Abstract
Background: The purpose of this prospective, investigator-initiated feasibility study is to evaluate the efficacy and safety of nonablative, cryogen-cooled, monopolar radiofrequency (CMRF) treatment for stress urinary incontinence (SUI).
Materials and Methods: Subjects meeting all the inclusion and exclusion criteria were enrolled and divided into two groups. Subjects in Group 1 received a single SUI treatment, and subjects in Group 2 received two SUI treatments ∼6 weeks apart. Follow-up visits are planned for 1, 4, 6, and 12 months post-treatment. At each study visit, subjects are asked to perform a 1-hour pad-weight test (PWT) and to complete the Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire-Short Form (IIQ-7), and International Consultation on Incontinence Modular Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF) questionnaires. In addition, subjects completed 7-day bladder voiding diary and safety assessments.
Results: Preliminary data indicate an improvement in SUI symptoms and quality of life for subjects, as determined by validated SUI-related patient-reported outcomes and the objective 1-hour PWT, with a >50% reduction in pad weight for 68.8% of the Group 1 subjects and 69.2% of the Group 2 subjects at 6 months. Initial review of the bladder voiding diaries suggests that subjects are having fewer urine leakage episodes per day. In addition to efficacy, the CMRF Viveve System was well tolerated and safe.
Conclusions: The endpoints evaluated indicate an improvement in SUI symptoms and quality of life. The sustained benefit of the CMRF vaginal treatment at 6 months suggests potential use as a nonsurgical approach to treat SUI.
Keywords: one-hour pad-weight test (1-hour PWT), radiofrequency (RF), stress urinary incontinence (SUI)
Introduction
Urinary incontinence is the involuntary loss of urine. There are two major types of female urinary incontinence: urgency urinary incontinence and stress urinary incontinence (SUI). Urgency incontinence is the complaint of involuntary loss of urine associated with the sudden need to pass urine.1 Stress incontinence is the involuntary loss of urine after a cough, sneeze, or physical activity.1 SUI is the most prevalent type of UI in women2 and has two major subtypes: intrinsic sphincter deficiency (ISD) and urethral hypermobility. Patients with ISD leak urine because the urethral sphincter does not effectively seal off the inner muscle of the bladder. Urethral hypermobility refers to the movement of the female urethra that occurs due to weakened pelvic floor muscles. In reality, many women have a mixed presentation.
SUI affects many women; especially during pregnancy, after childbirth, and during menopause. More than 55% of women who have delivered a child vaginally will show symptoms of SUI and are twice as likely to suffer from long-term SUI when compared with cesarean delivery.3 Furthermore, SUI can greatly impact a woman's health and quality of life.4 Depending on the severity of incontinence, some women may choose to avoid social or religious gathering, physical exercise, travel, and even sex.5,6
There are various treatment possibilities for women suffering from SUI; however, the current options have many limitations. Conservative, first-line treatment options include simple diet and exercise changes, and pelvic floor muscle training. Some women may find benefit from these,7 but long-term compliance and sustainability is difficult.8 Pharmacologic intervention9 or injectable bulking agents10 offer additional conservative treatment options but may pose efficacy or safety issues.11 At the other end of the treatment spectrum is surgery with mesh or a sling. Although synthetic sling placement has a proven success rate,12 complications of mesh surgery can occur, negatively impacting many patients. In addition, surgery often comes with several risks, including infection, voiding dysfunction, and anesthetic concerns,13 leading many women to use surgery as a last resort for treatment. The large gap between conservative and highly invasive treatment options presents an opportunity to provide more effective and less-invasive treatments for women suffering from SUI.
Radiofrequency (RF) energy has previously been used in various mucosal tissues, including pharynx, skin, cornea, and vagina.14,15 In addition, RF devices have been used to treat a variety of health-related issues, including SUI.16 However, these precedent devices are no longer commercially available as there have been some concerns about their safety profile.17–19 The Viveve System, a cryogen-cooled monopolar RF device, has a well-documented safety profile and has previously been used to treat sexual dysfunction.15,20 The device delivers monopolar RF with cryogen cooling to protect the upper epithelial layers of the mucosa while also enabling energy to reach the deeper tissue layers, resulting in volumetric heating of important connective tissue.
