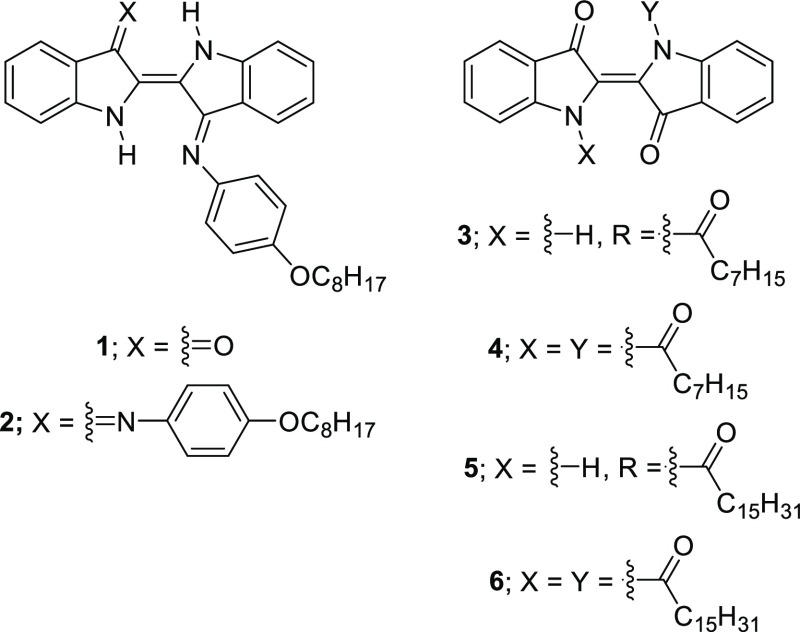
Abstract
This work describes the practical production of novel indigo derivatives from commercially available and economically friendly indigo and investigation for their potential use as diesel markers. Introduction of solubilizing long alkyl chains into an indigo molecule via formation of arylimino moieties at its carbonyl sites and amidation at its amino groups greatly enhances the solubility in diesel and several common organic solvents. Effects of the number and position of the alkyl chains on the absorption behavior of the compounds are discussed. Because of their superior absorption in a region where the diesel cannot absorb, indigo N-arylimine and N-monoacyl-substituted indigo derivatives can serve as diesel absorption markers at detection wavelengths of 590 and 575 nm, respectively. UV–visible spectrophotometric analysis suggested that this target marker is stable in diesel for at least 3 months under ambient conditions. Furthermore, physical testing according to the American Society for Testing and Materials standards indicates that addition of these markers at a concentration of 5 ppm does not significantly affect the physical properties of the original diesel, thus confirming the applicability of these compounds for marking of commercial diesels.
Introduction
Fuel tax is an excise tax imposed on the sale of fuels according to the government rates, depending on the types and applications of the fuels. Nowadays, fuel tax fraud, for example, by adulterating fuel products with low-cost components, diluting the higher taxed fuels with the lower taxed ones, and illegal oil smuggling, has become an increasingly serious problem. These illicit actions result in the loss of revenue to governments, poor fuel performance, and possible emission of harmful pollutants. One of the effective approaches to tackle this problem is the addition of silent markers into fuel products in order to track the sources of the fuels, to monitor the quality of the fuels, and to specify the types of the fuels in markets. Other than the possibility of low-cost scalable production, the required properties of fuel markers include: (1) solubility in the fuel products; (2) invisibility to the naked eye when added; (3) no adverse effect on the properties of the fuel products; (4) stability in the fuel products for at least 3 months, which is the average shelf-life of the fuel products under ambient conditions; and (5) detectability with good sensitivity by a simple analytical method.1 Many kinds of the markers have been proposed for using in petroleum products,2 such as porphyrins,1,3 anthraquinones,4 coumarins,5 phthalocyanines and naphthocyanines,6 and diazo compounds.2 However, the current number of suitable fuel markers is still too low to provide sufficient choices of markers to authorized organizations for regular change of marker types.
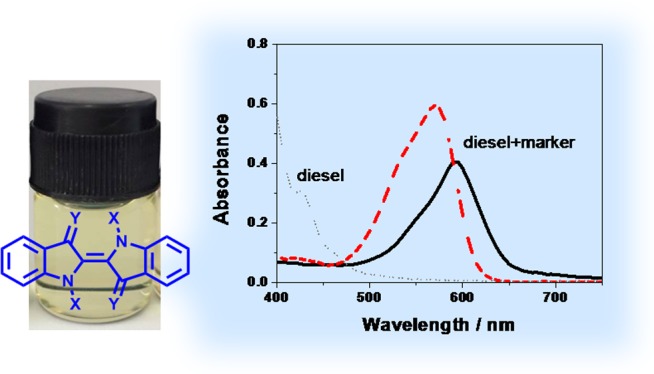
Owing to its natural abundance, strong extinction coefficients in the visible region, high stability at the ambient atmosphere, and amenability to structural modification via several well-established chemical approaches, indigo and its derivatives are one of the most well-known historic groups of organic compounds widely used for many applications such as dyes and pigments,7 medical active compounds,8 semiconducting materials,9,10 and so forth. According to previous studies, the indigo N,N′-diaryldiimine, or so-called “Nindigo,” and acyl-substituted indigo derivatives exhibited absorption in the range of 500–750 nm in which some kinds of fuels such as diesel does not absorb.11−13The incorporation of the aryl and acyl groups in these compounds allows introduction of solubilizing long alkyl chains into the indigo cores. Therefore, in this work, these two series of the indigo derivatives, whose structures contain the long alkyl chain, as shown in Chart 1, were synthesized and investigated for their potential use as diesel markers.
