Abstract
Background:
Trauma and symptoms of posttraumatic stress disorder (PTSD) have repeatedly been linked to impaired cardiovascular functioning. Poor fear extinction is a well-established biomarker of PTSD that may provide insight into mechanisms underlying cardiovascular risk. The current study probed the cardiovascular response to extinction in a sample of trauma-exposed individuals.
Methods:
Participants were 51 trauma-exposed women who underwent a fear conditioning paradigm. Heart rate (HR) during extinction was examined in response to a conditioned stimulus that was previously paired with an aversive unconditioned stimulus (CS+) and one that was never paired (CS−). Heart rate variability (HRV) was calculated at baseline and during the extinction session.
Results:
Consistent with fear bradycardia, initial HR deceleration (.5–2s) after CS+ onset occurred during early extinction and appeared to extinguish over time. Higher baseline HRV was significantly associated with greater fear bradycardia during early extinction.
Conclusions:
This is the first study to demonstrate a pattern of fear bradycardia in early extinction, which was associated with higher HRV levels and decreased over the course of the extinction phase. These results suggest that increased fear bradycardia may be indicative of greater vagal control (i.e., HRV), both of which are psychophysiological biomarkers that may influence cardiovascular and autonomic disease risk in trauma-exposed individuals.
Keywords: extinction, fear bradycardia, heart rate, cardiovascular, trauma
Introduction
Trauma exposure is extremely common among the general population, with lifetime prevalence rates of 89% in the United States (Kilpatrick et al., 2013) and 70% globally (Kessler et al., 2017). A subset of trauma-exposed individuals will subsequently develop symptoms of posttraumatic stress disorder (PTSD), such as re-experiencing of the event, avoidance of trauma-related reminders, emotional numbing, and hyperarousal (Kessler et al., 1995; Kilpatrick et al., 2013). PTSD has long been understood as a failure to inhibit fear in safe situations due to alterations in fear learning and extinction (e.g., Bremner et al., 2005; Grillon & Morgan, 1999; Jovanovic et al., 2012; Milad et al., 2009; for reviews, see Foa et al., 1989; Jovanovic and Ressler, 2010). This is characterized by exaggerated fear and sympathetic arousal to generalized reminders (e.g., increased heart rate and blood pressure, hyperventilation) in the absence of danger, and chronic hypervigilance. Chronic sympathetic arousal can strain the hypothalamic-pituitary-adrenal axis and thus alter neuroendocrine homeostasis. Over time, this enhanced sympathetic arousal and maladaptive autonomic regulation contribute to greater risk for cardiovascular disease in individuals exposed to trauma (Edmondson et al., 2013; Edmondson and von Känel, 2017; Myers, 2017).
A key mechanism underlying the risk of cardiovascular disease (CVD) following trauma is chronically elevated heart rate (HR) related to sympathetic arousal (Brudey et al., 2015). For example, studies have consistently reported elevated HR in response to threatening stimuli among individuals with PTSD compared to controls (Ehlers et al., 2010; Keane et al., 1998; Orr et al., 1993). Contemporaneous investigations focused on identifying differences in resting, rather than stimulus-evoked, cardiovascular activity (e.g., Jovanovic et al., 2009). A meta-analysis of 34 studies examining basal cardiovascular activity in PTSD found basal HR to be significantly higher in individuals with PTSD, compared with both traumatized and non-traumatized controls (Buckley and Kaloupek, 2001).
In addition to elevated HR resulting from sympathetic hyperarousal, PTSD is associated with decreased parasympathetic nervous system (PNS) activity. High frequency heart rate variability (HRV) is a commonly used measure of PNS activity that is thought to represent the influence of the vagus nerve on the heart’s sinoatrial node and is associated with emotion regulation and psychological health (Thayer et al., 2009). Among individuals with PTSD, HRV findings consistently point to significantly lower HRV both at baseline (Chang et al., 2013; Cohen et al., 1997; Hauschildt et al., 2011; Minassian et al., 2014, 2015) and in response to challenge (Jovanovic et al., 2009; Keary et al., 2009; Park et al., 2017; Sahar et al., 2001) compared to controls. Hopper and colleagues (2006) found that the only individuals in a PTSD population to exhibit elevated basal HR were those who also had relatively lower HRV, suggesting that despite marked sympathetic arousal, individuals with sufficiently high HRV (i.e. cardiac vagal control) may not present with elevated basal HR. These results support an earlier finding that low baseline HRV, as well as decreased HRV in response to challenge, is significantly correlated with sustained arousal (i.e. elevated HR) in individuals with PTSD (Sack et al., 2004). Understanding this regulatory effect is critical to identifying the autonomic nervous system (ANS) profile of trauma-exposed individuals at highest risk for developing CVD.
