Abstract
Background
Mobile-based interventions appear to be promising in ameliorating huge burdens experienced by patients with type 2 diabetes. However, it is unclear how effective mobile-based interventions are in glycemic management of patients with type 2 diabetes based on real-world evidence.
Objective
This study aimed to evaluate the effectiveness of a mobile-based intervention on glycemic control in patients with type 2 diabetes based on real-world population data.
Methods
This retrospective, propensity score-matched cohort study analyzed longitudinal data from a clinical electronic health database. The study population included 37,913 patients with type 2 diabetes at cohort entry between October 1, 2016, and July 31, 2018. A total of 2400 patients were matched 1:1, using propensity score matching, into the usual care and mobile health (mHealth) groups. The primary outcomes of glycemic control included control rates of glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and postprandial 2-hour blood glucose (P2BG). Mean values and variation trends of difference with 95% CI were the secondary outcomes. The general linear model was used to calculate repeated-measures analyses of variance to examine the differences between the two groups. Subgroup and sensitivity analyses were performed.
Results
Of the 2400 patients included in the analysis, 1440 (60.00%) were male and the mean age was 52.24 years (SD 11.56). At baseline, the control rates of HbA1c, FBG, and P2BG in the mHealth and usual care groups were 45.75% versus 47.00% (P=.57), 38.03% versus 32.76% (P=.07), and 47.32% versus 47.89% (P=.83), respectively. At the 3-, 6-, 9-, and 12-month follow-ups, the mHealth group reported higher control rates of HbA1c than did the usual care group: 69.97% versus 46.06% (P<.001), 71.89% versus 61.24% (P=.004), 75.38% versus 53.44% (P<.001), and 72.31% versus 46.70% (P<.001), respectively. At the four follow-up sessions, the control rates of FBG in the mHealth and usual care groups were statistically different: 59.24% versus 34.21% (P<.001), 56.61% versus 35.14% (P<.001), 59.54% versus 34.99% (P<.001), and 59.77% versus 32.83% (P<.001), respectively. At the four follow-up sessions, the control rates of P2BG in the mHealth group were statistically higher than in the usual care group: 79.72% versus 48.75% (P<.001), 80.20% versus 57.45% (P<.001), 81.97% versus 54.07% (P<.001), and 76.19% versus 54.21% (P=.001), respectively. At the four follow-up sessions, the percentages of HbA1c reduction in the mHealth group were 8.66% (95% CI 6.69-10.63), 10.60% (95% CI 8.66-12.54), 10.64% (95% CI 8.70-12.58), and 8.11% (95% CI 6.08-10.14), respectively. At the four follow-up sessions, the percentages of P2BG reduction in the mHealth group were 8.44% (95% CI 7.41-10.73), 17.77% (95% CI 14.98-20.23), 16.23% (95% CI 13.05-19.35), and 16.91% (95% CI 13.17-19.84), respectively. Starting from the sixth month, the mean HbA1c and P2BG values in the two groups increased slightly.
Conclusions
This mobile-based intervention delivered by a multidisciplinary team can better improve glycemic control rates of patients with type 2 diabetes than usual care. These effects were best sustained within the first 6 months. Starting from the sixth month, intensive management needs to be conducted to maintain long-term effectiveness of the mobile-based intervention.
Keywords: mobile health, glycemic control, type 2 diabetes, propensity score matching
Introduction
The number of adults with diabetes worldwide increased from 108 million to 422 million between 1980 and 2014 [1], with a projected increase to 642 million by 2040 [2]. In China, the overall prevalence of diabetes in the adult population was estimated to be 11.6% in 2010 and was less than 1.0% in 1980 [3]. Patients with type 2 diabetes mellitus account for 90%-95% of those with diabetes [4]. Diabetes not only results in blindness, cardiovascular disease, kidney failure, and other long-term consequences that substantially impact quality of life and years of life lived with disability [5,6], but also increases the risk of cancer and all-cause mortality [7-11]. Therefore, the prevention and control of diabetes is becoming more and more important.
For people with diabetes, a series of cost-effective interventions can improve their health outcomes, regardless of what type of diabetes they may have [12-17]. These interventions mainly include glycemic control, combined with diet, physical activity, and, if necessary, medication; control of blood pressure and lipids to reduce cardiovascular risk and other complications; and regular screening for damage to the eyes, kidneys, and feet to facilitate early treatment [12,13]. Glycemic control through quarterly physician visits with measurements of glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and postprandial 2-hour blood glucose (P2BG) were recommended in professional treatment guidelines [14]. In addition, health education, counseling, self-management, and consistent follow-up were also important for people with diabetes [15-17]. Therefore, people with diabetes require access to systematic, ongoing, and organized care delivered by a multidisciplinary team of skilled health care providers.
Mobile health (mHealth), which is defined as the use of mobile and wireless technologies for health (ie, mobile phones or sensor technologies), aims to capitalize on the rapid uptake of information and communication technologies to improve health system efficiency and health outcomes [18-20]. This includes simple apps and complex technologies, including voice, text messaging (ie, short message service), multimedia message service, Bluetooth technology, and others [21]. These advances, combined with changing patient attitudes toward self-testing, as well as an increased interest in wearable biosensors, are enticing health care providers to shift toward the paradigm of P4 medicine: predictive, pre-emptive, personalized, and participatory [22]. The characteristics of mobility, instantaneous access, and direct communication of mHealth allow for faster transfer of health information, which in turn supports patient management. mHealth is a promising tool for delivering interventions designed to promote lifestyle management of patients with type 2 diabetes. Use of mHealth often includes the possibility of sharing data between health professionals and their patients with diabetes, which could enhance the support to improve their self-management [23,24]. Successful use of mHealth technology requires an active user and cooperation among health professionals [25]. That is, the technology’s effectiveness is often determined by the way in which it is provided to patients or practitioners, how it is supported or taught, and how mHealth technology is added to clinical work or daily life [26].
Previous studies have shown that mobile-based interventions developed for diabetes holistic management have some effects [23-25,27,28]. A systematic review included a meta-analysis of 14 randomized trials aiming to investigate the effect of apps on HbA1c in the self-management of diabetes; these studies showed that the mean reduction in HbA1c among participants using an app compared with control group participants was 0.49% (95% CI 0.30-0.68; I2=10%) [23]. Another systematic review of high-quality review articles and meta-analysis, which focused on utilizing technology in diabetes self-management education and support services, found that technology-enabled diabetes self-management solutions significantly improved HbA1c and four key elements emerged as essential for improved HbA1c: (1) communication, (2) patient-generated health data, (3) education, and (4) feedback [24]. Although the majority of these interventions showed improvement on primary endpoints [25,27,28], whether results will drive substantial clinical adoption is unknown because small studies, even if randomized, are unlikely to be significantly powered to demonstrate meaningful real-world effects [20]. Therefore, real-world evidence and performance data of mobile-based interventions are needed to demonstrate value or motivate stakeholder adoption.
