Key Points
Angiotensin-converting enzyme (ACE) is a chloride-dependent metalloenzyme that catalyses the hydrolytic cleavage of dipeptides from the carboxyl terminus of many regulatory oligopeptides. ACE is central to the renin–angiotensin system that regulates blood pressure, fluid homeostasis, and renal and vascular function. It is therefore a major target for cardiovascular therapies.
ACE inhibitors (for example, captopril, enalaprilat and lisinopril) have been on the market for more than 20 years. Side effects of treatment with ACE inhibitors include cough and angioedema.
ACE comprises an N- and a C-domain, each containing an active site with distinct substrates and activation properties. The design of domain-selective inhibitors might produce new drugs with improved safety and efficacy — this endeavour will be facilitated by the recent determination of the three-dimensional structure of ACE.
The C-domain seems to be primarily responsible for the regulation of blood pressure. Data indicate that C-domain-selective inhibitors will have less severe side effects than current-generation inhibitors, which generally target both the N- and C-domains.
In contrast to the C-domain, the N-domain seems to have relatively low affinity for the peptides that control blood pressure. It preferentially hydrolyses at least three other physiologically important peptides, so targeted inhibition of the N-domain might have novel therapeutic applications.
Abstract
Current-generation angiotensin-converting enzyme (ACE) inhibitors are widely used for cardiovascular diseases, including high blood pressure, heart failure, heart attack and kidney failure, and have combined annual sales in excess of US $6 billion. However, the use of these ACE inhibitors, which were developed in the late 1970s and early 1980s, is hampered by common side effects. Moreover, we now know that ACE actually consists of two parts (called the N- and C-domains) that have different functions. Therefore, the design of specific domain-selective ACE inhibitors is expected to produce next-generation drugs that might be safer and more effective. Here we discuss the structural features of current inhibitors and outline how next-generation ACE inhibitors could be designed by using the three-dimensional molecular structure of human testis ACE. The ACE structure provides a unique opportunity for rational drug design, based on a combination of in silico modelling using existing inhibitors as scaffolds and iterative lead optimization to drive the synthetic chemistry.
Main
Recent advances in molecular biology, high-throughput crystallization techniques, high-energy synchrotron sources, combinatorial and fragment-based chemistries, and bioinformatics have had a dramatic effect on the drug discovery process, and have provided a new impetus to undertake 'structure-based drug discovery'1,2. At present, there is enormous interest in identifying and validating 'druggable' targets in the human proteome and applying structure-based drug design to the development of novel therapeutics1.
Angiotensin-converting enzyme (ACE) is a major target for cardiovascular therapies and ACE inhibitors have been on the market for more than 20 years. ACE is a central component of the renin–angiotensin system (RAS), which controls not only blood pressure and fluid and electrolyte homeostasis, but also renal and vascular function and myocardial remodelling3. As such, inhibitors of the RAS have found widespread application in cardiovascular disease, and current ACE inhibitors have a broad range of licensed indications, ranging from mild hypertension to post-myocardial infarction4.
Although very successful, the design of current-generation ACE inhibitors was the result of serendipity combined with brilliant insights, and was achieved without knowledge of the sequence or three-dimensional structure of the enzyme. Only after the introduction of ACE inhibitors did it become evident that somatic ACE is a complex two-domain enzyme, comprising an N- and a C-domain, each containing an active site with similar but distinct substrate specificities and chloride-activation requirements3. Therefore, if domain-selective inhibitors can be designed, this could produce next-generation drugs with altered safety and efficacy profiles.
The recent breakthrough in determining the high-resolution crystal structure of human testis ACE (tACE) has offered new details at the molecular level5. The structure of tACE provides an opportunity for the design of N- and C-domain-selective inhibitors by structure-guided drug design. This would be an interesting example of revisiting an 'old' drug target that is well established in the clinic by applying modern drug discovery tools. Successful examples of this strategy are the selective cyclooxygenase (COX)-2 inhibitors (for example, rofecoxib), the selective peripheral histamine H1-receptor antagonists (for example, loratadine), and the selective aldosterone receptor antagonist (eplerenone), all of which are 're-engineered' drugs active against well-understood targets6,7,8.
Here, we provide an overview of ACE and the RAS, current ACE inhibitors and their clinical utility, insights from the tACE crystal structure, and the rationale and prospects for developing second-generation, domain-selective inhibitors by structure-guided design.
ACE, the RAS and blood pressure regulation
The recognition that an elevated basal blood pressure can lead to a shortened life expectancy and higher morbidity (due to cardiovascular complications such as kidney failure, heart failure and stroke) only evolved during the first half of the twentieth century, when numerous clinical surveys demonstrated a normal distribution of blood pressures and a 10–20% prevalence of hypertension in an apparently healthy population9. Hypertension occurs in millions of people worldwide, the large majority of whom are unaware of their condition; a recent report indicated that prevalence rates are even higher than previously suspected, at 28% in North America and 44% in Europe10. Many long-term studies were required before the increased morbidity and mortality associated with hypertension became accepted and it was classified as a disease in need of treatment9. The underlying cause of hypertension in most cases was, and still is, unknown. Therapy was initially directed at reducing blood volume by the use of DIURETICS, SYMPATHETIC TONE by the use of adrenergic-blocking agents, or VASCULAR RESISTANCE by the use of vasodilators.
A major breakthrough in our understanding of blood pressure regulation came with the discovery of the RAS and ACE (reviewed in Ref. 11). On the basis of a suspected relationship between kidney and blood pressure, Goldblatt, Houssay, Page and others identified the renal enzyme renin and the pressor substance angiotensin in the 1930s and 1940s12,13,14. In the mid-1950s, Skeggs and co-workers purified angiotensin and found that it existed in two forms: a decapeptide called angiotensin I (Ang I) and an octapeptide called angiotensin II (Ang II)15. Two years later a chloride-dependent, metalloenzyme that could convert Ang I to Ang II was purified from horse plasma16, and was later referred to as ACE.
Properties of ACE
ACE, also known as peptidyl-dipeptidase A (EC 3.4.15.1), belongs to the M2 family of the MA clan (a protein clan contains all the modern-day polypeptides that have arisen from a single evolutionary progenitor) of zinc metallopeptidases17. It is a dipeptidyl carboxypeptidase that catalyses the hydrolytic cleavage of dipeptides from the carboxyl terminus of a wide variety of oligopeptides in vitro. Its best known function is the in vivo conversion of Ang I (DRVYIHPFHL), which circulates in plasma, into the potent vasopressor Ang II by removal of the C-terminal His–Leu (Fig. 1). Ang I is generated primarily by the renin-catalysed hydrolysis of the Leu10–Val11 peptide bond of angiotensinogen, a liver-derived 55-kDa plasma protein. ACE also affects blood pressure by cleaving bradykinin (BK, RPPGFSPFR), thereby abolishing its vasodilating activity (because of this, ACE is sometimes referred to as kininase II (kininase I is carboxypeptidase N)). BK is generated from a kininogen precursor by the action of plasma kallikrein (a serine proteinase) in a process analogous to that of Ang I formation (Fig. 1).
Figure 1. Metabolism of angiotensin peptides and bradykinin by ACE and other vasopeptidases.
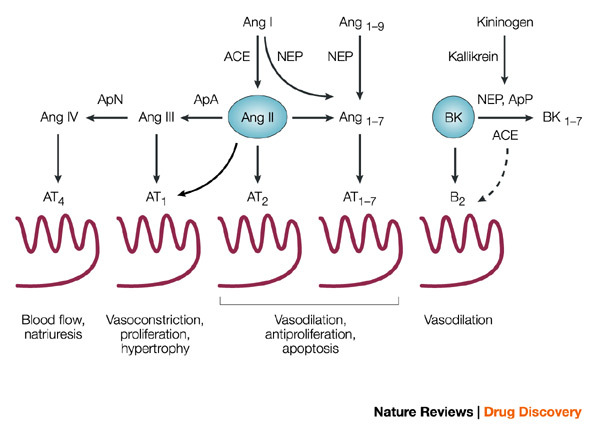
Also shown are the principal angiotensin (Ang) and bradykinin (BK) receptors and their downstream effects. The broken arrow from angiotensin-converting enzyme (ACE) to B2 BK receptor denotes evidence for crosstalk between the membrane-bound proteins. The conversion of Ang II to Ang1–7 can be mediated by ACE2; see text and Refs 3,37. Ap, aminopeptidase; NEP, neutral endopeptidase.
Human ACE is a monomeric zinc metalloenzyme that is synthesized as a 1,306-amino-acid polypeptide, is processed to a 1,277-residue mature form, is heavily glycosylated (30% by weight), and is localized to the plasma membrane of endothelial and absorptive epithelial and neuroepithelial cells18. A sequence of 22 hydrophobic amino acids located near the carboxyl terminus of the protein serves as a transmembrane domain that anchors ACE to the cell surface. This creates a 28-residue cytosolic domain and a 1,227-residue glycosylated extracellular domain. Its cellular orientation defines ACE as a type I transmembrane ectoprotein and, in the case of endothelial cells, positions it optimally for interaction with its circulating substrates. The amino acid sequence of human endothelial ACE provides clear evidence for a gene duplication event in its evolutionary history. It consists of an N-terminal domain of about 612 amino acids, a 15-residue interdomain sequence and a 650-residue C-terminal domain. A 357-amino-acid segment of the N-domain has more than 60% sequence identity to the corresponding segment of the C-domain (Fig. 2).