Another aspect of PNS activity relevant to trauma is fear bradycardia. Fear bradycardia is a decrease in HR that typically occurs within 0–2 seconds of stimulus onset and is followed by increased HR (Castegnetti et al., 2016; Sege et al., 2017). This response is controlled by the PNS, which influences HR very quickly due to the action of acetylcholine on the heart’s sinoatrial node, leading to rapid initial bradycardia (Berntson et al., 1997; Pumprla et al., 2002). Studies with healthy subjects have demonstrated that fear bradycardia can be conditioned in response to threatening stimuli (Castegnetti et al., 2016) and animal studies have shown that it can be extinguished (Burhans et al., 2010). Further, we recently found that fear extinction in rodents was associated with a reduction of conditioned bradycardia (Swiercz, Seligowski, Park, & Marvar). It is well-established that individuals with PTSD have deficits in fear extinction (Milad et al., 2008; Norrholm et al., 2011) as well as ANS function (e.g., Buckley and Kaloupek, 2001; Ehlers et al., 2010). However, to our knowledge, no prior studies have examined fear bradycardia (an important physiological and adaptive response to fear) within the context of extinction in trauma-exposed individuals. Better characterization of HR responses to extinction is necessary to elucidate mechanisms and identify individuals at risk for developing CVD, and it may inform ways to measure the cardiovascular response to psychotherapies that use the principles of extinction (i.e., exposure therapy).
In the current study we used fear conditioning to examine the cardiovascular response to extinction in trauma-exposed women with varying levels of PTSD symptoms (i.e., PTSD was examined as a dimensional construct rather than a dichotomous one, consistent with the research domain criteria; RDoC). We hypothesized that 1) HR in response to a previously fear conditioned stimulus (CS+) would initially decrease during early extinction, consistent with fear bradycardia, and this would decrease over the course of extinction (demonstrating extinction of the HR response); and 2) informed by the findings of Hopper et al. (2006), we hypothesized that there would be an interaction between HRV and HR response to the CS+, such that individuals with higher resting HRV would demonstrate greater fear bradycardia (i.e., stronger parasympathetic control) during early extinction compared to those with lower resting HRV.
Methods
Participants
The sample consisted of 51 trauma-exposed women who were recruited from a general medical hospital in Atlanta, GA as part of the Grady Trauma Project (see Gillespie et al., 2009 for a full study description). The average age of participants was 38.82 years (SD = 11.91). Nearly all participants identified their race as African American or Black (94.1%; n = 48); two participants identified as Asian or Pacific Islander (3.9%) and one participant identified their race as “other” (2.0%). All participants completed informed consent procedures and were administered self-report questionnaires prior to completing the fear conditioning paradigm. Study procedures were approved by the Emory University Institutional Review Board.
Measures
Modified PTSD Symptom Scale
(PSS; Falsetti et al., 1993). The modified PTSD Symptom Scale (PSS) is a 17-item self-report measure of DSM-IV PTSD symptoms experienced over the past two weeks. The PSS has demonstrated satisfactory internal consistency, high test-retest reliability, and good concurrent validity (Foa et al., 1993).
Fear Conditioning and Extinction Paradigm
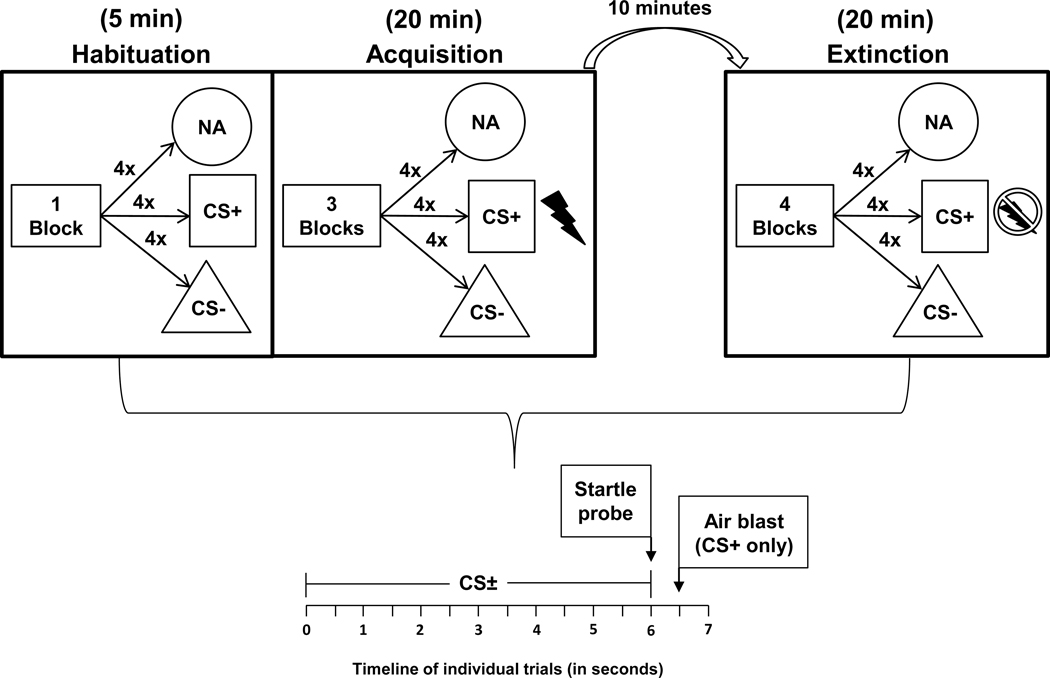
The FPS paradigm was based on classical conditioning principles, whereby an aversive unconditioned stimulus, US (140psi airblast to the larynx) was repeatedly paired with a shape conditioned stimulus (CS+, e.g., a blue square), while a different shape, (e.g., a purple triangle), was never paired with the aversive stimulus (CS−; see Figure 1 for diagram of experiment). An auditory startle probe (a 40-ms 106 dB white noise burst) was presented 6 seconds after onset of the CS+/−, and in the case of the CS+, was followed by the US 0.5 seconds later. Following a habituation block where no airblasts were delivered, the acquisition phase of the paradigm consisted of three conditioning blocks with four trials of each type (CS+, CS−, and noise alone, NA) in each block. The extinction phase occurred 10 min after acquisition and consisted of four blocks with four trials of each type, and the airblast was no longer paired with the CS+. The inter-trial interval was between 9 and 22 seconds.
