Abstract
Consumption of added sugars in the US is estimated to be approximately 1.5 times recommended levels and has been linked to increased risk for developing chronic diseases. We hypothesized that relative to sugar, honey would reduce energy intake and improve serum lipid profiles. To test this, we assessed the short-term (1-week) and relatively long-term (1-month) effects of honey versus sucrose on changes in dietary intake and serum lipid concentrations. Thirty-seven apparently healthy subjects (21 males; 16 females) aged 24–57 years (BMI = 17.6–37.2 kg/m2) completed two 4-week trials in a randomized, cross-over design separated by ≥4-week washout. During each trial, subjects consumed either clover honey or sucrose providing 1.2 g/kg/day of carbohydrate under free-living conditions with instructions to avoid changing their habitual food intake. Serum triglyceride (TG) concentrations were elevated (P < .05) after 1 week for both trials but only remained elevated (P < .05) at the 4-week time-point during sucrose consumption. The elevation after 1 week during the honey trial was concurrent with a transient increase (P < .05) in body weight. No effects on serum concentrations of insulin, total cholesterol, low density lipoprotein-cholesterol, or high density lipoprotein-cholesterol were detected for either trial. Subjects consumed significantly less energy (P < .05), carbohydrate (P < .005), sugars (P < .05), and saturated fat (P < .05) during the honey trial. These data suggest that honey may serve as a favorable substitute for sucrose with regard to reduced energy intake, carbohydrate and sugars, without negatively influencing serum lipid concentrations.
Keywords: Honey, Sucrose, Fructose, Sweetener, Blood lipids
1. Introduction
Restriction of sugar intake is among the most commonly advocated public health strategies, as it is believed to prevent the development of chronic diseases. Several studies have shown that higher intake of free sugars is associated with elevated risk and incidence of chronic diseases such pancreatic cancer [1, 2], hyperlipidemia [3, 4], diabetes [5] and obesity [6, 7]. The current Dietary Guidelines for Americans advise restricting added sugar intake to less than 10% of the total daily energy intake [8], which is in agreement with the approach set by the joint committee of the World Health Organization Guideline for Sugars Intake for Adults and Children [9].
Unlike sugar, honey is a heterogeneous material, believed to contain approximately 200 different substances, many of which may be bioactive [10, 11]. Results from several studies suggest that honey may favorably modulate chronic disease risk factors. For instance, honey has been shown to increase antioxidant status [12], improve blood lipid profiles in healthy, overweight, and hyperlipidemic patients [13, 14], decrease prostaglandin synthesis [15], and result in lower postprandial glycemia and insulinemia in healthy subjects, compared to responses of more highly refined sugar mixtures [16]. Taken together, honey may be a favorable sweetening alternative to refined sweeteners.
Abnormal blood lipids, including elevated triglycerides (TG), total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C) and low high density lipoprotein-cholesterol (HDL-C) are independent risk factors for cardiovascular diseases [17-19]. Very limited studies have examined the acute [20] and the relatively long–term [12, 21] effects of honey consumption on blood lipids in humans. Furthermore, there are limited data regarding whether consumption of honey favorably improves blood lipids, relative to other sweeteners. A parallel-arm study by Al-Waili et al [13] demonstrated an improvement in the blood lipid profiles of healthy subjects and patients with diabetes and dyslipidemia after consuming honey (1.2 g CHO/kg body weight/day) for only 14 days. Additionally, using a parallel-arm design, Rasad et al [21] demonstrated that honey (70 g/day) can favorably alter blood lipids relative to sucrose in a cohort of young adults (aged 18–30 years). However, more research is needed to determine the short-term and long-term effects of honey consumption on blood lipids and to determine if differences in blood lipid responses vary between honey and sucrose. Considering honey and sucrose are two commonly used sweeteners, it is important to determine the effects of each sweetener on risk factors for chronic disease. Therefore, the purpose of the present study was to assess responses to both short-term (1 week) and relatively long-term (1 month) ingestion of clover honey consumption versus sucrose on changes in dietary intake and serum lipid concentrations (key risk factors for chronic disease) including TG, TC, LDL-C and HDL-C in young to middle-aged adults. We hypothesized that consuming honey would reduce energy intake and favorably affect the blood lipid profile (decreased TG, TC, LDL-C; increased HDL-C) compared to sucrose.
2. Methods and materials
2.1. Recruitment/screening procedures
Participants were recruited from the university campus and the local community by flyers and internet posting. Prior to visiting the laboratory, subjects were screened. If subjects did not smoke, have honey allergy, were not pregnant, had not been previously diagnosed with any disease resulting in metabolic disorders, did not chronically use medications known to affect metabolism, and did not participate in greater than 3 hours of structured exercise per week, they were invited to the laboratory for further screening. Subjects were not excluded on the basis of oral contraceptive use; however, since oral contraceptives can alter the blood lipid profile, female subjects were requested to report any changes in oral contraceptive use. Female participants not using oral contraceptives were included only if they reported having a regularmenstrual cycle.
