Abstract
Background:
Tamoxifen (TAM) and aromatase inhibitor (AI) therapies have been associated with increased risk of thromboembolic and cardiovascular events, respectively, in addition to other side effects. This study analysed the risk of these events and the overall survival (OS) benefit in breast cancer patients treated with AI, compared with TAM-treated patients, in a large population-based cohort.
Methods:
This observational cohort study included women diagnosed with breast cancer and treated with TAM or AI. Data were extracted from primary care records in a population database (SIDIAP, System for the Development of Research in Primary Care). Incidence rates of study outcomes are reported. Survival analyses included Kaplan–Meier estimation and Cox proportional hazards models. Sensitivity analysis was carried out, using Fine and Gray models to account for competing risk of death. Confounding was minimized using propensity score adjustment and inverse probability weighting (IPW) adjustment.
Results:
Data from 3082 postmenopausal women treated with TAM, and 18,455 treated with AI, were available. Adjusted hazard ratios (HRs) [95% confidence interval (CI)] for AI users, compared with TAM group, were 0.93 (95%CI 0.69–1.26) for thromboembolic events (TEEs); 1.13 (95%CI 0.79–1.63) for cardiovascular events, and 0.76 (95%CI 0.70–0.82) for mortality. Additional analyses using competing risk analysis had similar results, while IPW adjustment showed a potential risk of pulmonary embolism (PE) [2.26 (95%CI 1.02–4.97)] in AI-treated patients.
Conclusions:
AI users had >20% lower all-cause mortality compared with TAM users, without increasing risk to experience cardiovascular and TEEs. This would locate AI therapy on the first line in clinical practice. Thus, AI might be the most preferable option in adjuvant hormonal therapy choice.
Keywords: aromatase inhibitor, breast cancer, cardiovascular events, overall mortality, tamoxifen, thromboembolic events
Introduction
Aromatase inhibitors (AIs) and tamoxifen (TAM) are known to be effective adjuvant endocrine therapies for patients with hormone-receptor-positive breast cancer. Generally, these patients have good prognosis, with an overall survival (OS) rate exceeding 80%.1,2 However, these therapies have been associated with side effects that can affect quality of life and could impact on mortality, among them, cardiovascular events (CVEs) and thromboembolic events (TEEs) are emerging as competing causes of death.3 A number of randomized controlled trials (RCT) have explored the cardiovascular effect, comparing AIs versus TAM, with heterogeneous results.4,5 These studies have provide evidence of increased CVEs associated with AI therapies, compared with TAM, likely due to high depletion of estradiol levels and alteration of lipid metabolism related to AIs,6 or to the cardioprotective role of tamoxifen per se.7
Although RCT and meta-analysis are gold standard experimental approaches for the study of efficacy and safety in ‘ideal’ conditions, they are sometimes not representative of clinical practice conditions or of the actual profile of the treated community,8 and, thus, they cannot address definitively safety issues, particularly for side effects with low incidence. We therefore aimed to analyze the risk of CVE and TEE, and OS benefit during AI therapy, compared with TAM, in real-world conditions.
Thus, the present study used the SIDIAP (System for the Development of Research in Primary Care) database, which provided anonymized clinical information as coded by primary care practitioners in Catalonia, Spain, covering more than 7 million patients.9 SIDIAP contains information on socio-demographics and extended clinical data. Moreover, SIDIAP is linked to pharmacy invoice data, which provides detailed information on drugs dispensed in community pharmacies under the universal health care system. Using this database, we performed a population-based study, including almost 28,000 women treated with AI or TAM for up to 10 years of follow up, to assess thromboembolic and cardiovascular events, and resulting OS in general clinical practice.
Methods
Data sources
SIDIAP (http://www.sidiap.org) is an anonymized clinical database of more than 7 million patient records collected from more than 370 primary care teams covering >80% of the total population of Catalonia. Among the available variables are socio-demographic data, lifestyle risk factors, prescriptions dispensed and comorbidities. Health professionals gather this information using ICD-10 codes and structured forms designed for the collection of clinical factors (alcohol use, smoking, body mass index, etc.). Migration out of the catchment area is also recorded, allowing for longitudinal follow up of patients.10 Death is also registered in the SIDIAP database, as provided by the universal health insurance database for Catalonia (in Catalan, ‘registre central de persones assegurades’).