Figure 2. Protein sequences of testis ACE and the N- and C-domains of somatic ACE.
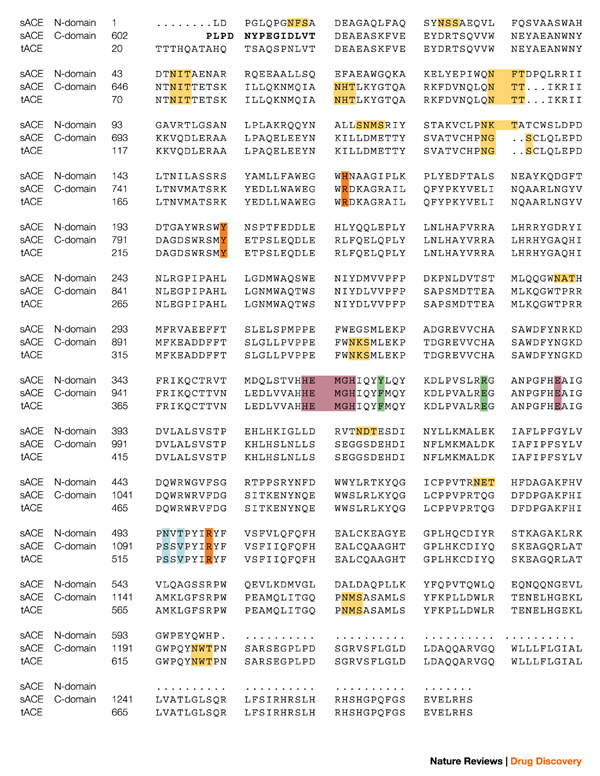
The sequences are aligned and numbered according to Refs 18,26. The bridge region is in bold and the zinc-binding motif, as well as the third zinc ligand (Glu), are in purple. The C-domain N-glycosylation sites are in yellow, the chloride ligands in orange, and the active site residues depicted in Fig. 4 are in blue and green. The secondary structure elements for tACE5 structure α-helices (α), β-strands (β) and 310 helices are: α1(40–71); α2(74–100); H1(101–107); α3(109–120); H2(122–127); α4(128–149); β1(150–153); β2(157–161); α5(163–172); α6(174–211); α7(215–222); H3(223–225); α8(228–260); β3(270–272); H4(283–285); α9(286–291); α10(300–308); α11(311–326); α12(332–339); β4(355–359); β5(364–368); α13(374–394); H5(398–402); α14(406–430); α15(439–473); α16(480–494); β6(495–496); H6(506–511); α17(520–541); H7(546–550); α18(555–568); α19(573–583); α20(589–610). sACE, somatic angiotensin-converting enzyme; tACE, testis angiotensin-converting enzyme.
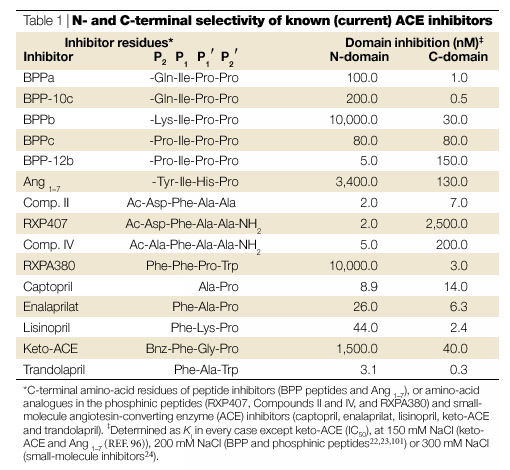
Each domain contains a five-residue sequence of amino acids, HEMGH, which is characteristic of catalytic zinc sites found in a large family of neutral endoproteinases. Detailed kinetic and mutational analyses have established that both of the zinc sites have catalytic activity19,20. The physiological consequence of such tandem active sites in an enzyme is unknown. Some differences in catalytic properties have been observed for these two sites: the N-domain site is notably 50-times more active toward the haemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro (AcSDKP)21, 1000-times more sensitive to inhibition by the phosphinic peptide RXP407 (Ref. 22), and more than 3000-times less sensitive to inhibition by RXPA380 (Ref. 23) (Table 1). In addition, the activity of the C-domain is highly dependent on chloride concentration, whereas that of the N-domain is much less so19,24. However, given the relative constancy of plasma chloride concentration, the significance of this chloride dependency is not obvious.
Table 1.
N- and C-terminal selectivity of known (current) ACE inhibitors
Human endothelial ACE, also known as somatic ACE, is encoded by a single gene that consists of 26 exons, all but one of which, exon 13, is transcribed into the corresponding messenger RNA25. A form of ACE found only in adult testis (tACE) is encoded by the same gene but its mRNA begins before exon 13 and continues through exon 26. It is transcribed by the interaction of a promoter with a site present in intron 12 that is active only in adult male germinal cells25. Translation of this mRNA results in a 701-amino-acid version of ACE that, except for the first 36 residues, is identical to the C-terminal domain of somatic ACE26. Testis or germinal ACE is found in developing sperm cells and mature sperm. Sperm lacking ACE are deficient in transport within the oviduct and in binding to the zonae pellucidae, and male ACE−/− mice have markedly diminished fertility27.
A polymorphism involving a 287-base-pair insertion corresponding to an Alu repetitive sequence has been found in intron 16 of the human ACE gene28. The insertion is all or none: in one study the (I)/(D) frequency ratio was 44:56. The deletion (D) allele of the ACE gene lacks the sequence that is present in the insertion (I) allele. The D allele is associated with a higher plasma and tissue ACE activity. The DD genotype is positively correlated with plasma ACE activity and numerous attempts have been made to search for associations between the DD genotype and specific cardiovascular diseases, but the conclusions remain controversial29. The association of allele I with athletic performance is equally controversial. Some studies have shown that this polymorphism is associated with endurance performance, whereas others have shown the D allele to be associated with that of elite short-distance athletes30,31.
ACE is released from the endothelial cell surface by the action of a so-called ACE-secretase, or sheddase, that cleaves the Arg1203–Ser1204 peptide bond near the transmembrane region32,33. Soluble ACE is a minor component of total ACE activity, and its physiological function is unknown. Activators of protein kinase C stimulate the release of ACE from endothelial and other cells in culture, and markedly elevated concentrations of plasma ACE are observed in certain diseases, particularly sarcoidosis.
Besides its role in blood pressure regulation, ACE also has been postulated to participate in 'local' or 'tissue' RASs. The Ang II arising from such systems is thought to act as a paracrine growth factor4. This activity has been implicated in the development of left ventricular dysfunction that can occur after a major heart attack, and seems to account for the beneficial effects of ACE inhibitor therapy in such situations.
ACE homologues
The structural and functional conservation of the gene encoding ACE are indications of its widespread evolutionary importance as a key metallopeptidase involved in the metabolism of regulatory peptides34. The Drosophila melanogaster genome contains six genes (Acer, Ance, Ance-2, Ance-3, Ance-4 and Ance-5) that encode ACE-like proteins. Two of these, angiotensin-converting enzyme (Ance) and angiotensin-converting enzyme-related (Acer), are enzymatically active and share 36% sequence identity with human ACE35. Ance displays properties very similar to those of the human C-domain, whereas Acer is inhibited by the N-domain-selective inhibitor RXP407 (Ref. 36).
ACE2 (also known as ACEH) is an ACE homologue found in humans and rodents that functions as a carboxypeptidase with a preference for C-terminal hydrophobic or basic residues (reviewed in Refs 3,37). It is expressed mainly in the heart, kidney and testis and is important for the regulation of blood pressure and cardiac function. Interestingly, ACE2 is not inhibited by ACE inhibitors such as lisinopril, captopril and enalaprilat.
Crystal structure of tACE
One of the main impediments to determining the structure of ACE by X-ray crystallography was the production of diffraction-quality crystals. The C-domain of ACE has seven potential N-linked glycosylation sites, six of which are located on the surface of the protein and the seventh in the juxtamembrane region. Two concurrent approaches succeeded in paving the way for the crystallization38 and successful X-ray structure determination of tACE5. Five of the N-linked sites were disrupted by substituting glutamines for each of the asparagine residues in the glycosylation sequences and a truncated form was expressed in the presence of a glucosidase inhibitor yielding crystals suitable for X-ray diffraction. The latter protein was used for the subsequent three-dimensional structure determination of tACE. High-resolution crystal structures of the human tACE and its complex with the widely used inhibitor lisinopril at 2.0 Å resolution were recently reported5.
Drosophila ACE (Ance) contains a single domain similar to tACE. However, it has only three potential N-linked glycosylation sites, which are not required for secretion and enzymatic activity39. Recently, the crystal structures of this homologue (which has considerable similarity to the tACE structure), bound to captopril and lisinopril, were reported40. The wild-type protein was expressed in a baculovirus expression system and the oligosaccharides did not hamper crystallization. However, it is unlikely that this will be the case with the ACE2 homologue, the ACE N-domain or the full-length somatic ACE structures — ACE structural milestones that are still eagerly awaited.