Recently, a pilot study at the Allan Centre in Calgary, Canada using the Viveve System to treat women with SUI reported a >90% improvement from baseline in SUI symptoms and quality of life (data on file). Following those positive results, this larger investigator-initiated early feasibility study is being conducted to further evaluate the use of the Viveve System to treat SUI. This study includes the objective 1-hour pad-weight test (PWT) in addition to subjective patient-reported outcome measures.
Materials and Methods
Study design and research subjects
This investigator-initiated feasibility study is a single-site, randomized, unblinded trial that is ongoing.
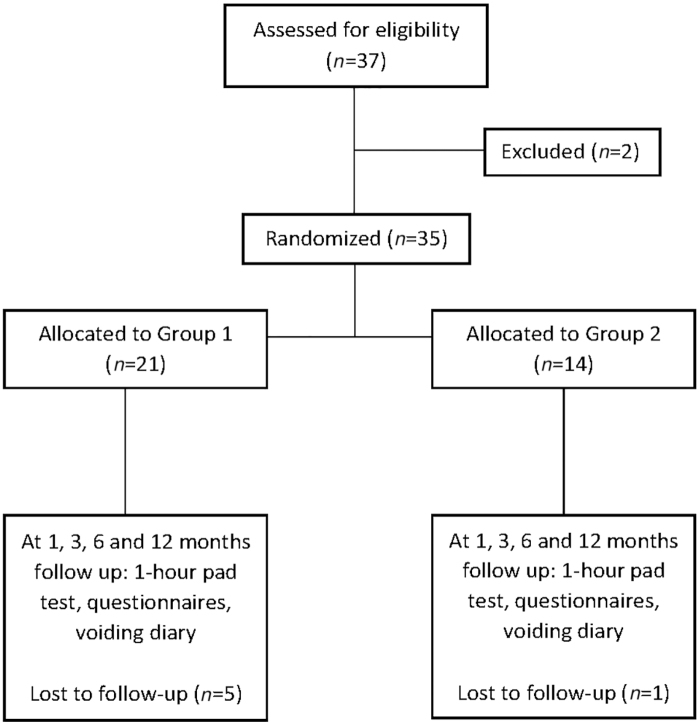
The trial included females (≥18 years of age) with a normal pelvic examination who were diagnosed with mild-to-moderate SUI as defined by the 1-hour PWT (1–50 g leakage).21 Women were excluded from the trial who were currently pregnant or discontinued breast feeding <6 months before enrollment; had a condition/illness that may confound the results of urinary incontinence assessment, including an abnormal pelvic examination, greater than stage II pelvic organ prolapse, or were morbidly obese; had a history of genital fistula or a thin rectovaginal septum (<2 cm); had a previous energy-based device treatment in the genitourinary area; and/or were taking any new medication that affects urination (Fig. 1).
FIG. 1.
CONSORT diagram. Note, the final follow-up visit is at 12 months, but this article only discusses the trial through 6-month follow-up visit.
Randomization and intervention
Subjects meeting the inclusion and exclusion criteria were randomized to receive either one or two treatments using a random number generator; odd numbers were placed into Group 1 and even numbers were placed into Group 2. The Viveve system protocol for sexual function15 was modified to provide additional energy to the tissue beyond the introitus for support of the urethra to improve SUI. A total of 220 pulses of 90 J/cm2 was applied during the treatment procedure. The treatment area was divided into quadrants of the vaginal introitus with the area directly beneath the urethra excluded. Each quadrant was treated with five consecutive passes of five locations of pulses for a total of 25 pulses per quadrant. The remaining 20 pulses are distributed equally in quadrants 1 and 4. If a subject was assigned to Group 2 (two SUI treatments), the treatment protocol was repeated 6 weeks after the initial treatment.