Study design and participants
Retrospective observational cohort study of women diagnosed with early breast cancer, defined as nonmetastatic breast cancer, stage I–III, and treated with monotherapy of TAM or AIs as registered in the SIDIAP database from January 2006 to December 2015. Therapeutic regimen in patients was identified by its anatomical therapeutic chemical classification in pharmacy dispensing records, coded as L02BG for AIs (L02BG03 for anastrozole, L02BG04 for letrozole, L02BG06 for exemestane), and L02BA01 for TAM.
Exclusion criteria were previous history of cancer (except nonmelanoma skin cancers) and patients who had received a switching therapy (TAM followed by AI or vice versa). No concomitant anticancer drugs other than TAM or AI were used.
Ethics statement
This study used only data collected routinely from the SIDIAP database. The Idiap Jordi Gol Research Ethics Committee and the SIDIAP Database Scientific Committee have approved the study protocol (P16/031). No human subjects or tissues were used in this study. Data provided by SIDIAP was anonymized and risk of identification was almost null according to Spanish law LO 15/ 1999 13 December. Thus, informed consent did not need to be obtained from participants.
Follow up
Participants were followed up from therapy initiation (first day of TAM or AI dispensing) until the earliest of three endpoints: treatment cessation (defined by a refill gap of 6 months or more with no dispensation of the index therapy), plus 1 month wash-out (for carry-over effects); evaluated outcomes date (as recorded in electronic medical records); or death, migration out of catchment area, or end of SIDIAP data availability (31 December 2015).
In the overall mortality assessment, patients were followed-up during all the study period (2006–2015).
Variables
Outcomes of the study
Analyzed outcomes were the first TEE [pulmonary embolism (PE) and deep vein thrombosis (DVT), including phlebitis and thrombophlebitis] and the first CVE [coronary artery disease (CAD), and cerebrovascular diseases (CVD), including stroke and intracerebral haemorrhage, among others] occurring during adjuvant therapy. In addition, PE, DVT, CAD and CVD were analysed separately as secondary outcomes. OS, expressed as mortality status during follow up, was also reported. ICD-10 codes used to identify the outcomes of the study are documented in Supplementary Table S1.
Confounders
A prespecified list of confounders was extracted from SIDIAP, informed by previous clinical knowledge and scientific literature. These confounding factors fell into five clusters:
Sociodemographics: age (at treatment initiation), body mass index (BMI), and socioeconomic status (assessed by MEDEA, a validated deprivation index).11
Menopausal status: defined as women >55 years old at diagnosis in the TAM group or all patients treated with AI. Menopausal status of patients <55 years old in TAM group is unknown.
Lifestyle factors: smoking, alcohol use (defined according to The Catalan Health Care System: none/low, as a mean of 0 g of alcohol per week; moderate, not exceeding 170 g of alcohol per week; high/alcoholic, 170 g of alcohol or more per week).
Past medical history: Charlson comorbidity index, and previous history of CVE and TEE.
Concomitant use of antiplatelets or anticoagulants or statins at cohort entry (i.e. TAM/AI initiation).
Statistical analysis
Data from SIDIAP were managed using MySQL. Differences in baseline characteristics between TAM and AI participants were described and imbalances analysed using t test and Chi-square test.
Incidence rates of study outcomes (during treatment for TEE/CVE and at any time for OS) were estimated.12
For each outcome, survival analysis was done by Kaplan–Meier estimation and Cox proportional hazards model to estimate cumulative probability plots and HRs according to treatment/exposure, respectively.
Additionally, a subanalysis using Fine and Gray regression models were fitted to estimate subdistribution hazard ratios (SHR) for TEE and CVE (separately) according to treatment arm, accounting for a competing risk of death.13
HR and SHR are reported with 95% CI, and using TAM as a reference group (and AI as the ‘exposed’ group). Moreover, the assumption of proportionality was verified though proportional hazards assumption for a Cox regression model test.