Prominent features of the tACE structure include an abundance of α-helices and a deep central cavity that divides the molecule into two halves, which pack together into an overall ellipsoid shape (Fig. 3). The active site, identified by the catalytic Zn2+ ion bound to the HEXXH sequence (and to lisinopril in its complex with the enzyme), is located in the deep cavity, some 10 Å from its entrance. The amino-terminal helices (α1–3) form a lid-like extension that partially covers the active-site channel, which limits the access of substrates and inhibitors. In fact, the aperture of the channel opening is approximately 3 Å in diameter, which is too small for most peptide substrates, indicating that tACE must undergo some conformational change, possibly associated with its unique chloride ion activation, for the substrate to enter the channel. From the structure of the lisinopril–tACE complex (Fig. 4) it is evident that the phenyl ring of the inhibitor interacts with the S1 sub-site in the active site, the lysine with the S1′ sub-site, and the proline occupies the S2′ sub-site. The carboxyl group, located between the phenylpropyl group and the lysine, binds to the Zn2+ in the active site and also forms a hydrogen bond with the side-chain carboxylate of Glu384. Other key interactions occur between the side-chain amino group of the inhibitor lysine and Glu162 of tACE, and between the C-terminal proline carboxyl group with Lys511 and Tyr520. The binding of the inhibitor to the S1, S1′ and S2′ pockets and its zinc coordination form the basis for the structure-guided design of improved domain-selective ACE inhibitors. In addition, two buried chloride ions that are important for the activation of the enzyme were identified in the crystal structure (outside the active site), both distant (20.7 Å and 10.4 Å, respectively) from the catalytic Zn2+ ion. The first is bound to Arg186, Arg489 and Trp485, whereas the second is bound to Arg522 and Tyr224. The structure is indicative of an indirect mechanism for chloride activation, possibly through effects on active-site structure. This study also revealed that the structure of tACE (MA sub-clan of M clan of peptidases (M clan peptidases are metalloenzymes and the metal is involved in catalysis)) resembles that of rat neurolysin41 and a newly identified carboxypeptidase from the hyperthermophilic archaeon Pyrococcus furiosus42 — both of which are members of the MA clan — despite low sequence similarity.
Figure 3. Overview of the tACE structure5.
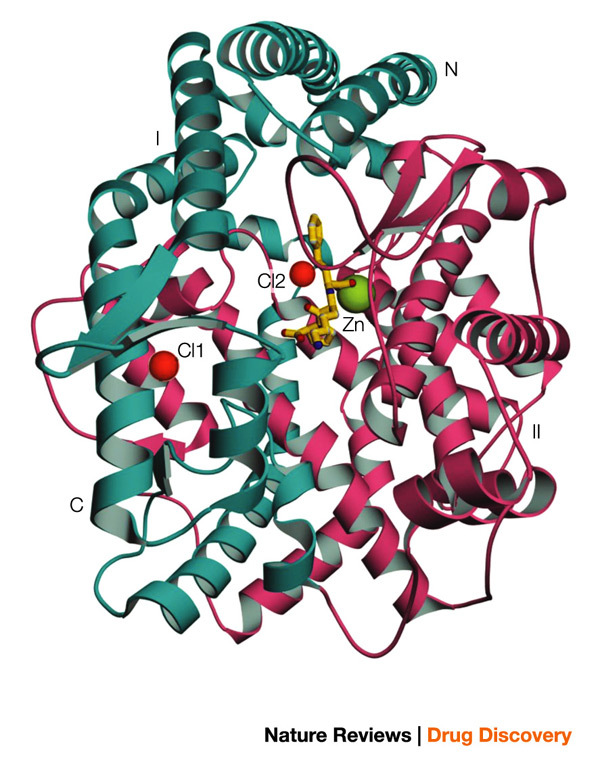
The molecule can be divided into two halves, sub-domains I and II (shown in cyan and pink colour, respectively). The two bound chloride ions are shown in red. The catalytic site zinc ion and the inhibitor lisinopril molecule are shown in green and yellow, respectively. tACE, testis angiotensin-converting enzyme.
Figure 4. Ball-and-stick representation of the active site of tACE with the inhibitor molecule (lisinopril) in yellow5.
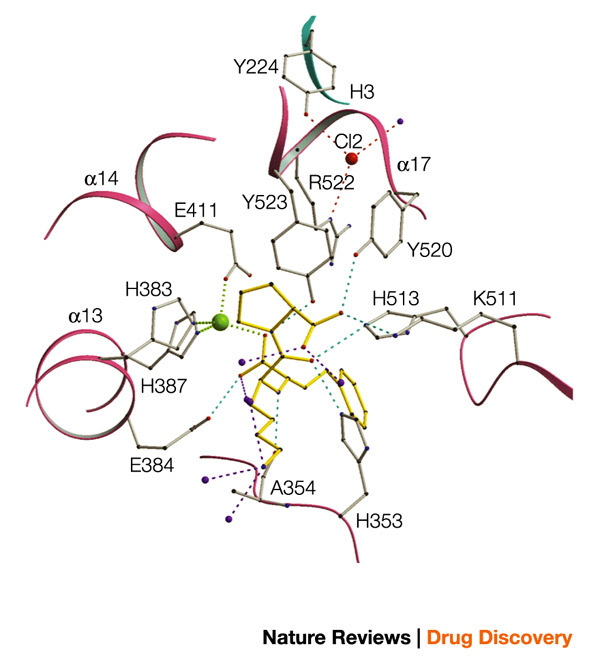
The zinc atom is in green, chloride ion in red and water molecules in purple. tACE, testis angiotensin-converting enzyme.
Structure-based modelling of the N-domain of somatic ACE (using the three-dimensional structure of tACE5, which has ∼60% amino-acid sequence identity to somatic ACE) reveals some interesting features. First, notable differences in hydrophobicity and charge are observed in the lid-like structure comprising helices α1, α2 and α3 (using tACE nomenclature; Fig. 2). This part of the structure seems to affect the substrate specificity of the N- or C-domain, as tACE mutants which had this region replaced by the corresponding N-domain sequence showed a preference for N-domain substrates43 (Z. L. Woodman et al., unpublished data). Second, the Zn2+-binding site with the canonical HEXXH motif is conserved. Third, it has been shown that the C-domain has greater chloride dependence than the N-domain, both in terms of substrate hydrolysis and inhibitor binding19,24. From the model we can predict that Arg186 (a key residue for binding one of the two chloride ions in tACE) is replaced by His164 in the N-domain. So, in the N-domain only one chloride-ion-binding pocket is plausible, involving Arg500 and Tyr202. Fourth, positioning of the lisinopril molecule in the active site of the N-domain model revealed that the full complement of structurally conserved residues was found as observed in the tACE structure, confirming that the N-domain of somatic ACE could also bind lisinopril with similar affinity, as previously reported24,44. Fifth, the S2 sub-site is formed by Asn494 and Thr496, which replace a serine and a valine, respectively, in the C-domain. Furthermore, the asparagine occurs in an N-GLYCOSYLATION SEQUON (NVT) that is unique to the N-domain; the attachment of any glycan to this residue would occlude the S2 pocket. This, in part, might explain how the bulky aromatic ring of the benzamido group in keto-ACE (an ACE inhibitor; see Fig. 5a) is accommodated by the S2 site in the C-domain (tACE), making keto-ACE more C-domain selective. Sixth, the ACE inhibitor RXP407 has an N-acetyl group that confers a certain degree of N-domain specificity22. The model of RXP407 and the N-domain reveals two residues, Arg381 and Tyr369, that form van der Waals interactions with the N-acetyl carbonyl/methyl groups (Fig. 5d). However, a glutamate and phenylalanine are substituted for these two residues in the C-domain and might exert repulsive forces in the binding of the inhibitor to the C-domain active site.
Figure 5. Models of interactions between inhibitors and the active sites of ACE.
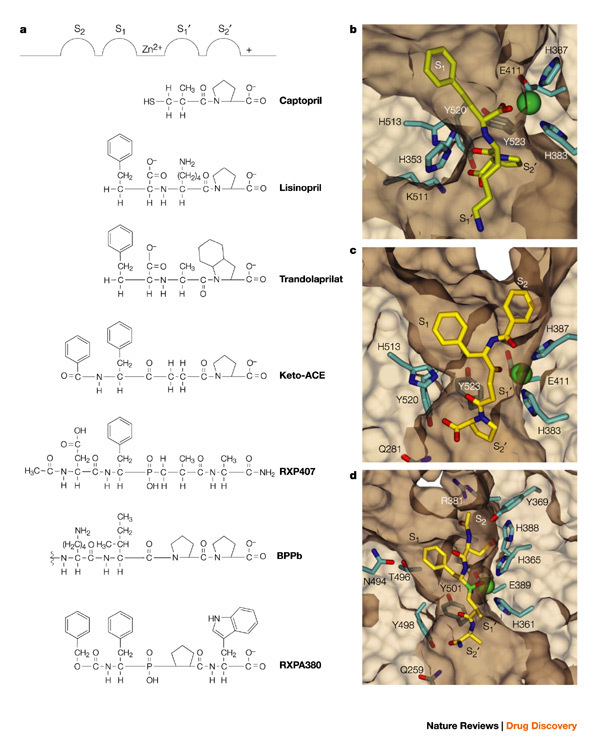
a | Classical representation of inhibitor binding to the 'generic' ACE active site53,62. b–d | Three-dimensional structural representation of inhibitor-binding pockets in the tACE–lisinopril complex (b)5, the modelled tACE–keto-ACE complex (c) and the modelled N-domain–RXP407 complex (d). The different sub-sites are marked as in Fig. 5a. ACE, angiotensin-converting enzyme; tACE, testis ACE.
