Abstract

Radiation chemical modeling of redox reactions of biologically active compounds from plant materials showed that coumarins possess strong antiradical properties. Data confirming the radioprotective properties of these compounds were obtained. Antioxidant activity has been shown for specific medicinal plant extracts—Melilotus officinalis and Ledum palustre cormus. The radiation chemical transformations of coumarins revealed that an unsubstituted coumarin has greater radioprotective activity.
1. Introduction
Environmental pollution caused by hazardous emissions from industrial production and energetics of radioactive materials causes our body to be constantly exposed to negative factors, one of which is ionizing radiation. It is known that exposure can cause the formation of reactive oxygen species.1−4 The human body possesses a variety of protection systems, including radioprotectors and antioxidants (AOs).5 The introduction of food or drinks with radioprotective properties reduces the effect of radiation exposure.6 Various radioprotectors are known to be used in the protection of living organisms, for example, biogenic amines without the sulfhydryl group, aminothiols (propamine, aminoethylisothiouronium, etc.), ascorbic acid,7 vitamin E,8 carotene, polysulfides of gallic acid,9 cysteine, cysteamine hydrochloride, coenzyme Q9,10,11 vitulin,12 dimethyl sulfoxide,13 some carbohydrates, biologically active plant and medicinal preparations,14−18 beech-tree oil Fagus orientalis,19 phenolic compounds,20−23 and others.
The main mechanisms of the protective effect exerted by radioprotectors during exposure to radiation20,24 are as follows: competition for the strong oxidizing agents and free radicals formed upon the radiolysis of water, solvents, and other media; protection of radiosensitive enzymes, hormones, and protein molecules; complexation of heavy metal ions25 and of cations with several common oxidation states; and inhibition of chain oxidation reactions.26 The role of secondary reactions in the radioprotective effect and the elucidation of the toxic properties of chemical radioprotectors and their reaction products is complex and contradictory. It may often altogether prevent the phenomenon of a radioprotective effect. At this point, a search for biologically active compounds (BACs) from plant materials which exhibit high radioresistance and which produce secondary reaction products with nontoxic properties is of great interest.
Investigation into the transformations of naturally occurring compounds exposed to ionizing radiation, the determination of their radiation stability and of the mechanism of their redox reactions is of great importance.27,28
One of the important and promising families of such compounds is the coumarin (Coum).29 Coums are the organic compounds which consist of a benzene ring joined to a pyrone ring. Coums are widely found in the plant kingdom. Depending on the structure, Coum has differing properties and a number of biological activities such as anti-inflammatory, anticoagulant, anticancer, anti-allergic, and photodynamic activities. The use of Coum as AOs and radioprotectors is of great interest.30−32 Wang et al.33 investigated the anticancer activity of a coumarin derivative—esculetin—extracted from the herb Cortex Fraxini. It was established that the exact Coum content in a medicinal plant material is related to the exhibition of its anticancer and antiradical activities. Esculetin was shown to have an inhibitory effect on the human colon carcinoma HT-29 cells.
A large number of plants comprise the organic BACs—Coums, flavonoids,34−36 carotenoids, porphyrins, chlorophylls, and so forth and their metal complexes.37−40 From the literature,41,42 it is known that various Coums are found in a high level; medicinal plants consist of Coum itself and dihydrocoumarin in Melilotus officinalis,43 and esculin, esculetin, scopoletin, and umbelliferone in Ledum palustre cormus.44 Moreover, it is important to notice that the plant material includes many micro-elements (e.g., manganese, copper, ferrum, selenium, magnesium, aluminum, and silver). Habitat, climate, soil composition, and other factors are known to impact the mineral composition of plants. The majority of plants containing the important mineral micro-elements play a significant role in exhibition of biological activity for living organisms.45
Application of a radiation chemical modeling of redox reactions in systems became very effective in investigation and prediction of the polyphenolic compounds’ AO and radioprotective properties.46,47 Methods for the determination of the reactivity of polyphenolic nature compounds can be divided into direct and indirect.30 The direct methods are based on detecting short-lived intermediate particles and free-radical formation of e̅solv, O2•, HO2, HO•, H2O2, R•, RO•, RO2• in liquid and solid systems and the study on its properties using modern physical chemistry methods such as pulse radiolysis, chemoluminescent analysis, electron paramagnetic resonance (EPR), and polarography. The indirect methods are based on a change in concentration of the phenolic compounds when exposed to ionizing radiation using different methods such as spectrophotometry, EPR, voltammetry, chromatography, and others.
Using a 2,2-diphenyl-1-picrylhydrazyl (DPPH)-stable free radical under normal conditions is quite common for the indirect methods. DPPH solution changes in color and in paramagnetic properties upon reduction. Thus, this makes it useful for the investigation of the polyphenolic compound reactivity using ultraviolet–visible spectrophotometry and EPR. The results of the work48,49 showed that the DPPH reducing rate is directly dependent on reactivity of AOs.
