Abstract
Viruses survive often harsh host environments, yet we know little about the strategies they utilize to adapt and subsist given their limited genomic resources. We are beginning to appreciate the surprising versatility of viral genomes and how replication-competent and -defective virus variants can provide means for adaptation, immune escape and virus perpetuation. This Review summarizes current knowledge of the types of defective viral genomes generated during the replication of RNA viruses and the functions that they carry out. We highlight the universality and diversity of defective viral genomes during infections and discuss their predicted role in maintaining a fit virus population, their impact on human and animal health, and their potential to be harnessed as antiviral tools.
Subject terms: Virology, Virus structures
This Review describes recent findings on the biogenesis and the role of defective viral genomes during replication of RNA viruses and discusses their impact on viral dynamics and evolution.
Main
Viruses are remarkably resilient microorganisms able to adapt and survive in the complex host physiological and immune environment. Accumulating evidence demonstrates that viruses can generate modified forms of their genome during replication as tools to adapt to environmental challenges. While the standard genome encodes all the viral proteins required for sustaining viral replication, viral variants contain random mutations that can serve to enhance the ability of the virus to adapt to new conditions1. In addition, defective viral genomes (DVGs) and an expanding family of sub-viral particles (Box 1) that result from either small mutations or drastic truncations and modifications of the viral genome render the virus unable to complete a full replication cycle in the absence of a helper full-length virus to complement the functions lost.
DVGs were identified by Preben Von Magnus in the late 1940s as incomplete influenza viruses able to interfere with the replication of the wild-type virus2. More than two decades after their discovery, Alice Huang and David Baltimore coined the term defective interfering (DI) particles, or DIPs, to define viral particles that contain normal structural proteins but only a part of the viral genome. In addition, they stipulated that DIPs can only replicate in the presence of helper virus and that they interfere with the intracellular replication of non-defective homologous virus3. Huang and Baltimore theorized that DIPs play a critical role in determining viral pathogenesis. Follow-up studies using viruses grown to contain either a large content of DIPs, or depleted of them, revealed that DIPs reduced virulence in vivo4,5, induced high levels of interferon (IFN) during infections in vitro6–8, and promoted viral persistence in vitro9–16 and in vivo17,18. However, despite their ubiquitous presence and important functions, the lack of appropriate technology to identify DIPs in infections in vivo led to a widespread belief that DIPs and their DVGs were largely a product of in vitro virus replication and were not relevant in natural viral infections19,20. By the late 1990s, the study of DVGs had slowed down drastically and was limited to their use as tools for studying viral replication or as potential antivirals.
Today, DVGs have been described in most RNA viruses (Table 1) and technological advances have contributed to establishing their role as de facto danger signals for triggering of antiviral immunity in many infections. In addition, we are beginning to appreciate their impact on the clinical outcome of natural infections and on the evolution of viruses. Moreover, we are witnessing rapid advancements in the understanding of the molecular mechanisms that regulate the generation of DVGs and those explaining their paradoxical roles in promoting antiviral immunity and viral persistence.
Table 1.
RNA virus families with described DVGs
| Virus | Type of DVG | Known DVG functions | References |
|---|---|---|---|
| psRNA viruses | |||
| Arteriviridae | |||
| Porcine reproductive and respiratory syndrome virus | Deletion | UN | 134 |
| Equine arteritis virus | Deletion | Interference | 40 |
| Closteroviridae | |||
| Citrus tristeza virus | Deletion | UN | 135,136 |
| Coronaviridae | |||
| Berne virus | Deletion | Interference | 137 |
| Bovine coronavirus | Deletion | UN | 138 |
| Infectious bronchitis virus | Deletion | UN | 139 |
| Mouse hepatitis virus | Deletion | Interference/persistence | 140,141 |
| Transmissible gastroenteritis virus | Deletion | Interference | 142 |
| Flaviviridae | |||
| Dengue virus | Deletion | Persistence | 75, 143,144 |
| Japanese encephalitis virus | Persistence | 15,145 | |
| Hepatitis C virus | Deletion | Persistence | 146,147 |
| Murray Valley encephalitis virus | Deletion | Persistence | 148 |
| Tick-borne encephalitis virus | Deletion | Modulate virulence | 149 |
| West Nile virus | Deletion | Interference/persistence | 82,150 |
| Nepoviridae | |||
| Tomato black ring virus | Deletion | Interference | 151 |
| Nodaviridae | |||
| Flock House virus | Deletion | Modulate virulence | 48 |
| Picornaviridae | |||
| Encephalomyocarditis virus | Deletion | Interference | 152 |
| Foot-and-mouth disease virus | Deletion | Interference | 153 |
| Mengo virus | Deletion | Interference | 154 |
| Polio virus | Deletion | Modulate virulence | 24, 35, 124,155 |
| Togaviridae | |||
| Rubella virus | Persistence | 156 | |
| Semliki Forest virus | Deletion | Interference/modulate virulence | 4, 93, 106, 157,158 |
| Sindbis virus | Deletion | Interference/IFN-induction | 6, 63,159 |
| Tombusviridae | |||
| Cucumber necrosis virus | Deletion | Modulate virulence | 160 |
| Tomato bushy stunt virus | Deletion | Interference/modulate virulence | 39 |
| Turnip crinkle virus | Deletion | Interference/modulate virulence | 161 |
| nsRNA viruses | |||
| Arenaviridae | |||
| Lymphocytic choriomeningitis mammarenavirus | Deletion | Interference/modulate virulence/persistence | 18, 162,163 |
| Filoviridae | |||
| Ebola virus | Deletion /copy-back | Persistence | 74 |
| Orthomyxoviridae | |||
| Influenza virus | Deletion | Interference/IFN-induction/persistence/modulate virulence | 2, 5, 10, 37, 51, 62, 97,164–170 |
| Paramyxoviridae | |||
| Human parainfluenza virus 3 | Deletion /copy-back | Interference | 112,171 |
| Parainfluenza virus 5 | Deletion/copy-back | IFN-induction | 57 |
| Measles virus | Deletion/copy-back | Interference/IFN-induction/persistence/modulate virulence | 76, 90, 96, 100, 123, 172,173 |
| Mumps virus | Persistence/modulate virulence | 9, 115,174 | |
| Sendai virus | Deletion/copy-back | Interference/IFN-induction/immune stimulation/persistence/modulate virulence | 7, 14, 23, 45, 54,87–89,92, 104, 175,176 |
| Peribunyaviridae | |||
| Bunyamwera virus | Deletion | Interference | 177 |
| Phenuiviridae | |||
| Rift Valley Fever virus (RVFV) | Deletion | Interference/modulate virulence | 19 |
| Toscana virus | Deletion | Interference | 178 |
| Pneumoviridae | |||
| Human metapneumovirus | Copy-back | IFN-induction | 52 |
| Human respiratory syncytial virus | Deletion/copy-back | Interference/IFN-induction/persistence | 55, 78,179 |
| Rhabdoviridae | |||
| Vesicular stomatitis virus | Deletion/copy-back | Interference/IFN-induction/persistence/modulate virulence | 3, 8, 16, 21, 38, 42, 46, 80, 109,180–185 |
| Rabies virus | Deletion | Interference/persistence | 11,79 |
| Tospoviridae | |||
| Tomato spotted wilt virus | Deletion | Modulate virulence | 186 |
| dsRNA viruses | |||
| Birnaviridae | |||
| Infectious necrotic pancreatic virus | UN | Persistence | 12 |
| Partitiviridae | |||
| Rosellinia necatrix virus | Deletion | Interference | 187 |
| Reoviridae | |||
| Type 3 reovirus | Deletion | Interference | 188 |
| Wound tumor virus | Deletion | UN | 189 |
| Retroviruses | |||
| Retroviridae | |||
| Human immunodeficiency virus 1 | Deletion/ hypermutation/frame shift | Persistence | 34, 190,191 |
UN, unknown.
Here, we review evidence accumulated over more than half a century of observations on DVG generation and activity during RNA virus infections. We highlight recent advances that illustrate their critical impact on viral dynamics and evolution during both acute and long-term virus–host interactions. See Box 2 for a glossary of relevant terms.
Box 1 The expanding family of sub-viral particles.
In addition to DVGs, a growing number of viral particle variants and sub-viral agents have been discovered in viruses of plants, arthropods and mammals. These include viroids, satellite viruses, virophages and viral-like extracellular vesicles (VLVs). The definition of these entities seems, at times, blurry, due to their largely intersecting properties. In general, sub-viral agents, similar to DVGs, depend on complementation with the standard virus to replicate and spread. However, differences in their requirements for complementation, target virus and sequence identity with their helper virus are used to sub-classify them. Here, we provide current definitions of these sub-viral entities and highlight those aspects that differentiate them from DIPs and their DVGs.
Satellite viruses were originally described in plant viruses as linear or circular RNAs (200–1,800 nt long) that require a helper virus to propagate but are unrelated in sequence to the helper virus. Satellite viruses are generally dispensable for the replication of the helper virus, with some exceptions192,193. Satellite viruses differ from satellite RNAs in that they encode a protein that packages the satellite RNA into virions. Satellite RNAs can interfere with the replication of its helper virus and either attenuate or exacerbate disease. An example of a satellite virus is the satellite tobacco necrosis virus194. This positive-sense, single-stranded RNA satellite virus suppresses the replication of its helper virus and ameliorates tobacco necrosis virus symptoms195.
Viroids differ from satellite viruses in that they do not encode any protein, do not require helper virus for replication, and are not encapsidated. Viroids have a circular RNA genome (200–400 nt long) that is highly complementary and structured, and are adapted to carry out their complete life cycle as a result from interactions with the host cell machinery196,197.
Virophages are 15–30 kbp long dsDNA viruses that normally produce icosahedral particles and parasitize from giant dsDNA viruses (mimiviruses and others) for propagation. Virophages infect the giant virus factory, thereby harming the giant virus198. The first virophage discovered, Sputnik, was found together with Acanthamoeba castellanii mamavirus and interferes with its propagation199. Several other virophages, as well as a large number of virophage candidates, have been identified since then195,198,200. Recent studies show that virophages could resemble bona fide DNA viruses201 and their reclassification as their own viral family has been proposed195.
VLVs are produced during viral infection and, in contrast to other extracellular vesicles, contain viral proteins and nucleic acids but lack capsid protein or viral genomes and, therefore, are not infectious. VLVs have been described in both RNA and DNA viruses, including herpes simplex virus-1, hepatitis C virus and Kaposi’s sarcoma-associated herpes virus. VLVs play functional roles during viral infections by facilitating communication among cells and enhancing viral infection202–204.
Box 2 Glossary.
Von Magnus particles. Defective influenza virus particles with the ability to interfere with the replication of homologous virus discovered by Preben Von Magnus in 1947.
DIPs. Defective interfering particles. Viral particles containing a fraction of the viral genome able to replicate only in the presence of helper virus and interfere with the intracellular replication of non-defective homologous virus.
DVGs. Defective viral genomes. Viral genomes with defective ability to replicate in the absence of a co-infecting standard virus. Viral genomes can become defective due to mutations, deletions or a variety of gene rearrangements.
RdRP. RNA-dependent RNA polymerase. Viral enzyme that copies the viral RNA during viral replication.
MOI. Multiplicity of infection. Ratio of infectious virus to target cells.
TIPs. Therapeutic interfering particles. Synthetic DVGs with strong interfering activity proposed as therapeutics to outcompete standard viruses.