Homology modelling of ACE2 using the atomic coordinates of the tACE structure revealed differences in the ligand-binding pockets of the two homologues that account for their substrate and inhibitor selectivity105. First, the accommodation of the S2′ sub-site in tACE increases the substrate-binding cavity and permits the binding of an additional amino acid to the obligatory binding site; second, Gln281, which interacts with the carboxyl terminus of lisinopril in tACE, is replaced by an arginine in ACE2. This represents an elongation of the side chain of residue 281, which causes steric conflict with the P2′ residue of lisinopril when it is docked in the active site of the ACE2 model. In addition, the substitution of the leucine and phenylalanine for the hydrogen-bonding Lys511 and Tyr520, respectively, and replacement of Thr282 in tACE by the more bulky Phe274 residue, probably account for the changes in substrate specificity. There are no striking differences in the large S1′ sub-sites of tACE and ACE2 except for a proline in ACE2 which corresponds to Ala354 in tACE.
First-generation ACE inhibitors
The story of the design and synthesis of the first orally active, potent inhibitors of ACE is one of the great success stories of modern medicinal chemistry. It has been described as one of the first examples of true 'rational drug design'3, and although this might not be true in the sense that we understand that term today, the design of the ACE inhibitors in the late 1970s and early 1980s was certainly based on a series of brilliant insights that, together with a sprinkling of serendipity, constituted what might now be called 'rational intuition'.
It is not our intention to provide a comprehensive review of the events leading to the design and synthesis of captopril, enalaprilat and lisinopril, the original group of potent ACE inhibitors that formed the basis for all subsequent compounds of what can now be termed first-generation ACE inhibitors. Several excellent reviews of this history have been published during the past two decades, especially by the inventors themselves45,46,47,48,49,50. However, it is instructive to consider some of the key insights that led to these drugs, especially in the context of the crystal structure that is now available and the structure-guided drug design of second-generation ACE inhibitors that can now be undertaken.
A role for a metal in the catalytic mechanism had been suspected since the discovery of the enzyme16 and was confirmed in the 1970s51,52, leading to the proposal that ACE was mechanistically similar to carboxypeptidase A (CPA)53,54. We now know that ACE is unrelated to the CPA class of enzymes, but instead falls into the group of metallo-endopeptidases characterized by the HEXXH zinc-binding motif55 (CPA possesses HXXE). Nevertheless, the use of CPA as a model led to key conceptual insights in inhibitor design, because CPA was much better understood and its crystal structure was known. Cushman and Ondetti reasoned that ACE was an exopeptidase like CPA, with the difference that ACE cleaved the penultimate peptide bond to release a dipeptide product.
The second major breakthrough in inhibitor design derived from an earlier observation that an extract from the South American pit viper Bothrops jararaca, known as bradykinin potentiating factor (BPF), could inhibit ACE56,57,58. BPF was a mixture of peptides59, which were shown to be potent and specific inhibitors of ACE (Table 1), and structure–activity studies indicated that the optimal C-terminal inhibitory sequence was Phe-Ala-Pro60. This work led to the proposal that the venom peptides were substrate analogues that bound competitively to the obligatory substrate-binding sites in the ACE active site (Fig. 5a). What was needed were orally active, non-peptide analogues of BPF.
The third key insight derived from work by Byers and Wolfenden61 describing a new design concept for inhibitors of CPA based on benzylsuccinic acid, referred to as by-product analogues. Cushman and Ondetti recognized that part of the binding affinity of benzylsuccinic acid derived from coordination of the active-site zinc by the carboxyl group, and predicted that a similar succinylamino acid derivative would inhibit ACE if its structure was analogous to the dipeptide product of ACE activity. On the basis of the Phe-Ala-Pro sequence derived from the BPF peptides, they synthesized methylsuccinyl-Pro (analogous to carboxy-Ala-Pro) and indeed found it to be a specific inhibitor, with an IC50 of 22 μM53. Cushman and Ondetti then searched for a superior zinc-binding group and the potency breakthrough was achieved by replacing the carboxyl with a sulphydryl group, yielding captopril with an IC50 of 23 nM53,54 (Fig. 5a).
Captopril became the first ACE inhibitor in clinical use (first approved in 1981) and rapidly established itself as a powerful new therapeutic agent in the treatment of hypertension and heart failure. Reports of captopril-related side effects, such as loss of taste and skin rash, prompted Patchett and colleagues to focus on the design of non-sulphydryl ACE inhibitors, by reverting to carboxyl compounds and introducing additional functionalities that would complete the by-product design. Captopril did not make use of at least two potential binding sites: the S1 binding site and a hydrogen-bonding site for the amide nitrogen of the (substrate) scissile bond (Fig. 5a). So, structure–activity studies were performed on a series of N-carboxyalkyl dipeptides of the general formula R-CHCO2H-A1-A2. The R group, occupying the S1 pocket, was best served by benzylmethylene, whereas A1-A2 was either Ala-Pro (as in the BPF peptides) or, surprisingly, Lys-Pro, leading to enalaprilat and lisinopril, respectively (Fig. 5a)62, which show nanomolar inhibition constants.
Enalaprilat and lisinopril are essentially tripeptide analogues with a Zn2+-coordinating carboxyl group substituting for the substrate scissile amide carbonyl. Enalaprilat closely resembles the Phe-Ala-Pro sequence that was found to be the optimal C-terminal sequence among the venom peptides. The structure of the ACE–lisinopril complex confirms that lisinopril makes extensive contacts with the active site, including occupation of the S1, S1′ and S2′ pockets, binding of a lysine by the C-terminal carboxylate, a hydrogen bond involving the (substrate) scissile amide nitrogen, and coordination of the active-site Zn2+ by the carboxyalkyl carboxylate5 (Fig. 4). Lisinopril binds with twofold greater affinity than enalaprilat63, probably because the lysine side chain makes better contacts with the deep S1′ pocket, including a weak hydrogen bond between the lysyl ε-amine and Glu162 (Ref. 5).
The design of captopril, enalaprilat and lisinopril was later extended by others, and a total of 17 ACE inhibitors have been approved for clinical use4. The later compounds are all variations on the original theme, with most of the differences residing in the functionalities that bind the active-site zinc and the S2′ pocket48. The different types of zinc-coordinating groups are of interest, because these have also found application in the design of inhibitors for other metalloproteases, such as the matrix metallopeptidases. On the basis of work first described by Holmquist and Vallee64, phosphonates have proven useful22,23, as have hydroxamates65, ketones66 and silanediols67.
Clinical profile of ACE inhibitors
Since their introduction in 1981, ACE inhibitors have been studied extensively (for recent reviews, see Refs 4,68,69). ACE inhibitors are first-line therapy for hypertension, congestive heart failure, left ventricular systolic dysfunction and myocardial infarction, and are recommended for slowing the progression of diabetic and non-diabetic nephropathy68,70. More recently, ACE inhibitors have also been shown to slow the progression of atherosclerotic vascular disease. This vascular benefit has been attributed to a direct vascular protective and anti-atherogenic effect of ACE inhibitors, because it has been observed even in normotensive individuals69.
A major debate concerns the mechanism by which cardiovascular benefits are conferred by ACE inhibitors. It is generally accepted that Ang II not only has direct pressor effects and stimulates salt and water retention (via aldosterone release), but also stimulates myocyte proliferation and exerts pro-atherogenic effects via the induction of oxidative stress, endothelial dysfunction and vascular inflammation71,72. However, there is also considerable evidence that some of the benefits of ACE inhibition derive from potentiation of BK signalling, which stimulates release of the vasodilator nitric oxide and of the fibrinolytic protein tissue plasminogen activator72,73. Indeed, co-administration of the specific BK-receptor antagonist icatibant significantly attenuated the hypotensive effect of captopril in both normotensive and hypertensive subjects74.
Further complicating this debate is the recent appreciation that the RAS is more complex than originally thought. There are multiple Ang peptides and at least three or four Ang receptors, some of which have opposing activities (Fig. 1). For instance, the principal Ang II receptor, the AT1 receptor receptor, mediates the well-known effects of Ang II described above, but the AT2 receptor, which has a more limited tissue distribution, mediates largely opposing effects. Similarly, Ang1–7, which is derived from both Ang I and Ang II by the action of various peptidases including ACE and ACE2, opposes the actions of Ang II3,37. Moreover, as already discussed, ACE also acts on non-Ang peptides, including BK, substance P, luteinizing hormone-releasing hormone (LH-RH) and the haemoregulator AcSDKP; with the exception of BK, the importance of these peptides for the cardiovascular effects of ACE inhibitors is unknown.
Some insights might derive from comparisons of the efficacy and side-effect profiles of ACE inhibitors and AT1-receptor blockers (ARBs). ACE inhibitors are generally well tolerated, but certain class-specific side effects have emerged, in particular cough and angioedema70. The incidence of cough has been estimated at 5–20% of patients and might result in the discontinuation of treatment70,75. Angioedema affects 0.1–0.5% of patients and can be life-threatening76. Both cough and angioedema have been attributed to alterations in levels of non-Ang peptides, especially raised BK concentration. Recently, an association has been found between ACE-inhibitor-related angioedema and low plasma levels of aminopeptidase P, an enzyme that is also involved in the metabolism of BK, indicating that these individuals are at risk for developing angioedema when treated with ACE inhibitors76. The potentiation of BK signalling by ACE inhibitors (Fig. 1) seems to result not only from reduced BK degradation but also from inhibition of desensitization of the B2 BK receptor, possibly by inducing crosstalk between ACE and the B2 receptor77,78,79. Therefore, both the benefits and side effects arising from increased BK signalling by ACE inhibitors seem to be mechanistically complex and have implications for the design of N- or C-selective inhibitors (see below).