Figure 1:
Overview of the fear-potentiated startle paradigm
Note. No stimuli present for first minute of habituation – resting baseline HRV is collected during this time. NA = noise alone; CS = conditioned stimulus.
Physiological Data Acquisition and Processing
Biopac MP150 for Windows (Biopac Systems, Inc.) was used to collect electrocardiogram (ECG) data at a sampling rate of 1 kHz, amplified and digitized using the Biopac system. HR and HRV were processed using MindWare software (MindWare Technologies, Inc), which identifies ECG R-waves and R-R intervals (i.e., the time period between heart beats), and detects artifacts, which were manually inspected and corrected. HRV was derived by spectral analysis of one-minute epochs with a Hamming windowing function and log transformed. Given that our primary interest was in parasympathetic contributions to HR, we chose to examine high frequency HRV because it best represents parasympathetic nervous system activity (Malliani et al., 1991, but also see Berntson et al., 1997). Settings for the high frequency band were based on standard recommendations for HRV data (0.12–0.40 Hz; Task Force, 1996), and transformed by natural log.
Data Analysis
Average HR in beats per minute (BPM) was calculated during habituation and during the last segment of acquisition to assess overall cardiovascular response to fear conditioning. Seven participants had unusable acquisition HR/HRV data due to motion artifact, bringing the acquisition sample size to 44. All 51 participants had usable HR/HRV data from extinction. During extinction, change in HR to the presentation of each CS was calculated in .5s interval bins starting from the onset of the CS (0.5s, 1.0s, 1.5s, 2.0s, 2.5s, 3.0s, 3.5s, 4.0s, 4.5s, 5.0s). HR during the 1-second period prior to CS+/CS− onset was used to calculate baseline HR and was subtracted from each HR interval post-CS+/CS− onset. While the CS+/CS− were presented for 6 seconds, only the first 5 seconds were used in order to minimize any potential effects of the sound probe, which was presented at 6 seconds. Consistent with prior research by Castegnetti et al. (2016), fear bradycardia was defined as HR during the .5 to 2s following stimulus onset (subtracting out the 1-second baseline period prior to stimulus onset). Thus, a fear bradycardia variable was created from the average of HR change .5 to 2s after CS+ and CS− onset. HRV values were derived during the habituation phase (one minute, prior to presentation of shapes, which was used as a resting baseline measurement), the last minute of acquisition, as well as during each block of extinction (each block contained five minutes that were averaged together). A median split was used to create higher and lower HRV groups based on average high-frequency HRV during habituation.
In order to statistically test for the curvilinear changes in HR across the 10 intervals of the CS+ and CS− for early and late extinction, we employed a polynomial contrast for the within-subject variable of time. Specifically, an omnibus 3-way repeated measures analyses of variance (ANOVA) was first used to test differences in HR response to the CS+ and CS− for early (block 1) and late (block 4) extinction, where the factors were CS type (CS+/CS−) by block (early/late) by time (0.5s, 1.0s, 1.5s, 2.0s, 2.5s, 3.0s, 3.5s, 4.0s, 4.5s, 5.0s). Follow up analyses were then conducted for each CS separately to assess extinction to each stimulus. For HRV analyses, we employed a 2-way (HRV group by time) ANOVA for early extinction and used a polynomial contrast to test the curvilinear changes in the HR time bins.
