. Antibodies have been used as targeting tools in various applications41,42, and many researchers are working on ways to capitalize on the diversity and wide availability of these reagents. Vectors have been genetically modified to allow coupling of monoclonal antibodies; for example, a region of a bacterial immunoglobulin (Ig)-binding protein (usually the Z-domain of the staphylococcus protein A) can be inserted into the viral attachment proteins of various vector systems14,43,44,45. In this way, the unmodified antibody can work like an adaptor, bridging the Ig-binding domain that is incorporated in the vector to the target receptor through its antibody specificity (Fig. 2Bc). This approach has been successfully used in vitro and in SCID (severe combined immunodeficiency) mice13. However, a significant limitation of this approach is that polyclonal Igs in the bloodstream might compete to displace the monoclonal antibody from the virally displayed Ig-binding protein before it reaches its target site. The approach has not yet been advanced to clinical testing and might be better suited to ex vivo gene therapy applications that require transduction of specific target cells in a mixed cell population (for example, stem cells in bone marrow).
Challenges facing adaptor systems. Most of the adaptor systems described above have disadvantages that detract from their potential use for gene therapy. One disadvantage relates to the potentially suboptimal stability of the vector–adaptor complex, especially in vivo, which might result from unforeseen interactions with factors that perturb the non-covalent binding. Systems that rely on either the BAP, with their strong binding of the targeting complex, or on chemical conjugation are the least likely to be affected by this. In addition, difficulties can arise in terms of scaling up adaptor protein production, and the coupling efficiency might vary between different batches. Finally, regulatory agencies favour single-component systems and consider the adaptor and the virus to be separate drugs. One perceived problem is that the crosslinker might elute from the surface of the virus in vivo; the adaptor approach is also considered more cumbersome for clinical applications.
None of the adaptor approaches has been tested extensively in vivo, which has limited our understanding of their potential utility. Systems based on the BAP have great flexibility and are expected to be widely applied, including potentially in patients. The other systems are likely to have their greatest potential in preclinical proof-of-principle studies.
Genetic incorporation of targeting ligands
To avoid the potential complexities of adaptor systems, researchers have investigated methods for the genetic incorporation of targeting ligands into viral vectors. Genetic fusion of these ligands into the capsid or the envelope protein yields a single virion molecule that recognizes the target cell. Despite being more technically challenging than the use of adaptors, such single-component systems provide homogenous retargeted vector particles, unlike adaptor-based approaches. As well as overcoming the regulatory issues of two-component systems, this approach facilitates high-titre production by eliminating the need to create a separate adaptor molecule.
Systems involving polypeptide ligands. Several promising single-component systems involve the genetic incorporation of polypeptide ligands into viral surface proteins, giving vectors new and highly specific tropisms for cells expressing the target antigen. This approach was pioneered in 1993 with the display of a single-chain antibody on the surface of an enveloped virus46. In this case, an anti-hapten antibody was genetically fused near the N-terminus of the MLV surface (SU) component of the envelope glycoprotein, and retroviral vectors incorporating the chimeric protein were shown to bind to hapten via the displayed antibody.
The single-chain antibody approach has been applied to several vectors — AAV47, adenovirus19, retrovirus48 (Fig. 2Ca), measles virus49 and herpes simplex virus50 — underlining the versatility of this approach. Many other complex polypeptide ligands, including growth factors and cytokines, have since been displayed on various gammaretroviral envelope glycoproteins, as either N-terminal fusions, insertions into the proline-rich hinge region or substitutions for the N-terminal protein domains51. Similar engineering has been attempted for other enveloped viruses: ligands have been fused proximal to the N-termini of herpesvirus proteins gC and gD52, influenza haemagglutinin53,54 and the VSV-G protein55, and a ligand has been inserted within the N-terminal receptor-binding domain of the E2 attachment protein of Sindbis virus14.
Despite the great potential of this approach in terms of specificity, one limitation to applying it more generally is that the introduction of large proteins can be deleterious to the structure of the viral protein into which they are inserted, or can impede the correct folding of the incorporated polypeptide. Incorporation of a single-chain antibody fusion into an Ad vector was initially impeded because of the different biosynthetic pathways that are used to produce the scFv (which is synthesized in the rough endoplasmic reticulum (ER), facilitating formation of disulphide bridges) and the Ad capsid proteins (which are synthesized in the cytosol, interfering with the formation of these bridges)56. In addition, incorporation of such large proteins into the Ad fibre can impede proper folding (trimerization) of the fibre and hence viral rescue. The use of cytosolically stabilized scFvs (intrabodies) and the generation of an artificial fibre allowed genetic coupling of the fibre and scFv in the Ad system19. The artificial fibre has the added advantage of ablating the native tropism of Ad. One challenge in using this system relates to identifying scFvs that will fold correctly in the cytosol, requiring expertise in scFv technology and complex fibre modifications. By contrast, the glycoproteins of enveloped vectors are routed through the ER, which supports the folding and post-translational modification of complex proteins fused to the envelope57,58. Thus, polypeptide ligands with multiple disulphide bonds, stringent glycosylation requirements or oligomeric structures can be more readily displayed on enveloped viruses than on non-enveloped viruses.
Targeted virus attachment does not necessarily lead to targeted entry. In the case of retroviral envelope glycoproteins, displayed targeting ligands usually impede infectivity because the normal functions of the viral attachment protein are altered. This can lead to direction of the virus into a non-functional entry pathway, steric blocking of its natural receptor interactions or prevention of conformational changes that are required for effective fusion triggering51. These problems led to the concept of inverse targeting, whereby the viral envelope glycoprotein is modified to selectively destroy its infectivity for cells expressing a targeted receptor59. Thus, amphotropic retroviral vectors that display epidermal growth factor (EGF), stem-cell factor (SCF) or insulin-like growth factor 1 (IGF1) are selectively non-infectious for cells that express the cognate receptors (EGFR, KIT or IGFR), but remain fully infectious for other (receptor-negative) human cells59,60,61. This inverse targeting provides a useful way to detarget the liver (EGFR positive) or marrow stem cells (KIT positive), and can be used to ameliorate specific vector toxicities against these targets, although the number of potential applications is limited.
Protease targeting is another concept that has emerged from early unsuccessful efforts to reprogramme retrovirus entry. Here virus infectivity is engineered to depend on the proteolytic maturation of a viral surface protein. This can be achieved through cleavage of a protease-susceptible linker that tethers an infectivity-blocking polypeptide to the viral surface. Another approach is cleavage of an engineered junctional sequence between the SU and transmembrane (TM) components of a retroviral envelope glycoprotein, or between the F1 and F2 components of a measles attachment glycoprotein62. In this way, the vector is targeted to cells that are bathed in the appropriate protease. Examples include the targeting of protease-rich tumours63 by vectors with an infectivity that is selectively activated by matrix metalloproteinases (MMPs) (Fig. 2) or by plasmin. Other potential protease-rich targets include the sites of new blood vessel formation in diabetic retinopathy and in the pannus tissue of joints affected by rheumatoid arthritis, as well as sites of acute and chronic inflammation.
Several animal studies have demonstrated the feasibility of in vivo transductional targeting using retroviral and lentiviral vectors with genetically incorporated polypeptide ligands. In one study, a retroviral vector that was activated by MMP and displayed a melanoma-targeting single-chain antibody was shown to target gene delivery to MMP-rich melanoma xenografts64. In another study, the liver was successfully detargeted by pseudotyping lentiviral vectors with an amphotropic MLV envelope glycoprotein that displayed EGF as an N-terminal fusion65. The reduced hepatic transduction in this case resulted from inverse targeting, which was possible because EGF receptors are expressed abundantly on hepatocytes.
