Abstract
Inflammatory molecules evolved partly to protect hosts from viruses, but increasing evidence suggests that they cause disease pathology and chronic conditions, and play a role in aging. By mitigating these effects, bats are able to both tolerate viral infections and live well beyond expectations.
Subject terms: Viral transmission
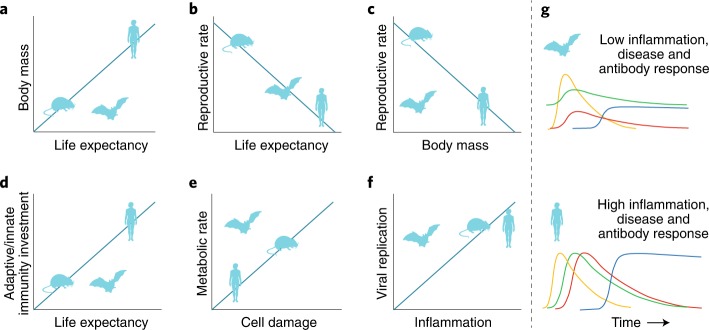
What is interesting about bats? Bats effectively live everywhere that humans do, from the Alaskan Arctic to remote Hawaiian Islands. They are the only mammals that can truly fly and are the most abundant nocturnal insect predators. For epidemiologists and virologists, bats are important because they host a range of viruses, a handful of which cause severe disease in humans and other mammals. Questions remain regarding whether bats directly transmit these viruses to humans or whether they are disproportionate hosts for such viruses1,2. However, viruses causing rabies, Ebola virus disease, severe acute respiratory syndrome and Middle East respiratory syndrome (MERS) all have viral ancestors in bats. For mammalogists and other biologists, bats are of interest because they age remarkably well; senescence, or ‘biological aging’, typically scales with body mass so that larger animals live longer. However, some bats with half the body mass of domestic mice have lifespans ten times that of the mice. Metabolic rates and other pace-of-life traits (Fig. 1a–f) are thought to drive this body mass–longevity relationship for most species; however, during flight, normal bat metabolism can reach twice that of exhaustion compared to mice3. Now, growing numbers of studies are linking bats’ abilities to age with their role as viral hosts through genetic adaptations to their immune responses4.
Fig. 1. Differences in traits related to longevity and infection between bats and other mammals.
a–f, Expected general relationships between mammals (diagonal lines). g, Bat viruses evolve in high IFN environments (green), so viral replication (yellow) continues when humans exhibit increased IFN expression following infection with bat viruses. Reduced inflammation (red) moderates disease pathology in bats and reduced B cell affinity maturation lowers antibody responses (blue).
In this issue of Nature Microbiology, Ahn et al. provide evidence that one mechanism bats have evolved to limit excessive inflammation during viral infection is through dampened transcriptional priming and lower functional capacity of the bat inflammasome sensor, NLR family pyrin domain containing 3 (NLRP3) (ref. 5). NLRP3 functions as a pathogen recognition receptor to activate inflammatory mediators. NLRP3-mediated inflammation has also been linked to aging and age-related chronic diseases. This study provides a mechanism for, and a provisional answer to, whether bats have evolved to tolerate or resist viral infections. This work suggests that bats have adapted to tolerate viral infection without development of pathology through inflammation (Fig. 1g). This finding helps explain another apparent feature of bats, which is why they seemingly don’t develop overt disease when infected, despite hosting a suite of viruses.
Previous studies have shown that parts of the innate immune system are ‘switched on’, even in unstimulated bat tissues, due to constitutive expression of three interferon (IFN)-α genes6. This constitutive IFN activation is hypothesized to have reduced the need for affinity maturation, a process during which B cells produce antibodies with increased antigen affinity during infection. These observations are relevant for epidemiologists because bats may limit viral replication without developing high antibody titres, thereby affecting serological assay interpretation through reduced antibody production (Fig. 1g). Further, the conditions bats provide for viruses are themselves hypothesized to facilitate high pathogenicity of bat viruses in other mammalian hosts, such as humans. Because of the constant high IFN activity, bat-borne viruses may be shed at low levels from bat cells without eliciting strong antibody responses7. Viral adaptation to these conditions through selection means that when other hosts (including humans) are infected by bat-derived viruses and produce similar immune responses, viral replication is not suppressed and pathology develops7.
Contrastingly, bats have been shown to have dampened IFN responses through substitutions in genes within the stimulator of IFN genes (STING) pathway8. In addition to reducing pathology during viral infection, this inhibitory IFN state, as well as the dampened NLRP3 inflammation5, may have evolved to reduce damage from the high metabolic demands of flight that cause DNA damage (Fig. 1e). These reductions in inflammation and adaptations for flight may have inadvertently increased bat lifespan4. This idea is supported by a recent field study which shows that telomeres, the protective caps of chromosomes, shorten more slowly within bat genera with the oldest living bat species9.