Adjustment in survival analysis was conducted using the propensity score (PS). PS was estimated using logistic regression models, where treatment group was the outcome and the previously listed confounders were adjusted for. The final list of variables used in PS adjustments are listed in Table 1, including statins, anticoagulants and antiplatelet drugs. Missing data were imputed before the PS estimates, using multiple imputation by chained equations, obtaining 10 imputed datasets that were combined using Rubin’s rules.14 Previous TEE and CVE history were included in their respective analyses, and in OS evaluation. Additional analysis censoring patients with previous TEE and CVE was performed to account for potential baseline higher risk.
Table 1.
Baseline characteristics of candidates in postmenopausal women.
| Variable | AI N = 18,455 |
TAM N = 3082 |
|---|---|---|
| Median age (years) [Q1;Q3] | 67.0 [59.0;77.0] | 69.0 [62.0;79.0] |
| Mean BMI (kg/m 2 ) ± (SD) | 29.7 (5.36) | 29.8 (5.09) |
| Missing, n (%) | 13,555 (73.45) | 2189 (71.03) |
| QMEDEA deprivation index, n (%): | ||
| Rural population | 3462 (18.8) | 579 (18.8) |
| Urban area #1 | 3498 (19.0) | 513 (16.6) |
| Urban area #2 | 2960 (16.0) | 446 (14.5) |
| Urban area #3 | 2692 (14.6) | 499 (16.2) |
| Urban area #4 | 2399 (13.0) | 409 (13.3) |
| Urban area #5 | 2012 (10.9) | 390 (12.7) |
| Missing | 1432 (7.76) | 246 (7.98) |
| Charlson comorbidity index, n (%): | ||
| 0 | 2315 (12.5) | 399 (12.9) |
| 1 | 704 (3.81) | 115 (3.73) |
| 2 | 9840 (53.3) | 1671 (54.2) |
| 3 | 3553 (19.3) | 575 (18.7) |
| ⩾4 | 2043 (11.1) | 322 (10.4) |
| Smoking status, n (%): | ||
| Never smokers | 10,269 (55.64) | 1579 (51.23) |
| Current smokers | 1343 (7.28) | 135 (4.38) |
| Ex-smokers (quit >1 year) | 997 (5.4) | 128 (4.15) |
| Missing, n (% of total) | 5846 (31.68) | 1240 (40.23) |
| Alcoholism, n (%): | ||
| None/Low | 2410 (13.06) | 268 (8.7) |
| Moderate | 390 (2.11) | 44 (1.43) |
| High/Alcoholic | 16 (0.09) | 1 (0.03) |
| Missing | 15,639 (84.74) | 2769 (89.84) |
| Antiplatelet drug users, n (%) | 1720 (9.32) | 308 (9.99) |
| Anticoagulant drug users, n (%) | 544 (2.95) | 70 (2.27) |
| Statin drug users, n (%) | 3518 (19.1) | 511 (16.6) |
| Previous TEE history, n (%) | 496 (2.69) | 38 (1.23) |
| Previous CVE history, n (%) | 693 (3.76) | 122 (3.96) |
Participants included in TAM group were older than 55 years old to ensure postmenopausal status.
AI, aromatase inhibitors; BMI, body mass index; CVE, cerebrovascular event; Q, quartile; QMEDEA, quintile MEDEA deprivation index; SDE, standard deviation; TAM, tamoxifen; TEE, thromboembolic event.
An additional analysis adjusting survival analysis by stabilized inverse probability weighting (IPW) and using a robust sandwich-type variance estimator was performed.15
In order to compare our results with an analysis not accounting for menopausal status, we repeated the same models using total TAM users, including those women younger than 55 years old.
All statistical analyses were conducted using R for Windows version 3.3.3 and the following R packages: foregin, Hmisc, compareGroups, survival and mice.
Results
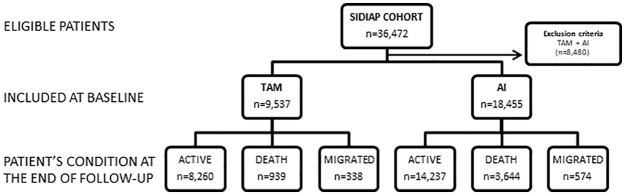
Of the 36,472 eligible participants, 21,537 (18,455 AI and 3082 TAM) monotherapy users were included in the analysis (see Figure 1), with a median (interquartile range) of treatment of 29 (10–53) months and a maximum of 119 months. Baseline characteristics of participants are presented in Table 1. Postmenopausal women treated with TAM were older, less likely to be current smokers, concomitant users of anticoagulants or statin therapy, and had lower prevalence of previous TEE than AI users; but had a similar BMI, Charlson comorbidity index, current alcohol drinker status, users of platelet inhibitors and prevalence of previous CVE.