Based on real-world population data from a clinical electronic health database, this study aimed to evaluate the effectiveness of a mobile-based intervention on glycemic control in patients with type 2 diabetes and to explore the change in trends of glycemic parameters in the short and long term.
Methods
Study Design
We conducted a retrospective, propensity score-matched cohort study using electronic health data from a clinical database in Tianjin, China. This clinical database was established in June 2014. The database contained longitudinal outpatient records of patients in five primary care practices and one tertiary care hospital specializing in diabetes, including their demographics, primary and secondary diagnoses, and clinical examination results.
Cohort Selection
The study cohort included 37,913 patients with type 2 diabetes who were registered in this clinical database between October 1, 2016, and July 31, 2018. The flowchart for cohort selection is shown in Figure 1.
Figure 1.
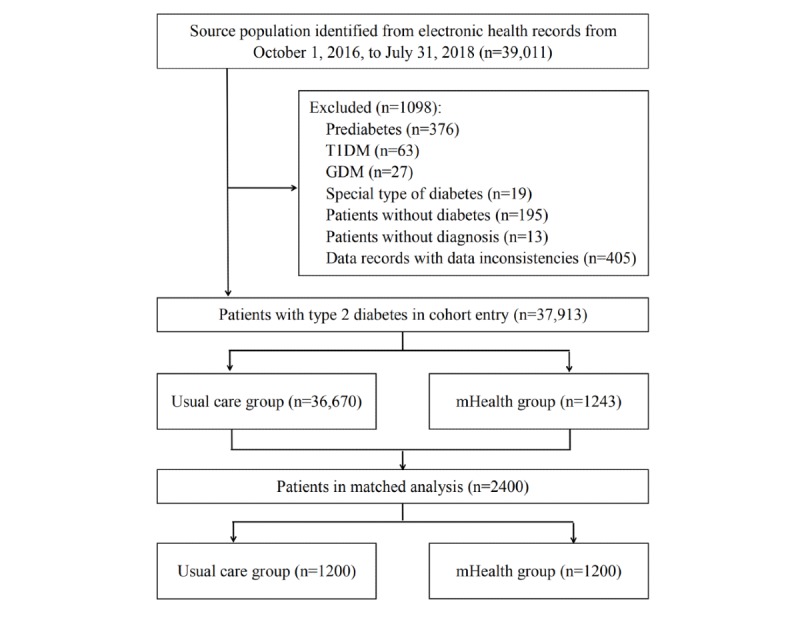
Flowchart for cohort selection. GDM: gestational diabetes mellitus; T1DM: type 1 diabetes mellitus.
All of the patients who were registered in the clinical database between October 1, 2016, and July 31, 2018, were included in our source population. In the end, we identified 39,011 individuals with 1,793,841 records from the clinical database. The unique ID numbers were used to identify the records of the patients with different outpatient numbers in different clinical settings. Using Microsoft Visual Basic 6.0, we developed sophisticated applications to extract and filter these data. After source population selection, the following exclusion criteria were applied: (1) prediabetes, (2) type 1 diabetes, (3) gestational diabetes, (4) special type of diabetes, (5) patients without diabetes, (6) patients without diagnosis, and (7) data records with data inconsistencies. Finally, we identified 37,913 patients with type 2 diabetes.
This unmatched cohort was divided into two groups, including the usual care group (n=36,670) and the mHealth group (n=1243). This was an observational study originating from the real world with no constraints on the cohort entry of participants in either group.
Interventions
The usual care group received standard medical care for patients with type 2 diabetes. Every 3 months, patients in this group underwent regular reviews to re-examine HbA1c, FBG, and P2BG levels. These lab examination results were considered as evidence to support doctors’ decisions to adjust medications. Meanwhile, patients in this group received routine health education at each session.
In addition to usual care, the mHealth group received a mobile-based intervention, which was continuous, real-time, personalized health care delivered by a multidisciplinary team consisting of doctors, nurses, health educators, and dietitians. This mobile-based intervention was based on a unified diabetes care system, which consisted of a mobile app, smart wearable medical devices (eg, wireless glucose monitor, wireless blood pressure monitor, pulse oximeter, and body composition scale), a Web platform, and a data-sharing cloud platform. Patients with type 2 diabetes in the mHealth group followed new flows in the clinical settings (see Multimedia Appendix 1). Patients’ management and education in the mHealth group extended from the clinic to home. They used the wireless glucose monitors and app to perform glucose checks at home. When they experienced hypoglycemia or hyperglycemia, the app could provide tips to help them regulate their glucose levels. Patients also sent their results immediately to the support team about what to do. The care team and the service support team members would be notified when the patient was experiencing abnormal glucose levels. They then phoned the patient to inquire about their recent medication, diet, and exercise, and to help the patient in analyzing possible reasons for the abnormal glucose level. If necessary, they would invite the patient for further in-clinic consultation or guide the patient to adjust their diet or exercise by phone. Patients could also record their meals in the app and get feedback from the service support team. According to an image or a description of the food, the team would provide an overall rating of the meal, comments on portion and nutrition, and suggestions on how to do better. Patients could log their exercise type and duration into the app. The service support team created updated knowledge covering blood glucose, blood pressure, food, fitness, oral medication, insulin, psychology, and complications in the form of articles, videos, and attractive posters. Patients had access to this educational information whenever and wherever possible.
Outcome Definition
The primary outcomes were control rates of HbA1c, FBG, and P2BG at baseline and at 3-, 6-, 9-, and 12-month follow-ups. We identified the control rates according to guidelines for the prevention and control of type 2 diabetes in China [29]. Control objectives were defined as follows: (1) HbA1c <7%, (2) 4.4 mmol/L< FBG <7.0 mmol/L, and (3) P2BG <10 mmol/L. We also considered mean values and variation trends of difference (VTD) with 95% CI, separately, as secondary outcomes. The formula for calculating VTD was as follows:
| VTD = (Valuen - Valuebaseline)/Valuebaseline × 100% |
where Valuen and Valuebaseline denoted the sample values of HbA1c, FBG, and P2BG at n-month (n=3, 6, 9, and 12) follow-up and baseline, respectively [30]. If VTD was positive, it represented the percentage of increase; if VTD was negative, it represented the percentage of reduction.
Covariates
Demographic and chronic disease covariates included sex, age, comorbidity (ie, hyperlipidemia and hypertension), P2BG, FBG, HbA1c, and low-density lipoprotein (LDL) cholesterol. App use-related covariates included times of FBG and P2BG self-testing, diet records, exercise records, and out-of-hospital follow-up.
