Abstract
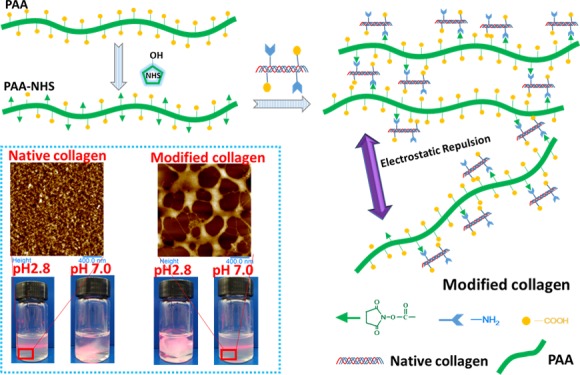
Because of poor water solubility and low thermostability, the application of collagen is limited seriously in fields such as injectable biomaterials and cosmetics. In order to overcome the two drawbacks simultaneously, a novel bifunctional modifier based on the esterification of polyacrylic acid (PAA) with N-hydroxysuccinimide (NHS) was prepared. The esterification degree of PAA-NHS esters was increased upon increasing the NHS dose, which was confirmed by Fourier-transform infrared (FTIR) and nuclear magnetic resonance spectrascopy. FTIR results indicated that the triple helix of the modified collagens remained integrated, whereas the molecular weight became larger, as reflected by the sodium dodecyl sulfate–polyacrylamide gel electrophoresis pattern. The modified collagens displayed excellent water solubility under neutral condition, owing to lower isoelectric point (3.1–4.3) than that of native collagen (7.1). Meanwhile, denaturation temperatures of the modified collagens were increased by 4.8–5.9 °C after modification. The modified collagen displayed hierarchical microstructures, as reflected by field-emission scanning electron microscopy, while atomic force microscopy further revealed a “fishing net-like” network in the nanoscale, reflecting a unique aggregation behavior of collagen macromolecules after modification. As a whole, the PAA-NHS ester as a bifunctional modifier endowed collagen with desired water solubility and thermostability in a conflict-free manner, which was beneficial to the process and application of the water-soluble collagen.
Introduction
Collagen, the main structural protein accounting for approximately one-third of all vertebrate body protein, is typically found in fibrous tissues such as tendons, skin, cartilages, bones, corneas, blood vessels, and ligaments.1,2 Nowadays, collagen is regarded as one of the most useful biomaterials as exemplified by current biomedical application such as surgery, prosthetic systems, pharmacology, drug formulation, and delivery3,4 due to excellent biocompatibility, safe biological characteristics, high mechanical strength, and weak antigenicity.5−7
Generally, native collagen has an isoelectric point (pI) near neutral pH, which means that it has poor water solubility under physiological conditions,8,9 resulting in the restrictions of application when used in the format of solution. In order to dissolve collagen into water to obtain a clear solution, traditionally anhydride was used as a modifier to decrease the pI via the introduction of carboxyl groups to collagen molecules. Another defect of native collagen is the weak thermostability (native collagen would be denatured at a temperature near 38 °C which is reported previously10). For the sake of strengthening the structural stability of collagen, a mass of methods include physical treating such as UV-light irradiation11,12 and chemicals or enzymes such as glutaraldehyde,13 formaldehyde,14 hexamethylene diisocyanate,15 carbodiimide/N-hydroxysuccinimide (NHS),16 tannin,17 genipin,18 transglutaminase,19 and so forth have been exploited to form the cross-linking among collagen molecules. However, acylation modification competes with cross-linking modification because both of them consume ε-amino groups of the collagen side chain in the course of the modification process.20 To avoid this contradiction, polycarboxylic acid with abundant free carboxyl groups has been used as a kind of a desired bifunctional modifier candidate. Specially, a collagen modifier can be prepared through the reaction between the carboxyl groups of polycarboxylic acid and hydroxyl groups of NHS to gain NHS-activated esters, which can react with the amino groups of collagen to endow improved water solubility and thermostability on collagen simultaneously.
Previously, we reported NHS-activated γ-polyglutamic acid esters (γ-PGA-NHS esters)9 and NHS-activated hyaluronic acid esters (HA-NHS esters)21 as a kind of bifunctional modifiers for collagen. Nevertheless, both γ-PGA (∼1000 kDa, respectively) and HA (∼24 kDa) had a molecular weight (MW) close to or even lower than that of native collagen (300 kDa). In these systems, collagen molecules were adjacent to each other, leading to the local attracting effect which might cause a barrier for a desired dissolution in neutral pH. Herein, we proposed that a polycarboxylic acid with a molecular weight much larger than that of native collagen could be a feasible candidate as a novel modifier to prepare water-soluble collagen. As a linear-chain polymer, polyacrylic acid (PAA) has a lot of carboxyl groups on the side chain just like γ-PGA or HA. Nevertheless, PAA used in our work possessed a high MW of up to 30,000 kDa, which is about 100 times higher than that of collagen. In recent years, PAA has been used as potential biomaterials because of its unique structure/function and nontoxicity.22 For instance, Gomaa23 prepared a PAA-functionalized quantum dot conjugates which was applied to detect biomolecules. It was shown that the conjugates exhibited nontoxicity and were biocompatible; Ghavamzadeh24 reported the hydrogels consisted of gelatin and PAA as biological glue for soft tissues, with an increased bonding strength and reduced gelatin time. Attractively, no sign of toxicity was observed in in vitro assays for the hydrogels. To the best of our knowledge, PAA or its derivatives were rarely reported in the modification of collagen.