Classes, types, structures and diversity
Different types of DVGs are defined by the type of genomic alterations present. Next generation sequencing (NGS) has revealed a large variety of DVG species present in some infections, and recent studies have begun to elucidate the distinct functions of these different DVGs.
Point mutations, hypermutations and frame shifts
While the first DVGs to be identified and distinguished from the wild-type full-length viral genome lacked large parts of the genome21–25, there are a number of DVG types that do not involve drastic genomic alterations. Point mutations in RNA viruses can result in detrimental alterations because of the highly constrained nature of their genome organization. Indeed, a majority of randomly introduced mutations are either lethal or confer a significant fitness cost, as observed for vesicular stomatitis virus (VSV)26, poliovirus27 and influenza virus28. While it is inherently understood that detrimental mutations give rise to defective genomes, such genome types have historically not been considered DVGs. A genome harbouring a detrimental mutation in a structural protein could replicate, but not assemble properly; while a detrimental mutation in the replicase would yield a genome that can produce proper structural and assembly proteins, but could not replicate. Either of these genomes could potentially hijack the lacking functions from (and thus, interfere with) a full-length co-infecting virus. Indeed, studies where mutation rates are increased to perturb a viral population from viability to non-viability, revealed that the defective RNAs that appear prior to population extinction interfere with replication of a wild-type viral genome29,30. Hypermutations and mutations leading to frame shifts also result in defective viruses31 (Fig. 1a). One example is mutations introduced by the cellular protein APOBEC3G into pro-retroviruses32. APOBEC3G deaminates deoxycytidine nucleotides in the viral DNA, leading to hypermutations that interfere with the ability of the viral genome to integrate into the host genome and replicate. Interestingly, in opposition to an interfering activity for these mutated pro-viruses, it has been proposed that defective proviruses enhance fitness and that complementation among them drives viral persistence and pathogenesis33,34.
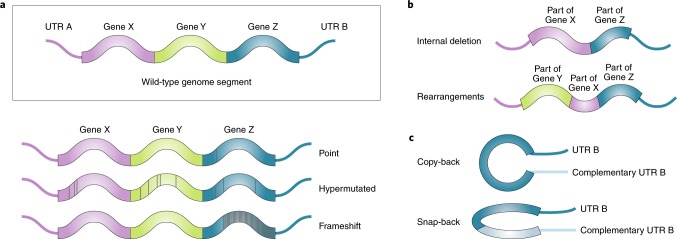
Fig. 1. Classes of DVGs.
a, Mutations in the viral genomes can lead to the generation of DVGs. These mutations can be single point mutations, hypermutations or frameshift mutations that alter virus replication, viral protein expression and/or viral protein function. b, Deletion-type DVGs occur when the polymerase skips part of the genome during replication and generates a truncated version of the genome. Deletion DVGs could also involve genomic rearrangements and gene duplication. Deletion generally results in lost or altered expression of one or more genes. c, Snap-back and copy-back DVGs are generated in nsRNA viruses when a sequence is duplicated in reverse complement to create theoretical panhandle structures for copy-back DVGs or hairpin structures for snap-back DVGs. Complementary sequence duplication occurs when the polymerase is released from the template strand and reattaches back to the nascent strand, copying back the end of the nascent genome. They can also occur from the polymerase copying back another complementary genome bound in trans. Most described snap-back and copy-back DVGs are generated from the 5’ end of the genome with the complementary end containing a duplication of the unstranslated region (UTR). Various degrees of complementation within Gene Z in the illustrated model can be found in copy-back DVGs. These types of genomes are not transcribed but can be replicated by the viral polymerase.
Deletions
The genomic viral species most commonly referred to as DVGs are truncated viral genomes resulting from large internal deletions occurring during viral replication. Deletion DVGs tend to bear single large truncations that remove several or all essential genes required for self-propagation while retaining 5′ and 3′ ends as well as other RNA structural elements required for polymerase binding, replication and/or packaging35–37. Multiple variants also exist, including DVGs that can be described as ‘mosaic’ in that they appear to be the result of multiple recombination and rearrangement events, including deletions, insertions, duplications and even inversions of parts of the genome38–40 (Fig. 1b).
Copy-backs
Copy-back and snap-back DVGs are rearranged genomes in which a sequence is duplicated in reverse complement to create theoretical stem-like structures (panhandle structures for copy-back DVGs or hairpin structures for snap-back DVGs)41–43. Copy-back DVGs have been reported in many negative-sense (ns)RNA viruses and are generated from the 5′ end of the genome in a process that produces DVGs with complementary 3′ and 5′ termini44,45. If the complementary region of the DVG comprises almost the entire sequence, the DVG can be further characterized as a snap-back DVG43 (Fig. 1c). Copy-back DVGs are predicted to be generated when the viral RNA-dependent RNA polymerase (RdRP) detaches from the template and reattaches to the nascent strand, copying back the end of the genome42,46.
DVG diversity during infection
Historically, it was thought that only certain viruses generated DVGs and that only one or a few distinct DVGs existed for any given virus. Most studies originally relied on high multiplicity of infection (MOI) passaging conditions that favoured the emergence of larger deletions (and thus, smaller DVGs) that could rapidly outcompete the longer full-length genomes owing to reduced replication times. Furthermore, these works relied on methods with low sensitivity of detection (such as visualization and purification on agarose gels) that would only isolate the most abundant DVGs. However, evidence for populations of distinct species of DVGs in a single infection has been reported since the 1980s45,47. We now know the diversity of DVGs to be much larger than initially appreciated and that certain DVGs are better than others at reaching high abundance, likely by retaining certain properties that confer replication advantage such as packaging signals and replication elements, or by acquiring the ability to interfere with the replication of other variants. NGS has revealed that DVGs are present in virtually any and every virus population, and that the diversity of DVGs is immense; however, the relative abundance of any given deletion or rearrangement varies, likely indicating that a variety of factors drive DVG generation and accumulation. The use of single-cell sequencing technology will allow precise quantification of the frequency and diversity of DVGs in an infected cell and will provide data on the distribution of DVG variants among a cell population.
Since most NGS alignment tools filter out reads with more than two mismatches, reads corresponding to deletion breakpoints or rearrangement junctions are rejected from a typical virus genome alignment. A re-analysis of rejected reads would identify these DVG hallmarks. With growing interest in this neglected part of the viral population, informatics tools such as ViReMa48, DI-tector49 and VODKA50 are emerging. These tools re-examine reads that potentially harbour jumbled or rearranged viral sequences and have enabled a broader appreciation of DVG diversity. A current challenge with NGS data is to determine how best to differentiate between true DVGs and background error of NGS, how to quantify the relative abundance of any given DVG with respect to full-length virus, and how best to normalize between samples and between sequencing runs—problems that are similar to transcriptome analysis of genetic isoforms. Furthermore, with increasing data sets and samples, it is becoming evident that DVG species are not necessarily the same if generated in different host and cell types, and the factors dictating these differences remain to be uncovered.
Mechanisms of DVG generation
DVGs have been long considered the result of stochastic mistakes introduced by the viral RNA polymerase that lacks proofreading activity. However, new evidence suggests that additional factors control DVG generation, opening the possibility of manipulating their generation for therapeutic purposes.
Random products or encoded in the viral genome?
While DVGs are generated by many viruses, the molecular mechanisms that govern their generation are poorly understood. A predominant theory is that DVGs arise from random errors that occur during viral replication at high viral titers due to the combination of a lack of proofreading activity of the viral polymerase and the presence of lower fidelity variants that favour the generation of deletions. In support, analyses using deep sequencing approaches revealed that multiple species of DVGs are generated during infection. For example, in infections with Flock house virus, a positive-sense (ps)RNA virus, ClickSeq and nanopore sequencing identified a large and seemingly random population of deletion DVGs early after infection48. In addition, sequencing analysis of nasopharyngeal samples from influenza virus-infected humans or infections in vitro with human metapneumovirus or measles virus (MeV) revealed multiple DVG species in these infections51–53. However, different from deletion and point mutation DVGs, copy-back DVGs are frequently found in discrete dominant populations in an infected cell or tissue, and the same copy-back DVG seems to arise in independent infections with the same parental virus54,55 or during infections with different virus strains56. The demonstration of hotspots for the generation of copy-back DVGs from respiratory syncytial virus (RSV) and the identification of specific nucleotides that determine where copy-back DVGs rejoin further demonstrate that the generation of copy-back DVGs is not completely random, but instead that specific sequences encoded in the viral genome direct or facilitate their formation50. In support, deep sequencing approaches have revealed discrete dominant DVG populations in infections with parainfluenza virus 5 and VSV57,58. Sequence similarities have been reported in the 5′ and 3′ regions flanking DVG deletion sites during influenza infection51,59, suggesting that there is also some degree of conservation in the generation of deletion DVGs. These observations indicate that, at least in some infections, DVG generation is not a completely stochastic process and, instead, virus-encoded sequences favour the production and/or amplification of predominant DVGs. It remains to be determined whether conservation is a property of certain DVG types and which specific sequences and/or RNA structures lead to DVG generation in these conditions.
Role of viral proteins
A number of viral proteins are implicated in the generation of DVGs, the best studied being the viral RdRP. Engineered viral polymerases with a decreased fidelity and an increased mutation rate produce highly attenuated viruses60,61 which, in many instances, correlate with enhanced production of DVGs62–65 (Fig. 2a). Although the mechanism mediating DVG generation by mutant RdRP remains speculative, a few models are emerging. For example, in viruses with low-fidelity RdRP, such as Sindbis virus or tombusvirus, an increased production of DVGs correlates with an enhanced rate of viral RNA recombination63,65. In addition, during influenza virus infection, variations in the elongation capacity of the polymerase associate with differential generation of DVGs64. A role for the polymerase multimerization activity was also recently proposed as a driver of DVG generation during influenza virus infection62.
Fig. 2. Mechanisms of DVG generation.