If subjects chose to participate, they were asked to arrive to the laboratory following an overnight fast (12 h). Upon arrival to the laboratory, subjects were asked to read the informed consent and researchers explained the contents. Subjects were advised to ask questions for clarification. After signing the informed consent, researchers randomly assigned subjects to begin the study in one of two trials (sucrose or honey). All experimental procedures were approved by the San Diego State University Institutional Review Board and in accordance with Federal and institutional regulations and policies for the protection of the rights and welfare of human subjects. Adherence to the protocol was enhanced by frequent contact with the participants by telephone and provision of monetary and gift incentives.
2.2. Study participants, design and treatment
Forty participants (male [n = 21] and female [n = 19]) between 25–57 years of age were initially recruited, of which 37 participants (male [n = 21] and female [n = 16]) completed the study. Subjects completed the study following randomized crossover design, which consisted of two phases for 4 weeks each separated by a 4-week washout period. Subjects were asked to maintain normal eating and physical activity habits throughout the study. A 3-day food record including two weekdays and one weekend day was used to assess the habitual food intake of the participants prior to initiation of each trial and again during the final week of each trial. All food records were analyzed by computer using My Diet Analysis 2.0 powered by ESHA Research Nutrition Software and Database. Subjects reported to the laboratory at baseline, after 1 week and after 4 weeks of each phase following 12-hour overnight fasts. Participants were asked to refrain from alcohol, caffeine, and structured exercise except for the walking required for daily living activities for the 24-hour period prior to each lab visit. This information was confirmed by researchers via use of a daily checklist prior to initiating data collection.
2.3. Test foods
Sucrose was purchased in bulk from a local grocery store. Clover honey was obtained from four different sources to account for variability in composition and to produce a composite food source. Three of the honey samples were processed clover honey obtained from Golden Heritage Foods (Hillsboro, KA), Smitty Bee Honey, Inc. (Defiance, IA), Millers Honey Company (Coulton, CA). The fourth sample was raw clover honey obtained from Marshall’s Farm Natural Honey (American Canyon, CA). Honey was stored in the dark between 2–8 ° C. The honey was pooled in equal amounts to formone composite sample for all feedings. Honey and sucrose were supplied as flavored beverages during their respective trials. Beverages were prepared to provide 1.2 g carbohydrate/kg body weight each day. Three drinks (250–300 ml each) containing 0.4 g carbohydrate/kg bodyweight were supplied for daily consumption. To provide variability and enhance adherence to the protocol, all beverages were flavored with 0.4 g/drink of food grade flavors; orange, strawberry, lime and fruit punch from Mission Flavors & Fragrance Inc. (Foothill Ranch, CA). To assess participant compliance, participants were provided with a checklist to indicate when beverages were consumed. Honey and granulated sugar were analyzed for monosaccharide and macronutrient contents by Q-Laboratories (Cincinnati, OH). Honey samples were tested for authenticity using total carbon SIRA and protein carbon SIRA tests by Kruger Food Laboratories, Inc. (Billerica, MA). Three-day food records (two weekdays and one weekend day) were collected and averaged.
2.4. Physical activity
To ensure that activity was similar between trials, participants were given pedometers (Optimal Health Products, San Antonio, TX) to monitor their steps counts on two weekdays and one weekend day each week.
2.5. Body composition
Participants were weighed on a double beam scale to the nearest±0.1 pound. Percent body fat was determined by hydrostatic weighing. Three subjects reported being uncomfortable with the hydrostatic weighing method and were evaluated for body composition by skinfold measurements, and percent body fat was calculated [22, 23].
2.6. Venous serum collection
Blood samples were collected using a 25-gaugebutterflyneedle in 13 mL vials containing gel separator and clot activator (Fisher Scientific, Pittsburgh, PA), allowed to clot for 15 minutes and centrifuged at 1200g for 10 minutes at 4 °C. Serum was stored at – 80 °C for batch analysis. For female subjects, all initial and 1-month blood collections occurred during the same phase of the menstrual cycle.
2.7. Biochemical assays
Serum concentrations of TG (Catalog #: 2100430), TC (Catalog #: 1010430), and HDL-C (Catalog #: 1010225) were analyzed colorimetrically with kits from Stanbio Laboratory (Boerne, TX). LDL-C was calculated using the Friedewald equation [24]. Serum insulin was analyzed by radioimmunoassay using Coat-a-Count kit from Diagnostic Products Corporation (Los Angeles, CA).
2.8. Statistical analyses
Based on previous work from our laboratory in healthy subjects who consumed fructose-rich snack foods for twoweeks, and considering changes in serum triglycerides was a primary outcome of this study, the number of subjects anticipated to detect a difference in serum triglyceride concentrations using a cross-over design is 15 per group to achieve 80% power with an alpha of .05. This calculation used conservative estimates of triglyceride difference of 22 ± 29 mg/dL. Data were analyzed using Statistical Package for Social Sciences computer software (SPSS, Version 16.0) and data were reported as means ± SEM. Accumulated steps data were analyzed using paired comparison’s t-tests. Dietary data were analyzed using a 2 (sweetener) × 2 (time-point) repeated measure ANOVA. Body weight, serum insulin, TG, TC, LDL-C and HDL-C concentrations were analyzed using a 2 (sweeteners) × 3 (time-points) repeated measures ANOVA. Significant main effects were followed by paired comparison’s t-tests when appropriate. Alpha was set at 0.05 and significance was determined at P < .05.