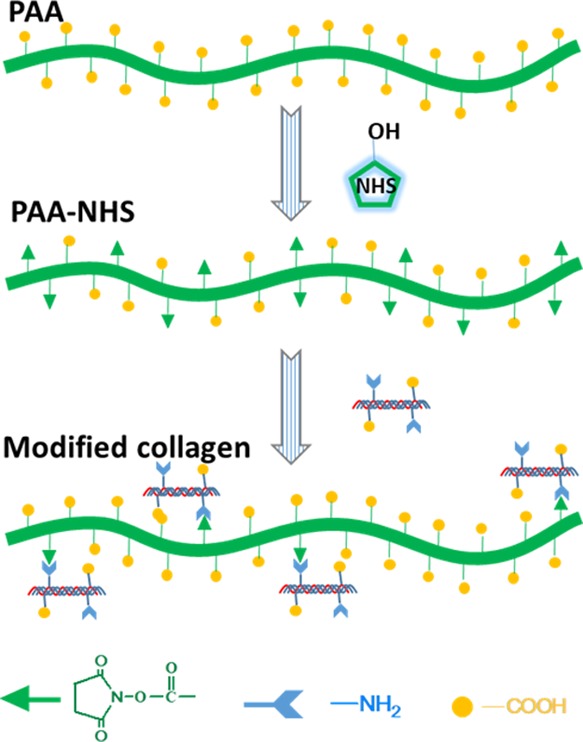
In this work, PAA-NHS esters were prepared and the structure was characterized by Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR). Afterward, the native collagen was modified using PAA-NHS esters to fabricate modified collagens, which were characterized by a series of methods such as FTIR, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), pI measurements, differential scanning calorimetry (DSC), field-emission scanning electron microscopy (FESEM), and atomic force microscopy (AFM). The synthesis process of the PAA-NHS ester and its reaction mechanism with collagen macromolecules are illustrated in Scheme 1. The results obtained confirmed that PAA-NHS esters as a novel bifunctional modifier endowed collagen with good water solubility and high thermostability simultaneously, which would broaden the application range of collagen.
Scheme 1. Schematic Illustrating the Synthesis of the PAA-NHS Ester and its Reaction Mechanism with Collagen Macromolecules.
Results and Discussion
Structural Characterizations of PAA-NHS Esters
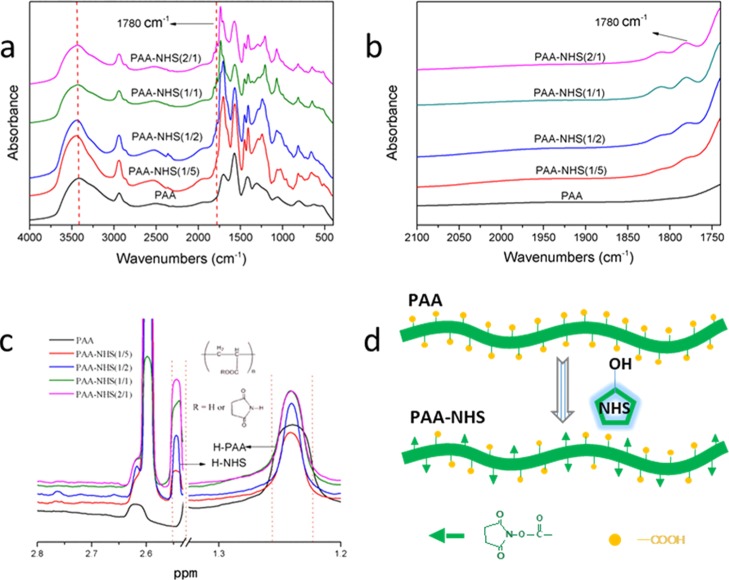
The FTIR spectra of PAA-NHS esters could be correlated to the backbone conformation. Figure 1a shows the FTIR spectra of neat PAA and PAA-NHS esters. PAA shows a strong and broad band at approximately 3415 cm–1 which was assigned to the stretching vibrations of O–H groups, while the stretching vibrations of C–H groups was found at about 2940 cm–1.25 The absorption bands at ∼ 1707, 1573, and 1421 cm–1 could be attributed to C=O, COO–, and CH2,26 respectively. Nevertheless, after esterification, the peak position of the stretching vibrations of O–H groups transfers to higher wavenumber at 3445 cm–1, which indicated that the intensity of the hydrogen interaction of the PAA-NHS ester became weaker. The peaks of PAA-NHS esters at about 1240 and 1450 cm–1 could be attributed to the vibration of C–N and CH2, respectively.9,27 Meanwhile, the new peak at 1780 cm–1 assigned to the ester group was observed clearly from Figure 1b.
Figure 1.
FTIR spectra of PAA and PAA-NHS esters in the range of 4000–400 cm–1 (a); FTIR spectra of PAA and PAA-NHS esters in the range of 2100–1720 cm–1 (b); 1H NMR spectra of PAA and PAA-NHS esters (c); and the synthesis mechanism of PAA-NHS ester (d).
1H NMR was further used as a quantitative method to estimate the molecular structure and the esterification degree of PAA-NHS esters. Figure 1c shows the 1H NMR spectra of PAA and PAA-NHS esters. The peak at 1.24 ppm was attributed to the methylene protons of PAA,28 while the peak of the protons of NHS was at about 2.60 ppm29 which verified that the PAA-NHS esters were prepared successfully. Positively correlated between the NHS/COOH molar ratios of 1/5, 1/2, 1/1, and 2/1, the esterification degrees of PAA-NHS esters were calculated to be 11.1 ± 0.2%, 18.1 ± 0.3%, 31.2 ± 0.1%, 39.2 ± 0.1%, respectively. Figure 1d displays the synthesis mechanism of the PAA-NHS ester via partial esterification of carboxyl groups of PAA with NHS.