A third example is represented by the first and so far only targeted vector that has been tested in the clinic: the retroviral vector Rexin-G, which expresses a cytocidal dominant-negative form of cyclin G1. This vector displays the collagen-binding portion of von Willebrand factor (vWF), which is required for the adhesion of platelets to sites of injured endothelium66, incorporated in its Env protein. Exploiting this targeting mechanism allows preferential vector delivery to the tumour site where angiogenesis and collagen matrix exposure occur (tumour neovessels)67. Rexin-G is targeted to the extracellular matrix of tumour tissue68 and has been tested for its anti-tumour activities in three clinical studies69. Several cases of partial responses and stable disease demonstrated the effectiveness of the vector.
The limitations that are encountered during retroviral or lentiviral vector targeting using engineered polypeptide ligands do not necessarily apply for other enveloped viruses. In contrast to retroviruses, the attachment and fusion functions of the measles virus are encoded on separate proteins, making it easier to manipulate binding specificity without negatively impacting the efficiency of fusion triggering (see below). However, measles is still the only virus that can be efficiently retargeted through a wide range of cellular receptors using a variety of cell-targeting polypeptides without significant reductions in its entry efficiency.
Small-peptide motifs. Small-peptide motifs are less likely to perturb the structure of the viral attachment protein, which permits their insertion at various regions of the protein. Despite their small size (generally 3–20 amino acids), they can change the targeting characteristics of a vector dramatically.
Small peptides containing an RGD motif, which targets vectors to integrins, have most often been used for this purpose, facilitating diverse applications that include targeting of the vasculature and of tumour cells. Such targeting has been achieved for AAV (in vitro)70, adenovirus (ex vivo in tissue-slice assays71, and in vivo)72, retroviral vectors (in vitro)73 (Fig. 2Cb), and for a phage–AAV hybrid vector (in vivo)20. Another useful small-peptide targeting moiety is the poly-lysine (pK7) peptide that targets vectors to heparan sulphates, which are overexpressed in a number of malignancies74 and other pathologies. Adenoviral vectors carrying pK7 in their fibre knob showed an increased transduction of various CAR-deficient targets, such as skeletal muscle in vivo75. In addition, RGD and pK7 have recently been used together in the Ad capsid to improve the efficiency of vector delivery (and hence survival) in a murine model of cancer76. Generally, both of these modifications broaden the vector tropism, which makes them especially useful for local administrations. An RGD-modified conditionally replicating Ad77 is soon to be used clinically for local applications in ovarian carcinoma at the University of Alabama at Birmingham, USA, following the recent completion of animal safety tests78.
Although small-peptide motifs are versatile and can be used to target viral vectors to several cell types, other cell types cannot be targeted in this way and require different targeting approaches.
Library-selection approaches. As described above, genetic targeting approaches are limited by the size, and sometimes the structure, of the ligand that can be incorporated without compromising assembly, stability or infectivity of the vector. It is also crucial that the displayed ligand maintains its bridging ability towards the target after fusion to the viral attachment protein. These considerations have driven library-selection approaches, which display the ligand in the context of the viral attachment protein and then select for the desired increase in infectivity by binding to a column that displays the targeting receptor79, by repeated cycles of binding to target cells80,81, or by serial passage through the target cells82.
In one example, the H and I sheets of the Ad fibre knob were inserted into the pIII protein of a bacteriophage. A random peptide library was introduced between the Ad knob sheets and selected against the target cells. This resulted in identification of a peptide that improved Ad transduction of mouse muscle cells 14-fold compared with unmodified Ad81. A different approach was used for library screening with AAV79. First, the AAV capsid protein CAP was subjected to PCR-based mutagenesis and recombination, followed by insertion into an AAV packaging plasmid and creation of an AAV library. This library was then screened for desired properties, for example, using heparin affinity chromatography to select for low or high heparin affinity, or incubation with neutralizing serum to select for mutants that evaded antibody responses. Library selection for changes in retroviral vector tropism has also been carried out. For example, in one study82, feline leukemia virus (FeLV) envelope glycoproteins were randomized in the cell-targeting region by oligonucleotide insertions83,84 and subjected to transduction-based selection strategies in cancer cells. The resulting FeLV vector was able to transduce prostate cancer cell lines, but required the presence of a murine retrovirus (4070A) helper envelope glycoprotein to facilitate virus entry. Although this example demonstrates the feasibility of selecting vectors with new tropisms from retroviral libraries, the selection strategy cannot be focused on a specific known receptor; it can only be focused on a particular cell type. However, in cases in which no targeting ligands are known, such library-selection approaches can provide one way forward, and proof of principle for this approach has been established for targeting all the major vector classes79,81,85,86.
Ablation of native vector tropism. For systemic applications, the native vector tropism of gene therapy vectors might need to be ablated to avoid the transduction of non-target tissue. In some instances, the addition of the targeting ligand reduces the native tropism sufficiently. For example, the incorporation of peptides that target human venous endothelial cells into AAV capsids resulted in significantly lower hepatocyte transduction, but greatly increased venous-cell transduction87. However, there are other cases in which additional steps must be taken to ablate the native tropism of the vector.
For example, for the Ad serotype 5 vector, which is the most commonly used Ad vector, detargeting is of central importance to enable successful systemic treatments that avoid effects on the liver; the addition of a targeting moiety alone is frequently insufficient. Many fibre mutants have been generated in attempts to achieve liver detargeting. The most dramatic effect has been seen for Ad fibres with deletions in a putative heparan-sulphate proteoglycan-binding motif that resulted in a 15-fold decrease in liver transduction and a 1,000-fold decrease when combined with a CAR-ablating mutation. However, the effects of detargeting mutations seem to be highly dependent on the strain and species of rodent88, highlighting the importance of using multiple and complementary preclinical model systems.
Examples for tropism ablation regarding retroviral vectors are discussed above in the context of inverse targeting, and an example for a lentiviral vector is discussed in Box 3. Generally, each vector requires specific changes in the viral attachment protein to ablate its native tropism. These modifications can then be used in a wide range of applications, with the exception of reverse targeting used for enveloped vectors, which is specific for a particular cell type.
An alternative to the above approaches is the use of a vector with no tropism in the target organism. When using a prokaryotic vector that normally does not infect eukaryotic cells, but can be modified with an RGD motif, systemic application becomes feasible and can lead to tumour-specific transduction, as shown for an AAV–phage hybrid20.
Outlook and future directions
Cell-type-specific targeting in vivo by gene therapy vectors is a milestone that has only recently been realized. For some applications, direct translation of that achievement to the clinic might be possible; for example, local application of genetically modified vectors with a broadened tropism or systemic administration of vectors with specific targeting and ablated native tropism could see application in a few years. Other therapeutic interventions will require further research to achieve the desired targeting specificity, which should be facilitated by several recent developments. For example, single-component systems (such as Ad with an artificial fibre that is genetically fused to a scFv) are easy to prepare in high titres and are not expected to face problems for clinical approval. By contrast, a lentiviral vector coupled to an adaptor might face more problems during large-scale preparation and in regard to the general safety issues of lentiviral vectors. As we have seen, in many cases the challenges to developing a successful targeting vector relate to the biology of the individual vector type.
Despite the vast range of experimental studies that have been carried out with targeted vectors, transition to a clinical setting has been slow. This delay is related to general problems in the field of gene therapy that concern high costs of vector production for clinical use and acquisition of financial support for this production. Several rounds of clinical trials will be needed to optimize a particular gene therapy approach, as is the case for other therapies (for example, monoclonal antibodies), which needed several trials to find their way into clinical practice. The important aspect of the clinical studies that have begun, and are beginning now, is that targeted vectors can gain regulatory (Food and Drug Administration (FDA)) approval in the USA69. With most western nations being geared to FDA procedures, this approval represents an encouraging sign for targeted gene therapy protocols worldwide.
