Abstract
Objective
The nucleus pulposus of the human intervertebral disc contains 2 cell types: notochordal (NC) and mature nucleus pulposus (MNP) cells. NC cell loss is associated with disc degeneration and this process may be initiated by mechanical stress and/or nutrient deprivation. This study aimed to investigate the functional responses of NC and MNP cells to hydrostatic pressures and glucose restriction.
Design
Bovine MNP and NC cells were cultured in 3-dimensional alginate beads under low (0.4-0.8 MPa) and high (1.6-2.4 MPa) dynamic pressure for 24 hours. Cells were cultured in either physiological (5.5 mM) glucose media or glucose-restriction (0.55 mM) media. Finally, the combined effect of glucose restriction and high pressure was examined.
Results
Cell viability and notochordal phenotypic markers were not significantly altered in response to pressure or glucose restriction. MNP cells responded to low pressure with an increase in glycosaminoglycan (GAG) production while high pressure significantly decreased ACAN gene expression compared with atmospheric controls. NC cells showed no response in matrix gene expression or GAG production with either loading regime. Glucose restriction decreased NC cell TIMP-1 expression but had no effect on MNP cells. The combination of glucose restriction and high pressure only affected MNP cell gene expression, with decreased ACAN, Col2α1, and ADAMTS-5 expression.
Conclusion
This study shows that NC cells are more resistant to acute mechanical stresses than MNP cells and provides a strong rationale for future studies to further our understanding the role of NC cells within the disc, and the effects of long-term exposure to physical stresses.
Keywords: hydrostatic pressure, glucose, notochordal cells, alginate culture, extracellular matrix
Introduction
The nucleus pulposus develops from the embryonic notochord.1-3 However, during aging and disease, notochordal (NC) cells are gradually replaced by “chondrocyte-like” mature nucleus pulposus (MNP) cells.1,3-5 The ratio of these cell types in the adult intervertebral discs (IVDs) varies with species, age, and disease.6-8 Humans retain a small proportion of NC cells, typically less than 20% of the total NP cell population into adulthood.5,9,10 While the adult human NP is a fibrous and opaque tissue, species that retain higher proportions of NC cells, such as rats, rabbits, and pigs, have a proteoglycan-rich, gelatinous, and translucent nucleus.6,11
Differences in extracellular matrix (ECM) composition across species and disease states are believed to underlie susceptibility to structural failure.1,8,12-14 It has therefore been hypothesized that loss of NC cells is an initiating factor of disc degeneration. Previous studies have suggested that mechanical stress or nutrient restriction in large animal discs initiate the death or differentiation of NC cells.15-18 In vivo, nucleus pulposus cells are exposed to dynamic hydrostatic pressures, ranging from 0.1 to 2.3 MPa for normal activity.19 In vivo disc-clamp and ex vivo studies have shown that in addition to a denser and more fibrous ECM, the proportion of NC cells decreases under excessive mechanical stress.17,20-22 Although numerous studies have investigated the effect of mechanical stimulation on nucleus pulposus cells, these studies have used cells separately isolated from either NC or MNP cell-rich tissues. Table 1 summarizes the findings from in vitro hydrostatic pressure studies using different animal models. While the studies differ in the magnitude and duration of loading, studies using predominately MNP cell-rich human or bovine NP cells, consistently show that high pressure causes a decrease in proteoglycan expression.23-25 In contrast, the effect of hydrostatic pressure on NC cells remains unclear with reports that both low and high pressure loading can increase proteoglycan expression.26-31
Table 1.
A Summary of Studies That Examined the Effects of Hydrostatic Pressure on Nucleus Pulposus Cells In Vitro from a Range of Species.
| Authors | Animal (Predominate Cell Type) | Pressures (MPa) Range Applied | Summary of Results |
|
|---|---|---|---|---|
| Collagena | Proteoglycanb | |||
| Kasra et al. (2003)30 | Rabbit (NC) | 0.75-3 | No change 0.75-2.5 MPa ↓ 3 MPa |
Not measured |
| Hutton et al. (2001)32 | Dog (NC) | 0.35 and 1 | ↑ 0.35 MPa ↓ 1 MPa |
↓ 0.35 MPa ↑ 1 MPa |
| Gokorsch et al. (2004)28 | Pig (NC) | 0.4, 3.4, and 6.0 | ↑ 0.4 MPa ↓ 6 MPa |
↑ 0.4 MPA ↓ 3.4 and 6 MPa |
| MacLean et al. (2004)31 | Rat (NC) | 0.2 and 1 | No change at 0.2 or 1 MPa | ↑ 1 MPa |
| Purmessur et al. (2013)33 | Pig (NC) | 0.5-2 | No change | ↑ PG staining No change in aggrecan gene expression |
| Wang et al. (2007)29 | Rabbit (NC) | 0.5 and 1 | ↑ 0.5 MPa ↑ 1 MPa |
↑ 0.5 MPa ↑ 1 MPa |
| Sowa et al. (2011)27 | Rabbit (NC) | 0.7, 2, and 4 | Not measured | ↑ 0.7 MPa No change at 2 or 4 MPa |
| Neidlinger-Wilke et al. (2006)22 | Human (MNP) | 0.25 and 2.5 | No change | ↓ 2.5 MPa |
| Neidlinger-Wilke et al. (2006)22 | Bovine (MNP) | 0.25 and 2.5 | ↑ 0.25 MPa ↓ 2.5 MPa |
↑ 0.25 MPa ↓ 2.5 MPa |
| Ishihara et al. (1996)23 | Bovine (MNP) | 2.5-10 | Not measured | ↓ PG with increasing pressure (2-10 MPa) |
| Le Maitre et al. (2008)25 | Human (healthy and degenerate) | 0.8-17 | No change | ↑ Healthy ↓ Degenerate |
MNP = mature nucleus pulposus; NC = notochordal; PG = proteoglycan.
Collagen measured by gene expression or collagen protein assay
Proteoglycan measured by aggrecan gene, glycosaminoglycan assay, or radiolabeled proteoglycan synthesis assay.
