Abstract
Gingival tissues are attacked by oral pathogens which can induce inflammatory reactions. The immune-inflammatory responses play essential roles in the patient susceptibility to periodontal diseases. There is a wealth of evidence indicating a link between chronic inflammation and risk of malignant transformation of the affected oral epithelium. Periodontitis is associated with an increased risk of developing chronic systemic conditions including autoimmune diseases and different types of cancers. Besides, some risk factors such as smoking, alcohol consumption and human papilloma virus have been found to be associated with both periodontitis and oral cancer. This review article aimed to study the current concepts in pathogenesis of chronic periodontitis and oral cancer by reviewing the related articles.
Key words: Inflammation, microbiota, mouth, neoplasms, periodontitis
Introduction
Rudolf Virchow was the first to indicate a possible link between inflammation and cancer in the 19th century. He noticed leukocyte infiltration in tumor microenvironment and proposed that chronic inflammation can drive cancer development.1 Genetic modifications can alter the normal control of cell growth and survival, therefore, result in cancer development. In head and neck area these genetic alterations can be induced by different factors including smoking, alcohol and sunlight.2 Accumulating evidence has also revealed the role of microorganisms in tumor growth in different organs.3 Gingival tissues are highly vulnerable to oral microbial attacks. The immune-inflammatory responses play essential roles in the patient susceptibility to periodontal diseases. 4-6 Keratinocytes of the gingival epithelium can produce and secret several immune response mediators such as cathelicidins, human defensins (hBDs), chemokines, pro-inflammatory cytokines, and angiogenetic proteins.7,8 Besides, gingival keratinocytes can recognize pathogen-associated molecular patterns (PAMPs) via receptors, such as toll-like receptors (TLRs).9
Periodontitis is associated with an increased risk of developing systemic conditions such as autoimmune diseases and different types of cancers.10,11 Different factors such as dental calculus, overhang restorations, tooth position, smoking, nutrition, diabetes mellitus, blood dyscrasia, age and genetic alterations have been considered as predisposing factors of periodontal disease.12 There is now a wealth of evidence indicating a link between chronic inflammation and malignant transformation of the affected oral epithelium.5,13-15 For instance, malignant transformation of oral lichen planus, a chronic inflammatory lesion, has been reported. 13,15 Oral squamous cell carcinoma (OSCC) represents up to 90% of all oral malignancies and is the main cause of cancer-related deaths.13-17 Some factors such as tobacco, alcohol, betel quid ingestion, malnutrition, viral infections and oral microbiome have been proposed as the risk factors.18-20 This review aimed to assess the link between periodontitis and oral cancer with a special focus on the recent advancements in the correlation between chronic periodontitis and oral cancer.
Search strategy
The literature search was conducted through PubMed, Scopus database, and Google Scholar. Studies published since 1995 to 2019, with full text available, were considered for inclusion. For further evaluation, research articles describing periodontitis, periodontalrelated disease drivers, periodontitis and systemic diseases, oral cancer and the mechanisms of tumor development were selected.
The contribution of microorganisms to carcinogenesis
Different viruses contribute to the development of cancers. For instance, the association between human papilloma virus (HPV) and cervical cancer and oropharyngeal cancer has been demonstrated. Also, the association between Epstein-Barr virus (EBV) and Hodgkin’s lymphoma has been reported.21,22 In addition to viruses, research has established a strong link between cancer and bacteria. Helicobacter pylori (H. pylori) is one the most well-recognized pathogenic bacteria associated with cancers such as stomach and intestine cancers.23,24 Also, the intestinal dysbiosis promotes hepatocarcinogenesis.25 Porphyromonas gingivalis (P. gingivalis) which colonizes in the oral epithelium is associated with digestive system cancers.26 Interestingly, P. gingivalis has been indicated in gingival SCC tissue samples.27
The role of oral microbiome and inflammation in periodontitis and oral cancer
Antony van Leeuwenhoek first recognized the oral microbiome in the late 1670s.28 Oral microbiome has an essential role in inflammatory responses in the head and neck area including oral cavity. 5,6,18,29 Oral microbime is the main cause of periodontitis. The effects of oral microbiota on oral epithelial barrier have been observed. In periodontitis, bacterial pathogens trigger inflammatory responses which result in the destruction of supporting tissues.29,30 Among the oral microbiome, three specific bacteria have been found as etiologic factors for periodontitis: Aggregatibacter actinomycetemcomitans (formerly Actinobacillus actinomycetemcomitans), P. gingivalis, and Tannerella forsythia formerly Bacteroides forsythus.31 However, P. gingivalis is suggested to be the major causative microorganism.32 A positive association between oral health and autoimmune disorders such as diabetes mellitus type-1 and rheumatic diseases has been demonstrated.33 Also, the role of periodontal infection in cancer risk has been recorded. For example, in an earlier report, the association between periodontal pathogens mainly A. actinomycetemcomitans and gastric precancerous lesions such as chronic atrophic gastritis, intestinal metaplasia, or dysplasia had been indicated.34 Moreover, it has been shown that some species of human oral bacteria such as Fusobacterium nucleatum or Bacteroides are associated with periodontitis, appendicitis and colorectal cancer.35 Interestingly, oral microbiome varies in patients with different types of cancer. For example, in patients with esophageal cancer, Treponema denticola, Streptococcus mitis, and Streptococcus anginosus are the main microorganisms, however, Fusobacterium nucleatum is the main component of oral microbiome in the patients with colorectal cancer.36 In addition, P. gingivalis and the Fusobacterium species have been found in esophageal cancer, colorectal carcinoma and pancreatic cancer.37-40 In a previously published paper, the positive association between periodontal diseases and non-Hodgkin lymphoma (NHL) has been demonstrated. The authors suggested the periodontal disease as a risk factor for NHL. Besides, they indicated an inverse association between tooth loss and NHL. They proposed that tooth loss may result in the resolution of local oral inflammation due to the oral microbiome and the immune responses.41 Nonetheless, a formerly published metaanalysis has found the tooth loss as a risk factor for esophageal cancer. 42 According to the Hill’s criteria, tooth loss is a marker for esophageal cancer not a causative factor.43 Also, a previously published work has indicated that bone loss associated with periodontitis is a risk factor for the development of oral cancer.44 Importantly, oral pathogens such as streptococci (Streptococcus intermedius, S. constellatus, S. oralis, S. mitis, S. sanguis, S. salivarius) have been isolated from cervical lymph nodes in patients with oral cancer.45 The association between H.pylori and oral cancer has also been suggested.18,46 In addition, a recent meta-analysis has demonstrated the association of H. pylori with periodontitis.47 Previous studies have suggested that the metabolism of alcohol and formation of acetaldehyde, a major carcinogene, by oral microbiome such as yeast, has a great impact on the risk of head and neck cancer, especially oral cancer.48 Bacterial species in the oral cavity of patients with periodontitis turn nitrate into nitrite or produce acetaldehyde which all are carcinogenic metabolites.49 Besides, in periodontal diseases, carcinogens produced by tobacco smoking and alcohol consumption, penetrate to the underlying tissues.50 It is suggested that microbiome induces cancer growth through the interaction of multiple signaling pathways.51 Table 111,33,41,52-64 shows a list of autoimmune disorders and different types of cancer associated with periodontitis.