Although this Review has focused largely on the final step of targeting — transduction of the target cell — other aspects are equally important, especially for systemic vector administration. We have briefly discussed other potential obstacles, such as the immune system (antibodies, complement system) or other blood factors that impact the vector in the blood stream, and ways to escape these problems that are currently being investigated (Box 1). The exit of vectors from the blood stream into the target tissue will also be an important step to address. Ensuring that vectors move beyond the vascular endothelial layer after systemic application, for example, by transcytosis, will be of crucial importance to achieve clinical success in many instances (such as disseminated metastases).
Strategies to enable transcytosis have recently moved to the forefront of vector targeting research. Whereas transcytosis for a lentivirus, HIV, has been described89, this area is only just beginning to be explored for viruses that form the basis of the other types of gene therapy vectors. In the context of AAV, three serotypes out of five that were tested showed transcytosis; this ability was both serotype and cell-type specific90. Serotype switching, which can be achieved by transfection of plasmids with different capsid backbones, can therefore be a valuable tool when transcytosis of AAV is required. Coupling the Ad vector with an adaptor that targets the transferrin-receptor pathway has enabled transcytosis, albeit at low efficiency91. Exploitation of other transcytosis pathways92 and elucidation of the natural transcytosis pathways of some viruses might allow for rational design of transcytosing vectors. Furthermore, the generation of vectors that are mosaic for their viral attachment protein (having two genetically distinct versions)93 could prove valuable (Fig. 2Cd). One attachment protein could carry a ligand for transcytosis, whereas the other could carry the ligand for transduction of the target cell. Alternatively, the transcytosis ligand could be placed onto other capsid proteins (for example, pIX in the case of Ad), while having the transduction ligand on all viral attachment proteins.
Besides improving established vector systems, new viruses are also being developed for targeted gene therapy. One promising example is the measles virus, an enveloped virus that has recently been retargeted to tumour cells to exploit its oncolytic potential. In contrast to retroviruses, because the attachment and fusion functions of the measles virus are encoded on separate proteins, it is easier to manipulate binding specificity without negatively impacting the efficiency of cell entry. Several large polypeptide ligands have been displayed on the surface of the measles virus as extensions of the viral attachment glycoprotein94. In most cases, the ligand-displaying measles viruses have been able to enter cells efficiently via the targeted receptor95,96. Mutations that are known to ablate the natural measles tropisms for CD46 and SLAM (signalling lymphocytic activation molecule) were subsequently incorporated into the chimeric viral attachment proteins, thereby generating fully retargeted measles viruses with entirely new receptor specificities49. Several fully retargeted measles viruses have been shown to mediate targeted in vivo destruction of receptor-positive tumours97,98. In this way, new gene-transfer vehicles can be created, combining established targeting principles (such as ablation of native tropism and targeting through single-chain antibodies) with the natural abilities of different vectors, thus continuously expanding the gene therapist's toolbox.
The concepts of vector targeting that are described in this Review are now recognized throughout the gene therapy field. In regard to non-viral vectors, the efficiencies of gene transfer after systemic delivery are low unless vectors are administered under high pressure (for example, hydrodynamic delivery in mice, which selectively transduces the liver). Non-viral vectors, which are essentially DNA-containing nanoparticles, can be retargeted by incorporating ligands into their lipid or protein shells, but to date there are no convincing studies to demonstrate the in vivo utility of retargeted non-viral vectors. However, once their efficiencies approach those of viral vectors, the targeting principles that are outlined in this Review are also likely to be applicable to non-viral vectors.
Box 1 | Obstacles to systemic targeting.
Many of the obstacles to systemic delivery have been studied most thoroughly for adenovirus (Ad), and still need to be addressed in other systems in terms of their impact and how they might be overcome. However, the areas discussed below are likely to be relevant to all vectors.
The first potential hurdles for a vector are found in the form of the immune system and other factors in the blood circulatory system. Reactions with the complement system99,100 and pre-existing antibodies101,102 (either naturally occurring or from previous vector applications) can impede the vector in reaching its target. Coating Ad vectors with polyethylene glycol (PEG) can help to escape both the antibody-mediated and innate immune responses, but should be combined with targeting as this modification can otherwise reduce the efficiency of gene transfer29,103. Blood factors (for example, coagulation factor IX and complement protein C4BP) can bind the adenoviral fibre and redirect the virus from the bloodstream into the liver via uptake through heparan-sulphate proteoglycans and LDL-receptor-related protein104,105. Furthermore, Ad can interact with human blood cells (erythrocytes, neutrophils and monocytes), which can prevent the vector from reaching its target106. Serotype switching and modifications of capsid proteins may circumvent such interactions.
The next hurdle is the endothelial cell layer: the vector must exit the bloodstream at the right tissue, move beyond the endothelial cell lining, and transduce the target cells. Some vascular beds are accessible for vector exit (for example, Ad can exit into the liver)107, and some vectors naturally possess mechanisms to allow transcytosis beyond the endothelium (for example, some AAV subtypes90 and HIV108 can cross certain monolayers). However, in most cases the vascular bed presents a barrier and the vector is unable to cross the endothelium109, necessitating vector modification. Engineering the ability to undergo transcytosis is one potential mechanism to achieve this transition; another method involves the use of cellular vehicles (such as stem cells) to carry the vector and home in to the target tissue110,111. Cellular vehicles might be especially helpful if one needs to overcome an extracellular matrix that separates the target cells (for example, tumour cells) from the endothelium112. After entering the correct target tissue, the final step for the vector is to infect the target cell (Fig. 1), as discussed in detail in the main text.
Additional obstacles can exist when tumours are targeted. Despite the fact that lesions are accessible on systemic treatment in some experimental models23, such models do not fully recapitulate a clinical scenario. Frequently, tumour-cell islets can be surrounded by a basal-membrane-like structure within stromal cells. In one animal model, blood vessels were observed to be in direct contact with only the stromal cells113. The vector would need to cross the stroma and the basal-membrane-like structure to reach the tumour. It might be possible to overcome these obstacles using stem cells carrying the vector or by first targeting the surrounding area of the tumour to weaken stroma- and basal-membrane-like structures (for example, with matrix metalloproteinases) and then targeting the tumour. However, targeting of the tumour environment might be sufficient for a therapeutic effect, at least in some cases114.
Box 2 | Model systems for evaluating vector targeting.
Cell-culture systems. The first step in evaluating the targeting capabilities of a vector is usually testing in cell lines. However, this has limited predictive value for in vivo scenarios (for example, cell lines can overexpress viral receptors relative to more clinically relevant primary cells115). Two-dimensional cell-culture systems are also limited in their predictive value as they usually represent only one or two cell types, and are grown on an artificial surface in an artificial two-dimensional context. As a first step towards three-dimensional culture, primary-tumour spheroids have been used to evaluate targeted Ad vectors. The vectors that were tested were replication competent in tumour cells and showed gradual penetration of the spheroids116. However, these spheroids were almost exclusively composed of tumour cells whereas, within a naturally occurring tumour, therapeutic effects can be modified by the presence of stromal cells and the extracellular matrix.
Another drawback of two-dimensional culture systems for assessing anti-cancer gene therapy mediated by viral vectors is that possible toxic effects on stromal cells cannot be evaluated. Three-dimensional cell-culture models can include endothelial cells, fibroblasts, immunocompetent cells and extracellular matrix. Although they are currently expensive, three-dimensional scaffolds provide a promising system to emulate the native structure of living tissue117 and could become a valuable tool for vector testing.
Tissue explants. The next step up in sophistication for model systems is the use of tissue explants. For example, tissue-slice systems can be used to evaluate targeting to any tissue. Although such systems do not directly resemble systemic administration, valuable data concerning the transduction of target and non-target tissues can be easily obtained. These systems have been available for different applications for some time, but their implementation in targeting studies is recent. In one example, this approach was used to analyse targeted Ad vector transduction of breast tumour and liver cells71. Another tissue explant model is represented by the human skin substrate system. Plastic surgery frequently yields skin that can be used ex vivo to evaluate vector targeting. This model has recently proved its potential usefulness for gene therapy assessment in the context of an Ad vector targeted to dendritic cells118.