Chart 1. Structures of the target compounds.
Results and Discussion
Synthesis and Absorption Properties of Target Indigo Derivatives
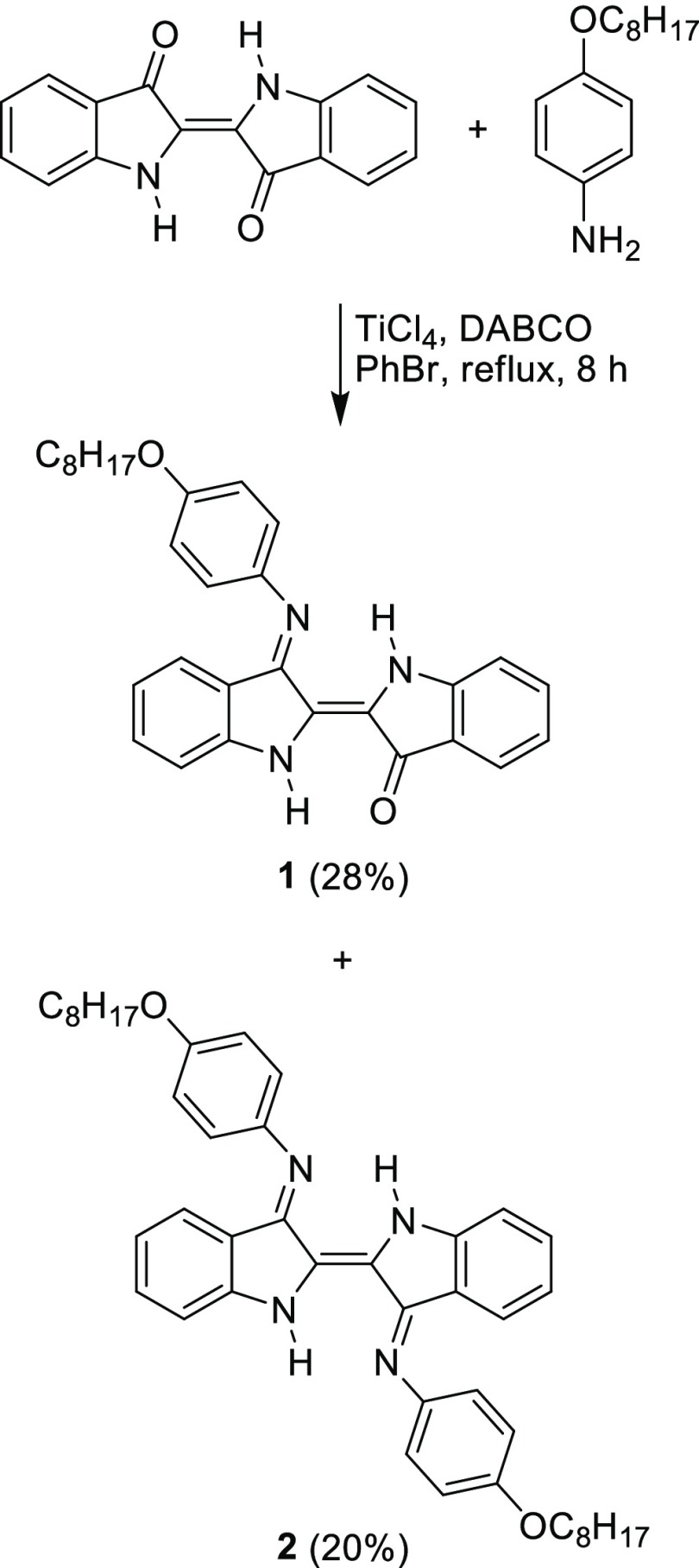
As shown in Scheme 1, compounds 1 and 2 were obtained in 28 and 20% yield, respectively, from condensation of indigo with 4-(octyloxy)aniline in the presence of TiCl4 and 1,4-diazabicyclo[2.2.2]octane (DABCO). According to 1H nuclear magnetic resonance (NMR) spectroscopy, alkyl and aromatic protons of these compounds showed signals at δ 0.78–1.80 and 6.59–7.81 parts per million (ppm), respectively. The presence of peaks at 3.87–3.96 ppm, which corresponded to the −OCH2– protons, confirmed the successful condensation of indigo with 4-(octyloxy)aniline. Moreover, two broad singlet signals at 9.12 and 9.61 ppm observed in a 1H NMR spectrum of compound 1 indicated the presence of amino protons on an indolinimine and an indolinone rings, respectively. However, a peak of the amino protons of compound 2 could not be clearly observed, possibly because of the steric effect from both long alkyl chains. 13C NMR spectra of compounds 1 and 2 showed the signals of sp3 carbons on the alkyl chain at 14.1–32.0 ppm with the signal of a −OCH2– carbon at 68.6–68.7 ppm. The signals of aromatic and C=C carbons appeared at 109.7–159.1 ppm, while the C=N and C=O carbons showed the signals at 160.2–161.3 and 185.6 ppm, respectively. Furthermore, high-resolution electrospray ionization mass spectrometry (HR–ESI–MS) also confirmed the successful formation of compounds 1 and 2 by showing their molecular ion peaks at m/z 466.2496 and 667.4011, respectively. As for the solubility, unlike indigo, both target compounds were found to have a satisfactory solubility (>15 mg/mL) in diesel and common organic solvents such as CH2Cl2, hexane, and methanol.
