Abstract
The influence of hammer mill screen size (4.5 and 8.5 mm) and enzyme addition (control and 500 ppm) on olive fruit cell wall breakdown and its consequences in terms of oil recovery and the phenolic content of olive oil was studied at the laboratory scale for “Arbequina” and “Koroneiki” at two different maturities. Water recovery and water-soluble carbohydrates in olive paste after malaxation were measured as an indicator of cell wall breakdown. Smaller screen size and enzymes increase oil recovery for Arbequina with a maturity index of 1.6 (6.3–6.6%); and for Koroneiki at a maturity index of 0.2 (15.0–38%) and 2.6 (1.3–4.3%). For both cultivars, the increase in oil recovery is larger in green fruits compared to more ripe fruit. Water recovery and water-soluble carbohydrates increase with small screen size and the enzyme treatments, even when no increment in oil recovery is observed. The water recovery range was 143–239% for Arbequina and 150–262% for Koroneiki; water-soluble carbohydrate range was 1.8–12.7 g/kg for Arbequina and 0.5–5.4 g/kg for Koroneiki. In general, smaller hammer mill screen size and enzymes increase total phenols in the oil, with a larger difference between control and treatment for green fruit than for the ripe fruit. For Arbequina, increases in total phenol content were in the range of 45–60 and 5–20% at maturity index 1.6 and 3.3, respectively. For Koroneiki, the increases were in the range of 31–121 and 7–9% at maturity index 0.2 and 2.6, respectively. Application of cell wall-degrading enzymes improves the cell wall breakdown caused by hammer mill, leading to higher oil recovery and total phenol content. The magnitude of the effect depends on the cultivar and olive fruit maturity.
Introduction
Crushing is the first operation for olive oil extraction. Fruit tissues need to be ruptured so that oil can be released from the oil bodies, where it is located inside the fruit cells. While several crusher types are available in the market, the hammer mill is the most widely used nowadays in the olive oil industry. The hammer mill screen size and screen open area have an impact on the minor components of olive oil. Previous works indicate that a smaller screen size produces higher yields and oils with higher concentration of total phenols and secoiridoid derivatives.1,2 Nonetheless, crushing is not completely efficient at breaking olive fruit cell wall and releasing oil bodies from the cellulose and lignin matrix;3 as a result, a portion of the oil is entrapped in olive pomace or emulsified in waste water,4 leading to product losses and decrements in the efficiency of the extraction process.
Enzymes have been suggested as an effective processing aid during olive oil extraction. In particular, the addition of cellulase, hemicellulose, and pectinase cocktails after crushing and during malaxation has been shown to increase both the yield and quality of virgin olive oil.5 Higher content of phenolic as well as other relevant minor components has been associated with the use of enzymes.6−11 From a mechanistic standpoint, the addition of enzymes after crushing may increase the release of phenolic compounds from the cell wall matrix, increasing their concentration in the olive paste.5 However, limited research has shown that cultivars and maturity of the olive fruit have a determinant impact on the magnitude of the effect of commercial enzymatic cocktails on both the yield and phenolic compounds.10−12
In this study, we explored the relative effect of hammer mill screen size and the addition of cell wall degrading enzymes on yield and phenolic compound content of olive oil. When olive fruit cell walls are broken down or degraded, water and soluble carbohydrates are released into the olive paste. Therefore, the impact of crushing and enzymes on cell wall breakdown was assessed by the measurement of water release and total water-soluble carbohydrates in the olive paste. Since changes in cell wall constitution and phenolic content of the olive fruit during ripening and across cultivars are known factors affecting extraction efficiency and phenolic content in olive oil, these factors were also considered in the experimental design. “Arbequina” and “Koroneiki” were selected for this experiment since both are common super-high-density cultivars used for olive oil production and have naturally different total content and profile of phenolic compounds.
Results and Discussion
Oil Recovery
Maturation and ripening index changes with time depending highly on the genotype of olive cultivars, which have a major impact on olive oil composition; the two cultivars studied in this experiment, Arbequina and Koroneiki, were considered separately for the analysis of variance (ANOVA). When the ANOVA was performed considering cultivars as a factor, the impact of this variable was significant for all of the combination levels of this experiment (data not shown). Therefore, the impact of maturity, screen size, and enzymes on the yield and phenolic content of olive oil will be discussed individually for each cultivar.