Animal models. Although their circulatory system is comparable to humans, animal models have limitations with respect to evaluating transductional targeting. For example, they do not express human receptors and, in the case of xenotransplantation models in cancer research, they lack a complete immune system. New immunocompetent transgenic mouse models that express human receptors are being developed for the evaluation of vector targeting. For example, transgenic mice have been generated that express human CD46 in an expression pattern that is similar to that in humans119; CD46 is the receptor for several viruses, and this mouse model has been used to evaluate a pseudotyped Ad5 vector120.
In some cases, ectopic transgenic expression of the human receptor in a mouse model will suffice, and relevant mouse models promise to reduce the effort and costs compared with those needed to generate a transgenic model for each target. Such a system was recently developed and used for the evaluation of targeted adenoviral vectors121,122,123.
Box 3 | Imaging technologies for evaluating vector targeting.
Until recently, testing the biodistribution of targeted vectors required sacrificing animals to evaluate transgene expression or viral particle concentration in various organs124,125. Several clinical trials have shown a lack of useful end points that would allow evaluation of vector targeting126,127. Approaches that allow imaging of viral vector distribution in vivo have the advantage that animals do not need to be sacrificed for analysis, enabling multiple real-time measurements of vector distribution. Vectors with novel modalities that allow in vivo imaging are now being developed. Imaging moieties are generally either expressed in the form of a reporter transgene from the viral genome or attached to the vector by genetic fusion to a capsid protein.
Gene-based imaging can make use of radioactive systems, such as the vector-mediated gene transfer of herpes simplex thymidine kinase, which can phosphorylate radiolabelled nucleoside analogues. Quantification is carried out using positron emission tomography (PET) imaging128, which is applicable in clinical settings. Alternatively, light-emitting systems can be used, including those using luciferase and green or red fluorescent proteins129,130.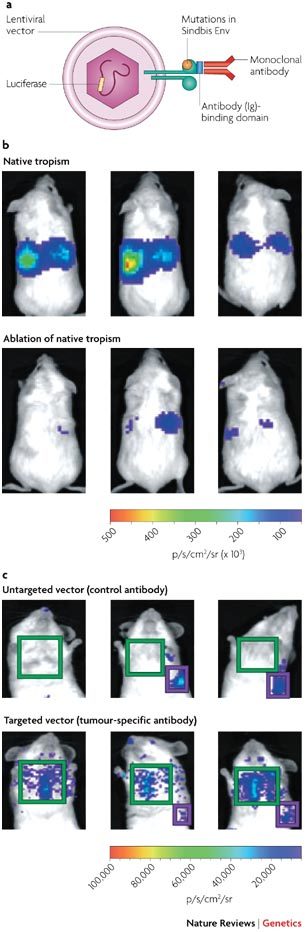
A recent example illustrates how imaging can greatly facilitate the evaluation of targeting approaches. A lentiviral vector that was pseudotyped with the envelope protein (Env) of the Sindbis virus was successfully retargeted to metastatic melanoma in vivo13. To achieve this, the Sindbis Env was mutated to ablate liver or spleen tropism, and the ZZ domain (Ig-binding domain) of protein A was genetically fused to this protein. This allowed coupling to a monoclonal antibody that targeted P-glycoprotein-expressing melanoma cells (see part a in the figure). Biodistribution was monitored through imaging, which was facilitated by vector-mediated luciferase expression. Part b in the figure shows ablation of the native tropism of the vector. SCID (severe combined immunodeficiency) mice were injected systemically with a lentivirus that was pseudotyped with a Sindbis Env protein that contained an antibody-binding domain. The upper panel shows strong liver and spleen tropism owing to domains within the Sindbis Env protein that target the vector to these tissues. Mutating these domains largely ablated this natural tropism (shown in the lower panel). Light emission is measured in photons (p) s −1 cm−2 steridian (sr)−1. In part c, mice were injected first with tumour cells that form lung metastases, and 12 days later with the vector. Use of a nonspecific control antibody did not lead to tumour-cell transduction (shown in the upper panel), whereas use of a tumour-cell-specific antibody leads to transduction of tumour cells in the lung (lower panel). This study shows how combining several targeting techniques (pseudotyping, ablation of native tropism and adaptor coupling) can lead to truly targeted gene transfer after systemic application, and how imaging is essential for the analysis.
For Ad vectors, it has recently become possible to incorporate imaging ligands into the capsid. Green and red fluorescent proteins131,132,133, herpes simplex virus thymidine kinase (HSVtk)134 and an HSVtk–luciferase fusion protein135 have been fused to the capsid protein pIX, allowing multi-modality imaging. In all cases, the imaging ligands retained their activity. Because of the localization of the imaging signal, capsid labelling seems to be especially promising in the context of tracking the intracellular fate of vectors, which would not be possible otherwise, and for observing the spread of targeted oncolytic vectors.
Another approach exploits magnetic resonance imaging (MRI), which has been used in a gene therapy context136 but only recently became applicable for direct vector imaging. Räty et al. used MRI-based imaging of an avidin-coated baculovirus that was conjugated with biotinylated superparamagnetic iron oxide particles137. This technique should be easily applicable to other capsid-coated viruses. The spatial resolution of MRI is in the millimetre (typical for medical MRI) to micrometre (typical for research MRI) range, which is much greater than for the other imaging methods (which generally have a resolution of several millimetres) and which will allow assignment of vector location to individual cells. However, MRI is considerably less sensitive than radioactive or light-based imaging systems. Parts b and c reproduced with permission from Nature Medicine Ref. 13 © (2005) Macmillan Publishers Ltd.
Acknowledgements
This work was supported with a grant by the German Research Foundation (DFG) to R.W., with grants from the US National Institutes of Health (NIH) to S.J.R. and grants from the NIH and the US Department of Defense (DOD) to D.T.C. The authors wish to thank J. Roth, J. T. Douglas, S. J. Hedley, S. Ponnazhagan, Q. L. Matthews, M. Everts and J. N. Glasgow for their critical reading of the manuscript.
Glossary
- Tropism
Affinity of a virus or vector for a particular tissue and cell type.
- Pseudotyping
Changing the tropism of a virus by replacing the viral attachment protein with that of a related virus.
- Viral attachment protein
A protein that is part of the virus or vector and can bind the cellular receptor.
- Vascular bed
An intricate network of minute blood vessels that extends through the tissues of the body or one of its parts.
- Gammaretroviruses
Simple viruses that are characterized by a C-type morphology; this genus has the most members in the retroviruses family, including murine leukaemia viruses, feline leukaemia viruses and the gibbon ape leukaemia virus.
- Serotypes
Antigenically distinct forms of microorganisms, including viruses, that elicit different antibody responses by the immune system.
- Adenoviral fibre
The attachment protein of an adenoviral vector.
- Primary-tumour spheroids
A three-dimensional aggregate of purified and unpassaged cancer cells.
- Oncolytic
An agent that induces lysis of tumour cells.
- B7.2
B7.2 (CD86) is a co-stimulatory transmembrane protein in the B7 family. It is found on antigen presenting cells (APCs) and interacts with receptors on T cells.
- Amphotropic
Describes a pathogen such as a virus or a bacteria that has a wide host range and can infect more than one species or cell-culture line.
- Complement system
A protein system in the blood that forms a defense against pathogens.
- Transcytosis
Transport of macromolecules (including pathogens) across a cell, which consists of the endocytosis of a macromolecule at one side of a monolayer and its exocytosis at the other side.
Biographies
Reinhard Waehler earned a M.S. in biochemistry at the University of Hanover, Germany, and a Ph.D. in natural sciences from the University of Hamburg, Germany. His research has focused on cytokine-mediated cancer gene therapy and is now devoted to cancer vaccination through targeted gene therapy. He began working in 2005 as a postdoctoral researcher at the University of Alabama at Birmingham, USA, in David T. Curiel's laboratory.