In addition to the high-pressure environment, nucleus pulposus cells reside in a nutrient-restricted environment.16 Because of the avascular nature of the IVD, nutrients and waste are transported in and out by diffusion and convection.16 The nucleus pulposus cells rely on nutrients supplied through the cartilaginous endplates. The glucose concentration gradient in a healthy adult human disc ranges from 5.5 mM at the endplates to 0.5 mM or lower in the center of the nucleus pulposus.16,34,35 Glucose concentration is primarily determined by the distance from capillaries; however, other factors such as endplate porosity can affect glucose supply.16,36-38 With age and disc degeneration, endplates calcify and glucose concentrations decrease.16,36,39 Previous work comparing bovine-MNP and porcine-NC cells found that porcine NC cells had a greater decrease in cell viability in low glucose culture conditions, compared with bovine MNP cells, suggesting that glucose restriction may be one of the causes of NC cell death in vivo.40
While several previous studies have investigated the effects of mechanical loading or nutrient restriction on MNP and NC cells, these studies have all used cell populations isolated from different species.17,24,32,33,40 Using our recently published method for isolating enriched fractions of NC cell-clusters (with an intact pericellular matrix) together with MNP cells from the same bovine discs,41 the present study aimed to examine how each cell type responded to different levels of hydrostatic intermittent pressure and glucose restriction. Our hypothesis was that high pressure and glucose restriction would be more detrimental to NC cells compared to MNP cells. Each cell fraction was cultured under dynamic low and high physiological range hydrostatic pressure for 24 hrs in a custom-built hydrostatic pressure vessel. Second, cells were cultured in either physiologically normal (5.5 mM) or glucose-restricted (0.55 mM) media under atmospheric or high hydrostatic pressure loading for 24 h. Following each treatment, cells were assessed for changes in viability, notochordal phenotypic markers and extracellular matrix production and gene expression.
Methods
Culture Media
To mimic the physiochemical environment of nucleus pulposus tissue, culture media was adjusted to 400 mOsm and pH 7.0. Media containing serum was used for cell isolation and all experimental media was serum-free and supplemented with glucose where appropriate. Ten percent serum media: 5.5 mM glucose Dulbecco’s modified Eagle medium (DMEM; cat no. D2902 Sigma-Aldrich, Auckland, New Zealand) was supplemented with 1% v/v penicillin/streptomycin (Life Technologies, Auckland New Zealand), 0.5% v/v fungizone (Life Technologies), 85 µM ascorbic acid (Sigma-Aldrich), 3.7 g/L NaHCO3, 10% v/v fetal bovine serum (FBS), and 19 g/L mannitol (Sigma-Aldrich). Serum-free control media: 5.5 mM glucose DMEM (cat no. D2902 Sigma-Aldrich, was supplemented with 1% v/v penicillin/streptomycin, 0.5% v/v fungizone, 85 µM ascorbic acid, and 19 g/L mannitol. Serum-free, glucose-restricted media: No glucose DMEM (cat no. D5030 Sigma-Aldrich) was supplemented with 100 mg/L (0.55 mM) glucose, 1% v/v penicillin/streptomycin/glutamine (Life Technologies), 0.5% fungizone, 85 µM ascorbic acid, 19 g/L mannitol.
Cell Isolation
Nucleus pulposus cell subpopulations were isolated from bovine caudal discs of 18-30-month-old animals. Bovine tails were obtained from the local slaughterhouse and isolation of NC cell– and MNP cell–rich fractions were performed as described previously.41 Briefly, NP tissue was separated from the surrounding annulus and finely minced. The tissue was then placed in a large volume of media and mechanically digested for 5 minutes to release the NC cell clusters. The mixture was then strained through a stainless steel mesh sieve (pore size 0.5 mm) to collect the NC cell–rich fraction. The remaining tissue was enzymatically digested in pronase solution (8 units/mL; Sigma-Aldrich) for 45 minutes, then in collagenase (245 units/ mL; Life Technologies) for 2.5 hours at 37°C to isolate the MNP cells. The isolated cells were seeded in 1.2% w/v alginate solution (Sigma-Aldrich) at a density of 0.3 × 106 cell/mL for the NC cells and 4 ×106 cells/mL for the MNP cell fraction to retain the in situ cell densities.41,42 Cell fractions from two caudal discs from three tails were pooled for each experiment, that is, n = 6 discs for each of the 3 biological replicates (N = 3). Cell-seeded alginate beads were cultured in 10% serum media overnight at 37°C and 5% CO2.
Hydrostatic Pressure
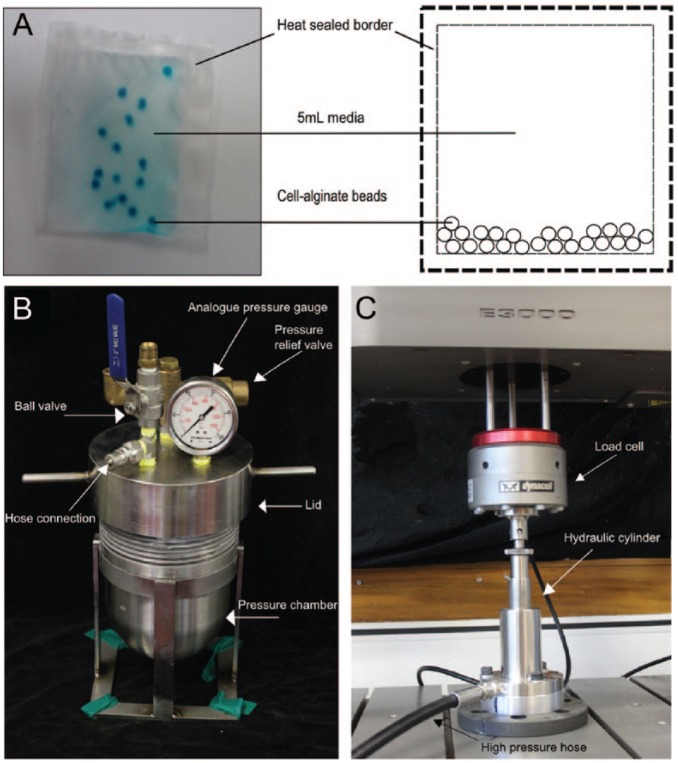
Following overnight culture, cell-seeded alginate beads were transferred to plastic pouches made from high-density polyethylene film, filled with 5-mL serum-free media. The air was removed from the plastic pouches and the edges sealed with a heat-sealer. This created a cell-culture system compatible with hydrostatic pressure as illustrated in Figure 1A . A custom-built hydrostatic pressure rig consisted of a 1-L capacity stainless steel pressure vessel ( Fig. 1B ), connected by a hose to a hydraulic piston ( Fig. 1C ). This was attached to an Instron Electropuls E3000 materials testing machine (Norwood, MA, USA). The cyclic compressive forces generated by the Instron were translated via a fluid-filled piston into an oscillating hydrostatic pressure within the water-filled pressure vessel. Instron Blue Hill 2 and Wave Matrix software were used to produce the loading regimes detailed in Table 1 . An analogue pressure gauge in the lid of the pressure vessel was used to monitor pressure throughout the experiment. The pressurization experiment had 2 phases to mimic a diurnal cycle; an 8-hour rest phase at 0.2 MPa and a 16-hour exercise phase. For the purposes of this study, a regime of 0.4 to 0.8 MPa at 1 Hz, was termed “low pressure” since it approximates the loads experienced during walking. The pressure regime of 1.6 to 2.4 MPa at 1 Hz, was termed “high pressure” since this represents the higher end of physiologically relevant pressure.19 For the pseudo-static rest phase of the experiment, a load-controlled compression protocol was used to maintain the vessel at 0.2 ± 0.1 MPa. A trimodal testing protocol was used to generate a cyclic loading regime for the dynamic 1 Hz oscillating pressure phase. All pressures are stated as gauge pressure; that is, atmospheric pressure = 0 MPa gauge pressure.