Scheme 1. Synthesis of Compounds 1 and 2.
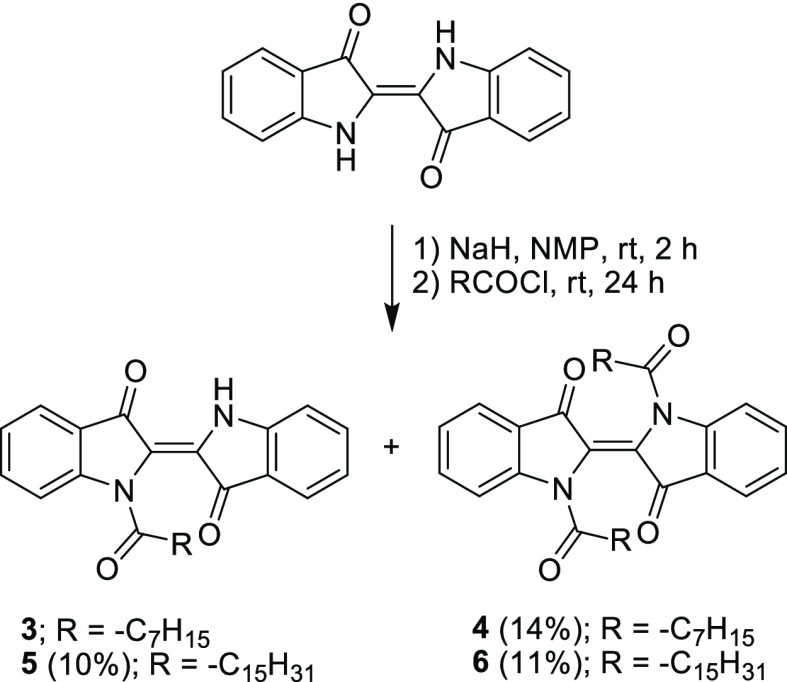
Compounds 3 and 4 were prepared from two-step one-pot amidation between indigo and octanoyl chloride, leading to the formation of compound 4 in 14% yield (Scheme 2). Because of the coexistence of unknown impurities having similar Rf values, compound 3 could not be completely separated from the reaction mixture and was obtained at >90% purity according to 1H NMR spectroscopy. Upon extension of the alkyl chains from C7 to C15 by using palmitoyl chloride instead of octanoyl chloride under similar conditions, compounds 5 and 6 could be purely isolated as dark blue and dark purple solids in 10 and 11% yield, respectively. The low yield was attributed to the indefinable side reaction(s) that became more competitive and suppressed the yields of the target compounds upon longer reaction time. However, approximately 20% of the starting indigo could be recovered from these syntheses. Similar to compounds 1 and 2, compounds 4–6 exhibited good solubility (>15 mg/mL) in diesel and common organic solvents.
Scheme 2. Synthesis of Compounds 3–6.
The formation of compounds 3–6 was confirmed by the presence of 1H NMR signals of the alkyl chains and aromatic protons at 0.78–2.88 and 7.00–8.24 ppm, respectively. In the case of N-monoacyl-substituted compounds 3 and 5, additional signals of the amino protons on the indolinone rings were found at 10.01–10.02 ppm. In the 13C NMR spectra, all compounds in this series, alkyl carbons, aromatic carbons, amido carbons, and carbonyl carbons on the indigo cores exhibited signals at 14.0–37.1, 112.5–152.0, 173.7–174.5, and 180.4–188.5 ppm, respectively. Moreover, their molecular ion peaks observed by the HR–ESI–MS were consistent with the calculated values.