A sham group was not included in the study design as this was a pilot study to obtain the first objective data using the Viveve Treatment, SUI protocol. In addition, the manufacture of a sham tip would have been cost prohibitive at this stage in the investigation.
The sample size (37 subjects) for this early feasibility study was determined based on considerations of such a feasibility study, confidence in outcome measures, and research risks, including the risk that the study aims may not be achieved.
Follow-up visits
The standardized 1-hour PWT,21 7-day bladder voiding diary and validated patient-reported outcome measures (Urogenital Distress Inventory-6 [UDI-6],22 Incontinence Impact Questionnaire-Short Form [IIQ-7],22 and International Consultation on Incontinence Modular Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF]23), was administered at the screening visit and at 1, 4, and 6 months post-treatment. These will also be completed at the final visit at 12 months. Adverse events and concomitant medications were collected at each of the follow-up visits.
Objective 1-hour PWT
The 1-hour PWT is a standardized series of activities (walking, coughing, climbing stairs, etc.) that the subject completes after ingestion of a set amount (500 mL) of sodium-free liquid. Subjects are asked to wear preweighed pads during the assessment. The pad is weighed again at the completion of the series of activities to determine the amount of leakage.
Subjective patient-reported outcomes
The questionnaires used to evaluate the status of the subject's SUI were UDI-6, IIQ-7, and ICIQ-UI-SF. These questionnaires have been used in several SUI clinical trials and have been validated for this use.22,23 Scoring was done per the respective questionnaire guidelines.
Seven-day bladder voiding diary
A site-developed 7-day bladder voiding diary was provided to subjects for completion. The voiding diary included questions about leakage and daily activities.
Ethics
Ethics Review Board approval was obtained from the Health Research Ethics Board of Alberta, and the study was done in compliance with Good Clinical Practices and International Conference on Harmonization (ICH) guidelines. Health Canada clearance was also obtained for this investigator-initiated study. Documentation and data management were conducted in a manner that aligns with local ethics review board guidelines.
Results
Participants
Between June and November 2017, 37 subjects were included in the study. Twenty-one and 14 subjects were randomized to receive one or two treatments, respectively; 2 subjects dropped out of the study before treatment. Twenty-nine subjects completed the baseline and 6-month follow-up visit. Table 1 shows the baseline characteristics for the remaining 29 subjects. Group 2 was slightly older than Group 1, 46.5 years of age versus 42.9, respectively. Both groups had similar BMI and number of pregnancies. A large majority (96%) of the subjects were of white race, and one subject was Asian.
Table 1.
Baseline Demographics, Clinical Characteristics, and Maternal History for Trial Subjects
| No. of subjects |
Group 1 |
Group 2 |
||
|---|---|---|---|---|
| 16 |
13 |
|||
| Demographic data | Mean | SD | Mean | SD |
| Age | 42.9 | 4.0 | 46.5 | 9.2 |
| Age categories, years | n | % | n | % |
| <35 | 1 | 6.3 | 1 | 7.7 |
| 35–39 | 4 | 25.0 | 2 | 15.3 |
| 40–44 | 4 | 25.0 | 3 | 23.1 |
| ≥45 | 7 | 43.7 | 7 | 53.9 |
| Clinical data | Mean | SD | Mean | SD |
| BMI | 25.1 | 4.6 | 25.9 | 4.7 |
| BMI categories | n | % | n | % |
| BMI <20 | 3 | 18.8 | 0 | 0 |
| BMI 20–24 | 5 | 31.2 | 5 | 38.5 |
| BMI 25–29 | 5 | 31.2 | 6 | 46.1 |
| BMI ≥30 | 3 | 18.8 | 2 | 15.4 |
| Maternal history | Mean | SD | Mean | SD |
| No. of pregnancies | 2.0 | 1.3 | 2.3 | 0.9 |
| No. of full-term deliveries | 1.7 | 1.0 | 2.3 | 0.8 |
| No. of vaginal deliveries | 1.5 | 1.1 | 2.0 | 1.1 |
| Race | n | % | n | % |
| White | 15 | 93.8 | 13 | 100 |
| Asian | 1 | 3.5 | 0 | 0 |
One-hour pad weight
Mean 1-hour pad-weight values are presented in Table 2. Baseline values differ between treatment groups; however, the 6-month pad-weight leakage volumes are similar, and the percentage of subjects with >50% reduction in pad weight is identical at 69%. The cure rate, defined here as ≤1 g of leakage and per FDA guidelines,24,25 is also comparable between groups. Overall mean change from baseline showed a decrease of 73% for all subjects.