3. Results
3.1. Subjects
Thirty-seven participants (16 women; 21 men; mean age: 32.9 ± 1.7 years; BMI: 25.4 ± 0.7 kg/m2; percent body fat: 26.2 ± 1.3%.) of the 40 that were recruited successfully completed both phases of the study and were included in the statistical analyses (Fig. 1). Participants followed instructions and adhered to the study protocol as reflected in the small number of reported missed drinks throughout the study. Overall, 98.3% of the honey drinks and 98.5% of the sucrose drinks were reportedly consumed.
Fig. 1 –
Flow chart of sample sizes for subject recruitment, drop-out and final analyses.
3.2. Test foods
The clover honeys from the different sources were all authentic, pure honey samples (Table 1). All passed the pure honey criteria set by Kruger Food Laboratories, Inc. (Billerica, MA). Pure honey should not include carbons from cane or corn sugars, and the difference of the total carbon SIRA from the protein carbon SIRA should be ≤1.0. The compositions of honeys as determined by Q Laboratories, Inc. (Cincinnati, OH) are provided in Table 2. Sucrose was found to be >99% sucrose as tested by the same laboratory.
Table 1 –
Purity results for clover honey samples
| Marshall’s Farm Honey | Miller’s Honey | Busy Bee Honey | Smitty Bee Honey | |
|---|---|---|---|---|
| % Carbon from Cane or Corn | None | None | None | None |
| Total Carbon SIRA δ13 C (% ο) | −26.9 | −26.8 | −26.9 | −26.7 |
| Protein Carbon SIRA δ13 C (% ο) | −27.1 | −25.5 | −25.9 | −25.4 |
| Difference of Carbon SIRA | 0.2 | −1.3 | −1.0 | −1.3 |
Table 2 –
Composition (percentages) of 4 clover honeysa
| Marshall’s Farm Honey | Miller’s Honey | Busy Bee Honey | Smitty Bee Honey | Average | |
|---|---|---|---|---|---|
| Moisture | 16.23 | 17.11 | 17.53 | 19.41 | 17.57 |
| Ash | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Protein | 0.26 | 0.24 | 0.25 | 0.25 | 0.25 |
| Fat | 0.12 | 0.69 | 1.54 | 1.12 | 0.87 |
| Total Carbohydrates a | 83.29 | 81.86 | 80.58 | 79.12 | 81.21 |
| Sugar Profile | |||||
| Fructose | 41.2 | 40.6 | 40.1 | 38.9 | 40.2 |
| Glucose | 35.7 | 34.8 | 34.8 | 33.3 | 34.7 |
| Sucrose | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Maltose | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Lactose | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Other sugars a | 6.39 | 6.46 | 5.68 | 6.92 | 6.4 |
= determined by calculation.
3.3. Dietary intake
Dietary intake data are provided in Table 3. Statistical analyses were performed on the dietary intake of 32 subjects. Five subjects were excluded from the analyses for providing incomplete dietary data, due to poor participant adherence to the food record protocol despite all subjects receiving identical training. Main effects for increasing energy (P < .001), carbohydrate (P < .001) and sugars intake (P < .01) over time were detected. Post-hoc analyses revealed that during the sucrose trial, subjects consumed more energy (P < .05), carbohydrate (P < .05), and sugars (P < .05) compared to the honey trial. Additionally, within the sucrose trial, the average intake of energy was significantly (P < .01) increased from baseline. Energy intake was not significantly different during the honey trial in comparison to baseline.
Table 3 –
Dietary intake within and across trials (n = 32)
| Nutrient Daily Intake |
Honey Trial |
Sucrose Trial |
||
|---|---|---|---|---|
| Baseline | 4 weeks | Baseline | 4 weeks | |
| Energy (Kcals) | 1998 ± 116 | 2112 ± 102 * | 2103 ± 145 a | 2428 ± 170 a* |
| CHO (g) | 251 ± 17 a | 306 ± 13 a * | 265 ± 17 a | 356 ± 21 a * |
| % CHO of energy intake | 51 ± 2 a | 59 ± 2 a | 53 ± 2 a | 60 ± 2 a |
| Sugars (g) | 77 ± 6 a | 152 ± 6a * | 83 ± 7 a | 168 ± 10 a * |
| % sugar of energy intake | 16 ± 1 a | 30 ± 1 a | 16 ± 1 a | 29 ± 1 a |
| Proteins (g) | 79 ± 6 | 75 ± 5 * | 80 ± 7 | 85 ± 8* |
| % protein of energy intake | 16 ± 1 a | 14 ± 1 a | 15 ± 1 | 14 ± 1 |
| Fat (g) | 73 ± 6 | 64 ± 5 | 77 ± 8 | 73 ± 8 |
| % fat of energy intake | 33 ± 1 a | 27 ± 1 a | 32 ± 2 a | 26 ± 1 a |
| Saturated fatty acids (g) | 25 ± 2 a | 21 ± 2 a | 25 ± 3 | 23 ± 2 |
| Cholesterol (mg) | 261 ± 37 | 248 ± 31 | 237 ± 38 | 243 ± 36 |
| Fiber (g) | 21 ± 3 | 19 ± 3 | 20 ± 2 | 21 ± 2 |
Values are means ± SEM, significant differences (P < .05) between groups within the same time-point. Letter superscripts represent significant differences (P < .05) between groups within group differences between baseline and 4-weeks.