Possible signaling pathways involved in microbial carcinogenesis
Several previous studies have demonstrated the chronic inflammation as a risk factor for malignant transformation in organs such as the oral cavity, head and neck area, esophagus, stomach, liver, colon, uterine cervix, ovaries, urinary bladder and lung.65 It is suggested that the inflammatory response and cellstimulating signals provide a microenvironment for cell proliferation and differentiation.66 Chronic inflammation can induce cell proliferation and mitogenic activities via the activation of signaling pathways such as MAPK/ ERK.67 Besides, chronic inflammation can inhibit apoptosis by modulation of the expression of Bcl- 2 family.68 It is proposed that persistent infections are able to induce DNA damage in proliferating cells through the production of toxic substances such as reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) by inflammatory cells. Consequently, tissue regeneration results in DNA damage and permanent genomic alterations in proliferating cells.69 Cytokines and chemokines have essential roles in tumor initiation and progression. 70 Activation of pro-inflammatory cytokines such as IL-6, IL- 8, IL-1β, and TNF-α has been demonstrated in cancers.71 Miroorganisms can induce cancer growth through different pathways. It has been proven that hepatitis B virus (HBV) and hepatitis C virus (HCV) cause hepatocellular carcinoma. Some of underlying mechanisms of carcinogenesis in HCV associated liver cancer include increased hepatocyte proliferation, induction of immune and inflammation responses and genomic mutations.72 In the oral cavity, traumatization of oral epithelium during mastication results in HPV infection of oral epithelial basal cells. Later, HPV reaches the superficial layers. Elevated expression of E6 and E7, the main contributors to microbial-induced cancer, has been noticed during HPV infection.73 In addition, HPV interrupts the initial phases of the immune response, including the expression of TLRs and cytokines which have great impacts on HPV recognition.74 Deep periodontal pocket is a niche for viral infections, such as HPV,75 EBV76 and HSV.77 Besides, the presence of HPV E6/E7 mRNA in periodontium may support the hypothesis that periodontal tissues function as a reservoir for latent HPV infection.78
Similar to viral infections, bacterial infections can cause inflammation and alterations in the microenvironment. Bacteria produce different mediators which promote cell proliferation, mutagenesis and angiogenesis. Additionally, bacteria inhibit cellular apoptosis. Some bacteria such as H. pylori invade the gastric mucosa and gastric lymph nodes, therefore, intiate and develop a chronic infection.18 H. pylori infection induces IL-1β production, the etiological agent for gastric cancer.79 IL-1β is a critical mediator of chronic inflammation and several cancers.80 The role of IL- 1β in tumorigenesis, tumor invasiveness, angiogenesis, and metastasis and tumor-host interactions has been demonstrated.81 The role of IL-1β in periodontitis has also been recorded.82 H.pylori has been indicated in the oral cavity83 and its prevalence in the oral cavity is related to the progression of periodontal diseases.84 A. actinomycetemcomitans is associated with increased secretion level of TNF-α, IL-1β and other cytokines involved in the inflammatory reactions.85,86 Inflammatory mediators can disseminate from the oral cavity to various extraoral sites and contribute to the development of different diseases such as cancers in other organs. A previously published work has demonstrated a higher prevalence of proximal colorectal neoplasms in patients with periodontitis.61 Also, a published paper has indicated that periodontal pathogens and/or inflammatory mediators may promote prostate cancer.60 These findings may suggest the role of some periodontal microorganisms including P. gingivalis in cancerogenesis.87
Table 1.
List of autoimmune disorders and different types of cancer associated with periodontitis.
| Autoimmune disease (reference no.) | Cancer type (reference no.) |
|---|---|
| Rheumatoid arthritis (11,33) | Pancreatic cancer (52) |
| Type 2 diabetes mellitus (33,53) | Orodigestive cancer (54) |
| Alzheimer’s disease (55) | non-Hodgkin lymphoma (41) |
| Systemic lupus erythematosus (56) | Lung Cancer (57) |
| Head and neck SCC (58) | |
| Breast cancer (59) | |
| Prostate cancer (60) | |
| Proximal colorectal neoplasms (61) | |
| Oral cancer (62,63) | |
| Gastrointestinal cancers (64) |
The mechanisms by which Porphyromonas gingivalis may promote head and neck cancer
Activation of some immunologic and inflammatory reactions in the host by P. gingivalis has been considered as the underlying mechanism. Besides, it has been found that P. gingivalis is capable to invade and penetrate different epithelial cells which enables it to alter some genes in response to chronic infection.88 A very recent investigation has demonstrated that P. gingivalis penetrates oral mucosa by targeting Grainyhead-like 2 (GRHL2), an epithelialspecific transcription factor. Later, GRHL2 causes epithelial barrier damage by inhibition of tight junction protein expression which results in increased periodontium tissue destruction.89 P. gingivalis also activates nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and MAPK pathways in human oral epithelial cells90 (Table 2). It is hypothesized that chronic infection by P. gingivalis can establish a microenvironment by targeting CD274 and programmed cell death 1 ligand 2 (PDCD1LG2) via the activation of STAT1. Moreover, P. gingivalis promotes the secretion of cytokines such as IL-6 which in turn activates tumorigenic transcription factors such as STAT1.70 Additionally, P. gingivalis induces the expression of the proenzyme matrix metalloproteinase 9 (proMMP9) and subsequently active form of MMP-9. It is believed that MMP-9 has a key role in the degaradation of tumor microenvironment to promote the invasion and metastasis of cancer cells including OSCC cells.91 P. gingivalis also induces epithelial- mesenchymal transition (EMT) of normal oral epithelial cells by increasing phospho-GSK3., an important regulator of EMT.92 Besides, the presence of P. gingivalis is related to high serum level of C-reactive protein (CRP).93 It has been reported that CRP opsonizes P. gingivalis for complement-binding and activates complement. 94 The association between circulating levels of CRP and an increased risk of epithelial cancers including ovarian cancer, breast cancer, metastatic gastric cancer and colorectal cancer has been proved.95,96 Table 397-119 summarizes the molecular mechanisms by which P. gingivalis may promote periodontitis and oral cancer.
The role of inflammatory cells in periodontitis and cancer development
Various inflammatory cell types contribute to the inflammatory responses. Macrophages are one of the most important inflammatory cells which have significant roles in the host innate response to periodontal pathogens. Macrophages are classified into two groups: M1-type and M2-type. M1 macrophages are produced in response to Th1-cell-related cytokines such as interferon-γ (IFN-γ) and lipopolysaccharide (LPS). M1-type macrophages secrete inflammatory cytokines such as tumor necrosis factor TNF-α, IL- 1β, IL-6, and the chemokines CXCL9 and CXCL10. Although M1 macrophages promote host immune defense and digest different groups of microorganisms, at the same time may cause tissue damage. Th2-cell-related cytokines, such as IL-4 and IL-13 can stimulate M2-type macrophages resulting in secretion of anti-inflammatory cytokines such as IL-10. Thus, activation of M2 cells has a great impact on local tissue repair and wound healing. However, M2 macrophages promote tumor development by producing IL- 10, IL-13 and TGF-β.97 Besides, the proportion of M1-type macrophages and M2-type macrophages plays a critical role in the status of gingival tissue and development of periodontitis.98,99 Inflammatory macrophages also take part in osteoclastogenesis, collagen degradation and alveolar bone resorption by secreting IL- 1β, IL-23, IL-6, and TNF-α and enzymes (MMPs).100 Additionally, interactions between tumor-associated macrophages (TAMs) and cancer cells play important roles in the regulation of tumor microenvironment. TAMs initiate and support tumor development via signaling molecules and pathways such as growth factors, cytokines and chemokines.97,101
Table 2.
A summary of molecular mechanisms by which Porphyromonas gingivalis may promote periodontitis and oral cancer.
| Molecular mechanism |
|---|
| Activation of immunologic and inflammatory reactions (IL-1, IL-6, IL-8, TNF-α) |
| Penetration of oral mucosa (GRHL2) |
| Establishment of a microenvironment (CD274) |
| Establishment of a microenvironment (PDCD1LG2) |
| Degaradation of tumor microenvironment (MMP9) |
| Induction of EMT (phospho-GSK3ß) |
| Opsonization of bacteria for complement-binding and activation of complement (CRP) |
| Promotion of alveolar bone loss (IL-17A) |
Table 3.
A summary of the role of inflammatory cells in development of periodontitis and secreted factors.