Table 2.
Mean Pad-Weight Data at 1, 4, and 6-Month Follow-Up Visits
| Group | Baseline pad weight | Subjects with a >50% reduction in pad weight from baseline |
Cure rate (≤1 g leak) |
||
|---|---|---|---|---|---|
| Mean pad-weight leakage volume (g) | |||||
| 1 month | 4 months | 6 months | 6 months | ||
| All subjects (n = 29) | 6.17 g | 55.2% | 75.9% | 69.0% | 65.5% |
| 2.14 g | 1.31 g | 1.69 g | |||
| Group 1 (n = 16) | 4.81 g | 56.3% | 68.8% | 68.8% | 68.8% |
| 2.19 g | 1.19 g | 1.81 g | |||
| Group 2 (n = 13) | 7.85 g | 53.8% | 84.6% | 69.2% | 61.5% |
| 2.08 g | 1.46 g | 1.54 g | |||
Percentage of subjects with a >50% reduction in pad weight (which represents a clinically meaningful decrease in leakage amount) and percentage of subjects considered cured at 6 months.
Patient-reported outcomes
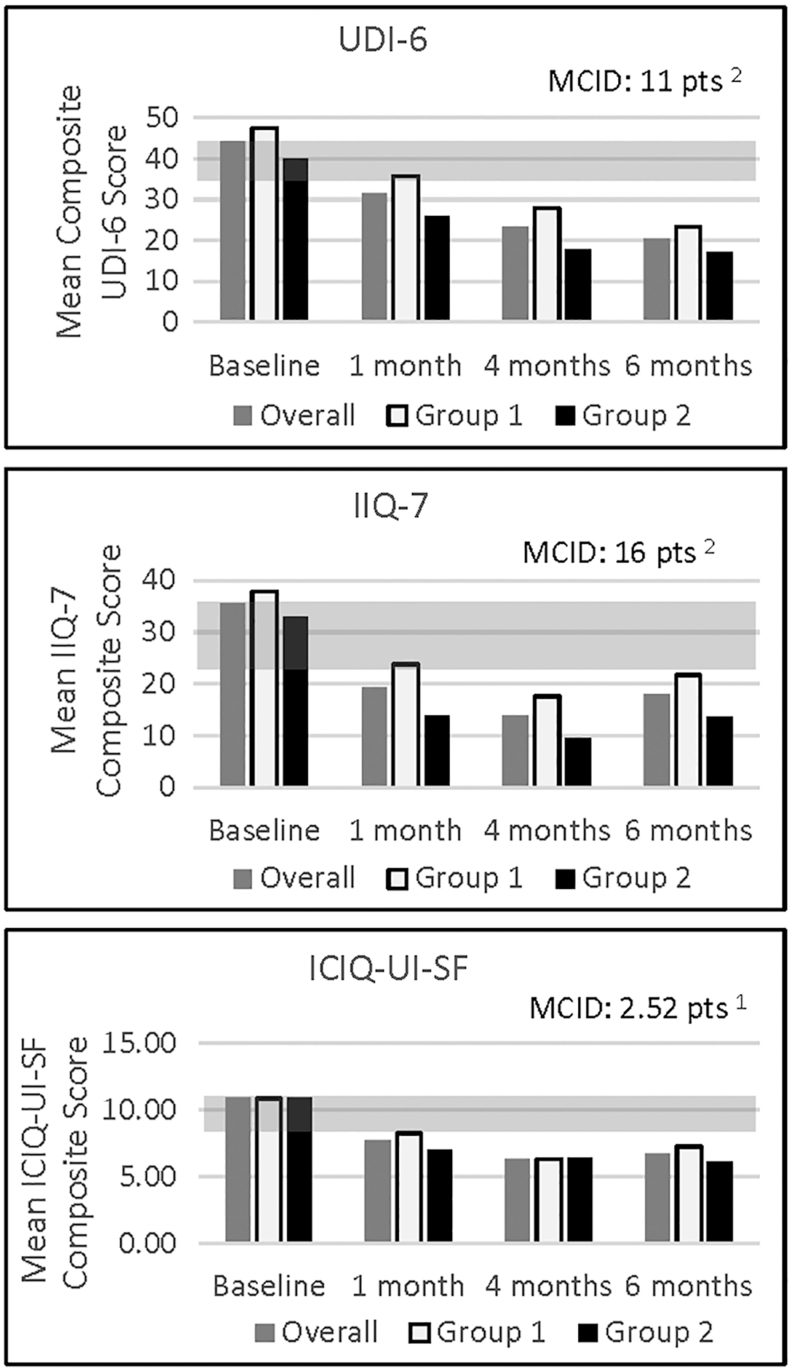
Clinically meaningful score decreases in subject's SUI symptoms, and improvement in quality of life was noted on two measures (UDI-6 and ICIQ-UI-SF) as early as 1-month post-treatment22,23 (Fig. 2). By 6-months post-treatment, all SUI-related subjective measures for both treatment groups show sustained improvement in mean composite scores. Group 1 reported greater mean composite scores than Group 2 at all measured time points for UDI-6 and IIQ-7.
FIG. 2.
Mean composite scores for SUI-related patient-reported outcomes. The gray bar in each graph denotes a published MCID from the literature. ICIQ-UI-SF, International Consultation on Incontinence Modular Questionnaire-Urinary Incontinence-Short Form; IIQ-7, Incontinence Impact Questionnaire-Short Form; MCID, minimal clinical important difference; SUI, stress urinary incontinence; UDI-6, Urogenital Distress Inventory-6.