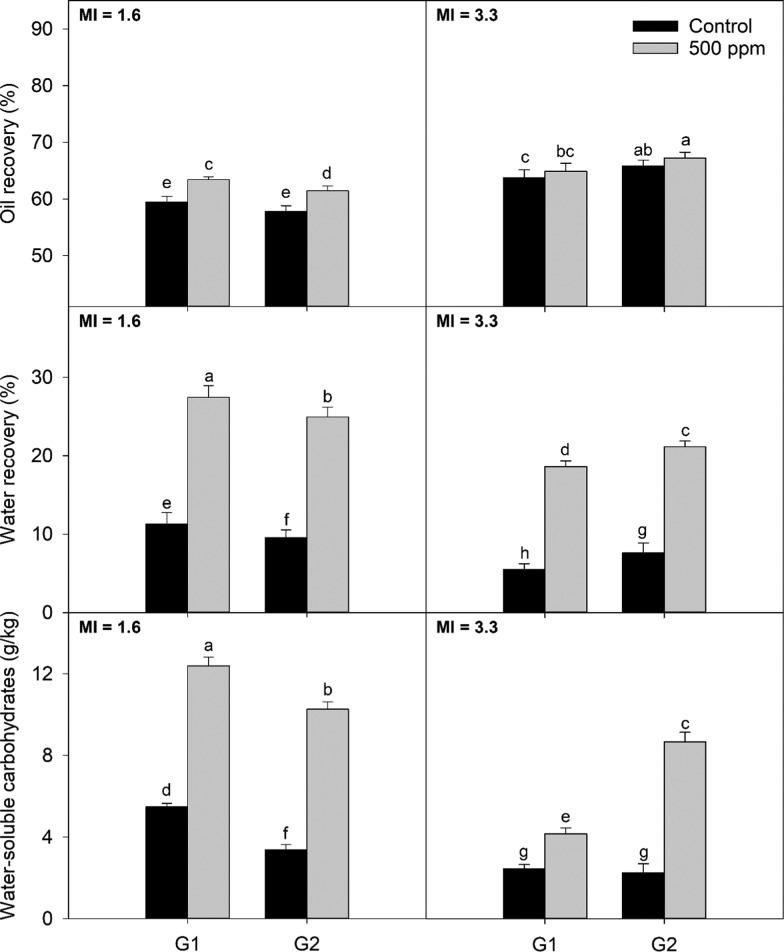
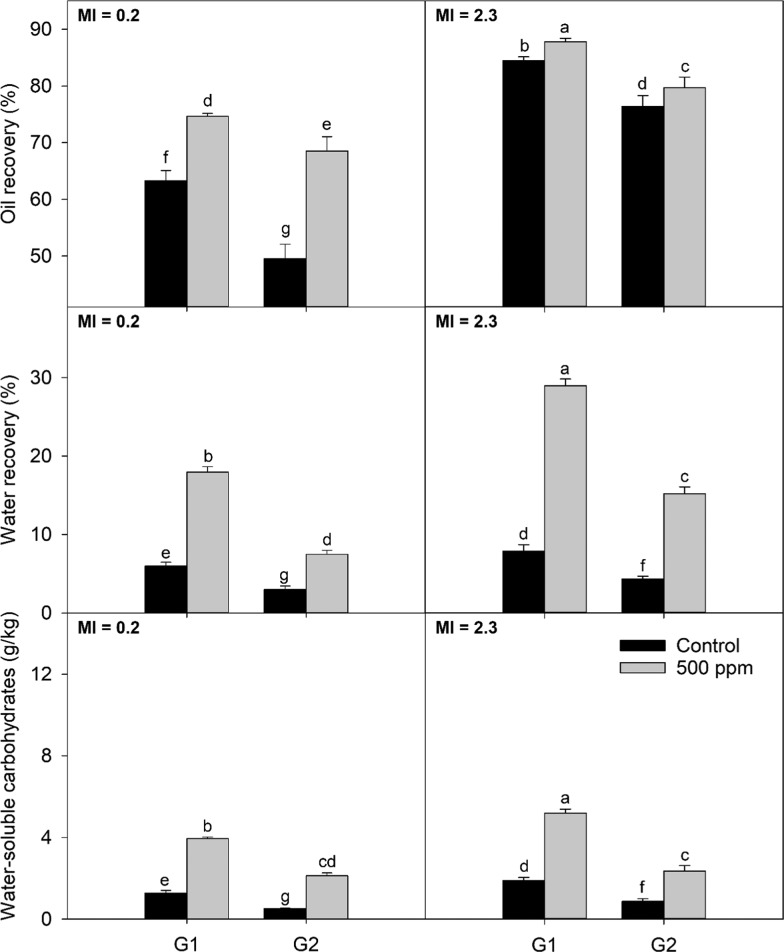
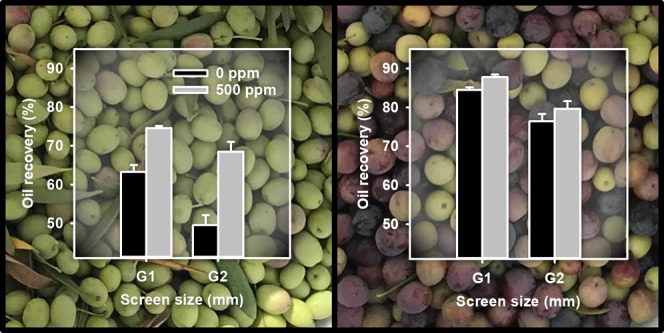
The maturity index (MI), moisture content, and fat content were measured for both cultivars are listed in Table 1. At the two different harvest times, MI was higher for Arbequina compared to Koroneiki, suggesting that Arbequina matured earlier compared with Koroneiki. Results for oil recovery are shown in (top) Figures 1 and 2 for Arbequina and Koroneiki cultivars, respectively. For Arbequina, values ranged between 57.3 and 68.1%. For Koroneiki, the range was from 48.1 to 88.4%. The lower moisture content of Koroneiki contributed to the higher oil recovery observed for this cultivar compared to Arbequina across all of the treatments.13 While oil recoveries increased with maturity and fat accumulation for both cultivars, a bigger increment was observed for Koroneiki.
Table 1. Olive Fruit Parametersa.
| cultivar | harvest date | maturity index | moisture content (g/100 g) | fat content, DB (g/100 g) | fat content, WB (g/100 g) |
|---|---|---|---|---|---|
| Arbequina | October 18, 2018 | 1.6 ± 0.1c | 59.4 ± 0.2a | 39.7 ± 0.3c | 16.1 ± 0.2c |
| November 19, 2018 | 3.3 ± 0.1a | 58.4 ± 0.3b | 46.8 ± 1.1a | 19.5 ± 0.4b | |
| Koroneiki | October 18, 2018 | 0.2 ± 0.1d | 49.5 ± 0.6c | 30.3 ± 0.4d | 15.3 ± 0.2d |
| November 19, 2018 | 2.3 ± 0.1b | 50.3 ±1.2c | 41.8 ± 0.5b | 20.7 ± 0.5a |
Values are expressed as the mean ± standard deviation; DB, dry basis; WB, wet basis. Within a column, different letters indicate significant differences according to the Fisher LSD test (α = 0.05).