Results
PTSD symptoms indicated by the PSS ranged from 20.00 to 50.00, M = 35.00, SD = 7.41. PSS total and subscale scores were not significantly related to any of the HR or HRV variables during acquisition or extinction (see Table 1 for descriptives and correlations). During the habituation period prior to US delivery, average HR was 77.58 BPM (SD = 9.15) and average HRV was 5.37 (SD = 1.52) among the total sample. Resting HR and HRV during the habituation phase were related to each other in the expected direction, such that higher HR was associated with lower HRV, r = −.48, p = .001. Resting HRV was significantly associated with greater fear bradycardia, r = −.36, p = .02, suggesting that stronger vagal control was related to a larger bradycardia response. Resting HR was not significantly associated with fear bradycardia.
Table 1.
Descriptives and bivariate correlations among study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. PSS total | -- | |||||||
| 2. PSS intrusions | .583** | -- | ||||||
| 3. PSS avoidance-numbing | .858** | .271 | -- | |||||
| 4. PSS hyperarousal | .781** | .321* | .460** | -- | ||||
| 5. Resting HR | −.043 | .050 | .004 | −.156 | -- | |||
| 6. Resting HRV | −.027 | −.215 | −.032 | .144 | −.481** | -- | ||
| 7. Early extinction bradycardia | −.203 | −.152 | −.190 | −.114 | .268 | −.362* | -- | |
| 8. Late extinction bradycardia | −.088 | −.237 | −.046 | .019 | −.155 | .271 | −.281* | -- |
| Mean | 35.00 | 10.12 | 14.20 | 10.69 | 77.58 | 5.37 | −1.05 | .42 |
| SD | 7.41 | 2.15 | 4.35 | 3.10 | 9.15 | 1.52 | 2.24 | 2.18 |
| Minimum | 20.00 | 8.00 | 4.00 | 2.00 | 58.77 | 2.46 | −7.72 | −3.89 |
| Maximum | 50.00 | 15.00 | 21.00 | 15.00 | 100.79 | 8.87 | 3.31 | 8.89 |
Note.
p < .05
p < .01
PSS = PTSD Symptom Scale; HR = heart rate; HRV = heart rate variability.
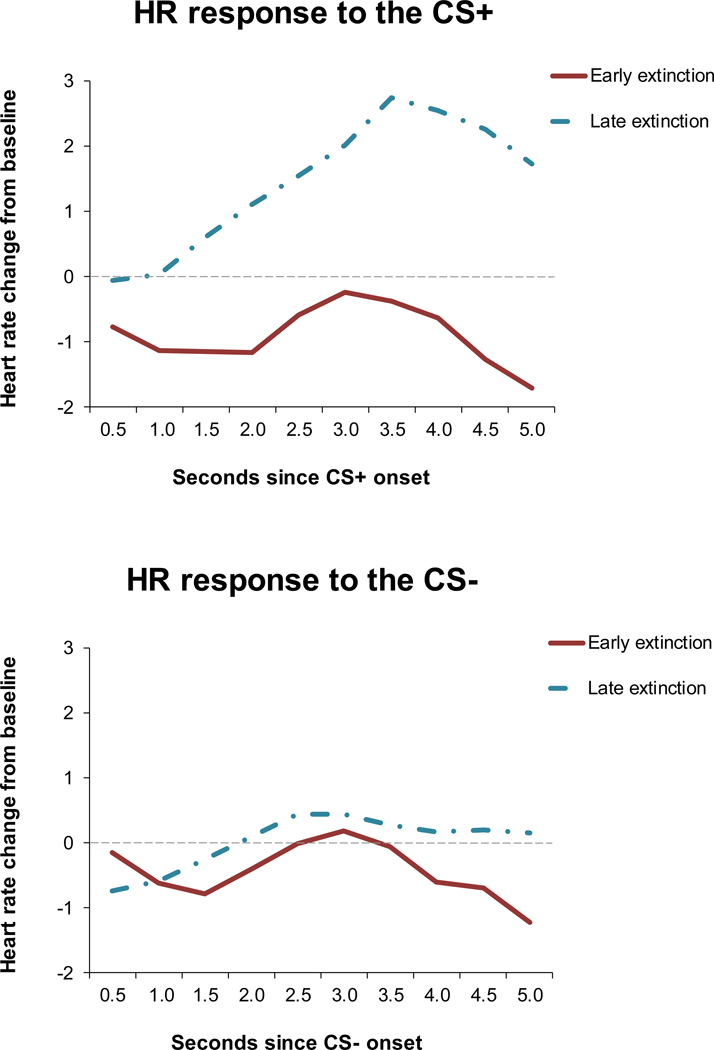
HR increased during fear acquisition such that HR during the final block of acquisition was significantly greater than HR during habituation, F(1,43) = 8.81, p = .005. An omnibus 3-way repeated measures analyses of variance (ANOVA) was first used to test differences in HR response to the CS+ and CS− for early (block 1) and late (block 4) extinction, where the factors were CS type (CS+/CS−) by block (early/late) by time (0.5s, 1.0s, 1.5s, 2.0s, 2.5s, 3.0s, 3.5s, 4.0s, 4.5s, 5.0s). There were significant main effects of extinction block, F(1,48) = 13.69, p = .001, and significant cubic contrast for time, F(1,48) = 17.16, p < .0001. In addition, the CS type by block interaction was significant, F(1,48) = 8.56, p = .005, suggesting that there were differences in the HR time course by CS type and extinction block. We then conducted a 2-way (block by time) ANOVA separately for each CS. For the CS+, there were significant main effects of block, F(1,50) = 18.32, p < .0001, and time, F(1,50) = 26.74, p < .0001 (cubic), as well as a significant interaction of block by time, F(1,50) = 25.22, p < .001. As depicted in Figure 2a, the cubic effect of time is consistent with fear bradycardia to the CS+ in early but not late extinction, suggesting that it diminished over the course of extinction. For the CS−, the effect of block was not significant, F(1,48) = 1.24, p = .27, suggesting that there was no effect of extinction for the CS−. In addition, the cubic contrast term was not significant, F(1,48) = 3.06, p = .09, indicating that the CS− did not induce bradycardia (Figure 2b).