Total carbohydrate intake was significantly (P < .001) higher by week four compared to baseline in both trials. This is expected as a result of the additional honey and sucrose provided by the investigators. Baseline carbohydrate intake was not significantly different between trials; however, carbohydrate intake was significantly (P < .005) lower at the end of honey trial compared to the sucrose trial.
There was a significant (P < .001) increase in total sugar intake by the end of both trials. Baseline sugar intake was not significantly different between trials (P = .35). Sugar intake by the end of the sucrose trial was significantly (P < .05) higher than intake during the honey trial.
Total fat, fiber, and cholesterol consumption were not significantly different within and between trials. However, within the honey trial, subjects significantly (P < .05) reduced saturated fat intake from baseline to week four, but saturated fat intake at 4 weeks was not different between the honey and sucrose groups.
3.4. Physical activity
Average daily steps taken were similar during each trial, which provides assurance that physical activity was relatively consistent. Step counts were not significantly different (P = .80) between trials (honey trial = 7433 ± 604 steps; sucrose trial = 7303 ± 520 steps).
3.5. Body weight and body composition
No significant main effects for change in bodyweight over time (P = .14) or by sweetener (P = .97) were observed, and no interactive effect (P = .53) of time and sweetener was detected (Fig. 2A). Body weight changes from baseline are depicted in Fig. 2B and indicate that a transient increase (P < .005) in body weight at week occurred during the honey trial. Body weight was similar to baseline for both trials at the end of week four.
Fig. 2 –
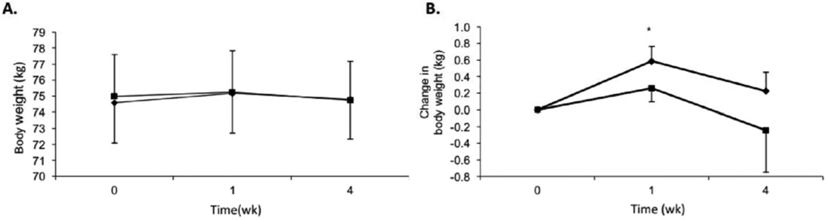
(A) Body weights (kg) and (B) change in body weights over time for honey (♦) and sucrose (■). Subjects n = 37, men: n = 21; women: n = 16. Data are presented as means ± SEM. *P < .05 from baseline within trial.
3.6. Biochemical analyses
A significant main effect for increasing fasting TG over time (P < .05) was detected (Fig. 3A). Neither a significant main effect for sweeteners (P = .60) nor interaction between sweeteners and time (P = .44) were observed. Paired comparison’s t-test revealed baseline fasting serum TG concentrations for the clover honey trial (120.7 ± 10.2 mg/dl) and sucrose trial (111.0 ± 7.4 mg/dl) were not significantly different (P = .40). Clover honey and sucrose significantly (P < .05) increased fasting serum TG concentrations from baseline to week one by 17.3 ± 8.3 mg/dL and 13.8 ± 6.6 mg/dL, respectively (Fig. 3B). By the end of week four, the TG concentration during the sucrose trial had increased from baseline by 18.0 ± 9.0 mg/dL, whereas the TG concentration declined to a level not significantly different from baseline during the honey trial. The elevated fasting serum TG concentration observed at the end of week one in both trials was accompanied by the transient increase in body weight (Fig. 2A and B) for the honey trial.
Fig. 3 –
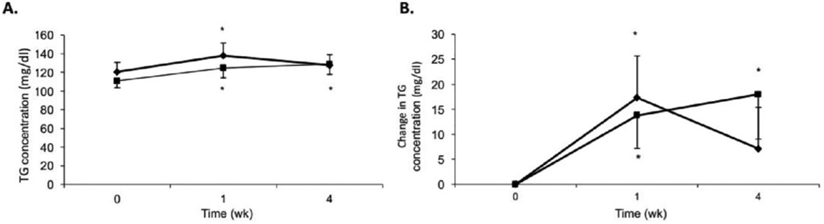
(A) Mean fasting serum TG concentrations and (B) change in mean fasting serum TG over time for honey (♦) and sucrose (■). Subjects n = 37, men: n = 21; women: n = 16. Data are presented as means ± SEM. *P < .05 from baseline within trial.
No significant main effects for change in TC over time (P = .72) or by sweetener (P = .86) were observed, and no interactive effect (P = .76) of time and sweetener was detected (Table 4). No significant main effects for change over time (P = .84) or by sweetener (P = .94) nor an interaction (P = .10) of time and sweetener were observed for fasting serum LDL-C. No significant main effects for change over time (P = .94) or by sweetener (P = .78) nor an interaction (P = .67) of time and sweetener were detected for HDL-C.