Figure 1.
(A) Pressure compatible cell culture system comprising alginate beads in media-filled plastic pouch (4 cm × 5 cm). (B) Custom-built hydrostatic pressure vessel. (C) Hydraulic piston attached to Instron.
Cells were exposed to 24 hours of dynamic pressure as detailed in Table 2 . Atmospheric control samples were placed in a water-filled 1-L glass bottle. Both the pressure and control vessel were maintained at 37°C in a water-bath. Each experimental condition was conducted on a separate occasion with a paired control sample (5.5 mM glucose at atmospheric pressure). Following treatment, the pouches were removed from the pressure vessel; the media was collected with a needle and syringe, the pouches opened, and the cell-alginate beads were removed for analysis.
Table 2.
Culture, Glucose Concentrations and Hydrostatic Pressure Conditions Used in This Study.
| Group | Glucose (mM) | Pressure (MPa) | Frequency | Time (h) |
|---|---|---|---|---|
| Pressure studies | ||||
| Control | 5.5 | Atmospheric | Static | 24 |
| Low pressure | 5.5 | 0.2, followed by 0.4-0.8 | Static 1 Hz |
8 + 16 |
| High pressure | 5.5 | 0.2, followed by 1.6-2.4 | Static 1 Hz |
8 + 16 |
| Glucose-restriction studies | ||||
| Control | 5.5 | Atmospheric | Static | 24 |
| Glucose-restricted | 0.55 | Atmospheric | Static | 24 |
| High pressure, glucose-restricted | 0.55 | 0.2, followed by 1.6-2.4 | Static 1 Hz |
8 + 16 |
Oxygen concentration in both the pressure vessel and control glass bottle was measured before and after the loading period using a Neofox oxygen probe (Ocean Optics, Auckland, New Zealand). The oxygen concentrations remained stable over the loading regime (6 mg/mL).
Viability Assay
Cell viability was assessed within 1 hour of the cessation of loading using an Apoptotic, Necrotic, and Healthy Cells Quantification Kit (Biotium, Fremont, CA, USA). Cell-alginate beads were rinsed once in PBS, then incubated in binding buffer, followed by incubation in a solution of FITC-annexin antibody (green), ethidium homodimer (red), and Hoechst (blue) according to the manufacturer’s instructions. The beads were then rinsed and imaged immediately on a Nikon TE2000 widefield fluorescence microscope using a 20× objective. Cells from 3 alginate beads (≥100 cells) per group were examined using Image J software grid counter. To determine cell viability, cells labeled with Hoechst only (blue) were counted as alive, while cells labeled annexin V (green) or ethidium (red) were counted as dead cells.
GAG/DNA Assays
The 1,9-dimethylmethylene blue (DMMB) dye binding assay was used to measure total sulfated glycosaminoglycan (GAG) content in cell-alginate beads, and a SYBR green–based plate assay was used to determine DNA content as previously described.41 GAG content was normalized to DNA content within each sample, then each condition was normalized to its paired atmospheric control.
Real-Time Polymerase Chain Reaction
Real-time polymerase chain reaction (PCR) was used to assess change in gene expression of the notochordal phenotypic marker T (brachyury) as well as key ECM relevant genes. Following treatment, approximately 25 beads per group were removed and preserved in RNA Later (Life Technologies) at −20°C. RNA was extracted using the TRIzol-chloroform method and real-time PCR was performed as described previously.41 Real-time PCR was performed using Taqman gene expression assays (Life Technologies) detailed in Table 3 . The tyrosine activation protein, YWHAZ, and ribosomal 18S were used as reference genes. Relative expression was calculated from mean expression of r18S and YWHAZ in each sample.
Table 3.
Taqman Gene Expression Assays Used in This Study.
| Protein | Gene | Taqman Assay Reference |
|---|---|---|
| Collagen type I | COL1α1 | Bt03225322_m1 |
| Collagen type II | COL2α1 | Bt03251861_m1 |
| Aggrecan | ACAN | Bt03212186_m1 |
| ADAMTS-5 | ADAMTS5 | Bt04230785_m1 |
| Tissue inhibitor of metalloproteinases 1 | TIMP1 | Bt03213713_m1 |
| Matrix metalloproteinase 3 | MMP3 | Bt04259490_m1 |
| Brachyury | T | Bt04313978_m1 |
| Ribosomal RNA 18sa | r18s | 4319413E / X03205.1 |
| Tyrosine activation protein 14-3-3a | YWHAZ | Bt03216375_g1 |
Indicates reference genes.
Immunocytochemistry
Cell morphology and phenotype was assessed by cytokeratin 8 and vimentin immunolabeling.41 Alginate beads were cross-linked in 100 mM BaCl2 for 10 minutes then fixed in 4% (w/v) paraformaldehyde for 20 minutes at 37°C. Beads were permeabilized in 0.5% (v/v) Triton X-100 (Sigma-Aldrich) for 10 minute, washed in PBS containing 0.1% (w/v) bovine serum albumin and 10 mM CaCl2 then incubated in 5% donkey serum (Sigma Aldrich) for 30 minutes for blocking. Finally, beads were incubated in goat polyclonal vimentin primary antibody (1:200; Santa Cruz Biotechnology Inc.) overnight at 4°C. The following day, beads were washed, incubated in donkey anti-goat Alexa-488 at room temperature for 2.5 hours (1:500; Life Technologies), washed again, blocked in 5% goat serum (Sigma Aldrich), and incubated overnight with mouse monoclonal cytokeratin-8 primary antibody (1:200; Acris Germany) at 4°C. The following day, beads were washed and incubated in goat anti-mouse Alexa-594. Finally, beads were counterstained with Hoechst (1:500; Sigma-Aldrich) for 15 minutes, washed and mounted by firmly pressing onto a slide under a coverslip with Prolong Gold (Life Technologies). A minimum of 100 cells per replicate were imaged using a Leica DMR fluorescence microscope and the number of CK8-positive cells was expressed as percentage of total cells.