Stephen J. Russell is currently professor of medicine and the Richard O. Jacobson Professor of Molecular Medicine at the Mayo Foundation in Rochester, USA. He obtained his Ph.D. from the University of London, UK, and received specialist accreditation in haematology from the Royal College of Pathologists in London. He has held research positions in the UK and USA. His research interests focus on developing injectable gene therapy vectors for the treatment of disseminated malignancies (particularly multiple myeloma) and demonstrating their efficacy in clinical trials. In 1998, he took the position of Director of the Molecular Medicine Program at the Mayo Clinic.
David T. Curiel heads the Gene Therapy Center (GTC) of the University of Alabama at Birmingham. In 1982, he graduated medical school at Emory University, Atlanta, USA, where he also completed his internship and residency in internal medicine. His scientific training includes tenureship at the National Institutes of Health in Bethesda, USA, at the Pulmonary Branch of the National Heart, Lung, and Blood Institute (NHLBI) from 1985–1989, and a fellowship in biotechnology at the National Cancer Institute, Navy Medical Oncology Branch from 1989–1990. Curiel has been at the University of Alabama at Birmingham since 1993. He received his Ph.D. in 2002 from the University of Groningen in the Netherlands.
In addition to his role as Director of the Gene Therapy Center, he is Director of the Division of Human Gene Therapy which is affiliated with the Departments of Medicine, Pathology, Surgery and Obstetrics and Gynecology at the University of Alabama at Birmingham, and the holder of the Jeanne and Ann Griffin chair of women's cancer research.
Curiel's current research interest relates to the development of vector systems for the achievement of targeted, cell-specific gene delivery.
Related links
DATABASES
OMIM
FURTHER INFORMATION
American Society of Gene Therapy
European Society of Gene and Cell Therapy
Gene and Virus Therapy Program, Mayo Clinic
Gene Therapy Clinical Trials Worldwide web site
The Gene Therapy Center, University of Alabama at Birmingham
Competing interests
The authors declare no competing financial interests.
References
- 1.Aghi M, Martuza RL. Oncolytic viral therapies — the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 2.Rocconi RP, et al. Targeted gene therapy for ovarian cancer. Curr. Gene Ther. 2005;5:643–653. doi: 10.2174/156652305774964668. [DOI] [PubMed] [Google Scholar]
- 3.Zeimet AG, Marth C. Why did p53 gene therapy fail in ovarian cancer? Lancet Oncol. 2003;4:415–422. doi: 10.1016/S1470-2045(03)01139-2. [DOI] [PubMed] [Google Scholar]
- 4.Sadeghi H, Hitt MM. Transcriptionally targeted adenovirus vectors. Curr. Gene Ther. 2005;5:411–427. doi: 10.2174/1566523054546189. [DOI] [PubMed] [Google Scholar]
- 5.Edelstein ML, Abedi MR, Wixon J, Edelstein RM. Gene therapy clinical trials worldwide 1989–2004 — an overview. J. Gene Med. 2004;6:597–602. doi: 10.1002/jgm.619. [DOI] [PubMed] [Google Scholar]
- 6.Boulaiz H, Marchal JA, Prados J, Melguizo C, Aranega A. Non-viral and viral vectors for gene therapy. Cell Mol. Biol. (Noisy-le-grand) 2005;51:3–22. [PubMed] [Google Scholar]
- 7.Rolland A. Nuclear gene delivery: the Trojan horse approach. Expert Opin. Drug Deliv. 2006;3:1–10. doi: 10.1517/17425247.3.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Burton EA, Fink DJ, Glorioso JC. Replication-defective genomic HSV gene therapy vectors: design, production and CNS applications. Curr. Opin. Mol. Ther. 2005;7:326–336. [PubMed] [Google Scholar]
- 9.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnierle BS, et al. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc. Natl Acad. Sci. USA. 1997;94:8640–8465. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zavada J. VSV pseudotype particles with the coat of avian myeloblastosis virus. Nature New Biol. 1972;240:122–124. doi: 10.1038/newbio240122a0. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi M, Iida A, Ueda Y, Hasegawa M. Pseudotyped lentivirus vectors derived from simian immunodeficiency virus SIVagm with envelope glycoproteins from paramyxovirus. J. Virol. 2003;77:2607–2614. doi: 10.1128/JVI.77.4.2607-2614.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morizono K, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nature Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 14.Ohno K, Sawai K, Iijima Y, Levin B, Meruelo D. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nature Biotechnol. 1997;15:763–767. doi: 10.1038/nbt0897-763. [DOI] [PubMed] [Google Scholar]
- 15.Schnell MJ, Johnson JE, Buonocore L, Rose JK. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell. 1997;90:849–857. doi: 10.1016/S0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 16.Endres MJ, et al. Targeting of HIV- and SIV-infected cells by CD4-chemokine receptor pseudotypes. Science. 1997;278:1462–1464. doi: 10.1126/science.278.5342.1462. [DOI] [PubMed] [Google Scholar]
- 17.Somia NV, Miyoshi H, Schmitt MJ, Verma IM. Retroviral vector targeting to human immunodeficiency virus type 1-infected cells by receptor pseudotyping. J. Virol. 2000;74:4420–4424. doi: 10.1128/JVI.74.9.4420-4424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercier GT, et al. A chimeric adenovirus vector encoding reovirus attachment protein σ1 targets cells expressing junctional adhesion molecule 1. Proc. Natl Acad. Sci. USA. 2004;101:6188–6193. doi: 10.1073/pnas.0400542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedley SJ, et al. An adenovirus vector with a chimeric fiber incorporating stabilized single chain antibody achieves targeted gene delivery. Gene Ther. 2006;13:88–94. doi: 10.1038/sj.gt.3302603. [DOI] [PubMed] [Google Scholar]
- 20.Hajitou A, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006;125:385–398. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Parrott MB, et al. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol. Ther. 2003;8:688–700. doi: 10.1016/S1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 22.Pereboev AV, et al. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol. Ther. 2004;9:712–720. doi: 10.1016/j.ymthe.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Li, H.-J. et al. Adenovirus tumor targeting and hepatic untargeting by a CAR ectodomain-antiCEA bi-specific adapter. Cancer Res.67, 5354–5361. [DOI] [PubMed]
- 24.Snitkovsky S, Young JA. Targeting retroviral vector infection to cells that express heregulin receptors using a TVA-heregulin bridge protein. Virology. 2002;292:150–155. doi: 10.1006/viro.2001.1314. [DOI] [PubMed] [Google Scholar]
- 25.Verheije MH, et al. Redirecting coronavirus to a nonnative receptor through a virus-encoded targeting adapter. J. Virol. 2006;80:1250–1260. doi: 10.1128/JVI.80.3.1250-1260.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanciotti J, et al. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol. Ther. 2003;8:99–107. doi: 10.1016/S1525-0016(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 27.Ogawara K, et al. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum. Gene Ther. 2004;15:433–443. doi: 10.1089/10430340460745766. [DOI] [PubMed] [Google Scholar]
- 28.Mok H, Palmer DJ, Ng P, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Eto Y, et al. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J. Gene Med. 2005;7:604–612. doi: 10.1002/jgm.699. [DOI] [PubMed] [Google Scholar]
- 30.Croyle MA, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J. Virol. 2004;78:912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreppel F, Gackowski J, Schmidt E, Kochanek S. Combined genetic and chemical capsid modifications enable flexible and efficient de- and retargeting of adenovirus vectors. Mol. Ther. 2005;12:107–117. doi: 10.1016/j.ymthe.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Diamandis EP, Christopoulos TK. The biotin-(strept)avidin system: principles and applications in biotechnology. Clin. Chem. 1991;37:625–636. [PubMed] [Google Scholar]