Figure 1.
Flow chart of SIDIAP cohort study.
AI, aromatase inhibitor; SIDIAP, System for the Development of Research in Primary Care; TAM, tamoxifen; ⩽55y, patients equal or less than 55 years old.
Thromboembolic adverse events
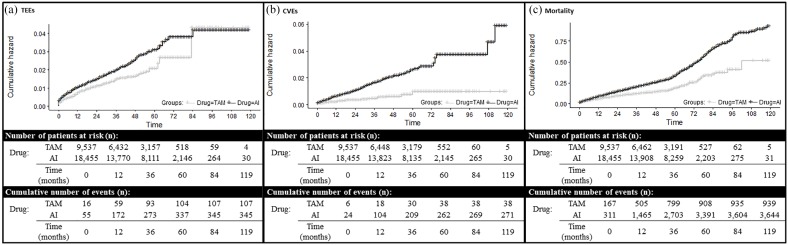
A total of 49 patients in the TAM group experienced TEEs (1.59%), whereas these were 345 (1.87%) patients in the AI group. This is equivalent to incidence rates of 8.16/1000 person-years (95%CI 6.10–10.69) in TAM users, and 6.93/1000 person-years (95%CI 6.23–7.69) in AI patients (Figure 2a). No significant differences in thromboembolic risk were observed between both therapies [adjusted HR 0.93 (95%CI 0.69–1.26)] (Table 2). Survival analysis, adjusted for competing risk, showed similar findings (Table 2). Sensitivity analyses excluding patients with previous TEE did not change the estimates (data not shown).
Figure 2.
Cumulative hazard plot of (a) TEEs, (b) CVEs and (c) mortality in SIDIAP cohort according to drug therapy. Graphs show Kaplan–Meier curves representing the outcomes of the study in terms of cumulative hazards.
AI, aromatase inhibitor; CVE, cardiovascular event; SIDIAP, System for the Development of Research in Primary Care; TAM, tamoxifen; TEE, thromboembolic event.
Table 2.
Thromboembolic, cardiovascular and mortality risk of AI treatment compared with TAM treatment in postmenopausal women.
| Hazard risk estimates | ||||
|---|---|---|---|---|
| Outcome | Number of events | Unadjusted HR (95%CI) | Adjusted HR (95%CI) | |
| TEEs | TAM AI |
49 345 |
0.92 (0.68–1.24) | 0.93 (0.69–1.26) |
| CVEs | TAM AI |
33 271 |
1.02 (0.71–1.47) | 1.13 (0.79–1.63) |
| Mortality | TAM AI |
696 3644 |
0.65 (0.60–0.71) | 0.76 (0.70–0.82) |
| Competing risk estimates | ||||
| Outcome | Number of events | Unadjusted HR (95%CI) | Adjusted HR (95%CI) | |
| TEEs | TAM AI |
49 345 |
0.99 (0.74–1.34) | 1.05 (0.78–1.42) |
| CVEs | TAM AI |
33 271 |
1.13 (0.79–1.62) | 1.31 (0.91–1.88) |
Adjusted results were obtained using continuous PS estimates.
AI, aromatase inhibitors; CI, confidence interval; CVEs, cardiovascular events; HR, hazard ratio; PS, propensity score; SHR, subdistribution hazard ratio; TAM, tamoxifen; TEEs, thromboembolic events.
Cardiovascular adverse events
A total of 33 (1.07%) TAM users had at least one CVE event during follow up, compared with 271 events (1.47%) in the AI user group. Incidence rates were therefore 5.50/1000 person-years (95%CI 3.85–7.63) in TAM, and 5.43/1000 person-years (95%CI 4.81–6.10) in AI users. Cumulative hazard plots of CVEs are shown in Figure 2b. No significant increase in cardiovascular risk was detected in AI-treated patients [adjusted HR 1.13 (95%CI 0.79–1.63)] (Table 2). Survival analysis, adjusted for competing risk, show similar findings (Table 2). Sensitivity analyses excluding patients with previous CVE did not change the estimates (data not shown).