Figure 2:
Extinction of the HR response.
Figure 2a: Extinction of the HR response to the CS+
Figure 2b: Extinction of the HR response to the CS−
Note. HR = heart rate; CS = conditioned stimulus.
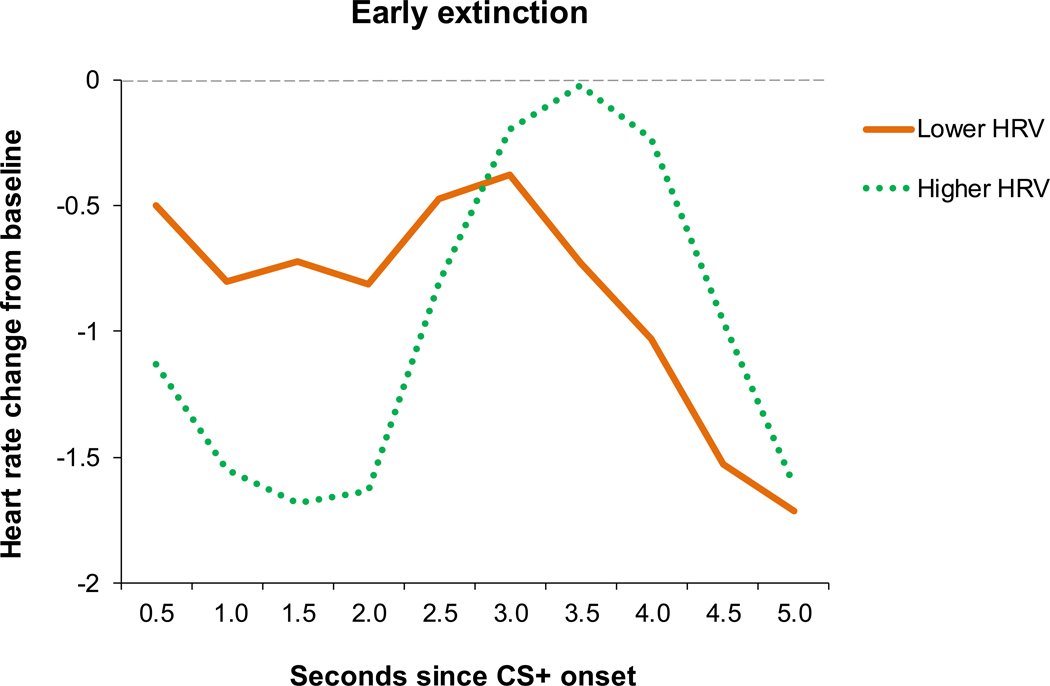
To examine differences in individuals with higher and lower HRV, we derived two groups based on the median split of resting HRV. Average HRV was 6.63 (SD = .89) among the higher HRV group and 4.11 (SD = .76) among the lower HRV group. Average HR was higher in the lower HRV group (M = 80.58, SD = 9.63) compared to the higher HRV group (M = 74.57, SD = 7.73), F(1,43) = 5.21, p = .03. The higher and lower HRV groups did not differ in terms of age, race, education, PSS total, or subscale scores (all p’s > .10 for ANOVA and chi-square tests). To test our hypothesis regarding the effect of HRV status on bradycardia-related HR changes during the presentation of the CS+ during early extinction, we analyzed a 2-way (HRV group by time) ANOVA, where time reflected binned HR change during the presentation of the CS+. To test for bradycardia, we examined the cubic contrast across the HR time bins. There was a significant main effect for a cubic trend of time, F(1,42) = 19.95, p < .00001, and an HRV group by time interaction, F(1,42) = 7.23, p = .01. Follow-up tests within each HRV group testing HR change over time resulted in a highly significant cubic term in the higher HRV group, F(1,21) = 18.01, p < .00001, but not in the the lower HRV group, F(1,21) = 2.73, p =.11. As depicted in Figure 3, individuals in the higher HRV group demonstrated a larger HR decrease to the CS+ during the fear bradycardia period followed by a greater HR increase in the post-bradycardia period, consistent with a cubic curve exhibited in bradycardia. The pronounced dip in HR at the onset of the CS+ suggests that the fear bradycardia effect was stronger among those with higher HRV.