Statistical Analysis
GAG measurements were normalized to DNA content within each sample to yield GAG/DNA (µg/ng) ratio. GAG/DNA ratios were then normalized to paired control samples (5.5 mM glucose atmospheric pressure) to calculate the relative change in response to each treatment condition. Statistical differences were calculated compared to control using a 2-sample t-test. Differences between treatment groups were compared using Sidak’s multiple comparisons test.
Gene expression data were analyzed using the Livak et al. 2−ΔΔCt method where target gene expression is normalized to averaged reference gene expression and normalized again to the paired controls (5.5 mM glucose atmospheric pressure), to allow the data to be expressed as fold-change from control. Differences between treatment groups were compared using Sidak’s multiple comparisons test.
For cell viability and cytokeratin 8 expression, data were expressed as mean ± standard deviation for all groups. Differences between groups were analyzed using Tukey’s multiple comparison test. A P value of <0.05 was considered statistically significant. All statistical analysis was performed using GraphPad Prism statistical software (v 6.01).
Results
Effect of Pressure
Viability and Phenotype
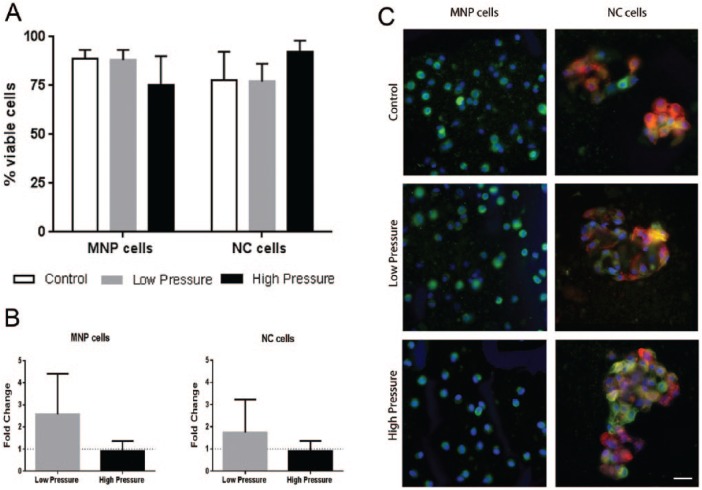
Cell viability was not significantly affected by either low or high pressure loading for either cell type compared to atmospheric controls. For MNP cells, the mean viability was 89%, 88%, and 75% for atmospheric control, low-, and high-pressure groups, respectively. The NC cell groups had a mean viability of 78%, 77%, and 92% for atmospheric control, low-, and high-pressure groups, respectively ( Fig. 2A ).
Figure 2.
(A) Viability of mature nucleus pulposus (MNP) and notochordal (NC) cells following 24-hour culture under control (atmospheric), low-, and high-pressure loading. (B) T (brachyury) gene expression relative to paired atmospheric control (y = 1). (C) MNP and NC cells labeled against vimentin (green) and cytokeratin 8 (red) counterstained with Hoechst (blue), scale bar = 20 µm (mean ± SD, N = 3) (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
The gene expression of the notochordal marker T (brachyury) was measured and found to be present in both cell-rich fractions. In accordance with our previous findings NC fractions expressed 4-fold more T than MNP fractions and these levels did not change in response to low or high pressure for either cell type ( Fig. 2B ).
Cell-alginate beads were immunolabeled against the mesenchymal marker vimentin and the notochordal marker cytokeratin-8, these abundantly expressed cytoskeletal proteins are most suited to clearly differentiate cell phenotype ( Fig. 2C ). Following pressurization, both cell types maintained their distinctive cell morphologies. MNP cells were seen as small individual spherical cells, which only expressed vimentin. NC cells were present as dense clusters which expressed both vimentin and CK8. The MNP fraction contained 2% to 3% CK8-positive cells across all conditions. The NC cell fractions had a frequency of ≥89% CK8-positive cells across all conditions.
GAG Production
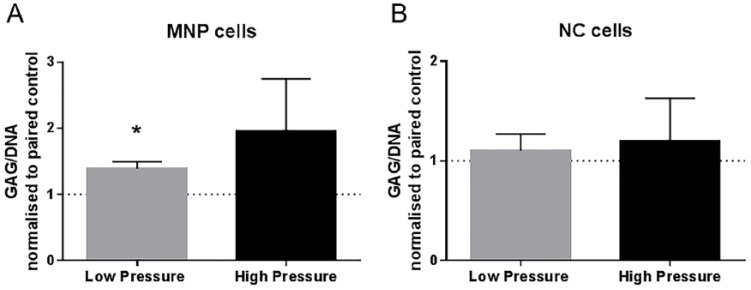
Change in GAG/DNA ratio was calculated relative to paired control (atmospheric pressure) samples. Following low-pressure loading, the MNP cells had a 1.4-fold increase in GAG/DNA ratio (P = 0.0250), while high-pressure loading had no significant effect on GAG/DNA ratio ( Fig. 3A ). Neither low- nor high-pressure loading significantly altered GAG/DNA ratio in the NC cells (Fig. 3B).
Figure 3.
GAG/DNA ratio normalized to paired controls (atmospheric pressure) for (A) mature nucleus pulposus (MNP) and (B) notochordal (NC) cells (mean ± SD, N = 3).