Figure 1.
Extraction parameters (oil recovery, water recovery, and water-soluble carbohydrates in paste) of Arbequina olive paste obtained from different screen sizes and addition of enzymes at two maturities; G1 and G2 are the screen sizes used for the experiment, 4.5 and 8.5 mm, respectively; MI: maturity index. Different letters indicate significant differences (α = 0.05) according to the Fisher LSD test.
Figure 2.
Extraction parameters (oil recovery, water recovery, and water-soluble carbohydrates in paste) from Koroneiki olive paste obtained from different screen sizes and addition of enzymes at two maturities; G1 and G2 are the screen sizes used for the experiment, 4.5 and 8.5 mm, respectively; MI: maturity index. Different letters indicate significant differences (α = 0.05) according to the Fisher LSD test.
Independently of the crushing conditions used, the addition of enzymes before malaxation produced a statistically significant increase in oil recovery for Arbequina at MI = 1.6 (6.6% for G1 and 6.3% for G2) and for Koroneiki at MI = 0.2 (15.0% for G1 and 38% for G2) and MI = 2.6 (1.3% for G1 and 4.3% for G2). The increment in oil recovery for Arbequina at MI = 3.3 was not statistically significant. These percentages of increment in oil recovery are in accordance with previous results involving other cultivars and enzymes.5 Oil recoveries for Koroneiki cultivar seemed to be more affected by the usage of enzymes than for Arbequina. This effect might be related to both a higher moisture content of the Arbequina fruit compared with Koroneiki and a lower maturity index of the Koroneiki fruit.14 While different concentrations of enzymes were not included during this experiment, enzyme dosage has shown a positive correlation with oil recovery and total phenols for “Coratina,”15 suggesting that the magnitude of the effect observed for Koroneiki might be reached for Arbequina at a higher enzyme dosage.
Regarding hammer mill screen size, the impact on oil recovery depended on both maturity and cultivar. For Arbequina, no effect of the screen size was observed for MI = 1.6 when no enzymes were added. On the contrary, when enzymes were used, a slight decrease in oil recovery was observed for larger screen sizes. For Arbequina at MI = 3.3, oil recovery increased with a larger grid size, with and without enzyme addition (3.3% without enzyme and 3.6% with enzyme). An opposite trend was observed for Koroneiki in which oil recovery decreased with larger grid sizes. For MI = 0.2 the decrement was 22.7 and 7.8%, for treatments without and with the addition of enzyme, respectively, and for MI = 2.3, the decrement was 10.3 and 7.6%, respectively. This behavior regarding the screen size had been reported for other cultivars.1,2 These findings suggest that crushing conditions should be chosen according to cultivar and maturity; however, more research including a wider range of maturities and cultivars is required to confirm these observations.
Water Recovery
When cell walls are broken by either mechanical or chemical means, water, which is the main component in plant cells, is released from the cytoplasm into the media. Therefore, water recovery can be a useful index for measuring cell wall breakdown during olive oil extraction. The results for water recovery are shown in (middle) Figures 1 and 2, for Arbequina and Koroneiki, respectively. Arbequina values ranged between 5.1 and 27.4%, whereas Koroneiki values ranged between 3.0 and 29.5%. For Arbequina, more mature fruits showed lower values for water recovery, while the opposite trend was observed for Koroneiki.
Enzymes significantly increased water recovery for Arbequina and Koroneiki at both studied maturities. For Arbequina at MI = 1.6, relative increments in water recovery of treatment compared to control were 143 and 162% for G1 and G2, respectively. For MI = 3.3, the increase in water recovery was 239% for G1 and 178% for G2. For Koroneiki at MI = 0.2, increment in water recovery when enzymes were used was 200% for G1 and 150% for G2. For MI = 2.6, the increase was 269 and 252% for G1 and G2, respectively.
Regarding the hammer mill screen size, the lower maturity index fruit yielded a lower water recovery with larger screen sizes, while the higher maturity index fruit presented higher values of water recovery with larger screen sizes. The same trend between these two variables was observed for oil recovery. The decrease in water recovery with larger screen sizes is consistent with a lower degree of tissue disruption expected from the lower retention time inside the hammer mill when larger screen sizes are used.2 However, more mature fruits may facilitate the generation of emulsions and the retention of water in the pomace when a more violent crushing is performed. Koroneiki presented the same trend for water recovery as the one observed for oil recovery; smaller screen sizes caused larger oil recoveries, independently of maturity and the use of enzymes.
Water-Soluble Carbohydrates
The measurement of water-soluble carbohydrates has been used to determine the effect of hydrolytic enzymes on several feedstock products,16 including olive paste.17 Results for water-soluble carbohydrates are shown in (bottom) Figures 1 and 2 for Arbequina and Koroneiki, respectively. For Arbequina, the values ranged between 1.8 and 12.7 g/kg. For Koroneiki, the range was between 0.5 and 5.4 g/kg.
For all treatments, values of water-soluble carbohydrates followed the same trend observed for water recovery: addition of enzymes significantly increased the concentration of water-soluble carbohydrates likely due to increased hydrolysis of the olive fruit cell wall by the enzymes. For Arbequina at MI = 1.6, the application of smaller screen sizes increased the water-soluble carbohydrates, supporting the idea that smaller screen size causes more damage to the olive fruit tissue. Nevertheless, the increase of water-soluble carbohydrates caused by the addition of enzymes was greater compared to the one exerted by the hammer mill screen size. This observation is consistent with the results of water recovery, where the application of enzymes was more impactful than changes in screen sizes.
