Abstract

Metallic nanoparticles (NPs) have enormous applications due to their remarkable physical and chemical properties. The synthesis of NPs has been a matter of concern because chemical methods are toxic. On the contrary, biological methods are considered eco-friendly. To compare the toxicity and the environment-friendly nature of the synthesis methodologies, cadmium NPs were synthesized through chemical (Ch) (co-precipitation) and biological (plant extracts as reducing agent) methods. Cadmium nitrate was reduced with NaOH, while in the biological method, the Cd ions were reduced by Artemisia scoparia (As) and Cannabis sativa (Cs) extracts. X-ray diffraction (XRD) analysis confirmed the pure single-phase cubic structure of green and chemically synthesized CdO NPs except As-CdO NPs that were crystalline cum amorphous in nature. The size of nanoparticles was 84 nm (Cs-CdO NPs) and 42.2 nm (Ch-CdO NPs). The scanning electron microscope (SEM) images exhibited an irregular disklike morphology of nanoparticles that agglomerated more in the case of green synthesis. The antioxidant and antimicrobial potential of NPs revealed that chemically synthesized NPs have better antimicrobial capability, while the antioxidative activities were better for green-synthesized NPs. However, the low yield, high ion disassociation, and waste (unreacted metal) production in the green synthesis of CdO NPs increase the risk of contamination to biosphere. Both types of NPs did not affect the seed germination of Dodonaea viscosa. However, chemically synthesized NPs were less toxic on plant morphological response. The study concludes that the chemically synthesized CdO NPs have better morphology, significant antimicrobial activity, and less toxicity to plant species compared to green-synthesized NPs. Moreover, during the green synthesis, unreacted metals are drained, which causes contamination to the ecosystem.
1. Introduction
Manipulation of matter to size less than 100 nm with at least one dimension changes the chemical and physical properties of a substance. Nanoparticles (NPs) are synthesized by biological, chemical, and physical methods. Biosynthesis encompasses the natural reduction property catalyzed by biomolecules in plants and microorganisms to transform metal ions into metal NPs. In 2019, more than 17 000 publications are focused on the green synthesis of nanoparticles, and this number is rising continuously. Variability in plants used for extract preparation is a major limiting factor to standardize conditions for the green synthesis of NPs due to variations in the type and concentration of biomolecules. However, yet plant-mediated green synthesis is considered cost-effective, simple, rapid, and eco-friendly regardless of the low yield of NPs.1 Quality, size, and morphology of green-synthesized NPs are influenced by various factors, including pH, temperature, reaction time, and concentration of metal salt.2
Chemical methods for the production of NPs mainly include sol–gel process, co-precipitation, microemulsion, hydrothermal, polyol, and chemical vapor deposition. In the metal reduction (co-precipitation) method, a solution containing a metal ion is treated with a reducing agent. In most of the cases, NaBH4, sodium citrate, ascorbic acid, and hydrazine are used as reducing agents, while in few cases, a stabilizing agent might be required for the synthesis of NPs.3 Each chemical method has its own advantages and disadvantages, i.e., the hydrothermal method gives specific shape and size to NPs under microwave radiation.4 Microemulsion requires a large amount of surfactant and is influenced by different external factors. Chemical-assisted reactions show high speed and yield and produce less side products. These methods also produce unique characteristics of size, shape, concentration, surface charge, and morphology, which determine their potential applications.5
Commercial NPs need rigorous standards that guarantee the quality and performance of NPs. The physiochemical conditions during the chemical, physical, and biological methods assist uniformity in size, shape, and surface properties to assure similar behavior.6 In the physical and chemical methodologies, these can be predetermined, but in the biological system, variation in biomolecules makes it impossible to reproduce the results. Biogenic synthesis of NPs is eco-friendly and well suited, with biological systems still a speculation because no comparisons are made between NPs synthesized through different routes and even the physiochemical and morphological properties are not compared.
CdO is an n-type semiconductor having a wide energy band gap (3.3 and 2.2 eV, respectively), ionic nature, low electrical resistivity, and high transmittance in the visible region of the solar spectrum. The most widely known applications of CdO NPs are in optoelectronics, phototransistors, photodiodes, solar cells, and gas sensors.6 CdO NPs opened new horizons to eliminate cancer cells and targeted drug delivery.7,8 CdS nanoparticles are also used as a fluorescence probe in the determination of nucleic acids based on the synchronous fluorescence quenching of functionalized CdS in the presence of DNA.9,10 The antimicrobial activity of CdO NPs is remarkable against several antibiotic-resistant pathogenic bacteria.11,12
There is a controversy that the green synthesis is eco-friendly and the NPs thus produced are biocompatible compared to those produced by other methods. In the current study, CdO nanostructures were synthesized by the chemical precipitation method and using plant extracts of Cannabis sativa and Artemisia scoparia. This study identifies any possible difference in the properties, yield, and toxicity of CdO NPs and the concentration of metal and waste synthesized through green and chemical routes, as well as determines the better route for the synthesis of CdO NPs on the basis of biological and toxicological studies.
2. Results and Discussion
2.1. Synthesis and Characterization
CdO NPs were synthesized by green and chemical routes under the same conditions and procedure (Table 1). Both methodologies resulted in the formation of brown-colored NPs. All of the syntheses were performed at the same experimental conditions, i.e., salt, salt concentration, temperature, and pH. The reduction of Cd ion was carried out by chemical reducing agents and plant extracts. The reducing agent NaOH and biomolecules, i.e., phenolics, flavonoids, alkaloids, proteins, etc., in the case of plant extracts, are capable of reducing the metal into NPs. The reduction in this case depends upon the choice of plant for extract preparation and the extraction methodology because each plant and method have their own biochemical and phytochemical properties. This is the reason that As-CdO NPs were crystalline cum amorphous while Cs-CdO NPs were crystalline in nature.