Molecular Structure of Collagens
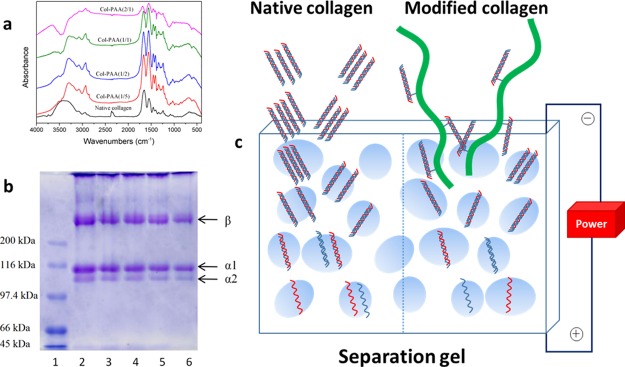
Figure 2a shows the FTIR spectra of the collagens. The peak at about 3400 cm–1 was mainly associated with the N–H stretching vibrations relating to amide A.30 The amide I band at about 1650 cm–1 was dominantly associated to the stretching vibrations of peptide C=O groups, the amide II band located at about 1550 cm–1 was due to the bending vibrations of N–H groups, and the amide III band at approximately 1240 cm–1 could be assigned to the stretching vibration of CH2.10 The spectra of collagens modified with PAA-NHS esters were similar to that of native collagen. However, blue shifts of the amide I band of modified collagens could be detected (from 1652 to 1692 cm–1 for Col-PAA(2/1)), and the position at 3400 cm–1 red-shifted to a lower frequency (3300 cm–1 for Col-PAA(2/1)). The changes on the bands could be ascribed to the enhanced hydrogen bond interaction among PAA-NHS-modified collagen molecules.30 Furthermore, the intensity ratio of amide III/1450 cm–1, which was regarded as an index to evaluate the structural integrity of collagen,31 was in the range of 0.85–0.90 for the native collagen and the collagens modified with PAA-NHS esters, indicating that the triple helix structure of modified collagens was not altered significantly as gelatin, which was the denatured collagen product, possessed a quite lower ratio of ∼0.6.32 Therefore, the FTIR results indicated that the PAA-NHS ester just played a role as a harmless modifier of collagen without damaging the structural integrity.
Figure 2.
FTIR spectra of the native collagen and collagens modified with PAA-NHS esters (a); SDS-PAGE of native collagen and collagens modified with PAA-NHS esters (b): lane (1) protein marker; (2) native collagen; (3) collagen modified with PAA-NHS(1/5); (4) collagen modified with PAA-NHS(1/2); (5) collagen modified with PAA-NHS(1/1); and (6) collagen modified with PAA-NHS(2/1); schematic illustrating the permeation of the native collagen and the modified collagen molecules through the micropores of electrophoresis gel (c).
The SDS-PAGE patterns of the collagens are shown in the Figure 2b. As we know, the native type I collagen monomer comprises two identical α1(I) as well as one α2 (I) chains, and each chain in this heterotrimer has a MW of ∼95 kDa.33 Just similar to previous reports,9,34 the traits of all collagens displayed the typical electrophoretic characteristic of type I collagen. In addition, there was no extra band with lower molecular weight on the gels, indicating that modified collagens retained the intact triple helix structure. Nevertheless, a weaker color of the bands for modified samples compared with those of the native collagen could be observed, which could be attributed to the increased MW of modified collagens, hindering the osmosis of collagen molecules when getting through the micropore of the polyacrylamide gel,35 as illustrated in Figure 2c. A comparison of the relative migration rate of the β component (higher-molecular-weight component consists of two α chains), as well as the relative band intensity between β component and α chains are presented in Table 1, from which a clear trend indicating greater migration rate of β, as well as the increase of β/α intensity ratios could be observed upon increasing the esterification degrees of PAA-NHS esters, testified the increase of the MW of collagen due to the modification.
Table 1. Relative Migration Rate and Intensity of the SDS-PAGE Bands.
| samples | relative migration rate of β chain (%) | intensity ratio of β/(α1 + α2) (%) |
|---|---|---|
| native collagen | 100 | 53.2 |
| Col-PAA(1/5) | 98.96 | 64.4 |
| Col-PAA(1/2) | 97.52 | 67.1 |
| Col-PAA(1/1) | 96.99 | 79.8 |
| Col-PAA(2/1) | 95.69 | 88.6 |
Influence of PAA-NHS Esters on the Water Solubility of Collagens
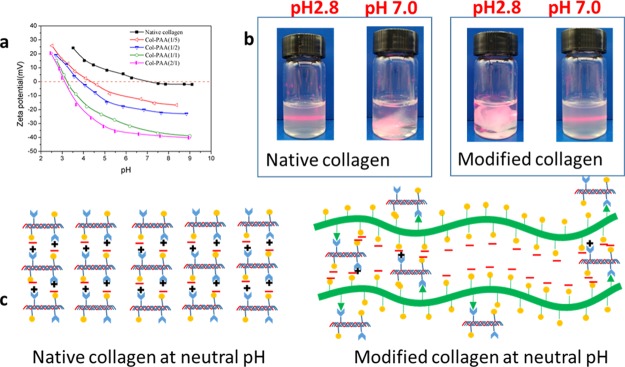
For proteins, the pI is an important parameter, reflecting the relative proportion of acid/basic amino residues.10 When the ampholyte does not migrate in an electric field (the zeta potential is zero), the pH of the solution or suspension is the pI of the ampholyte.36 It is well known that collagen could not be dissolved in aqueous solutions with a pH value close to its pI.37Figure 3a shows the zeta potential curves of all collagens. Under the same pH, the electric potential was lower upon increasing the esterification degree of PAA-NHS esters. According to the curves, the pI value of the native collagen was 7.1 ±0.2, which was similar to the previous result.38 Whereas, upon increasing the esterification degree, the pI values of the modified collagens decreased remarkably to 4.3 ± 0.2, 3.8 ± 0.2, 3.2 ± 0.1, and 3.1 ± 0.1, which indicated that a significantly improved water solubility was endowed by the modification on collagen with PAA-NHS esters. As shown in Figure 3b, the native collagen could be dissolved in acetic acidic solution and could not be dissolved in neutral phosphate-buffered saline (PBS) solution; nevertheless, the modified collagens had a dissolving capacity in contrast to that of native collagen in acetic acidic solution and neutral PBS solution. This result was well consistent with the analysis of pI measurements. Figure 3c illustrated the mechanism on the improved water solubility of modified collagen. In the case of the native collagen, nearly equal ionization occurred for both of the acid/basic amino groups at neutral pH; thus the compactly assembled collagen molecules could not be separated by water molecules. The reaction between ester groups of PAA-NHS esters and the amino groups of collagen directly resulted in the consumption of free amino groups. Furthermore, a lot of free carboxyl groups of PAA-NHS esters were introduced to the modified collagen molecules. When in neutral solution, the modified collagen molecules could be readily separated by the strong electrostatic repulsion between ionized carboxyl groups, leading to the desired dissolution capacity of collagen. On the contrary, the modified collagen molecules could not be segregated in acetic acidic solution because it was difficult for ionization of the carboxyl group at acidic pH.