Seven-day bladder voiding diary
Subjects report a decrease in leakage episodes per day as soon as 1-month post-treatment (Table 3). By 6 months, ∼80% of subjects report less leakage episodes compared with baseline, with most reporting a ≥50% reduction from baseline. In addition, some subjects report an ability to resume strenuous physical exercise (e.g., rock climbing) post-treatment.
Table 3.
Mean Number of Daily Incontinence Episodes at 1, 4, and 6-Month Follow-Up Visits*
| Group | Baseline mean # daily incontinence episodes | No. of incontinence episodes/day |
||
|---|---|---|---|---|
| % of subjects with >50% reduction from baseline in incontinence episodes/day | ||||
| 1 month | 4 months | 6 months | ||
| All subjects | 2.0 | 1.2 | 1.2 | 1.0 |
| (n = 29) | 48% | 69% | 64% | |
| Group 1 | 1.5 | 0.7 | 1.1 | 0.7 |
| (n = 16) | 50% | 75% | 60% | |
| Group 2 | 2.7 | 1.7 | 1.3 | 1.3 |
| (n = 13) | 46% | 62% | 69% | |
n = 28 at 6-month time point.
Safety
No unanticipated or serious adverse events have been reported in the trial to date. All other events were mild. One patient reported a urinary tract infection (UTI). The UTI occurred between the treatment visit and 1-month follow-up. The subject was treated with 1 week of antibiotics. The investigator assessed the UTI as unrelated to treatment.