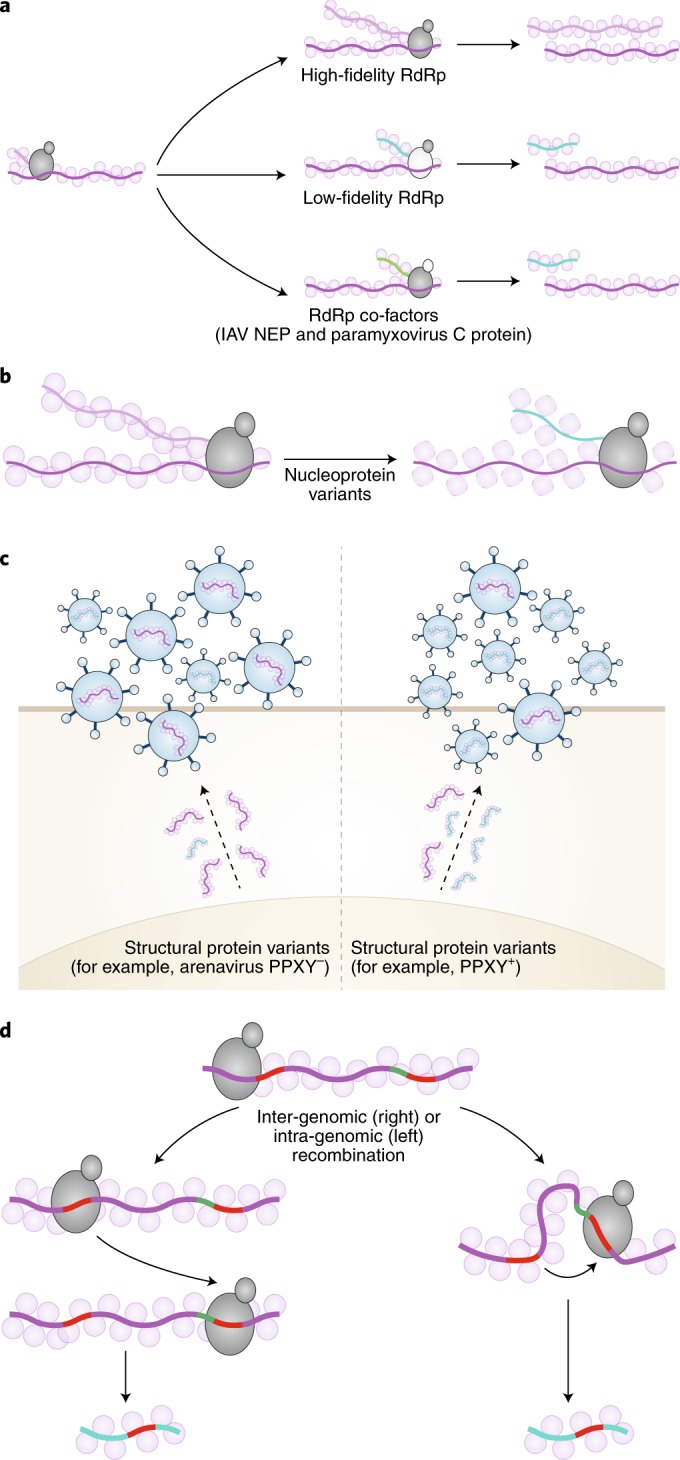
a, Variations in the viral RdRp fidelity due to mutations or effects of virus-encoded co-factors, such as the influenza A virus (IAV) NEP or the paramyxovirus C protein, can favour the generation of DVGs. b, Variants of the nucleoprotein with altered binding to viral RNA can promote DVG generation. c, Variants of structural proteins, such as the PPXY domain in the matrix protein of arenaviruses, can lead to DVG generation. d, Inter- and intra-recombination events using homologous sequences (red) can lead to the formation of deletion DVGs.
Viral proteins implicated in the regulation of viral transcription and replication are also associated with the generation of DVGs. Mutations in the influenza virus nuclear export protein (NEP, also known as NS2), which regulates the synthesis of complementary RNA, result in enhanced DVG production66. Similarly, deletion or mutations of the paramyxovirus C proteins that regulate template switching from antigenomic to genomic replication result in enhanced copy-back DVG production53,56 (Fig. 2a). Curiously, in infections with influenza virus lacking NEP or paramyxovirus lacking C, DVG production is observed in conditions that normally restrict the generation of DVGs, such as infections at low MOI. It is possible that the enhanced production of copy-back DVGs in these situations is related to higher template availability or to an indirect effect of viral polymerase activity, but the precise mechanism remains to be established. In addition, a single amino acid mutation in the Sendai virus (SeV) nucleoprotein that reduced the density of the ribonucleprotein associates with DVG generation67 (Fig. 2b). Lastly, in lymphocytic choriomeningitis mammarenavirus (LCMV) infection, the PPXY domain encoded within the matrix protein drives the production of viral particles containing defective genomes, but not those containing standard genomes68 (Fig. 2c).
Replication-driven template-switching
RNA recombination is a major driver of deletion DVG formation. A predominant model proposes that sequences at the break point or structural signals in the template RNA promote the replicase to switch to the acceptor RNA and resume synthesis (Fig. 2d). Replicase-driven recombination was proven in biochemical assays using RdRPs of a large number of RNA viruses69–71. Variations of this model include the forced template switch mechanism in which the replicase switches template after encountering the 5′ end of the template generating head-to-tail RNA dimers. If the templates are DVGs, these would lead to head-to-tail DVG dimers. The 5′ end could also be modified by endo- or exo-nucleases, leading to new versions of these genomes72.
RNA editing as a driver of DVG diversity
Editing of viral RNA leads to hypermutations and can result in the generation of DVGs, as reported in persistent measles infections31. In addition, a high rate of viral adenine-to-guanine (or uracil-to-cytosine) RNA editing by adenosine deaminase acting on RNA occurs in DVGs from VSV, human metapneumovirus and MeV38,52,53. In some cases, RNA editing regulates the immunostimulatory potential of DVGs53. However, the impact of DVG editing on viral replication seems to be virus-specific73. Whether DVGs have a higher rate of editing than the standard viral genome, as well as the impact of editing generated diversity in DVG evolution and selection, remains to be determined.
Roles in pathogenesis
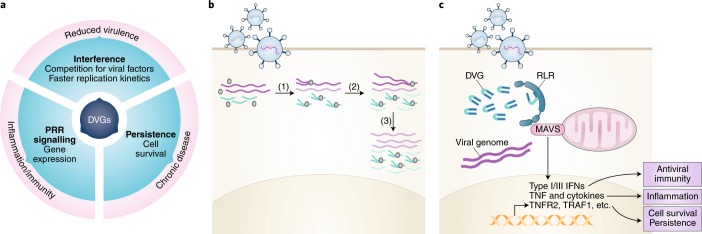
DVGs have three well-described functions that relate to their role in pathogenesis: interference with standard viral replication, immunostimulation and establishment of viral persistence (Fig. 3a).
Fig. 3. Functions and modes of actions of DVGs.
a, Overview of the known effects of DVGs on the standard virus and host cells, as well as their impact on viral pathogenesis. b, Proposed mechanism of competition for viral products in cells containing several copies of standard virus and DVGs, resulting in ‘interference’. (1) The viral polymerase replicates DVGs more efficiently than standard virus due to their shorter length and flanking trailer promoters. (2) These DVG properties lead to faster accumulation of DVGs in the infected cell. (3) DVGs eventually outcompete standard virus to become the predominant species and interfere with standard virus replication. c, Proposed mechanism for immunostimulation and cell survival induced by copy-back DVGs. Infected cells first detect DVGs through the RNA sensors RIG-I or MDA5, which signal through the adaptor protein MAVS for the production and secretion of type I and III IFNs pro-inflammatory cytokines and pro-survival proteins.
Interference with viral replication and viral production
DVGs were discovered during the search for factors responsible for reduced infectivity of influenza virus after passages at high titers2. DVGs with the ability to interfere with the replication of their parental virus both in vitro and in vivo are found in most positive and negative-sense (ns)RNA viruses54,74–82. DVGs accumulate at higher rates than full-length viral genomes in co-infected cells due to their shortened length and, in the case of copy-back species, their highly efficient flanking trailer promoters83,84. A predominant theory for how DVGs interfere with the replication of standard virus is based on the observed competition between defective and full-length viral genomes for viral components needed for replication (Fig. 3b). As DVGs accumulate to high levels, it is predicted that they can directly interfere with helper virus replication by monopolizing the viral polymerase and/or competing for structural proteins84,85.
It should be noted that while most evidence supports the notion that the shortest DVGs with largest deletions are best able to outcompete full-length virus because their much smaller size can be more rapidly replicated, most of these studies consist of high MOI cell culture conditions which favour competition dynamics based on replication kinetics. The question arises as to whether a longer deletion DVG that retains more coding sequence, yet contains mutated proteins (presumably replicating faster than full-length genomes, yet slower than the shortest DVGs), could be a better competitor of wild-type virus in certain conditions because it additionally expresses defective proteins that, in turn, interfere with wild-type proteins (for example, in multi-component structures such as capsids or replicases).
Furthermore, recent studies have demonstrated that DVGs and full-length viral genomes dominate in different cells during infection, conferring distinct functions to different infected cells86,87. While cells dominated with full-length viral genomes are the predominant producers of viral particles containing either full-length or defective genomes, cells enriched in DVGs do not produce many particles of any species86. These data highlight the need to consider the single cell versus population level impacts of DVGs during interference.
Triggers of antiviral immunity
DVGs, especially those of the copy-back type, strongly induce the expression of type I and III IFNs, tumour necrosis factor (TNF), interleukin (IL)-6, IL-1β and other pro-inflammatory cytokines, and are the primary stimuli of antiviral immunity in many infections6–8,52,54,55,88–90 (Fig. 3c). In addition, DVG stimulation optimizes the antigen presentation capacity of antigen-presenting cells91,92. Accumulating evidence indicates that the immunostimulatory activity of DVGs is maintained in vivo and during natural infections in humans. Increased survival of infected mice in infections containing DVGs have been reported for multiple viruses5,19,93. In mice infected with the respiratory viruses SeV, influenza or RSV, IFNs and pro-inflammatory cytokines are strongly induced only after DVGs have accumulated to detectable levels54,55. Detection of DVGs in respiratory secretions of children infected with RSV correlates with expression of antiviral genes55, and highly pathogenic influenza virus isolates that fail to induce potent antiviral responses in humans have an impaired ability to generate DVGs62.
Immunostimulatory DVGs can be recognized by pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and RIG-I-like receptors (RLRs). While TLR signalling is not essential for production of type I IFNs in vitro in response to DVGs, RLR signalling is required55,88,94–98. SeV copy-back DVGs are stronger immunostimulators than deletion DVGs89 and are among the strongest known inducers of the antiviral response. Therefore, much of what we know of DVGs immunostimulatory activity is based on the study of SeV copy-back DVGs. Copy-back DVG RNA binds RIG-I94,97,99 and DVGs from SeV, MeV and RSV viruses strongly stimulate RIG-I-dependent signalling55,92,95. RIG-I is triggered by binding of 5′ di- or triphosphates present on uncapped RNA or short regions of double-stranded (ds)RNA. Phosphatase treatment suppresses the ability of in vitro-transcribed copy-back DVGs to trigger IFN production following transfection supporting the role of RIG-I in DVG sensing91,94,96. SeV DVGs can also associate with melanoma differentiation-associated protein 5 (MDA5)88,94, but the role for MDA5 in sensing other DVGs is less clear99,100. Other cellular proteins can also associate with DVGs. For example, MeV DVGs associate with the dsRNA binding protein named protein activator of the interferon-induced protein kinase to optimally activate RIG-I96.
DVG-induced RLR signalling is not simply a result of higher viral RNA content in the infected cells, as increasing the amount of DVG-deficient virus does not increase the IFN response54,88,92. Importantly, efficient sensing of copy-back DVGs occurs even in the presence of virus-encoded antagonists of the cellular sensing pathways88,92. These observations suggest that unique features of DVGs favour their detection during infection. The predicted long dsRNA stretch formed by the reverse complementary ends of copy-back DVGs was thought to be a critical factor in their immunostimulatory activity8,99,101. However, recent evidence demonstrates the existence of additional features in DVGs that have a larger impact on their immunostimulatory potential. Structural modelling identified a 44 nucleotide (nt)-long stem-loop motif (DVG70–114) in a SeV DVG-546, a well-characterized and potent immunostimulatory copy-back DVG, which was absent in the SeV genome and spans the unique junction formed between the genomic break and rejoin points that form this copy-back DVG. Deletion of DVG70–114 reduces the DVG immunostimulatory activity, while introduction of the motif into an immunologically inert RNA improves its ability to induce the expression of type I IFNs and IFN-stimulated genes94. DVG70–114 acts in concert with the 5′-triphosphate motif to activate RIG-I and allows for enhanced RLR polymerization, a marker of activation94. DVG70–114 is active in the context of SeV infection, as recombinant viruses carrying DVGs that lack this motif significantly lose immunostimulatory potential94. Failure to detect dsRNA through immunostaining during SeV infection102 and normal immunostimulatory activity following disruption of the complementarity between the 5′ and 3′ ends of copy-back SeV DVGs94 further support the notion that the predicted long complementary ends of copy-back DVGs have a minor role in the onset of anti-viral immunity. It remains to be determined when and how DVG70-114 is exposed during infection, and whether similar motifs are present in other highly immunostimulatory DVGs.