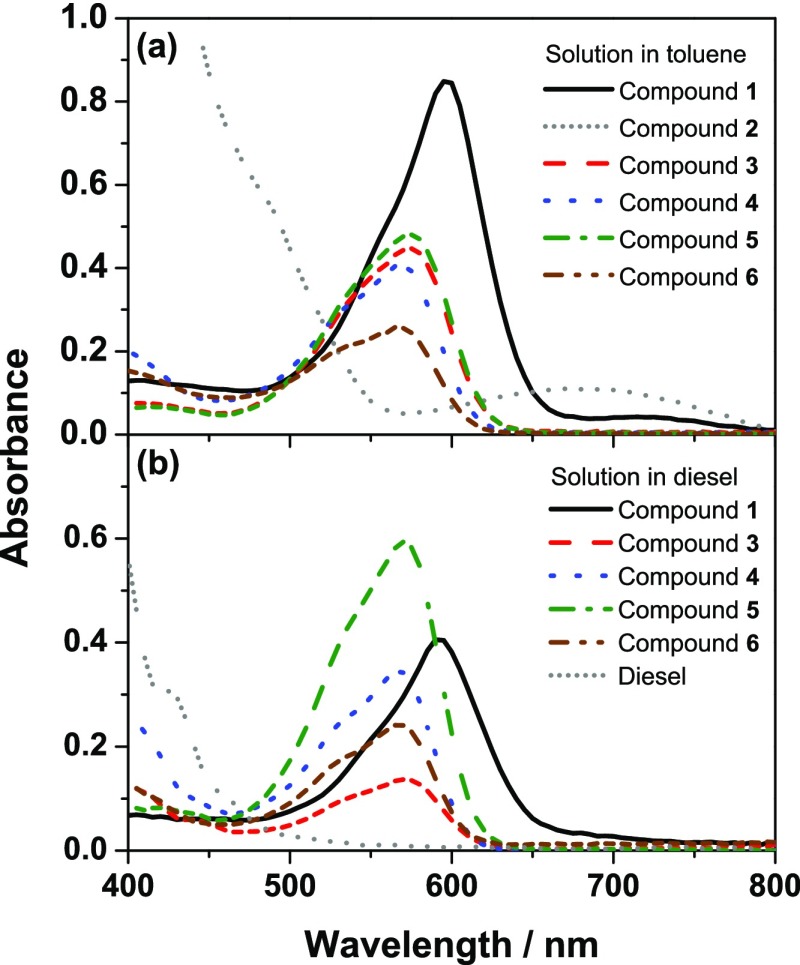
According to UV–visible spectrophotometry, solutions of compound 1 in toluene and diesel exhibited pronounced absorption peaks at 595 and 590 nm, respectively, as shown in Figure 1 and Table 1. However, the solution of compound 2 in toluene showed a broad and weak absorption peak at 675 nm, which could not be clearly observed in the diesel. Such a great difference in absorption behavior between these two compounds was possibly because of the electron-donating effect originating from the 4-alkoxyaryl groups as speculated by the previous study.11 As for compounds 3–6, their absorption in toluene and diesel could be observed in the range of 570–575 nm. It was obvious that the length of the alkyl chain did not significantly affect the absorption behavior of the compounds in this series. Moreover, the introduction of the long alkyl chains via the formation of the imino groups resulted in the longer absorption wavelength than the N-acylation on the indigo core. This bathochromic effect was attributed to the extension of π-conjugated systems because of the imino groups.
Figure 1.
Absorption spectra of the target compounds measured in (a) toluene and (b) diesel at a concentration of 40 ppm. Color code and line pattern for each compound: compound 1 (black solid line), compound 2 (gray densely dotted line), compound 3 (red-dashed line), compound 4 (blue-dotted line), compound 5 (green-dashed-dotted line), compound 6 (brown dashed dotted line), and diesel (gray densely dotted line).
Table 1. Absorption Characteristics of Target Compounds in Toluene and Diesel.
| λabs/nm (ε/M–1·cm–1) | ||
|---|---|---|
| compound | in toluene | in diesel |
| 1 | 595 (1 × 104) | 590 (5 × 103) |
| 2 | 675 (a) | a |
| 3b | 575 (5 × 103) | 570 (2 × 103) |
| 4 | 570 (5 × 103) | 570 (6 × 103) |
| 5 | 575 (7 × 103) | 575 (9 × 103) |
| 6 | 570 (6 × 103) | 570 (6 × 103) |
Because of the weak absorption, the value could not be determined.
The compound was investigated in the crude form at >90% purity.
In overall, as compared to the absorption feature of diesel, all compounds showed the absorption out of the absorption range of the diesel. In terms of absorptivity, compound 1 showed the highest molar extinction coefficient (ε) value in toluene, whereas compound 5 showed the superior absorption in diesel, among all compounds. Therefore, compounds 1 and 5 have been selected for further investigation on their potential use as the diesel markers in the next sections.
Quantitative Determination and the Stability Test of Compounds 1 and 5 in Diesel
As mentioned above, one of the requirements for the fuel marker is to be invisible to the eyes when added in the fuel products. To determine an appropriate working concentration of compounds 1 and 5 in the diesel, colors of the diesel samples containing compound 1 or 5 at the concentration of 5–10 ppm were visually compared to that of the pristine commercial diesel. The result showed that at the concentration of more than 5 ppm, the colors of the marked diesel samples became distinctive from the original diesel. Therefore, the concentration of compounds 1 and 5 for marking the diesel selected for this study was 5 ppm.
According to UV–visible spectrophotometry, standard calibration equations of the diesel solutions containing compounds 1 and 5 at their absorption maxima were determined and used for quantifying compounds 1 and 5 in the marked diesel samples. To be in accordance with the regular storage time of the commercial diesels in the market, a total period for this stability test was 3 months. As shown in Table 2, the results showed that the concentrations of compounds 1 and 5 in the diesel samples observed during the period of 3 months under the ambient condition were found to be almost constant, indicating the sufficient stability of these two compounds in the diesel.