Alternatives to ACE inhibitors
The ARBs were introduced more recently but have also been studied extensively4,80. As is the case for ACE inhibitors, ARBs have been shown to be effective in the treatment of hypertension and heart failure, in reducing cardiovascular morbidity and mortality, and in slowing the progression of nephropathy. Studies in myocardial infarction and heart failure have indicated a trend towards lower mortality in patients treated with ACE inhibitors versus ARBs, leading to the recommendation that ACE inhibitors remain the first-line agents in these conditions4,75,81,82. This difference might be due to the additional effect of ACE inhibitors on BK, although this comes at the cost of increased side effects75,81. Interestingly, and contrary to earlier assumptions, angioedema has also been reported with ARB therapy75,83, which might be related to unopposed activation of the AT2 receptor leading to increased BK concentrations73.
In light of the enormous importance of the RAS in cardiovascular pathophysiology, there is continued interest in novel compounds that target this system4. Eplerenone, the first selective aldosterone antagonist, was approved for the treatment of hypertension in September 2002 and will probably also find use in the treatment of severe heart failure7. An orally active renin inhibitor, aliskiren, is now in clinical development for hypertension84.
Of particular interest are the vasopeptidase inhibitors, which are dual ACE–neutral endopeptidase (NEP) inhibitors, of which omapatrilat is the most advanced in clinical development. NEP, also a zinc-metallopeptidase, is the principal enzyme responsible for the degradation of natriuretic peptides, which are vasodilatory and diuretic peptides that reduce volume loading and are therefore beneficial in both hypertension and heart failure85. As expected, omapatrilat was equivalent or superior to ACE inhibitors in clinical trials, but was found to be associated with a significantly higher incidence of angioedema, which has delayed its approval86. The higher incidence of angioedema is probably related to the fact that NEP also inactivates BK (Fig. 1), and its inhibition might result in even higher plasma BK levels, which together with raised concentrations of natriuretic peptides (and potentially other vasodilatory peptides) might promote vasodilation-related adverse events4,86. NEP might also be required for the removal of the neurotoxic amyloid β-peptide, and so chronic NEP inhibition could lead to neurodegeneration87.
The clinical results with omapatrilat have had a sobering effect on the field88 and have indicated that broad inhibition of vasoregulatory peptides should be approached with caution89. Indeed, overly vigorous inhibition of neuro-hormonal activation during heart failure, by simultaneous treatment with an ACE inhibitor, an ARB and a β-blocker was associated with excess mortality in the Valsartan Heart Failure Trial (Val-HeFT)90. So, efforts to develop triple inhibitors of ACE, NEP and endothelin-converting enzyme-1 (ECE-1)91 might need to be re-evaluated. ECE-1, which is structurally and functionally related to NEP, is the principal activating enzyme for the potent vasopressor peptides endothelin-1 and -3 (Ref. 92), and so triple inhibitors might show even greater efficacy than single ACE or dual ACE/NEP inhibitors. However, there is clearly an even greater potential for adverse events such as angioedema.
Second-generation ACE inhibitors
In light of these developments, highly specific, single-domain inhibitors of ACE offer an attractive alternative. As we have attempted to show in this review, our understanding of the RAS (and related vasoregulatory systems) has come a long way since the introduction of the first ACE inhibitors. The N- and C-domain sites of ACE hydrolyse Ang I and BK at comparable rates in vitro, but in vivo it seems that the C-domain is primarily responsible for regulating blood pressure93,94. This might indicate that a C-selective inhibitor would have a profile comparable to current mixed inhibitors, but this is not necessarily the case.
First, whereas Ang I is hydrolysed predominantly by the C-domain in vivo94, BK is hydrolysed by both domains23 and therefore selective inhibition of the C-domain site will allow some level of BK degradation to continue, catalysed by the N-domain. This could be sufficient to prevent the excessive BK accumulation that has been observed during attacks of angioedema86. Second, BK potentiation by B2 receptor resensitization is maximal when both the N- and C-domains are inhibited95, indicating that a pure C-selective inhibitor will have a lower propensity for excessive BK stimulation. Third, the multiple Ang and non-Ang peptides known to be vasoactive are not hydrolysed equally by the two domains3,96, making it likely that the ratio of vasopressor to vasodilator peptides will differ between C-selective and mixed inhibitors. So, a highly selective C-domain inhibitor has the potential for effective blood pressure control with reduced vasodilator-related side effects.
In contrast to a C-selective inhibitor, an N-selective inhibitor might open up novel therapeutic areas. As discussed, the N-domain seems to play a minor role in blood pressure control in vivo. At least three physiologically important peptides are hydrolysed preferentially or exclusively by the N-domain: LH-RH, Ang1–7 and AcSDKP21,44,96. The contribution of ACE to the metabolism of LH-RH and Ang1–7 in vivo is unclear, but there is increasing evidence that ACE is the principal metabolizing enzyme for AcSDKP, a natural haemoregulatory hormone97. AcSDKP has antiproliferative and antifibrotic activities, and might have utility in protecting haematopoietic stem cells against chemotherapy-induced injury97 and in limiting cardiac fibrosis98. Administration of ACE inhibitors results in a four to sixfold elevation of AcSDKP plasma levels94,97. This might be the basis for the observed association between ACE inhibitors and anaemia, and the effective treatment of altitude polycythaemia by the ACE inhibitor enalaprilat99.
Design of domain-selective ACE inhibitors
Current-generation ACE inhibitors in clinical use are essentially mixed N- and C-domain inhibitors48. Although a modest degree of domain selectivity can be observed in some cases, this is not likely to be clinically significant. Nevertheless, these differences might be instructive and can guide future attempts to develop highly domain-selective inhibitors. Captopril has been noted to be modestly N-selective, depending on Cl− concentration, whereas lisinopril and enalaprilat are more C-selective24,44. More recently, keto-ACE, originally described in 1980 (Ref. 66), was found to exhibit a 40–50-fold C-selectivity96; one of the BPP peptides, BPPb, was shown to be 300-fold more C-selective100; and the phosphinic tetrapeptide RXPA380 is 3,000-fold more C-selective23. By contrast, BPP-12b is 30-fold, and the phosphinic tetrapeptide RXP407 1,000-fold, more N-selective22,101 (Table 1).
Examination of these compounds reveals a number of features (Table 1 and Fig. 5a). Captopril is the smallest inhibitor and can be viewed as an N-thioalkyl derivative of the dipeptide Ala-Pro, whereas enalaprilat, lisinopril and keto-ACE are tripeptide analogues of Phe-Ala-Pro, Phe-Lys-Pro and Phe-Gly-Pro, respectively. This might indicate that a bulky P1 group — present in enalaprilat, lisinopril and keto-ACE, but absent in captopril — confers C-selectivity, and that a larger P1′ side chain also promotes C-selectivity (Fig. 5b), because lisinopril is more C-selective than enalaprilat. Interestingly, trandolaprilat, although a potent inhibitor for both domains, was tenfold more C-selective24 (Table 1). Trandolaprilat contains a C-terminal hexahydroindoline group, which also indicates that a bulky P2′ group confers C-selectivity. This is confirmed by results from radioligand-binding studies, which indicated that both perindoprilat and quinaprilat, which contain hexahydroindoline and tetrahydroisoquinoline groups, respectively, in the P2′ position, were 45–180-fold more C-selective102. Similarly, the highly C-selective tetrapeptide RXPA380 also contains a bulky methylindole group in the P2′ position. These studies also indicated that lisinopril and 351A (a hydroxybenzamidine analogue of lisinopril) were 110–146-fold more C-selective, reinforcing the importance of a bulky P1′ group103.
On the other hand, structure–activity studies performed with a series of phosphinic tetrapeptides indicated that a phenylalanine in the P1 position did not confer C-selectivity. Instead, the single most important determinant for N-selectivity was an amidated C-terminal carboxyl in the P2′ position, followed by an acidic group in the P2 position (Fig. 5d)22. The bradykinin potentiating peptides (BPPs) confirm the conclusion regarding the P2 group: BPPa, BPPb, BPPc and BPP2 all end with the sequence Ile-Pro-Pro, yet only BPPc is unselective. BPPc has a proline in the P2 position versus a lysine in the most C-selective peptide, BPPb100. Similarly, the C-selectivity of keto-ACE can probably be ascribed to a bulky P2 benzyl group (Fig. 5c); the selectivity of RXPA380 is also consistent with the proposed importance of a phenyl P2 group (Fig. 5a). Taken together, these data indicate that C-selectivity is conferred by bulky P1′ and P2′ groups and a large, neutral or basic P2 group, whereas N-selectivity is conferred by a blocked C-terminal carboxyl and an acidic P2 group.