Discussion
This early feasibility study highlights the promising efficacy and safety of the Viveve System for the treatment of SUI. The results show continued benefit out to 6 months post-treatment, the time point assessed to date, for all subjects. Although the results are encouraging, caution should be exercised when interpreting the data since this is a small study and lacks a control group. A larger scale, randomized, blinded, sham-controlled study is warranted.
While the objective pad-weight data are similar between treatment groups, the subjective, SUI-related patient-reported outcome measures show slight differences in the mean composite scores, with Group 2 reporting decreased scores at almost all time points. This could be due to a lower baseline value for Group 2; or also because this was an unblinded study, therefore subjects knew whether they received one or two treatments. An analysis of 130 clinical trials showed a significant placebo effect in studies with continuous subjective outcomes, however little to no placebo effect for objective measures.26 In addition, the lack of a study-wide retention plan and unblinded nature of the study could account for the larger dropout rate from Group 1 (n = 5) versus Group 2 (n = 1). Interestingly, of the five women who dropped out of Group 1 before 6-month visit, all had improvement from baseline on the 1-hour PWT at their last measured visit; and two of the five had no leakage at all (0 g of leakage on the 1-hour PWT). Furthermore, based upon the Viveve System's proposed mechanism of action, which includes fibroblast activation and restoration of connective tissue of the lamina propria tissue layer,27 and what is known about collagen restoration, a second RF treatment done later (e.g., 6 months vs. 6 weeks) may provide even greater benefit to women. A larger number of subjects, another study including a sham treatment group, a longer follow-up period, or a longer28 time between treatments may be necessary to determine the differences between one or two treatments and the optimal timing. Of note, this study will continue out to 12 months to assess safety and efficacy.
Although other RF devices are currently claiming to help treat SUI, no other studies with a nonablative, monopolar RF device have demonstrated a decrease in SUI symptoms as evaluated by objective measures (1-hour pad test and voiding diary) sustained out to 6-months post-treatment.16,29–32 This early feasibility study also includes validated SUI- and quality of life-related subjective questionnaires (UDI-6, IIQ-7, ICIQ-UI-SF) as study endpoints with >70% of women experiencing improvement (reduction from baseline) at 6 months. In addition, an earlier pilot study demonstrated the efficacy of the Viveve System for the treatment of SUI out to 12 months, with >90% response rate (reduction from baseline using validated SUI questionnaires). Although the IIQ-7 and ICIQ-UI-SF scores slightly increased from 4 to 6 months, the change was minimal and not clinically significant.28,33
These promising results were achieved with one treatment. Other RF devices require multiple (3+) sessions, typically at 1-month intervals.34 This incurs increased time and monetary cost to the patient, and increases the potential for a decrease in treatment compliance. It should also be noted that the Viveve System has a well-established safety profile. To date, thousands of women have been treated globally for sexual dysfunction with only mild adverse events reported.15,35,36 In the previously published, large-scale, randomized-controlled clinical trial using the Viveve System, the active and sham groups reported similar numbers of adverse events.20 In addition, recent ovine studies have confirmed tissue temperatures that would result in cellular responses related to the observed clinical outcomes with no thermal damage to the vaginal tissue following multiple pulses in the same area (Viveve internal data).
Conclusions
While this article summarizes data from an early investigator-initiated feasibility study, the results include the first 6-month objective outcome data for the vaginal RF treatment for SUI. The Viveve treatment shows promise as a viable option for patients searching for minimally invasive nonsurgical treatments. Initial experience merits a larger scale, randomized, blinded, and sham-controlled study to investigate this treatment for SUI.
Acknowledgment
The authors acknowledge Debbie Wilkerson, PhD, for her scientific input and editing assistance for the article.
Author Disclosure Statement
B.B.A. acquired support for the study from Viveve Investigator-sponsored Research Program, was a consultant to Viveve, Inc.; S.B. was an employee of Viveve, Inc., at the time of the study; and K.H. was an employee of Viveve, Inc.
