Abstract
In observational studies, left ventricular mass (LVM) and structure are strong predictors of mortality and cardiovascular (CV) events. However, the effect of hypertension treatment on LVM reduction and its relation to subsequent outcomes is unclear, particularly at lower blood pressure (BP) targets. In an ancillary study of SPRINT (Systolic BP Intervention Trial), where participants were randomly assigned to intensive BP control (target systolic BP target<120 mmHg) versus standard BP control (<140 mmHg), cardiac magnetic resonance imaging (CMRI) was performed at baseline and 18-month follow-up to measure: LVM, volumes, ejection fraction, and native T1 mapping for myocardial fibrosis. At baseline, 337 participants were examined (age: 64±9 years, 45% women); 300 completed the 18-month exam (153 intensive control, 147 standard control). In the intensive versus standard BP control group at 18-months, there was no difference in change in LVM (mean±SE = −2.7±0.5g versus −2.3±0.7g; p=0.368), ejection fraction, or native T1 (p=0.79), but there was a larger decrease in LVM/end-diastolic volume ratio (−0.04±0.01 versus −0.01±0.01; p=0.002) a measure of concentric LV remodeling. There were fewer CV events in the intensive control group, but no significant association between the reduced events and change in LVM or any other CMRI measure. In SPRINT-HEART, contrary to our hypothesis, there were no significant between-group differences in LVM, function, or myocardial T1 at 18-month follow-up. These results suggests that mediators other than these LV measures contribute to the improved CV outcomes with intensive BP control.
Keywords: Hypertension treatment, left ventricular mass, left ventricular remodeling, myocardial fibrosis, blood pressure, aging, elderly
Graphical Abstract:
Introduction
Left ventricular hypertrophy (LVH), abnormally increased LV mass (LVM), occurs commonly in hypertension, and in observational studies is an independent predictor of cardiovascular (CV) disease (CVD) events and mortality.1–3 However, relatively few randomized trials, have examined the effect of blood pressure (BP) lowering on LVM, and whether reducing LVM reduces CVD risk independent of BP changes.4–6 This information is needed before LVM can be considered a therapeutic target, independent of BP. Multiple lines of evidence suggest that LVM is partly independent of BP.2;7–10 One analysis indicated that BP may account for only 25% of the variability of LVM in a population.11 The uncertainty regarding the relationships between BP lowering, LVM reduction, and improved CVD outcomes is greatest at the lower ranges of target BP where there are almost no data.
In the Systolic BP Intervention Trial (SPRINT), intensive BP control to a target systolic BP (SBP) <120 mm Hg compared to standard BP control to a target of SBP < 140 mm of Hg reduced the risk of CV morbidity and mortality by 25%, total mortality by 27%,12 and acute decompensated heart failure (HF) by 36%.13 Recently, Soliman et al. reported that intensive BP control in SPRINT resulted in modest reduction of electrocardiogaphic LVH, however this did not explain the reduction in CVD events.14
In response to this work, Lewis et al recently commented that cardiac magnetic resonance imaging(CMRI), which has distinct advantages over ECG and echocardiography, might provide much needed mechanistic insight into the relationships between hypertension, LVM, and CVD.15 Assessment of LVH in most hypertension trials has relied on either ECG, which has lower sensitivity and does not measure actual LVM, or echocardiography, which has significant variability and relatively high rates of non-evaluability, resulting in potential assessment bias.3;4;16 CMRI has significantly greater accuracy, reproducibility, and rates of evaluability for LVM compared to echocardiography.17;18 In studies of LVM regression, these features of CMRI allow for sample sizes 90% smaller than for echocardiography.17;18 CMRI is also able to accurately assess other hypertension-related abnormalities, including LV remodeling and diffuse myocardial fibrosis.19
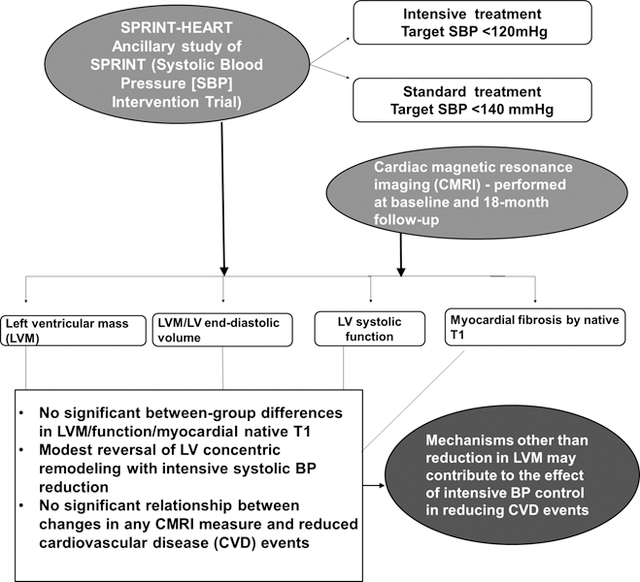
SPRINT-HEART was an ancillary study designed to prospectively examine the relationships between intensive BP control and CV structure and function by CMRI. We hypothesized that intensive BP control would result in reduced LVM and improved LV remodeling and myocardial fibrosis by native T1 mapping, and that improvements in thesemeasures would be associated with reduced CV events.
Methods
Study Population and Study measurements.
All data will be publicly available at the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC, https://biolincc.nhlbi.nih.gov/home/). Details of SPRINT’s design and primary outcomes have been published previously.12;20 SPRINT-HEART was a 340 participant ancillary study within the main SPRINT trial. All methods and inclusion and exclusion criteria were identical to SPRINT, except that participants with contraindications to MRI were excluded.20 Participants were recruited from four clinics and the CMRI exams were performed at Wake Forest School of Medicine. The study was approved by the Institutional Review Board and written informed consent was obtained.
The baseline CMRI exam was performed within 7–10 days of the randomization visit. Most LVM reduction typically occurs within 12 months of a change in BP.8 Similarly, regression of biopsy-proven established fibrosis is found within 12 months of treatment in hypertensive heart disease. 21;22 Accordingly, the follow-up CMRI exam was performed at the time of the 18-month follow-up visit. Since titration of BP medications was generally complete within 3–6 months of randomization, this strategy allowed a minimum intervention exposure of 12–15 months prior to the follow-up CMRI exam.