Table 2. Concentrations of Compounds 1 and 5 in Diesel after 1–3 Months.
| month | concentration in diesel/ppm | |
|---|---|---|
| compound 1 | compound 5 | |
| 1 | 5.02 ± 0.03 | 5.01 ± 0.05 |
| 2 | 5.02 ± 0.06 | 5.01 ± 0.05 |
| 3 | 5.02 ± 0.06 | 5.03 ± 0.07 |
Effects of Compounds 1 and 5 on Physical Properties of Diesel
In this study, physical properties of the diesel containing 5 ppm of compound 1 or 5 were investigated following the American Society for Testing and Materials (ASTM) test methods in comparison with those of the unmarked diesel to ensure that the addition of compounds 1 and 5 will not adversely affect the properties of the original diesel. The values in Table 3 indicated that the physical properties of the diesel marked by compounds 1 and 5 were very similar to those of the unmarked one, suggesting that compounds 1 and 5 did not significantly change the physical properties and quality of the diesel when used at this concentration.
Table 3. General Physical Properties of Marked and Unmarked Diesels.
| diesel |
|||||
|---|---|---|---|---|---|
| physical property | ASTMa method | limit | unmarked | marked by 1b | marked by 5b |
| APIc gravity at 15.6 °C | D 4052 | report | 38.1 | 38.1 | 38.1 |
| specific gravity at 15.6/15.6 °C | D 4052 | 0.81–0.87 | 0.8343 | 0.8344 | 0.8344 |
| calculated cetane index | D 976 | min. 50 | 55.63 | 55.57 | 55.44 |
| kinematic viscosity at 40 °C/cSt | D 445 | 1.8–4.1 | 3.068 | 3.079 | 3.083 |
| pour point/°C | D 5950 | max. 10 | –3 | –3 | –3 |
| flash point/°C | D 93 | min. 52 | 56.0 | 62.0 | 64.0 |
| sulfur content/%w/w | D 5453 | max. 50 | 44 | 42 | 41 |
| distillation at | D 86 | ||||
| initial boiling point (IBP) | report | 171.7 | 171.6 | 171.7 | |
| 10% (v/v) recovery/°C | report | 214.9 | 215.2 | 214.6 | |
| 50% (v/v) recovery/°C | report | 281.8 | 281.7 | 281.0 | |
| 90% (v/v) recovery/°C | max. 357 | 346.9 | 346.2 | 344.7 | |
| color | D 1500 | max. 4.0 | 1.0 | 1.0 | lower 1.5 |
American Society for Testing and Materials (ASTM).
Diesel was marked at the concentration of 5 ppm.
American Petroleum Institute (API).
As compared to the vast majority of fuel markers previously reported in the open literature,1,2 our marker type appeared to be one of a few practically useful kinds of markers1,2,14 that enabled onsite detection at a relatively low concentration by simple inexpensive UV–visible spectrophotometry without any extraction or sample preparation needed.
Conclusions
In this work, we successfully synthesized two series of the highly soluble indigo derivatives bearing one or two long alkyl chains by treating naturally abundant indigo with the commercially available 4-(alkyloxy)aniline and acid chloride via a simple one-pot process. All target compounds showed satisfactory solubilities in diesel, but only the monoalkyl-substituted derivatives of each series, that is, compounds 1 and 5, exhibited a pronounced absorption peak at 575–595 nm in toluene and the diesel, which is out of the absorption range of the diesel. The number and the position of the long alkyl substituents introduced on the indigo core played a significant role in the absorption properties of the resulting compounds. The proper working concentration of compounds 1 and 5 in the diesel was found to be 5 ppm to be uses as the silent absorption markers. At this concentration, quantitative UV–visible spectrophotometric analysis was carried out under the ambient condition to ascertain their stabilities in the diesel over 3 months. Finally, the effects of compounds 1 and 5 in the diesel were investigated under the ASTM test method conditions. These experiments showed that 1- and 5-marked diesel samples exhibited similar physical properties to the unmarked one and simultaneously passed all tests. These results suggest that indigo derivatives 1 and 5 had potential application in stealth marking of commercial diesels.
Experimental Section
Materials and Methods
All chemicals were of analytical grade, purchased from commercial sources, and used as received, unless noted otherwise. The 1H NMR and 13C NMR spectra were collected in deuterated chloroform (CDCl3) at 400 megahertz (MHz) for 1H nuclei and 100 MHz for 13C nuclei. Chemical shifts (δ) are reported in ppm relative to the residual CHCl3 peak (7.26 ppm for the 1H NMR spectra and 77.0 ppm for the 13C NMR spectra). Mass analysis was performed by using a HR-ESI-MS technique. Absorption spectra of the compounds were collected in toluene and the diesel at room temperature, and the ε values were reported in M–1·cm–1.