Table 4 –
Mean concentrations of TC, LDL-C and HDL-C across trials (n = 37)
| Honey Baseline |
Honey Week 1 |
Honey Week 4 |
Sucrose Baseline |
Sucrose Week 1 |
Sucrose Week 4 |
|
|---|---|---|---|---|---|---|
| TC (mg/dl) | 177.9 ± 5.4 | 180.1 ± 6.4 | 177.4 ± 5.2 | 175.7 ± 5.4 | 177.6 ± 5.5 | 178.7 ± 5.1 |
| LDL-C (mg/dl) | 109.9 ± 4.4 | 108.6 ± 5.4 | 108.9 ± 4.7 | 109.5 ± 4.9 | 108.2 ± 4.9 | 108.3 ± 4.5 |
| HDL-C (mg/dl) | 43.9 ± 1.8 | 43.9 ± 1.8 | 43.3 ± 1.6 | 44.0 ± 1.8 | 44.4 ± 1.8 | 44.6 ± 1.8 |
Statistical analysis of serum insulin concentrations was conducted on 34 subjects; 3 subjects were excluded from the analyses for missing data at a testing time point. No significant main effects for change in fasting serum insulin over time (P = .90) or by sweetener (P = .65) were detected. No interaction effect (P = .37) of time and sweetener was observed (Fig. 4).
Fig. 4 –
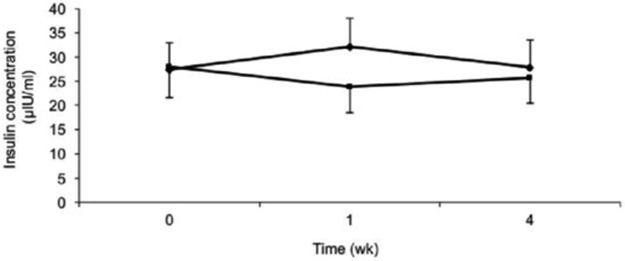
Fasting serum insulin concentrations over time for honey (♦) and sucrose (■). Subjects n = 34, men: n = 18; women: n = 16. Data are presented as means ± SEM.
4. Discussion
Results of this study only partially support our key hypotheses; therefore, we partially reject the original hypotheses. The primary purpose of the study was to assess responses to the short-term (1 week) and relatively long-term (1 month) effects of clover honey consumption compared to sucrose on changes in fasting serum lipid concentrations. Results revealed that consumption of clover honey at a rate of 1.2 g of carbohydrate per kilogram body weight for up to 1 month produced modestly positive dietary and triglyceride effects relative to sucrose; however, no positive lipid effects were detected within the clover honey trial. Compared to sucrose, clover honey consumption resulted in a significantly lower intake of energy, carbohydrate, sugars and fat as well as lower TG concentrations at the end of 4 weeks. However, no changes to insulin, total, LDL and HDL-C were observed within or between groups.
Although not measured, the observed lower food intake with honey consumption may be due to changes in leptin concentrations or sensitivity. Significantly lower food intake, leptin concentrations and weight gain were observed in rats fed a 20% clover honey diet compared to a 20% sucrose diet [25]. Significantly less weight gain was also observed in rats fed 10% honey diet compared to 8% sucrose and a mixed sugars diet [26]. It is reported that long-term high sucrose intake results in leptin resistance and lower circulating leptin concentration mediated by high levels of free fatty acids, hypertriglyceridemia, and insulin resistance [27]. The greater energy intake during the sucrose trial in this study might be partially explained by a sucrose-induced leptin resistance. It is also possible that the difference in honey sweetness or other honey components might have influenced satiety or increased leptin sensitivity to a greater extent than sucrose resulting in less energy intake during that trial. However, since leptin was not measured in the present study, it is not possible to confirm this putative mechanism.
It has been reported that high carbohydrate intake by healthy people can induce a transient increase in fasting TG that peaks in 1 week, and that this increase might be permanent for susceptible individuals [28]. In the present study, the transient increase in fasting TG was detected during the honey trial, but the change persisted throughout the sucrose trial. The return to approximately basal concentrations of TG observed during the honey trial could be partially explained by the significantly lower intake of energy, carbohydrate, sugars and/or fat compared to that observed during the sucrose trial. Additionally, changes could also be related to other constituents of honey such as non-digestible oligosaccharides, isomaltulose or other sugars (see Table 2) that can potentially influence TG production and/or clearance. Carbohydrate-induced hypertriglyceridemia is proposed to be mediated by hyperinsulinemia [28, 29]; however, in the absence of changes in fasting insulin concentration during both trials in this study, which is in line with other reports [4, 29-31], it is more likely that honey either modified TG clearance or induced lower postprandial insulin concentrations compared to those induced by sucrose.
A previous report has shown that a high carbohydrate diet with 25% of CHO from sucrose exerts a greater postprandial insulin response without detectable changes in fasting insulin levels [29]. Additionally, fasting insulin sensitivity did not change in healthy subjects consuming a 25% sucrose diet versus a 10% sucrose diet for 6 weeks [4]. Furthermore, a 75% CHO diet with 35% of energy from simple sugars for 2 weeks did not affect fasting insulin levels in healthy subjects [30]. However, all of these earlier studies reported higher fasting TG levels after consumption of these sugar-rich, high carbohydrate diets. Absence of changes in fasting plasma fatty acids and insulin after feeding a low-fat diet (15% fat) for 8 months suggests that the high TG levels were due to lower lipoprotein lipase activity and lower TG clearance rather than an increase in hepatic TG synthesis [31].