As previously described, a cohort of 60 healthy, age-matched, disease-free subjects ≥60 years old was recruited from the community and underwent CMRI using identical methods to the SPRINT-HEART participants.23 Subjects were excluded if they had any: chronic medical illness; chronic medications; current symptoms or complaints; abnormal physical examination (including BP≥140/90 mmHg); abnormal results on the screening tests (chemistry panel, complete blood count, echocardiogram, ECG and treadmill exercise test).23 This cohort served as a normative reference group for comparison to the SPRINT-HEART participants. Abnormally increased LVM or LVH was defined as LVM exceeding the upper 95th percentile confidence limit of this normative reference group, adjusted for age, sex, and body surface area.
CMRI measures
CMR protocol:
CMRI scans were performed on a 1.5 Tesla Avanto scanner (Siemens Medical Solutions, Erlangen, Germany) with a phased array chest coil. For assessment of LVM and volumes, cine short-axis white blood steady-state free precession images were acquired encompassing the LV in 8-mm thick planes separated by 2-mm gaps. Imaging parameters included a 40-cm field of view, 192×109 matrix, 10-ms repetition time, 1.12-ms echo time, 20° flip angle, 930 Hz/pixel bandwidth, and 40-ms temporal resolution. 19;24 After acquisition, all images were analyzed by individuals who were blinded to treatment group assignment and the study hypothesis.
LVM determination:
As previously described in our laboratory, the epicardial and endocardial borders of each LV slice were contoured using a semi-automatic method (QMASS 7.1, Medis, The Netherlands) at end-diastole.24 The difference between the epicardial and endocardial areas for all slices were multiplied by the slice thickness and section gap, and then multiplied by the specific gravity of myocardium (1.05 g/ml) to determine LVM.3;19;24 Papillary muscle mass was excluded from the measurement of LVM.19;24;25 Rather than ‘indexing’ to body surface area, LVM (and LV volumes, below) were adjusted statistically for age, sex, race, and body surface area to account for confounding demographic and anthorpomorphic variables.
LV volumes:
As previously described, endocardial borders of each slice were contoured at end-diastole and end-systole and volumes were calculated by summation using Simpson’s rule.3;19;24 To assess LV remodeling, the LVM/volume ratio was calculated as LVM divided by end diastolic volume (EDV). LV stroke volume (SV) was calculated by subtracting end systolic volume (ESV) from EDV. Significant mitral regurgitation was excluded. LV Ejection Fraction (LVEF) was calculated as the SV divided by EDV.19;24
Myocardial native T1:
Myocardial T1 mapping was acquired with a modified Look-Locker inversion (MOLLI) recovery sequence in a mid-cavity short-axis slice pre-contrast (native T1).26;27 The MOLLI imaging acquired 11 images during breaht-hold over 17 heartbeats with a 360×360 mm field of view collected with a 192×183 matrix, 35° flip angle, 8 mm slice thickness, 1.1 ms echo time, 2.2 ms repetition time, and an acceleration factor of 2.26;27 As previously published, a 3-parameter curve fit with the Levenberg–Marquardt algorithm was applied to the modified Look-Locker inversion source images to create a T1 map.26 Myocardial endocardial and epicardial borders were drawn manually on each cine series and on T1 maps to ensure exclusion of the LV blood pool and epicardial fat. Average T1 was calculated from the segmented myocardium; univariate linear modeling was performed to determine T1-related heart rate dependency.26
Because a key SPRINT trial inclusion criterion was significant chronic kidney disease (CKD), gadolinium contrast was not feasible. This limited our ability to measure extracellular volume fraction (ECV), a noninvasive marker for diffuse fibrosis. However it has been shown that non-contrast “native T1” measures have a linear correlation with percentage of myocardial fibrosis measured histologically on invasive myocardial biopsy.28 In addition, native T1 has comparable ability to ECV in the detection and quantification of histological collagen volume fraction, with high reproducibility.29 Furthermore, native T1 shows a stronger relationship with markers of structural and functional LV remodeling.
Statistical Methods
We estimated that a sample size of 340 subjects (170 per treatment group) would provide at least 300 evaluable CMRI scans at the 18-month follow-up visit. This assumed 4% loss to follow-up and that 92% of participants agreeing to a baseline MRI would agree to the follow-up MRI. With this sample size, we estimated that we would have > 80% power to detect a between group difference of 2.9 g for the primary outcome of change in LVM, assuming a standard deviation of 23.1 g and a correlation of 0.93 between the baseline and follow-up assessments. Therefore, we performed CMRs on 340 participants at baseline to have at least 300 evaluable participant CMR scan pairs at 18-months follow-up. The sample size of 300 evaluable data pairs will allow a correlation between MRI measures of LVM or change in LVM of 0.8 with a 95% confidence interval of (0.75, 0.84).
SAS v9.4 (Cary, NC) and The R Statistical Computing Environment were used for all analyses. Linear mixed models which included participant-specific and clinic site-specific random effects were used to compare longitudinal trajectories for BP between groups. Modeling was conducted using SAS proc mixed using minimum variance quadratic unbiased estimation. CMRI outcome measures were compared between groups using linear models, including adjustments for age, sex, body surface area, and baseline CMRI values. Exploratory mediation analyses were conducted using the mediation package for R. This involved fitting a linear regression (mediator model) for change in heart rate, and a similar linear regression for change in the LVM/LV EDV (outcome model), to estimate whether effects of intensive treatment on LVM/LV EDV were mediated via changes in heart rate. There was no adjustment of the multiple testing amongst the secondary end points; because of the potential for type I error, the findings from these analyses should be considered exploratory.
We estimated the incidence of the primary composite CVD outcome using Kaplan-Meier techniques, and used Cox proportional hazards regression to evaluate the association between the change in the CMRI outcome measures between baseline and the follow-up visit and incident CVD events. For analyses of CVD events, we excluded participants that experienced a CVD event prior to the 18 month follow-up visit (8 events excluded).
Results
Participant characteristics
A total of 337 SPRINT participants (mean age=64.3±8.9 years (Standard Deviation, SD), 45% female) completed the baseline exam, with 170 and 167 randomized to intensive treatment and standard treatment respectively. There were no significant baseline differences in characteristics between the treatment groups (Table 1). There were modest differences in some characteristics compared to the rest of the SPRINT cohort (Supplemental Table S1). Demographics of the normative reference group were previously published: mean age 69.3±7.4 years; 62% female).23
Table 1.