| Inflammatory cell type (no. of reference) | Role in periodontitis | Secreted factors |
|---|---|---|
| M1 Macrophages (98,119) | Promotion of host immune defense, digestion of microorganisms, initiation of inflammatory responses | TNF-α, IL‐1β, IL‐6, CXCL9 and CXCL10 |
| M1 Macrophages (100) | Alveolar bone resorption, osteoclastogenesis and collagen degradation | IL-1β, IL-23, IL-6, TNF-α and MMPs |
| M2 Macrophages (98,99) | Local tissue repair and wound healing | IL‐4 and IL‐10 |
| M2 Macrophages (101) | Promote tumor development | IL-10, IL-13 and TGF-β |
| TAMs (97) | Tumor migration, angiogenesis, invasion and metastasis | IL-4, MIP-1β, CCL18, MMP-9 and VEGF |
| Mast cells (103) | Enhancement of periodontitis | TLR4 |
| Neutrophil (106) | Improvement of host response to inflammation | LFA-1 |
| Neutrophil (109,110) | Enhancement of periodontal tissue breakdown | Lytic and proteolytic enzymes and MMP-8 |
| T helper cells (112) | Activation of other immune cells such as neutrophils and B cells | IL-1, IL-17E and IL-17 |
| B cells (113) | Promoting inflammatory responses and tumor microenvironment | Antibodies against pathogens |
| Both T cells and B cells (112) | Alveolar bone resorption | RANKL |
| Tregs CD4+ T lymphocytes (116) | Suppresion the immune responses and promotion of pathogen survival | CTLA-4), GITR, CD103, CD45RO and Foxp3 |
Mast cells are other key players in inflammatory responses. In a previously published work, a significant increase of mast cell density has been found in periodontitis compared to healthy gingiva. Notably, mast cells were mostly located next to mononuclear cells.102 A previous study has indicated an increased expression level of TLR4 on mast cells in periodontal tissues of patients with chronic periodontitis.103 In OSCC, mast cell density increases and is correlated with poor prognosis.104
In the healthy periodontium, keratinocytes protect the oral and sulcular epithelium by producing hBDs. In addition, neutrophils protect the junctional epithelium by secreting defensins.9 Neutrophils are short-lived cells and aged neutrophils undergo apoptosis. However, in inflamed periodontal tissues, apoptosis of neutrophils can be delayed.105A local homeostatic mechanism protects the periodontal tissue against the destructive potential of recruited inflammatory cells. One of these regulating mechanisms may be developmental endothelial locus-1 (Del-1). In embryonic development stages, Del-1 promotes embryonic vascular development. Del-1 is a ligand for lymphocyte function-associated antigen 1 (LFA-1). The adhesive interactions between LFA-1 on neutrophils and intercellular adhesion molecule 1 (ICAM-1) on endothelial cells promote the adhesion of neutrophils onto the vascular endothelium and extravasation of neutrophils.106 The neutrophil transmigration is an important event in immunity.107 In acute phase of inflammation, IL-17 enhances neutrophil influx to sites of inflammation.108 Neutrophils produce MMP-8 which has a great impact on the degradation of connective tissue in periodontal tissues.109 In periodontitis, neutrophil functions as a double-edged sword. While it is essential for host defense, it enhances periodontal tissue breakdown by producing lytic and proteolytic enzymes.110 P. gingivalis inhibits the synthesis of E-selectin, IL-8 (also known as CXCL8) and ICAM-1 to decrease neutrophil recruitment.105 In cancers, neutrophils display both anti- and protumor properties as they are able to kill tumor cells and regulate tumor growth and metastasis via pro-angiogenic factors.104 Very recent study has found a possible association between periodontitis and total leukocytes counts, neutrophil counts and hematocrit (HCT) levels.111
In healthy gingiva, CD4+ helper T cells are the most dominant T lymphocytes. These cells have important roles in the adaptive immune responses. CD8+ T cells are the second most abundant T lymphocytes in healthy gingiva. Under homeostatic conditions these cells can down-regulate the inflammatory responses. Therefore, they maintain gingival tissue integrity and suppress osteoclastogenesis.112 T helper cells such as Th1, Th2, Th9, Th17 and Th22 contribute to cell-mediated immune responses by producing different pro-inflammatory cytokines such as IL-1, IL-17E and IL-17 which activate other immune cells such as neutrophils and B cells. Activation of both T cells and B cells results in the production of RANKL which causes alveolar bone resorption by osteoclasts.112 Besides, memory B cells have been detected in the apical connective tissue of healthy gingiva. B cells contribute to gingival homeostasis and inflammatory responses by producing antibodies against periodontal pathogens.113 A growing body of evidence shows that B cells contribute to tumor promoting microenvironment through secretion of IL-10.114 A previously published study has shown the increased number of CD86+ B cells in the tumor microenvironment of head and neck SCC compared to normal mucosa.115
Tregs CD4+ T lymphocytes play a critical role in the maintenance of self-tolerance and immune homeostasis. Tregs suppress the naive T cell activation and expansion. During periodontitis, Tregs accumulate at inflamed tissues to limit the immune responses and promoting pathogen survival by secreting cytotoxic T-lymphocyte– associated antigen 4 (CTLA-4), Glucocorticoid-induced tumour necrosis factor receptor-related protein (GITR), CD103, CD45RO, and Foxp3.116 The role of regulatory CD4 T cells (Tregs) in cancers is controversial. Although some studies have observed a poor prognosis associated with increased number of Tregs, some other studies have reported a better prognosis.117,118
Involvement of some other cytokines and chemokines in periodontitis and oral cancer
Cytokines is classified as pro-inflammatory and anti-inflammatory groups. Any imbalances between their relative concentrations may result in tissue destruction. Chemokines recruit leukocytes and other immunological mediators in periodontal inflammatory foci. Besides, chemokines and their receptors are involved in the cell proliferation, cell motility, angiogenesis, cancer development and metastasis.120 In the oral cavity, different cell types synthesize chemokines including neutrophils, lymphocytes, monocytes/ macrophages, fibroblasts, osteoblasts, endothelial cells, epithelial cells and mast cells.120 For example, gingival epithelium produces some cytokines such as IL-1, IL-8, and TNF-α which in turn can recruit the macrophages.113 Pro-inflammatory interleukins such as IL-1 and IL-6 promote the secretion of hBDs from keratinocytes. 8,121 Additionally, IL-6 plays a crucial role in the development of periodontitis through a crosstalk of fibroblasts and macrophages.122 C-X-C motif chemokine 5 (CXCL5), a proinflammatory and pro-angiogenic chemokine, has been indicated in the serum of smokers with periodontitis.123 C-X-C motif chemokine receptor 2 (CXCR2), expressed on the surface of neutrophils, plays a crucial role in the recruitment of neutrophils.124 CXCL10 has been indicated in inflamed gingival tissues in response to interferon-γ, TNF-α, and IL-1β.120,125 One of the most abundant chemokines in the oral cavity is IL-8 (CXCL8) which can be found in both healthy individuals and patients with periodontitis. Besides, CXCL12 enhances the activity of MMP-9 in osteoclasts to promote bone resorption.120 TNF-α, IL-1β regulate osteoclastogenesis and induce bone resorption via enhanced expression of receptor activator of NF-kB ligand (RANKL).126,127 A previous animal study demonstrated that IL-17A promotes alveolar bone loss in a P. gingivalis induced model of periodontitis by activating NF-Kβ. In addition, IL-17A collaborates with TNF-α and IL-1β to enhance the expression of pro-inflammatory mediators by keratinocytes and fibroblasts.128 Furthermore, overexpression of some other chemokines such as CXCL10 promotes tumor associated inflammation.70 IL-8 and CXCR2 have been detected in OSCC.129 CXCL5, a chemotactic for neutrophils, drives oral cancer cell growth.120
Table 470,120,125,130-139 lists some cytokines and the signaling pathways involved in periodontitis and oral cancer.
The role of autophagy in periodontitis and oral cancer
Autophagy, an intracellular catabolic process, has a great impact on cellular homeostasis via the elimination of the damaged organelles and aggregated intracellular proteins. Autophagy process starts with the formation of autophagosomes which capture degraded components and later they fuse with lysosomes to recycle those components.140 Autophagy plays an essential role in inflammation, autoimmunity and cellular differentiation.141 Compared with healthy periodontal status, a higher level of autophagy activity has been found in periodontitis. It has been suggested that autophagy protects periodontal cells from apoptosis, promotes angiogenesis and facilitates oral bacteria to escape from the host’s responses.142 Oral bacteria invade the gingival epithelium by controlling the autophagy process. For example, P. gingivalis promotes its own survival and invasion of periodontal tissues by employing the autophagy processes.92 On the other hand, autophagy process is upregulated in many cancer types including head and neck cancer.143 In many cancers, autophagy has dual roles in tumor suppression and promotion. According to the recent study, oral cancer cells promote macro autophagy as an adaptive mechanism to invasion of P. gingivalis. This mechanism can limit the bacterial toxicity and help cancer cells to survive.92 Besides, autophagy modulates the characteristics of cancer stem cells.140
Genetic alterations and the risk of periodontitis development and carcinogenesis
Several genetic disorders affect periodontal tissues. While some disorders alter the host immune response to periodontal infections, some others cause defects in the gingiva or periodontal connective tissue.144,145 Severe periodontitis have been recorded in Down syndrome (trisomy 21), Chediak-Higashi syndrome and Papillon-Lefevre syndrome.145 Congenital neutropenia is associated with oral ulcerations and periodontitis.144 A previously published systematic review showed that polymorphisms in the IL-1α, IL-1β, IL-6, IL-10, MMP-3, and MMP-9 genes are significantly associated with an increased risk of development of periodontitis. 146 Also, increased expression of the human telomerase reverse transcription (hTERT) enzyme has been noticed in patients with periodontitis.147,148 Carcinogenesis is a multi-step process. Genetic alterations and different signaling pathways result in cell proliferation, resistance to apoptosis, invasion and metastasis.149 In the oral cavity, prolonged exposure to carcinogens results in the molecular alterations in the epithelium which lead to the formation of a pre-neoplastic (pre-cancerous) lesion.14 Overtime, this preneoplastic field develops to multiple cancers.14 A previously published work on the expression profile of hTERT has found a steadily increased expression level of hTERT from normal oral mucosa to oral epithelial dysplasia to OSCC.150 Another published paper has revealed that the earliest cytogenetic alterations in both nonsmokers and smokers are loss of heterozygosity (LOH) and loss of tumor suppressor genes in the oral epithelium.151 Chromosal instability detection could be a reliable technique for a risk assessment of oral pre-malignancies and may contribute to an appropriate treatment regimen.152
Role of microRNAs in the etiopathogenesis of periodontal disease and oral cancer
Altered expression of microRNAs contributes to many cellular immune responses.