DVG immunostimulation is also an important factor in the modulation of infections in insects. Similar to the RIG-I and MDA5 PRRs in mammals, insects sense viral RNA through the protein Dicer-2. This protein processes the viral RNA into small interfering RNAs that protect insects from re-infection with the same virus103. DVGs are the primary targets for Dicer-2 (ref. 103). These observations indicate that the immunostimulatory activity of DVGs is widespread and may have a significant impact on the spread of pathogens within and across species.
Facilitators of RNA virus persistence
DIPs facilitate the establishment of persistently infected cell cultures in a variety of RNA virus infections, including influenza, SeV, Ebola, mumps and Semliki Forest virus10,13–15,74,104–107. Such in vitro evidence is accompanied by studies in mice showing that infection with Semliki Forest virus or LCMV containing DIPs establish persistent infections18,108, as well as one study reporting DVGs in the brain of human patients who had died due to post-MeV subacute sclerosing panencephalitis17.
DIPs and full-length viruses cycle asynchronously in many persistent infections in vitro109,110 and in vivo19,109. Cycling occurs in a predictable pattern and has been mathematically modelled using variations of the predator–prey model111. A theory to explain the asynchronous cycling of full-length viral genomes and DVGs during persistency was put forward in 1970 (ref. 3). This theory, which is based on the interference effect of DVGs on the replication of the full-length genome, proposed the following: DIPs accumulate during virus replication until they reach high concentrations and become predominant. In this condition, DVGs interfere with the replication of the full-length viral genome by competing for essential replication machinery, driving a reduction in the full-length virus. During this process, some previously uninfected cells are infected by standard virus and re-initiate the cycle. Interestingly, in some persistent infections the amount of DIPs appears constant112. What drives these cyclic patterns in some viruses but not others, and whether host factors such as the infected cell type influences the cycling pattern, remain unknown.
Recent evidence indicates that the mechanisms involved in the establishment of persistence are more complex than simple intracellular competition for the replication machinery among different types of viral genomes87. Using RNA in situ fluorescent hybridization, Xu et al. showed that during infection with SeV or RSV containing DIPs, there is heterogeneity in the content of viral genomes in the infected population87. While some cells are enriched in DVGs, others are enriched in standard full-length viral genomes. The mechanisms for this heterogeneity are currently unknown, however, striking functional differences among these cell populations are beginning to emerge86,87. Cells enriched in DVGs engage the RLR sensing pathway and produce IFNs and other pro-inflammatory molecules, including TNF. In addition, these cells induce a pro-survival program, also dependent on signalling through the RLR pathway. These programs protect DVG-high cells from TNF-mediated death, while cells lacking DVGs die during infection. Surviving DVG-high cells can be propagated for months as a persistent infection. This mechanism provides an explanation for the paradoxical stimulation of both antiviral immunity and establishment of persistence by DVGs. It remains unclear how the enhanced survival of DVG-high cells leads to persistence, and how this survival fits into the cycling of DVG and standard virus observed in many infections. Cycling may occur at the intracellular level (where each infected cell goes through cycles of standard viral genome or DVG enrichment driven by competition and interference with the viral replication machinery) and/or at the population level, where individual infected cells will determine the composition of the pool of standard virus or DIPs available for infection of new cells.
Use as antivirals and vaccine adjuvants
The strong interfering and immunostimulatory activities of DVGs make them attractive candidates for vaccine adjuvants and antivirals113,114. The ability of DIPs to interfere with the replication of standard viruses and diminish virus-associated disease has been extensively demonstrated in mice during infection with virus stocks containing DIPs4,54,75,77,115, even when the vaccine contained standard virus inactivated by ultraviolet treatment116. A similar protection has been reported in ferrets vaccinated with an influenza vaccine containing only DIPs117. Synthetically engineered DIPs with strong interfering potential, or ‘therapeutic interfering particles (TIPs)’, have been more recently proposed as a possible strategy to control viral infections. TIPs would theoretically replicate faster than the wild-type virus and therefore outcompete the virus hindering spread and transmission. TIPs would have the advantage of being active only in organisms already infected with the wild-type virus due to their dependence on a helper virus to replicate118,119. Although still in the exploratory phase, it remains to be determined how TIPs would impact virus persistence, the generation of adaptive mutations and the generation of new infectious viruses by complementation.
Whether protection elicited by natural or synthetic DIPs is due to direct interference with the standard virus replication or through strong immunostimulatory ability of DVGs is unclear. SeV copy-back DVGs augment the antigen presentation capacity of mouse and human dendritic cells, resulting in enhanced activation of T cells91. In addition, experimental vaccines against influenza virus and RSV adjuvanted with in vitro-transcribed SeV DVGs delivered subcutaneously, intramuscularly or intranasally show improved antibody production and increased protection from virus challenge91,120. SeV DVG-derived oligonucleotides (DDOs) containing the immunostimulatory motif DVG70–114 are effective adjuvants able to bias the humoral and cellular responses against inactivated virus and protein vaccines towards type I immune responses including antibodies of the IgG2a/c isotypes, Th1 CD4+ T cells and cytotoxic CD8+ T cells in mice91,121. DDOs also synergize with the emulsion antibody AddaVax and enhance its type I immunity-driving potential by mechanisms that depends on type I IFNs121. Notably, a DIP influenza vaccine conferred protection to an unrelated virus through stimulation of type I IFN production122, suggesting that they can be used as prophylactic or therapeutic antivirals.
DVGs are present in live attenuated vaccines against polio, measles and influenza viruses76,123–126. However, their impact in the development of protective immunity and vaccine efficacy has not been formally assessed. Based on their interfering and immunostimulatory ability, it is speculated that DVGs may enhance the efficiency of the vaccine while enhancing safety of the virus by reducing its replication and spread. If correct, it would be important to carefully regulate the amount of DVGs in vaccine preparations to avoid complete interference and drastic reduction of the virus to a point of ineffectiveness.
Impact on viral evolution and dynamics
While recent NGS data reveal that hundreds of DVGs can arise within a single viral infection, the fact that a smaller subset of dominant DVGs are repeatedly detected in different samples50 indicates that complex dynamics are at play within the viral population. These complexities include competition (and possibly compensation or cooperation) between different DVGs and positive selection of the best competitors that implicates their relative fitness in relation to the wild-type parental virus and other DVGs. Parameters such as replication fitness, packaging, immunoregulation and other traits determine the virus dynamics. It is important to stress that most events leading to DVG formation, including mutations, deletions, recombination and translocations, are either non-viable or deleterious to the virus. In addition, although hundreds or even thousands of different DVGs are generated during a virus infection, the vast majority of these will be lost during the population bottlenecks that occur in vivo, for example when crossing anatomical barriers or during transmission from host to host127. However, there are instances where these genomes could make it through bottlenecks, such as during infections of hosts that are immunosuppressed or have comorbidities where founding populations are increased. In addition, infections may occur with virions that have co-packaged wild-type genomes and DVGs128 or virions may aggregate during infection enhancing co-transmission, as seen for VSV129 and poliovirus130. Current mathematical models have generally only considered one dominant DVG131,132; further development is required to incorporate the potential cooperation and competition among others.
While historically DVGs have been considered replication waste, an artifact of cell culture passaging conditions or simply a nuisance to laboratory experimentation, a renewed interest in this part of the viral population may reveal a more functional or biological relevance to their existence. Do DVGs exist for a reason? Why keep waste lying around? Given the notoriously high recombination and reassortment rates of some viruses, it is possible that for viruses that undergo recombination, DVGs can provide a repertoire of mutations that could feed back into the viable virus population to promote adaptation. It remains to be seen whether DVGs can provide a selective advantage to the virus, and whether the high MOI or localized co-infection conditions are biologically relevant outside of cell culture.
Recent work in insects suggests that DVGs, which are a preferred template for the generation of the viral DNA form of RNA viruses, provide additional substrate to help boost the RNA interference response that is responsible for viral persistence in insects103. Indeed, it was shown that a change in the amount of viral DNA generated during RNA virus infection in Drosophila, altered the persistence and kinetics of a wild-type RNA virus infection. The authors suggested that evolution has perhaps fine-tuned the production of DVGs to balance wild-type infection and promote persistence (and ultimately, transmission of viruses, including arboviruses in mosquitoes).
Additionally, a closer examination of DVG dynamics within viral populations can help better understand the biology of standard viruses. Indeed, in addition to distinguishing between the dispensable and indispensable nucleotide sequences, proteins and RNA structures, we can identify what viral components can operate in cis or in trans. A recent study of HCV DVGs, for example, identified novel cis-acting RNA structures that were required for replication and packaging133.
Concluding remarks
Emerging technologies that allow the identification of defective and standard viruses in natural infections, as well as those allowing the establishment of specific associations of DVGs with functional outcomes, have been crucial in reviving the interest in studying DVGs. Recent work has provided new appreciation for DVG diversity and the potentially critical role of DVGs in defining the clinical outcome of infections; however, a number of questions remain unanswered: what are the molecular mechanisms driving the generation of DVGs? Can DVGs be harnessed for the control of virus pathogenesis and spread? How do alterations in the DVG population impact virus evolution and adaptation to new hosts? How do host factors impact DVG accumulation and activity? Further technological developments and interdisciplinary research will be required to obtain these answers.