Gene Expression
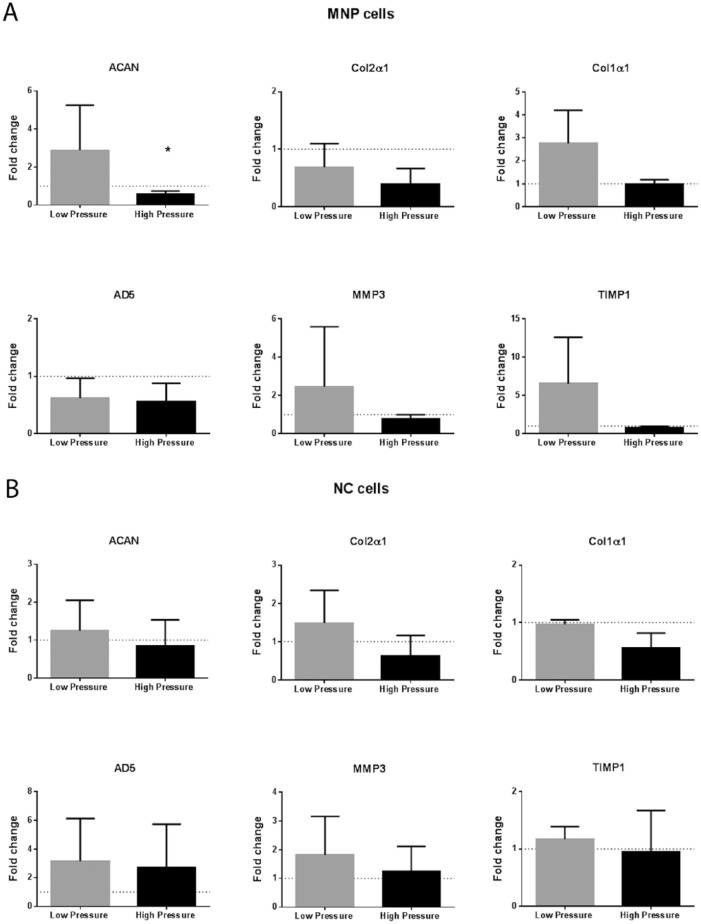
For MNP cells under low pressure, there was a nonsignificant increase in gene expression compared to atmospheric pressure for all genes except ADAMTS-5 ( Fig. 4A ). High-pressure treatment resulted in a significant decrease in aggrecan gene expression for MNP cells compared with atmospheric control, P = 0.0478 ( Fig. 4A ). Col2α1 expression was nonsignificantly decreased, P = 0.0600. The NC cells had no significant change in gene expression for any of the target genes studied when comparing pressurized with atmospheric controls ( Fig. 4B ).
Figure 4.
Extracellular matrix (ECM) gene expression. Fold change in gene expression relative to paired atmospheric control for (A) mature nucleus pulposus (MNP) cells and (B) notochordal (NC) cells in response to low- and high-pressure loading (mean ± SD, N = 3).
Effect of Glucose Restriction
Viability and Phenotype
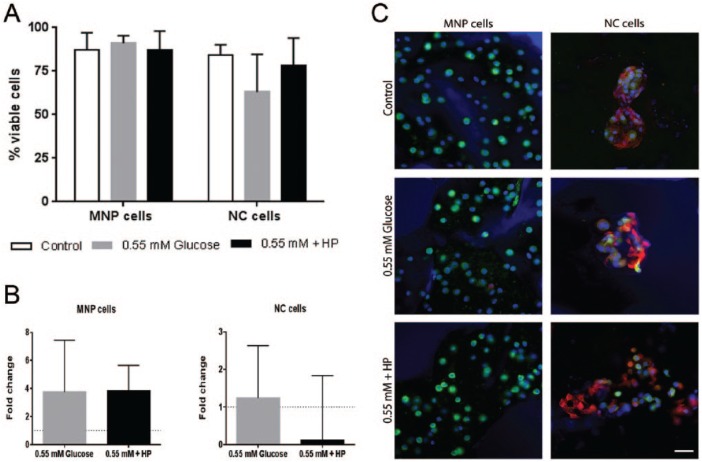
Cell viability was not significantly reduced for either cell type under glucose restriction. MNP cell viability remained high ranging from 75% to 91% across all conditions ( Fig. 5 ). NC cells showed greater variability, ranging from 63% in the 0.55 mM group to 92% in the 5.5 mM + HP group ( Fig. 5 ). Despite these fluctuations, neither glucose concentration nor high pressure had a statistically significant effect on cell viability for NC cells compared with control conditions. NC cells remained in clusters expressing both vimentin and CK8, while the MNP cells remained as single cells expressing vimentin. Less than 3% of cells in the MNP fraction expressed CK8, while 90% of the cells in the NC fraction expressed CK8. There was no significant change in the frequency of CK8+ cells in either cell fraction under any of the tested conditions ( Fig. 5 ).
Figure 5.
(A) Viability of mature nucleus pulposus (MNP) and notochordal (NC) cells following 24-hour culture under control (5.5 mM glucose), 0.55 mM glucose and 0.55M glucose + HP (high pressure) loading. (B) T (brachyury) gene expression relative to paired atmospheric control (y = 1) (mean ± SD, N = 3). (C) MNP and NC cells labeled against vimentin (green) and cytokeratin 8 (red) counterstained with Hoechst (blue), scale bar = 20 µm (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
GAG Production
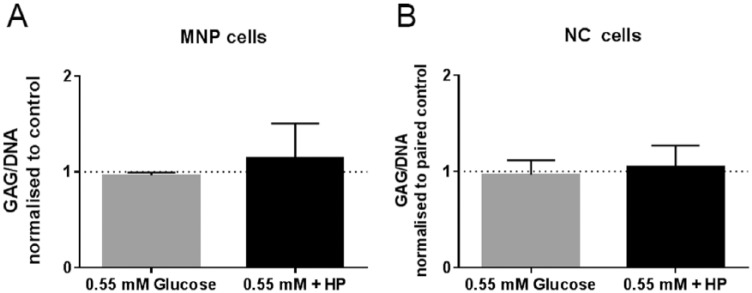
GAG/DNA ratios did not significantly change for either cell type in response to glucose restriction or the combination of glucose restriction and high hydrostatic pressure ( Fig. 6 ).
Figure 6.
GAG/DNA ratio normalized to paired 5.5 mM glucose atmospheric controls for (A) mature nucleus pulposus (MNP) cells and (B) notochordal (NC) cells in response to 0.55 mM glucose and 0.55 mM glucose + HP (high pressure) loading (mean ± SD, N = 3).