Figure 3.
Zeta potential of native collagen and collagens modified with PAA-NHS esters (a); digital photos for the solubility of the native collagen and modified collagen in PBS (pH = 7.0) and acetic acid solution (pH = 2.8), respectively (b); schematic illustrating the insolubility and solubility of the native collagen and the modified collagen in neutral aqueous solution (c).
Thermal Stability of Collagens
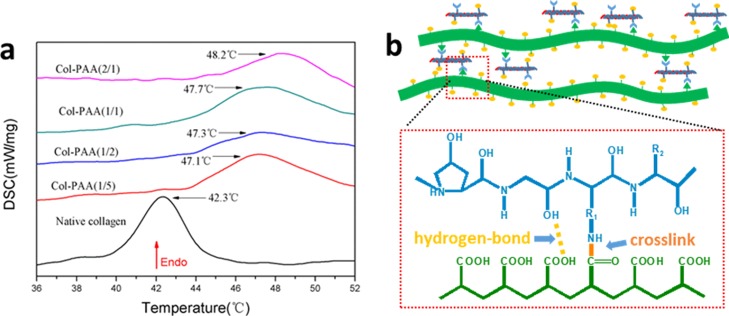
The DSC thermograms of collagens are shown in Figure 4a. During temperature programming, the triple helix structure of collagen molecules shifts into a disorganized form due to the destruction of the interchain hydrogen bonding which stabilizes the triplex of collagen.39,40 In the DSC method, the endothermic peaks were associated with the above-mentioned transformation. The denaturation temperature (Td) of collagens modified with PAA-NHS esters increased by 4.8–5.9 °C compared with that of native collagen (42.3 ± 0.1 °C). Moreover, the Td of modified collagen increased upon increasing the esterification degree of PAA-NHS esters, which was related to more crosslinking points between collagen and PAA. It should be pointed out that the pre-existing traditional water-soluble collagen prepared with succinic anhydride as the modifier possessed Td a few degrees lower than that of the native collagen.41 The changes in Td of collagen solutions modified with various chemicals are shown in Table 2. It could be detected that collagen modified with PAA-NHS possessed denaturation temperature not only higher than those of traditional modifiers such as succinic anhydride and glutaraldehyde listed in this table but also superior to that modified by adipic acid-NHS, which was an NHS-activated small molecule modifier of collagen previously reported.42 As indicated in Figure 4b, the superior thermal stability of collagen modified with PAA-NHS was attributed to the stable chemical cross-linking among modified collagen molecules, which formed the stable amide bonds between the activated ester groups of PAA-NHS and amino groups of collagen, plus the hydrogen-bond interaction between the non-ionized carboxyl groups of PAA and the hydroxyl, amino, and carboxyl groups of collagen macromolecules. The improved thermal stability was quite beneficial to the process and application of this water-soluble collagen. For example, it was favorable for the usage of the cosmetics containing collagen in summer.
Figure 4.
DSC thermograms of the native collagen and collagens modified with PAA-NHS esters (a); schematic illustrating the improved thermostability of collagen by PAA-NHS esters (b).
Table 2. Variation on the Td of Collagen Solutions Modified with Various Chemicals.
Micro–Nano Structure and Aggregation Morphology of Collagens
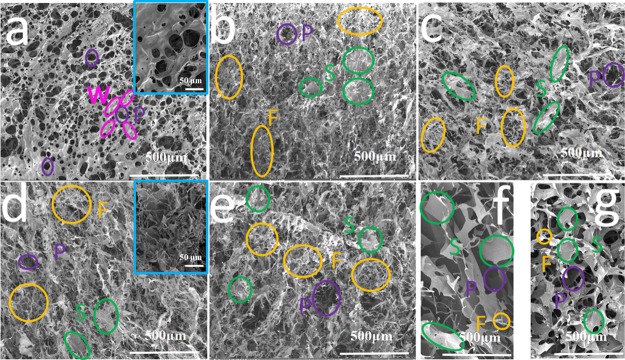
Figure 5 displays the visualized morphologies of the lyophilized collagens observed via FESEM. All of the sponges possessed numerous pores, which reflected a superior permeability. The porous morphology could be ascribed to the sublimation of the ice crystal in the frozen hydrogels during the lyophilization process. As shown in Figure 5a, walls around the pores were observed to be the dominant sector of the sponges for native collagen. In addition, it seemed that the native collagen exhibited a relatively homogeneous morphology, which was due to the isotropous aggregation of collagen molecules. As for modified collagens (Figure 5b–e), however, all of them showed hierarchical pores, which was believed to be helpful for cell proliferation when used as scaffolds.43 In addition, the sponges consisted of abundant fibrils and sheets with different sizes but not walls. These morphologies suggested the polydispersity of the aggregates of the modified collagens in solution. The differences in the microstructure could be observed more clearly in the enlarged images (the insert of Figure 5a,d). As a control, the neat PAA (Figure 5f) and PAA-NHS ester (Figure 5g) displayed a homogeneous three-dimensional morphology, suggesting their uniform aggregation state in solution. Therefore, it seemed that the hierarchical morphology of the modified collagen was in relevance of the interactions between collagen and the PAA-NHS ester.
Figure 5.
FESEM images of the surface of freeze-dried samples of native collagen (a); Col-PAA(1/5) (b); Col-PAA(1/2) (c); Col-PAA(1/1) (d); Col-PAA(2/1) (e); the neat PAA (f); and PAA-NHS(1/2) ester (g). Bars indicate 500 μm in images (a–g) and 50 μm in the insets of (a,d). (P-pores; W-wall; F-fibrils; S-sheets).