It has been reported that hypertriglyceridemia induced by low fat, high CHO diets is due to reduced clearance not overproduction of VLDL-TG [32]. As TC secretion is proportional to VLDL production, and VLDL is a precursor to LDL [33], and given also that reduced VLDL-TG clearance does not increase plasma cholesterol [32], the absence of changes in total cholesterol and LDL-C levels observed in this study strongly suggests that the persisted high TG levels during the sucrose trial might be related to decreased TG clearance rather than its increased production. Additionally, it is possible that clover honey modified the level or activity of LPL, which resulted in a transient TG increase. However, we did not measure lipoprotein lipase activity in this study to confirm this potential mechanism.
Some reports have related the lack of changes in the blood lipids with low fat diets of moderate to high amounts of sugars to the weight loss accompanying the feeding period [34-36]. In the present study, although the subjects’ weight almost returned to baseline by the end of both trials, the TG levels were persistently elevated for the sucrose trial. This elevation might support the speculation of decreased clearance of TG rather than an increased production of VLDL. A previous study which assessed the prolonged effects of consuming honey versus sucrose, found that feeding 70 g of sucrose to obese subjects (>30 kg/m2) for 30 days did not increase TG levels but promoted a 4% reduction in TG in the subjects with elevated TG (>150 mg/dL) [14], which is contrary to the previous literature and to the findings in the present study. Additionally, the reduction in TG levels after feeding unprocessed honey for 30 days was more pronounced (19% vs.11%) in the subjects with elevated TG values (>150 mg/dL) compared to the reductions obtained for the whole cohort [14]. Frayn and Kingman [37] stated that the dose of the sugars does not solely determine the response of sucrose on fasting blood lipids. Those researchers suggest that the potential response should be viewed within the context of many factors other than the diet such as presence of obesity, level of physical activity, sensitivity in responses to diet changes, and genetic and metabolic abnormalities.
Busserolles et al [38] suggested that the fructo-oligosaccharides (FOS) content of honey might be responsible for the hypotriglyceridemic effect of honey observed in rats. The FOS-related hypothesis is based on the capacity of FOS to modulate the activity of lipogenic enzymes [39]. Although we cannot dismiss the possibility that FOS may play a role in the hypotriglyceridemic effect observed in that study, it is unlikely that FOS are the major components of honey that drive the hypotriglyceridemic effect for several reasons: 1) Aside from the marked species differences between rodents and humans as reflected in lipid metabolism and the intensity of the factors that would modulate these processes in these two species, the dose that might induce changes in animals may be too low to impart a physiological response in humans; 2) Studies on human subjects who were fed doses comparable to and even higher than those fed to animal models did not demonstrate similar effects; 3) No changes in concentrations of TG, total cholesterol, LDL-C or HDL-C were observed after feeding 30 mildly hypercholesterolemic subjects 10.6 g FOS/day for 2 months [40]; 4) No changes in blood lipids were detected after feeding 12 normal healthy subjects 20 g FOS/day for 4 weeks [41]. Important to note, the average FOS content in honey is 3–4% [38]. The dose that imparted changes in rats is 10 g/100 g diet [39].
The lack of changes in total cholesterol, LDL-C and HDL- C in this study with increasing sucrose intake by about 13–14% of total energy intake above the subjects’ usual intake of 16% are in line with previous reports [28-30], which may be due to several factors. First, the portion of extra sweeteners given might not be sufficient to induce changes in the blood cholesterol profile of healthy subjects who are moderately active. Additionally, consuming a diet composed of 35% of total energy from simple sugars for 2 weeks did not alter total cholesterol concentration of healthy subjects [30]. Furthermore, switching from a high fat diet (49% of total energy) to a low fat diet (70% CHO) in healthy people by increasing sucrose intake reduced total cholesterol and LDL-C when sucrose comprised up to 18% of total energy intake [28]. In fact, the concentrations of these lipids were maintained and not elevated when diets containing more than 36% of energy from sucrose diets were consumed [28]. Second, it has been reported that high intake of cholesterol and saturated fatty acids raise total cholesterol and LDL-C [42, 43]. The higher total cholesterol and LDL-C levels observed when healthy subjects consumed diets comparable in composition to the food intake of our study (29% fat, 59% CHO and 23.2% sucrose) were partially explained by the higher saturated fat and cholesterol content in that diet compared to the other high CHO, 2.5% sucrose diet [34]. Moreover, the 2.5% and 23.2% sucrose diets failed to induce changes in HDL-C levels. It was concluded that high CHO diets decrease HDL-C levels due to lower fat content not due to a sucrose effect, per se [28, 29, 31, 34]. It is likely that the subjects’ moderate physical activity and the 26–27% fat in their diets were sufficient to prevent a decline in HDL-C levels in the present study.
The strengths of the present study are the inclusion of both men and women, employment of a crossover design, and monitoring average daily physical activity to verify no major differences in physical activity between trials. Although conducting the study under free-living conditions could be considered a strength relative to other studies, this study is not without limitations. Limitations of the study include: i) the different food habits and the amount of food intake among subjects might have made it more difficult to identify specific dietary and or other environmental factors underlying nonsignificant outcomes; ii) the amount of honey given to subjects in the present study almost doubled the subjects’ usual sugar intake (16% to 29–30% of total energy intake). Although the design of this study allowed us to evaluate differences between feedings of honey and sucrose, the amount of honey used in this study is likely not practical on a daily basis; therefore, whether substitution of lower amounts of honey for sucrose would yield similar results is unknown; iii) this study used clover honey and was conducted on healthy people; therefore, the results cannot be generalized to all types of honey or to specific disease populations; iv) FFA, LPL activity, and adipokines were not monitored in this study, which may have allowed for us to understand the possible mechanisms behind the observed positive outcomes.