In the present work, Coum and its water–ethanolic extracts from the plant material are investigated in order to search and create new BACs with strong AO and radioprotective properties. Reactions with active particles of radiolysis are considered as basis of interactions. Yeast cells “Feodosiya-7” were applied to determine the Coum radioprotective effect.
2. Results and Discussion
2.1. Micro- and Macro-elemental Composition of M. officinalis and L. palustre Cormus by the ICP–MS Method
It is known50 that functional activity of the studied plants is associated with the content of certain elements in plant materials. Using the inductively coupled plasma mass spectrometry (ICP–MS) analysis method, it was established (Table 1) that magnesium is more plentiful in L. palustre cormus, while the same is true for iron, copper, and zinc—in M. officinalis. Microquantities of selenium, which is thought to be responsible for some AO properties, were also registered in the studied plants.
Table 1. Content of Some Macro- and Micro-elements in Dried Samples of M. officinalis and L. palustre Cormus (mg/g of Plant Materials).
| element | M. officinalis (mg/g) | L. palustre cormus (mg/g) | element | M. officinalis (mg/g) | L. palustre cormus (mg/g) |
|---|---|---|---|---|---|
| Mg | 8.39 × 10–1 | 2.65 × 100 | Ge | 2.97 × 10–5 | 3.09 × 10–5 |
| Mn | 2.61 × 10–2 | 1.09 × 100 | As | 1.13 × 10–4 | 7.68 × 10–5 |
| Sr | 6.84 × 10–2 | 1.58 × 10–2 | Se | 2.88 × 10–4 | 1.58 × 10–4 |
| Ba | 3.11 × 10–2 | 1.26 × 10–1 | Mo | 5.05 × 10–4 | 2.75 × 10–4 |
| Fe | 8.09 × 10–1 | 2.92 × 10–1 | Ag | 5.44 × 10–5 | 1.56 × 10–4 |
| Ni | 4.73 × 10–3 | 1.53 × 10–3 | Cs | 3.86 × 10–5 | 1.58 × 10–4 |
| Cu | 6.41 × 10–3 | 4.93 × 10–3 | Tl | 3.64 × 10–6 | 9.21 × 10–5 |
| Zn | 2.71 × 10–2 | 1.73 × 10–2 | Pb | 1.73 × 10–3 | 2.29 × 10–3 |
2.2. Antiradical Activity of Coum and Plant Extracts (on the Base DPPH)
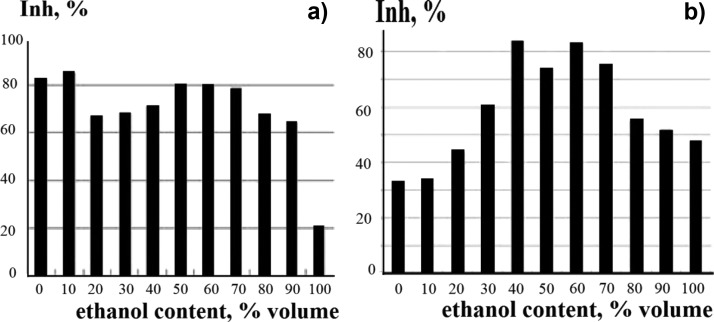
It was established that one of the mechanisms of AO action is the ability of an AO of polyphenolic nature to easily donate hydrogen, forming a phenoxyl radical (PhO•), which is much less active (reaction 2). Free-radical DPPH51 was used to detect the AO properties of the studied plants (Figure 1). The PhO• compound with an unpaired electron, which appeared as a result of hydrogen detachment, is involved in the formation of the inactive reaction products48 (reactions 3 or 4)
| 1 |
 |
2 |
| 3 |
| 4 |
Figure 1.
(a) Effect of the DPPH reaction with M. officinalis extracts on ethanol content. Measurements taken after introducing DPPH into the system for 30 min. (b). Effect of the DPPH reaction with L. palustre cormus extracts on the ethanol content in the system. A 30-fold dilution of extracts was applied. Measurements taken after introducing DPPH into the system for 30 min.
We have registered (Table 2) a decrease of approximately 37 ± 2% of the effect of the DPPH reaction with L. palustre cormus as the irradiation dose increased; it was noted that the effect of the DPPH reaction with the irradiated extracts of L. palustre cormus remains above 50%. Therefore, a decrease of DPPH efficiency in the reaction with L. palustre cormus extracts upon exposure to ionizing radiation may indicate the protective properties of L. palustre cormus.
Table 2. Dependence of Radioprotective Activity (as Defined by [PhO•] Content in the Studied Extracts) of L. palustre cormus Extracts Diluted 30 times on the Absorbed Dosea.
| % inh. DPPH extracts L. palustre cormus that were diluted 30 times |
||
|---|---|---|
| D, kGy | 40% ethanolic solution | 60% ethanolic solution |
| 0 | 84.0 | 83.0 |
| 0.07 | 45.6 | 52.2 |
| 0.14 | 51.7 | 56.6 |
| 0.21 | 54.7 | 52.4 |
| 0.28 | 63.9 | 56.3 |
| 0.56 | 59.0 | 61.0 |
| 0.85 | 56.8 | 53.5 |
Measurements taken after introducingDPPH into the system for 30 min.