AFM possessed some advantages in exploring the microscopic aggregation state of the biomacromolecule, as very dilute solutions could be applied during sample preparation. Here, 20 μg/mL of the sample solution was added dropwise to the mica prior to air drying, and then collagen macromolecules assembled each other to form aggregates which could be observed using AFM. The AFM images of native collagen and collagen modified with PAA-NHS(2/1) are shown in the Figure 6. The AFM images of the native collagen (Figure 6a1) exhibited a homogeneous fibrillar structure with a lot of microfibrils overlapped each other, in accordance with the previous results.44,45 Nevertheless, the modified collagen sample (Figure 6b1) displayed a microscopic morphology distinct from that of the native one; that is, each radial sheet with lager size (a few hundreds of nanometers) was interconnected by several nanofibrils to form a heterogeneous network. A similar morphology could be observed from sample solutions with a higher collagen concentration (400 μg/mL), although it seemed that the aggregates possessed a larger size (Figure 6a2, b2). The results from AFM further verified the speculation that the uniform and isotropous aggregation could be formed among the native collagen molecules in acidic solution as expected (Figure 6a3), while the aggregation behavior of the modified collagens was deeply influenced by the introduction of a PAA backbone and the newly generated cross-linking (Figure 6b3). In brief, most of the modified collagens could assemble to form sheets with size larger than that of the native collagen due to both of the high molecular weight (100 times that of collagen) of PAA and the covalent bond formation between PAA-NHS and collagen. The sheets were interlinked with each other by nanofibrils (the aggregates in a quite small size) to generate the “fishing net-like” nanostructure.
Figure 6.
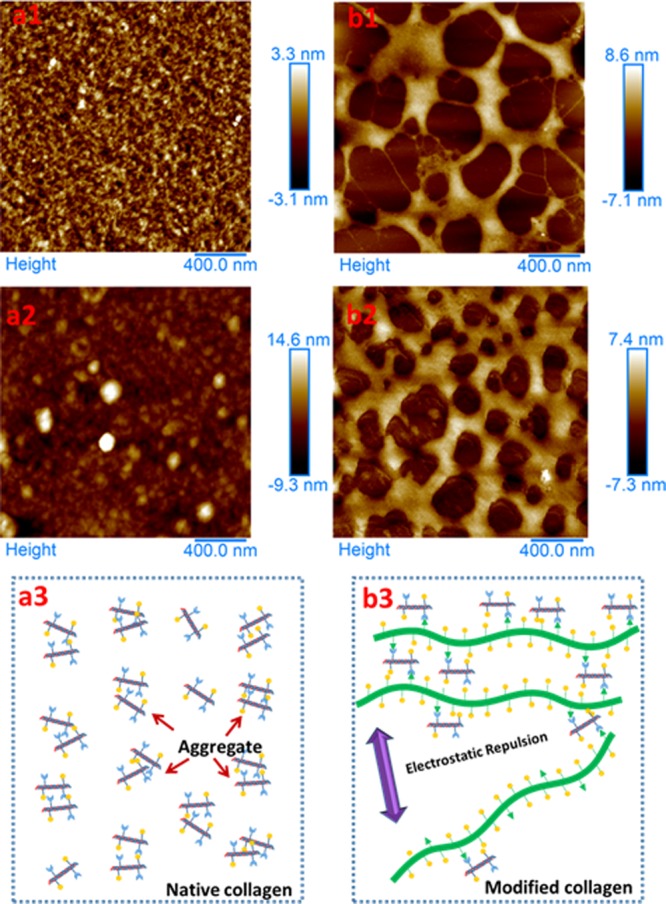
AFM images of aqueous native collagen with concentration of 20 (a1) and 400 μg/mL (a2) and aqueous Col-PAA(2/1) with concentration of 20 (b1) and 400 μg/mL (b2); schematic illustrating the aggregation behavior of the native collagen (a3) and the modified collagen (b3). Bars in images indicate 400 nm.
Conclusions
In summary, we reported the facile fabrication of water-soluble collagen using an NHS-esterified derivative of high molecular weight PAA (PAA-NHS ester) as a novel bifunctional modifier. Such modified collagen designs provided unique advantages compared with the established strategies such as succinylation modification, including intact secondary structures, facile water solubility in water at neutral pH, improved thermostability, and the hierarchical microstructures/“fishing net-like” nanostructure simultaneously. Note that PAA possessed an ultra-high molecular weight (100 times that of collagen), a plenty of negative charge could be introduced when PAA was linked with collagen via the amide bond formation between the amino groups of collagen and the ester groups of PAA-NHS esters. We believed that this work could stimulate new opportunities in the facile preparation of polyanions but not limited to the water-soluble collagen, using the PAA-NHS ester as a novel modifier for a wide range of applications.
Materials and Methods
Materials
Collagen was extracted from calf hide referring to the previous method.46 Calf split was swollen in 0.5 mol/L acetic acid (HAc) and then treated with pepsin (0.3%, w/w) for 72 h. After enzyme treatment, calf pieces were centrifuged and salted by sodium chloride (NaCl). Afterward, crude collagen was dissolved and dialyzed against 0.1 mol/L HAc for 72 h. Finally, the depurated collagen sponge was obtained via lyophilization and stored until use. PAA sodium salt (PAA-Na) with MW of ∼30,000 kDa was provided by Sinopharm Chemical Reagent Co., Ltd. (China). Protein marker (6.6–200 kDa), NHS, EDC, dimethyl sulfoxide (DMSO), ethanol, and n-hexane were purchased from Aladdin Co., Ltd. (China). Deionized water was used for all the experiments.