Dysregulation of miRNA expression has been reported in biofluids such as serum, saliva and crevicular fluid of the gingiva. 17,153,154 The role of miRNAs in pathobiology of periodontal diseases and oral cancer has been demonstrated. For example, miR-1226-5p is down regulated in patients with periodontal diseases and it has been suggested that this miRNA could be a biomarker of periodontitis.155 Moreover, miR-21 expression is up-regulated in P. gingivalis lipopolysaccharide (LPS) stimulated macrophages; therefore, miR-21 could be a target for the control of periodontitis. MiR-21 deficiency increases the synthesis of proinflammatory cytokines and promotes activation of NF-κB in P. gingivalis LPS - stimulated cells.156 Also, the expression level of miRNA-146 was increased in human gingival fibroblasts after P. gingivalis LPS stimulation. This finding indicated that miRNA- 146 inhibits pro-inflammatory cytokine secretion via regulating IL-1 receptor-associated kinase 1(IRAK1), IL-1β, IL-6 and TNF-α production.157
Elevated expression of miR-143-3p was demonstrated in periodontitis. K-RAS is the target gene for miR-143-3p.158 Up-regulation of miR-15a, miR-29b, miR-125a, miR-146a, miR-148/148a, miR-223, and down-regulation of miR-92 have been noticed in patients with periodontitis.4 Furthermore, down-regulation of miR- 100, miR-125b and up-regulation of miR-Let-7a and miR-21 have been reported in periodontitis. NFκB is the common target for aforementioned miRNAs.159 Neutrophils express some miRNAs in periodontitis. In a mouse periodontitis model, up-regulation of miR-155, and miR-223 and down-regulation of miR-17 and miR- 31 in neutrophils has been noticed.4 IL-8 was a target of miR-155 and CXCL2, C–C motif chemokine 3 (CCL3) and IL-6 were the main targets of miR-223. ICAM-1 and E-selectin expression were detected as the potential targets of miR-31 and miR-17-3p, respectively. 4 On the other hand, elevated expression of miR-21, miR- 181b, and miR-345 is associated with malignant transformation of oral leukoplakia.153 Additionally, upregulation of miR-31 is negatively associated with oral leukoplakia progression.17,160 Altered expression of several miRNAs has also been recorded in oral cancer. For example, down-regulation of miR-143 and miR- 145 and miR-590 has been reported in oral cancer cells.161 Decreased expression level of miR-100 and miR-125b elevates oral cancer cells proliferation.162 Detailed information about the miRNAs involved in periodontitis and potential targets is presented in Table 5.4,155-158
Figure 1 summarizes the involved factors in chronic periodontitis which promote the oral cancer development.
Table 4.
List of some cytokines and the signaling pathways involved in periodontitis and oral cancer.
| Type of cytokine (reference no.) | Mechanism/signaling pathway |
|---|---|
| IL-6 (70,130-133) | Classic (specific membrane-bound IL-6 receptor) and trans-signaling (sIL-6R), TLR, NF-κB/ ERK1/2, PI3K/AKT/NF-κB, STAT1 |
| IL-1β (130,134) | Induction of IL-6 and osteoclastogenesis-mediated bone loss by targeting osteoclasts |
| IL-8 (132,133) | NF-κB/ ERK1/2, PI3K/AKT/NF-κB |
| TNF-α (135,136) | TNF-α/HIF-1α/VEGF, RANK and RANKL |
| IL-17A (137) | TLR |
| CXCL5 (138,139) | Increasing the number of neutrophils, AKT/NF-κB |
| CXCL12(120) | Activation of MMP-9 |
| CXCL10(125) | RANKL, osteoclastogenesis |
Salivary proteins can be used as biomarkers of periodontitis and oral cancer
Many studies have attempted to find potential biomarkers in the periodontitis. For instance, salivary matrix metalloproteinase-8 (MMP8) is higher in patients with periodontitis and can be used as a biomarker for periodontitis.163 Recently, higher concentrations of CXCL10 have been identified in the saliva of patients with chronic periodontitis and severe bone loss.125 Some other salivary biomarkers in periodontitis are IL-1β, MMP9,164 mucin 4 (MUC4) and matrix metalloproteinase 7 (MMP7).165 MMP-8 (or collagenase 2) which is found in oral fluid of patients with periodontitis can be considered as one of the most promising biomarkers.166 Salivary extracellular vesicles (EVs) can be used as non-invasive sources of different miRNAs for OSCC diagnosis.167 Also, altered concentrations of choline, betaine, pipecolinic acid and l-carnitine can help in the early detection of OSCC.168
Some similarities and differences between periodontal diseases and oral squamous cells cancer
Clinically, advanced periodontitis and oral cancer, especially gingival cancer, share some signs and symptoms such as swelling, bleeding, deep periodontal pockets, bone destruction and tooth mobility.4,169 However, there are some differences which can help to differentiate gingival SCC from periodontal diseases: i) periodontal diseases are not stable and respond to therapies; ii) unlike gingival SCC, periodontal diseases are more generalized; iii) in both diseases, bone loss and widening of periodontal ligament space occur but in gingival SCC more aggressive pattern of bone destruction can be noticed. However, in both cancer lesions and periodontal diseases, severe bone resorption and tooth mobility can be seen.170
Table 5.
The detail of miRNAs involved in periodontitis and potential targets.
| Involved miRNAs (reference no.) | Potential targets |
|---|---|
| ImiR-1226-5p (155) | Not mentioned |
| miR-21(156) | NF-κB |
| miRNA-146(157) | IRAK1, IL-1β, IL-6 and TNF-α |
| miR-143-3p (158) | K-RAS |
| miR-15a, miR-29b, miR-125a, miR-146a, miR-148/148a, miR-223, miR-92, miR-100, miR-125b, miR-Let-7a and miR-21(4) | NFκB |
| miR-155(4) | IL-8 |
| miR-223(4) | CXCL2, CCL3 and IL-6 |
| miR-31(4) | ICAM-1 |
| miR-17-3p (4) | E-selectin |
Figure 1.
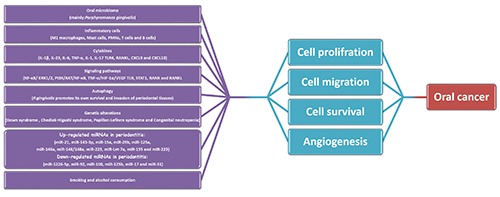
Overview of the major mechanisms that chronic periodontitis promotes oral cancer. Oral microbiome induces inflammation, chemokine production and autophagy which enhance genetic alterations. In addition, some miRNAs, smoking and alcohol consumption are involved in cell proliferation, cell migration, cell survival and angiogenesis which play essential roles in oral cancer development.