Synthesis of Indigo Derivatives 1 and 2
Following a published method,11,12 a 1.0 M solution of TiCl4 in toluene (1.02 mL, 5.20 mmol) was added dropwise to bromobenzene (53.4 mL) containing DABCO (2.26 g, 20.2 mmol) and 4-(octyloxy)aniline (0.496 g, 2.24 mmol), leading to the immediate generation of a white fume and light-brown precipitate. After the fuming subsided, indigo (0.588 g, 2.24 mmol) was added, and the resulting dark blue mixture was refluxed overnight (approximately 14 h). The resulting red-brown reaction mixture was then filtered while still warm, and bromobenzene was removed from the filtrate under vacuum. After that, a crude product was dissolved in CH2Cl2 (400 mL), washed with deionized (ID) water (3 × 200 mL), dried over anhydrous Na2SO4, and concentrated to dryness. Subsequent purification using column chromatography [silica, CH2Cl2/hexanes (2:1)] yielded compounds 1 and 2.
Compound 1 (0.294 g, 28%): dark-blue gummy solid. 1H NMR (δ): 0.78–0.87 (m, 3H), 1.44–1.61 (m, 8H), 1.73–1.80 (m, 4H), 3.96 (t, J = 4.0 Hz, 2H), 6.60 (t, J = 8.0 Hz, 1H), 6.85–6.99 (m, 6H), 7.19–7.25 (m, 1H), 7.35 (t, J = 8.0 Hz, 1H), 7.45–7.47 (m, 1H), 7.63–7.65 (m, 1H), 7.71 (d, J = 8.0 Hz, 1H), 9.12 (br s, 1H), 9.61 (br s, 1H); 13C NMR (δ): 14.1, 22.6, 23.4, 25.5, 26.2, 29.2, 29.3, 29.7, 31.8, 32.2, 68.7, 114.4, 115.3, 119.7, 121.1, 122.7, 125.0, 125.5, 126.2, 126.3, 128.9, 132.6, 135.2, 137.5, 138.1, 150.6, 151.4, 155.5, 160.2, 185.6; HR-ESI-MS m/z: [M + H]+ calcd for C30H31N3O2, 466.2495; found, 466.2496; λabs(ε) (toluene) 595(1 × 104) nm, (diesel) 590(5 × 104) nm.
Compound 2 (0.206 g, 20%): reddish brown gummy solid. 1H NMR (δ): 0.81–0.84 (m, 6H), 1.23–1.28 (m, 13H), 1.37–1.44 (m, 6H), 1.68–1.79 (m, 5H), 3.87–3.96 (m, 4H), 6.59 (t, J = 4.0 Hz, 1H), 6.86–6.93 (m, 8H), 7.00 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 4.0 Hz, 1H), 7.17 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 2H), 7.81 (s, 1H); 13C NMR (δ): 14.1, 22.7, 25.9, 26.0, 29.2, 29.4, 31.8, 68.6, 109.7, 111.1, 114.7, 115.0, 119.1, 120.6, 121.7, 122.1, 122.8, 123.2, 124.3, 124.8, 126.1, 128.7, 132.0, 134.1, 134.4, 134.8, 136.3, 136.6, 139.1, 152.2, 156.3, 159.1, 160.4, 161.3; HR-ESI-MS m/z: [M – H]+ calcd for C44H52N4O2, 667.4012; found, 667.4011; λabs (toluene) 675 nm.
General Synthesis Procedure of Indigo Derivatives 3–6
Following a published procedure,13 a solution of indigo (0.803 g, 3.10 mmol) in N-methyl-2-pyrrolidone (NMP, 38.25 mL) was treated with sodium hydride (0.294 g, 12.2 mmol) at room temperature for 2 h. Then, octanoyl chloride (1.984 g, 12.20 mmol) or palmitoyl chloride (3.364 g, 12.24 mmol) was added, and the reaction was continued at room temperature for additional 24 h. After that, the reaction mixture was poured into the deionized (DI) water and extracted with ethyl acetate. To remove NMP, the organic layer was washed with a saturated solution of NaCl and then with DI water. The resulting organic phase was dried over anhydrous Na2SO4 and concentrated to dryness. Purification by a silica column [hexanes/ethyl acetate (4:1)] yielded the desired compounds.
(E)-1-Octanoyl-[2,2′-biindolinylidene]-3,3′-dione (3)
Because of the presence of unknown byproducts having similar Rf values, compound 3 could not be completely separated using column chromatography. According to 1H NMR spectroscopy, the resulting crude containing compound 3 (0.143 g) was obtained at >90% purity. 1H NMR δ: 0.78–0.91 (m, 3H), 1.14–1.38 (m, 8H), 1.61–1.75 (m, 2H), 2.69 (t, J = 15.2, 2H), 7.00–7.07 (m, 2H), 7.25 (t, J = 14.8, 1H), 7.53 (t, J = 15.2 Hz, 1H), 7.63 (t, J = 15.6 Hz, 1H), 7.70 (d, J = 7.6, 1H), 7.81 (d, J = 7.6 Hz, 1H), 8.12 (d, J = 8.4 Hz, 1H), 10.02 (s, 1H); 13C NMR δ: 14.1, 22.7, 24.8, 29.0, 29.2, 31.8, 34.2, 112.7, 116.6, 120.1, 122.2, 123.6, 124.3, 125.2, 125.5, 130.0, 136.2, 137.1, 149.9, 152.0, 174.5, 180.4, 188.4; HR-ESI-MS m/z: [M + H]+ calcd for C24H24N2O3, 389.1865; found, 389.1869; λabs(ε) (toluene) 575(5 × 103) nm, (diesel) 570(2 × 103) nm.