The ACE C-domain crystal structure revealed that the S1′ pocket was surprisingly deep, easily accommodating the lysyl group of lisinopril, with a hydrogen bond between Glu162 and the ε-amine (Figs 4 and 5b)5. Additional modifications to the P1′ group will potentially further enhance C-selectivity. Moreover, the C-terminal carboxylate of lisinopril was found to bind to Lys511, explaining the importance of a free C-terminal (P2′) carboxyl for binding to the C-domain active site22. Binding to Lys511, instead of to an arginine (as originally predicted), might prompt investigation of functionalities other than carboxylates in this position. Since N-selectivity is conferred by a blocked P2′ carboxylate, it can also be predicted that this sub-site is significantly different in the N-domain active site. Modelling of the S2 sub-site in the N-domain has also revealed differences, as expected (see earlier), confirming its potential utility for conferring domain selectivity (Fig. 5c,d). These considerations can form the starting point for the structure-guided design of domain-selective inhibitors, which will be refined further once the N-domain structure becomes available.
An important caveat in considering the design and pharmacological utility of domain-selective ACE inhibitors is the potential for conformational effects that have not yet been observed in the tACE crystal structure. For instance, it is unknown whether chloride binding and dissociation result in significant movement of the N-terminal 'lid' formed by helices α1, α2 and α3, and thereby restrict substrate access5. Even more importantly, the physical orientation of the N- and C-domains in somatic ACE is unknown, as is whether there is any significant degree of domain interaction or cooperativity. Inhibitor titrations in vitro44 and studies with domain-selective inhibitors in vivo23 have provided indirect evidence for some form of domain interaction, which could have significant effects on the pharmacological profile of domain-selective inhibitors.
Conclusion
The past decade has seen major advances in structure-based drug design approaches, including technologies such as mass spectrometry, X-ray crystallography and nuclear magnetic resonance. These are important tools in structural proteomics and to some extent have eliminated the scepticism about the feasibility and value of the structure-based approach. In particular, high-throughput structure-based drug design using protein crystallography has become a very attractive proposition for the pharmaceutical industry. Many examples exist today in which a combination of the three-dimensional structure of the target protein, computer-aided drug design (in silico or virtual screening), and a rational approach using high-throughput screening have produced important lead compounds that are now being evaluated in clinical trials (for recent reviews see Refs 1,2,103,104). In addition to mining the untapped riches of the human proteome, the application of modern structure-based drug design methods to existing drug targets will generate more selective compounds for known disease targets, such as ACE. We expect that next-generation, domain-selective ACE inhibitors will be a result of such endeavours.
Acknowledgements
The ACE research in K.R.A.'s and E.D.S.'s laboratories is supported by the Wellcome Trust, UK. We would like to acknowledge the help of R. Natesh and S. Iyer in the preparation of this manuscript.
Glossary
- DIURETICS
Drugs that increase the net renal excretion of solute and water.
- SYMPATHETIC TONE
The neural activity of the sympathetic nervous system, regulating (through adrenergic receptors) cardiac and vascular function.
- VASCULAR RESISTANCE
The resistance to blood flow, which is directly proportional to the extent of vasoconstriction, is one of the primary determinants of blood pressure.
- GLYCOSYLATION SEQUON
Consensus sequence Asn-X-Ser/Thr whose core glycosylation generally occurs at the Asn residue.
Biographies
K. Ravi Acharya is a Professor of Structural Biology in the Department of Biology and Biochemistry, University of Bath, UK. He received his Ph.D. from Bangalore University, India, in X-ray crystallography and went to the Laboratory of Molecular Biophysics, Oxford, UK, on a postdoctoral fellowship where he was involved in the structural studies of glycogen phosphorylase (with Louise Johnson), α-lactalbumin (with David Phillips) and foot and mouth disease virus (with David Stuart). In 1990 he moved to Bath, where his research is focused on the study of structure–function relationships of proteins involved in inflammatory processes. Earlier this year, his group, in collaboration with Edward Sturrock (UCT, South Africa), determined the three-dimensional structure of human angiotensin-converting enzyme (ACE).
Edward D. Sturrock is a Wellcome Trust International Senior Research Fellow in the Institute of Infectious Disease and Molecular Medicine at the University of Cape Town, South Africa. He received his Ph.D. in 1993 working on the synthesis and metabolism of bile pigments, and went on to do a postdoctoral fellowship at Harvard Medical School, where he investigated the glycosylation and disulphide requirements of ACE. His research interests include structure–function aspects of ACE; structure-based design of ACE inhibitors; the post-translational processing of the membrane-anchored form of the enzyme; and the role of urinary proteins in urolithiasis.
James F. Riordan is a Professor of Biochemistry in the Center for Biochemical and Biophysical Sciences and Medicine at Harvard Medical School, Boston, Massachusetts, USA. He has a long-standing interest in zinc metalloenzymes, particularly carboxypeptidase A and ACE. More recently, he has been focusing on the molecular and cellular biochemistry of angiogenin, a unique member of the ribonuclease family that targets the nucleolus of endothelial cells to induce ribosomal RNA synthesis and ultimately induces angiogenesis.
Mario R. N. Ehlers received his M.B.Ch.B. and Ph.D. (medicine) degrees at the University of Cape Town Medical School, South Africa. He performed postdoctoral work and was appointed Instructor in the Center for Biochemical and Biophysical Sciences and Medicine, Harvard Medical School, and was then appointed Professor and Head, Department of Medical Biochemistry, UCT Medical School; he is now Chief Medical Officer at Pacific Biometrics Inc., Seattle, USA. He has worked extensively on the structure and function of ACE, notably chloride activation, zinc- and inhibitor-binding stoichiometries, and shedding of membrane-bound ACE. More recently he has directed the clinical development of endocrine peptides for metabolic and cardiovascular indications.
Related links
DATABASES
LocusLink
References
- 1.Blundell TL, Jhoti H, Abell C. High-throughput crystallography and lead discovery in drug design. Nature Rev. Drug Discov. 2001;1:45–53. doi: 10.1038/nrd706. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn P, Wilson K, Patch MG, Stevens RC. The genesis of high-throughput structure-based drug discovery. Curr. Opin. Chem. Biol. 2002;6:704–710. doi: 10.1016/S1367-5931(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 3.Turner AJ, Hooper NM. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol. Sci. 2002;23:177–183. doi: 10.1016/S0165-6147(00)01994-5. [DOI] [PubMed] [Google Scholar]
- 4.Zaman MA, Oparil S, Calhoun DA. Drugs targeting the renin–angiotensin–aldosterone system. Nature Rev. Drug Discov. 2002;1:621–636. doi: 10.1038/nrd873. [DOI] [PubMed] [Google Scholar]
- 5.Natesh R, Schwager SLU, Sturrock ED, Acharya KR. Crystal structure of the human angiotensin-converting enzyme–lisinopril complex. Nature. 2003;421:551–554. doi: 10.1038/nature01370. [DOI] [PubMed] [Google Scholar]
- 6.Flower RJ. The development of COX2 inhibitors. Nature Rev. Drug Discov. 2003;2:179–191. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- 7.Brown R, Quirk J, Kirkpatrick P. Eplerenone. Nature Rev. Drug Discov. 2003;2:177–178. doi: 10.1038/nrd1039. [DOI] [PubMed] [Google Scholar]
- 8.Simons, F. E. Comparative pharmacology of H1 antihistamines: clinical relevance. Am. J. Med.113 (Suppl. 9A), S38–S46. [DOI] [PubMed]
- 9.Pickering G. Hypertension: Pathophysiology, Diagnosis, and Management. 1990. pp. 3–16. [Google Scholar]
- 10.Wolf-Maier K, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 11.Sealey JE, Laragh JH. Hypertension: Pathophysiology, Diagnosis, and Management. 1990. pp. 1287–1317. [Google Scholar]
- 12.Goldblatt H, Lynch J, Hanzal RF, Summerville WN. Studies on experimental hypertension. I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J. Exp. Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houssay BA, Fasciolo JC. Secretion hipertensora del rinon isquemaido. Rev. Soc. Argent. Biol. 1937;13:284–294. [Google Scholar]
- 14.Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the interaction between renin and renin activator. J. Exp. Med. 1940;71:29. doi: 10.1084/jem.71.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skeggs LT, Marsh WH, Kahn JR, Shumway NP. The purification of hypertensin I. J. Exp. Med. 1954;100:363–370. doi: 10.1084/jem.100.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skeggs LT, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J. Exp. Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corvol P, Williams TA, et al. Handbook of Proteolytic Enzymes. 1998. pp. 1066–1076. [Google Scholar]