Synthesis of PAA-NHS Esters
First, PAA was prepared through the dialysis of PAA-Na in HCl solution (1 × 10–4 mol/L). Then, the pretreated PAA was placed into DMSO and was allowed to stand in the solution for 24 h with stirring. After PAA was dissolved, NHS and EDC were added (the molar ratios of NHS/COOH were 1/5, 1/2, 1/1, and 2/1; the constant molar ratio of EDC/COOH was 1/1). When reaction finished, the mixed solution was precipitated with ethanol and n-hexane for three times. Ultimately, the products were dried at vacuum and stored at room temperature until use. The activated esters were denoted as PAA-NHS(1/5), PAA-NHS(1/2), PAA-NHS(1/1), and PAA-NHS(2/1), respectively.
Characterization of PAA-NHS Esters
PAA or PAA-NHS esters were prepared as pellets via tableting with potassium bromide (KBr). The FTIR spectra were recorded with a Thermo Fisher Scientific instrument (Nicolet IS10, USA). All spectra were obtained at the resolution of 4 cm–1 in the wavenumber region of 4000–400 cm–1, and each spectra pot represented the average of 64 scans.
1H NMR spectra were further applied to determine the esterification degree of PAA-NHS esters. First, samples were put into a 2 mL reaction tube. After the addition of deuterated D2O or deuterated DMSO, these tubes vibrated and were capped. The tube was analyzed by a Bruck instrument (ADVANCE III 500, Germany) under 500 MHz. The esterification extent was calculated based on the relative peaks area, corresponding to the protons of NHS and those binding to the carbon atom of PAA, respectively.
Modification of Collagen with PAA-NHS Esters
First, the lyophilized collagen was dissolved into 1 × 10–3 mol/L HCl solution to 5 mg/mL, followed by adjusting the pH to 9 with 1 mol/L NaOH. Then, PAA-NHS esters were dissolved in DMSO to gain a concentration of 50 mg/mL, which was added dropwise into collagen solution with stirring subsequently (the mass ratio of PAA-NHS ester to collagen was 1/2). The pH value of collagen solution was maintained at 9 with the supplement of 1 mol/L NaOH during the reaction. After 24 h, the rough-wrought modified collagen was dialyzed against deionized (DI) water for 72 h. Finally, the modified collagens were freeze-dried and stored until use. For short, collagens modified with PAA-NHS(1/5), PAA-NHS(1/2), PAA-NHS(1/1), and PAA-NHS(2/1) were named Col-PAA(1/5), Col-PAA(1/2), Col-PAA(1/1) and Col-PAA(2/1), respectively.
FTIR Analysis of Collagen
The FTIR spectra of collagens were recorded according to the method similar to the section “Characterization of PAA-NHS Esters”.
SDS-PAGE Pattern
The SDS-PAGE patterns of collagens were analyzed according to the method of Lamemmli.47 First, collagens were mixed with the sample buffer and boiled for 10 min and then were loaded on 2.5% stacking gel and 7.5% running gel in a vertical slab. At a constant current of 12 mA, SDS-PAGE was performed and subjected to electrophoresis, running on a Mini Protein II unit. When the indicatrix reached the bottom, the gel was dyed with coomassie brilliant blue for 45 min and faded with methanol/HAc. A protein marker with the MW in the range of 6.6–200 kDa was used as the standard. Gel images were analyzed with ImageJ 1.42 software (developed by Wayne Rasband, National Institutes of Health, U.S.A.).
pI Measurements
Collagen sponges of the native and modified collagens were dissolved in DI water or dilute HCl solution to reach a final concentration of 1 mg/mL, and then the zeta potential values were recorded by a Malvern Zeta potential analyzer (Zetasizer Nano ZS90, UK). After the collagen solution was transferred to a capillary cell, the pH value was adjusted using 0.1 mol/L HCl or 0.1 mol/L NaOH. The zeta potential of each solution was measured in quintuplicates.
DSC Measurements
The thermostability of collagens was evaluated by DSC (Netzsch DSC 200PC, Germany). The native and modified collagens with a concentration of 10 mg/mL was prepared by dissolving lyophilized samples in 0.1 mol/L HAc and 0.2 mol/L PBS, respectively. The collagen solutions were weighted and put into aluminium pans with covers, followed by scanning from 36 to 52 °C with the heating rate of 2 °C/min in a nitrogen atmosphere. Pan with 0.1 mol/L HAc or 0.2 mol/L PBS (pH 7.0) was used as the reference. The denaturation temperature (Td) values were the mean value from three replicates.
FESEM Observations
The morphology of collagens was observed with a Navo FEI field-emission electron microscope (NanoSEM 230, USA). Lyophilized collagen sponges were mounted on stubs, sputter-coated with gold and then observed at various magnifications. The morphology was collected at five different points for each sample.
AFM Observations
AFM was used to reveal the microstructure change of collagen fibrils in the presence of PAA. First, the native collagen and modified collagens were dissolved in 0.1 mol/L acetic acid or 0.2 mol/L PBS (pH 7.0) to 20 or 400 μg/mL. After dissolution completely, 20 μL of collagen solution was added dropwise onto a fresh mica and dried for 12 h at room temperature. Afterward, the mica slice was applied on a Bruker AFM (Multimode 8, Germany) for observations.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant no. 21606046), the Fund for University Leading Talents Plan (grant no. 660150010), the Fund for Distinguished Young Scientific Research Talents Plan in Universities of Fujian Province (grant no. KLA18064A), the Natural Science Foundation of Fujian Province (grant no. 2019J05044), and the Special Fund for Science and Technology Innovation of FAFU (grant no. KFA17217A).
The authors declare no competing financial interest.