(E)-1,1′-Dioctanoyl-[2,2′-biindolinylidene]-3,3′-Dione (4) (0.216 g, 14%)
The product is obtained as a dark purple solid. mp 90–91 °C; 1H NMR δ: 0.80 (t, J = 12.8 Hz, 6H), 1.10–1.32 (m, 16H), 1.68–1.80 (m, 4H), 2.74–2.87 (m, 4H), 7.22 (t, J = 14.8 Hz, 2H), 7.61 (t, J = 15.6 Hz, 2H), 7.71 (d, J = 7.2 Hz, 2H), 8.20 (d, J = 8.0 Hz, 2H); 13C NMR δ: 14.0, 22.5, 25.6, 29.0, 29.2, 31.6, 36.2, 117.0, 121.9, 124.3, 124.9, 126.0, 136.8, 149.4, 173.7, 184.1; HR-ESI-MS m/z: [M + Na]+ calcd for C32H38N2O4, 537.2729; found, 537.2739; λabs(ε) (toluene) 570(5 × 103) nm, (diesel) 570(6 × 103) nm.
(E)-1-Palmitoyl-[2,2′-biindolinylidene]-3,3′-dione (5) (0.164 g, 10%)
The product is obtained as a dark blue solid. mp 89–91 °C; 1H NMR δ: 0.87 (t, J = 13.2 Hz, 3H), 1.11–1.31 (m, 24H), 1.57–1.75 (m, 2H), 2.69 (t, J = 14.8 Hz, 2H), 7.00–7.07 (m, 2H), 7.25 (t, J = 14.4 Hz, 1H), 7.53 (t, J = 15.6 Hz, 1H), 7.62 (t, J = 15.6 Hz, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.81 (d, J = 7.6 Hz, 1H), 8.12 (d, J = 8.4 Hz, 1H), 10.01 (s, 1H); 13C NMR δ: 14.2, 22.8, 25.8, 29.2, 29.4, 29.5, 29.6, 29.7, 29.8, 32.0, 37.1, 112.5, 116.6, 119.9, 122.2, 122.9, 123.5, 124.3, 125.5, 129.1, 129.7, 136.2, 137.1, 149.9, 151.9, 174.4, 186.5, 188.5; HR-ESI-MS m/z: [M + Na]+ calcd for C32H40N2O3, 523.2937; found, 523.2935; λabs(ε) (toluene) 575(7 × 103) nm, (diesel) 575(9 × 103) nm.
(E)-1,1′-Dipalmitoyl-[2,2′-biindolinylidene]-3,3′-Dione (6) (0.261 g, 11%)
The product is obtained as a dark purple solid. mp 95–96 °C; 1H NMR δ: 0.87 (t, J = 13.2 Hz, 6H), 1.12–1.33 (m, 48H), 1.70–1.81 (m, 4H), 2.77–2.88 (m, 4H), 7.25 (t, J = 15.2 Hz, 2H), 7.64 (t, J = 15.6 Hz, 2H), 7.75 (d, J = 7.6 Hz, 2H), 8.24 (d, J = 7.6 Hz, 2H); 13C NMR δ: 14.2, 22.8, 25.8, 29.4, 29.5, 29.6, 29.7, 29.8, 32.1, 36.4, 117.1, 122.0, 124.5, 125.1, 126.2, 137.0, 149.6, 173.8, 184.3; HR-ESI-MS m/z: [M + Na]+ calcd for C48H70N2O4, 761.5233; found, 761.5238; λabs(ε) (toluene) 570(6 × 103) nm, (diesel) 570(6 × 103) nm.
Investigation of Working Concentrations of Markers 1 and 5 in Diesel
A 100 ppm stock solution of each marker in diesel was prepared by dissolving compound 1 or 5 (0.005 g) with diesel in a 50 mL volumetric flask. Then, this solution (0.25 and 0.50 mL) was further diluted with the diesel in 5 mL volumetric flasks to obtain solutions with final concentrations of 5 and 10 ppm, respectively. After that, the color of the resulting solutions was visually compared with that of the commercial diesel.
Quantitative Determination and the Stability Test of Compounds 1 and 5 in Diesel
A series of calibration solutions of compounds 1 and 5 in the diesel was prepared at the concentrations of 5, 10, 15, 20, 40, and 50 ppm by diluting the aforementioned 100 ppm stock solutions (0.5, 1.0, 1.5, 3.0, 4.0, and 5.0 mL, respectively) with the diesel in 10 mL volumetric flasks. The absorption spectrum of each calibration solution was recorded by a UV–visible spectrophotometer. The calibration equations for compounds 1 and 5 were obtained from the slopes of plots between absorbance at 590 nm for compound 1 and at 575 nm for compound 5 (y-axis) and the concentration of compounds 1 and 5 in ppm (x-axis). These equations were used to quantify compounds 1 and 5 in the solution samples analyzed in the stability test.