Baseline characteristics of SPRINT- HEART participants by treatment group
| Intensive Treatment | Standard Treatment | |
|---|---|---|
| Characteristics | N=170 | N=167 |
| Age (years) | 64.1±8.3 | 64.5±9.4 |
|
| ||
| Age ≥75 years, N (%) | 20(11.8) | 27(16.2) |
|
| ||
| History of CVD, N (%) | 22(12.9) | 25(15.0) |
|
| ||
| 10-year Framingham CVD risk score ≥15%, N (%) | 118(69.4) | 116(69.5) |
|
| ||
| Female sex, N (%) | 82(48.2) | 68(40.7) |
|
| ||
| Race or Ethnicity- N (%) | ||
| White | 98(57.6) | 85(50.9) |
| Black | 67(39.4) | 78(46.7) |
| Hispanic | 3(1.8) | 2(1.2) |
| Other | 2(1.2) | 2(1.2) |
|
| ||
| Smoking status, N (%) | ||
| Current smoker | 31(18.2) | 34(20.4) |
| Former smoker | 59(34.7) | 61(36.5) |
| Never smoker | 80(47.1) | 72(43.1) |
|
| ||
| Body mass index (kg/m2) | 30.5±5.9 | 30.7±6.1 |
|
| ||
| Body surface area (m2) | 2.0±0.2 | 2.0±0.2 |
|
| ||
| Gait speed (m/s) | 1.1±0.2 | 1.2±0.2 |
|
| ||
| Systolic blood pressure (mm Hg) | 140.8±16.5 | 141.5±17.0 |
|
| ||
| Diastolic blood pressure (mm Hg) | 81.3±12.6 | 80.5±13.4 |
|
| ||
| Systolic blood pressure tertile, N (%) | ||
| ≤132 mm Hg | 59(34.7) | 47(28.1) |
| 133 to <145 mm Hg | 47(27.6) | 59(35.3) |
| ≥145 mm Hg | 64(37.6) | 61(36.5) |
|
| ||
| No. of antihypertensive agents | 1.7±1.1 | 1.6±1.2 |
|
| ||
| Not using antihypertensive agents, N (%) | 25(14.7) | 36(21.6) |
|
| ||
| Use of statins, N (%) | 59(34.7) | 44(26.3) |
|
| ||
| Use of aspirin -no (%) | 84(49.4) | 66(39.5) |
|
| ||
| Serum creatinine (mg/dl), median [IQR] | 1.0(0.8 to 1.2) | 1.0(0.8 to 1.2) |
|
| ||
| eGFR (ml/min/1.73 m2) | 75.5±19.6 | 75.6±19.1 |
|
| ||
| eGFR<60 ml/min/1.73 m2, N (%) | 35(20.6) | 35(21.0) |
|
| ||
| Urine albumin to creatinine ratio (mg/g), median [IQR] | 8.1(4.9 to 14.8) | 7.8(5.3 to 15.8) |
|
| ||
| Fasting plasma glucose, mg/dl | 98.7±11.4 | 100.0±12.6 |
|
| ||
| Fasting triglycerides (mg/dl), median [IQR] | 109.0(81.0 to 147.8) | 104.0(73.0 to 150.5) |
|
| ||
| Fasting total cholesterol (mg/dl) | 192.4±40.0 | 200.0±42.4 |
|
| ||
| Fasting HDL cholesterol (mg/dl) | 51.9±14.6 | 51.9±14.0 |
Values are mean±standard deviation unless otherwise indicated. CVD indicates cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IQR, Interquartile Range.
At baseline, the SPRINT-HEART subgroup had reasonably well-controlled BP (mean SBP=141.2±16.7 mm Hg). Based on the 95% upper limit (mean+2 SD) from the healthy normative control group, 9.1% of men and 13.3% of women in SPRINT-HEART had LVH (abnormally increased LVM) by CMRI. By electrocardiograph (Cornell voltage criteria), 5.7% of SPRINT HEART participants had LVH at baseline.
Achieved BP
Figure 1 displays SBP by treatment group through the 18-month study visit. The least square mean SBPs during follow-up were 123.2 mm Hg and 135.8 mm Hg in the intensive treatment and standard treatment groups respectively. The between-group difference in SBP was 12.7 mm Hg (95% CI:11.1–14.3 mm Hg). Most (11.4 mm Hg, 90%) of this between-group difference was achieved by 3 months follow-up, thereby allowing >15 months exposure to observe changes in the CMRI measures. We also compared the SBP and DBP at the time of baseline and 18 month follow-up CMRI scan. As shown in Supplemental Table S2, this did not significantly change the separation in BP between the 2 groups.
Figure 1.
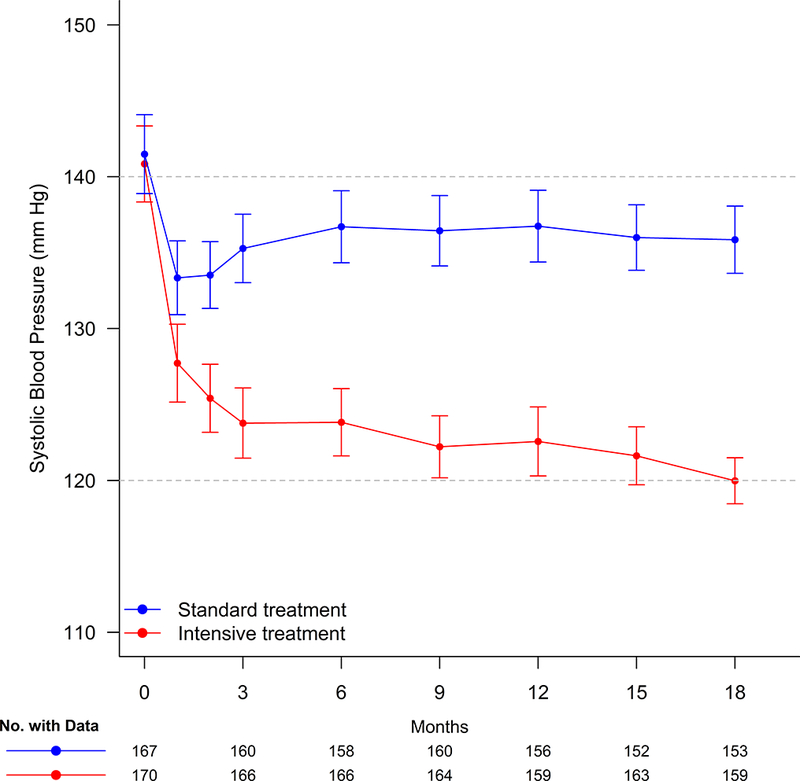
Systolic Blood Pressure (SBP) for participants in SPRINT-HEART through the-18 month follow-up visit. Points denote mean SBP, 95% confidence intervals.