References
- Mu C.; Liu F.; Cheng Q.; Li H.; Wu B.; Zhang G.; Lin W. Collagen cryogel cross-linked by dialdehyde Starch. Macromol. Mater. Eng. 2010, 295, 100–107. 10.1002/mame.200900292. [DOI] [Google Scholar]
- Yang Q.; Guo C.; Deng F.; Ding C.; Yang J.; Wu H.; Ni Y.; Huang L.; Chen L.; Zhang M. Fabrication of highly concentrated collagens using cooled urea/HAc as novel binary solvent. J. Mol. Liq. 2019, 291, 111304. 10.1016/j.molliq.2019.111304. [DOI] [Google Scholar]
- Lee C. H.; Singla A.; Lee Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- Meena C.; Mengi S. A.; Deshpande S. G. Biomedical and industrial applications of collagen. Proc. Indian Acad. Sci. Chem. Sci. 1999, 111, 319–329. [Google Scholar]
- Bazrafshan Z.; Stylios G. K. Spinnability of collagen as a biomimetic material: A review. Int. J. Biol. Macromol. 2019, 129, 693–705. 10.1016/j.ijbiomac.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Liu W.; Li D.; Li G. Physicochemical properties of succinylated calfskin pepsin-solubilized collagen. Biosc. Biotech. Biochem. 2007, 71, 2057–2060. 10.1271/bbb.70055. [DOI] [PubMed] [Google Scholar]
- Sorushanova A.; Delgado L. M.; Wu Z.; Shologu N.; Kshirsagar A.; Raghunath R.; Mullen A. M.; Bayon Y.; Pandit A.; Raghunath M.; Zeugolis D. I. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. 10.1002/adma.201801651. [DOI] [PubMed] [Google Scholar]
- He L.; Mu C.; Shi J.; Zhang Q.; Shi B.; Lin W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. 10.1016/j.ijbiomac.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Yang J.; Ding C.; Huang L.; Zhang M.; Chen L. The preparation of poly(γ-glutamic acid)-NHS ester as a natural cross-linking agent of collagen. Int. J. Biol. Macromol. 2017, 97, 1–7. 10.1016/j.ijbiomac.2016.12.070. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Yang J.; Ding C.; Huang L.; Chen L. A novel strategy to fabricate water-soluble collagen using poly(γ-glutamic acid)-derivatives as dual-functional modifier. React. Funct. Polym. 2018, 122, 131–139. 10.1016/j.reactfunctpolym.2017.11.012. [DOI] [Google Scholar]
- Sionkowska A. Modification of collagen films by ultraviolet irradiation. Polym. Degrad. Stabil. 2000, 68, 147–151. 10.1016/s0141-3910(99)00176-7. [DOI] [Google Scholar]
- Sionkowska A.; Kozłowska J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. 10.1016/j.ijbiomac.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Huang G. P.; Shanmugasundaram S.; Masih P.; Pandya D.; Amara S.; Collins G.; Arinzeh T. L. An investigation of common crosslinking agents on the stability of electrospun collagen scaffolds. J. Biomed. Mater. Res. 2015, 103, 762–771. 10.1002/jbm.a.35222. [DOI] [PubMed] [Google Scholar]
- Hawkins C. L.; Davies M. J. Generation and propagation of radical reactions on proteins. Biochim. Biophys. Acta 2001, 1504, 196–219. 10.1016/s0005-2728(00)00252-8. [DOI] [PubMed] [Google Scholar]
- van Luyn M. J. A.; van Wachem P. B.; Damink L. O.; Dijkstra P. J.; Feijen J.; Nieuwenhuis P. Relations between in vitro cytotoxicity and crosslinked dermal sheep collagens. J. Biomed. Mater. Res. 1992, 26, 1091–1110. 10.1002/jbm.820260810. [DOI] [PubMed] [Google Scholar]
- Kozłowska J.; Sionkowska A. Effects of different crosslinking methods on the properties of collagen-calcium phosphate composite materials. Int. J. Biol. Macromol. 2015, 74, 397–403. 10.1016/j.ijbiomac.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Vidal C. M. P.; Leme A. A.; Aguiar T. R.; Phansalkar R.; Nam J.-W.; Bisson J.; Mcalpine J. B.; Chen S.-N.; Pauli G. F.; Bedran-Russo A. Mimicking the hierarchical functions of dentin collagen cross-links with plant derived phenols and phenolic acids. Langmuir 2014, 30, 14887–14893. 10.1021/la5034383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyam A.; Subramanian G. S.; Raghunath M.; Pandit A.; Zeugolis D. I. In vitro evaluation of Ficoll-enriched and genipin-stabilised collagen scaffolds. J. Regen. Med. Tissue Eng. 2014, 8, 233–241. 10.1002/term.1522. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Zheng M.; Liu X.; Zhang S.; Wang X.; Chen Y.; Hou M.; Zhu J. Feasibility Study of Tissue Transglutaminase for Self-Catalytic Cross-Linking of Self-Assembled Collagen Fibril Hydrogel and Its Promising Application in Wound Healing Promotion. ACS Omega 2019, 4, 12606–12615. 10.1021/acsomega.9b01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petite H.; Rault I.; Huc A.; Menasche P.; Herbage D. Use of the acyl azide method for cross-linking collagen-rich tissues such as pericardium. J. Biomed. Mater. Res. 1990, 24, 179–187. 10.1002/jbm.820240205. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Yang J.; Deng F.; Guo C.; Yang Q.; Wu H.; Ni Y.; Huang L.; Chen L.; Ding C. Dual-functionalized hyaluronic acid as a facile modifier to prepare polyanionic collagen. Carbohydr. Polym. 2019, 215, 358–365. 10.1016/j.carbpol.2019.03.086. [DOI] [PubMed] [Google Scholar]
- Jing X.; Mi H.-Y.; Peng X.-F.; Turng L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 2018, 136, 63–72. 10.1016/j.carbon.2018.04.065. [DOI] [Google Scholar]
- Gomaa O. M.; Okasha A.; Hosni H. M.; El-Hag Ali A. Biocompatible water soluble pot conjugates for biomolecule detection. J. Fluoresc. 2018, 28, 41–49. 10.1007/s10895-017-2158-3. [DOI] [PubMed] [Google Scholar]
- Ghavamzadeh R.; Haddadi-Asl V.; Mirzadeh H. Bioadhesion and biocompatibility evaluations of gelatin and polyacrylic acid as a crosslinked hydrogel in vitro. J. Biomater. Sci. Polym. Ed. 2004, 15, 1019–1031. 10.1163/1568562041526478. [DOI] [PubMed] [Google Scholar]
- Kirwan L. J.; Fawell P. D.; van Bronswijk W. In situ FTIR-ATR examination of poly (acrylic acid) adsorbed onto hematite at low pH. Langmuir 2003, 19, 5802–5807. 10.1021/la027012d. [DOI] [PubMed] [Google Scholar]
- Cárdenas G.; Muñoz C.; Carbacho H. Thermal properties and TGA–FTIR studies of polyacrylic and polymethacrylic acid doped with metal clusters. Eur. Polym. J. 2000, 36, 1091–1099. 10.1016/s0014-3057(99)00187-1. [DOI] [Google Scholar]
- Mckittrick P. T.; Katon J. E. Infrared and raman group frequencies of cyclic imide. Appl. Spectrosc. 1990, 44, 812–817. 10.1366/0003702904087000. [DOI] [Google Scholar]
- Yanjie C.; Jiangong Z.; Fengqi L.; Zhaorang H. Synthesis of Poly (methyl acrylate) by SET-LRP Method and ATRP Method. J. Jilin Univ. 2018, 56, 173–177. [Google Scholar]
- Zhang H.Research Progress in Synthesis of N-hydroxysuccinimide. Acetaldehyde Acetic Acid Chemical Industry, 2014; Vol. 1, pp 21–24.