Gene Expression
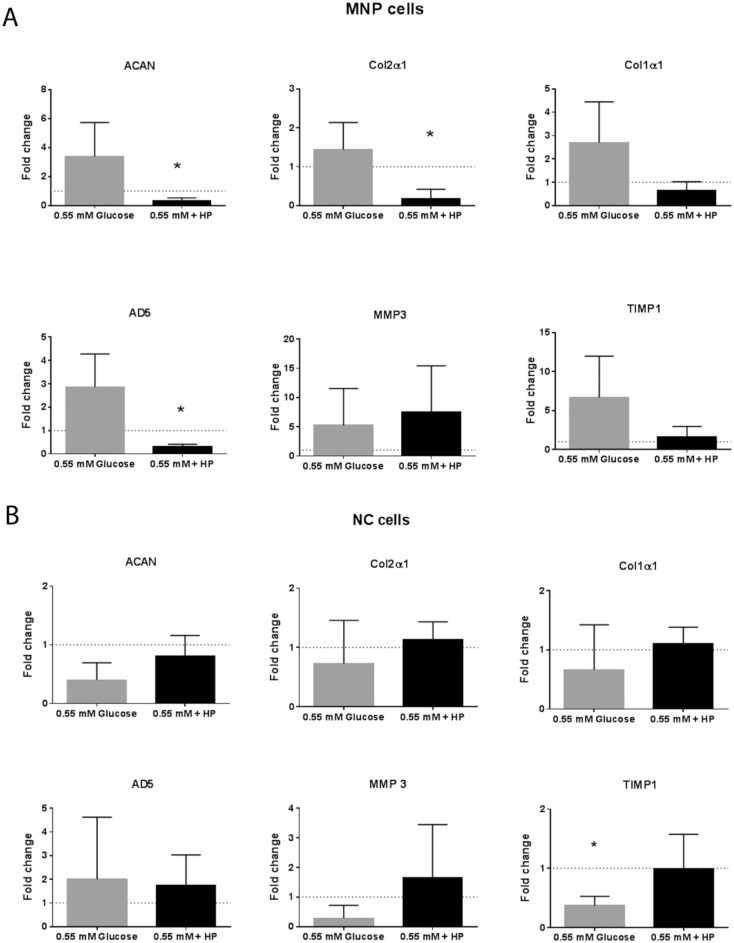
MNP cells showed no significant change in gene expression in response to 0.55 mM glucose alone, however, in combination with high hydrostatic pressure, there was a significant decrease in the expression of collagen type II (P = 0.0274), aggrecan (P = 0.0251), and a highly significant decrease in ADAMTS-5 expression (P = 0.0087) compared with the 5.5 mM glucose atmospheric pressure controls ( Fig. 7A ). Glucose restriction induced a significant decrease in TIMP-1 expression in NC cells compared with paired control (P = 0.0198). However, in the combined 0.55 mM glucose and high-pressure group, TIMP-1 expression levels returned to control levels. All other genes did not change in response to either treatment ( Fig. 7B ).
Figure 7.
Extracellular matrix (ECM) gene expression. Fold change in gene expression relative to paired 5.5 mM glucose paired atmospheric control for (A) mature nucleus pulposus (MNP) cells and (B) notochordal (NC) cells in response to 0.55 mM glucose and 0.55 mM glucose + high pressure loading (mean ± SD, N = 3).
Discussion
To examine the functional differences between NC and MNP cells, without the interference of species-derived differences, our study isolated both cell types from the same discs. Since mechanical stress and nutrient deprivation have both been hypothesized to cause NC cell loss,15-18 the effects of both of these factors were investigated. Both cell types were cultured under physiologically relevant hydrostatic pressures and glucose concentrations for 24 hours and subsequently assessed for changes in viability, NC phenotypic markers and ECM-related gene expression.
Response to Low and High Pressure
In vivo disc-clamp studies, which have loaded NC cell–rich discs over days or weeks, have reported changes in ECM composition and an accompanying decrease in the proportion of NC cells.17,18,20 In contrast, in vitro studies, which exposed isolated NC cells to pressure (between minutes and hours), reported both anabolic and catabolic changes in ECM expression in response to hydrostatic pressure (see Table 1 ).28-30,32 In the present study, we used physiologically relevant magnitudes and frequencies of loading19 to compare the response of bovine MNP and bovine NC cells in 3-dimensional culture. We found no decrease in cell viability for either cell-type compared to controls. Additionally, we found no evidence of cell differentiation in response to mechanical stress. The MNP cell-rich fraction had a small fraction (2%-3%) of CK8-positive cells and an extremely low T gene expression (Ct = 37-40) across all conditions, presumably due to contaminating NC cells.41 There was no significant change in these phenotypic markers following 24 hours of low- or high-pressure dynamic loading.
Using a mixed MNP and NC population of bovine as well as human cells in vitro, Neidlinger-Wilke et al.24 showed that low-range hydrostatic pressure (0.25 MPa) promoted ECM synthesis, while high-magnitude pressure (2.5 MPa) decreased anabolic gene expression. In the present study, we found that MNP cells had a small but significant increase in GAG/DNA ratio in response to low-pressure loading. Also, in agreement with Neidlinger-Wilke et al.,24 we found that high pressure resulted in a significant decrease in ACAN expression and a nonsignificant decrease in Col2α1 expression. Low-pressure loading did not significantly alter the expression of any of the genes examined.
Notably, NC cells showed no change in GAG/ DNA ratio or ECM related gene expression in response to either the low- or high-pressure loading conditions used in this study. While this finding does not prove that NC cells are mechanically insensitive, it does indicate that NC cells behave differently to their MNP counterparts and are possibly less sensitive to acute mechanical stress. Additionally, our finding may be species specific since no previous pressure study has used NC cells isolated from bovine samples. Since both cattle and humans naturally lose NC cells with age, the bovine model may be a more relevant model for testing medical interventions.
Response to Glucose Restriction
Since NC cells in this model showed no significant response to pressure, we next investigated the effect of glucose restriction alone and in combination with high-pressure loading. The MNP cells retained high cell viability with glucose restriction while the NC cells had a nonsignificant decrease in viability. Bibby et al.2 reported a decrease in MNP cell viability only when cultured in 0 mM glucose, while Guehring et al.40 found a decrease in porcine NC viability in response to 0.5 mM glucose after 3 days. With a longer culture duration, we may have also seen a significant decrease. Alternatively, the discrepancy in NC viability could be due to species differences since Guehring et al.40 used porcine NC cells and bovine MNP cells, while our cells where both isolated from bovine discs. Additionally, we found no change in cell morphology or phenotype for either cell-type under glucose restriction or glucose restriction combined with high pressure.