The stability of both compounds in the diesel was evaluated over a period of 3 months. A set of nine sealed vials containing 5 ppm solutions of each compound in the diesel (5 mL) was stored under the ambient condition. After 1, 2, and 3 months, the solutions from 3 vials were measured for their absorbance at 590 nm for compound 1 and at 575 nm for compound 5 by the UV–visible spectrophotometer. The quantities of compounds 1 and 5 in the diesel were then determined by the abovementioned calibration equations.
Effect of Compounds 1 and 5 on the Physical Properties of Diesel
The 5 ppm solutions of compounds 1 and 5 in the diesel were prepared by dissolving the aforementioned 100 ppm stock solutions (50 mL) with the diesel in a 1 L volumetric flask. The physical properties of these marked diesel samples were examined in comparison with those of an unmarked one according to the ASTM test methods.
Acknowledgments
This research was supported by the Ratchadaphiseksomphot Endowment Fund (2018), Chulalongkorn University (CU-GR_61_019_23_006), Graduate School of Chulalongkorn University (The 90th Anniversary of Chulalongkorn University Fund, Ratchadaphiseksomphot Endowment Fund), and Thailand Research Fund (RTA6080005). Physical properties data of the marked and unmarked diesels were obtained from the PTT Public Company Limited, Thailand.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04449.
Synthesis procedure of target compounds, standard calibration curves, spectral data, including 1H NMR, 13C NMR, HR-ESI mass, and absorption spectra for new compounds (PDF)
Author Contributions
⊥ S.M. and P.P. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Puangmalee S.; Petsom A.; Thamyongkit P. A porphyrin derivative from cardanol as a diesel fluorescent marker. Dyes Pigm. 2009, 82, 26–30. 10.1016/j.dyepig.2008.10.015. [DOI] [Google Scholar]
- Orzel J.; Daszykowski M. Recent trends in the use of liquid fuel taggants and their analysis. Trends Anal. Chem. 2017, 87, 98–111. 10.1016/j.trac.2016.11.010. [DOI] [Google Scholar]
- Figueira A. C. B.; de Oliveira K. T.; Serra O. A. New porphyrins tailored as biodiesel fluorescent markers. Dyes Pigm. 2011, 91, 383–388. 10.1016/j.dyepig.2011.05.020. [DOI] [Google Scholar]
- Orelup R. B.Colored petroleum markers. U.S. Patent 4,735,631 A, Apr 3, 1988.
- Friswell M. R.Silent markers for petroleum, method of tagging, and method of detection. U.S. Patent 5,156,653 A, Oct 20, 1992.
- Krutak J. J.; Cushaman M. R.; Waever M. A.. Method for tagging petroleum products. U.S. Patent 5,525,516 A, Nov 6, 1996; Chem. Abstr. 1996, 125, 37859n.
- Clark R. J. H.; Cooksey C. J.; Daniels M. A. M.; Withnall R. Indigo, woad, and Tyrian Purple: important vat dyes from antiquity to the present. Endeavour 1993, 17, 191–199. 10.1016/0160-9327(93)90062-8. [DOI] [Google Scholar]
- Stasiak N.; Kukula-Koch W.; Glowniak K. Modern industrial and pharmacological applications of indigo dye and its derivatives—A review. Acta Pol. Pharm. 2014, 71, 21–221. [PubMed] [Google Scholar]
- Głowacki E. D.; Voss G.; Leonat L.; Irimia-Vladu M.; Bauer S.; Sariciftci N. S. Indigo and Tyrian Purple - from ancient natural dyes to modern organic semiconductors. Isr. J. Chem. 2012, 52, 540–551. 10.1002/ijch.201100130. [DOI] [Google Scholar]
- Głowacki E. D.; Voss G.; Sariciftci N. S. 25th anniversary article: progress in chemistry and applications of functional indigos for organic electronics. Adv. Mater. 2013, 25, 6783–6800. 10.1002/adma.201302652. [DOI] [PubMed] [Google Scholar]
- Nawn G.; Waldie K. M.; Oakley S. R.; Peters B. D.; Mandel D.; Patrick B. O.; McDonald R.; Hicks R. G. Redox-active bridging ligands based on indigo diimine (“Nindigo”) derivatives. Inorg. Chem. 2011, 50, 9826–9837. 10.1021/ic200388y. [DOI] [PubMed] [Google Scholar]
- Oakley S. R.; Nawn G.; Waldie K. M.; MacInnis T. D.; Patrick B. O.; Hicks R. G. ‘‘Nindigo’’: synthesis, coordination chemistry, and properties of indigo diimines as a new class of functional bridging ligands. Chem. Commun. 2010, 46, 6753–6755. 10.1039/c0cc01736a. [DOI] [PubMed] [Google Scholar]
- Guo C.; Sun B.; Quinn J.; Yan Z.; Li Y. Synthesis and properties of indigo based donor-acceptor conjugated polymer. J. Mater. Chem. C 2014, 2, 4289–4296. 10.1039/c3tc32276a. [DOI] [Google Scholar]
- Orzel J.; Daszykowski M.; Grabowski I.; Zaleszczyk G.; Sznajder M.; Walczak B. Simultaneous determination of Solvent Yellow 124 and Solvent Red 19 in diesel oil using fluorescence spectroscopy and chemometrics. Talanta 2012, 101, 78–84. 10.1016/j.talanta.2012.08.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.