Statistical Analysis
To control for the nonrandom assignment of patients, a logistic regression model that predicted the likelihood of being included in the mHealth group was constructed and used as the propensity score. Patients were then matched 1:1, using propensity score matching, into the usual care group and mHealth group. We selected all of the common available variables for two-group matching [31], including sex, age group, comorbidity (ie, hyperlipidemia and hypertension), HbA1c level, and LDL cholesterol level. The propensity score-matching tolerance was 0.005. No replacement was allowed, and patients were matched only once. Standardized differences with mirror histograms before and after matching are shown in Figure 2 and Multimedia Appendix 2. We evaluated the balances of matched covariates with standardized differences [29] and considered differences of less than 10% to be matched sufficiently [32,33].
Figure 2.
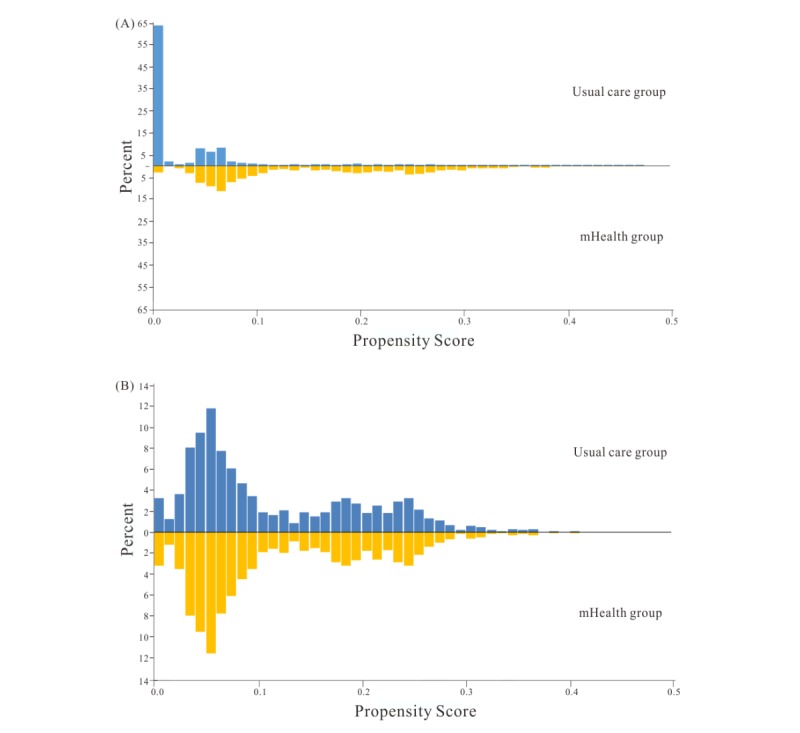
Mirror histograms. (A) Before match. (B) After match.
We presented categorical variables as numbers (percentages) and continuous variables as means (SDs) or 95% CIs, or as medians (IQRs), as appropriate. Descriptive statistics were used to analyze the patient demographics. Binary or categorical outcome measures were analyzed using the chi-square test and continuous measures were analyzed using the t test or a nonparametric equivalent (eg, Wilcoxon rank test). We used the general linear model to calculate repeated-measures analyses of variance to examine mean differences of two groups at baseline and at 3-, 6-, 9-, and 12-month follow-ups. Subgroup analyses to explore the effects of this mobile-based intervention in different patient subgroups were undertaken for the primary and secondary outcomes. The subgroups, specified in the statistical analysis, included patient demographics: sex, age group, hyperlipidemia, and hypertension. The total proportion of missing values at the 12-month follow-up was 12.3%. The proportions of missing data at each data point were 4.5% (3-month follow-up), 7.1% (6-month follow-up), and 9.5% (9-month follow-up). Expectation maximization was used to estimate the missing values of continuous variables. A sensitivity analysis was performed by repeating our primary analysis but excluding patients with hyperlipidemia or hypertension.
We determined statistical significance using a two-tailed P value of less than .05. All of the statistical analyses were carried out using SPSS Statistics for Windows, version 25.0 (IBM Corp).
Results
Patient Demographics
Of the 39,011 patients, 37,913 met the selection criteria for additional analysis (see Figure 1). In the unmatched cohort, the proportion of male patients was 53.41% (20,248/37,913), patients’ mean age was 57.94 years (SD 12.10), and 88.13% of patients were 36-74 years of age. The proportion of patients with hyperlipidemia was 20.52% (7779/37,913), and the proportion of patients with hypertension was 8.11% (3073/37,913). The mean HbA1c level was 7.86% (SD 1.25) and the mean LDL cholesterol level was 3.37 mmol/L (SD 0.65). Table 1 shows the baseline demographics of patients with type 2 diabetes in unmatched and propensity score-matched cohorts. There were significant differences in demographics or glycemic parameters between the usual care group and the mHealth group.
Table 1.
Baseline demographics of patients with type 2 diabetes in unmatched and propensity score-matched cohorts.
| Characteristic | Unmatched cohort | Propensity score-matched cohort | |||||||
|
|
mHealth group (n=1243) | Usual care group (n=36,670) | P value | Std diffa | mHealth group (n=1200) | Usual care group (n=1200) | P value | Std diff | |
| Sex, n (%) |
|
|
|
|
|
|
|
|
|
|
|
Male | 777 (62.51) | 19,471 (53.10) | <.001 | 19.13 | 738 (61.50) | 702 (58.50) | .15 | 3.80 |
|
|
Female | 466 (37.49) | 17,199 (46.90) |
|
|
462 (38.50) | 498 (41.50) |
|
|
| Age group (years), n (%) |
|
|
|
|
|
|
|
|
|
|
|
≤35 | 80 (6.44) | 2086 (5.69) | <.001 | 29.17 | 73 (6.08) | 96 (8.00) | .07 | 7.31 |
|
|
36-59 | 657 (52.85) | 15,914 (43.40) |
|
19.10 | 631 (52.58) | 634 (52.83) |
|
0.40 |
|
|
60-74 | 464 (37.33) | 16,379 (44.66) |
|
15.10 | 454 (37.84) | 444 (37.00) |
|
1.67 |
|
|
≥75 | 42 (3.38) | 2291 (6.25) |
|
13.13 | 42 (3.50) | 26 (2.17) |
|
7.78 |
| Comorbidity, n (%) |
|
|
|
|
|
|
|
|
|
|
|
Hyperlipidemia | 647 (52.05) | 7132 (19.45) | <.001 | 72.67 | 604 (50.33) | 561 (46.75) | .09 | 7.00 |
|
|
Hypertension | 593 (47.71) | 2480 (6.76) | <.001 | 102.25 | 550 (45.83) | 515 (42.92) | .16 | 5.80 |
| Biochemical indicator, mean (SD) |
|
|
|
|
|
|
|
||
|
|
HbA1cb level (%) | 7.82 (1.60) | 7.86 (1.24) | <.001 | 3.16 | 7.83 (1.60) | 7.70 (1.15) | .26 | 9.35 |
|
|
LDLc cholesterol (mmol/L) | 3.26 (0.88) | 3.38 (0.64) | <.001 | 15.10 | 3.29 (0.88) | 3.25 (0.69) | .71 | 5.06 |
aStd diff: standardized difference.
bHbA1c: glycated hemoglobin.
cLDL: low-density lipoprotein.