Acknowledgements
This work was supported by the US National Institutes of Health National Institute of Allergy and Infectious Diseases (grants nos. NIH AI083284, AI137062 and AI134862 to C.B.L.) and the DARPA INTERCEPT program (to M.V.) managed by J. Gimlett and administered though DARPA Cooperative Agreement (grant no. HR0011-17-2-0023). In addition, this work was supported by a Fulbright US Scholar award to C.B.L. The views expressed in this article do not necessarily represent the position or the policy of the US government, and no official endorsement should be inferred.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poirier EZ, Vignuzzi M. Virus population dynamics during infection. Curr. Opin. Virol. 2017;23:82–87. doi: 10.1016/j.coviro.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Von Magnus P, Gard S. Studies on interference in experimental influenza. Ark. Kemi. Mineral. Geol. 1947;24:4. [Google Scholar]
- 3.Huang AS, Baltimore D. Defective viral particles and viral disease processes. Nature. 1970;226:325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- 4.Barrett AD, Dimmock NJ. Modulation of Semliki Forest virus-induced infection of mice by defective-interfering virus. J. Infect. Dis. 1984;150:98–104. doi: 10.1093/infdis/150.1.98. [DOI] [PubMed] [Google Scholar]
- 5.Rabinowitz SG, Huprikar J. The influence of defective-interfering particles of the PR-8 strain of influenza A virus on the pathogenesis of pulmonary infection in mice. J. Infect. Dis. 1979;140:305–315. doi: 10.1093/infdis/140.3.305. [DOI] [PubMed] [Google Scholar]
- 6.Fuller FJ, Marcus PI. Interferon induction by viruses. IV. Sindbis virus: early passage defective-interfering particles induce interferon. J. Gen. Virol. 1980;48:63–73. doi: 10.1099/0022-1317-48-1-63. [DOI] [PubMed] [Google Scholar]
- 7.Johnston MD. The characteristics required for a Sendai virus preparation to induce high levels of interferon in human lymphoblastoid cells. J. Gen. Virol. 1981;56:175–184. doi: 10.1099/0022-1317-56-1-175. [DOI] [PubMed] [Google Scholar]
- 8.Marcus PI, Sekellick MJ. Defective interfering particles with covalently linked [±]RNA induce interferon. Nature. 1977;266:815–819. doi: 10.1038/266815a0. [DOI] [PubMed] [Google Scholar]
- 9.Andzhaparidze OG, Bogomolova NN, Boriskin Yu S, Drynov ID. Chronic non-cytopathic infection of human continuous cell lines with mumps virus. Acta Virol. 1983;27:318–328. [PubMed] [Google Scholar]
- 10.De BK, Nayak DP. Defective interfering influenza viruses and host cells: establishment and maintenance of persistent influenza virus infection in MDBK and HeLa cells. J. Virol. 1980;36:847–859. doi: 10.1128/jvi.36.3.847-859.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai A, Matsumoto S, Tanabe K. Characterization of rabies viruses recovered from persistently infected BHK cells. Virology. 1975;67:520–533. doi: 10.1016/0042-6822(75)90452-3. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy JC, Macdonald RD. Persistent infection with infectious pancreatic necrosis virus mediated by defective-interfering (DI) virus particles in a cell line showing strong interference but little DI replication. J. Gen. Virol. 1982;58:361–371. doi: 10.1099/0022-1317-58-2-361. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann-Grube F, Slenczka W, Tees R. A persistent and inapparent infection of L cells with the virus of lymphocytic choriomeningitis. J. Gen. Virol. 1969;5:63–81. doi: 10.1099/0022-1317-5-1-63. [DOI] [PubMed] [Google Scholar]
- 14.Roux L, Waldvogel FA. Establishment of Sendai virus persistent infection: biochemical analysis of the early phase of a standard plus defective interfering virus infection of BHK cells. Virology. 1981;112:400–410. doi: 10.1016/0042-6822(81)90287-7. [DOI] [PubMed] [Google Scholar]
- 15.Schmaljohn C, Blair CD. Persistent infection of cultured mammalian cells by Japanese encephalitis virus. J. Virol. 1977;24:580–589. doi: 10.1128/jvi.24.2.580-589.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekellick MJ, Marcus PI. Persistent infection. I Interferon-inducing defective-interfering particles as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1978;85:175–186. doi: 10.1016/0042-6822(78)90422-1. [DOI] [PubMed] [Google Scholar]
- 17.Baczko K, et al. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J. Virol. 1986;59:472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popescu M, Lehmann-Grube F. Defective interfering particles in mice infected with lymphocytic choriomeningitis virus. Virology. 1977;77:78–83. doi: 10.1016/0042-6822(77)90407-x. [DOI] [PubMed] [Google Scholar]
- 19.Mims CA. Rift Valley Fever virus in mice. IV. Incomplete virus; its production and properties. Brit. J. Exp. Pathol. 1956;37:129–143. [PMC free article] [PubMed] [Google Scholar]
- 20.Viola MV, Scott C, Duffy PD. Persistent measles virus infection in vitro and in man. Arthritis Rheum. 1978;21:S47–51. [PubMed] [Google Scholar]
- 21.Huang AS, Greenawalt JW, Wagner RR. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966;30:161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- 22.Duesberg PH. The RNA of influenza virus. Proc. Natl Acad. Sci. USA. 1968;59:930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingsbury DW, Portner A, Darlington RW. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970;42:857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- 24.Cole CN, Smoler D, Wimmer E, Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J. Virol. 1971;7:478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Repik P, Bishop DH. Determination of the molecular weight of animal RNA viral genomes by nuclease digestions. I. Vesicular stomatitis virus and its defective T particle. J. Virol. 1973;12:969–983. doi: 10.1128/jvi.12.5.969-983.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanjuan R, Moya A, Elena SF. The distribution of fitness effects caused by single-nucleotide substitutions in an RNA virus. Proc. Natl Acad. Sci. USA. 2004;101:8396–8401. doi: 10.1073/pnas.0400146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acevedo A, Brodsky L, Andino R. Mutational and fitness landscapes of an RNA virus revealed through population sequencing. Nature. 2014;505:686–690. doi: 10.1038/nature12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thyagarajan B, Bloom JD. The inherent mutational tolerance and antigenic evolvability of influenza hemagglutinin. eLife. 2014;3:e03300. doi: 10.7554/eLife.03300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perales C, Mateo R, Mateu MG, Domingo E. Insights into RNA virus mutant spectrum and lethal mutagenesis events: replicative interference and complementation by multiple point mutants. J. Mol. Biol. 2007;369:985–1000. doi: 10.1016/j.jmb.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 30.Grande-Perez A, Lazaro E, Lowenstein P, Domingo E, Manrubia SC. Suppression of viral infectivity through lethal defection. Proc. Natl Acad. Sci. USA. 2005;102:4448–4452. doi: 10.1073/pnas.0408871102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cattaneo R, et al. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Q, et al. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat. Struct. Mol. Biol. 2004;11:435–442. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]
- 33.Imamichi H, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl Acad. Sci. USA. 2016;113:8783–8788. doi: 10.1073/pnas.1609057113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomoto A, Jacobson A, Lee YF, Dunn J, Wimmer E. Defective interfering particles of poliovirus: mapping of the deletion and evidence that the deletions in the genomes of DI(1), (2) and (3) are located in the same region. J. Mol. Biol. 1979;128:179–196. doi: 10.1016/0022-2836(79)90125-6. [DOI] [PubMed] [Google Scholar]
- 36.Perrault J, Semler BL. Internal genome deletions in two distinct classes of defective interfering particles of vesicular stomatitis virus. Proc. Natl Acad. Sci. USA. 1979;76:6191–6195. doi: 10.1073/pnas.76.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis AR, Hiti AL, Nayak DP. Influenza defective interfering viral RNA is formed by internal deletion of genomic RNA. Proc. Natl Acad. Sci. USA. 1980;77:215–219. doi: 10.1073/pnas.77.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hara PJ, Nichol ST, Horodyski FM, Holland JJ. Vesicular stomatitis virus defective interfering particles can contain extensive genomic sequence rearrangements and base substitutions. Cell. 1984;36:915–924. doi: 10.1016/0092-8674(84)90041-2. [DOI] [PubMed] [Google Scholar]
- 39.Hillman BI, Carrington JC, Morris TJ. A defective interfering RNA that contains a mosaic of a plant virus genome. Cell. 1987;51:427–433. doi: 10.1016/0092-8674(87)90638-6. [DOI] [PubMed] [Google Scholar]
- 40.Molenkamp R, Rozier BC, Greve S, Spaan WJ, Snijder EJ. Isolation and characterization of an arterivirus defective interfering RNA genome. J. Virol. 2000;74:3156–3165. doi: 10.1128/jvi.74.7.3156-3165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976;8:547–555. doi: 10.1016/0092-8674(76)90223-3. [DOI] [PubMed] [Google Scholar]
- 42.Lazzarini RA, Keene JD, Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981;26:145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- 43.Nichol ST, O’Hara PJ, Holland JJ, Perrault J. Structure and origin of a novel class of defective interfering particle of vesicular stomatitis virus. Nucleic Acids Res. 1984;12:2775–2790. doi: 10.1093/nar/12.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perrault J, Leavitt RW. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J. Gen. Virol. 1978;38:35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- 45.Re GG, Gupta KC, Kingsbury DW. Genomic and copy-back 3′ termini in Sendai virus defective interfering RNA species. J. Virol. 1983;45:659–664. doi: 10.1128/jvi.45.2.659-664.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrault J. Origin and replication of defective interfering particles. Curr. Top. Microbiol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- 47.Barrett AD, Crouch CF, Dimmock NJ. Defective interfering Semliki Forest virus populations are biologically and physically heterogeneous. J. Gen. Virol. 1984;65:1273–1283. doi: 10.1099/0022-1317-65-8-1273. [DOI] [PubMed] [Google Scholar]
- 48.Jaworski E, Routh A. Parallel ClickSeq and Nanopore sequencing elucidates the rapid evolution of defective-interfering RNAs in Flock House virus. PLoS Pathog. 2017;13:e1006365. doi: 10.1371/journal.ppat.1006365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beauclair G, et al. DI-tector: defective interfering viral genomes detector for next generation sequencing data. RNA. 2018;24:1285–1296. doi: 10.1261/rna.066910.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y, et al. A specific sequence in the genome of respiratory syncytial virus regulates the generation of copy-back defective viral genomes. PLoS Pathog. 2019;15:e1007707. doi: 10.1371/journal.ppat.1007707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saira K, et al. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J. Virol. 2013;87:8064–8074. doi: 10.1128/JVI.00240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Hoogen BG, et al. Excessive production and extreme editing of human metapneumovirus defective interfering RNA is associated with type I IFN induction. J. Gen. Virol. 2014;95:1625–1633. doi: 10.1099/vir.0.066100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfaller CK, et al. Measles virus defective interfering RNAs are generated frequently and early in the absence of C protein and pan be destabilized by adenosine deaminase acting on RNA-1-like hypermutations. J. Virol. 2015;89:7735–7747. doi: 10.1128/JVI.01017-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tapia K, et al. Defective viral genomes arising in vivo provide critical danger signals for the triggering of lung antiviral immunity. PLoS Pathog. 2013;9:e1003703. doi: 10.1371/journal.ppat.1003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y, et al. Immunostimulatory defective viral genomes from respiratory syncytial virus promote a strong innate antiviral response during Infection in mice and humans. PLoS Pathog. 2015;11:e1005122. doi: 10.1371/journal.ppat.1005122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez-Aparicio MT, et al. Loss of Sendai virus C protein leads to accumulation of RIG-I immunostimulatory defective interfering RNA. J. Gen. Virol. 2017;98:1282–1293. doi: 10.1099/jgv.0.000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Killip MJ, et al. Deep sequencing analysis of defective genomes of parainfluenza virus 5 and their role in interferon induction. J. Virol. 2013;87:4798–4807. doi: 10.1128/JVI.03383-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Timm C, Akpinar F, Yin J. Quantitative characterization of defective virus emergence by deep sequencing. J. Virol. 2014;88:2623–2632. doi: 10.1128/JVI.02675-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jennings PA, Finch JT, Winter G, Robertson JS. Does the higher order structure of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA? Cell. 1983;34:619–627. doi: 10.1016/0092-8674(83)90394-x. [DOI] [PubMed] [Google Scholar]