- 18.Soubrier F, et al. Two putative active centers in human angiotensin 1-converting enzyme revealed by molecular cloning. Proc. Natl Acad. Sci. USA. 1988;85:9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei L, Alhenc-Gelas F, Corvol P, Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J. Biol. Chem. 1991;266:9002–9008. [PubMed] [Google Scholar]
- 20.Jaspard E, et al. Differences in properties and enzymatic specificities between the two active sites of angiotensin 1-converting enzyme. J. Biol. Chem. 1993;268:9496–9503. [PubMed] [Google Scholar]
- 21.Rousseau A, Michaud A, Chauvet M-T, Lenfant M, Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J. Biol. Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 22.Dive V, et al. RXP 407, a phosphinic peptide, is a potent inhibitor of angiotensin I converting enzyme able to differentiate between its two active sites. Proc. Natl Acad. Sci. USA. 1999;96:4330–4335. doi: 10.1073/pnas.96.8.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geordiadis D, Beau F, Czarny B, Cotton J, Yiotakis A, Dive V. Roles of the two active sites of somatic angiotensin-converting enzyme in the cleavage of angiotensin I and bradykinin. Circ. Res. 2003;93:148–154. doi: 10.1161/01.RES.0000081593.33848.FC. [DOI] [PubMed] [Google Scholar]
- 24.Wei L, Clauser E, Alhenc-Gelas F, Corvol P. The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J. Biol. Chem. 1992;267:13398–13405. [PubMed] [Google Scholar]
- 25.Hubert C, Houot AM, Corvol P, Soubrier F. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J. Biol. Chem. 1991;266:15377–15383. [PubMed] [Google Scholar]
- 26.Ehlers MRW, Fox EA, Strydom DJ, Riordan JF. Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc. Natl Acad. Sci. USA. 1989;86:7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagaman JR, et al. Angiotensin-converting enzyme and male fertility. Proc. Natl Acad. Sci. USA. 1998;95:2552–2557. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigat B, et al. An insertion/deletion polymorphism in the angiotensin 1-converting enzyme gene accounting for half of the variance of serum enzyme levels. J. Clin. Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu T, Chen X, Xu X. Angiotensin converting enzyme gene insertion/deletion polymorphism and cardiovascular disease: therapeutic implications. Drugs. 2002;62:977–993. doi: 10.2165/00003495-200262070-00001. [DOI] [PubMed] [Google Scholar]
- 30.Jones A, Montgomery HE, Woods DR. Human performance: a role for the ACE genotype? Exerc. Sport Sci. Rev. 2002;30:184–190. doi: 10.1097/00003677-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Woods DR, Brull D, Montgomery HE. Endurance and the ACE I/D polymorphism. Sci. Prog. 2000;83:317–336. [PubMed] [Google Scholar]
- 32.Ehlers MRW, et al. Proteolytic release of membrane-bound angiotensin-converting enzyme: role of the juxtamembrane stalk sequence. Biochemistry. 1996;35:9549–9559. doi: 10.1021/bi9602425. [DOI] [PubMed] [Google Scholar]
- 33.Woodman ZL, et al. Shedding of somatic angiotensin-converting enzyme (ACE) is inefficient compared with testis ACE despite cleavage at identical stalk sites. Biochem. J. 2000;347:711–718. doi: 10.1042/bj3470711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isaac RE, Ekbote U, Coates D, Shirras AD. Insect angiotensin-converting enzyme. A processing enzyme with broad substrate specificity and a role in reproduction. Ann. NY Acad. Sci. 1999;897:342–347. doi: 10.1111/j.1749-6632.1999.tb07904.x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor CA, Coates D, Shirras AD. The Acer gene of Drosophila codes for an angiotensin-converting enzyme homologue. Gene. 1996;181:191–197. doi: 10.1016/S0378-1119(96)00503-3. [DOI] [PubMed] [Google Scholar]
- 36.Coates D, et al. Functional conservation of the active sites of human and Drosophila angiotensin I-converting enzyme. Biochemistry. 2000;39:8963–8969. doi: 10.1021/bi000593q. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson U, Danilczyk U, Penninger JM. Just the beginning: novel functions for angiotensin-converting enzymes. Curr. Biol. 2002;12:R745–R752. doi: 10.1016/S0960-9822(02)01255-1. [DOI] [PubMed] [Google Scholar]
- 38.Gordon K, et al. Deglycosylation, processing and crystallization of human testis angiotensin converting enzyme. Biochem. J. 2003;371:437–442. doi: 10.1042/bj20021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams TA, et al. Drosophila melanogaster angiotensin I-converting enzyme expressed in Pichia pastoris resembles the C domain of the mammalian homologue and does not require glycosylation for secretion and enzymic activity. Biochem J. 1996;318:125–131. doi: 10.1042/bj3180125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HO, et al. Crystal structure of Drosophila angiotensin I-converting enzyme bound to captopril and lisinopril. FEBS Lett. 2003;538:65–70. doi: 10.1016/S0014-5793(03)00128-5. [DOI] [PubMed] [Google Scholar]
- 41.Brown CK, et al. Structure of neurolysin reveals a deep channel that limits substrate access. Proc. Natl Acad. Sci. USA. 2001;98:3127–3132. doi: 10.1073/pnas.051633198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arndt JW, et al. Crystal structure of a novel carboxypeptidase from the hyperthermophilic archaeon Pyrococcus furiosus. Structure. 2002;10:215–224. doi: 10.1016/S0969-2126(02)00698-6. [DOI] [PubMed] [Google Scholar]
- 43.Marcic B, et al. Effects of the N-terminal sequence of ACE on the properties of its C-domain. Hypertension. 2000;36:116–121. doi: 10.1161/01.HYP.36.1.116-a. [DOI] [PubMed] [Google Scholar]
- 44.Ehlers MRW, Riordan JF. Angiotensin-converting enzyme: zinc- and inhibitor-binding stoichiometries of the somatic and testis isozymes. Biochemistry. 1991;30:7118–7126. doi: 10.1021/bi00243a012. [DOI] [PubMed] [Google Scholar]
- 45.Ondetti MA, Cushman DW. Enzymes of the renin–angiotensin system and their inhibitors. Annu. Rev. Biochem. 1982;51:283–308. doi: 10.1146/annurev.bi.51.070182.001435. [DOI] [PubMed] [Google Scholar]
- 46.Patchett AA, Cordes EH. The design and properties of N-carboxyalkyldipeptide inhibitors of angiotensin-converting enzyme. Adv. Enzymol. Relat. Areas Mol. Biol. 1985;57:1–84. doi: 10.1002/9780470123034.ch1. [DOI] [PubMed] [Google Scholar]
- 47.Cushman DW, Ondetti MA. Design of angiotensin converting enzyme inhibitors. Nature Med. 1999;5:1110–1113. doi: 10.1038/13423. [DOI] [PubMed] [Google Scholar]
- 48.Menard J, Patchett AA. Angiotensin-converting enzyme inhibitors. Adv. Prot. Chem. 2001;56:13–75. doi: 10.1016/s0065-3233(01)56002-7. [DOI] [PubMed] [Google Scholar]
- 49.Patchett AA. Natural products and design: interrelated approaches in drug discovery. J. Med. Chem. 2002;45:5609–5616. doi: 10.1021/jm020424z. [DOI] [PubMed] [Google Scholar]
- 50.Smith CG, Vane JR. The discovery of captopril. FASEB J. 2003;17:788–789. doi: 10.1096/fj.03-0093life. [DOI] [PubMed] [Google Scholar]
- 51.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 52.Das M, Soffer RL. Pulmonary angiotensin-converting enzyme. Structural and catalytic properties. J. Biol. Chem. 1975;250:6762–6768. [PubMed] [Google Scholar]
- 53.Cushman DW, Cheung HS, Sbo EF, Ondetti MA. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry. 1977;16:5484–5491. doi: 10.1021/bi00644a014. [DOI] [PubMed] [Google Scholar]
- 54.Ondetti MA, Rubin B, Cushman DW. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977;196:441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 55.Vallee BL, Auld DS. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 56.Ng KK, Vane JR. Conversion of angiotensin I to angiotensin II. Nature. 1967;216:762–766. doi: 10.1038/216762a0. [DOI] [PubMed] [Google Scholar]
- 57.Ng KK, Vane JR. Fate of angiotensin I in the circulation. Nature. 1968;218:144–150. doi: 10.1038/218144a0. [DOI] [PubMed] [Google Scholar]
- 58.Bakhle YS. Conversion of angiotensin I to angiotensin II by cell-free extracts of dog lung. Nature. 1968;220:919–921. doi: 10.1038/220919a0. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira SH, Greene LH, Alabaster VA, Bakhle YS, Vane JR. Activity of various fractions of bradykinin potentiating factor against angiotensin I converting enzyme. Nature. 1970;225:379–380. doi: 10.1038/225379a0. [DOI] [PubMed] [Google Scholar]
- 60.Ondetti MA, Williams NJ, Sabo EF, Pluscec J, Weaver ER, Kocy O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry. 1971;10:4033–4039. doi: 10.1021/bi00798a004. [DOI] [PubMed] [Google Scholar]
- 61.Byers LD, Wolfenden R. Binding of the by-product analog benzylsuccinic acid by carboxypeptidase A. Biochemistry. 1973;12:2070–2078. doi: 10.1021/bi00735a008. [DOI] [PubMed] [Google Scholar]
- 62.Patchett AA, et al. A new class of angiotensin-converting enzyme inhibitors. Nature. 1980;288:280–283. doi: 10.1038/288280a0. [DOI] [PubMed] [Google Scholar]