Glucose restriction alone did not alter MNP cell ECM gene expression; however, glucose restriction combined with high pressure decreased ACAN, Col2α1 and ADAMTS 5. This result is an exacerbation of the pattern of ACAN and Col2α1 gene expression decrease following high pressure alone and is in line with the findings of Neidlinger-Wilke et al.24 for bovine NP cells. The reduction in ADAMTS 5 was unexpected, however, since the absolute expression was relatively low (Ct > 30), we believe this reduction would have a significant effect on secreted matrix composition. In the degenerated disc, glucose restriction and high pressure loading often occur together, and our results predict this would lead to a cumulative effect in vivo. Aggrecan and collagen II are the most abundant anabolic proteins of the nucleus pulposus; reduced gene expression for these proteins would substantially disrupt ECM homeostasis.
The NC cells had a nonsignificant decrease in viability under glucose restriction as well as a significant decrease in the anti-catabolic factor TIMP-1, suggesting that glucose restriction has a detrimental influence on NC cells. However, in the combined glucose restricted and high hydrostatic pressure group, TIMP-1 expression levels returned to control levels. This result suggests that pressure is inhibiting the effects of glucose restriction in NC cells.
The interaction between pressure and glucose restriction could be due to differential glucose transporter (GLUT) expression. Hydrostatic pressure has been shown to reduce glucose uptake in chondrocytes by inhibiting GLUT function,43 whereas osmotic pressure increases glucose uptake in skeletal muscle,44 hepatocytes,45 and adipocytes.46 Glucose transporters GLUT-1, -3, and -9 have been identified in adult human nucleus pulposus47 and only GLUT-1 in the NC cell–rich rat nucleus pulposus48; however, their precise function in IVD homeostasis still remains unclear.
While the present study examined the differences between NC and MNP cell populations in situ, NC and MNP cells coexist. While the majority of co-culture studies have focused on NC cell signaling and its influence on MNP cells, signaling is likely bidirectional. Gantenbein-Ritter and Chan49 cultured porcine NC cells and bovine MNP cells separated by a membrane under standard conditions and found that in a 50:50 NC:MNP ratio, NC cells stimulated increased GAG production in MNP cells. In contrast, MNP cells stimulated aggrecan and collagen II expression at culture ratios of 25%, 50%, and 75%, indicating robust MNP cell to NC cell signaling at a wide range of cell concentrations. Therefore, extensive co-culture studies are needed to understand if the cell-specific response to loading and nutrient deprivation is influenced by cell signaling and how this response changes as cell ratios change from development through to disease states.
While in vivo disc-clamp studies report substantial changes in cell populations due to mechanical stress, these studies require devices to be inserted into the vertebrae; where the applied mechanical stress causes changes to the bone, vertebral endplates and other tissues. It is possible that these changes in the surrounding structures influence the loss of NC cells in in vitro models.17,50
Conclusion
Using a same-species in vitro cell model, this study found that MNP and NC cells respond differently to hydrostatic pressure and glucose restriction. These functional differences could explain why species and individuals that retain a higher NC:MNP cell ratio into adulthood are less likely to develop disc degeneration. Additionally, our findings underline the importance of species and, as such, cell type selection in IVD biological research.
Footnotes
Authors’ Note: All work reported in this article was conducted at the University of Auckland.
Acknowledgments and Funding: We thank the support of Auckland Medical Research Foundation and the staff at the Biomedical Imaging Research Unit. The work was funded by an Auckland Medical Research Foundation Doctoral Scholarship (TS), the School of Medical Sciences, University of Auckland Graduate Student Fund and the University of Auckland’s Doctoral PReSS account.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
Ethical Approval: Ethical approval was not sought for the present study because animal tissue was obtained from a local met processing plant.
ORCID iD: Susan Read McGlashan  https://orcid.org/0000-0002-8666-2456
https://orcid.org/0000-0002-8666-2456
References
- 1. Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J. 2014;23(9):1803-14. [DOI] [PubMed] [Google Scholar]
- 2. Urban JP, Roberts S. Development and degeneration of the intervertebral discs. Mol Med Today. 1995;1(7):329-35. [DOI] [PubMed] [Google Scholar]
- 3. Peacock A. Observations on the prenatal development of the intervertebral disc in man. J Anat. 1951;85(3):260-74. [PMC free article] [PubMed] [Google Scholar]
- 4. Peacock A. Observations on the postnatal structure of the intervertebral disc in man. J Anat. 1952;86(2):162-79. [PMC free article] [PubMed] [Google Scholar]
- 5. Stosiek P, Kasper M, Karsten U. Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation. 1988;39(1):78-81. [DOI] [PubMed] [Google Scholar]
- 6. Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9(4):667-77. [DOI] [PubMed] [Google Scholar]
- 7. Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smolders LA, Meij BP, Onis D, Riemers FM, Bergknut N, Wubbolts R, et al. Gene expression profiling of early intervertebral disc degeneration reveals a down-regulation of canonical wnt signaling and caveolin-1 expression: Implications for development of regenerative strategies. Arthritis Res Ther. 2013;15(1):R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weiler C, Nerlich AG, Schaaf R, Bachmeier BE, Wuertz K, Boos N. Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J. 2010;19(10):1761-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun Z, Wang HQ, Liu ZH, Chang L, Chen YF, Zhang YZ, et al. Down-regulated CK8 expression in human intervertebral disc degeneration. Int J Med Sci. 2013;10(8):948-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miyazaki T, Kobayashi S, Takeno K, Meir A, Urban J, Baba H. A phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails; which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng Part A. 2009;15(12):3835-46. [DOI] [PubMed] [Google Scholar]
- 12. Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, et al. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res. 2015;33(3):283-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams MA, McNally DS, Dolan P. “Stress” distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78(6):965-72. [DOI] [PubMed] [Google Scholar]
- 14. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976). 2006;31(18):2151-61. [DOI] [PubMed] [Google Scholar]