Author Contributions
B.B.A. performed protocol/project development, data collection, and article writing/editing; S.B. implemented protocol/project development, data collection, data analysis, and article writing/editing; and K.H. accomplished protocol/project development, data collection, data analysis, and article writing/editing.
Clinical Trial Registration
clinicaltrials.gov Identifier: NCT03066180.
Funding Information
The study was funded within the Allan Centre and supported as part of the Viveve Investigator-Sponsored Research Program (which also provided study supplies and administrative assistance).
References
- 1. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20 [DOI] [PubMed] [Google Scholar]
- 2. Markland AD, Richter HE, Fwu CW, Eggers P, Kusek JW. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol 2011;186:589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tahtinen RM, Cartwright R, Tsui JF, et. al. Long-term impact of mode of delivery on stress urinary incontinence and urgency urinary incontinence: A systematic review and meta-analysis. Eur Urol 2016;70:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saarni SI. The impact of 29 chronic conditions on health-related quality of life: A general population survey in Finland using 15D and EQ5D. Qual Life Res 2006;15:1403–1414 [DOI] [PubMed] [Google Scholar]
- 5. Salonia A. Sexual dysfunction is common in women with lower urinary tract symptoms and urinary incontinence: Results of a cross-sectional study. Eur Urol 2004;45:642–648 [DOI] [PubMed] [Google Scholar]
- 6. Yip SK. The impact of urodynamic stress incontinence and detrusor overactivity on marital relationship and sexual function. Am J Obstet Gynecol 2003;188:1244–1248 [DOI] [PubMed] [Google Scholar]
- 7. Guralnick ML, Kelly H, Engelke H, Koduri S, O'Connor RC. InTone: A novel pelvic floor rehabilitation device for urinary incontinence. Int Urogynecol J 2015;26:99–106 [DOI] [PubMed] [Google Scholar]
- 8. Beyar N, Groutz A: Pelvic floor muscle training for female stress urinary incontinence: Five years outcomes. Neurourol Urodyn 2015;36:132–135 [DOI] [PubMed] [Google Scholar]
- 9. Norton PA, Zinner NR, Yalcin I, Bump RC. Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol 2002;187:40–48 [DOI] [PubMed] [Google Scholar]
- 10. Toozs-Hobson P, Al-Singary W, Fynes M, Tegerstedt G, Lose G. Two-year follow-up of an open-label multicenter study of polyacrylamide hydrogel (Bulkamid(R)) for female stress and stress-predominant mixed incontinence. Int Urogynecol J 2012;23:1373–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kerr LA. Bulking agents in the treatment of stress urinary incontinence: History, outcomes, patient populations, and reimbursement profile. Rev Urol 2005;7:S3–S11 [PMC free article] [PubMed] [Google Scholar]
- 12. Ford AA, Rogerson L, Cody JD, Ogah J. Midurethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev 2015;7:CD006375. [DOI] [PubMed] [Google Scholar]
- 13. Aoki Y, Brown HW, Brubaker L, Cornu JN, Daly JO, Cartwright R. Urinary incontinence in women. Nat Rev Dis Primers 2017;3:17042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berjano EJ, Ribera ENV, Gorris J, Alió JL. Radiofrequency heating of the cornea: An engineering review of electrodes and applicators. Open Biomed Eng J 2007;1:71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: The VIVEVE I randomized controlled trial. J Sex Med 2017;14:215–225 [DOI] [PubMed] [Google Scholar]
- 16. Lordelo P, Vilas Boas A, Sodre D, Lemos A, Tozetto S, Brasil C. New concept for treating female stress urinary incontinence with radiofrequency. Int Braz J Urol 2017;43:896–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dmochowski R, Appell RA. Advancements in minimally invasive treatments for female stress urinary incontinence: Radiofrequency and bulking agents. Curr Urol Rep 2003;4:350–355 [DOI] [PubMed] [Google Scholar]