A propensity score match was then performed and 2400 patients were matched 1:1. After matching, covariates were well balanced and we did not observe any significant differences between groups (see Table 1). In the propensity score-matched cohort, the proportion of male patients was 60.00% (1440/2400), the mean age of patients was 52.24 years (SD 11.56), and 90.13% of patients were 36-74 years of age. A total of 48.54% (1165/2400) of patients had a comorbidity of hyperlipidemia, and 44.38% (1065/2400) of patients had a comorbidity of hypertension. The mean HbA1c level was 7.76% (SD 1.39) and the mean LDL cholesterol level was 3.27 mmol/L (SD 0.79).
Until July 31, 2018, the total number of times of starting up the app, self-monitoring of glycemic parameters, diet recording, exercise recording, and out-of-hospital follow-ups were 80,129; 172,355; 17,860; 4464; and 5264, respectively; the median follow-up time was 457 days.
Control Rates of Glycemic Parameters
At baseline, the control rates of HbA1c, FBG, and P2BG in the mHealth and usual care groups were 45.75% versus 47.00% (P=.57), 38.03% versus 32.76% (P=.07), and 47.32% versus 47.89% (P=.83), respectively. The control rates of HbA1c, FBG, and P2BG in both groups at different follow-up sessions are shown in Figure 3.
Figure 3.
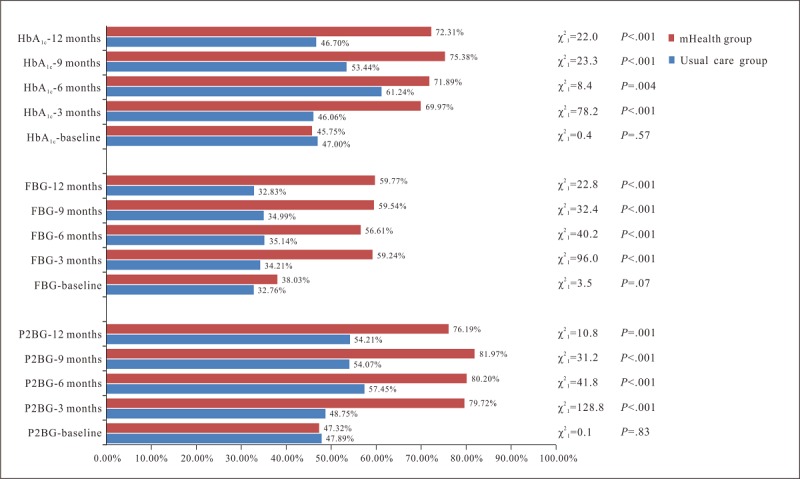
Control rates of glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and postprandial 2-hour blood glucose (P2BG) in the mHealth and usual care groups at different follow-up sessions.
At the 3-, 6-, 9-, and 12-month follow-ups, the mHealth group reported higher control rates of HbA1c than usual care, which were 69.97% versus 46.06% (P<.001), 71.89% versus 61.24% (P=.004), 75.38% versus 53.44% (P<.001), and 72.31% versus 46.70% (P<.001), respectively. Differences in the control rates between the two groups at these four follow-up sessions were 23.91%, 10.65%, 21.94%, and 25.61%, respectively. At the 9-month follow-up, the mHealth group reported the highest control rate of HbA1c, which was 75.38%.
Additionally, at the 3-, 6-, 9-, and 12-month follow-ups, the control rates of FBG in the mHealth and usual care groups were statistically different, which were 59.24% versus 34.21% (P<.001), 56.61% versus 35.14% (P<.001), 59.54% versus 34.99% (P<.001), and 59.77% versus 32.83% (P<.001), respectively. The control rates of P2BG in the mHealth group were statistically higher than in the usual care group, which were 79.72% versus 48.75% (P<.001), 80.20% versus 57.45% (P<.001), 81.97% versus 54.07% (P<.001), and 76.19% versus 54.21% (P=.001), respectively.
Mean Values of Glycemic Parameters
Table 2 shows the effects of this mobile-based intervention on glycemic parameters. The mean values of HbA1c, FBG, and P2BG in the mHealth group were significantly lower than those in the usual care group at the 3-, 6-, 9-, and 12-month follow-ups (P<.01). The P values of the month factor were less than .001, which meant that the HbA1c, FBG, and P2BG levels changed with time. The group and month factors had interaction effects in the mean values of HbA1c, FBG, and P2BG (P<.01), which meant that the effect of the time factor varied with the group. These results identified improved effects due to this mobile-based intervention on changes of the HbA1c, FBG, and P2BG mean values.
Table 2.
Effects of the mobile-based intervention on glycemic parameters.