Mortality
Overall mortality was 22.58% (696 participants) in the TAM group, and 19.75% (3644 subjects) in the AI group. Crude mortality rates were 40.68/1000 person-years (95%CI 37.74–43.78) in TAM, and 40.25/1000 (95%CI 38.95–41.57) in AI users. Cumulative hazard plots of mortality are shown in Figure 2c. Adjusted Cox models showed a better prognostic for AI users [HR of 0.76 (95%CI 0.70–0.82)] compared with TAM users (Table 2). Similar findings were observed after competing risk adjustment (Table 2).
Secondary outcomes
TEEs: PE and DVT
In our cohort, 100 PE events [7 in TAM group, incidence rate 1.17 (95%CI: 0.51–2.31); and 93 in AI group, incidence rate 1.87 (95%CI: 1.52–2.28)] and 294 DVTs [42 in TAM group, incidence rate 6.99 (95%CI: 5.10–9.36); and 252 in AI group, incidence rate 5.06 (95%CI: 4.47–5.72)] were reported. No differences in DVT risk were found between both groups. A nonsignificant increased risk of PE (Adjusted SHR of 2.15 [95%CI 0.99–4.64]) in AI group was observed (Table 3).
Table 3.
Risk of PE, DVT, CAD and CVD of AI treatment compared with TAM treatment in postmenopausal woman.
| Hazard ratio estimates | |||||
|---|---|---|---|---|---|
| Outcome | Subtype | Number of events | Unadjusted HR (95%CI) | Adjusted HR (95%CI) | |
| TEEs | PE | TAM AI |
7 93 |
1.77 (0.82–3.82) | 1.91 (0.88–4.13) |
| DVT | TAM AI |
42 252 |
0.92 (0.68–1.24) | 0.81 (0.58–1.13) | |
| CVEs | CAD | TAM AI |
32 260 |
1.02 (0.71–1.47) | 1.12 (0.77–1.62) |
| CVD | TAM AI |
1 11 |
1.02 (0.71–1.48) | 1.49 (0.19–11.66) | |
| Competing risk estimates | |||||
| Outcome | Subtype | Number of events | Unadjusted SHR (95%CI) | Adjusted SHR (95%CI) | |
| TEEs | PE | TAM AI |
7 93 |
1.91 (0.88–4.12) | 2.15 (0.99–4.64) |
| DVT | TAM AI |
42 252 |
0.84 (0.61–1.17) | 0.89 (0.64–1.24) | |
| CVEs | CAD | TAM AI |
32 260 |
1.12 (0.77–1.61) | 1.29 (0.89–1.87) |
| CVD | TAM AI |
1 11 |
1.52 (0.20–11.76) | 1.75 (0.22–13.71) | |
Adjusted results were obtained using continuous PS estimates.
AI, aromatase inhibitors; CAD, coronary artery disease; CI, confidence interval; CVD, cerebrovascular diseases, including stroke and intracerebral hemorrhage; CVEs, cardiovascular events; DVT, deep vein thrombosis, phlebitis and thrombophlebitis; HR, hazard ratio; PE, pulmonary embolism; PS, propensity score; SHR, subdistribution hazard ratio; TAM, tamoxifen; TEEs, thromboembolic events.
CVEs: CAD and CVD
Of 304 CVEs, 292 were CAD [32 in TAM, incidence rate 5.33 (95%CI: 3.71–7.44); and 260 in AI users, incidence rate 5.21 (95%CI: 4.60–5.87)] and 12 were CVD [1, incidence rate 0.17 (95%CI:0.01–0.82) in TAM; and 11, 0.22 (95%CI: 0.12–0.38) in AI users]. No increased risk of CAD nor CVD was detected between both groups (Table 3).
A secondary analysis where patients with previous CVE and TEE were censored showed similar results (data not shown).
Inverse probability weighting analysis
Survival analyses adjusted for IPW are reported in Table 4. CVEs were similar to those obtained in Cox regression models, with no significant difference in risk between AI and TAM users. Likewise, lower mortality risk was detected in AI users. On the contrary, an increased risk of PE was detected in AI users [stabilized IPW HR 2.26 (95%CI 1.02–4.97)].
Table 4.