- 60.Van Slyke GA, et al. Sequence-specific fidelity alterations associated with West Nile virus attenuation in mosquitoes. PLoS Pathog. 2015;11:e1005009. doi: 10.1371/journal.ppat.1005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rozen-Gagnon K, et al. Alphavirus mutator variants present host-specific defects and attenuation in mammalian and insect models. PLoS Pathog. 2014;10:e1003877. doi: 10.1371/journal.ppat.1003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vasilijevic J, et al. Reduced accumulation of defective viral genomes contributes to severe outcome in influenza virus infected patients. PLoS Pathog. 2017;13:e1006650. doi: 10.1371/journal.ppat.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poirier EZ, et al. Low-Fidelity Polymerases of Alphaviruses Recombine at Higher Rates To Overproduce Defective Interfering Particles. J. Virol. 2015;90:2446–2454. doi: 10.1128/JVI.02921-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fodor E, Mingay LJ, Crow M, Deng T, Brownlee GG. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase promotes the generation of defective interfering RNAs. J. Virol. 2003;77:5017–5020. doi: 10.1128/JVI.77.8.5017-5020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panaviene Z, Nagy PD. Mutations in the RNA-binding domains of tombusvirus replicase proteins affect RNA recombination in vivo. Virology. 2003;317:359–372. doi: 10.1016/j.virol.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 66.Odagiri T, Tobita K. Mutation in NS2, a nonstructural protein of influenza A virus, extragenically causes aberrant replication and expression of the PA gene and leads to generation of defective interfering particles. Proc. Natl Acad. Sci. USA. 1990;87:5988–5992. doi: 10.1073/pnas.87.15.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida A, et al. A single amino acid substitution within the paramyxovirus Sendai virus nucleoprotein is a critical determinant for production of interferon-beta-inducing copyback-type defective interfering genomes. J. Virol. 2018;92:e02094-17. doi: 10.1128/JVI.02094-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziegler CM, et al. The lymphocytic choriomeningitis virus matrix protein PPXY late domain drives the production of defective interfering particles. PLoS Pathog. 2016;12:e1005501. doi: 10.1371/journal.ppat.1005501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White KA, Morris TJ, Nonhomologous RNA. recombination in tombusviruses: generation and evolution of defective interfering RNAs by stepwise deletions. J. Virol. 1994;68:14–24. doi: 10.1128/jvi.68.1.14-24.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim MJ, Kao C. Factors regulating template switch in vitro by viral RNA-dependent RNA polymerases: implications for RNA-RNA recombination. Proc. Natl Acad. Sci. USA. 2001;98:4972–4977. doi: 10.1073/pnas.081077198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wierzchoslawski R, Bujarski JJ. Efficient in vitro system of homologous recombination in brome mosaic bromovirus. J. Virol. 2006;80:6182–6187. doi: 10.1128/JVI.02447-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jaag HM, Nagy PD. Silencing of Nicotiana benthamiana Xrn4p exoribonuclease promotes tombusvirus RNA accumulation and recombination. Virology. 2009;386:344–352. doi: 10.1016/j.virol.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 73.Ward SV, Sternsdorf T, Woods NB. Targeting expression of the leukemogenic PML-RARalpha fusion protein by lentiviral vector-mediated small interfering RNA results in leukemic cell differentiation and apoptosis. Hum. Gene Ther. 2011;22:1593–1598. doi: 10.1089/hum.2011.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calain P, Monroe MC, Nichol ST. Ebola virus defective interfering particles and persistent infection. Virology. 1999;262:114–128. doi: 10.1006/viro.1999.9915. [DOI] [PubMed] [Google Scholar]
- 75.Li D, et al. Defective interfering viral particles in acute dengue infections. PLoS ONE. 2011;6:e19447. doi: 10.1371/journal.pone.0019447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calain P, Roux L. Generation of measles virus defective interfering particles and their presence in a preparation of attenuated live-virus vaccine. J. Virol. 1988;62:2859–2866. doi: 10.1128/jvi.62.8.2859-2866.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roux L, Simon AE, Holland JJ. Effects of defective interfering viruses on virus replication and pathogenesis in vitro and in vivo. Adv. Virus Res. 1991;40:181–211. doi: 10.1016/S0065-3527(08)60279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Treuhaft MW, Beem MO. Defective interfering particles of respiratory syncytial virus. Infect. Immun. 1982;37:439–444. doi: 10.1128/iai.37.2.439-444.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiktor TJ, Dietzschold B, Leamnson RN, Koprowski H. Induction and biological properties of defective interfering particles of rabies virus. J. Virol. 1977;21:626–635. doi: 10.1128/jvi.21.2.626-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang AS, Wagner RR. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966;30:173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- 81.Sekellick MJ, Marcus PI. Viral interference by defective particles of vesicular stomatitis virus measured in individual cells. Virology. 1980;104:247–252. doi: 10.1016/0042-6822(80)90385-2. [DOI] [PubMed] [Google Scholar]
- 82.Brinton MA. Analysis of extracellular West Nile virus particles produced by cell cultures from genetically resistant and susceptible mice indicates enhanced amplification of defective interfering particles by resistant cultures. J. Virol. 1983;46:860–870. doi: 10.1128/jvi.46.3.860-870.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li T, Pattnaik AK. Replication signals in the genome of vesicular stomatitis virus and its defective interfering particles: identification of a sequence element that enhances DI RNA replication. Virology. 1997;232:248–259. doi: 10.1006/viro.1997.8571. [DOI] [PubMed] [Google Scholar]
- 84.Calain P, Roux L. Functional characterisation of the genomic and antigenomic promoters of Sendai virus. Virology. 1995;212:163–173. doi: 10.1006/viro.1995.1464. [DOI] [PubMed] [Google Scholar]
- 85.Portner A, Kingsbury DW. Homologous interference by incomplete Sendai virus particles: changes in virus-specific ribonucleic acid synthesis. J. Virol. 1971;8:388–394. doi: 10.1128/jvi.8.4.388-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Genoyer E, Lopez CB. Defective viral genomes alter how Sendai virus interacts with cellular trafficking machinery leading to heterogeneity in the production of viral particles among infected cells. J. Virol. 2018;93:e01579-18. doi: 10.1128/JVI.01579-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu J, et al. Replication defective viral genomes exploit a cellular pro-survival mechanism to establish paramyxovirus persistence. Nat. Commun. 2017;8:7996. doi: 10.1038/s41467-017-00909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yount JS, Gitlin L, Moran TM, Lopez CB. MDA5 participates in the detection of paramyxovirus infection and is essential for the early activation of dendritic cells in response to Sendai virus defective interfering particles. J. Immunol. 2008;180:4910–4918. doi: 10.4049/jimmunol.180.7.4910. [DOI] [PubMed] [Google Scholar]
- 89.Strahle L, Garcin D, Kolakofsky D. Sendai virus defective-interfering genomes and the activation of interferon-beta. Virology. 2006;351:101–111. doi: 10.1016/j.virol.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 90.Shivakoti R, Siwek M, Hauer D, Schultz KL, Griffin DE. Induction of dendritic cell production of type I and type III interferons by wild-type and vaccine strains of measles virus: role of defective interfering RNAs. J. Virol. 2013;87:7816–7827. doi: 10.1128/JVI.00261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mercado-Lopez X, et al. Highly immunostimulatory RNA derived from a Sendai virus defective viral genome. Vaccine. 2013;31:5713–5721. doi: 10.1016/j.vaccine.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yount JS, Kraus TA, Horvath CM, Moran TM, Lopez CB. A novel role for viral-defective interfering particles in enhancing dendritic cell maturation. J. Immunol. 2006;177:4503–4513. doi: 10.4049/jimmunol.177.7.4503. [DOI] [PubMed] [Google Scholar]
- 93.Barrett AD, Dimmock NJ. Modulation of a systemic Semliki Forest virus infection in mice by defective interfering virus. J. Gen. Virol. 1984;65:1827–1831. doi: 10.1099/0022-1317-65-10-1827. [DOI] [PubMed] [Google Scholar]
- 94.Xu J, et al. Identification of a natural viral RNA motif that optimizes sensing of viral RNA by RIG.-I. mBio. 2015;6:e01265-15. doi: 10.1128/mBio.01265-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strahle L, et al. Activation of the beta interferon promoter by unnatural Sendai virus infection requires RIG-I and is inhibited by viral C proteins. J. Virol. 2007;81:12227–12237. doi: 10.1128/JVI.01300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ho TH, et al. PACT- and RIG-I-dependent activation of Type I interferon production by a defective interfering RNA derived from measles virus vaccine. J. Virol. 2015;90:1557–1568. doi: 10.1128/JVI.02161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baum A, Sachidanandam R, Garcia-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc. Natl Acad. Sci. USA. 2010;107:16303–16308. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopez CB, et al. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 2004;173:6882–6889. doi: 10.4049/jimmunol.173.11.6882. [DOI] [PubMed] [Google Scholar]
- 99.Runge S, et al. In vivo ligands of MDA5 and RIG-I in measles virus-infected cells. PLoS Pathog. 2014;10:e1004081. doi: 10.1371/journal.ppat.1004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mura M, et al. Nonencapsidated 5′ Copy-Back Defective Interfering Genomes Produced by Recombinant Measles Viruses Are Recognized by RIG-I and LGP2 but Not MDA5. J. Virol. 2017;91:e00643-17. doi: 10.1128/JVI.00643-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel JR, et al. ATPase-driven oligomerization of RIG-I on RNA allows optimal activation of type-I interferon. EMBO Rep. 2013;14:780–787. doi: 10.1038/embor.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poirier EZ, et al. Dicer-2-dependent generation of viral DNA from defective genomes of RNA viruses modulates antiviral immunity in insects. Cell Host Microbe. 2018;23:353–365. doi: 10.1016/j.chom.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roux L, Holland JJ. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979;93:91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- 105.Andzhaparidze OG, Bogomolova NN, Boriskin YS, Bektemirova MS, Drynov ID. Comparative study of rabies virus persistence in human and hamster cell lines. J. Virol. 1981;37:1–6. doi: 10.1128/jvi.37.1.1-6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barrett AD, et al. Subclinical infections in mice resulting from the modulation of a lethal dose of Semliki Forest virus with defective interfering viruses: neurochemical abnormalities in the central nervous system. J. Gen. Virol. 1986;67:1727–1732. doi: 10.1099/0022-1317-67-8-1727. [DOI] [PubMed] [Google Scholar]