| Variables and group | Measurement session | F months | P value | F groups | P value | F months × group | P value | |||||
|
|
Baseline | 3 months | 6 months | 9 months | 12 months |
|
|
|
|
|
|
|
| HbA1ca (%), mean (SD) |
|
|
|
|
|
|
|
|
|
|
||
|
|
mHealth group | 7.83 (1.60) | 6.70 (0.73) | 6.60 (0.61) | 6.44 (0.59) | 6.75 (0.76) | 11.822 | <.001 | 0.058 | .08 | 5.905 | .003 |
|
|
Usual care group | 7.70 (1.15) | 7.36 (1.17) | 6.82 (0.64) | 6.97 (0.63) | 7.12 (0.64) |
|
|
|
|
|
|
|
|
Z | -1.123 | -20.382 | -18.592 | -19.922 | -9.657 |
|
|
|
|
|
|
|
|
P value | .26 | <.001 | <.001 | <.001 | <.001 |
|
|
|
|
|
|
| FBGb (mmol/L), mean (SD) |
|
|
|
|
|
|
|
|
|
|||
|
|
mHealth group | 8.34 (2.41) | 6.51 (1.27) | 6.74 (2.11) | 6.68 (1.49) | 6.89 (1.52) | 9.614 | <.001 | 16.425 | <.001 | 5.762 | <.001 |
|
|
Usual care group | 8.68 (2.34) | 8.53 (2.37) | 8.45 (2.45) | 8.38 (2.39) | 8.47 (2.68) |
|
|
|
|
|
|
|
|
Z | -1.326 | -15.268 | -17.315 | -14.831 | -5.755 |
|
|
|
|
|
|
|
|
P value | .20 | <.001 | <.001 | <.001 | <.001 |
|
|
|
|
|
|
| P2BGc (mmol/L), mean (SD) |
|
|
|
|
|
|
|
|
|
|||
|
|
mHealth group | 11.14 (4.40) | 8.03 (1.75) | 7.75 (1.90) | 7.89 (1.77) | 8.29 (2.38) | 12.424 | <.001 | 9.566 | .002 | 7.193 | <.001 |
|
|
Usual care group | 10.36 (2.94) | 9.99 (2.99) | 9.85 (2.56) | 9.93 (2.83) | 9.97 (2.91) |
|
|
|
|
|
|
|
|
Z | -1.575 | -23.995 | -20.921 | -16.243 | -3.149 |
|
|
|
|
|
|
|
|
P value | .12 | <.001 | <.001 | <.001 | .002 |
|
|
|
|
|
|
aHbA1c: glycated hemoglobin.
bFBG: fasting blood glucose.
cP2BG: postprandial 2-hour blood glucose.
Multimedia Appendices 3-7 show that, compared with usual care, at the 3-, 6-, 9-, and 12-month follow-ups, both sexes in the mHealth group reported significantly lower mean values of HbA1c, FBG, and P2BG (P<.05), although we did not observe any significant differences in the P2BG mean values of female participants between the two groups at the 12-month follow-up (P=.20). Patients aged 36-74 years in the mHealth group had steadily lower HbA1c, FBG, and P2BG mean values than those in the usual care group (P<.05). No statistically significant difference was observed in P2BG mean values of patients aged 36-59 years between the two groups at the 12-month follow-up (P=.09). Patients younger than 35 or older than 75 years of age in the mHealth group reported unstable variation trends of mean values.
Variation Trends of Difference for Glycemic Parameters
Figure 4 shows the variation trends of difference for glycemic parameters between the two groups. At the 3-, 6-, 9-, and 12-month follow-ups, the percentages of HbA1c reduction in mHealth group were 8.66% (95% CI 6.69-10.63), 10.60% (95% CI 8.66-12.54), 10.64% (95% CI 8.70-12.58), and 8.11% (95% CI 6.08-10.14), respectively; the percentages of P2BG reduction in the mHealth group were 8.44% (95% CI 7.41-10.73), 17.77% (95% CI 14.98-20.23), 16.23% (95% CI 13.05-19.35), and 16.91% (95% CI 13.17-19.84), respectively. Equally important was that, after 6 months, the declines in HbA1c and P2BG of the two groups decreased, whereas the mHealth group experienced larger decreases in HbA1c and P2BG than the usual care group. At the 3-month follow-up, the reduction of FBG in the mHealth group was larger than in the usual care group (4.83% vs 1.38%). However, starting from the sixth month, the reductions of FBG in the usual care group were larger than in the mHealth group.
Figure 4.
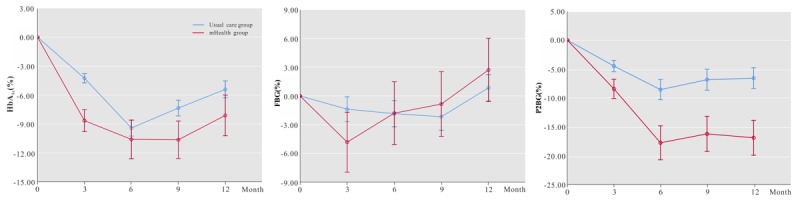
Variation trends of glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and postprandial 2-hour blood glucose (P2BG) differences.
Sensitivity Analysis
We conducted sensitivity analysis, where we excluded 1587 patients with hyperlipidemia or hypertension. In the mHealth group at the baseline and the four follow-up sessions, the control rates of HbA1c were 36.57%, 72.77%, 76.92%, 80.00%, and 75.00%, respectively; the control rates of FBG were 44.44%, 65.79%, 65.74%, 59.38%, and 65.63%, respectively; and the control rates of P2BG were 54.02%, 82.63%, 77.45%, 83.33%, and 72.41%, respectively. There were no significant differences between the sensitivity analysis and the primary analysis (χ2=10.0 P=.35), so we presented only the results of the primary analysis.
Discussion
Principal Findings
In this study, a total of 2400 patients with type 2 diabetes were matched 1:1, using propensity score matching, into the usual care and mHealth groups. A total of 60% of the patients were male and more than half were 36-59 years of age. These demographics were similar to the population in previous studies [29,34]. Our results showed the improvement in control rates of HbA1c, FBG, and P2BG for patients with type 2 diabetes in the mHealth group compared with those in the usual care group. These effects were best sustained within the first 6 months. Starting from the sixth month, the mean HbA1c and P2BG values in the two groups increased slightly.
Comparison With Prior Work
The role of glycemic control in preventing the development and progression of complications has been proven in diabetes [35-38], with an especially strong relationship identified between intensive glycemic control and diabetic complications and mortality. In general, a target HbA1c level of less than 7% is optimal, according to diabetes guidelines [14]. Each 1% of mean HbA1c value reduction has been associated with a 21% reduction in the risk of diabetes-related complications [39]. A recent study on the legacy effect of early glycemic control on future complications in type 2 diabetes showed that, compared with an HbA1c of less than 6.5% for the 0-1-year early exposure period, HbA1c levels of 6.5% or higher were associated with increased microvascular and macrovascular events, and HbA1c levels of 7.0% or higher were associated with increased mortality [40]. However, a recent meta-analysis demonstrated that HbA1c target achievement is low, with a pooled average of 43% worldwide [41], both in primary and secondary care settings. In 2013, among Chinese patients with diabetes, only 39.7% of those treated had adequate glycemic control [34]. The reason for this low target achievement, despite the expanding arsenal of glucose-lowering interventions, remains unclear [42]. Our study found that the control rates of HbA1c, FBG, and P2BG in the mHealth group were higher than those in the usual care group, which were much higher than the average level worldwide [41]. These findings confirmed the effectiveness of this mobile-based intervention on glycemic control in patients with type 2 diabetes. Our study also found that at different follow-up sessions, both sexes in the mHealth group reported significantly lower mean values of HbA1c, FBG, and P2BG. Patients aged 36-74 years in the mHealth group had steadily lower HbA1c, FBG, and P2BG mean values than those in the usual care group, and patients younger than 35 or older than 75 years old in the mHealth group reported unstable variation trends of mean values. There may be many reasons for poor glycemic control in patients older than 75 years of age in the mHealth group, including decreased self-management ability, inadequate exercise, irregular glycemic monitoring, and poor convenience in using apps, among other reasons [21,23,43]. For patients younger than 35 years old, the main reason may be poor compliance of patients and insufficient understanding of the importance of glycemic monitoring [24,25]. Especially for young patients, poor parental health literacy is the main reason [44,45].