In conclusion, data from the present study support previous research indicating that honey produces limited, modest health benefits relative to sucrose. Clover honey failed to produce similar negative serum TG responses (change in TG concentration from 1 to 4 weeks) that were detected during the sucrose trial. Importantly, this response should be considered as very modest, since there were no differences between the honey and sucrose trials at the end of 4 weeks. Furthermore, compared to sucrose, honey promoted lower intake of energy, carbohydrate, and sugars. Additionally, honey displaced a small amount of saturated fat from the trials, although saturated fat intake was not statistically different between trials. The lower energy intake during honey trial may partially explain the difference in TG responses; however, body weight changes were not different and we cannot exclude the possibility of metabolic roles that other honey components may have played. Insulin, total cholesterol, LDL-C, and HDL-C responses did not change within trials or differ between the trials. Future studies are needed to investigate the possible mechanisms by which honey influences TG production and/or clearance and the metabolic and hormonal regulators of food intake.
Acknowledgment
Authors express no conflict of interest. This work was supported by a grant from the National Honey Board. ZSC is currently supported by the National Institutes of Health award T32 DK007135-44.
Abbreviations:
- TG
Triglycerides
- TC
total cholesterol
- LDL-C
low density lipoprotein-cholesterol
- VLDL-C
very low density lipoprotein-cholesterol
- HDL-C
high density lipoprotein cholesterol
- CHO
carbohydrate
- Kg
kilogram
- Mg
milligram
- dL
deciliter
- SPSS
statistical package for the social sciences
- ANOVA
analysis of variance
- SEM
standard error of the mean
- SIRA
stable isotope ratio analysis
- FOS
fructo-oligosaccharide
REFERENCES
- [1].Larsson SC, Bergkvist L, Wolk A. Consumption of sugar and sugar-sweetened foods and the risk of pancreatic cancer in a prospective study. Am J Clin Nutr 2006;84:1171–6. [DOI] [PubMed] [Google Scholar]
- [2].Michaud DS, Liu S, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Dietary sugar, glycemic load, and pancreatic cancer risk in a prospective study. J Natl Cancer Inst 2002;94: 1293–300. [DOI] [PubMed] [Google Scholar]
- [3].Daly ME, Vale C, Walker M, Alberti KG, Mathers JC. Dietary carbohydrates and insulin sensitivity: a review of the evidence and clinical implications. Am J Clin Nutr 1997;66:1072–85. [DOI] [PubMed] [Google Scholar]
- [4].Black RN, Spence M, McMahon RO, Cuskelly GJ, Ennis CN, McCance DR, et al. Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: a randomized controlled trial. Diabetes 2006;55:3566–72. [DOI] [PubMed] [Google Scholar]
- [5].Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC. Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- [6].Malik VS, Schulze MB. Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33: 2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. Available at: http://health.gov/dietaryguidelines/2015/guidelines/. Accessed on June 12, 2019. [Google Scholar]
- [9].World Health Organization. Guidelines for sugar intake for adults and children. 2015.
- [10].Erejuwa OO, Sulaiman SA, Wahab MS. Honey—a novel antidiabetic agent. Int J Biol Sci 2012;8:913–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Küçük M, Kolaylı S. Karaoğlu Ş, Ulusoy E, Baltaci C, Candan F. biological activities and chemical composition of three honeys of different types from Anatolia. Food chemistry: science. Director 2007:526–34. [Google Scholar]
- [12].Al-Waili NS. Effects of daily consumption of honey solution on hematological indices and blood levels of minerals and enzymes in normal individuals. J Med Food 2003;6:135–40. [DOI] [PubMed] [Google Scholar]
- [13].Al-Waili NS. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: comparison with dextrose and sucrose. J Med Food 2004;7:100–7. [DOI] [PubMed] [Google Scholar]
- [14].Yaghoobi N, Al-Waili N, Ghayour-Mobarhan M, Parizadeh SM, Abasalti Z, Yaghoobi Z, et al. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. ScientificWorldJournal 2008;8:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Al-Waili NS. Effects of honey on the urinary total nitrite and prostaglandins concentration. Int Urol Nephrol 2005;37:107–11. [DOI] [PubMed] [Google Scholar]
- [16].Al Khalidi AJ, Faruk H. Tawfiq Naji H. Effects of bees honey, zahdi date and its syrup on blood glucose and serum insulin of diabetics. Food and Agricultural Organization of the United Nations; 1982. p. 631–43. [Google Scholar]
- [17].Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47. [DOI] [PubMed] [Google Scholar]
- [18].Liu J, Hong Y, D’Agostino RB, Wu Z, Wang W, Sun J, et al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese multi-provincial cohort study. JAMA 2004;291:2591–9. [DOI] [PubMed] [Google Scholar]