LV mass
In the intensive treatment group, 153 (90%) participants completed the follow-up CMRI exam, and 147 (88.0%) in the standard treatment group (p=0.56). At 18-months follow-up, there was a modest decline in LVM that was similar between treatment groups (intensive:−2.7 ±6.4 g versus standard:−2.3±8.4 g; p=0.368, Table 2, Figure 2). There were no significant differences in percentage of participants with >5% (p=0.5) or >10% (p=0.8) decline in LVM (Supplemental Table S3). In both treatment groups, participants with higher baseline LVM had larger declines in LVM, but there was no indication of heterogeneity in the effect of intensive BP control by baseline tertile of LVM (Table 3).
Table 2.
Effect of intensive blood pressure control on cardiac structure and function
| Baseline | Follow-up | Delta* | Follow-up | |||
|---|---|---|---|---|---|---|
| Measure | Group | Mean±SD | Mean±SD | Mean±SD | LS Mean (95% CI) † | p-value |
| LV Mass (g) | INT | 98.0±22.6 | 94.4±21.74 | −2.7±6.4 | 96.8(95.6, 97.9) | 0.37 |
| STD | 103.1±26.8 | 99.9±25.34 | −2.3±8.4 | 97.5(96.4, 98.7) | ||
|
| ||||||
| LV End Diastolic Volume (ml) | INT | 124.7±26.9 | 125.3±26.4 | 2.4±11.5 | 127.6(125.7, 129.4) | 0.01 |
| STD | 128.8±30.0 | 126.7±28.4 | −1.6±12.8 | 124.3(122.4, 126.2) | ||
|
| ||||||
| LV Mass/LV End Diastolic Volume | INT | 0.8±0.1 | 0.8±0.13 | −0.04±0.09 | 0.76(0.75, 0.78) | 0.002 |
| STD | 0.8±0.2 | 0.8±0.16 | −0.01±0.09 | 0.79(0.78, 0.81) | ||
|
| ||||||
| LV End Systolic Volume (ml) | INT | 48.6±15.3 | 49.4±14.64 | 1.3±6.9 | 50.5(49.4, 51.5) | 0.18 |
| STD | 50.7±16.6 | 50.5±15.88 | 0.0±7.0 | 49.5(48.4, 50.5) | ||
|
| ||||||
| Stroke Volume (ml) | INT | 76.3±15.1 | 75.8±14.69 | 0.9±7.8 | 77.1(75.9, 78.3) | 0.02 |
| STD | 78.2±16.8 | 76.4±15.96 | −1.7±8.4 | 75.0(73.8, 76.3) | ||
|
| ||||||
| Cardiac Output (ml/min) | INT | 4863±1301 | 4778±1170 | 26.5±796.5 | 4779(4663, 4895) | 0.15 |
| STD | 4797±1068 | 4905±1129 | 154.1±715.2 | 4900(4781, 5020) | ||
|
| ||||||
| LV Ejection Fraction (%) | INT | 61.7±6.1 | 61.0±5.23 | −0.6±3.3 | 60.8(60.4, 61.3) | 0.89 |
| STD | 61.2±6.0 | 60.7±5.84 | −0.6±3.3 | 60.8(60.3, 61.3) | ||
|
| ||||||
| Myocardial native T1 values (msc) | INT | 947.4±25.9 | 956.2±25.3 | 10.0±26.2 | 958.6(954.2, 962.9) | 0.79 |
| STD | 952.8±29.3 | 960.1±30.0 | 7.2±26.5 | 959.5(955.0, 963.9) | ||
SD indicates standard deviation; LS, Least Squares; CI, Confidence Interval; EDV, end diastolic volume; ESV, end systolic volume; LV, left ventricular; msc, milliseconds; INT, Intensive Treatment; STD, Standard treatment.
Difference of follow-up measure minus baseline.
Adjusted for baseline value, age, sex, and body surface area.
Figure 2.
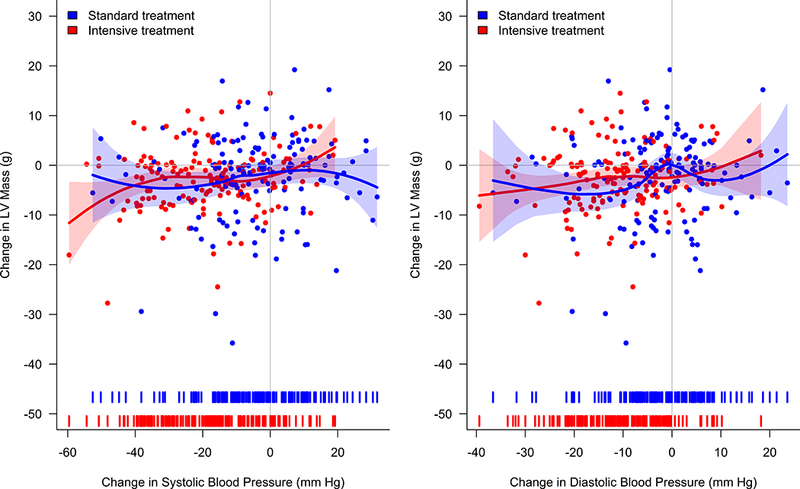
Change in Left Ventricle (LV) Mass versus Change in Blood Pressure by Treatment Group. Change in blood pressure defined as the difference between the mean blood pressure over the 6, 9, 12, 15, and 18 month study visits and baseline blood pressure. Solid lines denote estimated regression fit based on local polynomial regression with point-wise 95% confidence intervals (shaded areas). Tick marks on x-axis represent density of changes in blood pressure.
Table 3.
Effect of intensive blood pressure control on change in left ventricular mass (LVM) by baseline LVM tertile
| Adjusted LS Mean for Change in LVM in g (95% CI) | Interaction | ||
|---|---|---|---|
|
| |||
| Intensive Treatment | Standard Treatment | p-value | |
| Baseline LV Mass Tertile | 0.137 | ||
| ≤87.2 g | −0.19(−2.38, 2.00) | 0.97(−1.27, 3.21) | |
| >87.2 to ≤110.1 g | −3.01(−4.90, −1.12) | −0.87(−2.89, 1.16) | |
| >110.1 g | −5.14(−7.57, −2.74) | −6.96(−9.15, −4.76) | |
LS indicates Least Squares; CI, Confidence Interval.