Treatment protocols for periodontitis and oral cancer
The primary treatment for oral cancer patients is surgery. Despite the development of effective drugs for some cancers, there is no effective chemotherapeutic agent for OSCC.171 Unfortunately, no established role of adjuvant chemotherapy has been identified for oral cancer. Most chemotherapeutic drugs utilized for oral cancer treatment, only decrease the tumor size before surgery.171 Adjuvant chemotherapy after surgery may reduce the incidence of recurrence and metastasis. In addition, adjuvant chemotherapy may reduce radiation resistance. Cisplatin is associated with an increased risk for acute and late toxicities. Cetuximab combined with radiation has been studied for the primary treatment of locally advanced unresectable head and neck cancer but the role of Cetuximab is not recommended for oral cancer patients.172 Recent studies indicate that inflammatory mediators have a great impact on the development of resistance to chemotherapeutic agents and tumor progression. Thus, cancer cells which are continuously exposed to inflammatory signals acquire chemoresistance and more aggressive behaviors. Therefore, it can be concluded that infection of cancer cells with oral microbiome is the main cause of chemoresistance in the oral cavity.171 As both periodontitis and oral cancer are common in adults, thus, periodontitis- related pathogens and mediators are considered as the main cause of chemoresistance of cancer cells.171 According to the recent investigation, inflammatory mediators especially IL-6 provoke resistance to paclitaxel.171 Interestingly, the prophylactic administration of ibuprofen improves the resistance of OSCC to paclitaxel.171 For that reason, it is logical to treat periodontitis as an inflammatory lesion and a promoter of cancers. Therapeutic protocol includes improvement of individual oral-hygiene, smoking- cessation, dietary adjustment, subgingival scaling, local and systemic pharmacotherapy; and different types of surgery.173
Discussion and Conclusions
Periodontitis is an inflammatory response to oral microbiotome and is associated with bone loss and tooth loss.10 Exposure to inflammatory conditions promotes the infiltration of immune cells to the oral mucosa. Inflammation and immune related mediators have been accepted as the hallmarks of malignant transformation.70 There are some reports about the association between periodontitis and different cancers such as breast cancer and head and neck cancer.59,174 P. gingivalis is the main cause of periodontitis. Due to genetic alterations in response to chronic infection P. gingivalis could contribute to malignant transformation. 88 Several life style factors, including poor oral hygiene, poor nutrition, alcohol consumption, cigarette smoking and obesity are important risk factors for both periodontitis and oral cancer.175-177 Also, genetic alterations have been considered to be involved in periodontitis and oral cancer.20 The recent genetic and molecular studies can explain the high risk of development of cancers from pre-existing inflammatory lesions such as periodontitis. Due to high prevalance of periodontitis and the risk of developing of different cancers including oral cancer around the world, diagnostic and therapeutic strategies for periodontal treatment need to be considered. Similarities in clinical presentations among patients with periodontitis and oral cancer encourage the clinicians to find more reliable and non-aggressive tools for early detection of oral cancer. Early detection of oral cancer has a great impact on the patient prognosis and salivary biomarkers and chemokines are the most promising tools for the early detection of oral cancer.
In conclusion, periodontitis is a common disease around the world. Oral microbiome is the main causative factor for periodontal diseases. Inflammatory responses can increase the risk of genetic alterations and malignant transformation. Oral cancer is a multistep process which involves many signaling pathways. Understanding the signaling pathways and using biomarkers can help to the early detection of oral cancer. Doctors should consider that patients with periodontitis have been linked to an increased risk of oral cancer. Education and training of oral health practitioners can reduce the consequences of both periodontitis and oral cancer.178
Funding Statement
Funding: the authors would like to thank Hamadan University of Medical Sciences for financial support.
References
- 1.Cao X, Xu J. Insights into inflammasome and its research advances in cancer. Tumori 2019;105:456-64. [DOI] [PubMed] [Google Scholar]
- 2.van Elsland D, Neefjes J. Bacterial infections and cancer. EMBO Rep 2018;19:pii: e46632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose M, Mukherjee P. Role of Microbiome in Modulating Immune Responses in Cancer 2019;2019:4107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luan X, Zhou X, Naqvi A, Francis M, Foyle D, Nares S, et al. MicroRNAs and immunity in periodontal health and disease. Int J Oral Sci 2018;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irani S. Orofacial Bacterial Infectious Diseases: An Update. J Int Soc Prev Commun Dentistry 2017;7:S61-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irani S. Oral Health and Related Factors: An Update. J Int Oral Health 2016;8:140-4. [Google Scholar]
- 7.Kasnak G, Kononen E, Syrjanen S, et al. NFE2L2/NRF2, OGG1, and cytokine responses of human gingival keratinocytes against oxidative insults of various origin. Mol Cell Biochem 2019;452:63-70. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Du X, Chen J, Hu L, Chen L. The induction expression of human β-defensins in gingival epithelial cells and fibroblasts. Archiv Oral Biol 2013;58:1415-21. [DOI] [PubMed] [Google Scholar]
- 9.Song B, Zhang Y, Chen L, et al. The role of Toll like receptors in periodontitis. Oral Dis 2017;23:168-80. [DOI] [PubMed] [Google Scholar]
- 10.Jensen A, Ladegaard Gronkjaer L, Holmstrup P, et al. Unique subgingival microbiota associated with periodontitis in cirrhosis patients. Sci Rep 2018;8:10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa JD, Fernandes GR. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep 2019;9:8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight ET, Liu J, Seymour GJ, et al. Risk factors that may modify the innate and adaptive immune responses in periodontal diseases. Periodontology 2016;71:22-51. [DOI] [PubMed] [Google Scholar]
- 13.Irani S, Monsef Esfahani A, Ghorbani A. Dysplastic change rate in cases of oral lichen planus: A retrospective study of 112 cases in an Iranian population. Journal of oral and maxillofacial pathology. JOMFP 2016;20:395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irani S. Pre-Cancerous Lesions in the Oral and Maxillofacial Region: A Literature Review with Special Focus on Etiopathogenesis. Iran J Pathol 2016;11:303-22. [PMC free article] [PubMed] [Google Scholar]
- 15.Irani S. Squamous Cell Carcinoma arising in Oral Lichen Planus. DJH 2010;1:1-6. [Google Scholar]
- 16.Irani S. Distant metastasis from oral cancer: A review and molecular biologic aspects. J Int Soc Prevent Commun Dentistry 2016;6:265-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irani S. miRNAs Signature in Head and Neck Squamous Cell Carcinoma Metastasis: A Literature Review. J Dentistry (Shiraz, Iran) 2016;17:71-83. [PMC free article] [PubMed] [Google Scholar]
- 18.Irani S, Monsef Esfahani A, Bidari Zerehpoush F. Detection of Helicobacter pylori in Oral Lesions. J Dental Res Dental Clin Dental Prospects 2013;7:230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpinski TM. Role of Oral Microbiota in Cancer Development. Microorganisms 2019;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irani S. New insights into Oral Cancer: Risk factors and Prevention: A Review of Literature. Int J Prevent Med 2019. [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speicher DJ, Ramirez-Amador V, Dittmer DP, et al. Viral infections associated with oral cancers and diseases in the context of HIV: a workshop report. Oral Dis. 2016;22 Suppl 1:181-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales-Sanchez A, Fuentes-Panana EM. Human viruses and cancer. Viruses 2014;6:4047-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correa P, Piazuelo MB. Helicobacter pylori Infection and Gastric Adenocarcinoma. US Gastroenterol Hepatol 2011;7:59-64. [PMC free article] [PubMed] [Google Scholar]
- 24.Irani S, Monsef Esfahani A, Sabeti S, Bidari Zerehpoush F. Detection of Helicobacter pylori in Oral Lichen Planus and Oral Lichenoid Reaction. Avicenna J Dent Res 2014;6:e23213. [Google Scholar]
- 25.Gupta H, Youn GS, Shin MJ, Suk KT. Role of Gut Microbiota in Hepatocarcinogenesis. Microorganisms 2019;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Luo GH. Porphyromonas gingivalis and digestive system cancers. World J Clin Cases 2019;7:819-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz J, Onate MD, Pauley KM, et al. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci 2011;3:209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjunatha BS, Mahajan A, Mody BM, Shah V. Adenomatoid Odontogenic Tumor (AOT) Arising from a Dentigerous Cyst: Literature Review and Report of a Case. J Maxillofac Oral Surg 2015;14:393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irani S. Herbal Medicine and Oral Health: A Review. J Int Oral Health 2016;8:989-94. [Google Scholar]