All these fascinating insights have been inspired through field observations but have relied on genomic, transcriptomic and in vitro studies. Despite these advances, there are numerous open questions in bat research. Ahn et al. showed that the bat-derived virus Pteropine orthoreovirus 3 and MERS coronavirus (MERS-CoV) cause NLRP3-mediated inflammation in mouse and human cell lines. Interestingly, the pathogenesis of these viruses (all RNA viruses) in humans can mainly be ascribed to aberrant innate immune responses. In vivo virus challenge studies with high doses of Ebola virus and MERS-CoV caused limited pathology in bats, despite high viral tissue titres10,11. However, questions remain as to whether bats are adapted to tolerate all viral infections or simply those they have co-evolved with. Rabies and related viruses persist in bat populations, but can kill bats. Are these viruses really the only exceptions? The study of MERS-CoV is valuable because, though sometimes thought of as a bat-derived virus, only MERS-CoV ancestors have been detected in bats, and MERS-CoV appears to be well established in camel populations with limited camel disease12. Therefore, bat NLRP3 responses to MERS-CoV hint that these responses may be general among bats. However, more cross-species studies using bat and non-bat viruses (including viruses distantly related to bat-derived viruses) in bat and non-bat cell lines are required5,10,11. Furthermore, while bats challenged with viruses may rarely develop disease, even in the presence of high viral loads, understanding disease in wild animals (in particular, small nocturnal flying bats) is difficult.
The dampened NLRP3-mediated inflammatory responses in bat tissues to three different types of RNA viruses5 support the hypothesis that innate immune tolerance, rather than resistance, is a mechanism bats have evolved to host a diverse suite of viral infections with limited disease. Bat longevity and high viral diversity suggest that there are general patterns, but bats are diverse, and whether bats are overrepresented reservoir hosts of zoonotic viral infections is still arguable1,2. The fact that bats are extremely long-lived compared to other mammalian species given their size is not debatable, but how linked this is to their ability to host viruses remains to be seen. Recent work demonstrating temperature-independent filovirus (for example, Ebola virus) replication in bat cells10, in contrast to that predicted by the ‘flight as fever’ hypothesis3 and the study by Ahn et al., are excellent examples of in vitro hypothesis testing. However, bats also use torpor, sometimes so deep that hibernating temperate bats may lower their body temperature to just above freezing for weeks, and even tropical bats have been shown to reduce their heart rates from over 1,000 beats per minute to less than 200 (ref. 13). What roles these physiological conditions have on bat longevity and viral dynamics within bats, and their consequences at the population level, have yet to be fully explored. Increasingly, however, hypotheses are being tested and each reveals fascinating insights into bats as animals and their role as viral reservoirs.
Competing interests
The author declares no competing interests.
References
- 1.Han BA, Kramer AM, Drake JM. Trends Parasitol. 2016;32:565–577. doi: 10.1016/j.pt.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olival KJ, et al. Nature. 2017;546:646. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Shea TJ, et al. Emerg. Infect. Dis. 2014;20:741. doi: 10.3201/eid2005.130539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G, et al. Science. 2013;339:456–460. doi: 10.1126/science.1230835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn Matae, Anderson Danielle E., Zhang Qian, Tan Chee Wah, Lim Beng Lee, Luko Katarina, Wen Ming, Chia Wan Ni, Mani Shailendra, Wang Loo Chien, Ng Justin Han Jia, Sobota Radoslaw M., Dutertre Charles-Antoine, Ginhoux Florent, Shi Zheng-Li, Irving Aaron T., Wang Lin-Fa. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nature Microbiology. 2019;4(5):789–799. doi: 10.1038/s41564-019-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou, P. et al. Proc. Natl Acad. Sci. USA113, 2696–2701 (2016).
- 7.Schountz T, Baker ML, Butler J, Munster V. Front. Immunol. 2017;8:1098. doi: 10.3389/fimmu.2017.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlovich SS, et al. Cell. 2018;173:1098–1110. doi: 10.1016/j.cell.2018.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foley NM, et al. Science Advances. 2018;4:eaao0926. doi: 10.1126/sciadv.aao0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munster VJ, et al. Sci. Rep-UK. 2016;6:21878. doi: 10.1038/srep21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanepoel R, et al. Emerg. Infect. Dis. 1996;2:321–325. doi: 10.3201/eid0204.960407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudas G, Carvalho LM, Rambaut A, Bedford T. eLife. 2018;7:e31257. doi: 10.7554/eLife.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Mara MT, et al. eLife. 2017;6:e26686. doi: 10.7554/eLife.26686. [DOI] [PMC free article] [PubMed] [Google Scholar]