Risk of TEEs and CVEs in AI treatment compared with TAM treatment in postmenopausal women using stabilized IPW adjustment.
| Outcome and subtypes | Number of events | Stabilized IPW HR (95%CI) | |
|---|---|---|---|
| TEEs | TAM AI |
49 345 |
0.96 (0.70–1.32) |
| PE | TAM AI |
7 93 |
2.26 (1.02–4.97) |
| DVT | TAM AI |
42 252 |
0.79 (0.55–1.11) |
| CVEs | TAM AI |
33 271 |
1.05 (0. 71–1.54) |
| CAD | TAM AI |
32 260 |
1.05 (0.71–1.54) |
| CVD | TAM AI |
1 11 |
1.97 (0.25–15.54) |
| Mortality | TAM AI |
696 3644 |
0.79 (0.72–0.86) |
AI, aromatase inhibitors; CAD, coronary artery disease; CI, confidence interval; CVD, cerebrovascular diseases, including stroke and intracerebral hemorrhage; CVEs, cardiovascular events; DVT, deep vein thrombosis, phlebitis and thrombophlebitis; IPW HR, Inverse probability weighting hazard ratio; PE, pulmonary embolism; TAM, tamoxifen; TEEs, thromboembolic events.
Survival analysis not accounting for menopausal status (including TAM women younger than 55 years old) showed an increased risk of CVE in AI patients [adjusted SHR 1.96 (95%CI 1.37–2.81)]. Nevertheless, the IPW analysis did not found differences between groups [HR 0.87 (95%CI 0.56–1.38)] (Supplementary Tables S2–5).
Discussion
In our cohort study, including 21,537 women with a breast cancer diagnosis treated in actual practice conditions, similar CVE and TEE risk was observed in both AI and TAM treatment groups. In addition, AI use seems to be a beneficial choice in terms of overall mortality reduction, with a >20% lower rate in AI users after adjusting for potential confounders.
A further analysis adjusting for IPW corroborated these findings but suggested a potential increase of PE associated with AI use.
In general, previous data on cardiovascular risk provided by RCT and meta-analyses, despite being heterogeneous, have identified a potential excess CVE risk associated with AI therapy, with slightly increasing odds of developing CVD, compared with patients receiving TAM therapy (OR 1.26, 95%CI = 1.10–1.43).16 In this regard, Abdel-Qadir and colleagues published a similar population-based study using routinely collected data from Canada that observed an increased risk of myocardial infarction in AI users (HR 2.02; 95%CI = 1.16–3.53), but, exploring a lower-risk subgroup of patients aged <74 years, with stage I–II breast cancer and no prior ischaemic heart disease, this excess risk was not detected (HR 1.53; 95%CI = 0.41–5.71, P = 0.53).17
In order to minimize imbalances when comparing treatment groups, we adjusted for a long list of potential confounders using propensity score equations, which is recommended in this type of study.18 An additional analysis adjusting for IPW was performed to correct for potential attrition bias, confirming that AI-treated women did not experience an increased risk of CVE compared with postmenopausal women in the TAM group. Consistent with this, other population studies selecting older women also reported similar incidences in stroke and several heart diseases between treatment groups.19,20 All together, these findings suggest that the increased risk detected in AI users compared with total TAM users is driven mainly by menopausal status.
In contrast with previous RCT findings,3 an increasing risk of PE was observed after IPW adjustment of our data: patients treated with AIs had twice the risk of PE compared with TAM users. However, this was a post hoc analysis based on a limited number of events, and needs further confirmation in external cohorts. Further research is needed to explore this potential association between AI treatment and PE risk.
As a side note, and similar to the results of Abdel-Qadir and colleagues,17 our supplemental survival analysis adjusted by computing risk of death including all TAM users (lower and older than 55 years old) suggested the AI group has nearly twice the risk of CVEs compared with the total TAM group, but later selection of postmenopausal women has shown similar hazard risks for all events in both treatment groups, proposing that menopause status is not a confounder but a potential interaction.