- 33.Laitinen OH, Nordlund HR, Hytonen VP, Kulomaa MS. Brave new (strept)avidins in biotechnology. Trends Biotechnol. 2007;25:269–277. doi: 10.1016/j.tibtech.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Roux P, Jeanteur P, Piechaczyk M. A versatile and potentially general approach to the targeting of specific cell types by retroviruses: application to the infection of human cells by means of major histocompatibility complex class I and class II antigens by mouse ecotropic murine leukemia virus-derived viruses. Proc. Natl Acad. Sci. USA. 1989;86:9079–9083. doi: 10.1073/pnas.86.23.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etienne-Julan M, Roux P, Carillo S, Jeanteur P, Piechaczyk M. The efficiency of cell targeting by recombinant retroviruses depends on the nature of the receptor and the composition of the artificial cell-virus linker. J. Gen. Virol. 1992;73:3251–3255. doi: 10.1099/0022-1317-73-12-3251. [DOI] [PubMed] [Google Scholar]
- 36.Campos SK, Parrott MB, Barry MA. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol. Ther. 2004;9:942–954. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campos SK, Barry MA. Comparison of adenovirus fiber, protein IX, and hexon capsomeres as scaffolds for vector purification and cell targeting. Virology. 2006;349:453–462. doi: 10.1016/j.virol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 38.Maguire CA, et al. Recombinant adenovirus type 5 vectors that target DC-SIGN, ChemR23 and αvβ3 integrin efficiently transduce human dendritic cells and enhance presentation of vectored antigens. Vaccine. 2006;24:671–682. doi: 10.1016/j.vaccine.2005.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold GS, Sasser AK, Stachler MD, Bartlett JS. Metabolic biotinylation provides a unique platform for the purification and targeting of multiple AAV vector serotypes. Mol. Ther. 2006;14:97–106. doi: 10.1016/j.ymthe.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Purow B, Staveley-O'Carroll K. Targeting of vaccinia virus using biotin–avidin viral coating and biotinylated antibodies. J. Surg. Res. 2005;123:49–54. doi: 10.1016/j.jss.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Hohlfeld R, Wekerle H. Drug Insight: using monoclonal antibodies to treat multiple sclerosis. Nature Clin. Pract. Neurol. 2005;1:34–44. doi: 10.1038/ncpneuro0016. [DOI] [PubMed] [Google Scholar]
- 42.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nature Rev. Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 43.Gigout L, et al. Altering AAV tropism with mosaic viral capsids. Mol. Ther. 2005;11:856–865. doi: 10.1016/j.ymthe.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Korokhov N, et al. Targeting of adenovirus via genetic modification of the viral capsid combined with a protein bridge. J. Virol. 2003;77:12931–12940. doi: 10.1128/JVI.77.24.12931-12940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tai CK, et al. Antibody-mediated targeting of replication-competent retroviral vectors. Hum. Gene Ther. 2003;14:789–802. doi: 10.1089/104303403765255174. [DOI] [PubMed] [Google Scholar]
- 46.Russell SJ, Hawkins RE, Winter G. Retroviral vectors displaying functional antibody fragments. Nucleic Acids Res. 1993;21:1081–1085. doi: 10.1093/nar/21.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Q, et al. Development of novel cell surface CD34-targeted recombinant adenoassociated virus vectors for gene therapy. Hum. Gene Ther. 1998;9:1929–1937. doi: 10.1089/hum.1998.9.13-1929. [DOI] [PubMed] [Google Scholar]
- 48.Chowdhury S, Chester KA, Bridgewater J, Collins MK, Martin F. Efficient retroviral vector targeting of carcinoembryonic antigen-positive tumors. Mol. Ther. 2004;9:85–92. doi: 10.1016/j.ymthe.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura T, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nature Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 50.Menotti L, Cerretani A, Campadelli-Fiume G. A herpes simplex virus recombinant that exhibits a single-chain antibody to HER2/neu enters cells through the mammary tumor receptor, independently of the gD receptors. J. Virol. 2006;80:5531–5539. doi: 10.1128/JVI.02725-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larochelle A, Peng KW, Russell SJ. Lentiviral vector targeting. Curr. Top. Microbiol. Immunol. 2002;261:143–163. doi: 10.1007/978-3-642-56114-6_7. [DOI] [PubMed] [Google Scholar]
- 52.Zhou G, Roizman B. Characterization of a recombinant herpes simplex virus 1 designed to enter cells via the IL13Rα2 receptor of malignant glioma cells. J. Virol. 2005;79:5272–5277. doi: 10.1128/JVI.79.9.5272-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hatziioannou T, Valsesia-Wittmann S, Russell SJ, Cosset FL. Incorporation of fowl plague virus hemagglutinin into murine leukemia virus particles and analysis of the infectivity of the pseudotyped retroviruses. J. Virol. 1998;72:5313–5317. doi: 10.1128/jvi.72.6.5313-5317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verhoeyen E, et al. Novel lentiviral vectors displaying 'early-acting cytokines' selectively promote survival and transduction of NOD/SCID repopulating human hematopoietic stem cells. Blood. 2005;106:3386–3395. doi: 10.1182/blood-2004-12-4736. [DOI] [PubMed] [Google Scholar]
- 55.Dreja H, Piechaczyk M. The effects of N-terminal insertion into VSV-G of an scFv peptide. Virol. J. 2006;3:69. doi: 10.1186/1743-422X-3-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magnusson MK, Hong SS, Henning P, Boulanger P, Lindholm L. Genetic retargeting of adenovirus vectors: functionality of targeting ligands and their influence on virus viability. J. Gene Med. 2002;4:356–370. doi: 10.1002/jgm.285. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu Y, Hendershot LM. Organization of the functions and components of the endoplasmic reticulum. Adv. Exp. Med. Biol. 2007;594:37–46. doi: 10.1007/978-0-387-39975-1_4. [DOI] [PubMed] [Google Scholar]
- 58.Maggioni C, Braakman I. Synthesis and quality control of viral membrane proteins. Curr. Top. Microbiol. Immunol. 2005;285:175–198. doi: 10.1007/3-540-26764-6_6. [DOI] [PubMed] [Google Scholar]
- 59.Fielding AK, Maurice M, Morling FJ, Cosset FL, Russell SJ. Inverse targeting of retroviral vectors: selective gene transfer in a mixed population of hematopoietic and nonhematopoietic cells. Blood. 1998;91:1802–1809. [PubMed] [Google Scholar]
- 60.Cosset FL, et al. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J. Virol. 1995;69:6314–6322. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chadwick MP, Morling FJ, Cosset FL, Russell SJ. Modification of retroviral tropism by display of IGF-I. J. Mol. Biol. 1999;285:485–494. doi: 10.1006/jmbi.1998.2350. [DOI] [PubMed] [Google Scholar]
- 62.Sandrin V, Russell SJ, Cosset FL. Targeting retroviral and lentiviral vectors. Curr. Top. Microbiol. Immunol. 2003;281:137–178. doi: 10.1007/978-3-642-19012-4_4. [DOI] [PubMed] [Google Scholar]
- 63.Szecsi J, et al. Targeted retroviral vectors displaying a cleavage site-engineered hemagglutinin (HA) through HA-protease interactions. Mol. Ther. 2006;14:735–744. doi: 10.1016/j.ymthe.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Martin F, Chowdhury S, Neil S, Phillipps N, Collins MK. Envelope-targeted retrovirus vectors transduce melanoma xenografts but not spleen or liver. Mol. Ther. 2002;5:269–274. doi: 10.1006/mthe.2002.0550. [DOI] [PubMed] [Google Scholar]
- 65.Peng KW, et al. Organ distribution of gene expression after intravenous infusion of targeted and untargeted lentiviral vectors. Gene Ther. 2001;8:1456–1463. doi: 10.1038/sj.gt.3301552. [DOI] [PubMed] [Google Scholar]