- 63.Bull HG, Thornberry NA, Cordes MH, Patchett AA, Cordes EH. Inhibition of rabbit lung angiotensin-converting enzyme by N α-[(S)-1-carboxy-3-phenylpropyl] l-alanyl-l-proline and N α-[(S)-1-carboxy-3-phenylpropyl] l-lysyl-l-proline. J. Biol. Chem. 1985;260:2952–2962. [PubMed] [Google Scholar]
- 64.Holmquist B, Vallee BL. Metal-coordinating substrate analogs as inhibitors of metalloenzymes. Proc. Natl Acad. Sci. USA. 1979;76:6216–6220. doi: 10.1073/pnas.76.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris RB, Strong PD, Wilson IB. Dipeptide-hydroxamates are good inhibitors of the angiotensin I-converting enzyme. Biochem. Biophys. Res. Commun. 1983;116:394–399. doi: 10.1016/0006-291X(83)90535-1. [DOI] [PubMed] [Google Scholar]
- 66.Almquist RG, Chao WR, Ellis ME, Johnson HL. Synthesis and biological activity of a ketomethylene analogue of a tripeptide inhibitor of angiotensin converting enzyme. J. Med. Chem. 1980;23:1392–1398. doi: 10.1021/jm00186a020. [DOI] [PubMed] [Google Scholar]
- 67.Mutahi MW, Nittoli T, Guo L, Sieburth SM. Silicon-based metalloprotease inhibitors: synthesis and evaluation of silanol and silanediol peptide analogues as inhibitors of angiotensin-converting enzyme. J. Am. Chem. Soc. 2002;124:7363–7375. doi: 10.1021/ja026158w. [DOI] [PubMed] [Google Scholar]
- 68.Sleight P. The renin–angiotensin system: a review of trials with angiotensin-converting enzyme inhibitors and angiotensin receptor blocking agents. Eur. Heart J. 2002;4(Suppl. A):A53–A57. doi: 10.1016/S1520-765X(02)90074-X. [DOI] [Google Scholar]
- 69.Lonn E, Gerstein HC, Smieja M, Mann JFE, Yusuf S. Mechanisms of cardiovascular risk reductions with ramipril: insights from HOPE and HOPE substudies. Eur. Heart J. 2003;5(Suppl. A):A43–A48. doi: 10.1016/S1520-765X(03)90063-0. [DOI] [Google Scholar]
- 70.Bicket DP. Using ACE inhibitors appropriately. Am. Fam. Physician. 2002;66:461–468. [PubMed] [Google Scholar]
- 71.Nickening G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherogenesis. Part I: oxidative stress and atherogenesis. Circulation. 2002;105:393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 72.De Caterina R, Manes C. Inflammation in early atherogenesis: impact of ACE inhibition. Eur. Heart J. 2003;5(Suppl. A):A15–A24. doi: 10.1093/oxfordjournals.ehjsupp.a000771. [DOI] [Google Scholar]
- 73.Murphey L, Vaughan D, Brown N. Contribution of bradykinin to the cardioprotective effects of ACE inhibitors. Eur. Heart J. 2003;5(Suppl. A):A37–A41. doi: 10.1016/S1520-765X(03)90062-9. [DOI] [Google Scholar]
- 74.Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N. Engl. J. Med. 1998;339:1285–1292. doi: 10.1056/NEJM199810293391804. [DOI] [PubMed] [Google Scholar]
- 75.Dickstein K, et al. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomized trial. Lancet. 2002;360:752–760. doi: 10.1016/S0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 76.Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angio-oedema on ACE inhibitors. Lancet. 2002;359:2088–2089. doi: 10.1016/S0140-6736(02)08914-6. [DOI] [PubMed] [Google Scholar]
- 77.Minshall RD, et al. Potentiation of the actions of bradykinin by angiotensin I-converting enzyme inhibitors. The role of expressed human bradykinin B2 receptors and angiotensin-converting enzyme in CHO cells. Circ. Res. 1997;81:848–856. doi: 10.1161/01.RES.81.5.848. [DOI] [PubMed] [Google Scholar]
- 78.Marcic B, Deddish PA, Jackman HL, Erdös EG. Enhancement of bradykinin and resensitization of its B2 receptor. Hypertension. 1999;33:835–843. doi: 10.1161/01.HYP.33.3.835. [DOI] [PubMed] [Google Scholar]
- 79.Benzing T, Fleming I, Blaukat A, Müller-Esterl W, Busse R. Angiotensin-converting enzyme inhibitor ramiprilat interferes with the sequestration of the B2 kinin receptor within the plasma membrane of native endothelial cells. Circulation. 1999;99:2034–2040. doi: 10.1161/01.CIR.99.15.2034. [DOI] [PubMed] [Google Scholar]
- 80.Brunner HR, Gavras H. Angiotensin blockade for hypertension: a promise fulfilled. Lancet. 2002;359:990–992. doi: 10.1016/S0140-6736(02)08062-5. [DOI] [PubMed] [Google Scholar]
- 81.Pitt B, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomized trial — the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355:1582–1587. doi: 10.1016/S0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 82.McKelvie RS, et al. Comparison of candesartan, enalapril, and their combination in congestive heart failure. Randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. Circulation. 1999;100:1056–1064. doi: 10.1161/01.CIR.100.10.1056. [DOI] [PubMed] [Google Scholar]
- 83.Howes LG, Tran D. Can angiotensin receptor antagonists be used safely in patients with previous ACE inhibitor-induced angioedema? Drug Saf. 2002;25:73–76. doi: 10.2165/00002018-200225020-00001. [DOI] [PubMed] [Google Scholar]
- 84.Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–E8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- 85.Weber MA. Vasopeptidase inhibitors. Lancet. 2001;358:1525–1532. doi: 10.1016/S0140-6736(01)06584-9. [DOI] [PubMed] [Google Scholar]
- 86.Messerli FH, Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet. 2000;356:608–609. doi: 10.1016/S0140-6736(00)02596-4. [DOI] [PubMed] [Google Scholar]
- 87.Carson JA, Turner AJ. β-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases? J. Neurochem. 2002;81:1–8,. doi: 10.1046/j.1471-4159.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- 88.Coats AJ. Omapatrilat — the story of Overture and Octave. Int. J. Cardiol. 2002;86:1–4. doi: 10.1016/S0167-5273(02)00389-3. [DOI] [PubMed] [Google Scholar]
- 89.Campbell DJ. Vasopeptidase inhibition: a double-edged sword? Hypertension. 2003;41:383–389. doi: 10.1161/01.HYP.0000054215.71691.16. [DOI] [PubMed] [Google Scholar]
- 90.Cohn JN, et al. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 91.Inguimbert N, et al. N-[2-(Indan-1-yl)-3-mercapto-propionyl] amino acids as highly potent inhibitors of the three vasopeptidases (NEP, ACE, ECE): in vitro and in vivo activities. Bioorg. Med. Chem. Lett. 2002;12:2001–2005. doi: 10.1016/S0960-894X(02)00248-2. [DOI] [PubMed] [Google Scholar]
- 92.Johnson GD, Stevenson T, Ahn K. Hydrolysis of peptide hormones by endothelin-converting enzyme-1. A comparison with neprilysin. J. Biol. Chem. 1999;274:4053–4058. doi: 10.1074/jbc.274.7.4053. [DOI] [PubMed] [Google Scholar]
- 93.Esther CR, et al. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J. Clin. Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Junot C, et al. RXP 407, a selective inhibitor of the N-domain of angiotensin I-converting enzyme, blocks in vivo the degradation of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro with no effect on angiotensin I hydrolysis. J. Pharmacol. Exp. Ther. 2001;297:606–611. [PubMed] [Google Scholar]
- 95.Tom B, de Vries R, Saxena PR, Danser AHJ. Bradykinin potentiation by angiotensin-(1–7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension. 2001;38:95–99. doi: 10.1161/01.HYP.38.1.95. [DOI] [PubMed] [Google Scholar]
- 96.Deddish PA, et al. N-domain-specific substrate and C-domain inhibitors of angiotensin-converting enzyme. Angiotensin-(1–7) and keto-ACE. Hypertension. 1998;31:912–917. doi: 10.1161/01.HYP.31.4.912. [DOI] [PubMed] [Google Scholar]
- 97.Azizi M, Junot C, Ezan E, Ménard J. Angiotensin I-converting enzyme and metabolism of the haematological peptide N-acetyl-seryl-aspartyl-lysyl-proline. Clin. Exp. Pharmacol. Physiol. 2001;28:1066–1069. doi: 10.1046/j.1440-1681.2001.03560.x. [DOI] [PubMed] [Google Scholar]
- 98.Rhaleb NE, et al. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension. 2001;37:827–832. doi: 10.1161/01.HYP.37.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Plata R, et al. Angiotensin-converting-enzyme inhibition therapy in altitude polycythaemia: a prospective randomised trial. Lancet. 2002;359:663–666. doi: 10.1016/S0140-6736(02)07812-1. [DOI] [PubMed] [Google Scholar]
- 100.Cotton J, et al. Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry. 2002;41:6065–6071. doi: 10.1021/bi012121x. [DOI] [PubMed] [Google Scholar]
- 101.Hayashi, et al. The C-type natriuretic peptide precursor of snake brain contains highly specific inhibitors of the angiotensin-converting enzyme. J. Neurochem. 2003;85:969–977. doi: 10.1046/j.1471-4159.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- 102.Perich RB, Jackson B, Johnston CI. Structural constraints of inhibitors for binding at two active sites on somatic angiotensin converting enzyme. Eur. J. Pharmacol. 1994;266:201–211. doi: 10.1016/0922-4106(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 103.Bajorath J. Integration of virtual and high-throughput screening. Nature Rev. Drug Discov. 2002;1:882–894. doi: 10.1038/nrd941. [DOI] [PubMed] [Google Scholar]
- 104.van Dongen M, et al. Structure-based screening and design in drug discovery. Drug Discov. Today. 2002;7:471–478. doi: 10.1016/S1359-6446(02)02233-X. [DOI] [PubMed] [Google Scholar]
- 105.Guy, J. L., Jackson, R. M., Acharya, K. R., Sturrock, E. D., Hooper, N. M. & Turner, A. J. Angiotensin-converting enzyme-2 (ACE-2): comparative modelling of the active site, specificity requirements and chloride dependence. Biochemistry (in the press) [DOI] [PubMed]