- Madhan B.; Subramanian V.; Rao J. R.; Nair B. U.; Ramasami T. Stabilization of collagen using plant polyphenol: Role of catechin. Int. J. Biol. Macromol. 2005, 37, 47–53. 10.1016/j.ijbiomac.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Liu X.; Dan N.; Dan W. Preparation and characterization of an advanced collagen aggregate from porcine acellular dermal matrix. Int. J. Biol. Macromol. 2016, 88, 179–188. 10.1016/j.ijbiomac.2016.03.066. [DOI] [PubMed] [Google Scholar]
- Figueiro S.; Goes J.; Moreira R.; Sombra A. On the physico-chemical and dielectric properties of glutaraldehyde crosslinked galactomannan–collagen films. Carbohydr. Polym. 2004, 56, 313–320. 10.1016/j.carbpol.2004.01.011. [DOI] [Google Scholar]
- Wu J.; Li Z.; Yuan X.; Wang P.; Liu Y.; Wang H. Extraction and isolation of type I, III and V collagens and their SDS-PAGE analyses. Trans. Tianjin Univ. 2011, 17, 111–117. 10.1007/s12209-011-1543-2. [DOI] [Google Scholar]
- Wollensak G.; Redl B. Gel electrophoretic analysis of corneal collagen after photodynamic cross-linking treatment. Cornea 2008, 27, 353–356. 10.1097/ico.0b013e31815cf66a. [DOI] [PubMed] [Google Scholar]
- Cao Q.; He N.; Wang Y.; Lu Z. Self-assembled nanostructures from amphiphilic globular protein–polymer hybrids. Polym. Bull. 2018, 75, 2627–2639. 10.1007/s00289-017-2176-y. [DOI] [Google Scholar]
- Hardy W. B. A preliminary investigation of the conditions which determine the stability of irreversible hydrosols. Proc. R. Soc. London, Ser. A 1900, 66, 110–125. 10.1098/rspl.1899.0081. [DOI] [Google Scholar]
- Dawes H.; Boyes S.; Keene J.; Heatherbell D. Protein instability of wines: influence of protein isoelectric point. Am. J. Enol. Vitic. 1994, 45, 319–326. [Google Scholar]
- Thomas A. W.; Kelly M. W. The isoelectric point of collagen. J. Am. Chem. Soc. 1922, 44, 195–201. 10.1021/ja01422a025. [DOI] [Google Scholar]
- Zeugolis D. I.; Khew S. T.; Yew E. S. Y.; Ekaputra A. K.; Tong Y. W.; Yung L.-Y. L.; Hutmacher D. W.; Sheppard C.; Raghunath M. Electro-spinning of pure collagen nano-fibres-Just an expensive way to make gelatin?. Biomaterials 2008, 29, 2293–2305. 10.1016/j.biomaterials.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Mintál T.; Patczai B.; Wiegand N.; Kereskai L.; Lőrinczy D. The effect of deep-freezing on the structure of patellar and Achilles tendon allografts used for ACL reconstruction. J. Therm. Anal. Calorim. 2017, 127, 1171–1175. 10.1007/s10973-016-5338-5. [DOI] [Google Scholar]
- Tian Z.; Li C.; Duan L.; Li G. Physicochemical properties of collagen solution cross-linked by glutaraldehyde. Connect. Tissue Res. 2014, 55, 239–247. 10.3109/03008207.2014.898066. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Li J.; Ding C.; Liu W.; Li G. The rheological and structural properties of fish collagen cross-linked by N-hydroxysuccinimide activated adipic acid. Food Hydrocolloids 2013, 30, 504–511. 10.1016/j.foodhyd.2012.07.015. [DOI] [Google Scholar]
- Shen X.; Chen L.; Cai X.; Tong T.; Tong H.; Hu J. A novel method for the fabrication of homogeneous hydroxyapatite/collagen nanocomposite and nanocomposite scaffold with hierarchical porosity. J. Mater. Sci.: Mater. Med. 2011, 22, 299–305. 10.1007/s10856-010-4199-x. [DOI] [PubMed] [Google Scholar]
- Maeda H. An dtomic force microscopy study of ordered molecular assemblies and concentric ring patterns from evaporating droplets of collagen solutions. Langmuir 1999, 15, 8505–8513. 10.1021/la981738l. [DOI] [Google Scholar]
- Li D.; Mu C.; Cai S.; Lin W. Ultrasonic irradiation in the enzymatic extraction of collagen. Ultrason. Sonochem. 2009, 16, 605–609. 10.1016/j.ultsonch.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Wu K.; Li G. Interactions of collagen molecules in the presence of N-hydroxysuccinimide activated adipic acid (NHS-AA) as a crosslinking agent. Int. J. Biol. Macromol. 2011, 49, 847–854. 10.1016/j.ijbiomac.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]