- 66.Denis CV. Molecular and cellular biology of von Willebrand factor. Int. J. Hematol. 2002;75:3–8. doi: 10.1007/BF02981972. [DOI] [PubMed] [Google Scholar]
- 67.Morse M. Technology evaluation: Rexin-G, Epeius Biotechnologies. Curr. Opin. Mol. Ther. 2005;7:164–169. [PubMed] [Google Scholar]
- 68.Gordon EM, et al. Systemic administration of a matrix-targeted retroviral vector is efficacious for cancer gene therapy in mice. Hum. Gene Ther. 2001;12:193–204. doi: 10.1089/104303401750061258. [DOI] [PubMed] [Google Scholar]
- 69.Gordon EM, et al. Pathotropic nanoparticles for cancer gene therapy Rexin-G IV: three-year clinical experience. Int. J. Oncol. 2006;29:1053–1064. [PubMed] [Google Scholar]
- 70.Stachler MD, Bartlett JS. Mosaic vectors comprised of modified AAV1 capsid proteins for efficient vector purification and targeting to vascular endothelial cells. Gene Ther. 2006;13:926–931. doi: 10.1038/sj.gt.3302738. [DOI] [PubMed] [Google Scholar]
- 71.Stoff-Khalili MA, et al. Gene transfer to carcinoma of the breast with fiber-modified adenoviral vectors in a tissue slice model system. Cancer Biol. Ther. 2005;4:1203–1210. doi: 10.4161/cbt.4.11.2084. [DOI] [PubMed] [Google Scholar]
- 72.Wu H, et al. Preclinical evaluation of a class of infectivity-enhanced adenoviral vectors in ovarian cancer gene therapy. Gene Ther. 2004;11:874–878. doi: 10.1038/sj.gt.3302249. [DOI] [PubMed] [Google Scholar]
- 73.Gollan TJ, Green MR. Redirecting retroviral tropism by insertion of short, nondisruptive peptide ligands into envelope. J. Virol. 2002;76:3558–3563. doi: 10.1128/JVI.76.7.3558-3563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fjeldstad K, Kolset SO. Decreasing the metastatic potential in cancers — targeting the heparan sulfate proteoglycans. Curr. Drug Targets. 2005;6:665–682. doi: 10.2174/1389450054863662. [DOI] [PubMed] [Google Scholar]
- 75.Bouri K, et al. Polylysine modification of adenoviral fiber protein enhances muscle cell transduction. Hum. Gene Ther. 1999;10:1633–1640. doi: 10.1089/10430349950017635. [DOI] [PubMed] [Google Scholar]
- 76.Mahasreshti PJ, et al. Ovarian cancer targeted adenoviral-mediated mda-7/IL-24 gene therapy. Gynecol. Oncol. 2006;100:521–532. doi: 10.1016/j.ygyno.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 77.Bauerschmitz GJ, et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res. 2002;62:1266–1270. [PubMed] [Google Scholar]
- 78.Page JG, et al. Identifying the safety profile of a novel infectivity-enhanced conditionally replicative adenovirus, Ad5-δ24-RGD, in anticipation of a phase I trial for recurrent ovarian cancer. Am. J. Obstet. Gynecol. 2007;196:e1–e9. doi: 10.1016/j.ajog.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 79.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nature Biotechnol. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 80.Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nature Med. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 81.Ghosh D, Barry MA. Selection of muscle-binding peptides from context-specific peptide-presenting phage libraries for adenoviral vector targeting. J. Virol. 2005;79:13667–13672. doi: 10.1128/JVI.79.21.13667-13672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bupp K, Sarangi A, Roth MJ. Selection of feline leukemia virus envelope proteins from a library by functional association with a murine leukemia virus envelope. Virology. 2006;351:340–348. doi: 10.1016/j.virol.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 83.Bupp K, Roth MJ. Altering retroviral tropism using a random-display envelope library. Mol. Ther. 2002;5:329–335. doi: 10.1006/mthe.2002.0546. [DOI] [PubMed] [Google Scholar]
- 84.Bupp K, Sarangi A, Roth MJ. Probing sequence variation in the receptor-targeting domain of feline leukemia virus envelope proteins with peptide display libraries. J. Virol. 2005;79:1463–1469. doi: 10.1128/JVI.79.3.1463-1469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noureddini SC, et al. Generation and selection of targeted adenoviruses embodying optimized vector properties. Virus Res. 2006;116:185–195. doi: 10.1016/j.virusres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Soong NW, et al. Molecular breeding of viruses. Nature Genet. 2000;25:436–439. doi: 10.1038/78132. [DOI] [PubMed] [Google Scholar]
- 87.White SJ, et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation. 2004;109:513–519. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- 88.Nicklin SA, Wu E, Nemerow GR, Baker AH. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol. Ther. 2005;12:384–393. doi: 10.1016/j.ymthe.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 89.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nature Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 90.Di Pasquale G, Chiorini JA. AAV transcytosis through barrier epithelia and endothelium. Mol. Ther. 2006;13:506–516. doi: 10.1016/j.ymthe.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 91.Zhu ZB, et al. Transport across a polarized monolayer of Caco-2 cells by transferrin receptor-mediated adenovirus transcytosis. Virology. 2004;325:116–128. doi: 10.1016/j.virol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Tang Y, et al. Directing adenovirus across the blood–brain barrier via melanotransferrin (P97) transcytosis pathway in an in vitro model. Gene Ther. 2007;14:523–532. doi: 10.1038/sj.gt.3302888. [DOI] [PubMed] [Google Scholar]
- 93.Pereboeva L, Komarova S, Mahasreshti PJ, Curiel DT. Fiber-mosaic adenovirus as a novel approach to design genetically modified adenoviral vectors. Virus Res. 2004;105:35–46. doi: 10.1016/j.virusres.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 94.Nakamura T, Russell SJ. Oncolytic measles viruses for cancer therapy. Expert Opin. Biol. Ther. 2004;4:1685–1692. doi: 10.1517/14712598.4.10.1685. [DOI] [PubMed] [Google Scholar]
- 95.Hallak LK, Merchan JR, Storgard CM, Loftus JC, Russell SJ. Targeted measles virus vector displaying echistatin infects endothelial cells via αvβ3 and leads to tumor regression. Cancer Res. 2005;65:5292–5300. doi: 10.1158/0008-5472.CAN-04-2879. [DOI] [PubMed] [Google Scholar]
- 96.Peng KW, et al. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood. 2003;101:2557–2562. doi: 10.1182/blood-2002-07-2195. [DOI] [PubMed] [Google Scholar]
- 97.Hasegawa K, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin. Cancer Res. 2006;12:6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 98.Allen C, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66:11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- 99.Breun S, Salmons B, Gunzburg WH, Baumann JG. Protection of MLV vector particles from human complement. Biochem. Biophys. Res. Commun. 1999;264:1–5. doi: 10.1006/bbrc.1999.1474. [DOI] [PubMed] [Google Scholar]
- 100.Kiang A, et al. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 2006;14:588–598. doi: 10.1016/j.ymthe.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 101.Moskalenko M, et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J. Virol. 2000;74:1761–1766. doi: 10.1128/JVI.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhi Y, et al. Efficacy of severe acute respiratory syndrome vaccine based on a nonhuman primate adenovirus in the presence of immunity against human adenovirus. Hum. Gene Ther. 2006;17:500–506. doi: 10.1089/hum.2006.17.500. [DOI] [PubMed] [Google Scholar]
- 103.Croyle MA, et al. PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- 104.Parker AL, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 105.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lyons M, et al. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 107.Li Q, Kay MA, Finegold M, Stratford-Perricaudet LD, Woo SL. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum. Gene Ther. 1993;4:403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- 108.Parry S, Zhang J, Koi H, Arechavaleta-Velasco F, Elovitz MA. Transcytosis of human immunodeficiency virus 1 across the placenta is enhanced by treatment with tumour necrosis factor alpha. J. Gen. Virol. 2006;87:2269–2278. doi: 10.1099/vir.0.81071-0. [DOI] [PubMed] [Google Scholar]