However, some studies have found that even if blood glucose is effectively controlled, the occurrence and development of complications cannot be improved or reversed [46-48]. Researchers believe that this is due to the “metabolic memory” effect of hyperglycemia [46-48]. “Metabolic memory” effect refers to the persistent damage of early hyperglycemia to tissues and organs of diabetic patients, even though the glycemic control is good [48]. A growing body of experimental evidence supports the concept that the risk for diabetes complications may be linked to oxidative stress, nonenzymatic glycosylation of proteins, epigenetic changes, and chronic inflammation, laying the foundation for the “metabolic memory” theory [46]. From a clinical standpoint, the “metabolic memory” theory supports the need for very early aggressive treatment, with the goal of normalizing metabolic control as soon as possible, especially blood glucose. Therefore, achieving glycemic control targets as soon as possible and maintaining glycemic control for a long time have significantly positive effects in the prevention of complications [14]. The treatment strategy of diabetes should be changed from strict glycemic control to strict glycemic control at the early stage. The mobile-based intervention in this study seems to offer a promising option to implement this strategy. One meta-analysis of 35 randomized controlled trials found that an internet-based or mobile-based intervention duration of 3 months or less yielded optimal performance [49]. Our study also found that the glycemic control rates of the mHealth group were higher than those of the usual care group at the 3-, 6-, 9-, and 12-month follow-ups. These findings were not only consistent with previous studies, but also illustrated that the mobile-based intervention had generated a statistically significant improvement on glycemic control in the short and long term [47,49]. Therefore, implementation of mobile-based interventions could be a promising strategy for glycemic control of patients with diabetes not only at the early stage, but also in the long term.
In our study, the mobile-based intervention was designed to provide continuous, real-time, personalized health care for patients with diabetes, and it was delivered by a multidisciplinary team consisting of doctors, nurses, health educators, and dietitians. With the help of mobile technologies, this intervention provides a solution for diabetes management that includes the following: (1) a simple and intuitive way of vital data collection, (2) automatic in-hospital exam data and at-home data consolidation, (3) convenient and timely communication with care team professionals, and (4) continuous and vivid diabetes education, both in person and through multimedia. Based on the hardware equipment and professional support team, we have realized real-time guidance and management for diabetic patients; meanwhile, we have also collected a large amount of sample data. These data from the real world reflected the effectiveness of this mobile-based intervention. Notably, we found that, starting from the sixth month, the glycemic control of patients with type 2 diabetes in the mHealth group began to fluctuate slightly. This is a reminder that intensive management needs to be conducted to maintain the long-term effectiveness of this mobile-based intervention from the sixth month.
Strengths and Limitations
A major strength of this study was the high-quality, continuously updated, clinical database of electronic medical records that provided a large sample size and reflected real-world clinical conditions. In addition, in our study, propensity score matching was used to control the confounding factors between the two groups. Propensity score matching could reduce the bias resulting from confounding variables; this approach attempted to mimic randomization by creating a sample of units that received the mobile-based intervention that is comparable, on all observed covariates, to a sample of units that received usual care.
This study had several limitations. First, as a result of its retrospective nature, we may not have addressed unobserved confounders in propensity score matching. Therefore, selection bias may exist in this research. For this reason, we used the propensity score matching to balance the common available covariates of the two groups, including sex, age, comorbidity (ie, hyperlipidemia and hypertension), HbA1c level, and LDL cholesterol level. Second, there are inherent limitations as to what data are recorded in the clinical medical records. For instance, the clinical medical records of patients in the usual care group did not include some demographic information, such as education level, economic level, and occupation, among others, as well as anthropometry data, such as height, weight, systolic blood pressure, and diastolic blood pressure, among others. Cognitive function was not evaluated for either group. Third, in this study, the total proportion of missing values was 12.3%, and the missing values were on continuous variables, including HbA1c, FBG, P2BG, and LDL cholesterol. In order to decrease the amount of bias in the data, we used expectation maximization to estimate the missing values of continuous variables.
Conclusions
This mobile-based intervention delivered by a multidisciplinary team to promote glycemic control of patients with type 2 diabetes led to increases in the control rates of HbA1c, FBG, and P2BG. These effects were best sustained within the first 6 months. It is noteworthy that, starting from the sixth month, intensive management might need to be conducted to maintain long-term effectiveness of this mobile-based intervention.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (#91746205 and #71673199), the Tianjin Science and Technology Project (#15ZXHLSY00460 and #18ZXZNSY00280), the Natural Science Foundation of Tianjin City (#18JCYBJC26100), and key social science projects of the Tianjin Education Commission (#2019JWZD54).
Abbreviations
- FBG
fasting blood glucose
- HbA1c
glycated hemoglobin
- LDL
low-density lipoprotein
- mHealth
mobile health
- P2BG
postprandial 2-hour blood glucose
- VTD
variation trends of difference
Appendix
In-clinic user flow of the mHealth group.
Standardized differences before and after propensity score matching.
Variation trends of glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and postprandial 2-hour blood glucose (P2BG) mean values (sex groups).
Variation trends of glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and postprandial 2-hour blood glucose (P2BG) mean values (age groups).
Subgroup analysis of glycated hemoglobin (HbA1c) (%) between usual care and mHealth groups.
Subgroup analysis of fasting blood glucose (FBG) (mmol/L) between usual care and mHealth groups.
Subgroup analysis of postprandial 2-hour blood glucose (P2BG) (mmol/L) between usual care and mHealth groups.