The effect of the interaction of Coum with DPPH was not registered. At the same time, it was found that individual Coums,esculletin, esculin, and scopoletin, which are part of the extract of L. palustre cormus, participate in the reaction with DPPH after irradiation (Table 3).
Table 3. Effect of the Irradiation Dose on the Potency of the Reactions of Interaction between DPPH and the Products of Radiolysis of Couma.
| D, kGy | esculetin | esculin | scopoletin | umbelliferone |
|---|---|---|---|---|
| 0 | 82.2 | 12.6 | 7.3 | 0.7 |
| 0.14 | 79.5 | 20.3 | 10.5 | 1.8 |
| 0.28 | 82.1 | 14.3 | 2.4 | 12.7 |
| 0.56 | 84.3 | 16.2 | 8.3 | 16.6 |
The concentration of coumarins was 0.01 M in 40% ethanol solution. Measurements taken after introducing DPPH into the system for 30 min.
To start the reaction, the DPPH water–ethanolic solution was added into irradiated solutions of Coum. The concentration of Coums was 0.01 M in 40% ethanol solution. It is important to note that an increase in antiradical activity with an increase in the absorbed dose (based on the reaction with DPPH) was recorded for umbelliferone. It can be assumed that umbelliferone has a greater AO activity because of the interaction with the products of ethanol radiolysis.
2.3. Determination of the Concentration of Coum from M. officinalis Extracts after Exposure of Ionizing Radiation
The effect of dilution of the extracts on radiation sensitivity was confirmed by the influence of ethanol concentration on the extraction of Coum from M. officinalis.
Then, for research, the 30, 50, and 70% extracts of M. officinalis were de-aerated. Concentrations of Coum from M. officinalis extracts after irradiation (dose of 6 kGy) were calculated by calibration with naphthalene (Table 4).
Table 4. Concentrations of Coum in 30, 50, and 70% Extracts of M. officinalis before and after Irradiation with a Dose of 6 kGy.
| content of ethanol in the system (% volume) | initial [Coum]in × 10–4 (mol/L) | irradiated [Coum]irrad × 10–4 (D = 6 kGy) | degree of
Coum transformation  (%) (%) |
|---|---|---|---|
| 30 | 3.2 | 2.3 | 28.1 |
| 50 | 2.5 | 2.4 | 4.0 |
| 70 | 2.0 | 1.6 | 20.0 |
It can be assumed that the low radiation stability of the 30% solution is associated with the unique structure of ethanol in the initial 30–40% Coum solution.
2.4. Determination of the Oxidative Activity of Extracts in Reaction with Carbon-Centered α-hydroxyethyl Radicals CH3C•HOH
Gas chromatography was used to determine the radiation chemical yields of ethanal (EA) (reaction 5) during the radiolysis of 70% de-aerated ethanol extracts of M. officinalis and L. palustre cormus after the reaction with free carbon-centered α-hydroxyethyl radicals (HERs). The main molecular products of de-aerated ethanol radiolysis are EA (CH3CHO) and butanediol-2,352−57
 |
5 |
If a BAC is capable of intercepting an HER, then EA would not be accumulated in the system with increasing irradiation and the oxidizing properties of the radicals will be reduced. Figure 2 shows the accumulation curves of EA in the system as a function of the increase in absorbed dose for the studied 70% de-aerated extracts of M. officinalis and L. palustre cormus. The radiation chemical yield of EA was calculated using the first derivatives of these functions, according to formula 6(58)
| 6 |
where CEA is the concentration of EA, mol/L; ρ is the density of the solvent, g/L; D is absorbed dose, Gy.
Figure 2.
Dependence of EA accumulation on the absorbed dose for de-aerated 70% water–ethanolic extracts from plant materials: 1—70% water–ethanolic solution without components (control), 2—M. officinalis, 3—L. palustre cormus.
The radiation chemical yield of EA in ethanol is 1 molecule/100 eV,53 which correlates with the published data. As it can be seen in Figure 2, the curves showing the dependence of EA concentration from the absorbed dose for the studied herbal extracts lie higher than the ones for ethanol, which demonstrates EA accumulation, and, therefore, it corresponds to larger radiation chemical yields (G). It is known53 that the maximum value of G (EA) does not exceed 5.5 molecules/100 eV. For L. palustre cormus in the dose range from 0 to 120 Gy, G (EA) was equal to 20 molecules/100 eV, which may indicate the contribution of chain processes occurring in the extract immediately after irradiation. In fact, in the postirradiation period, an occurrence of uncontrolled oxidation processes was recorded.
It was found that oxidative activity of the studied extracts from plant materials decreases in the order L. palustre cormus > M. officinalis. Under the same irradiation conditions, M. officinalis extracts have insignificant oxidizing properties.