Table 1. Properties and Routes Used for the Synthesis of CdO NPs.
| chemical synthesis | green synthesis |
||
|---|---|---|---|
| route 1 | route 1 | route 2 | |
| source | Cd(NO3)2·4H2O | Cd(NO3)2·4H2O | Cd(NO3)2·4H2O |
| reducing agents | NaOH | aqueous leaves extract | aqueous leaves extract |
| plant source | C. sativa | A. scoparia | |
| molarity (M) | 0.5 | 0.5 | 0.5 |
| temperature (°C) | 60 | 70 | 70 |
| color | brown | brown | brown |
| nature | crystalline | crystalline | crystalline cum amorphous |
| morphology | irregular disk | irregular disk | |
| size by XRD (nm) | 42.2 | 84 | |
| size by SEM (nm) | 10–30 | 50 | |
XRD patterns of CdO NPs showed absorbance of the diffraction peaks at 2θ values. The estimated particle size using the relative intensity peak for Ch-CdO-NPs (chemically synthesized CdO NPs) was 42.2 nm, and sharp peaks indicate that particles were crystalline in nature. The peaks at the diffraction angles of 33.325, 55.658, and 66.169° are indexed to the (111), (220) and (311) planes, respectively (Figure 1). The estimated size of the Cs-CdO NPs (CdO NPs synthesized by C. sativa) was found to be 84 nm, and sharp peaks show that particles were crystalline in nature. The peaks at the diffraction angles of 33.273, 38.591, 55.606, and 66.282° are indexed to the (111), (200), (220), and (311) planes, respectively (Figure 1). Only one peak at 31.8° was observed for As-CdO NPs (CdO NPs synthesized by A. scoparia) (Figure 1) and that represents the crystalline cum amorphous nature of NPs. The XRD patterns of chemically and biologically synthesized NPs match the reference patterns for CdO nanoparticles in the published data.13−15
Figure 1.
XRD pattern of CdO nanoparticles synthesized through the green chemistry and co-precipitation method: Ch-CdO NPs (nanoparticles synthesized by chemical method), Cs-CdO NPs (nanoparticles synthesized by C. sativa), and As-CdO NPs (nanoparticles synthesized by A. scoparia).
Figure 1 also shows that the intensity and quality of XRD peaks vary depending upon the procedure and also the plant used for extraction and extraction procedure. Due to the variation in plant metabolites among species and extraction procedure, the morphology of NPs also varies. A. scoparia extract was prepared by heating, which might degrade biomolecules responsible for the conversion of ions into NPs. Therefore, the NPs produced by A. scoparia were crystalline cum amorphous in nature compared to those produced by C. sativa that were only crystalline in nature.
Fourier-transform infrared (FTIR) spectra confirmed the attachment of biomolecules responsible for capping and stabilization of the NPs. The FTIR spectra of Ch-CdO NPs (Figure 2) show prominent peaks at 3561, 3245, 1739, 983, and 691. The peak at 3561 shows the presence of N–H symmetric stretching. The peak at 3245 displays the presence of O–H stretching. The peak at 1739 illustrates the presence of carbonyl stretching, while the peaks at 983 and 691 show the presence of the CdO bond. The FTIR spectra of Cs-CdO NPs showed prominent peaks at 3369, 3171, 1460, 714, and 613 (Figure 2). The broad FTIR absorption band at 3369 shows O–H stretching, while the peak at 3171 indicates the presence of N–H stretching. The peak at 1460 depicts the presence of C–H bonding of hydrocarbons. The broad bands observed at 714 and 613 are due to the metal–oxygen bond. Prominent peaks at 2938, 2355, 1482, 1062, and 614 were shown by the FTIR spectra of As-CdO NPs (Figure 2). These peaks depict the stretching of C–H, C–C, and C–O as mentioned for Cs-CdO NPs. The plant extracts containing a higher amount of phenolics and flavonoids that are responsible for their antioxidative response might have a role in the reduction as well as capping and stabilization of NPs. Therefore, diverse functional groups are observed on NPs synthesized by plant extract; however, variation is due to distinct phytocomponents in both plant extracts. FTIR spectra also show that green synthesis procedures result in the attachment of functional group on the surface of NPs and the variation in the intensity of each peak depending upon the extract used.
Figure 2.

FTIR spectra of CdO nanoparticles synthesized through green chemistry and co-precipitation method: Ch-CdO NPs (nanoparticles synthesized by chemical method), Cs-CdO NPs (nanoparticles synthesized by C. sativa), and As-CdO NPs (nanoparticles synthesized by A. scoparia).
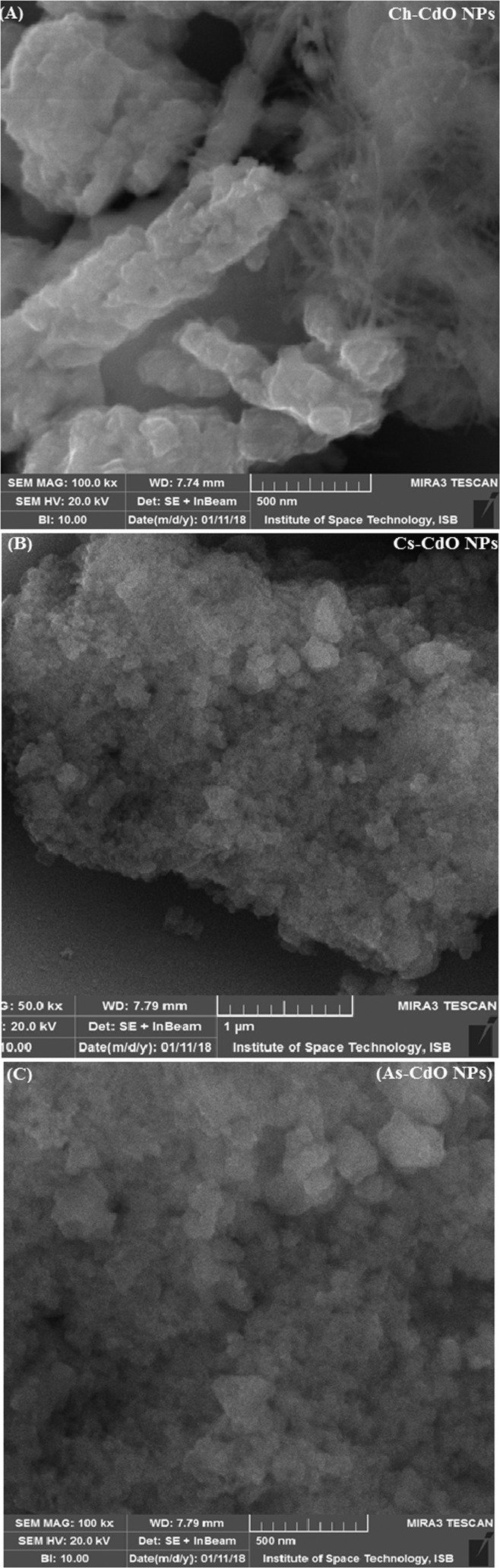
Scanning electron microscopy (SEM) disclosed the clear and distinct images of green and chemically synthesized CdO NPs (Figure 3). They have irregular disklike morphologies with Ch-CdO NPs and Cs-CdO NPs in the sizes of 10–30 and 50 nm, respectively.