Footnotes
Authors' Contributions: LC and YW (Yaogang Wang) contributed to the conception and design of the study. JL, LG, DL, CL, NS, and ZX acquired the data. JS, LS, SL, YJ, YW (Yuan Wang), and SZ analyzed the data and all authors interpreted the data. JL, LS, YW (Yaogang Wang), and LC drafted the manuscript and all authors were involved in critical revision and approval of the final manuscript. YW (Yaogang Wang) and LC contributed equally to this paper as corresponding authors. (Yaogang Wang, PhD, School of Public Health, Tianjin Medical University No 22, Qixiangtai Road, Heping District Tianjin, 300070, China, Phone: 86 13820046130, Email: wyg@tmu.edu.cn) The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Conflicts of Interest: None declared.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016 Apr 09;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017 Jun;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 3.National Diabetes Research Group A mass survey of diabetes mellitus in a population of 300,000 in 14 provinces and municipalities in China (author's transl) [Article in Chinese] Zhonghua Nei Ke Za Zhi. 1981 Nov;20(11):678–683. [PubMed] [Google Scholar]
- 4.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014 Jan;37 Suppl 1:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 5.The Lancet Type 2 diabetes: The urgent need to protect young people. Lancet. 2018 Dec 01;392(10162):2325. doi: 10.1016/S0140-6736(18)33015-0. [DOI] [PubMed] [Google Scholar]
- 6.Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, Bao W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: Population based study. BMJ. 2018 Sep 04;362:k1497. doi: 10.1136/bmj.k1497. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=30181166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: Disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015 Feb;38(2):264–270. doi: 10.2337/dc14-1996. [DOI] [PubMed] [Google Scholar]
- 8.Preis SR, Hwang S, Coady S, Pencina MJ, D'Agostino RB, Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009 Apr 07;119(13):1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. http://europepmc.org/abstract/MED/19307472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baena-Díez JM, Peñafiel J, Subirana I, Ramos R, Elosua R, Marín-Ibañez A, Guembe MJ, Rigo F, Tormo-Díaz MJ, Moreno-Iribas C, Cabré JJ, Segura A, García-Lareo M, Gómez de la Cámara A, Lapetra J, Quesada M, Marrugat J, Medrano MJ, Berjón J, Frontera G, Gavrila D, Barricarte A, Basora J, García JM, Pavone NC, Lora-Pablos D, Mayoral E, Franch J, Mata M, Castell C, Frances A, Grau M, FRESCO Investigators Risk of cause-specific death in individuals with diabetes: A competing risks analysis. Diabetes Care. 2016 Nov;39(11):1987–1995. doi: 10.2337/dc16-0614. [DOI] [PubMed] [Google Scholar]
- 10.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014 Apr 17;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 11.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ. 2015 Jan 02;350:g7607. doi: 10.1136/bmj.g7607. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=25555821. [DOI] [PubMed] [Google Scholar]
- 12.Gaede P, Lund-Andersen H, Parving H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008 Feb 07;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 13.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000 Apr 18;132(8):605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 14.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2019 executive summary. Endocr Pract. 2019 Jan;25(1):69–100. doi: 10.4158/CS-2018-0535. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Wang P, Dai Z, Liu K, Jin Y, Li A, Wang S, Zheng J. Increased self-care activities and glycemic control rate in relation to health education via WeChat among diabetes patients: A randomized clinical trial. Medicine (Baltimore) 2018 Dec;97(50):e13632. doi: 10.1097/MD.0000000000013632. doi: 10.1097/MD.0000000000013632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluml BM, Kolb LE, Lipman R. Evaluating the impact of year-long, augmented diabetes self-management support. Popul Health Manag. 2019 Dec;22(6):522–528. doi: 10.1089/pop.2018.0175. http://europepmc.org/abstract/MED/30668228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayre J, Bonner C, Bramwell S, McClelland S, Jayaballa R, Maberly G, McCaffery K. Factors for supporting primary care physician engagement with patient apps for type 2 diabetes self-management that link to primary care: Interview study. JMIR Mhealth Uhealth. 2019 Jan 16;7(1):e11885. doi: 10.2196/11885. https://mhealth.jmir.org/2019/1/e11885/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal S, LeFevre AE, Lee J, L'Engle K, Mehl G, Sinha C, Labrique A, WHO mHealth Technical Evidence Review Group Guidelines for reporting of health interventions using mobile phones: Mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. 2016 Mar 17;352:i1174. doi: 10.1136/bmj.i1174. [DOI] [PubMed] [Google Scholar]
- 19.Fedele DA, Cushing CC, Fritz A, Amaro CM, Ortega A. Mobile health interventions for improving health outcomes in youth: A meta-analysis. JAMA Pediatr. 2017 May 01;171(5):461–469. doi: 10.1001/jamapediatrics.2017.0042. http://europepmc.org/abstract/MED/28319239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CE, Harrington RA, Desai SA, Mahaffey KW, Turakhia MP. Characteristics of digital health studies registered in ClinicalTrials.gov. JAMA Intern Med. 2019 Jun 01;179(6):838–840. doi: 10.1001/jamainternmed.2018.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn CC, Swasey KK, Torain JM, Shardell MD, Terrin ML, Barr EA, Gruber-Baldini AL. An mHealth diabetes intervention for glucose control: Health care utilization analysis. JMIR Mhealth Uhealth. 2018 Oct 15;6(10):e10776. doi: 10.2196/10776. https://mhealth.jmir.org/2018/10/e10776/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood CS, Thomas MR, Budd J, Mashamba-Thompson TP, Herbst K, Pillay D, Peeling RW, Johnson AM, McKendry RA, Stevens MM. Taking connected mobile-health diagnostics of infectious diseases to the field. Nature. 2019 Feb;566(7745):467–474. doi: 10.1038/s41586-019-0956-2. http://europepmc.org/abstract/MED/30814711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and grade of 14 randomized trials. Diabetes Care. 2016 Nov;39(11):2089–2095. doi: 10.2337/dc16-0346. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A systematic review of reviews evaluating technology-enabled diabetes self-management education and support. J Diabetes Sci Technol. 2017 Sep;11(5):1015–1027. doi: 10.1177/1932296817713506. http://europepmc.org/abstract/MED/28560898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garabedian LF, Ross-Degnan D, Wharam JF. Mobile phone and smartphone technologies for diabetes care and self-management. Curr Diab Rep. 2015 Dec;15(12):109. doi: 10.1007/s11892-015-0680-8. http://europepmc.org/abstract/MED/26458380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auerbach AD. Evaluating digital health tools: Prospective, experimental, and real world. JAMA Intern Med. 2019 Jun 01;179(6):840–841. doi: 10.1001/jamainternmed.2018.7229. [DOI] [PubMed] [Google Scholar]