Figure 3:
HR response to the CS+ by HRV group in early extinction
Note. Early extinction refers to extinction Block 1; CS = conditioned stimulus; HRV = heart rate variability.
Discussion
This is the first study, to our knowledge, to examine the HR response during fear extinction among trauma-exposed individuals. As hypothesized, we observed an initial rapid HR deceleration to the CS+ (fear bradycardia), typically associated with the fear response, that decreased over the course of extinction and was associated with higher resting HRV. This is consistent with prior fear research with HR (e.g., Lang et al., 2011; Panitz et al., 2015; Sege et al., 2017) and contributes to the understanding of cardiac responses to fear in trauma, which may accelerate cardiovascular disease risk. Overall, these results suggest that fear bradycardia was associated with better cardiac vagal control in a trauma-exposed sample.
As expected, we found evidence for a conditioned fear bradycardia response to the CS+ during early extinction, which diminished over time. Prior research has demonstrated that HR may initially slow and then increase in response to neutral stimuli as well, which appears to be associated with attentional orienting to novelty (Bradley, 2009; Bradley & Lang, 2007; Urry, 2010). This would suggest that regardless of fear, the CS+ presented without the US in extinction may elicit cardiac deceleration due to novelty alone (i.e., the unpaired CS+ is novel in comparison to the CS+ paired with the US). However, given that the HR response to the CS+ appeared stronger than that of the CS− in the current study, it is not likely that our findings were due to attentional orienting alone.
At the bivariate level, higher resting HRV was associated with increased fear bradycardia, which was expected given that both of these indicators are controlled by the PNS. This may suggest that cardiac vagal control is an indicator of cardiac extinction performance (indexed via fear bradycardia) and could help to explain prior findings of elevated CVD risk in PTSD patients (Edmonson et al., 2013; Edmonson and von Känel, 2017). Specifically, low HRV may be a mechanism through which elevated HR confers greater CVD risk, and higher HRV may represent a protective factor. Consistent with the research of Hopper and colleagues (2006), individuals in our study with lower HRV demonstrated significantly higher HR than those with higher HRV. This may suggest that elevated HR is only be a risk for PTSD in the presence of low HRV and that cardiac vagal control is a more salient indicator of disease severity than HR alone. However, given that HR and HRV were not related to PTSD symptoms in the current study, additional research is needed to fully replicate the Hopper et al. (2006) findings. Our analyses also suggest that the pattern of fear bradycardia to the CS+ in early extinction may be driven by those with higher HRV, such that those in the higher HRV group demonstrated the expected pattern of bradycardia while those in the lower HRV group did not. Thus, individuals with better resting cardiac vagal control may demonstrate more adaptive fear responding through greater PNS activation.
Based on these findings, we suggest that the current study may have clinical implications. In particular, our finding regarding HRV and fear bradycardia suggests that individuals may respond differently to extinction-based psychotherapy depending on the strength of their cardiac vagal control, and that fear bradycardia may be a relevant biomarker in predicting how individuals will respond to these treatments. Future research testing treatment outcomes based on resting HRV could inform whether certain individuals benefit more or less than others from extinction-based approaches. If so, it will be important to determine whether these approaches could be modified for those with low HRV or if alternative approaches should be considered. One possibility is to provide interventions that are known to increase HRV, such as mindfulness meditation (Ditto et al., 2006; Krygier et al., 2013), in preparation for starting exposure. This could allow individuals with low resting HRV to gain more from the exposure portion of treatment.
In terms of study limitations, a lack of information about pre-trauma cardiovascular function and CVD incidence prevents causal inference. For example, it is possible that individuals with poor cardiovascular function (e.g., high resting HR) are more likely to develop PTSD (Morris et al., 2016; see Pole et al., 2009 for alternate findings). Future replication with prospective studies and specific measures of CVD would help to elucidate causality between cardiovascular function and trauma-related sequalae. Second, the current study was limited in its reliance on a one-minute epoch for resting HRV. As discussed by Baeg and colleagues (2015), a short epoch may present a threat to reliability even when using the high frequency range. A minimum of five minutes is recommended for more robust estimates of resting HRV. Additionally, the current study included only trauma-exposed individuals, which precludes us from comparing our findings to those of a non-trauma-exposed control group. While it is a strength that we examined PTSD dimensionally in a trauma-exposed sample, future studies should include comparison to a group without trauma exposure to further evaluate the clinical significance of our findings. Finally, it is important to note that using a median split for HRV groups does not necessarily result in groups that truly have high or low HRV, but relies on the specific sample distribution. This procedure can result in false negatives due to power loss and doesn’t account well for individuals who are close to the median.