- [19].Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309–16. [DOI] [PubMed] [Google Scholar]
- [20].Katsilambros NL, Philippides P, Touliatou A, Georgakopoulos K, Kofotzouli L, Frangaki D, et al. Metabolic effects of honey (alone or combined with other foods) in type II diabetics. Acta Diabetol Lat 1988;25:197–203. [DOI] [PubMed] [Google Scholar]
- [21].Rasad H, Entezari MH, Ghadiri E, Mahaki B, Pahlavani N. The effect of honey consumption compared with sucrose on lipid profile in young healthy subjects (randomized clinical trial). Clin Nutr ESPEN 2018;26:8–12. [DOI] [PubMed] [Google Scholar]
- [22].Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr 1978;40:497–504. [DOI] [PubMed] [Google Scholar]
- [23].Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc 1980; 12:175–81. [PubMed] [Google Scholar]
- [24].Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- [25].Nemoseck TM, Carmody EG, Furchner-Evanson A, Gleason M, Li A, Potter H, et al. Honey promotes lower weight gain, adiposity, and triglycerides than sucrose in rats. Nutr Res 2011;31:55–60. [DOI] [PubMed] [Google Scholar]
- [26].Chepulis LM. The effect of honey compared to sucrose, mixed sugars, and a sugar-free diet on weight gain in young rats. J Food Sci 2007;72:S224–9. [DOI] [PubMed] [Google Scholar]
- [27].Rossi AS, Lombardo YB, Lacorte JM, Chicco AG, Rouault C, Slama G, Rizkalla SW. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol 2005; 289:R486–R94. [DOI] [PubMed] [Google Scholar]
- [28].Albrink MJ, Ullrich IH. Interaction of dietary sucrose and fiber on serum lipids in healthy young men fed high carbohydrate diets. Am J Clin Nutr 1986;43:419–28. [DOI] [PubMed] [Google Scholar]
- [29].Coulston AM, Liu GC, Reaven GM. Plasma glucose, insulin and lipid responses to high-carbohydrate low-fat diets in normal humans. Metabolism 1983;32:52–6. [DOI] [PubMed] [Google Scholar]
- [30].Mittendorfer B, Sidossis LS. Mechanism for the increase in plasma triacylglycerol concentrations after consumption of short-term, high-carbohydrate diets. Am J Clin Nutr 2001;73: 892–9. [DOI] [PubMed] [Google Scholar]
- [31].Kasim-Karakas SE, Almario RU, Mueller WM, Peerson J. Changes in plasma lipoproteins during low-fat, high-carbohydrate diets: effects of energy intake. Am J Clin Nutr 2000;71: 1439–47. [DOI] [PubMed] [Google Scholar]
- [32].Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest 1999;104:1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Parks EJ. Effect of dietary carbohydrate on triglyceride metabolism in humans. J Nutr 2001;131:2772S–74S. [DOI] [PubMed] [Google Scholar]
- [34].Marckmann P, Raben A, Astrup A. Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: effects on blood lipids, factor VII coagulant activity, and fibrinogen. Metabolism 2000;49:731–5. [DOI] [PubMed] [Google Scholar]
- [35].Saris WH, Astrup A, Prentice AM, Zunft HJ, Formiguera X, Verboeket-van de Venne WP, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. The carbohydrate ratio Management in European National diets. Int J Obes Relat Metab Disord 2000; 24:1310–8. [DOI] [PubMed] [Google Scholar]
- [36].Surwit RS, Feinglos MN, McCaskill CC, Clay SL, Babyak MA, Brownlow BS, et al. Metabolic and behavioral effects of a high-sucrose diet during weight loss. Am J Clin Nutr 1997;65: 908–15. [DOI] [PubMed] [Google Scholar]
- [37].Frayn KN, Kingman SM. Dietary sugars and lipid metabolism in humans. Am J Clin Nutr 1995;62:250S–61S; discussion 61S–63S. [DOI] [PubMed] [Google Scholar]
- [38].Busserolles J, Gueux E, Rock E, Mazur A, Rayssiguier Y. Substituting honey for refined carbohydrates protects rats from hypertriglyceridemic and prooxidative effects of fructose. J Nutr 2002;132:3379–82. [DOI] [PubMed] [Google Scholar]
- [39].Delzenne NM, Kok NN. Biochemical basis of oligofructose-induced hypolipidemia in animal models. J Nutr 1999;129: 1467S–70S. [DOI] [PubMed] [Google Scholar]
- [40].Giacco R, Clemente G, Luongo D, Lasorella G, Fiume I, Brouns F, et al. Effects of short-chain fructo-oligosaccharides on glucose and lipid metabolism in mild hypercholesterolaemic individuals. Clin Nutr 2004;23:331–40. [DOI] [PubMed] [Google Scholar]
- [41].Luo J, Rizkalla SW, Alamowitch C, Boussairi A, Blayo A, Barry JL, et al. Chronic consumption of short-chain fructooligosac-charides by healthy subjects decreased basal hepatic glucose production but had no effect on insulin-stimulated glucose metabolism. Am J Clin Nutr 1996;63:939–45. [DOI] [PubMed] [Google Scholar]
- [42].Fielding CJ, Fielding PE. Cholesterol and caveolae: structural and functional relationships. Biochim Biophys Acta 2000; 1529:210–22. [DOI] [PubMed] [Google Scholar]
- [43].Spady DK, Woollett LA, Dietschy JM. Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Annu Rev Nutr 1993;13:355–81. [DOI] [PubMed] [Google Scholar]