2.5. Evaluation of the Radioprotective Activity of Coum Found in Extracts from Plant Materials Using Yeast Cells Saccharomyces cerevisiae of the Race Feodosiya-7
In this work, we studied the radioprotective activity of Coum obtained from the extracts of M. officinalis and L. palustre cormus on the yeast cells S. cerevisiae of the Feodosiya-7 race, which is a convenient model subject for studying the effects of ionizing exposure on living systems. The concentration of K+ ions in the medium with yeast irradiated in the absence of Coum was taken as 100%. Radioprotective activity corresponds to the concentration of potassium ions in solution after irradiation: the smaller the loss of ions K+, the more pronounced the antiradical and protective properties of Coum.59
Coums obtained from the herbal extracts were isolated according to the protocol.60 The results of studies of the radioprotective activity of Coum obtained from the herbal extracts (Figure 3) made clear that the water solutions of Coum isolated from M. officinalis demonstrated radioprotective properties, while the Coum extracted from L. palustris cormus did not demonstrate them.
Figure 3.
Yield of [K+] % ions from yeast cells irradiated after Coum was introduced into them 1—control, 2—coumarins from M. officinalis, and 3—coumarins from L. palustre cormus. Dose = 0.4 kGy.
Information on the radioprotective properties of Coum itself can be found in ref (30). Coum has a protective effect in the range of concentrations from 0.003 mg/cm3 (3 × 10–3 g/L) to 0.03 mg/cm3 (3 × 10–2 g/L) and retains the ability of yeast cells to divide. At the same time, the re-activating effect of Coum is weakly expressed: only a slight increase in the number of viable yeast cells was noted. Radioprotective properties of M. officinalis are associated with the presence of Coum in it.
3. Experimental Section
Dried samples were used from the pharmacy network: M. officinalis herb from “Kameliya-LT” series “Force of nature”, Russia, (Figure 4a) and L. Palustre cormus shoots of the company “Krasnogorskmedfacilities”, Russia, (Figure 4b).
Figure 4.

Photos of the samples: (a)—herbs of medicinal sweet clover (Latin, M. officinalis (L.) Lam.) and (b)—shoots of wild rosemary (Latin, L. palustre cormus). The photos were taken by one of the authors.
Samples of coumarin (99%) (coumarin, esculetin, esculin, scopoletin, and umbelliferone) and DPPH were obtained from Sigma-Aldrich. Structural formulas for Coum are shown in Figure 5.
Figure 5.

Structural formulas for coumarin: (a) coumarin, (b) esculetin, (c) esculin, (d) scopoletin, and (e) umbelliferone.
3.1. Inductively Coupled Plasma Mass Spectrometry
Samples of dry plants were crushed before the analysis. Crushed samples (100 mg) were placed in fluoroplastic autoclaves; then, 4 mL of nitric and 0.10 mL of hydrofluoric acids were added. The autoclave was sealed and kept in a microwave sample preparation system-6 (180 °C, 20 atm) for 15 min. Sample solution after dilution with 2% HNO3 was analyzed by ICP–MS (Thermo Scientific Fisher mass spectrometer iCAP-Qc) using argon as a plasma-forming gas.61
3.2. Chromatography Conditions for Ethanal Concentration Determination in Extracts from Herb Raw
Extracts (1:20, 70% ethanol by volume) from raw materials were prepared. The solutions were de-aerated and irradiated. Analysis was carried out immediately after opening the irradiated glass ampules in the postradiation period. A Chromatek Crystal 5000 (Russia) chromatograph equipped with a 2 m × 2 mm column filled with Polysorb-1 with a size of 0.1–0.25 mm was used. The volume of the injected sample was 1 μL. Detection was carried out on a flame ionization detector (FID) at a temperature of 160 °C with an air flow of 250 mL/min and a hydrogen flow of 25 mL/min. The carrier gas flow rate (helium grade A as the gradient mobile phase) was as follows: 30 mL/min (9–10 min), 40 mL/min (13 min), and 30 mL/min (0 min).
3.3. Chromatography Conditions for Concentration of Coumarin Determination in M. officinalis
Varian column VF-5 ms (length 60 m, diameter 0.25 mm, and layer thickness 0.25 μm) was used. The flow of carrier gas (helium grade 6.0) was 2 mL/min. Temperature setting were as follows: evaporator 250 °C, detector (FID) 250 °C, the thermostat 200 °C (4 min), and heating to 290 °C at a rate of 20 °C/min.