To our knowledge, this is the first study examining the cardiovascular response to fear extinction in trauma-exposed individuals. We observed a pattern of fear bradycardia early in extinction and demonstrated that this response decreased or was extinguished over time, and that it appeared stronger in those with higher resting HRV. Overall, our results indicate that fear bradycardia may be indicative of greater vagal control (i.e., HRV), which could be a useful psychophysiological biomarker to assess change in PNS function over the course of treatment, such as exposure therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, fifth ed. American Psychiatric Publishing, Virginia. [Google Scholar]
- Baek HJ, Cho CH, Cho J, Woo JM, 2015. Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemed. J. E. Health 21, 404–14. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW, 1997. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34, 623–648. 10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ, 2008. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299, 1291–1305. 10.1001/jama.299.11.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, 2009. Natural selective attention: Orienting and emotion. Psychophysiology 46, 1–11. doi: 10.1111/j.1469-8986.2008.00702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, 2007. Emotion and motivation In Cacioppo JT, Tassinary LG, & Berntson GG (Eds.), Handbook of Psychophysiology (pp. 581–607). New York, NY, US: Cambridge University Press. doi: 10.1017/CBO9780511546396.025 [DOI] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS, 2005. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol. Med. 35, 791–806. 10.1017/s0033291704003290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar PJ, 2015. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R315–21. 10.1152/ajpregu.00343.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Kaloupek DG, 2001. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom. Med. 63, 585–594. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell C, Schreurs BG, 2010. Effects of extinction on classical conditioning and conditioning-specific reflex modification of rabbit heart rate. Behav. Brain Res. 206, 127–134. 10.1016/j.bbr.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castegnetti G, Tzovara A, Staib M, Paulus PC, Hofer N, Bach DR, 2016. Modeling fear-conditioned bradycardia in humans. Psychophysiology 53, 930–939. 10.1111/psyp.12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H-A, Chang C-C, Tzeng N-S, Kuo TB, Lu R-B, Huang S-Y, 2013. Decreased cardiac vagal control in drug-naïve patients with posttraumatic stress disorder. Psychiatry Investig. 10, 121–130. 10.4306/pi.2013.10.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y, 1997. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol. Psychiatry 41, 627–629. 10.1016/S0006-3223(96)00525-2 [DOI] [PubMed] [Google Scholar]
- Ditto B, Eclache M, Goldman N, 2006. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann. Behav. Med. 32, 227–234. 10.1207/s15324796abm3203_9 [DOI] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM, 2013. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am. Heart J. 166, 806–814. 10.1016/j.ahj.2013.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, von Känel R, 2017. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry 4, 320–329. 10.1016/S2215-0366(16)30377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Suendermann O, Boellinghaus I, Vossbeck-Elsebusch A, Gamer M, Briddon E, Martin MW, Glucksman E, 2010. Heart rate responses to standardized trauma-related pictures in acute posttraumatic stress disorder. Int. J. Psychophysiol. 78, 27–34. 10.1016/j.ijpsycho.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG, 2013. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. Behav. Ther. 16, 161–162. [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO, 1993. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J. Trauma. Stress 6, 459–473. 10.1002/jts.2490060405 [DOI] [Google Scholar]
- Foa EB, Steketee G, Rothbaum BO, 1989. Behavioral/cognitive conceptualizations of posttraumatic stress disorder. Behav. Ther. 20, 155–176. 10.1016/S0005-7894(89)80067-X [DOI] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ, 2009. Trauma exposure and stress-related disorders in inner city primary care patients. Gen. Hosp. Psychiatry 31, 505–514. 10.1016/j.genhosppsych.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA 3rd, 1999. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J. Abnorm. Psychol. 108, 134–142. [DOI] [PubMed] [Google Scholar]
- Hauschildt M, Peters MJV, Moritz S, Jelinek L, 2011. Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biol. Psychol. 88, 215–222. 10.1016/j.biopsycho.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Hopper JW, Spinazzola J, Simpson WB, van der Kolk BA, 2006. Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J. Psychosom. Res. 60, 83–90. 10.1016/j.jpsychores.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M, 2012. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62, 695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozarić-Kovacić D, 2009. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. Int. J. Psychophysiol. 71, 264–268. 10.1016/j.ijpsycho.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ, 2010. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry 167, 648–662. 10.1176/appi.ajp.2009.09071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, Hsieh FY, Lavori PW, 1998. Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. J. Consult. Clin. Psychol. 66, 914–923. [DOI] [PubMed] [Google Scholar]