For Arbequina at MI = 3.3, screen sizes caused no effect in the absence of enzymes. However, when enzymes were added, the concentration of water-soluble carbohydrates was higher for larger screen sizes, showing the same interaction observed for oil and water recoveries. Nonetheless, the same interaction was not observed in the case of Koroneiki, where water-soluble carbohydrates increased with the addition of enzymes and smaller screen sizes.
Cell wall composition has been shown to change during the ripening process.18,19 In particular, pectic and hemicellulosic polymers are solubilized and lost within the cell wall matrix when the fruit matures.20 In the advanced ripening stage, cell wall polysaccharides are degraded and solubilized. This degradation of the cell wall might expose layers with different polysaccharide compositions that are not targeted by the enzymes used for this experiment.
Total Phenol Content in Olive Paste
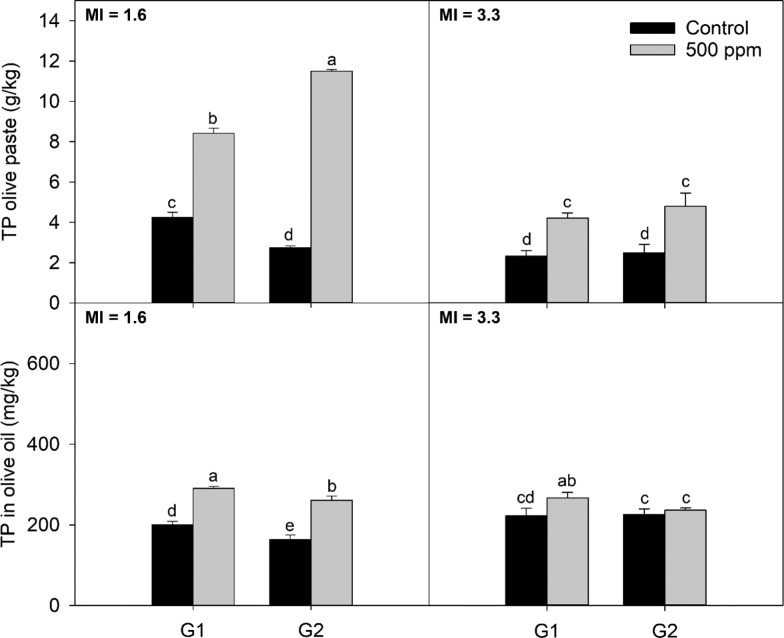
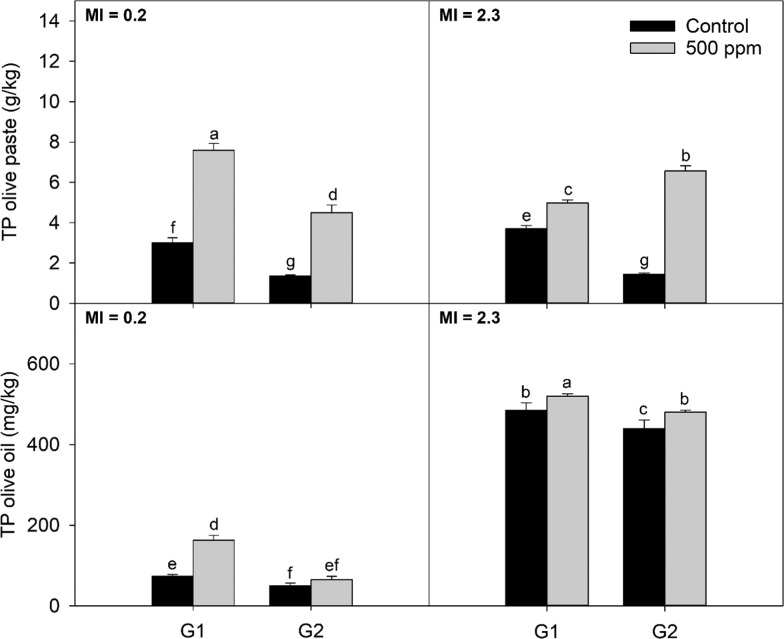
Results for total phenol content in the olive paste are shown in (upper) Figures 3 and 4, for Arbequina and Koroneiki, respectively. For Arbequina values ranged between 2.1 and 11.6 g/kg, whereas for Koroneiki the range was from 1.3 to 8.0 g/kg.
Figure 3.
Total phenol content in Arbequina olive oil and paste obtained using different screen sizes and addition of enzymes at two maturities; G1 and G2 are the screen sizes used for the experiment 4.5 and 8.5 mm, respectively; MI: maturity index. Different letters indicate significant differences (α = 0.05) according to the Fisher LSD test.
Figure 4.
Total phenol content in Koroneiki olive oil and paste obtained using different screen sizes and addition of enzymes at two maturities; G1 and G2 are the screen sizes used for the experiment 4.5 and 8.5 mm, respectively; MI: maturity index. Different letters indicate significant differences (α = 0.05) according to the Fisher LSD test.
Enzymes increased the concentration of phenols in the olive paste and had a larger effect compared to the hammer mill screen size. For Arbequina at MI = 1.6, total phenol concentration was higher when the addition of enzymes was combined with a larger screen size. The same was observed for Koroneiki at MI = 2.3. Koroneiki at MI = 0.2 presented the highest phenolic content when the smaller screen size was combined with enzymes. The interaction between maturity, crushing, and enzymes might be explained by the generation of emulsions, changes in cell wall constitution, and/or the activities of phenol-related enzymes during the extraction process.