LS means are adjusted for age, sex, and body surface area.
LV volumes
LV EDV increased modestly from baseline to 18 months in the intensive treatment group and decreased in the standard treatment group (2.4±11.5 versus −1.6±12.8 ml, p=0.015; Table 2). The ratio of LVM/LV EDV declined more in the intensive treatment group (−0.04±0.09 versus −0.01±0.09; p=0.002), consistent with less concentric hypertrophic LV remodeling. LV SV increased slightly in the intensive treatment group but decreased in the standard treatment group (0.9±7.8 versus −1.7±8.4 ml; p=0.021). There were no between-group differences for change in LV ESV, LVEF, heart rate, or cardiac output. By mediation analysis only 4.4% (p=0.45) of the total effect of intensive treatment on change in LVM/LV EDV was explained by changes in heart rate.
Myocardial native T1
At 18-month follow-up, there was no between-group difference in change in myocardial native T1 values (p=0.78; Table 2).
CMRI measures by sex
Sex-specific analyses showed similar results as with both sexes combined (Supplemental Table S4).
Cardiovascular events
Although the sample size of this ancillary study provides limited power to examine CVD events, the results in SPRINT-HEART were consistent with the overall trial.12 Compared to standard treatment, intensive treatment had a lower incidence of the primary composite CVD endpoint (9 versus 18 events, Hazard Ratio =0.48, 95% CI:0.22–1.06) and all-cause mortality (3 versus 8 deaths, Hazard Ratio =0.32, 95% CI:0.08–1.22). There was no significant association between change in any CMRI measure and CVD events subsequent to the 18-month visit (Table 4).
Table 4.
Association between 18-month change in cardiac MRI measures and incidence of the primary composite cardiovascular outcome following the 18-month study visit
| HR (95% CI) | p-value | |
|---|---|---|
| Measure | per 1 SD decrease | |
| Change in LV Mass (g) | 1.39(0.95, 2.03) | 0.094 |
| Change in LV End Diastolic Volume (ml) | 0.84(0.52, 1.37) | 0.486 |
| Change in LV Mass/LV End Diastolic Volume | 1.15(0.71, 1.87) | 0.561 |
| Change in LV End Systolic Volume (ml) | 0.91(0.57, 1.47) | 0.707 |
| Change in Stroke Volume (ml) | 0.81(0.50, 1.31) | 0.391 |
| Change in Cardiac Output (ml/min) | 0.72(0.45, 1.16) | 0.652 |
| Change in LV Ejection Fraction (%) | 0.82(0.50, 1.36) | 0.450 |
| Change in Myocardial native T1 (milliseconds) | 0.88(0.51, 1.52) | 0.652 |
HR indicates Hazard Ratio based on Cox proportional hazards regression adjusting for baseline value; LV, Left Ventricular; SD, Standard Deviation. Primary cardiovascular composite endpoint includes myocardial infarction, acute coronary syndrome, stroke, heart failure, or death from cardiovascular causes.
DISCUSSION
In SPRINT-HEART, a prospective, ancillary study to SPRINT, we utilized CMRI to examine the effect of intensive SBP control on LVM, structure, function, and myocardial native T1 indicative of diffuse fibrosis. Contrary to our hypothesis, there were no significant between-group differences in LVM, function, or myocardial T1 values at 18-months follow-up. There was a modest but significant decrease in the LVM/EDV ratio, indicating improved LV concentric remodeling. Consistent with the overall trial, participants randomized to intensive treatment in SPRINT-HEART had a considerably lower incidence of CVD events and all-cause mortality. Given this disparity between reduction in events that was similar to that observed in the overall trial but with no reduction in LVM, it is not surprising that we found no significant association between reduced event rates and changes in LVM or any other CMRI measure. This observation is in accord with a prior report from the entire SPRINT cohort which showed that change in ECG-LVH was not associated with reduction in CVD events.14 Taken together, these data suggest that the reduction in CVD morbidity and mortality in SPRINT was likely not driven by changes in LVM, structure, function, or fibrosis.
There have been surprisingly few reports from randomized trials that have examined the effect of BP lowering on LVM and whether reducing LVM reduces risk independent of BP changes.4;14;30–35 In contrast to the present study, nearly all previously reported trials examined BP levels much higher than SPRINT, did not compare two different treatment BP targets, assessed LVH by electrocardiography or echocardiography, and were unable to examine relationships of LVM with events.4;14;30–35 For example, a meta-analysis of randomized trials showed regression of echocardiographic LVH with BP lowering, however, they compared different classes of antihypertensive therapy and not different SBP targets.33 In addition, BP averaged 165 mm Hg at baseline and decreased by 14 mm of Hg with treatment; thus baseline and final BP levels were much higher than in SPRINT.33 Two prior randomized trials suggested regression of LVM using CMRI, however comparisions were between 2 different agents, the final achieved BP was similar between groups, and the BP levels were much higher than in SPRINT.31;32
Thus, to our knowledge, SPRINT-HEART is the first prospective evaluation of the effect of intensive BP control (<140 mm Hg) using CMRI to measure LVM, structure, function, and fibrosis in a randomized, controlled trial. The present study is also unique in that it is able to relate changes in clinical events with changes in CMRI measures in the lower BP range.