Figure 3.
SEM analysis of CdO NPs synthesized through green chemistry and co-precipitation method: (A) Ch-CdO NPs, (B) Cs-CdO NPs, and (C) As-CdO NPs.
2.2. Biological Activities
2.2.1. Antioxidative Response of CdO NPs
Compounds having a high reducing power act as primary and secondary antioxidants to reduce the oxidized intermediates of biochemical processes.16C. sativa-synthesized CdO NPs showed significant antioxidant capacity and reducing power 6.4 ± 0.38 and 12.7 ± 0.38 μg AAE/mg, respectively, in comparison to As- and Ch-CdO NPs (Figure 4). Ch-CdO NPs, As-CdO NPs, and Cs-CdO NPs showed 4.2, 5.4, and 4.2% radical scavenging potential, respectively. Phenols have redox properties which make them serve as reducing agents, hydrogen donors, and singlet oxygen quenchers. Flavonoids are groups of polyphenolic compounds that act as scavenging and chelating agents. NPs show biological activities due to the presence of functional groups on the surface of NPs. Cs-CdO NPs displayed better phenolic (3.5 ± 0.81 μgGAE/mg) and flavonoid (3.1 ± 0.19 μg QE/mg) like contents as compared with As-CdO NPs and Ch-CdO NPs (Figure 4). The figure also shows that C. sativa is rich with flavonoids compared to A. scoparia, which produced crystalline NPs. This depicts that flavonoids play a more important role in the synthesis of NPs compared to flavonoids although flavonoids are a subclass of phenolics.
Figure 4.

Antioxidative properties of CdO NPs and plant extract. As: A. scoparia, Cs: C. sativa, As-CdO NPs: Artemesia-synthesized CdO NPs, Cs-CdO NPs: Cannabis-synthesized CdO NPs, Ch-CdO NPs: chemically synthesized CdO NPs. Values are presented as mean ± standard error. Different small letters on each column show a significant difference between values.
The nanoparticles behave as antioxidative agents due to their chemical nature, the presence of the metal itself, and the attachment of different functional groups on the surface of NPs.17 Through the chemical procedure, the nature of reactants defines the attachment of functional groups that might change little during each synthesis process. However, in the green synthesis approach, variation of biomolecules in plants by the extraction procedure changes the attachment of functional groups that determine the strength of NPs during activities.18
2.2.2. Antimicrobial Activity
Chemically synthesized CdO NPs exhibited a significant zone of inhibition against all bacterial strains compared to green-synthesized CdO NPs (Table 2). Green-synthesized Cs-CdO NPs showed mild to moderate behavior against all bacterial strains except Bacillus subtilis and Klebsiella pneumoniae, i.e., 13 and 12 mm, respectively. As-CdO NPs showed a significant zone of inhibition against Pseudomonas aeruginosa and K. pneumoniae. In the case of Escherichia coli and Staphylococcus aureus, all tested samples showed mild to moderate antibacterial behavior. Similar studies have demonstrated that CdO nanoparticles can be effective in the treatment of infectious diseases caused by E. coli and other bacterial strains.19−21
Table 2. Antimicrobial Activity of CdO NPs and Plant Extracts of C. sativa and A. scopariaa.
| zone of inhibition (mm) |
|||||
|---|---|---|---|---|---|
| species | A. scoparia | C. sativa | As-CdO-NPs | Cs-CdO-NPs | Ch-CdO-NPs |
| Staphylococcus aureus | 7 ± 0.51 | 8 ± 0.57 | 8 ± 0.4 | 9 ± 0.51 | 10 ± 0.5 |
| Bacillus subtilis | 7 ± 0.51 | 7 ± 0.51 | 8 ± 0.5 | 13 ± 0.8 | 14 ± 1.1 |
| Escherichia coli | 8 ± 0.57 | 8 ± 0.57 | 7 ± 0.51 | 8.5 ± 0.57 | 10 ± 0.5 |
| Pseudomonas aeruginosa | 20 ± 1.2 | 7 ± 0.51 | 19 ± 1.2 | 9 ± 0.59 | 17.5 ± 1.13 |
| Klebsiella pneumoniae | 7 ± 0.51 | 8 ± 0.57 | 14 ± 1.1 | 12 ± 0.89 | 15.5 ± 1.12 |
| Fusarium solani | 21 ± 1.2 | 18 ± 1.13 | 21 ± 1.2 | ||
| Aspergillus flavus | 9 ± 0.51 | 9 ± 0.51 | 10 ± 0.5 | ||
| Aspergillus fumigatus | 12 ± 0.89 | 15 ± 1.1 | 12 ± 0.89 | ||
| Aspergillus niger | 7 ± 0.51 | 7 ± 0.51 | |||
Values are presented as mean ± standard error. As-CdO NPs (A. scoparia-synthesized), Cs-CdO NPs (C. sativa-synthesized), and Ch-CdO NPs (chemically synthesized CdO-NPs).