The use of smaller screen size increased the concentration of phenols in the olive paste, supporting the hypothesis that smaller screen sizes release phenolic compounds more efficiently by breaking down the olive fruit cell wall where phenols have formed a complex.1,17 Arbequina at MI = 3.3 showed no statistically significant difference between the two studied screen sizes. It can be suggested that changes in cell wall constitution, in addition to changes in phenol-related enzymatic activities and overall phenolic concentration, affect the way screen size affects the concentration of phenol during crushing according to cultivar and maturity.
Phenolic Profile in Olive Oil
All treatments resulted in olive oils with quality parameters within the limits for the extra virgin category according to USDA21 and IOC22 standards (results not shown). Total phenol content results are shown in (lower) Figures 3 and 4. Values ranged between 149 and 294 mg/kg for Arbequina and between 42.7 and 522 mg/kg for Koroneiki.
As reported elsewhere,1,2 total phenols decreased with larger screen sizes, following a similar trend as the one observed for oil recovery. For Arbequina at MI = 1.6, smaller screen sizes yielded higher content in total phenols. However, no effect was observed for MI = 3.3. Enzymes significantly increased total phenol content in olive oil for all maturities and screen sizes except for Arbequina at MI = 3.3. However, the effect of enzyme addition was more pronounced with fruits of lower maturity index. For Arbequina at MI = 1.6, relative increments in total phenol content for treatment compared to control were 45 and 60% for G1 and G2, respectively. For MI = 3.3, the increase in total phenols was 20% for G1 and 5% for G2. Koroneiki at MI = 0.2 presented an increment of 121% for G1 and 31% for G2. For MI = 2.6, the increase was 7 and 9% for G1 and G2, respectively.
Smaller screen sizes increased total phenol content for Koroneiki at both maturities. The addition of enzymes increased the concentration of total phenols in Koroneiki oil in all conditions except for greener Koroneiki crushed with larger grid sizes. As observed for Arbequina, the increase in the total phenol content with the addition of enzymes was higher for the greener Koroneiki fruit. Total phenol content dramatically increased with ripening in the case of Koroneiki, as described before for other cultivars and regions.23,24 Results for individual phenolic compounds are detailed in Tables 2 and 3, for Arbequina and Koroneiki, respectively. In accordance with previous studies using the above-mentioned cultivars,25,26 the main phenolic compounds detected for all treatments were the dialdehydic form of oleuropein and ligstroside aglycones (3,4-DHPEA-EDA and p-HPEA-EDA) along with the aldehydic forms (3,4-DHPEA-EDA and p-HPEA-EDA, respectively) and 1-pinoresinol. 3,4-DHPEA-EDA, 3,4-DHPEA-EA, p-HPEA-EDA, and p-HPEA-EA presented the higher concentrations and wider range among all of the treatments.
Table 2. Main Phenolic Compound Concentration (mg/kg) in Arbequina Olive Oils Obtained Using Different Processing Conditionsa.
| maturity | MI = 1.6 |
MI = 3.3 |
||||||
|---|---|---|---|---|---|---|---|---|
| screen size (mm) | 4.5 |
8.5 |
4.5 |
8.5 |
||||
| enzyme | control | 500 ppm | control | 500 ppm | control | 500 ppm | control | 500 ppm |
| caffeic acid | 5.2 ± 0b | 6.6 ± 0.3a | 4.3 ± 0.1c | 6.3 ± 0.1a | 3.9 ± 0.2d | 5.3 ± 0.2b | 4.4 ± 0.1c | 5.1 ± 0.1b |
| vanillin | 0.9 ± 0.5ab | 0.9 ± 0.6ab | 0.4 ± 0.4b | 1.1 ± 0.1a | 0.6 ± 0.1ab | 0.8 ± 0ab | 0.5 ± 0.3b | 0.9 ± 0ab |
| p-coumaric acid | 0.7 ± 0b | 0.9 ± 0a | 0.6 ± 0c | 0.9 ± 0.1a | 0.8 ± 0b | 0.9 ± 0.1a | 0.8 ± 0b | 0.9 ± 0a |
| ferulic acid | 11.9 ± 0.9d | 35.3 ± 2.6ab | 5.9 ± 1.5d | 24.7 ± 4.1c | 27.2 ± 9.1bc | 40.2 ± 6.1a | 41.4 ± 8.7a | 43.2 ± 1.7a |
| vanillic acid | 0.4 ± 0a | 0.3 ± 0b | 0.2 ± 0cd | 0.2 ± 0c | 0.1 ± 0e | 0.2 ± 0de | 0.2 ± 0c | 0.2 ± 0cd |