- 18. Appell RA, Juma S, Wells WG, et al. Transurethral radiofrequency energy collagen micro-remodeling for the treatment of female stress urinary incontinence. Neurourol Urodyn 2006;25:331–336 [DOI] [PubMed] [Google Scholar]
- 19. Appell RA. Transurethral collagen denaturation for women with stress urinary incontinence. Curr Urol Rep 2008;9:373–379 [DOI] [PubMed] [Google Scholar]
- 20. Krychman M, Rowan CG, Allan BB, Durbin S, Yacoubian A, Wilkerson D. Effect of single-session, cryogen-cooled monopolar radiofrequency therapy on sexual function in women with vaginal laxity: The VIVEVE I Trial. J Womens Health (Larchmt) 2018;27:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krhut J, Zachoval R, Smith PP, et al. Pad weight testing in the evaluation of urinary incontinence. Neurourol Urodyn 2014;33:507–510 [DOI] [PubMed] [Google Scholar]
- 22. Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Andrew Fantl J; and the Continence Program for Women Research Group: Short forms to assess life quality and symptom distress for urinary incontinence in women: The incontinence impact questionnaire and the urogenital distress inventory. Neurourol Urodyn 1995;14:131–139 [DOI] [PubMed] [Google Scholar]
- 23. Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322–330 [DOI] [PubMed] [Google Scholar]
- 24. Tubaro A, Artibani W, Bartram C, Delancey J, Khullar V, Vierhout M.. Imaging and other investigations. In: Abrams P, Cardozo L, Khoury S, Wein A eds. Incontinence, 4th Ed. Wiley Online, 2009. [Google Scholar]
- 25. FDA Center for Devices and Radiological Health Office of Device Evaluation. Guidance for Industry and Food and Drug Administration Staff Clinical Investigations of Devices Indicated for the Treatment of Urinary Incontinence 2011. www.fda.gov/MedicalDevices/DeviceRegulationsGuidance/GuidanceDocuments/ucm07 Accessed March8, 2011
- 26. Hróbjartsson A, Gøtzsche PC: Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001;344:1594–1602 [DOI] [PubMed] [Google Scholar]
- 27. Vos JA, Ryan TP, Livengood RH, Jessop M, Coad JE. Non-ablative hyperthermic mesenchymal regeneration: A proposed mechanism of action based on the Vivev model. SPIE 2011;7901:790104-790101–790104-790108. [Google Scholar]
- 28. Huber SA, Chinthakanan O, Hawkins S, Miklos JR, Moore RD. Laparoscopic Burch urethropexy at time of mesh sling removal: A cohort study evaluating functional outcomes and quality of life. World J Obstet Gynecol 2016;5:210–217 [Google Scholar]
- 29. Caruth J. Evaluation of the safety and efficacy of a novel radiofrequency device for vaginal treatment. Surg Technol Int 2018;32:145–149 [PubMed] [Google Scholar]
- 30. Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol 2017;16:230–234 [DOI] [PubMed] [Google Scholar]
- 31. Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int 2016;29:149–159 [PubMed] [Google Scholar]
- 32. Vicariotto F, Raichi M. Technological evolution in the radiofrequency treatment of vaginal laxity and menopausal vulvo-vaginal atrophy and other genitourinary symptoms: First experiences with a novel dynamic quadripolar device. Minerva Ginecol 2016;68:225–236 [PubMed] [Google Scholar]
- 33. Nystrom E, Sjostrom M, Stenlund H, Samuelsson E. ICIQ symptom and quality of life instruments measure clinically relevant improvements in women with stress urinary incontinence. Neurourol Urodyn 2015;34:747–751 [DOI] [PubMed] [Google Scholar]
- 34. Magon N, Alinsod R. ThermiVa: The revolutionary technology for vulvovaginal rejuvenation and noninvasive management of female SUI. J Obstet Gynaecol India 2016;66:300–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: Nonsurgical vaginal tightening. J Sex Med 2010;7:3088–3095 [DOI] [PubMed] [Google Scholar]
- 36. Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: Nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt) 2013;22:775–781 [DOI] [PubMed] [Google Scholar]