- 109.Fechner H, et al. Expression of coxsackie adenovirus receptor and av-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 110.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 111.Stoff-Khalili Mariam A., Rivera Angel A., Mathis J. Michael, Banerjee N. Sanjib, Moon Amanda S., Hess A., Rocconi Rodney P., Numnum T. Michael, Everts M., Chow Louise T., Douglas Joanne T., Siegal Gene P., Zhu Zeng B., Bender Hans Georg, Dall Peter, Stoff Alexander, Pereboeva Larissa, Curiel David T. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Research and Treatment. 2007;105(2):157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 112.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002;62:1063–1068. [PubMed] [Google Scholar]
- 113.Kuppen PJ, et al. Tumor structure and extracellular matrix as a possible barrier for therapeutic approaches using immune cells or adenoviruses in colorectal cancer. Histochem. Cell Biol. 2001;115:67–72. doi: 10.1007/s004180000224. [DOI] [PubMed] [Google Scholar]
- 114.Wang L, et al. Prolonged and inducible transgene expression in the liver using gutless adenovirus: a potential therapy for liver cancer. Gastroenterology. 2004;126:278–289. doi: 10.1053/j.gastro.2003.10.075. [DOI] [PubMed] [Google Scholar]
- 115.Rivera AA, et al. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 2004;11:1694–1702. doi: 10.1038/sj.gt.3302346. [DOI] [PubMed] [Google Scholar]
- 116.Lam JT, et al. Inter-patient variation in efficacy of five oncolytic adenovirus candidates for ovarian cancer therapy. J. Gene Med. 2004;6:1333–1342. doi: 10.1002/jgm.635. [DOI] [PubMed] [Google Scholar]
- 117.Kim JB. Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 2005;15:365–377. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 118.Korokhov N, et al. A single-component CD40-targeted adenovirus vector displays highly efficient transduction and activation of dendritic cells in a human skin substrate system. Mol. Pharm. 2005;2:218–223. doi: 10.1021/mp050002w. [DOI] [PubMed] [Google Scholar]
- 119.Marie JC, et al. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nature Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- 120.DiPaolo N, et al. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Mol. Ther. 2006;13:756–765. doi: 10.1016/j.ymthe.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Everts M, et al. Selective induction of tumor-associated antigens in murine pulmonary vasculature using double-targeted adenoviral vectors. Gene Ther. 2005;12:1042–1048. doi: 10.1038/sj.gt.3302491. [DOI] [PubMed] [Google Scholar]
- 122.Izumi M, et al. In vivo analysis of a genetically modified adenoviral vector targeted to human CD40 using a novel transient transgenic model. J. Gene Med. 2005;7:1517–1525. doi: 10.1002/jgm.806. [DOI] [PubMed] [Google Scholar]
- 123.Tallone T, et al. A mouse model for adenovirus gene delivery. Proc. Natl Acad. Sci. USA. 2001;98:7910–7915. doi: 10.1073/pnas.141223398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakayama M, et al. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology. 2006;350:103–115. doi: 10.1016/j.virol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 125.Pan D, et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol. Ther. 2002;6:19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
- 126.Makower D, et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin. Cancer Res. 2003;9:693–702. [PubMed] [Google Scholar]
- 127.Galanis E, et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- 128.Luker GD, et al. Noninvasive imaging of protein–protein interactions in living animals. Proc. Natl Acad. Sci. USA. 2002;99:6961–6966. doi: 10.1073/pnas.092022399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Soling A, Theiss C, Jungmichel S, Rainov NG. A dual function fusion protein of Herpes simplex virus type 1 thymidine kinase and firefly luciferase for noninvasive in vivo imaging of gene therapy in malignant glioma. Genet. Vaccines Ther. 2004;2:7. doi: 10.1186/1479-0556-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shah K, Weissleder R. Molecular optical imaging: applications leading to the development of present day therapeutics. NeuroRx. 2005;2:215–225. doi: 10.1602/neurorx.2.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Le LP, et al. Fluorescently labeled adenovirus with pIX-EGFP for vector detection. Mol. Imaging. 2004;3:105–116. doi: 10.1162/1535350041464874. [DOI] [PubMed] [Google Scholar]
- 132.Le LP, et al. Dynamic monitoring of oncolytic adenovirus in vivo by genetic capsid labeling. J. Natl Cancer Inst. 2006;98:203–214. doi: 10.1093/jnci/djj022. [DOI] [PubMed] [Google Scholar]
- 133.Le LP, Li J, Ternovoi VV, Siegal GP, Curiel DT. Fluorescently tagged canine adenovirus via modification with protein IX-enhanced green fluorescent protein. J. Gen. Virol. 2005;86:3201–3208. doi: 10.1099/vir.0.80968-0. [DOI] [PubMed] [Google Scholar]
- 134.Li J, Le L, Sibley DA, Mathis JM, Curiel DT. Genetic incorporation of HSV-1 thymidine kinase into the adenovirus protein IX for functional display on the virion. Virology. 2005;338:247–258. doi: 10.1016/j.virol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 135.Matthews QL, et al. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol. Imaging. 2006;5:510–519. doi: 10.2310/7290.2006.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waehler R, et al. Low-dose adenoviral immunotherapy of rat hepatocellular carcinoma using single-chain interleukin-12. Hum. Gene Ther. 2005;16:307–317. doi: 10.1089/hum.2005.16.307. [DOI] [PubMed] [Google Scholar]
- 137.Raty JK, et al. Magnetic resonance imaging of viral particle biodistribution in vivo. Gene Ther. 2006;13:1440–1446. doi: 10.1038/sj.gt.3302828. [DOI] [PubMed] [Google Scholar]
- 138.Berk AJ. Fields Virology. 2007. pp. 2355–2436. [Google Scholar]
- 139.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 141.Goff SP. Fields Virology. 2007. pp. 2000–2069. [Google Scholar]
- 142.Pizzato M, Marlow SA, Blair ED, Takeuchi Y. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J. Virol. 1999;73:8599–8611. doi: 10.1128/jvi.73.10.8599-8611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haynes C, Erlwein O, Schnierle BS. Modified envelope glycoproteins to retarget retroviral vectors. Curr. Gene Ther. 2003;3:405–410. doi: 10.2174/1566523034578267. [DOI] [PubMed] [Google Scholar]
- 144.Harrison SC. Mechanism of membrane fusion by viral envelope proteins. Adv. Virus Res. 2005;64:231–261. doi: 10.1016/S0065-3527(05)64007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bartlett JS, Kleinschmidt J, Boucher RC, Samulski RJ. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab′γ)2 antibody. Nature Biotechnol. 1999;17:181–186. doi: 10.1038/6185. [DOI] [PubMed] [Google Scholar]
- 146.Harding TC, et al. Enhanced gene transfer efficiency in the murine striatum and an orthotopic glioblastoma tumor model, using AAV-7- and AAV-8-pseudotyped vectors. Hum. Gene Ther. 2006;17:807–820. doi: 10.1089/hum.2006.17.807. [DOI] [PubMed] [Google Scholar]
- 147.Reynolds PN, et al. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nature Biotechnol. 2001;19:838–842. doi: 10.1038/nbt0901-838. [DOI] [PubMed] [Google Scholar]
- 148.Wurdinger T, et al. Targeting non-human coronaviruses to human cancer cells using a bispecific single-chain antibody. Gene Ther. 2005;12:1394–1404. doi: 10.1038/sj.gt.3302535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stone D, et al. Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 2005;79:5090–5104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