- 30.Vieira Colombo AP, Magalhaes CB, Hartenbach FA, et al. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb Pathogen 2016;94:27-34. [DOI] [PubMed] [Google Scholar]
- 31.How KY, Song KP, Chan KG. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front Microbiol 2016;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asteriou E, Gkoutzourelas A, Mavropoulos A, et al. Curcumin for the Management of Periodontitis and Early ACPAPositive Rheumatoid Arthritis: Killing Two Birds with One Stone. Nutrients 2018;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julkunen A, Heikkinen AM, Soder B. Autoimmune Diseases and Oral Health: 30-Year Follow-Up of a Swedish Cohort. Dent J (Basel) 2017;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salazar CR, Sun J, Li Y, et al. Association between selected oral pathogens and gastric precancerous lesions. PLoS One 2013;8:e51604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordero OJ, Varela-Calvino R. Oral hygiene might prevent cancer. Heliyon 2018;4:e00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin JM, Luo T, Kamarajan P, et al. Microbial Communities Associated with Primary and Metastatic Head and Neck Squamous Cell Carcinoma - A High Fusobacterial and Low Streptococcal Signature. Sci Rep 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binder Gallimidi A, Fischman S, Revach B, et al. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015;6:22613-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao S, Li S, Ma Z, et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agents Cancer 2016;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukugaiti MH, Ignacio A, Fernandes MR, et al. High occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Braz J Microbiol 2015;46:1135-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan X, Alekseyenko AV, Wu J, Peters BA. Human oral microbiome and prospective risk for pancreatic cancer: a population- based nested case-control study. Gut 2018;67:120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertrand KA, Shingala J, Evens A, et al. Periodontal disease and risk of non-Hodgkin lymphoma in the Health Professionals Follow-Up Study. Int J Cancer J 2017;140:1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen QL, Zeng XT, Luo ZX, et al. Tooth loss is associated with increased risk of esophageal cancer: evidence from a meta-analysis with dose-response analysis. Sci Rep 2016;6:18900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HJ, Yoo YS, Park K, et al. Genomic aberrations in salivary duct carcinoma arising in Warthin tumor of parotid gland: DNA microarray and HER2 fluorescence in situ hybridization. Archiv Pathol Lab Med 2011;135:1088-91. [DOI] [PubMed] [Google Scholar]
- 44.Michaud DS, Fu Z, Shi J, Chung M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol Rev 2017;39:49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakamoto H, Naito H, Ohta Y, et al. Isolation of bacteria from cervical lymph nodes in patients with oral cancer. Archiv Oral Biol 1999;44:789-93. [DOI] [PubMed] [Google Scholar]
- 46.Dayama A, Srivastava V, Shukla M, et al. Helicobacter pylori and oral cancer: possible association in a preliminary case control study. Asian Pacific J Cancer Prevent 2011;12:1333-6. [PubMed] [Google Scholar]
- 47.Chen Z, Cai J, Chen YM, et al. A meta-analysis of the association between the presence of Helicobacter pylori and periodontal diseases. Medicine 2019;98:e15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lachenmeier DW, Monakhova YB. Short-term salivary acetaldehyde increase due to direct exposure to alcoholic beverages as an additional cancer risk factor beyond ethanol metabolism. J Exp Clin Cancer Res 2011;30:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajeev R, Choudhary K, Panda S, Gandhi N. Role of bacteria in oral carcinogenesis. South Asian J Cancer 2012;1:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tezal M, Grossi SG, Genco RJ. Is periodontitis associated with oral neoplasms? J Periodontol 2005;76:406-10. [DOI] [PubMed] [Google Scholar]
- 51.Whisner CM, Athena Aktipis C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another’s Growth. Curr Nutr Rep 2019;8:42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Archibugi L, Signoretti M, Capurso G. The Microbiome and Pancreatic Cancer: An Evidence-based Association? Proceedings from the 9th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition & Human and Microbiota Health Meeting, held in Rome, Italy from September 10 to 12. J Clin Gastroenterol. 2018;52 Suppl 1:S82-s5. [DOI] [PubMed] [Google Scholar]
- 53.Farina R, Severi M, Carrieri A, et al. Whole metagenomic shotgun sequencing of the subgingival microbiome of diabetics and non-diabetics with different periodontal conditions. Archiv Oral Biol 2019;104:13-23. [DOI] [PubMed] [Google Scholar]
- 54.Olsen I, Yilmaz O. Possible role of Porphyromonas gingivalis in orodigestive cancers. J Oral Microb 2019;11:1563410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singhrao SK, Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J Oral Microbiol 2019;11:1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Correa JD, Calderaro DC, Ferreira GA, et al. Subgingival microbiota dysbiosis in systemic lupus erythematosus: association with periodontal status. Microbiome 2017;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng XT, Xia LY, Zhang YG, et al. Periodontal Disease and Incident Lung Cancer Risk: A Meta-Analysis of Cohort Studies. J Periodontol 2016;87:1158-64. [DOI] [PubMed] [Google Scholar]
- 58.Gondivkar SM, Gondivkar RS, Gadbail AR, et al. Chronic periodontitis and the risk of head and neck squamous cell carcinoma: facts and figures. Exper Oncol 2013;35:163-7. [PubMed] [Google Scholar]
- 59.Sfreddo CS, Maier J, De David SC, et al. Periodontitis and breast cancer: A case-control study. Commun Dentist Oral Epidemiol 2017;45:545-51. [DOI] [PubMed] [Google Scholar]
- 60.da Silva APB, Alluri LSC, Bissada NF, Gupta S. Association between oral pathogens and prostate cancer: building the relationship. Am J Clin Exper Urol 2019;7:1-10. [PMC free article] [PubMed] [Google Scholar]
- 61.Kim GW, Kim YS. Periodontitis is associated with an increased risk for proximal colorectal neoplasms. Sci Rep 2019;9:7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin YJ, Choung HW, Lee JH, et al. Association of Periodontitis with Oral Cancer: A Case-Control Study. J Dent Res 2019;98:526-33. [DOI] [PubMed] [Google Scholar]
- 63.Chang C, Geng F, Shi X, et al. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl Microbiol Biotechnol 2019;103:1393-404. [DOI] [PubMed] [Google Scholar]
- 64.Chou SH, Tung YC, Wu LS, et al. Severity of chronic periodontitis and risk of gastrointestinal cancers: A populationbased follow-up study from Taiwan. Medicine 2018;97:e11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niture S, Dong X, Arthur E, et al. Oncogenic Role of Tumor Necrosis Factor α-Induced Protein 8 (TNFAIP8). Cells 2018;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corbella S, Veronesi P, Galimberti V, et al. Is periodontitis a risk indicator for cancer? A meta-analysis. PLoS One 2018;13:e0195683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L, Zhao GD, Shi Z, et al. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol Lett 2016;12:3045-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermouet S, Bigot-Corbel E, Gardie B. Pathogenesis of Myeloproliferative Neoplasms: Role and Mechanisms of Chronic Inflammation. Mediat Inflamm 2015;2015:145293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morgillo F, Dallio M, Della Corte CM, et al. Carcinogenesis as a Result of Multiple Inflammatory and Oxidative Hits: a Comprehensive Review from Tumor Microenvironment to Gut Microbiota. Neoplasia (New York, NY) 2018;20:721-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geng F, Wang Q, Li C, et al. Identification of Potential Candidate Genes of Oral Cancer in Response to Chronic Infection With Porphyromonas gingivalis Using Bioinformatical Analyses. Front Oncol 2019;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bent R, Moll L, Grabbe S, Bros M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int J Mol Sci 2018;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ringelhan M, McKeating JA. Viral hepatitis and liver cancer. Philos Trans R Soc Lond B Biol Sci 2017;372:1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang SS, Tang YL, Pang X, et al. The maintenance of an oral epithelial barrier. Life Sci 2019;227:129-36. [DOI] [PubMed] [Google Scholar]
- 74.Barros MR, de Oliveira THA, de Melo CML. Viral Modulation of TLRs and Cytokines and the Related Immunotherapies for HPV-Associated Cancers. J Immunol Res 2018;2018:2912671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ortiz AP, Gonzalez D, Vivaldi-Oliver J, et al. Periodontitis and oral human papillomavirus infection among Hispanic adults. Papillomavirus Res 2018;5:128-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao Z, Lv J, Wang M. Epstein-Barr virus is associated with periodontal diseases: A meta-analysis based on 21 case-control studies. Medicine 2017;96:e5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kazi M, Bharadwaj R. Role of herpesviruses in chronic periodontitis and their association with clinical parameters and in increasing severity of the disease. Eur J Dent 2017;11:299-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shipilova A, Dayakar MM, Gupta D. High risk human papillomavirus in the periodontium : A case control study. J Indian Soc Periodontol 2017;21:380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hong JB, Zuo W, Wang AJ, Lu NH. Helicobacter pylori Infection Synergistic with IL-1beta Gene Polymorphisms Potentially Contributes to the Carcinogenesis of Gastric Cancer. Int J Med Sci 2016;13:298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Apte RN, Dotan S, Elkabets M, et al. The involvement of IL- 1 in tumorigenesis, tumor invasiveness, metastasis and tumorhost interactions. Cancer Metastasis Rev 2006;25:387-408. [DOI] [PubMed] [Google Scholar]