Reports by Soliman et al examined LVH by electrocardiography in two prior trials that achieved goal BPs <140mmHg.14;35 In the Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD) trial of patients with hypertension and diabetes mellitus, intensive BP lowering (<120 mmHg) was associated with 39% lower risk of electrocardiographic LVH compared to standard BP lowering (SBP <140 mmHg).35 In a recent report from SPRINT, intensive BP lowering in SPRINT resulted in lower rates of developing new electrocardiographic LVH and higher rates of regression of existing LVH.14
SPRINT-HEART significantly expands upon the reports by Soliman et al by evaluating the effect of intensive BP reduction to <120 mm Hg on measured LVM (rather than electrocardiographic LVH) and also LV structure, function, and fibrosis using modern CMRI techniques. Although electrocardiographic LVH has reasonable correlations with LVM measured by autopsy, CMRI or echocardiography in cross-sectional studies, ECG LVH has fundamentally different determinants than measured LVM (electrical vs anatomical), and because of this, changes over time in electrocardiographic LVH and measured LVM are less closely related than in cross-sectional correlations.14 Furthermore, changes in LVM can result from diverse etiologies, including number of and size of cardiomyocytes, edema, fatty infiltration and ischemic cellular changes.15;36 These changes have variable, sometimes opposite, effects on the electrical signal.3;36 Electrcardiographic LVH can also reflect, instead of increased LVM, increased myocardial tension, or neurohormonal and/or biochemical changes in the myocardium.36 These and other reasons led Lewis et al, who commented on the above electrocardiography-based LVH report by Soliman et al, to call for a study such as SPRINT-HEART to quantitatively assess LVM and fibrosis with intensive BP lowering.15
Several factors support the credibility of our finding of no significant reduction in measured LVM with intensive BP control. Participants in SPRINT, by design, already had relatively well-controlled BP at baseline, and thus likely had lower LVM and less LVH as compared to patients in prior hypertension studies where BPs were higher.31;32 Indeed, in SPRINT- HEART, only 9% of men and 13% of women had abnormal LVM by CMRI when compared to a cohort of healthy, non-hypertensive controls studied in our laboratory using identical CMRI methods.23 Our participants also had a low rate of electrocardiographic LVH (6%) at baseline, in accord with the low rates in the entire SPRINT cohort.14 Furthermore, the mean change in LVM (−0.4 g), and 95% confidence intervals of −2.0 to 1.3 g, generally exclude a clinically significant effect on LVM. This lack of LVM reduction occurred despite an adequate BP reduction stimulus, since an average of 13 mmHg SBP reduction was achieved at 3 months in the intensive treatment group, and despite adequate exposure time since CMRI measurements were performed at 18-month follow-up.
Other studies have shown that LVH regression is not uniformly observed with BP reduction. In a longitudinal study of anti-hypertensive treatment, among hypertensive patients with LVH at baseline, only 14% had LVH regression at follow-up.37 Furthermore, several patient characteristics known to predict lower likelihood of LVM regression, including older age, kidney disease, female sex, obesity and metabolic syndrome, were present in a large proportion of the SPRINT population.38–40 Indeed, contrary to conventional belief, BP may account for only 25% of the variability of LVM in a population.40 This concept is supported by the observation that increased LVM can precede development of hypertension,11 perhaps via neuro-hormonal activation that can directly promote myocyte hypertrophy and matrix deposition independent of BP.41;42
We also did not observe significant changes in LV function or in myocardial native T1 values, which correlates with diffuse fibrosis, with intensive BP control. While we don’t have a normative reference population for myocardial native T1 values, comparison with other studies suggests that values in SPRINT-HEART were generally within the normal range.26;43 Consistent with this finding, a recent CMRI study in patients with hypertensive heart disease found that patients with concentric LV remodeling had normal native T1, suggesting that the development of myocardial fibrosis in hypertensive heart disease may not be linearly related to BP.44 Also, SPRINT utilized a BP reduction strategy with a menu of recommended anti-hypertensive agents, rather than a specific medication, and not all antihypertensive agents have anti-fibrotic effects.45 Finally, other reports indicate LVH-related fibrosis may not be highly responsive to antihypertensive treatment.22;46
The present study is unique in that it is the only trial that has examined the relationship between change in LVM and events with intensive BP reduction. There are only two prior reports that have examined relationships between change in LVM and events and these had multiple differences in design from SPRINT-HEART, including use of electrocardiography and echocardiography to assess LVM, and BP ranges that were much higher than SPRINT.4;6 Several factors support the credibility of our finding of a lack of relationship between lower CV events and changes in LVM, structure, function, and fibrosis. The LVM findings are in accord with electrocardiographic findings which were measured in the entire SPRINT cohort, which showed that changes in LVH accounted for only 4% of the reduction in CV events.14 Despite having relatively modest levels of LVH at baseline, participants had a high rate of CVD events during follow-up. The magnitude of CV event reduction during follow-up in the intensive control group in SPRINT was large, about 25% overall, leading to early trial termination, and the event reduction in SPRINT-HEART was similar or even larger. In contrast, the effect size of intensive BP reduction on LVM was very small (−0.4 gm) and was non-significant. Prior studies have also suggested that even when there is LVH reduction, CV event risk, particularly for HF, persists.5 Taken together, these findings suggest that, in this BP range and this patient population, LVM may not play a major role in mediating BP treatment-related reduction in CVD events.
These results, while contrary to our initial hypothesis, are valuable since they suggest that mediators other than changes in LVM, structure, function, and fibrosis are primarily responsible for the improved outcomes from intensive BP reduction and thereby help direct future research directions in this important area. While the present study did not assess other potential mechanisms of benefit, other candidates include amelioration of neurohormonal activation, especially excess sympathetic activity, which may drive myocardial changes independently of BP, as well as increased arterial stiffness,47 oxidative stress,48 systemic inflammation,49 and changes in the microvasculature, including microvascular rarefaction/remodelling,50 and endothelial dysfunction.51 Identification of novel therapeutic targets beyond LVH and fibrosis could have significant impact on future management of hypertension.14
Our study has several strengths, including a racially diverse population, large number of patients >75 years old, random treatment assignment, adjudication of clinical events, use of modern CMRI techniques which are highly accurate and reproducible for assessing changes in LVM, structure, function, and myocardial fibrosis,17;18 and centralized, blinded image analyses. To our knowledge, this is the largest study using CMRI embedded in a hypertension clinical trial, and the only one embedded in a clinical events trial with intensive SBP reduction.
Our study also has some limitations. Since SPRINT was a treatment strategy trial based on different BP goals, we are unable to separate the impact of lowering BP from the impact of individual medications. The SPRINT trial population was restricted to older patients with relatively high CVD risk, high rates of CKD, and no diabetes mellitus. Thus, our results may not apply to other populations. Previous studies indicate that most LVM reduction occurs within 12 months of a change in BP.7 With 18-month follow-up and an average 11.4 mmHg SBP between-group difference at 3-months, the intensive control group participants had at least 15-months treatment exposure. Still, we cannot exclude that even longer term exposure may have resulted in greater change in LVM and other CMRI measures. Because advanced kidney disease was an inclusion criterion in SPRINT, we were unable to use gadolinium contrast to measure myocardial fibrosis. We also did not assess the impact of BP reduction on patterns of LV geometry.