- 107.Bangham CR, Kirkwood TB. Defective interfering particles: effects in modulating virus growth and persistence. Virology. 1990;179:821–826. doi: 10.1016/0042-6822(90)90150-p. [DOI] [PubMed] [Google Scholar]
- 108.Atkinson T, Barrett AD, Mackenzie A, Dimmock NJ. Persistence of virulent Semliki Forest virus in mouse brain following co-inoculation with defective interfering particles. J. Gen. Virol. 1986;67:1189–1194. doi: 10.1099/0022-1317-67-6-1189. [DOI] [PubMed] [Google Scholar]
- 109.Cave DR, Hendrickson FM, Huang AS. Defective interfering virus particles modulate virulence. J. Virol. 1985;55:366–373. doi: 10.1128/jvi.55.2.366-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frensing T, et al. Continuous influenza virus production in cell culture shows a periodic accumulation of defective interfering particles. PLoS ONE. 2013;8:e72288. doi: 10.1371/journal.pone.0072288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stauffer Thompson KA, Rempala GA, Yin J. Multiple-hit inhibition of infection by defective interfering particles. J. Gen. Virol. 2009;90:888–899. doi: 10.1099/vir.0.005249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moscona A. Defective interfering particles of human parainfluenza virus type 3 are associated with persistent infection in cell culture. Virology. 1991;183:821–824. doi: 10.1016/0042-6822(91)91018-c. [DOI] [PubMed] [Google Scholar]
- 113.Dimmock NJ, Easton AJ. Defective interfering influenza virus RNAs: time to reevaluate their clinical potential as broad-spectrum antivirals? J. Virol. 2014;88:5217–5227. doi: 10.1128/JVI.03193-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vasou A, Sultanoglu N, Goodbourn S, Randall RE, Kostrikis LG. Targeting pattern recognition receptors (PRR) for vaccine adjuvantation: from synthetic PRR agonists to the potential of defective interfering particles of viruses. Viruses. 2017;9:186. doi: 10.3390/v9070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Santak M, et al. Accumulation of defective interfering viral particles in only a few passages in Vero cells attenuates mumps virus neurovirulence. Microbes Infect. 2015;17:228–236. doi: 10.1016/j.micinf.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 116.Dimmock NJ, Marriott AC. In vivo antiviral activity: defective interfering virus protects better against virulent Influenza A virus than avirulent virus. J. Gen. Virol. 2006;87:1259–1265. doi: 10.1099/vir.0.81678-0. [DOI] [PubMed] [Google Scholar]
- 117.Mann A, et al. Interfering vaccine (defective interfering influenza A virus) protects ferrets from influenza, and allows them to develop solid immunity to reinfection. Vaccine. 2006;24:4290–4296. doi: 10.1016/j.vaccine.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 118.Notton T, Sardanyes J, Weinberger AD, Weinberger LS. The case for transmissible antivirals to control population-wide infectious disease. Trends Biotechnol. 2014;32:400–405. doi: 10.1016/j.tibtech.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 119.Rast LI, et al. Conflicting selection pressures will constrain viral escape from interfering particles: principles for designing resistance-proof antivirals. PLoS Comput. Biol. 2016;12:e1004799. doi: 10.1371/journal.pcbi.1004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martinez-Gil L, et al. A Sendai virus-derived RNA agonist of RIG-I as a virus vaccine adjuvant. J. Virol. 2013;87:1290–1300. doi: 10.1128/JVI.02338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fisher DG, Coppock GM, Lopez CB. Virus-derived immunostimulatory RNA induces type I IFN-dependent antibodies and T-cell responses during vaccination. Vaccine. 2018;36:4039–4045. doi: 10.1016/j.vaccine.2018.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Easton AJ, et al. A novel broad-spectrum treatment for respiratory virus infections: influenza-based defective interfering virus provides protection against pneumovirus infection in vivo. Vaccine. 2011;29:2777–2784. doi: 10.1016/j.vaccine.2011.01.102. [DOI] [PubMed] [Google Scholar]
- 123.Bellocq C, Mottet G, Roux L. Wide occurrence of measles virus subgenomic RNAs in attenuated live-virus vaccines. Biologicals. 1990;18:337–343. doi: 10.1016/1045-1056(90)90039-3. [DOI] [PubMed] [Google Scholar]
- 124.McLaren LC, Holland JJ. Defective interfering particles from poliovirus vaccine and vaccine reference strains. Virology. 1974;60:579–583. doi: 10.1016/0042-6822(74)90352-3. [DOI] [PubMed] [Google Scholar]
- 125.Xue J, Chambers BS, Hensley SE, Lopez CB. Propagation and characterization of influenza virus stocks that lack high levels of defective viral genomes and hemagglutinin mutations. Front. Microbiol. 2016;7:326. doi: 10.3389/fmicb.2016.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gould PS, Easton AJ, Dimmock NJ. Live attenuated influenza vaccine contains substantial and unexpected amounts of defective viral genomic RNA. Viruses. 2017;9:269. doi: 10.3390/v9100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCrone JT, et al. Stochastic processes constrain the within and between host evolution of influenza virus. eLife. 2018;7:e35962. doi: 10.7554/eLife.35962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Loney C, Mottet-Osman G, Roux L, Bhella D. Paramyxovirus ultrastructure and genome packaging: cryo-electron tomography of sendai virus. J. Virol. 2009;83:8191–8197. doi: 10.1128/JVI.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cuevas JM, Duran-Moreno M, Sanjuan R. Multi-virion infectious units arise from free viral particles in an enveloped virus. Nat. Microbiol. 2017;2:17078. doi: 10.1038/nmicrobiol.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aguilera ER, Erickson AK, Jesudhasan PR, Robinson CM, Pfeiffer JK. Plaques formed by mutagenized viral populations have elevated coinfection frequencies. mBio. 2017;8:e02020-16. doi: 10.1128/mBio.02020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laske T, Heldt FS, Hoffmann H, Frensing T, Reichl U. Modeling the intracellular replication of influenza A virus in the presence of defective interfering RNAs. Virus Res. 2016;213:90–99. doi: 10.1016/j.virusres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 132.Rouzine IM, Weinberger LS. Design requirements for interfering particles to maintain coadaptive stability with HIV-1. J. Virol. 2013;87:2081–2093. doi: 10.1128/JVI.02741-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Q, Tong Y, Xu Y, Niu J, Zhong J. Genetic analysis of serum-derived defective Hepatitis C virus genomes revealed novel viral cis elements for virus replication and assembly. J. Virol. 2018;92:e02182-17. doi: 10.1128/JVI.02182-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xiao CT, et al. Identification of new defective interfering RNA species associated with porcine reproductive and respiratory syndrome virus infection. Virus Res. 2011;158:33–36. doi: 10.1016/j.virusres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 135.Mawassi M, et al. Multiple species of defective RNAs in plants infected with citrus tristeza virus. Virology. 1995;214:264–268. doi: 10.1006/viro.1995.9930. [DOI] [PubMed] [Google Scholar]
- 136.Che X, Mawassi M, Bar-Joseph M. A novel class of large and infectious defective RNAs of Citrus tristeza virus. Virology. 2002;298:133–145. doi: 10.1006/viro.2002.1472. [DOI] [PubMed] [Google Scholar]
- 137.Snijder EJ, den Boon JA, Horzinek MC, Spaan WJ. Characterization of defective interfering RNAs of Berne virus. J. Gen. Virol. 1991;72:1635–1643. doi: 10.1099/0022-1317-72-7-1635. [DOI] [PubMed] [Google Scholar]
- 138.Hofmann MA, Sethna PB, Brian DA. Bovine coronavirus mRNA replication continues throughout persistent infection in cell culture. J. Virol. 1990;64:4108–4114. doi: 10.1128/jvi.64.9.4108-4114.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Penzes Z, Wroe C, Brown TD, Britton P, Cavanagh D. Replication and packaging of coronavirus infectious bronchitis virus defective RNAs lacking a long open reading frame. J. Virol. 1996;70:8660–8668. doi: 10.1128/jvi.70.12.8660-8668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Makino S, Yokomori K, Lai MM. Analysis of efficiently packaged defective interfering RNAs of murine coronavirus: localization of a possible RNA-packaging signal. J. Virol. 1990;64:6045–6053. doi: 10.1128/jvi.64.12.6045-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van der Most RG, Bredenbeek PJ, Spaan WJ. A domain at the 3′ end of the polymerase gene is essential for encapsidation of coronavirus defective interfering RNAs. J. Virol. 1991;65:3219–3226. doi: 10.1128/jvi.65.6.3219-3226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mendez A, Smerdou C, Izeta A, Gebauer F, Enjuanes L. Molecular characterization of transmissible gastroenteritis coronavirus defective interfering genomes: packaging and heterogeneity. Virology. 1996;217:495–507. doi: 10.1006/viro.1996.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aaskov J, Buzacott K, Thu HM, Lowry K, Holmes EC. Long-term transmission of defective RNA viruses in humans and Aedes mosquitoes. Science. 2006;311:236–238. doi: 10.1126/science.1115030. [DOI] [PubMed] [Google Scholar]
- 144.Juarez-Martinez AB, et al. Detection and sequencing of defective viral genomes in C6/36 cells persistently infected with dengue virus 2. Arch. Virol. 2013;158:583–599. doi: 10.1007/s00705-012-1525-2. [DOI] [PubMed] [Google Scholar]
- 145.Yoon SW, et al. Characterization of homologous defective interfering RNA during persistent infection of Vero cells with Japanese encephalitis virus. Mol. Cells. 2006;21:112–120. [PubMed] [Google Scholar]
- 146.Noppornpanth S, et al. Characterization of Hepatitis C virus deletion mutants circulating in chronically infected patients. J. Virol. 2007;81:7. doi: 10.1128/JVI.01059-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pacini L, Graziani R, Bartholomew L, De Francesco R, Paonessa G. Naturally occurring Hepatitis C virus subgenomic deletion mutants replicate efficiently in huh-7 Cells and are trans-packaged in vitro to generate infectious defective particles. J. Virol. 2009;83:14. doi: 10.1128/JVI.00308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lancaster MU, Hodgetts SI, Mackenzie JS, Urosevic N. Characterization of defective viral RNA produced during persistent infection of Vero cells with Murray Valley encephalitis virus. J. Virol. 1998;72:2474–2482. doi: 10.1128/jvi.72.3.2474-2482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mandl CW, et al. Spontaneous and engineered deletions in the 3′ noncoding region of tick-borne encephalitis virus: construction of highly attenuated mutants of a flavivirus. J. Virol. 1998;72:2132–2140. doi: 10.1128/jvi.72.3.2132-2140.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brinton MA. Characterization of West Nile virus persistent infections in genetically resistant and susceptible mouse cells. I. Generation of defective nonplaquing virus particles. Virology. 1982;116:84–98. doi: 10.1016/0042-6822(82)90405-6. [DOI] [PubMed] [Google Scholar]
- 151.Hasiow-Jaroszewska B, Minicka J, Zarzynska-Nowak A, Budzynska D, Elena SF. Defective RNA particles derived from Tomato black ring virus genome interfere with the replication of parental virus. Virus Res. 2018;250:87–94. doi: 10.1016/j.virusres.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 152.Radloff RJ, Young SA. Defective interfering particles of encephalomyocarditis virus. J. Gen. Virol. 1983;64:1637–1641. doi: 10.1099/0022-1317-64-7-1637. [DOI] [PubMed] [Google Scholar]