- 81.Baker KJ, Houston A, Brint E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front Immunol 2019;10:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh H, Hirano J, Takai H, Ogata Y. Effects of initial periodontal therapy on interleukin-1beta level in gingival crevicular fluid and clinical periodontal parameters. J Oral Sci 2015;57:67-71. [DOI] [PubMed] [Google Scholar]
- 83.Yee JK. Helicobacter pylori colonization of the oral cavity: A milestone discovery. World J Gastroenterol 2016;22:641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flores-Trevino CE, Urrutia-Baca VH, Gomez-Flores R, et al. Molecular detection of Helicobacter pylori based on the presence of cagA and vacA virulence genes in dental plaque from patients with periodontitis. J Dental Sci 2019;14:163-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zadeh HH, Nichols FC, Miyasaki KT. The role of the cellmediated immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontitis. Periodontology 1999;20:239-88. [DOI] [PubMed] [Google Scholar]
- 86.Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology 1999;20:136-67. [DOI] [PubMed] [Google Scholar]
- 87.Khoi Anh Nguyen KHTT, Giang CT. Malignant Transformation in a Parotid Warthin’s Tumor: Clinical Features and Histopathological Examination. J Cancer Biol Res 2018;6:1115. [Google Scholar]
- 88.Sayehmiri F, Sayehmiri K, Asadollahi K, et al. The prevalence rate of Porphyromonas gingivalis and its association with cancer: A systematic review and meta-analysis. Int J Immunopathol Pharmacol 2015;28:160-7. [DOI] [PubMed] [Google Scholar]
- 89.Chen W, Alshaikh A, Kim S, et al. Porphyromonas gingivalis Impairs Oral Epithelial Barrier through Targeting GRHL2. J Dent Res 2019:22034519865184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Groeger S, Jarzina F, Domann E, Meyle J. Porphyromonas gingivalis activates NFkappaB and MAPK pathways in human oral epithelial cells. BMC Immunol 2017;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inaba H, Sugita H, Kuboniwa M, et al. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol 2014;16:131-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olsen I, Yilmaz O. Possible role of Porphyromonas gingivalis in orodigestive cancers. 2019;11:1563410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dye BA, Choudhary K, Shea S, Papapanou PN. Serum antibodies to periodontal pathogens and markers of systemic inflammation. J Clin Periodontol 2005;32:1189-99. [DOI] [PubMed] [Google Scholar]
- 94.Gani DK, Lakshmi D, Krishnan R, Emmadi P. Evaluation of C-reactive protein and interleukin-6 in the peripheral blood of patients with chronic periodontitis. J Indian Soc Periodontol 2009;13:69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J, Jiao X, Yuan Z, et al. C-reactive protein and risk of ovarian cancer: A systematic review and meta-analysis. Medicine 2017;96:e7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimura T, Kitagawa M, Yamada T, Ebi M, Mizoshita T, Tanida S, et al. C-reactive protein is a potential prognostic factor for metastatic gastric cancer. Anticancer Res 2012;32:491-6. [PubMed] [Google Scholar]
- 97.Chen Y, Song Y, Du W, et al. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci 2019;26:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang J, Zhu Y, Duan D, et al. Enhanced activity of macrophage M1/M2 phenotypes in periodontitis. Archiv Oral Biol 2018;96:234-42. [DOI] [PubMed] [Google Scholar]
- 99.Kononen E, Gursoy M, Gursoy UK. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J Clin Med 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hienz SA, Paliwal S, Ivanovski S. Mechanisms of Bone Resorption in Periodontitis. 2015;2015:615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen Y, Song Y, Du W, et al. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci 2019;26:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Batista AC, Rodini CO, Lara VS. Quantification of mast cells in different stages of human periodontal disease. Oral Dis 2005;11:249-54. [DOI] [PubMed] [Google Scholar]
- 103.Huang B, Dai Q, Huang SG. Expression of Toll like receptor 4 on mast cells in gingival tissues of human chronic periodontitis. Mol Med Rep 2018;17:6731-5. [DOI] [PubMed] [Google Scholar]
- 104.Irani S. Emerging insights into the biology of metastasis: A review article. Iranian J Basic Med Sci 2019;22:833-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olsen I, Hajishengallis G. Major neutrophil functions subverted by Porphyromonas gingivalis. J Oral Microbiol 2016;8:30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hajishengallis G, Sahingur SE. Novel inflammatory pathways in periodontitis. Adv Dental Res 2014;26:23-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olsen I, Hajishengallis G. Major neutrophil functions subverted by Porphyromonas gingivalis. J Oral Microbiol 2016;8:30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 2014;35:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Franco C, Patricia HR, Timo S, et al. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int J Mol Sci 2017;18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scott DA, Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol 2012;15:56-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nibali L, Darbar U, Rakmanee T, Donos N. Anemia of inflammation associated with periodontitis: Analysis of two clinical studies. J Periodontol 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 112.Figueredo CM, Lira-Junior R, Love RM. T and B Cells in Periodontal Disease: New Functions in A Complex Scenario. Int J Mol Sci. 2019;20:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoare A, Soto C, Rojas-Celis V, Bravo D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediators Inflamm 2019;2019:1029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607-12. [DOI] [PubMed] [Google Scholar]
- 115.Lechner A, Schlo.er HA, Thelen M, et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology 2019;8:1535293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alvarez C, Rojas C. Regulatory T Lymphocytes in Periodontitis: A Translational View. Mediators Inflamm 2018;2018:7806912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gasparoto TH, de Souza Malaspina TS, Benevides L, et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother 2010;59:819-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wolf GT, Chepeha DB, Bellile E, et al. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol 2015;51:90-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atri C, Guerfali FZ. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci 2018;19:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sahingur SE, Yeudall WA. Chemokine function in periodontal disease and oral cavity cancer. Front Immunol 2015;6:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hiroshima Y, Bando M, Kataoka M, Iet al. Regulation of antimicrobial peptide expression in human gingival keratinocytes by interleukin-1alpha. Archiv Oral Biol 2011;56:761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naruishi K, Nagata T. Biological effects of interleukin-6 on Gingival Fibroblasts: Cytokine regulation in periodontitis. J Cell Physiol 2018;233:6393-400. [DOI] [PubMed] [Google Scholar]
- 123.Arenberg DA, Keane MP, DiGiovine B, et al. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest 1998;102:465-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Vries TJ, Andreotta S, Loos BG, Nicu EA. Genes Critical for Developing Periodontitis: Lessons from Mouse Models. Front Immunol 2017;8:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aldahlawi S, Youssef AR, Shahabuddin S. Evaluation of chemokine CXCL10 in human gingival crevicular fluid, saliva, and serum as periodontitis biomarker. J Inflamm Res 2018;11:389-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luo G, Li F, Li X, et al. TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol Med Rep 2018;17:6605-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Motedayyen H, Ghotloo S, Saffari M, et al. Evaluation of MicroRNA-146a and Its Targets in Gingival Tissues of Patients With Chronic Periodontitis. J Periodontol 2015;86:1380-5. [DOI] [PubMed] [Google Scholar]
- 128.Awang RA, Lappin DF, MacPherson A, et al. Clinical associations between IL-17 family cytokines and periodontitis and potential differential roles for IL-17A and IL-17E in periodontal immunity. Inflamm Res 2014;63:1001-12. [DOI] [PubMed] [Google Scholar]
- 129.Sahibzada HA, Khurshid Z, Khan RS, et al. Salivary IL-8, IL- 6 and TNF-alpha as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics (Basel, Switzerland). 2017;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Naruishi K, Nagata T. Biological effects of interleukin 6 on gingival fibroblasts: cytokine regulation in periodontitis. J Cell Physiol 2018;233:6393-400. [DOI] [PubMed] [Google Scholar]
- 131.Morandini AC, Chaves Souza PP, Ramos-Junior ES, et al. Toll-like receptor 2 knockdown modulates interleukin (IL)-6 and IL-8 but not stromal derived factor-1 (SDF-1/CXCL12) in human periodontal ligament and gingival fibroblasts. J Periodontol 2013;84:535-44. [DOI] [PubMed] [Google Scholar]