Chemically synthesized CdO NPs did not show any antifungal activity in the case of A. niger. None of the plant extracts showed antifungal activity against all tested fungal strains. However, the antifungal activities of C. sativa- and A. scoparia-mediated green-synthesized nanoparticles were greater than those of chemically synthesized nanoparticles (Table 2). Swain et al. reported a comparative study of green- and chemically synthesized AuNPs with respect to their antifungal properties with few variations in size and antifungal properties.22 It is believed that there is electromagnetic attraction between microbe and NPs surface that make microbes oxidized and cause instant death. CdO NPs interact with the cell membrane of bacteria and cause disruption in the mesosome function of DNA replication, cell division, etc. Nanomaterials inactivate protein function and decrease cell permeability, leading to the death of the microbe.23,24
2.3. Quantification of Total Cd Metal Content and Yield of NPs
The yield of nanoparticles is not contented to 100% in spite of the high yield of NPs via chemical and physical processes.25 The yield of NPs was significantly higher (840 mg) by using the chemical method of CdO NPs synthesis. A. scoparia extract procured 390 mg of CdO NPs relative to the C. sativa extract that yielded only 304 mg of NPs. Table 3 shows that the Cd content was higher in chemically synthesized NPs so the theoretical waste was less. However, by green synthesis, higher waste depicts that the Cd content is drained into the water as waste that causes metal contamination to water and soil. By the green synthesis approach more waste produces. In present study, we presumed the formation of pure CdO NPs, although the FTIR analysis showed attachment of different functional groups on the surface of NPs.
Table 3. Metal Analysis of Green- and Chemically Synthesized CdO NPs Yield and Total Waste in NPs Synthesisa,b.
| sample | yield of NPs (mg) | conc. of Cd in CdO NPs (μM)c | theoretical waste Cd (μM)d |
|---|---|---|---|
| Ch-CdO NPs | 840 | 1756.8 | 1053.2 |
| Cs-CdO NPs | 304 | 631.19 | 2178.81 |
| As-CdO NPs | 390 | 805.83 | 2004.17 |
Concentration of Cd in salt used for the synthesis of NPs was 2810 μM.
As-CdO-NPs (A. scoparia-synthesized), Cs-CdO-NPs (C. sativa-synthesized), and Ch-CdO-NPs (chemically synthesized CdO-NPs).
Assuming that the NPs were purely CdO.
Cd in salt – Cd in NPs.
The dissolution (Table 4) of Cd from NPs shows that increasing the concentration of NPs increases the dissolution of Cd from NPs. However, the route of synthesis of NPs also influences the dissolution power. Chemically synthesized NPs released less Cd compared to the green route. The plants used for the synthesis of CdO NPs also effect the release of metal from NPs. Most probably, the NPs synthesized by the chemical route are compact, and the strong interaction between elements makes it hard to release metal. While in the case of green synthesis, attachment of functional groups around the metal makes them less compact. There are few studies that depict the release of metal ions from NPs; however, the release is dependent on the concentration, shape, and route of synthesis of NPs.26,27 CdO is dreadfully toxic, soluble in vivo, and our results specified dissolution of green-synthesized NPs was significantly higher compared to Ch-CdO NPs. This increases the contamination level in the ecosystem, which is a threat to living beings.
Table 4. Dissolution of Cd from NPsa.
| sample | conc. | Cd content (mg/L) |
|---|---|---|
| Ch-CdO | 2.5 | 0.209 |
| 5 | 0.246 | |
| 10 | 0.323 | |
| 20 | 0.407 | |
| Cs-CdO | 2.5 | 0.215 |
| 5 | 0.353 | |
| 10 | 0.322 | |
| 20 | 0.552 | |
| As-CdO | 2.5 | 0.233 |
| 5 | 0.541 | |
| 10 | 0.495 | |
| 20 | 0.638 |
As-CdO NPs (A. scoparia-synthesized), Cs-CdO NPs (C. sativa-synthesized), and Ch-CdO NPs (chemically synthesized CdO NPs).
2.4. Toxicity of CdO NPs Synthesized by Both Chemical and Green Means on Dodonaea viscosa
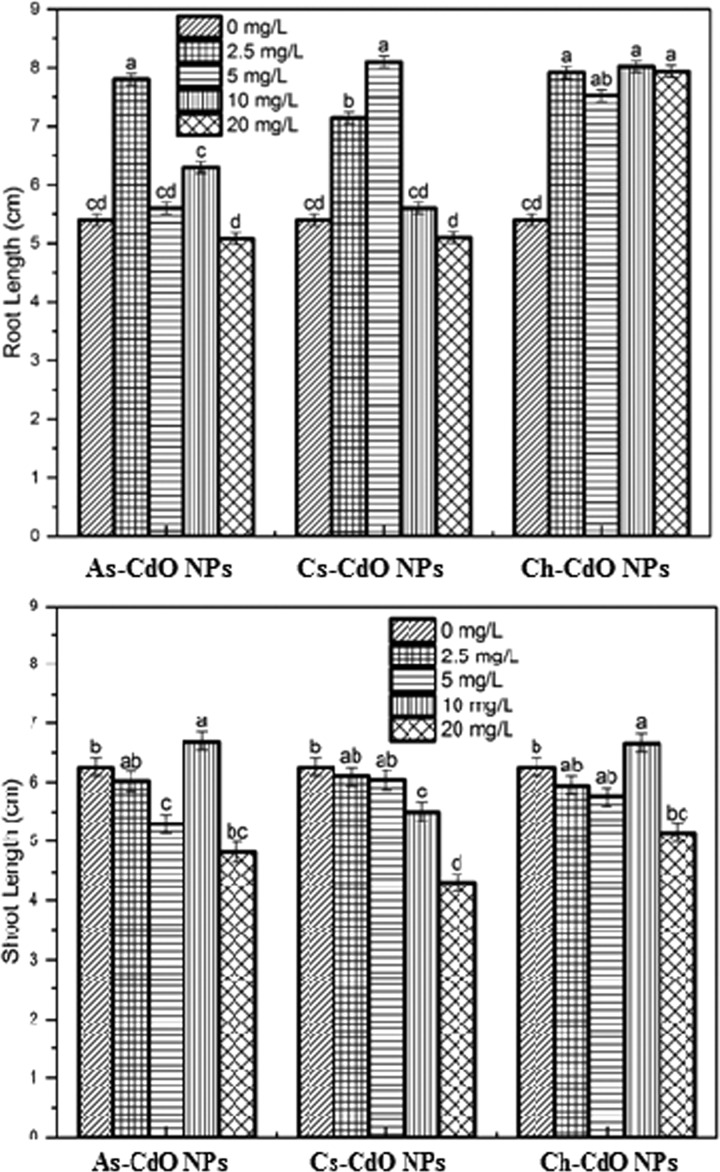
CdO NPs showed toxicity on the morphological characteristics (root length, shoot length, fresh weight, and dry weight) of D. viscosa depending on the concentration of NPs. Nanoparticles synthesized through both routes did not affect the seed germination rate (>90%). The application of a low concentration of NPs significantly affected the root length and shoot length (Figure 5). However, an increase in the concentration of NPs in the media negatively influenced the root length and a decrease was observed. NPs accumulate at the cell surface and translocate to other parts of the plant.28 In the plasma membrane of plant roots, many ion and protein transporters are present, which function as Cd transport.29,30 The plants grown in the presence of chemically synthesized CdO NPs had better root length than green-synthesized NPs. Furthermore, at lower concentration(s), CdO NPs also functioned as a growth enhancer. The figure also shows that green-synthesized NPs retorted significant root growth.