| hydroxytyrosol | 1.5 ± 0.2d | 3.3 ± 0.3a | 1.6 ± 0.3cd | 3 ± 0.3ab | 1.6 ± 0.2cd | 3.1 ± 0.7a | 2.3 ± 0.4bc | 3.4 ± 0.6a |
| 3,4-DHPEA-EDA | 77.2 ± 6.7d | 129.8 ± 5.7a | 47.9 ± 9.7e | 113.6 ± 10.2b | 77.1 ± 7.9d | 101.5 ± 4.7c | 73.8 ± 3.8d | 82.1 ± 3.6d |
| 3,4-DHPEA-EA | 9.6 ± 0.1b | 10.6 ± 0.2a | 7.7 ± 0.6d | 10.3 ± 0.2a | 8.5 ± 0.3c | 8.2 ± 0.4c | 6.5 ± 0e | 6.6 ± 0.4e |
| tyrosol | 3.2 ± 0.1bc | 2.7 ± 0.1d | 2.7 ± 0d | 2.9 ± 0.1cd | 3.4 ± 0.2a | 3.3 ± 0.2ab | 3.2 ± 0ab | 3 ± 0.2bc |
| p-HPEA-EDA | 31.8 ± 1.3d | 37.6 ± 0.2b | 28.4 ± 0.8e | 39.6 ± 1.2a | 34.3 ± 0.8c | 36.4 ± 1.4b | 32.5 ± 0.6d | 33.2 ± 0.3cd |
| p-HPEA-EA | 4.0 ± 0.6abc | 4.3 ± 0.5ab | 3.1 ± 0.4d | 3.9 ± 0.3abc | 3.6 ± 0.6bcd | 4.6 ± 0.3a | 3.5 ± 0.3cd | 4.6 ± 0.1a |
| apigenin | 1.4 ± 0.1bc | 1.4 ± 0.1bc | 1.2 ± 0.2c | 1.2 ± 0.1bc | 1.5 ± 0.1ab | 1.7 ± 0.3a | 1.7 ± 0.3a | 1.4 ± 0.2bc |
| luteolin | 0.8 ± 0b | 0.8 ± 0.1b | 0.8 ± 0.1b | 1 ± 0.1b | 1.7 ± 0.4a | 1.8 ± 0.4a | 1.6 ± 0.3a | 1.4 ± 0.2a |
| 1-pinoresinol | 51.9 ± 0.3cd | 52.4 ± 0.7c | 58 ± 1.5ab | 60 ± 1.1a | 57.6 ± 0.6b | 58.9 ± 1.5ab | 53.1 ± 2c | 50.1 ± 1.3d |
Values are expressed as the mean ± standard deviation. Within a row, different letters indicate significant differences according to the Fisher LSD test (α = 0.05). 3,4-DHPEA-EDA, the dialdehydic form of oleuropein aglycone; 3,4-DHPEA-EA, the aldehydic form of oleuropein aglycone; p-HPEA-EDA, the dialdehydic form of ligstroside aglycone; and p-HPEA-EA, the aldehydic form of ligstroside aglycone.
Table 3. Main Phenolic Compound Concentration (mg/kg) in Koroneiki Olive Oils Obtained Using Different Processing Conditionsa.
| maturity | MI = 0.2 |
MI = 2.3 |
||||||
|---|---|---|---|---|---|---|---|---|
| screen size (mm) | 4.5 |
8.5 |
4.5 |
8.5 |
||||
| enzyme | control | 500 ppm | control | 500 ppm | control | 500 ppm | control | 500 ppm |
| caffeic acid | 4.7 ± 0.1b | 5.7 ± 0.4a | 4.5 ± 0.3b | 6.2 ± 0.2a | 3.5 ± 0.3c | 3.5 ± 0.2c | 3.6 ± 0.2c | 4.3 ± 0.1b |
| vanillin | 0.2 ± 0c | 0.3 ± 0a | 0.1 ± 0c | 0.1 ± 0c | 0.2 ± 0c | 0.2 ± 0b | 0.1 ± 0c | 0.1 ± 0c |
| p-coumaric acid | 0.2 ± 0e | 0.2 ± 0de | 0.2 ± 0e | 0.2 ± 0.1cde | 0.3 ± 0abc | 0.3 ± 0ab | 0.3 ± 0bcd | 0.3 ± 0a |
| ferulic acid | 0.2 ± 0.1e | 4.2 ± 0.6a | 0.1 ± 0e | 0.2 ± 0e | 3.5 ± 0.4b | 3.6 ± 0.1b | 1.8 ± 0.1d | 2.9 ± 0.1c |
| vanillic acid | 0.8 ± 0.1b | 1 ± 0.1a | 0.9 ± 0.1b | 1 ± 0.1a | 0.4 ± 0c | 0.4 ± 0cd | 0.3 ± 0d | 0.4 ± 0cd |
| hydroxytyrosol | 0.7 ± 0f | 1.5 ± 0.1e | 0.9 ± 0.1f | 0.9 ± 0f | 2.7 ± 0.2d | 3.3 ± 0.1c | 3.6 ± 0.1b | 4.2 ± 0.2a |
| 3,4-DHPEA-EDA | 4.8 ± 1.6e | 60.1 ± 9.6d | 1.4 ± 0.4e | 3 ± 0.2e | 281.6 ± 8.2b | 305.5 ± 3.9a | 240 ± 18.5c | 274.7 ± 2.7b |
| 3,4-DHPEA-EA | 5.0 ± 0.9d | 17.3 ± 1.5c | 1.0 ± 0.1d | 1.3 ± 0.1d | 78.9 ± 5.2abc | 82.5 ± 1.3a | 74.5 ± 4.8b | 76.4 ± 1.1b |
| tyrosol | 1.9 ± 0.2e | 3.1 ± 0d | 1 ± 0f | 1.7 ± 0.3e | 5 ± 0.1c | 5.5 ± 0.2b | 6.2 ± 0a | 6.5 ± 0.1a |
| p-HPEA-EDA | 28.9 ± 0.8c | 39.2 ± 1.6a | 16.9 ± 0e | 23 ± 2.1d | 34.6 ± 0.8b | 39.6 ± 0.6a | 30.1 ± 3.3c | 34.2 ± 1.6b |
| p-HPEA-EA | 6.7 ± 0.4e | 7.9 ± 0.6d | 4.3 ± 0g | 5.5 ± 0.7f | 20.7 ± 1.2c | 22.1 ± 0.2b | 22.1 ± 0.1b | 24 ± 0.5a |
| apigenin | 0.7 ± 0.1c | 0.8 ± 0.1c | 0.5 ± 0c | 0.6 ± 0.1c | 3.8 ± 0.8b | 4.2 ± 0.4ab | 4.7 ± 0.3a | 4.6 ± 0.3a |
| luteolin | 0.3 ± 0c | 0.3 ± 0.1c | 0.4 ± 0c | 0.3 ± 0c | 16.4 ± 0.3b | 17.8 ± 0.5a | 18.2 ± 1.7a | 18.5 ± 0.6a |
| 1-pinoresinol | 18.6 ± 0.3e | 21.4 ± 0.4c | 20.0 ± 0.6d | 19.6 ± 0.1d | 29.8 ± 0.1b | 29.6 ± 0.6b | 31.0 ± 0.2a | 30.3 ± 0.4ab |
Values are expressed as the mean ± standard deviation. Within a row, different letters indicate significant differences according to Fisher LSD test (α = 0.05). 3,4-DHPEA-EDA, dialdehydic form of oleuropein aglycone; 3,4-DHPEA-EA, aldehydic form of oleuropein aglycone; p-HPEA-EDA, dialdehydic form of ligstroside aglycone; p-HPEA-EA, aldehydic form of ligstroside aglycone.