In addition to the lack of association between AI and CVE observed in our population, the significant OS in AI treated patients places these drugs in front to TAM in terms of cardiovascular safety and efficacy on recurrence incidence.21 It is noteworthy that selective oestrogen receptor modulators (SERMs) have been associated with higher proportion of adverse drug reaction (ADR) reports related to QT prolongation, Torsade de Pointes, and ventricular arrhythmias compared with AIs, in the European database of suspected ADR reports. Nonetheless, the overall number of these events was very small.22
One limitation of the study is that data of previous exposure to chemotherapy or radiotherapy were not available. In any case, these treatments are given independently of endocrine therapy election.23 Hence, potential toxic effects in heart would be allocated randomly among patients. In addition, we performed a sensitivity analysis using IPW to minimize the presence of a potential bias by indication due to other factors. Likewise, data of severity, grade and the clinical stage of breast cancer were not accessible. However, TAM and AI monotherapies are recommended and used mainly for hormone receptor-positive early breast cancer.24 In our study, we excluded patients with sequential therapies (TAM/AI) and further studies analysing outcomes in these patients might provide additional safety data.
Additionally, the SIDIAP data were collected during routine clinical practice (not by an expert researcher), potentially limiting the validity of coded outcomes. However, high accuracy in coding for all of the study outcomes was previously validated in the SIDIAP database.10
The main strength of the study is the sample size, almost 22,000 participants, reflecting real population conditions. Likewise, the SIDIAP dataset includes all treatment centres and has the potential to include all patients in the source population, increasing the external validity of our findings.
In summary, no difference in CVD was observed between postmenopausal AI and TAM users. Furthermore, AI users had >20% lower all-cause mortality, yielding a positive risk–benefit for long-term use of these therapies.
Supplemental Material
Supplemental material, 21-11-2019_Supplementary_data for Thromboembolic, cardiovascular and overall mortality risks of aromatase inhibitors, compared with tamoxifen treatment: an outpatient-register-based retrospective cohort study by Marta Pineda-Moncusí, Natalia Garcia-Giralt, Adolfo Diez-Perez, Ignasi Tusquets, Sonia Servitja, Joan Albanell, Daniel Prieto-Alhambra and Xavier Nogués in Therapeutic Advances in Medical Oncology
Acknowledgments
Authors Marta Pineda-Moncusí and Natalia Garcia-Giralt contributed equally to this work.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health. The research was supported by Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (grant number CB16/10/00245); Formación en Investigación en Salud grants (grant numbers PI13/00444, PI16/00818) from Instituto de Salud Carlos III; and European Regional Development Funds.
Conflict of interest statement: ADP reports grants from Amgen, personal fees from UCB, Mereo, Sandoz, Gilead, and other from Active Life Sci, outside the submitted work. DPA reports unrestricted research funding from UCB, Amgen, and Servier Laboratoires outside of the submitted work; DPA’s department has received fees for speaker services from Amgen outside of the submitted work. MPM, NGG, IT, SS, JA and XN have nothing to disclose.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Marta Pineda-Moncusí, IMIM (Hospital del Mar Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona, Spain.
Natalia Garcia-Giralt, IMIM (Hospital del Mar Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona, Spain.
Adolfo Diez-Perez, IMIM (Hospital del Mar Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona, Spain; Internal Medicine Department, Hospital del Mar, Department of Medicine Autonomous University of Barcelona, Spain.
Ignasi Tusquets, Cancer Research Program, IMIM (Hospital del Mar Research Institute), Barcelona, Spain.
Sonia Servitja, Cancer Research Program, IMIM (Hospital del Mar Research Institute), Barcelona, Spain.
Joan Albanell, Cancer Research Program, IMIM (Hospital del Mar Research Institute), Barcelona, Spain Medical Oncology Department, Hospital del Mar-CIBERONC, Barcelona, Spain Universitat Pompeu Fabra, Barcelona, Spain.
Daniel Prieto-Alhambra, Botnar Research Centre, Nuffield Orthopaedic Centre, Windmill Road, Headington, Oxford OX3 7LD, UK; Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, UK; Grup de Recerca en Malalties Prevalents de l’Aparell Locomotor (GREMPAL) Research Group and CIBERFes, University Institute for Primary Care Research (IDIAP) Jordi Gol, Universitat Autonoma de Barcelona and Instituto de Salud Carlos III, Barcelona, Spain.
Xavier Nogués, IMIM (Hospital del Mar Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona, Spain; Internal Medicine Department, Hospital del Mar, Department of Medicine Autonomous University of Barcelona, Spain.