Figure 5.
Morphological response ofD. viscosa grown in the presence of chemically and green-synthesized CdO nanoparticles. The NPs showed varied toxicity on shoot length and root length depending upon the type and concentration of NPs. The similar small letters shown on bars show nonsignificant difference at p < 0.05.
Most probably, green-synthesized NPs have more functional groups on their surface that bind strictly with biomolecules present on the root surface or inside the cell, increasing the toxicity (Figure 6). Additionally, the dissolution data showed more release of Cd from green-synthesized NPs, making them more toxic for plant growth. Almost same results are found in the case of shoot length; however, lesser variation is because the effect on shoot is more dependent on the transport of NPs to the shoot and dissolution power of NPs. Growth also retards due to protein denaturation, and the reduced uptake of nutrients is due to the existence of NPs around vascular bundles. The decrease of shoot length might be due to the more release of ions from particles that accumulate in the shoot, so more exposure to ions retards their growth.31,32 The decrease in root length might also be due to the lower activity of H+-ATPase, which participates in the uptake of elements by roots.
Figure 6.

Toxicity mechanism of chemically and biologically synthesized CdO NPs. Ch-CdO NPs release a Cd ion that interacts with the surface biomolecules and causes more damage due to influx, where it causes ROS and inactivation of proteins, lipids, and DNA. The green-synthesized CdO NPs interact more on the surface due to compatibility with biological system along with release of ions.
Variations in the weight of plants grown in the presence of NPs show that fresh weight and dry matter are not directly affected by the concentration and type of NPs (Table 5). Fresh weight is a property of an individual plant that shows biomass and water content of the plant, while dry matter is based on the water retention capability of the plant. Therefore, a variation in fresh and dry weights can be observed. This behavior is due to the overcompensation response, in which there is a disruption of homeostasis by chemical substances called the “hormetic effect”. Biomass accumulation also associates with the primary metabolism, mainly the nitrogen and carbon metabolism that contributes to cellular growth and cell components.33−35 Biomass reduction can also be due to the reduced availability of nutrients and water, which disturbs the normal cellular function required for proper growth.36
Table 5. Fresh Weight (FW) and Dry Weight (DW) of D. viscosa under CdO NPs Stressa.
| Cs-CdO
NPs |
As-CdO NPs |
Ch-CdO NPs |
||||
|---|---|---|---|---|---|---|
| conc. (mg/L) | FW (mg) | DW (mg) | FW (mg) | DW (mg) | FW (mg) | DW (mg) |
| 0 | 139 ± 1.1d | 50 ± 0.9d | 139 ± 1.1d | 50 ± 0.9c | 139 ± 1.1c | 50 ± 0.9c |
| 2.5 | 184 ± 1.2b | 83 ± 0.9b | 192 ± 1.0a | 90 ± 0.5a | 163 ± 0.4b | 70 ± 1.3b |
| 5 | 199 ± 1.1a | 95 ± 0.5a | 142 ± 0.6c | 48 ± 1.1c | 123 ± 1.2d | 44 ± 0.1d |
| 10 | 151 ± 0.2c | 60 ± 0.4c | 163 ± 0.8b | 66 ± 1.2b | 188 ± 0.4a | 90 ± 1.3a |
| 20 | 117 ± 0.9e | 24 ± 1.2e | 123 ± 1.1e | 40 ± 0.7d | 110 ± 0.4e | 33 ± 0.9e |
Values represent mean ± standard error from triplicate. Different letters show a significant difference between them. As-CdO NPs (A. scoparia-synthesized), Cs-CdO NPs (C. sativa-synthesized), and Ch-CdO NPs (chemically synthesized CdO NPs).
3. Experimental Section
3.1. Synthesis of CdO NPs
CdO NPs were prepared by chemical synthesis through the co-precipitation method described by Shanmugam et al. with slight modifications.37 For the synthesis of CdO NPs, 50 mL of 0.5 M Cd (NO3)2·4H2O was mixed dropwise with 50 mL of a NaOH (0.5 M) solution. The reaction was continued for 2 h at 60 °C. The NPs were collected by centrifugation at 10 000 rpm for 10 min at 25 °C and washed thrice. The NPs were dried at 70 °C and calcinated at 400 °C for 2 h to obtain the CdO nanoparticles.
For the green synthesis of CdO NPs, fresh leaves of C. sativa were collected from Quaid-i-Azam University, Islamabad, Pakistan, and an A. scoparia plant was collected from Pa̅ṛachina̅r, Khyber Pakhtunkhwa, Pakistan. The plants were identified by Taxonomist Department of Plant Sciences, Quaid-i-Azam University, Islamabad, following authentic plant sheets preserved in herbarium and flora of Pakistan. Fresh leaves of C. sativa were washed repeatedly with water to remove dirt, rinsed with distilled water thoroughly, and then air-dried. Distilled water (200 mL) and air-dried leaves (20 g) were blended to prepare the aqueous extract. After that, the extract was collected by filtration through a Whatman No. 1 filter paper, followed by centrifugation at 8000 rpm for 5 min at 25 °C. A supernatant was used for the synthesis of CdO nanoparticles.