3.4. 60Co γ-Source
The RCHM-γ-20 plant housing a 60Co γ-ray source (D. Mendeleev University of Chemical Technology of Russia) was used as the 60Co γ-source. A Fricke dosimeter was used to estimate the dose rate of the absorbed radiation. The dose rate was determined to be 0.078 ± 0.002 Gy/s.62,63
3.5. Experimental Approach
Antiradical properties of extracts from M. officinalis, L. palustre cormus, and Coum were estimated by reaction with stabile free-radical DPPH. The concentration of DPPH in ethanol was 0.2 mM. Spectrophotometry was used, and the optical density values were measured at λ = 517 nm, with 50% ethanol solution as a comparison sample.48
The added volume of water–ethanol extracts was 200 μL, and the composition was brought to the total 50% ethanol content in the system. After adding DPPH (2 mL) in the system, it was stored in a dark place and measured after 30 min. The percentage of inhibition of DPPH (Inh., %) extracts was calculated using formula 7
| 7 |
where Ac is the optical density of water–ethanol DPPH solution in the absence of additives (control) and Ao is the optical density of water–ethanol DPPH solution in the presence of additives.
Ethanol solution (40%) of 0.01 M coumarins were irradiated. The added volume of coumarin solution was 200 μL, the composition was brought to the total 50% ethanol content in the system, and DPPH (2 mL) was added in the system, and then, the system was stored for 30 min in a dark place and measured.
The coumarin concentrations in water–ethanol (1:20 mass extracts of M. officinalis and L. palustre cormus) were determined. Afterward, the following procedures were carried out:53 samples of 25 mL are taken and then transferred to a round-bottom flask, 200 mL of deionized water and 25 mL of chloroform were diluted and mixed well, 0.3 g of anhydrous sodium sulfate was added, and the given solution was stored at room temperature in a dark place for 24 h. After a day, 10 mL of the selected lower layer of the extract was vacuumed using a water jet pump; the dried residue was dissolved in a solution of 500 μL of naphthalene in CHCl3 (internal standard) with stirring. The resulting solution (250 μL) is used to determine the concentration of coumarin. The ratio of coumarin peak areas to the naphthalene area was determined by the results of chromatogram analysis (Figure 6).
Figure 6.
Chromatogram of Coum solution in naphthalene with chloroform. Chromatographic peaks: 1—chloroform, 2—naphthalene, and 3—Coum.
A calibration graph for concentrations of coumarin in M. officinalis extracts using the internal naphthalene standard is shown in Figure 7. This technique was applied to water–ethanolic solutions of M. officinalis depending on the concentration of ethanol.
Figure 7.

Dependence of the chromatographic peak area ratio SCoum/Sstandard on concentrations of Coum.
Results on Figure 8 demonstrate the nonlinear dependence of Coum concentrations in M. officinalis on the content of ethanol. It was found that the maximum concentration of Coum contained in 30–40% alcoholic solutions of M. officinalis, and it is equal to 2.8–3.2 × 10–4 g/L.
Figure 8.
Dependence of coumarin concentrations in extracts from M. officinalis on the content of ethanol (% by volume).
3.6. Radioprotective Activity Determination of Coum: Diploid Yeast S. cerevisiae was Used in the Logarithmic Growth Phase22
Coum from plant extracts was isolated by a previous method.60 Yeast was exposed to γ-radiation 60Co on RCHM-γ-20 at a dose of 0.4 kGy and measured a day after irradiation, and the concentration of K+ ions in the medium was estimated using a potassium-selective electrode (pH meter ionomer “ECOTEST-2000”, Russia).
The concentration of K+ ions in the medium with yeast irradiated in the absence of Coum was taken as 100%. Radioprotective activity corresponds to the concentration of potassium ions in solution after irradiation: the smaller the loss of K+ ions, the more pronounced the antiradical and protective properties of Coum.59
4. Conclusions
The ionizing radiation is successfully used in high energy chemistry as a source of initiation in water–ethanolic solution of highly reactive redox particles: e̅solv, O2•, HO2, HO•, H2O2, R•, RO•, and RO2•. This allows us to study the radiation chemical transformations of water–ethanolic extracts from plant materials. As a result of studies of radiation chemical redox reactions in ethanolic extracts of BACs from plant materials, the dependence of oxidative activity on HERs for the herbal extracts was established: L. palustre cormus > M. officinalis. It was found that BACs of the M. officinalis extract show moderate pro-oxidant properties.
The antioxidant activity of extracts from plant materials was studied on the samples of L. palustre cormus and M. officinalis. For M. officinalis and Coum, no antiradical activity was recorded in the reaction with DPPH. However, for the L. palustre cormus extract, high values of protective activity were recorded (on the base of the reaction with DPPH).
It was found that coumarins obtained from the M. officinalis extract have radioprotective activity, which was absent for coumarins obtained from L. palustre cormus. As a result, it was shown that not all BACs of a herbal origin have radioprotective activity. However, on the other hand, it can be assumed that all radioprotectors of a plant origin can act as AOs.
Acknowledgments
This research was supported by the Dmitry Mendeleev University of Chemical Technology of Russia. Project 043-2018.