References
- 1. Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010; 11: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 2. Regan MM, Neven P, Giobbie-Hurder A, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol 2011; 12: 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryden L, Heibert Arnlind M, Vitols S, et al. Aromatase inhibitors alone or sequentially combined with tamoxifen in postmenopausal early breast cancer compared with tamoxifen or placebo - Meta-analyses on efficacy and adverse events based on randomized clinical trials. Breast 2016; 26: 106–114. [DOI] [PubMed] [Google Scholar]
- 4. Group ATAC, Forbes JF, Cuzick J, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 2008; 9: 45–53. [DOI] [PubMed] [Google Scholar]
- 5. Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005; 366: 455–462. [DOI] [PubMed] [Google Scholar]
- 6. Foglietta J, Inno A, de Iuliis F, et al. Cardiotoxicity of aromatase inhibitors in breast cancer patients. Clin Breast Cancer 2017; 17: 11–17. [DOI] [PubMed] [Google Scholar]
- 7. Braithwaite RS, Chlebowski RT, Lau J, et al. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med 2003; 18: 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reyes C, Pottegard A, Schwarz P, et al. Real-life and RCT participants: alendronate users versus FITs’ trial eligibility criterion. Calcif Tissue Int 2016; 99: 243–249. [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Gil Mdel M, Hermosilla E, Prieto-Alhambra D, et al. Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP). Inform Prim Care 2011; 19: 135–145. [DOI] [PubMed] [Google Scholar]
- 10. Ramos R, Ballo E, Marrugat J, et al. Validity for use in research on vascular diseases of the SIDIAP (Information System for the Development of Research in Primary Care): the EMMA study. Rev Esp Cardiol 2012; 65: 29–37. [DOI] [PubMed] [Google Scholar]
- 11. Caro-Mendivelso J, Elorza-Ricart JM, Hermosilla E, et al. Associations between socioeconomic index and mortality in rural and urban small geographic areas of Catalonia, Spain: ecological study. J Epidemiol Res 2016; 2: 80–86. [Google Scholar]
- 12. Alexander LK, Lopes B, Ricchetti-Masterson K, et al. Calculating Person-Time. ERIC Notebook. 2nd ed. Chapel Hill: UNC, 2015, pp.1–3. [Google Scholar]
- 13. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016; 133: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubin DB. Underlying Bayesian Theory. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, 1987, pp. 75–112. [Google Scholar]
- 15. Mansournia MA, Altman DG. Inverse probability weighting. BMJ 2016; 352: i189. [DOI] [PubMed] [Google Scholar]
- 16. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 2011; 103: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 17. Abdel-Qadir H, Amir E, Fischer HD, et al. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Cancer 2016; 68: 11–21. [DOI] [PubMed] [Google Scholar]
- 18. Freemantle N, Marston L, Walters K, et al. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ 2013; 347: f6409. [DOI] [PubMed] [Google Scholar]
- 19. Ligibel JA, O’Malley AJ, Fisher M, et al. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat 2012; 131: 589–597. [DOI] [PubMed] [Google Scholar]
- 20. Haque R, Shi J, Schottinger JE, et al. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol 2016; 2: 1590–1597. [DOI] [PubMed] [Google Scholar]
- 21. Group EBCTC. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 22. Grouthier V, Lebrun-Vignes B, Glazer AM, et al. Increased long QT and torsade de pointes reporting on tamoxifen compared with aromatase inhibitors. Heart 2018; 104: 1859–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ayala de la Pena F, Andres R, Garcia-Saenz JA, et al. SEOM clinical guidelines in early stage breast cancer (2018). Clin Transl Oncol 2019; 21: 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO Clinical Practice Guideline Focused Update. 2019; 37: 423–438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 21-11-2019_Supplementary_data for Thromboembolic, cardiovascular and overall mortality risks of aromatase inhibitors, compared with tamoxifen treatment: an outpatient-register-based retrospective cohort study by Marta Pineda-Moncusí, Natalia Garcia-Giralt, Adolfo Diez-Perez, Ignasi Tusquets, Sonia Servitja, Joan Albanell, Daniel Prieto-Alhambra and Xavier Nogués in Therapeutic Advances in Medical Oncology