- 15. Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976). 2004;29(23):2700-9. [DOI] [PubMed] [Google Scholar]
- 17. Guehring T, Nerlich A, Kroeber M, Richter W, Omlor GW. Sensitivity of notochordal disc cells to mechanical loading: an experimental animal study. Eur Spine J. 2010;19(1):113-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, Kroeber MW, et al. Changes in gene expression and protein distribution at different stages of mechanically induced disc degeneration—an in vivo study on the New Zealand white rabbit. J Orthop Res. 2006;24(3):385-92. [DOI] [PubMed] [Google Scholar]
- 19. Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Phila Pa 1976). 1999;24(8):755-62. [DOI] [PubMed] [Google Scholar]
- 20. Hirata H, Yurube T, Kakutani K, Maeno K, Takada T, Yamamoto J, et al. A rat tail temporary static compression model reproduces different stages of intervertebral disc degeneration with decreased notochordal cell phenotype. J Orthop Res. 2014;32(3):455-63. [DOI] [PubMed] [Google Scholar]
- 21. Kuo YJ, Wu LC, Sun JS, Chen MH, Sun MG, Tsuang YH. Mechanical stress-induced apoptosis of nucleus pulposus cells: an in vitro and in vivo rat model. J Orthop Sci. 2014;19(2):313-22. [DOI] [PubMed] [Google Scholar]
- 22. Yurube T, Takada T, Suzuki T, Kakutani K, Maeno K, Doita M, et al. Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther. 2012;14(2):R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishihara H, McNally DS, Urban JP, Hall AC. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol (1985). 1996;80(3):839-46. [DOI] [PubMed] [Google Scholar]
- 24. Neidlinger-Wilke C, Wurtz K, Urban JP, Borm W, Arand M, Ignatius, et al. Regulation of gene expression in intervertebral disc cells by low and high hydrostatic pressure. Eur Spine J. 2006;15(Suppl 3):S372-S378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Maitre CL, Frain J, Fotheringham AP, Freemont AJ, Hoyland JA. Human cells derived from degenerate intervertebral discs respond differently to those derived from non-degenerate intervertebral discs following application of dynamic hydrostatic pressure. Biorheology. 2008;45(5):563-75. [PubMed] [Google Scholar]
- 26. Hutton WC, Elmer WA, Boden SD, Hyon S, Toribatake Y, Tomita K, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine (Phila Pa 1976). 1999;24(15):1507-15. [DOI] [PubMed] [Google Scholar]
- 27. Sowa GA, Coelho JP, Bell KM, Zorn AS, Vo NV, Smolinski P, et al. Alterations in gene expression in response to compression of nucleus pulposus cells. Spine J. 2011;11(1):36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gokorsch S, Nehring D, Grottke C, Czermak P. Hydrodynamic stimulation and long term cultivation of nucleus pulposus cells: a new bioreactor system to induce extracellular matrix synthesis by nucleus pulposus cells dependent on intermittent hydrostatic pressure. Int J Artif Organs. 2004;27(11):962-70. [DOI] [PubMed] [Google Scholar]
- 29. Wang DL, Jiang SD, Dai LY. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine (Phila Pa 1976). 2007;32(23):2521-8. [DOI] [PubMed] [Google Scholar]
- 30. Kasra M, Goel V, Martin J, Wang ST, Choi W, Buckwalter J. Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J Orthop Res. 2003;21(4):597-603. [DOI] [PubMed] [Google Scholar]
- 31. Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22(6):1193-200. [DOI] [PubMed] [Google Scholar]
- 32. Hutton WC, Elmer WA, Bryce LM, Kozlowska EE, Boden SD, Kozlowski M. Do the intervertebral disc cells respond to different levels of hydrostatic pressure? Clin Biomech (Bristol, Avon). 2001;16(9):728-34. [DOI] [PubMed] [Google Scholar]
- 33. Purmessur D, Guterl CC, Cho SK, Cornejo MC, Lam YW, Balliff BA, et al. Dynamic pressurization induces transition of notochordal cells to a mature phenotype while retaining production of important patterning ligands from development. Arthritis Res Ther. 2013;15(5):R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13(8):695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8(2):101-19. [DOI] [PubMed] [Google Scholar]
- 36. Grunhagen T, Shirazi-Adl A, Fairbank JC, Urban JP. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42(4):465-77. [DOI] [PubMed] [Google Scholar]
- 37. Shirazi-Adl A, Taheri M, Urban JP. Analysis of cell viability in intervertebral disc: effect of endplate permeability on cell population. J Biomech. 2010;43(7):1330-6. [DOI] [PubMed] [Google Scholar]
- 38. Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(Suppl 2):30-5. [DOI] [PubMed] [Google Scholar]
- 39. Hristova GI, Jarzem P, Ouellet JA, Roughley PJ, Epure LM, Antoniou J, et al. Calcification in human intervertebral disc degeneration and scoliosis. J Orthop Res. 2011;29(12):1888-95. [DOI] [PubMed] [Google Scholar]
- 40. Guehring T, Wilde G, Sumner M, Grünhagen T, Karney GB, Tirlapur UK, et al. Notochordal intervertebral disc cells: Sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60(4):1026-34. [DOI] [PubMed] [Google Scholar]
- 41. Saggese T, Redey P, McGlashan SR. Same-species phenotypic comparison of notochordal and mature nucleus pulposus cells. Eur Spine J. 2015;24(9):1976-85. [DOI] [PubMed] [Google Scholar]
- 42. Gilson A, Dreger M, Urban JP. Differential expression level of cytokeratin 8 in cells of the bovine nucleus pulposus complicates the search for specific intervertebral disc cell markers. Arthritis Res Ther. 2010;12(1):R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Windhaber RA, Wilkins RJ, Meredith D. Functional characterisation of glucose transport in bovine articular chondrocytes. Pflugers Arch. 2003;446(5):572-7. [DOI] [PubMed] [Google Scholar]
- 44. Farlinger CM, Lui AJ, Harrison RC, Leblanc PJ, Peters SJ, Roy BD. Extracellular hyperosmotic stress stimulates glucose uptake in incubated fast-twitch rat skeletal muscle. Appl Physiol Nutr Metab. 2013;38(6):605-12. [DOI] [PubMed] [Google Scholar]
- 45. Hwang DY, Ismail-Beigi F. Stimulation of GLUT-1 glucose transporter expression in response to hyperosmolarity. Am J Physiol Cell Physiol. 2001;281(4):C1365-72. [DOI] [PubMed] [Google Scholar]
- 46. Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25(2):177-204. [DOI] [PubMed] [Google Scholar]
- 47. Richardson SM, Knowles R, Tyler J, Mobasheri A, Hoyland JA. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1α in normal and degenerate human intervertebral disc. Histochem Cell Biol. 2008;129(4):503-11. [DOI] [PubMed] [Google Scholar]
- 48. Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308(3):401-7. [DOI] [PubMed] [Google Scholar]
- 49. Gantenbein-Ritter B, Chan SC. The evolutionary importance of cell ratio between notochordal and nucleus pulposus cells: an experimental 3-D co-culture study. Eur Spine J. 2011;21(6):819-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yurube T, Hirata H, Kakutani K, Maeno K, Takada T, Zhang Z, et al. Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther. 2014;16(1):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]