- 153.Garcia-Arriaza J, Domingo E, Escarmis C. A segmented form of foot-and-mouth disease virus interferes with standard virus: a link between interference and competitive fitness. Virology. 2005;335:155–164. doi: 10.1016/j.virol.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 154.McClure MA, Holland JJ, Perrault J. Generation of defective interfering particles in picornaviruses. Virology. 1980;100:408–418. doi: 10.1016/0042-6822(80)90532-2. [DOI] [PubMed] [Google Scholar]
- 155.Lundquist RE, Sullivan M, Maizel JV., Jr. Characterization of a new isolate of poliovirus defective interfering particles. Cell. 1979;18:759–769. doi: 10.1016/0092-8674(79)90129-6. [DOI] [PubMed] [Google Scholar]
- 156.Derdeyn CA, Frey TK. Characterization of defective-interfering RNAs of rubella virus generated during serial undiluted passage. Virology. 1995;206:216–226. doi: 10.1016/S0042-6822(95)80036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dimmock NJ, Kennedy SI. Prevention of death in Semliki Forest virus-infected mice by administration of defective-interfering Semliki Forest virus. J. Gen. Virol. 1978;39:231–242. doi: 10.1099/0022-1317-39-2-231. [DOI] [PubMed] [Google Scholar]
- 158.Barrett AD, Dimmock NJ. Properties of host and virus which influence defective interfering virus mediated-protection of mice against Semliki Forest virus lethal encephalitis. Brief report. Arch. Virol. 1984;81:185–188. doi: 10.1007/BF01309308. [DOI] [PubMed] [Google Scholar]
- 159.Fuller FJ, Marcus PI. Interferon induction by viruses. Sindbis virus: defective-interfering particles temperature-sensitive for interferon induction. J. Gen. Virol. 1980;48:391–394. doi: 10.1099/0022-1317-48-2-391. [DOI] [PubMed] [Google Scholar]
- 160.Finnen RL, Rochon DM. Sequence and structure of defective interfering RNAs associated with cucumber necrosis virus infections. J. Gen. Virol. 1993;74:1715–1720. doi: 10.1099/0022-1317-74-8-1715. [DOI] [PubMed] [Google Scholar]
- 161.Li XH, Heaton LA, Morris TJ, Simon AE. Turnip crinkle virus defective interfering RNAs intensify viral symptoms and are generated de novo. Proc. Natl Acad. Sci. USA. 1989;86:9173–9177. doi: 10.1073/pnas.86.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Welsh RM, O’Connell CM, Pfau CJ. Properties of defective lymphocytic choriomeningitis virus. J. Gen. Virol. 1972;17:355–359. doi: 10.1099/0022-1317-17-3-355. [DOI] [PubMed] [Google Scholar]
- 163.Meyer BJ, Southern PJ. A novel type of defective viral genome suggests a unique strategy to establish and maintain persistent lymphocytic choriomeningitis virus infections. J. Virol. 1997;71:6757–6764. doi: 10.1128/jvi.71.9.6757-6764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Isaacs A, Edney M. Interference between inactive and active influenza viruses in the chick embryo. II. The site of interference. Aust. J. Exp. Biol. Med. 1950;28:231–238. doi: 10.1038/icb.1950.21. [DOI] [PubMed] [Google Scholar]
- 165.Ada GL, Perry BT. Influenza virus nucleic acid: relationship between biological characteristics of the virus particle and properties of the nucleic acid. J. Gen. Microbiol. 1956;14:623–633. doi: 10.1099/00221287-14-3-623. [DOI] [PubMed] [Google Scholar]
- 166.Chanda PK, Chambers TM, Nayak DP. In vitro transcription of defective interfering particles of influenza virus produces polyadenylic acid-containing complementary RNAs. J. Virol. 1983;45:55–61. doi: 10.1128/jvi.45.1.55-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bean WJ, Kawaoka Y, Wood JM, Pearson JE, Webster RG. Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNAs in nature. J. Virol. 1985;54:151–160. doi: 10.1128/jvi.54.1.151-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chambers TM, Webster RG. Defective interfering virus associated with A/Chicken/Pennsylvania/83 influenza virus. J. Virol. 1987;61:1517–1523. doi: 10.1128/jvi.61.5.1517-1523.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Morgan DJ, Dimmock NJ. Defective interfering influenza virus inhibits immunopathological effects of infectious virus in the mouse. J. Virol. 1992;66:1188–1192. doi: 10.1128/jvi.66.2.1188-1192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scott PD, Meng B, Marriott AC, Easton AJ, Dimmock NJ. Defective interfering influenza virus confers only short-lived protection against influenza virus disease: evidence for a role for adaptive immunity in DI virus-mediated protection in vivo. Vaccine. 2011;29:6584–6591. doi: 10.1016/j.vaccine.2011.06.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Murphy DG, Dimock K, Kang CY. Defective interfering particles of human parainfluenza virus 3. Virology. 1987;158:439–443. doi: 10.1016/0042-6822(87)90217-0. [DOI] [PubMed] [Google Scholar]
- 172.Sidhu MS, et al. Defective measles virus in human subacute sclerosing panencephalitis brain. Virology. 1994;202:10. doi: 10.1006/viro.1994.1384. [DOI] [PubMed] [Google Scholar]
- 173.Whistler T, Bellini WJ, Rota PA. Generation of defective interfering particles by two vaccine strains of measles virus. Virology. 1996;220:480–484. doi: 10.1006/viro.1996.0335. [DOI] [PubMed] [Google Scholar]
- 174.Andzhaparidze OG, Boriskin Yu,S, Bogomolova NN, Drynov ID. Mumps virus-persistently infected cell cultures release defective interfering virus particles. J. Gen. Virol. 1982;63:499–503. doi: 10.1099/0022-1317-63-2-499. [DOI] [PubMed] [Google Scholar]
- 175.Portner A, Kingsbury DW. Identification of transcriptive and replicative intermediates in Sendai virus-infected cells. Virology. 1972;47:711–725. doi: 10.1016/0042-6822(72)90561-2. [DOI] [PubMed] [Google Scholar]
- 176.Calain P, Curran J, Kolakofsky D, Roux L. Molecular cloning of natural paramyxovirus copy-back defective interfering RNAs and their expression from DNA. Virology. 1992;191:62–71. doi: 10.1016/0042-6822(92)90166-m. [DOI] [PubMed] [Google Scholar]
- 177.Patel AH, Elliott RM. Characterization of Bunyamwera virus defective interfering particles. J. Gen. Virol. 1992;73:389–396. doi: 10.1099/0022-1317-73-2-389. [DOI] [PubMed] [Google Scholar]
- 178.Marchi A, Nicoletti L, Accardi L, Di Bonito P, Giorgi C. Characterization of Toscana virus-defective interfering particles generated in vivo. Virology. 1998;246:125–133. doi: 10.1006/viro.1998.9195. [DOI] [PubMed] [Google Scholar]
- 179.Sarmiento RE, Tirado R, Gomez B. Characteristics of a respiratory syncytial virus persistently infected macrophage-like culture. Virus Res. 2002;84:45–58. doi: 10.1016/s0168-1702(01)00420-8. [DOI] [PubMed] [Google Scholar]
- 180.Cooper PD, Bellett AJ. A transmissible interfering component of vesicular stomatitis virus preparations. J. Gen. Microbiol. 1959;21:485–497. doi: 10.1099/00221287-21-3-485. [DOI] [PubMed] [Google Scholar]
- 181.Palma EL, Huang A. Cyclic production of vesicular stomatitis virus caused by defective interfering particles. J. Infect. Dis. 1974;129:402–410. doi: 10.1093/infdis/129.4.402. [DOI] [PubMed] [Google Scholar]
- 182.Schubert M, Keene JD, Lazzarini RA, Emerson SU. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978;15:103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- 183.Rao DD, Huang AS. Interference among defective interfering particles of vesicular stomatitis virus. J. Virol. 1982;41:210–221. doi: 10.1128/jvi.41.1.210-221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.DePolo NJ, Holland JJ. Very rapid generation/amplification of defective interfering particles by vesicular stomatitis virus variants isolated from persistent infection. J. Gen. Virol. 1986;67:1195–1198. doi: 10.1099/0022-1317-67-6-1195. [DOI] [PubMed] [Google Scholar]
- 185.Marcus PI, Gaccione C. Interferon induction by viruses. XIX. Vesicular stomatitis virus—New Jersey: high multiplicity passages generate interferon-inducing, defective-interfering particles. Virology. 1989;171:630–633. doi: 10.1016/0042-6822(89)90637-5. [DOI] [PubMed] [Google Scholar]
- 186.Resende Rde O, et al. Generation of envelope and defective interfering RNA mutants of tomato spotted wilt virus by mechanical passage. J. Gen. Virol. 1991;72:2375–2383. doi: 10.1099/0022-1317-72-10-2375. [DOI] [PubMed] [Google Scholar]
- 187.Chiba S, Lin YH, Kondo H, Kanematsu S, Suzuki N. A novel betapartitivirus RnPV6 from Rosellinia necatrix tolerates host RNA silencing but is interfered by its defective RNAs. Virus Res. 2016;219:62–72. doi: 10.1016/j.virusres.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 188.Nonoyama M, Watanabe Y, Graham AF. Defective virions of reovirus. J. Virol. 1970;6:226–236. doi: 10.1128/jvi.6.2.226-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Anzola JV, Xu ZK, Asamizu T, Nuss DL. Segment-specific inverted repeats found adjacent to conserved terminal sequences in wound tumor virus genome and defective interfering RNAs. Proc. Natl Acad. Sci. USA. 1987;84:8301–8305. doi: 10.1073/pnas.84.23.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bruner KM, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016;22:1043–1049. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sanchez G, Xu X, Chermann JC, Hirsch I. Accumulation of defective viral genomes in peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected individuals. J. Virol. 1997;71:2233–2240. doi: 10.1128/jvi.71.3.2233-2240.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Palukaitis P. Satellite RNAs and satellite viruses. Mol. Plant Microbe. 2016;29:181–186. doi: 10.1094/MPMI-10-15-0232-FI. [DOI] [PubMed] [Google Scholar]
- 193.Simon AE, Roossinck MJ, Havelda Z. Plant virus satellite and defective interfering RNAs: new paradigms for a new century. Annu. Rev. Phytopathol. 2004;42:415–437. doi: 10.1146/annurev.phyto.42.040803.140402. [DOI] [PubMed] [Google Scholar]
- 194.Sivanandam V, Mathews D, Rao AL. Properties of satellite tobacco mosaic virus phenotypes expressed in the presence and absence of helper virus. Virology. 2015;483:163–173. doi: 10.1016/j.virol.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 195.Krupovic M, Kuhn JH, Fischer MG. A classification system for virophages and satellite viruses. Arch. Virol. 2016;161:233–247. doi: 10.1007/s00705-015-2622-9. [DOI] [PubMed] [Google Scholar]
- 196.Rao AL, Kalantidis K. Virus-associated small satellite RNAs and viroids display similarities in their replication strategies. Virology. 2015;479–480:627–636. doi: 10.1016/j.virol.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 197.Palukaitis P. What has been happening with viroids? Virus Genes. 2014;49:175–184. doi: 10.1007/s11262-014-1110-8. [DOI] [PubMed] [Google Scholar]
- 198.Bekliz M, Colson P, La Scola B. The expanding family of virophages. Viruses. 2016;8:317. doi: 10.3390/v8110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.La Scola B, et al. The virophage as a unique parasite of the giant mimivirus. Nature. 2008;455:100–104. doi: 10.1038/nature07218. [DOI] [PubMed] [Google Scholar]
- 200.Roux S, et al. Ecogenomics of virophages and their giant virus hosts assessed through time series metagenomics. Nat. Commun. 2017;8:858. doi: 10.1038/s41467-017-01086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Born D, et al. Capsid protein structure, self-assembly, and processing reveal morphogenesis of the marine virophage mavirus. Proc. Natl Acad. Sci. USA. 2018;115:7332–7337. doi: 10.1073/pnas.1805376115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gong D, et al. Virus-Like Vesicles of Kaposi’s Sarcoma-Associated Herpesvirus Activate Lytic Replication by Triggering Differentiation Signaling. J. Virol. 2017;91:e00362-17. doi: 10.1128/JVI.00362-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gastaminza P, et al. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J. Virol. 2010;84:10999–11009. doi: 10.1128/JVI.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Deschamps T, Kalamvoki M. Extracellular vesicles released by herpes simplex virus 1 infected cells block virus replication in recipient cells in a STING-dependent manner. J. Virol. 2018;92:e01102-18. doi: 10.1128/JVI.01102-18. [DOI] [PMC free article] [PubMed] [Google Scholar]