Glossary
Abbreviations
- ROS
reactive oxygen species
- BAC
biologically active compound
- AO
antioxidant
- AOA
antioxidant activity
- EPR
electron paramagnetic resonance
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- HERs
carbon-centered α-hydroxyethyl radicals
- EA
ethanal
- BD
butanediol-2,3
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Harman D. Free Radical Theory of Aging. Mutat. Res. 1992, 275, 257. 10.1016/0921-8734(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Halliwell B.; Gutteridge J. M. C.. Free Radicals in Biology and Medicine, 3rd ed.; Halliwell B., Gutteridge J. M. C., Eds.; Oxford University Press: Oxford, 1999; pp 1–25. [Google Scholar]
- Koyama S.; Kodama S.; Suzuki K.; Matsumoto T.; Miyazaki T.; Watanabe M. Radiation-induced longlived radicals which cause mutation and transformation. Mutat. Res., Fundam. Mol. Mech. Mutagen. 1998, 421, 45–54. 10.1016/s0027-5107(98)00153-5. [DOI] [PubMed] [Google Scholar]
- Santoke H.; Song W.; Cooper W. J.; Greaves J.; Miller G. E. Free-radical-induced oxidative and reductive degradation of fluoroquinolone pharmaceuticals: Kinetic studies and degradation mechanism. J. Phys. Chem. A 2009, 113, 7846–7851. 10.1021/jp9029453. [DOI] [PubMed] [Google Scholar]
- Tang Q.; Zhao F.; Yu X.; Wu L.; Lu Z.; Yan S. The role of radioprotective spacers in clinical practice: a review. Quant. Imag. Med. Surg. 2018, 8, 514. 10.21037/qims.2018.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. F.; Landauer M. R. Radioprotection by antioxidants. Ann. N.Y. Acad. Sci. 2000, 899, 44–60. [PubMed] [Google Scholar]
- Kashino G.; Kodama S.; Nakayama Y.; Suzuki K.; Fukase K.; Goto M.; Watanabe M. Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radical Biol. Med. 2003, 35, 438. 10.1016/s0891-5849(03)00326-5. [DOI] [PubMed] [Google Scholar]
- Sree K. K.; Srinivasan V.; Toles R.; Jobe L.; Seed T. M. Nutritional approachts to radioprotection: Vitamin E. Mil. Med. 2002, 167, 57–59. 10.1093/milmed/167.suppl_1.57. [DOI] [PubMed] [Google Scholar]
- Abramyan A. K.; Oganesyan N. M.. Therapeutic and prophylactic effect of legal in radiation exposure of rats. VI International Conference “Bioantioxidant”, 16–19 April Moscow: Moscow, 2002.
- Zaslavsky Y. S.. The role of ubiquinone as an antioxidant in the structure and function of membranes in normal and under the action of irradiation. Ph. D. (Biol.) Dissertation, Press Institut of biological Physics, Institut of Chemical Physics USSR Academy of Science, Puschino, 1984. [Google Scholar]
- Ulyanova E. V.; Larionov O. G.; Revina A. A.; Andryevskaya D. V.; Urusova L. M.; Fenin A. A. Protective properties of wine products and the role of high performance liquid chromatography in the study of these properties. Russ. Chem. Rev. 2013, 82, 1117–1134. 10.1070/rc2013v082n12abeh004332. [DOI] [Google Scholar]
- Degtyarev M. V.Radioprotective properties of the drug “Vitulin”. Ph.D. (Biol.) Dissertation, Press Saint-Petersburg State Academy of Veterinary Medicine, Saint Petersburg, 2006. [Google Scholar]
- Kashino G.; Liu Y.; Suzuki M.; Masunaga S.-i.; Kinashi Y.; Ono K.; Tano K.; Watanabe M. An alternative Mechanism for radioprotection by dimethyl sulfoxide; possible facilitation of DNA double-strand break repair. J. Radiat. Res. 2010, 51, 733–740. 10.1269/jrr.09106. [DOI] [PubMed] [Google Scholar]
- Jagetia G. C. Radioprotective potential of plants and herbs against the effects of ionizing radiation. J. Clin. Biochem. Nutr. 2007, 40, 74. 10.3164/jcbn.40.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havsteen B. Flavonoids: A class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983, 32, 1141. 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Maffei Facino R.; Carini M.; Aldini G.; Bombardelli E.; Morazzoni P.; Morelli R. Free Radicals Scavenging Action and Anti-Enzyme Activities of Procyanidines From Vitis Vinifera. A Mechanism for Their Capillary Protective Action. Arzneimittelforsch. 1994, 44, 592–601. [PubMed] [Google Scholar]
- Menschikova E. B.; Lankin V. Z.; Kandalintseva N. V.. Phenolic antioxidants in biology and medicine. Structure, Properties, Mechanisms of Action; Press LAP Lambert Academic Publishing: AV Akademikerverlag GmbH and Co. KG, 2012. [Google Scholar]
- Ho C.-T.; Chen Q.; Shi H.; Zhang K.-Q.; Rosen R. T. Antioxidative effect of polyphenol extract prepared from various Chinese teas. Prev. Med. 1992, 21, 520–525. 10.1016/0091-7435(92)90059-q. [DOI] [PubMed] [Google Scholar]
- Agabeyli R. A.Antimutagenic Effect of Lipid Containing Compounds Obtained from Fagus orientalis on the Mutability Induced by Aging and X-Rays. In Proceedings of 5th International Conference on Mechanisms of Antimutagenesis and Anticarcinogenesis, Okayama, 1996; p 39.