For Arbequina, 3,4-DHPEA-EDA was the most abundant phenolic compound at both maturities, with a range between 47.9 and 129.8 mg/kg. 3,4-DHPEA-EA range was between 6.5 and 10.6 mg/kg. p-HPEA-EDA ranged between 28.4 and 39.6 mg/kg. p-HPEA-EA range was between 3.1 and 4.6 mg/kg. 1-Pinoresinol was mostly affected by hammer mill screen size and ranged between 50.1 and 60.0 mg/kg. For the greener fruit 1-pinoresinol concentration increased with larger screen sizes, while for the more mature fruit the trend was inverted. At MI = 1.6, relative increments in 3,4-DHPEA-EDA of treatment compared to control were 68 and 137% for G1 and G2, respectively. For MI = 3.3, the increase in 3,4-DHPEA-EDA was 32% for G1 and 11% for G2.
Koroneiki showed p-HPEA-EDA as the main phenolic compound at MI = 0.2, with a range between 16.9 and 39.6 mg/kg. For MI = 2.3, the main phenolic compound was 3,4-DHPEA-EDA, with a range between 1.4 and 305.5 mg/kg. 3,4-DHPEA-EA range was between 1.0 and 82.5 mg/kg. p-HPEA-EA ranged between 4.3 and 24.0 mg/kg. 1-Pinoresinol was mostly affected by maturity and ranged between 18.6 and 31.0 mg/kg. Green Koroneiki was particularly affected by the addition of enzymes: at MI = 0.2, the increment in 3,4-DHPEA-EDA when enzymes were used was 1150% for G1 and 115% for G2. For MI = 2.6, the increase was 9 and 15% for G1 and G2, respectively.
Overall, ligstroside derivatives presented higher concentrations when smaller grid size was used, these results being consistent with previously published results for Arbequina and Cornicabra by Inarejos-García et al. Furthermore, the increase in the concentration of ligstrosides caused by the addition of enzymes was described previously by Vierhuis et al. for “Moraiolo” treated with Olivex and Novoferm 12, two alternative commercial enzymatic aids for the olive oil industry.
The impact exerted by the addition of 500 ppm of enzymes was larger compared to the one exerted by changes in hammer mill screen size, suggesting the potential of enzymatic aids to obtain olive oils beyond the possibilities of the traditional mechanical extraction process. Moreover, the increment in individual ligstroside derivatives was larger in greener fruits compared to more ripe fruits, further demonstrating the relevance of the changes in cell wall composition during ripening on the olive oil extraction process.
The comparative effect of hammer mill screen size and enzymes on oil recovery and the phenolic composition of olive oil was studied for super-high-density Arbequina and Koroneiki at two maturities. Oil quality parameters such as free fatty acidity, peroxide value, and UV absorbance were measured for all of the treatments; no differences were found in the different treatments of crushing conditions and enzyme additions (data not shown). Treatments that increased oil recoveries and phenolic content also presented higher water recoveries, water-soluble carbohydrates, and total phenols in the olive paste, demonstrating a greater degree of cell wall breakdown during the extraction process. Enzyme addition increased the yield and phenolic content of the olive oil, suggesting that the mechanical action of crushing did not disrupt all of the olive fruit cells and release the oil from the oil bodies.
Finally, the effect of crushing and enzymes depended on the cultivar and maturity of the olive fruit; thus, both factors should be considered when choosing optimal processing conditions. Enzymes were more effective for lower maturity indexes, not showing an effect for Arbequina at a maturity index of 3.3. Hence, while different concentrations and composition of the enzymatic cocktail were not tested in this research, these factors should be considered in future studies to understand the full potential and limitations of enzymatic aids for the olive oil industry.
Materials and Methods
Olive Samples
This study was carried out with olives harvested from the commercial orchards of California Olive Ranch (Artois, CA). Olives from super-high-density Arbequina and Koroneiki cultivars were hand-harvested in October and November 2018. Approximately 15 kg of fruits was harvested for each cultivar and time, and the oil was extracted using an Abencor system. All quality and compositional measurements were carried out in triplicate. Fruit parameters are shown in Table 1.