Whole plant of A. scoparia was washed with distilled water and dried under sunlight. The dried plant was ground into a fine powder; 30 g of the plant was soaked into 150 mL of distilled water and kept at room temperature for 12 h. The mixture was heated for 10 min at 50–60 °C to make the extract. The extract was collected by filtration through a Whatman No. 1 filter paper, followed by centrifugation at 8000 rpm for 5 min at 25 °C. A supernatant was used for the synthesis of CdO nanoparticles.
CdO NPs were synthesized by mixing 25 mL of C. sativa extract and 50 mL of A. scoparia extract with 0.5 M Cd(NO3)2·4H2O separately. The mixture was heated at 70 °C for 3 h with constant stirring on a magnetic stirrer. Particles were collected by centrifugation at 10 000 rpm for 10 min at 25 °C. The NPs were washed thrice, dried in an oven at 80 °C for 10 h, and calcinated at 400 °C for 2 h.
3.2. Characterization of CdO NPs
X-ray diffraction of CdO NPs was carried out using a D8 Advance having a Cu Kα radiation source of 1.54 Å with 1200 W X-ray energy. The scanning was done in the 2θ range of 10–70°. The Scherrer equation was used to calculate the size, i.e., D = 0.9λ/β cos θ, where D is the diffraction angle, λ is the Cu Kα wavelength (1.54 Å), θ is the diffraction angle, and β is the full width at half-maximum (FWHM) of the diffraction peak. NicoletTM380 was used for the FTIR spectroscopy of CdO NPs. The dried pellet samples were used to test the surface functional groups by FTIR spectroscopy 1000–3500 cm–1 in the transmittance mode. The morphology of CdO NPs was investigated by a scanning electron microscope (MIRA3 TESCAN) operating at an acceleration voltage of 20 kV. Thin films of the samples were prepared on a silicon wafer by dropping a very small amount of sample onto the silicon wafer.
3.3. Quantification of Cd Metal Content
To quantify the Cd content in NPs, 10 mg of each NPs was acid-digested using a 4:1 mixture of nitric acid (HNO3) and perchloric acid (HClO4) in a conical flask at 110 °C. The remaining was diluted with 50 mL of 1% nitric acid. The contents were then transferred to properly rinsed plastic bottles for the quantification of total Cd metal content by atomic absorption spectroscopy. To investigate the dissolution of Cd metal from NPs, four concentrations (2.5, 5, 10, and 20 mg/L) were used. NPs were suspended in distilled water (pH 6.5) for 18 h at room temperature. After centrifugation, the supernatant was used to check the dissolution of Cd metal from NPs by atomic absorption spectroscopy.
3.4. Biological Properties of Cd NPs
The plant extracts and nanoparticles, 4 mg each, were dissolved in 1 mL of dimethyl sulfoxide (DMSO) and used for characterizing the antibacterial, antifungal, and antioxidative activities (2,2-diphenyl-1-picrylhydrazyl (DPPH)-based free-radical scavenging activity, total antioxidant potential, total reducing power) and nonenzymatic antioxidant properties (phenolics- and flavonoids-like properties). The detailed protocols have already been published.38,39
3.5. Toxicological Assessment of CdO NPs
Four concentrations of NPs (2.5, 5, 10, and 20 mg/L) were added in MS media. The MS media was augmented with 3% sucrose and solidified with 0.44% gelrite. The medium was sonicated for 30 min to avoid aggregation of NPs, and pH was adjusted to 5.6–5.8. The media was poured into conical flasks (30/100 mL) by constant shaking for equal distribution of NPs in every flask. After that, the flasks were autoclaved at 121 °C, 15 psi for 20 min and allowed to cool up to 45 °C; shook well, and kept at −4 °C until solidification to avoid settling of NPs at base.
Seeds of D. viscosa were collected from Quaid-i-Azam University, Islamabad. The seeds were cleaned to make them free of foreign materials. The germination efficiency of the seeds was >90%. The seeds were sterilized under aseptic conditions by immersing in a freshly prepared 0.1% mercuric chloride solution for 20 s and then washed thrice with distilled water. The seeds blotted on a filter paper were inoculated in MS medium augmented with CdO NPs flasks. Four seeds per flask were inoculated, and the flasks were kept in a growth room at 25 °C with a photoperiod of 8/16 h. Seed germination started after 1 week and kept for 4 weeks to obtain full growth of plantlets. The morphological characters of the plantlets were measured and kept in an incubator at 40 °C for 24 h for drying to calculate dry biomass.
3.6. Statistical Analysis
To investigate the effect of NPs on D. viscosa (collected from the vicinity of Quaid-i-Azam University after authentication of taxonomist), five flasks of each concentration were inoculated, each containing four seeds. Data were expressed as mean ± standard deviation. All antioxidative tests and other assays were performed in triplicate. The means were further analyzed by least significant difference (LSD) using Origin (8.5) software.
4. Conclusions
In summary, CdO NPs were successfully synthesized by the chemical precipitation method and green approach using C. sativa and A. scoparia extracts. CdO NPs were crystalline in nature except As-CdO NPs that were crystalline cum amorphous in nature. The size of nanoparticles varied depending upon the mode and reactants used for synthesis. Green-synthesized CdO NPs increase the risk of contamination in the ecosystem because of high dissolution power and low yield with maximum waste. Furthermore, there was a minor variation in response to biological activities and toxicological study. Based on the results, it can be concluded that although green-synthesized NPs might be less compatible to the living system, there is variation in the characteristics of nanoparticles. The green synthesis approach also produces more waste (unreacted metal) that is drained into the water flow. This causes contamination in water, soil, and, of course, the food chain, thereby causing a serious threat to humans.
Author Contributions
All authors contributed equally in designing and performing the experiments, interpreting the results, and writing the manuscript
The authors declare no competing financial interest.