- Beckman I. N.Radiochemistry: Teaching Manual. Radiation and Nuclear Medicine: Physical and Chemical Aspects; Press Marhotin P. Yu: Moscow, 2012; Vol. VII. [Google Scholar]
- Revina A. A.Abstracts of Papers the III-d International Symposium on Natural Colourants, Princeton, USA, 1998; pp 278–292.
- Kostyuk V. A.; Potapovitch A. I. Superoxide-driven Oxidation of Quercetin and a Sim-ple Sensitive Assay for Determination of Superoxide Dismutase. Biochem. Int. 1989, 19, 1117–1124. [PubMed] [Google Scholar]
- Roginsky V. A.Phenolic Antioxidants: Reactivity and Effectiveness; Press Science: Moscow, 1988. [Google Scholar]
- Vladimirov V. K.; Krasilnikov I. I.; Arapov O. V.. Radioprotectors: Structure and Functions; Press Naukova Dumka: Kiev, 1989. [Google Scholar]
- Tarumov R. A.; Basharin V. A.; Grebenyuk A. N. Radioprotective properties of modern antioxidants. Radiol. Radioecol. 2012, 13, 682–700. [Google Scholar]
- Kang U.; Han A.-R.; So Y.; Jin C. H.; Ryu S. M.; Lee D.; Seo E. K. Furanocoumarins from the Roots of Angelica dahurica with Inhibitory Activity against Intracellular Reactive Oxygen Species Accumulation. J. Nat. Prod. 2019, 82, 2601. 10.1021/acs.jnatprod.9b00547. [DOI] [PubMed] [Google Scholar]
- Revina A. A.Radiation-chemical modeling of fast processes with the participation of the intermediate oxygen-containing reaction centers in different systems. Doctoral (Chem.) Dissertation, Press Institute of Physical Chemistry, Russian Academy of Sciences, Moscow, 1995. [Google Scholar]
- Ghiselli A.; Serafini M.; Natella F.; Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radical Biol. Med. 2000, 29, 1106–1114. 10.1016/s0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Traven V. F.; Vorobjeva L. I.; Chibisova T. A.; Carberry E. A.; Beyer N. J. Electronic absorption spectra and structure of hydroxycoumarin derivatives and their ionized forms. Can. J. Chem. 1997, 75, 365–376. 10.1139/v97-042. [DOI] [Google Scholar]
- Antropova I. G.Radiation-Chemical Transformations of Coumarin and Its Derivatives in Water-Organic Solutions. Ph.D. (Chem) Dissertation, Press Mendeleev University of Chemical Technology of Russia, Moscow, 2010. [Google Scholar]
- Antropova I. G.; Fenin A. A.; Semenistaya E. N.; Larionov O. G.; Revina A. A.; Parfenov E. A. Effect of Metal Ions on Radiation-Chemical Transformations of Coumarins in Solutions. High Energy Chem. 2008, 42, 507. 10.1134/S0018143908060179. [DOI] [Google Scholar]
- Antropova I. G.; Fenin A. A.; Revina A. A. Radiation-chemical transformations of coumarins in organic solvents. High Energy Chem. 2007, 41, 61. 10.1134/S0018143907020026. [DOI] [Google Scholar]
- Wang K.; Zhang Y.; Ekinwe S. I. N.; Yi X.; Liu X.; Wang H.; Pan Y. Antioxidant activity and inhibition effect on the growth of human colon carcinoma (HT-29) cells of esculetin from Cortex Fraxini. Med. Chem. Res. 2011, 20, 968. 10.1007/s00044-010-9426-y. [DOI] [Google Scholar]
- Pietta P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035. 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Jovanovic S. V.; Steenken S.; Tosic M.; Marjanovic B.; Simic M. G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. 10.1021/ja00090a032. [DOI] [Google Scholar]
- Heim K. E.; Tagliaferro A. R.; Bobilya D. J. Flavonoid antioxidants: chemistry, metabo-lism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Brown E. J.; Khodr H.; Hider C. R.; Rice-Evans C. A. Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem. J. 1998, 330, 1173–1178. 10.1042/bj3301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel I.; Cillard P.; Cillard J.. Flavonoid-metal interactions in biological systems. In Flavonoids in Health and Disease; Rice-Evans C. A., Packer L., Eds.; Marcel Dekker, Inc.: New York, 1998; pp 163–177. [Google Scholar]
- Makris D. P.; Rossiter J. T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 2000, 48, 3830–3838. 10.1021/jf0001280. [DOI] [PubMed] [Google Scholar]
- Halliwell B.; Gutteridge J. M. C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy A.; O’Kennedy R. Studies on Coumarins and Coumarin-Related Compounds to Determine Their Therapeutic Role in the Treatment of Cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. 10.2174/1381612043382693. [DOI] [PubMed] [Google Scholar]