Was the present sample size adequate to address the hypothesis ? To our knowledge, this is the largest study using CMRI to assess the relationship between changes in LVM and events, and CMRI has much lower variability than echocardiography for assessing changes in LVM.17;18 The sample size was based on formal power analyses using data in the literature and from our laboratory. Furthermore, the effect size of LVM (−0.4 g; 95% confidence intervals −2.0–1.3 g) indicate that even if the sample size were many fold larger, the likely LVM reduction would be of minimal clinical relevance. Finally, the disparity between the size of the event reduction verus change in LVM was marked.
Conclusions
Randomization to intensive BP control was not associated with a significant difference in LVM, structure, function, or diffuse myocardial fibrosis but was associated with a modest reversal of LV concentric remodeling. There was no significant relationship between changes in any CMRI measure and reduced CV events. These data, combined with electrocardiographic data from SPRINT and data from other studies, suggest that mechanisms other than reduction in LVM and/or fibrosis may contribute to the effect of intensive BP control in reducing CV events. Examining other potential mechanisms may help idenitfy novel therapeutic targets.
Perspectives
Despite high CV risk at baseline, SPRINT-HEART participants had modest levels of LVH by both CMRI and electrocardiography. Also, despite having modest levels of LVH, they had high rates of CVD events during follow-up. Although intensive BP control resulted in a lower event rate, this was not significantly associated with changes in any CMRI measure. Therefore, in this BP range and patient population, LVM may not play a major role in determining CVD risk or treatment benefit.
Novelty and Significance
What Is New?
To our knowledge, SPRINT-HEART is the first prospective evaluation of the effect of intensive BP control (SBP < 120 mm of Hg) on LVM, structure, and fibrosis in a large randomized, controlled trial using CMRI.
Despite high CVD risk at baseline, SPRINT-HEART participants had modest levels of LVH by both CMRI and electrocardiography. Also, despite having modest levels of LVH, they had high rates of CVD events during follow-up.
There were no significant between-group differences in LVM, function, or myocardial T1 at 18-month follow-up.
Although intensive BP control resulted in a lower event rate, this was not significantly associated with changes in any CMRI measure.
What Is Relevant?
These data suggest that mechanisms other than reduction in LVM and/or diffuse fibrosis contribute to the effect of intensive BP control in reducing CV events.
Future mechanistic studies should seek to identify these other mechanisms and determine their potential as independent therapeutic targets for improving outcomes in hypertension.
Summary
Randomization to intensive BP control was not associated with a significant difference in LVM, structure, function, or diffuses myocardial fibrosis but was associated with favorable reversal of LV concentric remodeling
Supplementary Material
Acknowledgments
Source of Funding
The SPRINT-HEART Ancillary study was supported by R01HL107257 as well as R01AG18917, R01AG045551, P30-AG21331, and the Kermit G. Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine. SPRINT was by National Institutes of Health (NIH) under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13–002-001. It was also supported partly through the Department of Veterans Affairs and contribution of study medications from Takeda Pharmaceuticals International, Inc. For a full of contributors to SPRINT, please see: https://www.sprinttrial.org/public/dspScience.cfm.
Footnotes
A complete list of the members of the Systolic Blood Pressure Intervention Trial (SPRINT) Research Group was provided in the Supplementary Appendix, available at NEJM.org.
Supplement to: The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16. DOI: 10.1056/NEJMoa1511939
Financial disclosures
Dr. Kitzman: Consultant for Abbvie, Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer-Ingelheim, GSK, Astra Zeneca, CinRx, Novartis, and Actavis, grant funding from Novartis, Bayer, and GSK, and stock ownership in Gilead Sciences.
Dr. Upadhya: research funding from Novarits and Corvia.
Dr Oparil: personal fees from Forest Laboratories Inc, Boehringer-Ingelheim, GlaxoSmithKline, Amgen (Onyx is subsidiary); grants, personal fees, and nonfinancial support from Medtronic; grants and personal fees from AstraZeneca and Bayer Healthcare Pharmaceuticals Inc; personal fees and grants from Merck and Co; and serving as cochair for the Eighth Joint National Committee.
Other authors report no conflicts.
References
- (1).Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- (2).JM, McClelland R, Kitzman DW et al. M-mode echocardiographic predictors of six-to-seven year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort: The Cardiovascular Health Study. Am J Cardiol 2001;87:1051–1057. [DOI] [PubMed] [Google Scholar]
- (3).Bluemke DA, Kronmal RA, Lima JA et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Okin PM, Devereux RB, Jern S et al. Regression of Electrocardiographic Left Ventricular Hypertrophy During Antihypertensive Treatment and the Prediction of Major Cardiovascular Events. JAMA 2004;292:2343–2349. [DOI] [PubMed] [Google Scholar]
- (5).Devereux RB, Wachtell K, Gerdts E et al. Prognostic Significance of Left Ventricular Mass Change During Treatment of Hypertension. JAMA 2004;292:2350–2356. [DOI] [PubMed] [Google Scholar]
- (6).Mathew J, Sleight P, Lonn E et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001;104:1615–1621. [DOI] [PubMed] [Google Scholar]
- (7).Gottdiener JS, Arnold AM, Aurigemma GP et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 2000;35:1628–1637. [DOI] [PubMed] [Google Scholar]
- (8).Simpson HJ, Gandy SJ, Houston JG, Rajendra NS, Davies JI, Struthers AD. Left ventricular hypertrophy: reduction of blood pressure already in the normal range further regresses left ventricular mass. Heart 2010;96:148–152. [DOI] [PubMed] [Google Scholar]
- (9).Lauer MS, Anderson KM, Levy D. Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: The Framingham Heart Study. J Am Coll Cardiol 1991;18:1287–1294. [DOI] [PubMed] [Google Scholar]
- (10).Savage DD, Levy D, Dannenberg AL, Garrison RJ, Castelli WP. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study). Am J Cardiol 1990;65:371–376. [DOI] [PubMed] [Google Scholar]
- (11).Fraser R Studying genes and the development of cardiac hypertrophy: convenient intermediate phenotypes in man. [Editorial]. Journal of Hypertension 2003;21:873–874. [DOI] [PubMed] [Google Scholar]