References
- Das R. K.; Pachapur V. L.; Lonappan L.; Naghdi M.; Pulicharla R.; Maiti S.; Cledon M.; Dalila L. M. A.; Sarma S. J.; Brar S. K. Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18 10.1007/s41204-017-0029-4. [DOI] [Google Scholar]
- Rastogi A.; Singh P.; Haraz F. A.; Barhoum A.. Biological Synthesis of Nanoparticles: An Environmentally Benign Approach. In Fundamentals of Nanoparticles; Elsevier, 2018; pp 571–604. [Google Scholar]
- Ali A.; Zafar H.; Zia M.; ul Haq I.; Phull A. R.; Ali J. S.; Hussain A. Synthesis, characterization, applications, challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. 10.2147/NSA.S99986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta-Videa J. R.; Huang Y.; Parsons J. G.; Zhao L.; Lopez-Moreno L.; Hernez-Viezcas J. A.; Gardea-Torresdey J. L. Plant-based green synthesis of metallic nanoparticles: scientific curiosity or a realistic alternative to chemical synthesis?. Nanotechnol. Environ. Eng. 2016, 1, 4 10.1007/s41204-016-0004-5. [DOI] [Google Scholar]
- Ealia S. A. M.; Saravanakumar M. P. A review on the classification, characterisation, synthesis of nanoparticles their application. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 263, 032019 10.1088/1757-899x/263/3/032019. [DOI] [Google Scholar]
- Aldwayyan A. S.; Al-Jekhedab F. M.; Al-Noaimi M.; Hammouti B.; Hadda T. B.; Suleiman M.; Warad I. Synthesis characterization of CdO nanoparticles starting from organometalic dmphen-CdI2 complex. Int. J. Electrochem. Sci. 2013, 8, e10514. [Google Scholar]
- Heidari A. H.; Brown C. P. Study of composition morphology of cadmium oxide (CdO) nanoparticles for eliminating cancer cells. J. Nanomed. Res. 2015, 2, 20. 10.15406/jnmr.2015.02.00042. [DOI] [Google Scholar]
- Pratheepa M. I.; Lawrence M. Synthesis of pure, Cu Zn doped CdO nanoparticles by co-precipitation method for supercapacitor applications. Vacuum 2019, 162, 208–213. 10.1016/j.vacuum.2019.01.042. [DOI] [Google Scholar]
- Zafar H.; Abbasi B. H.; Zia M. Physiological and antioxidative response of Brassica nigra (L.) to ZnO nanoparticles grown in culture media and soil. Toxicol. Environ. Chem. 2019, 101, 281–299. 10.1080/02772248.2019.1691555. [DOI] [Google Scholar]
- Zahera M.; Khan S. A.; Khan I. A.; Sharma R. K.; Sinha N.; Al-Shwaiman H. A.; Al-Zahrani R. R.; Elgorban A. M.; Syed A.; Khan M. S. Cadmium oxide nanoparticles: An attractive cidate for novel therapeutic approaches. Colloids Surf., A 2020, 585, 124017 10.1016/j.colsurfa.2019.124017. [DOI] [Google Scholar]
- Abd A. N.; Al Marjani M. F.; Kadham Z. A. Synthesis of CdO NPs for antimicrobial activity. Int. J. Thin Films Sci. Technol. 2018, 7, 43–47. 10.18576/ijtfst/070106. [DOI] [Google Scholar]
- Ghotekar S. A review on plant extract mediated biogenic synthesis of CdO nanoparticles their recent applications. Asian J. Green Chem. 2019, 3, 187–200. 10.22034/ajgc.2018.140313.1084. [DOI] [Google Scholar]
- Prabu K. M.; Anbarasan P. M.; Janarthanan S. Preparation characterization of CDO/PVP nanoparticles by precipitation method. Asian J. Eng. Appl. Technol. 2014, 2, 66–70. [Google Scholar]
- Permana Y. N.; Yulizar Y. Potency of Parkia speciosa Hassk seed extract for green synthesis of CdO nanoparticles its characterization. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 188, 012018 10.1088/1757-899x/188/1/012018. [DOI] [Google Scholar]
- Nagabhushana H.; Basavaraj R. B.; Prasad B. D.; Sharma S. C.; Premkumar H. B.; Udayabhanu; Vijayakumar G. R. Facile EGCG assisted green synthesis of raspberry shaped CdO nanoparticles. J. Alloys Compd. 2016, 669, 232–239. 10.1016/j.jallcom.2016.01.201. [DOI] [Google Scholar]
- Kalita P.; Tapan B. K.; Pal T. K.; Kalita R. Estimation of total flavonoids content (TFC) anti oxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. J. Drug Delivery Ther. 2013, 3, 33–37. 10.22270/jddt.v3i4.546. [DOI] [Google Scholar]
- Demirbas A.; Welt B. A.; Ocsoy I. Biosynthesis of red cabbage extract directed Ag NPs their effect on the loss of antioxidant activity. Mater. Lett. 2016, 179, 20–23. 10.1016/j.matlet.2016.05.056. [DOI] [Google Scholar]
- Karatoprak G. Ş.; Aydin G.; Altinsoy B.; Altinkaynak C.; Koşar M.; Ocsoy I. The Effect of Pelargonium endlicherianum Fenzl. root extracts on formation of nanoparticles their antimicrobial activities. Enzyme Microb. Technol. 2017, 97, 21–26. 10.1016/j.enzmictec.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari T. S.; Thirugnanam T.; Thirumurugan V. Phytomediated synthesis of silver nanoparticles using L: Evaluation Cassia auriculata of antibacterial antifungal activity. Asian J. Pharm. Pharmacol. 2019, 5, 326–331. 10.31024/ajpp.2019.5.2.16. [DOI] [Google Scholar]
- Salehi B.; Mehrabian S.; Ahmadi M. Investigation of antibacterial effect of Cadmium Oxide nanoparticles on Staphylococcus aureus bacteria. J. Nanobiotechnol. 2014, 12, 26 10.1186/s12951-014-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocsoy I.; Temiz M.; Celik C.; Altinsoy B.; Yilmaz V.; Duman F. A green approach for formation of silver nanoparticles on magnetic graphene oxide highly effective antimicrobial activity reusability. J. Mol. Liq. 2017, 227, 147–152. 10.1016/j.molliq.2016.12.015. [DOI] [Google Scholar]