- Sharova E. I.Plant Antioxidants: A Textbook; Press Saint Petersburg State University: St. Petersburg, 2016. [Google Scholar]
- Efremov A. A.; Zykova I. D.; Tselukovskaya M. M. Component composition of biologically active substances of Melilotus officinalis. Chem. Plant Mater. 2012, 3, 111–114. [Google Scholar]
- Basova E. V.Chemical and pharmacological study of Ledum palustre cormus. Ph.D. Dissertation, Press Sib. Gov. Univ. of Med., 2004. [Google Scholar]
- Sorenson J. R. J.; Soderberg L. S. F.; Chang L. W.; Walker R. B. Essential metalloelement chelates facilitate repair of radiation injury. Met.-Based Drugs 2001, 8, 215–234. 10.1155/mbd.2001.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhorova L. I.; Revina A. A. The Role of Labile Complexes With Oxygen in Antioxidant Activity of Carotenoids. Radiats. Biol. Radioecol. 2001, 41, 24–32. [PubMed] [Google Scholar]
- Revina A. A.; Amiragova M. I.; Volod’ko V. V.; Vannikov A. V. Kinetics and mechanism of superoxide radical reactions with some biologically important compounds in aqueous solutions. Pulse radiolysis. J. Radiat. Phys. Chem. 1989, 34, 653. 10.1016/1359-0197(89)90075-1. [DOI] [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- El-Hajjaji F.; Abdellaoui A.; Taleb M.; Hammouti B.; Zarrouk A. Chemical composition, anticorrosion and antioxidant activity of clove (Syzygium aromaticum) oil. J. Nat. Prod. 2017, 10, 45–57. [Google Scholar]
- Bubenchikova V. N.; Logutev S. V.; Sukhomlinov Y. A.; Malyutina A. Y. Bul. Voronezh State Univ.: Chem. Bio. Pharm. 2011, 2, 181–184. [Google Scholar]
- Nonhebel D.; Walton J.. Chemistry of free radicals. Structure and Mechanism of Reactions; Press MIR: Moscow, 1977. [Google Scholar]
- Brinkevich S. D.; Shadyro O. I. The effects of ascorbic acid on homolytic processes involving α-hydroxyl-containing carbon-centered radicals. Bioorg. Med. Chem. Lett. 2008, 18, 6448–6450. 10.1016/j.bmcl.2008.10.073. [DOI] [PubMed] [Google Scholar]
- Pikaev A. K.The Modern Radiation Chemistry: Radiolysis of Gases and Liquids; Press Science: Moscow, 1986. [Google Scholar]
- Petryaev E. P.; Shadyro O. I.. Radiation Chemistry of Bifunctional Organic Compounds; Press Belarusian State University: Minsk, 1986. [Google Scholar]
- Marfak A.; Trouillas P.; Allais D. P.; Calliste C. A.; Cook-Moreau J.; Duroux J.-L. Reactivity of flavonoids with 1-hydroxyethyl radical: a γ-radiolysis study. Biochim. Biophys. Acta, Gen. Subj. 2004, 1670, 28. 10.1016/j.bbagen.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Antropova I. G.; Kurakina E. S.; Magomedbekov E. P.; Oo P. M. Coumarin reactivity in free radical reactions. J. Radioanal. Nucl. Chem. 2019, 321, 823–829. 10.1007/s10967-019-06666-8. [DOI] [Google Scholar]
- Freeman G. R.Radiation Chemistry of Ethanol; Press NBS: Washington, 1974. [Google Scholar]
- Experimental Methods of High Energy Chemistry: Study Guide; Melnikov M. Y., Ed.; Press Moscow State University: Moscow, 2009. [Google Scholar]
- Andrievskaya D. V.Improvement of technology of table wines on the basis of regulation of their protective properties. Ph.D. Dissertation, Press All-Russian Scientific Research Institute of the Brewing, Non-Alcoholic and Wine Industry, Moscow, 2009. [Google Scholar]
- Korenskaya I. M.; Ivanovskaya N. P.; Izmalkova I. E.. Medicinal Plants and Medicinal Plant Raw Materials Containing Flavonoids, Coumarins, Chromons; Press SKO-Polygraphic Center of Voronezh State University, 2007. [Google Scholar]
- Pupyshev A. A.; Surikov V. T.. Inductively Coupled Plasma Mass Spectrometry. Ion Formation; Press Institute of Plant and Animal Ecology of the Ural Branch of the Russian Academy of Sciences, 2006. [Google Scholar]
- Pikaev A. K.The modern radiation chemistry: General Aspects, Experimental Equipment and Methods; Press Science: Moscow, 1985. [Google Scholar]
- Kaushanskii D. A. The RCHM-γ-20 multichamber γ-irradiator for radiation research. Sov. Atom. Energy 1970, 29, 1162. 10.1007/bf01666714. [DOI] [Google Scholar]