- Keary TA, Hughes JW, Palmieri PA, 2009. Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. Int. J. Psychophysiol. 73, 257–264. 10.1016/j.ijpsycho.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet EJ, Cardoso G, Degenhardt L, de Girolamo G, Dinolova RV, Ferry F, Florescu S, Gureje O, Haro JM, Huang Y, Karam EG, Kawakami N, Lee S, Lepine J-P, Levinson D, Navarro-Mateu F, Pennell B-E, Piazza M, Posada-Villa J, Scott KM, Stein DJ, Ten Have M, Torres Y, Viana MC, Petukhova MV, Sampson NA, Zaslavsky AM, Koenen KC, 2017. Trauma and PTSD in the WHO World Mental Health Surveys. Eur. J. Psychotraumatol. 8, 1353383. 10.1080/20008198.2017.1353383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048–1060. 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM‐IV and DSM‐5 criteria. J. Trauma. Stress 26, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krygier JR, Heathers JAJ, Shahrestani S, Abbott M, Gross JJ, Kemp AH, 2013. Mindfulness meditation, well-being, and heart rate variability: a preliminary investigation into the impact of intensive Vipassana meditation. Int. J. Psychophysiol. 89, 305–313. 10.1016/j.ijpsycho.2013.06.017 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Wangelin BC, Bradley MM, Versace F, Davenport PW, Costa VD, 2011. Threat of suffocation and defensive reflex activation. Psychophysiology 48, 393–396. 10.1111/j.1469-8986.2010.01076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S, 1991. Cardiovascular neural regulation explored in the frequency domain. Circulation 84, 482–492. 10.1161/01.cir.84.2.482 [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK, 2008. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J. Psychiatr. Res. 42, 515–520. 10.1016/j.jpsychires.2008.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66, 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Geyer MA, Baker DG, Nievergelt CM, O’Connor DT, Risbrough VB, Marine Resiliency Study Team, 2014. Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom. Med. 76, 292–301. 10.1097/PSY.0000000000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB, Marine Resiliency Study Team, 2015. Association of Predeployment Heart Rate Variability With Risk of Postdeployment Posttraumatic Stress Disorder in Active-Duty Marines. JAMA Psychiatry 72, 979–986. 10.1001/jamapsychiatry.2015.0922 [DOI] [PubMed] [Google Scholar]
- Morris MC, Hellman N, Abelson JL, Rao U, 2016. Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clin. Psychol. Rev. 49, 79–91. doi: 10.1016/j.cpr.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, 2017. Corticolimbic regulation of cardiovascular responses to stress. Physiol. Behav. 172, 49–59. 10.1016/j.physbeh.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ, 2011. Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biol. Psychiatry 69, 556–563. 10.1016/j.biopsych.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR, 1993. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J. Abnorm. Psychol. 102, 152–159. 10.1037/0021-843X.102.1.152 [DOI] [PubMed] [Google Scholar]
- Panitz C, Hermann C, Mueller EM, 2015. Conditioned and extinguished fear modulate functional corticocardiac coupling in humans. Psychophysiology 52, 1351–1360. 10.1111/psyp.12498 [DOI] [PubMed] [Google Scholar]
- Park JE, Lee JY, Kang S-H, Choi JH, Kim TY, So HS, Yoon I-Y, 2017. Heart rate variability of chronic posttraumatic stress disorder in the Korean veterans. Psychiatry Res. 255, 72–77. 10.1016/j.psychres.2017.05.011 [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR, 2009. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biol. Psychiatry 65, 235–240. doi: 10.1016/j.biopsych.2008.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumprla J, Howorka K, Groves D, Chester M, Nolan J, 2002. Functional assessment of heart rate variability: physiological basis and practical applications. Int. J. Cardiol. 84, 1–14. 10.1016/s0167-5273(02)00057-8 [DOI] [PubMed] [Google Scholar]
- Sack M, Hopper JW, Lamprecht F, 2004. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: heart rate dynamics and individual differences in arousal regulation. Biol. Psychiatry 55, 284–290. 10.1016/S0006-3223(03)00677-2 [DOI] [PubMed] [Google Scholar]
- Sahar T, Shalev AY, Porges SW, 2001. Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biol. Psychiatry 49, 637–643. 10.1016/S0006-3223(00)01045-3 [DOI] [PubMed] [Google Scholar]
- Sege CT, Bradley MM, Lang PJ, 2017. Escaping aversive exposure. Psychophysiology 54, 857–863. 10.1111/psyp.12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz AP, Seligowski AV, Park J, Marvar PJ, 2018. Extinction of fear memory attenuates conditioned cardiovascular fear reactivity. Front. Behav. Neurosci. 12, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology, 1996. Heart Rate Variability. Circulation. 10.1161/01.CIR.93.5.1043 [DOI]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH, 2009. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 37, 141–153. 10.1007/s12160-009-9101-z [DOI] [PubMed] [Google Scholar]
- Urry HL (2010). Seeing, thinking, and feeling: Emotion-regulating effects of gaze-directed cognitive reappraisal. Emotion 10, 125–135. doi: 10.1037/a0017434 [DOI] [PubMed] [Google Scholar]