- Swain S.; Barik S. K.; Behera T.; Nayak S. K.; Sahoo S. K.; Mishra S. S.; Swain P. Green synthesis of gold nanoparticles using root leaf extracts of Vetiveria zizanioidesCannabis sativa its antifungal activities. BioNanoScience 2016, 6, 205–213. 10.1007/s12668-016-0208-y. [DOI] [Google Scholar]
- DeAlba-Montero I.; Guajardo-Pacheco J.; Morales-Sánchez E.; Araujo-Martínez R.; Loredo-Becerra G. M.; Martínez-Castañón G. A.; Ruiz F.; Compeán Jasso M. E. Antimicrobial properties of copper nanoparticles amino acid chelated copper nanoparticles produced by using a soya extract. Bioinorg. Chem. Appl. 2017, 2017, 1064918 10.1155/2017/1064918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocsoy I.; Demirbas A.; McLamore E. S.; Altinsoy B.; Ildiz N.; Baldemir A. Green synthesis with incorporated hydrothermal approaches for silver nanoparticles formation enhanced antimicrobial activity against bacterial fungal pathogens. J. Mol. Liq. 2017, 238, 263–269. 10.1016/j.molliq.2017.05.012. [DOI] [Google Scholar]
- Shang L.; Nienhaus K.; Nienhaus G. U. Engineered nanoparticles interacting with cells: size matters. J. Nanobiotechnol. 2014, 12, 5 10.1186/1477-3155-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoso E.; Cadore S. Development of a digestion method for the determination of inorganic contaminants in polyvinyl acetate (PVAc). J. Braz. Chem. Soc. 2008, 19, 1284–1289. 10.1590/S0103-50532008000700009. [DOI] [Google Scholar]
- Mudunkotuwa I. A.; Anthony T. R.; Grassian V. H.; Peters T. M. Accurate quantification of TiO2 nanoparticles collected on air filters using a microwave-assisted acid digestion method. J. Occup. Environ. Hyg. 2016, 13, 30–39. 10.1080/15459624.2015.1072278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D.; Xie G.; Luo J. Mechanical properties of nanoparticles: basics applications. J. Phys. D: Appl. Phys. 2013, 47, 013001 10.1088/0022-3727/47/1/013001. [DOI] [Google Scholar]
- Rossi L.; Zhang W.; Schwab A. P.; Ma X. Uptake, Accumulation, in Planta Distribution of Coexisting Cerium Oxide Nanoparticles Cadmium in Glycine max (L.) Merr. Environ. Sci. Technol. 2017, 51, 12815–12824. 10.1021/acs.est.7b03363. [DOI] [PubMed] [Google Scholar]
- Strayer A.; Ocsoy I.; Tan W.; Jones J. B.; Paret M. L. Low concentrations of a silver-based nanocomposite to manage bacterial spot of tomato in the greenhouse. Plant Dis. 2016, 100, 1460–1465. 10.1094/PDIS-05-15-0580-RE. [DOI] [PubMed] [Google Scholar]
- Zaka M.; Abbasi B. H.; Rahman L. U.; Shah A.; Zia M. Synthesis characterisation of metal nanoparticles their effects on seed germination seedling growth in commercially important Eruca sativa. IET Nanobiotechnol. 2016, 10, 134–140. 10.1049/iet-nbt.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocsoy I.; Tasdemir D.; Mazicioglu S.; Celik C.; Katı A.; Ulgen F. Biomolecules incorporated metallic nanoparticles synthesis their biomedical applications. Mater. Lett. 2018, 212, 45–50. 10.1016/j.matlet.2017.10.068. [DOI] [Google Scholar]
- Zahir A.; Ahmad W.; Nadeem M.; Giglioli-Guivarc’h N.; Hano C.; Abbasi B. H. In vitro cultures of Linum usitatissimum L.: Synergistic effects of mineral nutrients photoperiod regimes on growth biosynthesis of lignans neolignans. J. Photochem. Photobiol., B 2018, 187, 141–150. 10.1016/j.jphotobiol.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Gonçalves J. F.; Antes F. G.; Maldaner J.; Pereira L. B.; Tabaldi L. A.; Rauber R.; Rossato L. V.; Bisognin D. A.; Dressler V. L.; de Moraes Flores É. M.; Nicoloso F. T. Cadmium mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol. Biochem. 2009, 47, 814–821. 10.1016/j.plaphy.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Akhtar M. S.; Panwar J.; Yun Y. S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustainable Chem. Eng. 2013, 1, 591–602. 10.1021/sc300118u. [DOI] [Google Scholar]
- Hussain I.; Singh N. B.; Singh A.; Singh H.; Singh S. C. Green synthesis of nanoparticles its potential application. Biotechnol. Lett. 2016, 38, 545–560. 10.1007/s10529-015-2026-7. [DOI] [PubMed] [Google Scholar]
- Shanmugam N.; Saravanan B.; Reagan R.; Kannadasan N.; Sathishkumar K.; Cholan S. Effect of thermal annealing on the Cd(OH)2 preparation of CdO nanocrystals. Mod. Chem. Appl. 2014, 02, 1–5. 10.4172/2329-6798.1000124. [DOI] [Google Scholar]
- Zia M.; Gul S.; Akhtar J.; Ul Haq I.; Abbasi B. H.; Hussain A.; Naz S.; Chaudhary M. F. Green synthesis of silver nanoparticles from grape tomato juices evaluation of biological activities. IET Nanobiotechnol. 2017, 11, 193–199. 10.1049/iet-nbt.2015.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A.; Ambreen S.; Javed R.; Tabassum S.; ul Haq I.; Zia M. ZnO nanostructure fabrication in different solvents transforms physio-chemical, biological photodegradable properties. Mater. Sci. Eng., C 2017, 74, 137–145. 10.1016/j.msec.2017.01.004. [DOI] [PubMed] [Google Scholar]