- 27.Desveaux L, Shaw J, Saragosa M, Soobiah C, Marani H, Hensel J, Agarwal P, Onabajo N, Bhatia RS, Jeffs L. A mobile app to improve self-management of individuals with type 2 diabetes: Qualitative realist evaluation. J Med Internet Res. 2018 Mar 16;20(3):e81. doi: 10.2196/jmir.8712. https://www.jmir.org/2018/3/e81/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hood M, Wilson R, Corsica J, Bradley L, Chirinos D, Vivo A. What do we know about mobile applications for diabetes self-management? A review of reviews. J Behav Med. 2016 Dec;39(6):981–994. doi: 10.1007/s10865-016-9765-3. [DOI] [PubMed] [Google Scholar]
- 29.Chinese Diabetes Society Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition) Chin J Pract Intern Med. 2018;38(4):292–344. [Google Scholar]
- 30.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009 Apr 09;38(6):1228–1234. doi: 10.1080/03610910902859574. [DOI] [Google Scholar]
- 31.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 32.Ramos R, Comas-Cufí M, Martí-Lluch R, Balló E, Ponjoan A, Alves-Cabratosa L, Blanch J, Marrugat J, Elosua R, Grau M, Elosua-Bayes M, García-Ortiz L, Garcia-Gil M. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: Retrospective cohort study. BMJ. 2018 Sep 05;362:k3359. doi: 10.1136/bmj.k3359. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=30185425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson DB, Rice DC, Niu J, Atay S, Vaporciyan AA, Antonoff M, Hofstetter WL, Walsh GL, Swisher SG, Roth JA, Tsao A, Gomez D, Giordano SH, Mehran R, Sepesi B. Long-term survival outcomes of cancer-directed surgery for malignant pleural mesothelioma: Propensity score matching analysis. J Clin Oncol. 2017 Oct 10;35(29):3354–3362. doi: 10.1200/JCO.2017.73.8401. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen C, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G, 2010 China Noncommunicable Disease Surveillance Group Prevalence and control of diabetes in Chinese adults. JAMA. 2013 Sep 04;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 35.Mohan V, Mapari JA, Karnad PD, Mann JS, Maheshwari VK. Reduced diabetes mellitus-related comorbidities by regular self-monitoring of blood glucose: Economic and quality of life implications. Indian J Endocrinol Metab. 2018;22(4):461–465. doi: 10.4103/ijem.IJEM_216_17. http://www.ijem.in/article.asp?issn=2230-8210;year=2018;volume=22;issue=4;spage=461;epage=465;aulast=Mohan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altschul DM, Starr JM, Deary IJ. Cognitive function in early and later life is associated with blood glucose in older individuals: Analysis of the Lothian Birth Cohort of 1936. Diabetologia. 2018 Sep;61(9):1946–1955. doi: 10.1007/s00125-018-4645-8. http://europepmc.org/abstract/MED/29860628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan DM, Cleary PA, Backlund JC, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–2653. doi: 10.1056/NEJMoa052187. http://europepmc.org/abstract/MED/16371630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liese AD, Ma X, Reid L, Sutherland MW, Bell BA, Eberth JM, Probst JC, Turley CB, Mayer-Davis EJ. Health care access and glycemic control in youth and young adults with type 1 and type 2 diabetes in South Carolina. Pediatr Diabetes. 2019 May;20(3):321–329. doi: 10.1111/pedi.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000 Aug 12;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. http://europepmc.org/abstract/MED/10938048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, Karter AJ. The legacy effect in type 2 diabetes: Impact of early glycemic control on future complications (the Diabetes & Aging Study) Diabetes Care. 2019 Mar;42(3):416–426. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khunti K, Ceriello A, Cos X, De Block C. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: A meta-analysis. Diabetes Res Clin Pract. 2018 Mar;137:137–148. doi: 10.1016/j.diabres.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Jalving AC, Gant CM, Binnenmars SH, Soedamah-Muthu SS, Bakker SJ, Navis G, Laverman GD. Glycaemic control in the diabetes and Lifestyle Cohort Twente: A cross-sectional assessment of lifestyle and pharmacological management on HbA1c target achievement. Diabetes Obes Metab. 2018 Oct;20(10):2494–2499. doi: 10.1111/dom.13399. http://europepmc.org/abstract/MED/29862616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almutairi N, Hosseinzadeh H, Gopaldasani V. The effectiveness of patient activation intervention on type 2 diabetes mellitus glycemic control and self-management behaviors: A systematic review of RCTs. Prim Care Diabetes. 2020 Feb;14(1):12–20. doi: 10.1016/j.pcd.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Vloemans AF, Eilander MM, Rotteveel J, Bakker-van Waarde WM, Houdijk EC, Nuboer R, Winterdijk P, Snoek FJ, De Wit M. Youth with type 1 diabetes taking responsibility for self-management: The importance of executive functioning in achieving glycemic control: Results from the longitudinal DINO study. Diabetes Care. 2019 Feb;42(2):225–231. doi: 10.2337/dc18-1143. [DOI] [PubMed] [Google Scholar]
- 45.Pulgarón ER, Sanders LM, Patiño-Fernandez AM, Wile D, Sanchez J, Rothman RL, Delamater AM. Glycemic control in young children with diabetes: The role of parental health literacy. Patient Educ Couns. 2014 Jan;94(1):67–70. doi: 10.1016/j.pec.2013.09.002. http://europepmc.org/abstract/MED/24091252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A. The "metabolic memory" theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients. 2017 Apr 28;9(5):437. doi: 10.3390/nu9050437. http://www.mdpi.com/resolver?pii=nu9050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vervloet M, van Dijk L, de Bakker DH, Souverein PC, Santen-Reestman J, van Vlijmen B, van Aarle MC, van der Hoek LS, Bouvy ML. Short- and long-term effects of real-time medication monitoring with short message service (SMS) reminders for missed doses on the refill adherence of people with type 2 diabetes: Evidence from a randomized controlled trial. Diabet Med. 2014 Jul;31(7):821–828. doi: 10.1111/dme.12439. [DOI] [PubMed] [Google Scholar]
- 48.Yin H, Liang S, He H, Liu XQ. Progress in therapeutic strategy for high glucose-induced metabolic memory. Prog Pharm Sci. 2018;42(8):599–607. [Google Scholar]
- 49.Shen Y, Wang F, Zhang X, Zhu X, Sun Q, Fisher E, Sun X. Effectiveness of internet-based interventions on glycemic control in patients with type 2 diabetes: Meta-analysis of randomized controlled trials. J Med Internet Res. 2018 May 07;20(5):e172. doi: 10.2196/jmir.9133. https://www.jmir.org/2018/5/e172/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In-clinic user flow of the mHealth group.
Standardized differences before and after propensity score matching.
Variation trends of glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and postprandial 2-hour blood glucose (P2BG) mean values (sex groups).
Variation trends of glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and postprandial 2-hour blood glucose (P2BG) mean values (age groups).
Subgroup analysis of glycated hemoglobin (HbA1c) (%) between usual care and mHealth groups.
Subgroup analysis of fasting blood glucose (FBG) (mmol/L) between usual care and mHealth groups.
Subgroup analysis of postprandial 2-hour blood glucose (P2BG) (mmol/L) between usual care and mHealth groups.