- 132.Liu J, Wang Y, Ouyang X. Beyond toll-like receptors: Porphyromonas gingivalis induces IL-6, IL-8, and VCAM-1 expression through NOD-mediated NF-kappaB and ERK signaling pathways in periodontal fibroblasts. Inflamm 2014;37:522-33. [DOI] [PubMed] [Google Scholar]
- 133.Wang Q, Zhang B, Yu JL. Farrerol inhibits IL-6 and IL-8 production in LPS-stimulated human gingival fibroblasts by suppressing PI3K/AKT/NF-kappaB signaling pathway. Archiv Oral Biol 2016;62:28-32. [DOI] [PubMed] [Google Scholar]
- 134.Sawada S, Chosa N, Ishisaki A, Naruishi K. Enhancement of gingival inflammation induced by synergism of IL-1β and IL- 6. Biomed Res 2013;34:31-40. [DOI] [PubMed] [Google Scholar]
- 135.Afacan B, Ozturk VO, Pasali C, et al. Gingival crevicular fluid and salivary HIF-1alpha, VEGF, and TNF-alpha levels in periodontal health and disease. J Periodontol 2019;90:788-97. [DOI] [PubMed] [Google Scholar]
- 136.Algate K, Haynes DR, Bartold PM, et al. The effects of tumour necrosis factor-alpha on bone cells involved in periodontal alveolar bone loss; osteoclasts, osteoblasts and osteocytes. J Period Res 2016;51:549-66. [DOI] [PubMed] [Google Scholar]
- 137.Abusleme L, Moutsopoulos NM. IL-17: overview and role in oral immunity and microbiome. Oral Dis 2017;23:854-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lappin DF, Murad M, Sherrabeh S, Ramage G. Increased plasma levels epithelial cell-derived neutrophil-activating peptide 78/CXCL5 in periodontitis patients undergoing supportive therapy. J Clin Periodontol 2011;38:887-93. [DOI] [PubMed] [Google Scholar]
- 139.Chen C, Xu Z-Q, Zong Y-P, et al. CXCL5 induces tumor angiogenesis via enhancing the expression of FOXD1 mediated by the AKT/NF-κB pathway in colorectal cancer. Cell Death Dis 2019;10:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yun CW, Lee SH. The Roles of Autophagy in Cancer. Int J Mol Sci 2018;19:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nagar R. Autophagy: A brief overview in perspective of dermatology. Indian J Dermatol Venereol Leprol 2017;83:290-7. [DOI] [PubMed] [Google Scholar]
- 142.Wei W, An Y, An Y, et al. Activation of autophagy in periodontal ligament mesenchymal stem cells promotes angiogenesis in periodontitis. J Periodontol 2018;89:718-27. [DOI] [PubMed] [Google Scholar]
- 143.New J, Arnold L, Ananth M, et al. Secretory Autophagy in Cancer-Associated Fibroblasts Promotes Head and Neck Cancer Progression and Offers a Novel Therapeutic Target. Cancer Res 2017;77:6679-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Albandar JM, Susin C, Hughes FJ. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J Periodontol. 2018;89 Suppl 1:S183-s203. [DOI] [PubMed] [Google Scholar]
- 145.Nualart Grollmus ZC, Morales Chavez MC, Silvestre Donat FJ. Periodontal disease associated to systemic genetic disorders. Med Oral Patol Oral Cir Bucal 2007;12:E211-5. [PubMed] [Google Scholar]
- 146.da Silva MK, de Carvalho ACG, Alves EHP, et al. Genetic Factors and the Risk of Periodontitis Development: Findings from a Systematic Review Composed of 13 Studies of Meta- Analysis with 71,531 Participants 2017;2017:1914073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maisonneuve P, Amar S, Lowenfels AB. Periodontal disease, edentulism, and pancreatic cancer: a meta-analysis. Ann Oncol 2017;28:985-95. [DOI] [PubMed] [Google Scholar]
- 148.Katarkar A, Saha A, Mukherjee S, et al. Telomerase expression in individuals with chronic and aggressive periodontitis. J Periodontol 2015;86:656-65. [DOI] [PubMed] [Google Scholar]
- 149.Sasahira T, Kirita T. Hallmarks of Cancer-Related Newly Prognostic Factors of Oral Squamous Cell Carcinoma. Int J Mol Sci 2018;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Raghunandan BN, Sanjai K, Kumaraswamy J, et al. Expression of human telomerase reverse transcriptase protein in oral epithelial dysplasia and oral squamous cell carcinoma: An immunohistochemical study. J Oral Maxillofac Pathol 2016;20:96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de la Oliva J, Larque AB, Marti C, et al. Oral premalignant lesions of smokers and non-smokers show similar carcinogenic pathways and outcomes. A clinicopathological and molecular comparative analysis. J Oral Pathol Med 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 152.Siebers TJ, Bergshoeff VE, Otte-Holler I, et al. Chromosome instability predicts the progression of premalignant oral lesions. Oral Oncol 2013;49:1121-8. [DOI] [PubMed] [Google Scholar]
- 153.Cervigne NK, Reis PP, Machado J, et al. Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Human Mol Genet 2009;18:4818-29. [DOI] [PubMed] [Google Scholar]
- 154.Turchinovich A, Cho WC. The origin, function and diagnostic potential of extracellular microRNA in human body fluids. Front Genet 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mico-Martinez P, Garcia-Gimenez JL, Seco-Cervera M, et al. miR-1226 detection in GCF as potential biomarker of chronic periodontitis: A pilot study. Med Oral Patol Oral Cir Bucal 2018;23(3):e308-e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou W, Su L, Duan X, et al. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol Immunol 2018;101:608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xie YF, Shu R, Jiang SY, et al. MicroRNA-146 inhibits proinflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J Inflamm (London, England) 2013;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nisha KJ, Janam P, Harshakumar K. Identification of a novel salivary biomarker miR-143-3p for periodontal diagnosis: A proof of concept study. J Periodontol 2019;90:1149-59. [DOI] [PubMed] [Google Scholar]
- 159.Venugopal P, Koshy T, Lavu V, et al. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J Cell Physiol 2018;233:5877-84. [DOI] [PubMed] [Google Scholar]
- 160.Xiao W, Bao ZX, Zhang CY, et al. Upregulation of miR-31 *is negatively associated with recurrent/newly formed oral leukoplakia. PLoS One 2012;7:e38648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Irani S, Shokri G. The Role of miR-143, miR-145, and miR- 590 in Expression Levels of CD44 and Vascular Endothelial Cadherin in Oral Squamous Cell Carcinoma. Middle East J Cancer 2019;10:194-204. [Google Scholar]
- 162.Henson BJ, Bhattacharjee S, O’Dee DM, et al. Decreased expression of miR-125b and miR-100 in oral cancer cells contributes to malignancy. Genes Chromos Cancer 2009;48:569-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang L, Li X, Yan H, Huang L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: A PRISMA- compliant systematic review and meta-analysis. Medicine 2018;97:e9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu YC, Ning L, Tu YK, et al. Salivary biomarker combination prediction model for the diagnosis of periodontitis in a Taiwanese population. J Formosan Med Assoc Taiwan yi zhi 2018;117:841-8. [DOI] [PubMed] [Google Scholar]
- 165.Lundmark A, Johannsen G, Eriksson K, et al. Mucin 4 and matrix metalloproteinase 7 as novel salivary biomarkers for periodontitis. J Clin Periodontol 2017;44:247-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sorsa T, Gursoy UK, Nwhator S, et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2016;70:142-63. [DOI] [PubMed] [Google Scholar]
- 167.Gai C, Camussi F, Broccoletti R, et al. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018;18:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Q, Gao P, Wang X, Duan Y. Investigation and identification of potential biomarkers in human saliva for the early diagnosis of oral squamous cell carcinoma. Clin Chim Acta Int J Clin Chem 2014;427:79-85. [DOI] [PubMed] [Google Scholar]
- 169.Bornstein MM, Andreoni C, Meier T, Leung YY. Squamous Cell Carcinoma of the Gingiva Mimicking Periodontal Disease: A Diagnostic Challenge and Therapeutic Dilemma. Int J Periodont Restor Dentistry 2018;38:253-9. [DOI] [PubMed] [Google Scholar]
- 170.Yoon TY, Bhattacharyya I, Katz J, et al. Squamous cell carcinoma of the gingiva presenting as localized periodontal disease. Quintess Int (Berlin, Germany: 1985) 2007;38:97-102. [PubMed] [Google Scholar]
- 171.Song JM, Woo BH, Lee JH, et al. Oral Administration of Porphyromonas gingivalis, a Major Pathogen of Chronic Periodontitis, Promotes Resistance to Paclitaxel in Mouse Xenografts of Oral Squamous Cell Carcinoma. Int J Mol Sci 2019;20:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hartner L. Chemotherapy for Oral Cancer. Dental Clin N Am 2018;62:87-97. [DOI] [PubMed] [Google Scholar]
- 173.Graziani F, Karapetsa D, Alonso B, Herrera D. Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontology 2017;75:152-88. [DOI] [PubMed] [Google Scholar]
- 174.Galvao-Moreira LV, da Cruz MC. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol 2016;53:17-9. [DOI] [PubMed] [Google Scholar]
- 175.Shekarchizadeh H, Khami MR, Mohebbi SZ, et al. Oral health status and its determinants among opiate dependents: a crosssectional study. BMC Oral Health 2019;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol 2016;34:4270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.AlJehani YA. Risk factors of periodontal disease: review of the literature. Int J Dent 2014;2014:182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 178.Irani S, Meschi M, Goodarzi A. Influence of Education on Oro-dental Knowledge among School Hygiene Instructors. J Dental Res Dental Clin Dental Prospects 2009;3:56-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