- (12).Wright JT Jr, Williamson JD, Whelton PK et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Upadhya B, Rocco M, Lewis CE et al. Effect of intensive blood pressure treatment on heart failure events in the systolic blood pressure reduction intervention trial. Circ Heart Fail 2017;10:e003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Soliman EZ, Ambrosius WT, Cushman WC et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation 2017;136:440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lewis GA, Schelbert EB, Miller CA. Letter by Lewis et al. Regarding Article, “Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients With Hypertension: SPRINT (Systolic Blood Pressure Intervention Trial)”. Circulation 2018;137:1297–1298. [DOI] [PubMed] [Google Scholar]
- (16).Gottdiener JS, Bednarz J, Devereux RB et al. American Society of Echocardiography Recommendations for Use of Echocardiography in Clinical Trials: A report from the American Society of Echocardiography’s Guidlines and Standards Committee and the Task Force on Echocardiography in Clinical Trials. Journal of the American Society of Echocardiography (ECHO) 2004;17:1086–1119. [DOI] [PubMed] [Google Scholar]
- (17).Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2000;2:271–278. [DOI] [PubMed] [Google Scholar]
- (18).Grothues F, Smith GC, Moon JC et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29–34. [DOI] [PubMed] [Google Scholar]
- (19).Hundley WG, Bluemke DA, Finn JP et al. ACCF/ACR/AHA/NASCI/SCMR 2010 Expert Consensus Document on Cardiovascular Magnetic Resonance. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2462–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ambrosius WT, Sink KM, Foy CG et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 2002;105:2512–2517. [DOI] [PubMed] [Google Scholar]
- (22).Brilla CG. Regression of myocardial fibrosis in hypertensive heart disease: diverse effects of various antihypertensive drugs. Cardiovasc Res 2000;46:324–331. [DOI] [PubMed] [Google Scholar]
- (23).Haykowsky MJ, Nicklas BJ, Brubaker PH et al. Regional Adipose Distribution and its Relationship to Exercise Intolerance in Older Obese Patients Who Have Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2018;6:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Jordan JH, Castellino SM, Melendez GC et al. Left Ventricular Mass Change After Anthracycline Chemotherapy. Circ Heart Fail 2018;11:e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Vogel-Claussen J, Finn J, Gomes A et al. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr 2006;30:426–432. [DOI] [PubMed] [Google Scholar]
- (26).Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141–146. [DOI] [PubMed] [Google Scholar]
- (27).Jordan JH, D’Agostino RB Jr, Hamilton CA et al. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2014;7:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bull S, White SK, Piechnik SK et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013;99:932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Nakamori S, Dohi K, Ishida M et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc Imaging 2018;11:48–59. [DOI] [PubMed] [Google Scholar]
- (30).Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation 2004;110:1456–1462. [DOI] [PubMed] [Google Scholar]
- (31).Burns J, Ball SG, Worthy G, Struthers AD, Mary DA, Greenwood JP. Hypertensive left ventricular hypertrophy: a mechanistic approach to optimizing regression assessed by cardiovascular magnetic resonance. J Hypertens 2012;30:2039–2046. [DOI] [PubMed] [Google Scholar]
- (32).Schmieder RE, Wagner F, Mayr M et al. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur Heart J 2017;38:3308–3317. [DOI] [PubMed] [Google Scholar]
- (33).Fagard RH, Celis H, Thijs L, Wouters S. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension 2009;54:1084–1091. [DOI] [PubMed] [Google Scholar]
- (34).Verdecchia P, Staessen JA, Angeli F et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009;374:525–533. [DOI] [PubMed] [Google Scholar]
- (35).Soliman EZ, Byington RP, Bigger JT et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with diabetes mellitus: action to control cardiovascular risk in diabetes blood pressure trial. Hypertension 2015;66:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bacharova L, Chen H, Estes EH et al. Determinants of discrepancies in detection and comparison of the prognostic significance of left ventricular hypertrophy by electrocardiogram and cardiac magnetic resonance imaging. Am J Cardiol 2015;115:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Lonnebakken MT, Izzo R, Mancusi C et al. Left Ventricular Hypertrophy Regression During Antihypertensive Treatment in an Outpatient Clinic (the Campania Salute Network). J Am Heart Assoc 2017;6:e004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).de Simone G, Okin PM, Gerdts E et al. Clustered metabolic abnormalities blunt regression of hypertensive left ventricular hypertrophy: the LIFE study. Nutr Metab Cardiovasc Dis 2009;19:634–640. [DOI] [PubMed] [Google Scholar]
- (39).Gerdts E, Okin PM, de Simone G et al. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension 2008;51:1109–1114. [DOI] [PubMed] [Google Scholar]
- (40).de Simone G, Devereux RB, Izzo R et al. Lack of reduction of left ventricular mass in treated hypertension: the strong heart study. J Am Heart Assoc 2013;2:e000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).de Simone G, De Marco M. Sodium, left ventricular mass, and arterial hypertension: is it time to look for a new paradigm? Hypertension 2011;58:349–351. [DOI] [PubMed] [Google Scholar]
- (42).Devereux RB. Does increased blood pressure cause left ventricular hypertrophy or vice versa? Ann Intern Med 1990;112:157–159. [DOI] [PubMed] [Google Scholar]
- (43).Liu CY, Liu YC, Wu C et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;62:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Rodrigues JC, Amadu AM, Dastidar AG et al. Comprehensive characterisation of hypertensive heart disease left ventricular phenotypes. Heart 2016;102:1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Johnson DB, Dell’Italia LJ. Cardiac hypertrophy and failure in hypertension. Curr Opin Nephrol Hypertens 1996;5:186–191. [DOI] [PubMed] [Google Scholar]
- (46).Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 2000;102:1388–1393. [DOI] [PubMed] [Google Scholar]
- (47).Seravalle G, Lonati L, Buzzi S et al. Sympathetic nerve traffic and baroreflex function in optimal, normal, and high-normal blood pressure states. J Hypertens 2015;33:1411–1417. [DOI] [PubMed] [Google Scholar]
- (48).Baradaran A, Nasri H, Rafieian-Kopaei M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J Res Med Sci 2014;19:358–367. [PMC free article] [PubMed] [Google Scholar]
- (49).Ikdahl E, Rollefstad S, Hisdal J et al. Sustained Improvement of Arterial Stiffness and Blood Pressure after Long-Term Rosuvastatin Treatment in Patients with Inflammatory Joint Diseases: Results from the RORA-AS Study. PLoS One 2016;11:e0153440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension 2006;48:1012–1017. [DOI] [PubMed] [Google Scholar]
- (51).Felmeden DC, Spencer CG, Belgore FM, Blann AD, Beevers DG, Lip GY. Endothelial damage and angiogenesis in hypertensive patients: relationship to cardiovascular risk factors and risk factor management. Am J Hypertens 2003;16:11–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