Maturity Index
Maturity index (MI) was measured by evaluating the color of the olive fruit skin and flesh.27 Briefly, a hundred olives were randomly chosen and categorized into seven groups according to the epicarp color, from right-green skin (group N = 0) to black skin with 100% purple flesh (group N = 7). Maturity index is given by
where N is the group number and n is the number of olives in that group.
Moisture Content
Olives were crushed with a hammer mill, and the paste (60 ± 0.1 g) was weighed in a beaker and placed in the oven at 105 °C until attaining a constant weight. The beaker was transferred to a desiccator, and the weight of the dry paste was registered after 2 h.
Fat Content
The dried olive sample (20.0 ± 0.1 g) was weighed in a cellulose extraction thimble and extracted using n-hexane for 6 h in a Soxhlet apparatus. When the extraction finished, the solvent was removed in a rotary evaporator and the residual solvent was eliminated by placing the sample in the oven at 105 °C for 3 h. The fat content in wet basis was calculated as follows:
where MC is the moisture content and FCdry basis is the fat content of the sample expressed on a dry basis.
Olive Oil Extraction
Olive oil was extracted using an Abencor analyzer (MC2 Ingenieria y Sistemas S.L., Seville, Spain) consisting of a hammer crusher equipped with exchangeable sieves, a malaxer, and a basket centrifuge. Olive samples (700 g) were crushed in triplicate using two different screen sizes (G1 = 4.5 mm and G2 = 8.5 mm). After malaxation (45 min at 27 °C), the olive paste was centrifuged for 2 min in the basket centrifuge. Oil was separated from vegetation waters by decantation and centrifuged at 3000g for 10 min before being stored at −20 °C in plastic bottles without headspace until analysis. For the trials including enzymes, 500 ppm of Novozymes Pectinex Ultra Olio was added right after crushing and before malaxation.
Oil Recovery
Oil recovery was calculated according to the fat content of the olive fruits and the volume of olive oil obtained according to
where 0.915 is the oil density, Voil is the volume of oil extracted, molives is the mass of processed olives, and FColives is the fat content of the olives in the wet basis.
Water Recovery
Water recovery was calculated according to the moisture content of the olive fruit and the volume of water obtained after centrifugation according to
where Vwater is the volume of water obtained after centrifugation, molives is the mass of processed olives, and MColives is the moisture content of the olives. δwater is the density of the water and was considered as 1 g/mL.
Analysis of Fruit Cell Wall Degradation
The level of cell wall breakdown was evaluated by measuring water-soluble carbohydrates in olive paste samples at the end of the malaxation period, before the centrifugation step, following the extraction protocol described by Slominski et al.28 Total water-soluble carbohydrate quantification was performed using the anthrone method.29
Analysis of Total Phenol Content in Olive Paste
Total phenol content in the olive paste after the malaxation period was quantified according to the method suggested by Gómez-Rico et al.30 with some modifications. Briefly, the sample (4.0 g) was homogenized with a mixture of methanol/water (40 mL, 80:20 v/v) for 2 min with vortex agitation for 1 min. The suspension obtained was shaken (20 min, 150 rpm) and then centrifuged (2000g, 10 min). The hydro-methanolic phase was recovered and filtered with a 0.45 μm nylon syringe filter. Phenolic compounds were quantified by the Folin–Ciocalteu method using a calibration curve of caffeic acid and expressed as gram per kilogram of olive paste.
Quality Parameters
Free fatty acids, peroxide value, and UV absorbances (K232, K270, and ΔK) were determined according to AOCS standard methods Ca 5a-40(09), Cd 8b-90(09), and Ch 5-91(09), respectively.
Total Phenol Determination in Oil Samples
The sample (2.0 ± 0.1 g) was dissolved in hexane (1 mL) and extracted three times with methanol/water (2 mL; 60:40 v/v). After centrifugation (3000g, 6 min), the supernatants were collected, and total phenol content was determined using the Folin–Ciocalteu colorimetric method. An aliquot (0.2 mL) of the phenolic extract was diluted with distilled water up to 5 mL and mixed with the Folin–Ciocalteu reagent (0.5 mL) and sodium carbonate (1 mL; 35% w/v). After bringing it to a final volume of 10 mL, the mixture was stored in the dark for 2 h. The absorbance at 725 nm was measured, and the concentration of phenolic compounds was calculated using an external calibration curve prepared with caffeic acid.
Phenolic Compounds Profile in Oil Samples
The sample (2.500 ± 0.001 g) was weighed in a 10 mL caramel-colored vial containing the internal standard solution (0.5 mL, 6.75 × 10–2 mg/mL of p-hydroxyphenyl-acetic acid) and n-hexane (6 mL). A 1000 mg/6 mL diol-bonded phase cartridge (Thermo Scientific, Waltham, MA) was put in a vacuum elution apparatus and conditioned with methanol (6 mL) and hexane (6 mL), consecutively. After the oil solution was applied onto the diol cartridge, the cartridge was washed with hexane (6 mL) twice and hexane/ethyl acetate (6 mL, 90:10, v/v) once. The cartridge was then eluted with methanol (10 mL), and the solvent was evaporated in a rotatory evaporator at room temperature under vacuum until dry. Finally, the residue was reconstituted with methanol/water (1 mL, 1:1, v/v) for injection.
A 5 μm, 250 mm × 4.6 mm C18 column (Agilent Technologies, Santa Clara, CA) was used for UPLC (Infinity 1290, Agilent Technologies, Santa Clara, CA) analysis. The sample injection volume was 20 μL, and the flow rate was 1.0 mL/min. In this analysis, the mobile phase A was water/acetic acid (98:2, v/v) and B was methanol/acetonitrile (1:1, v/v). The solvent gradient changed according to the following conditions: from 0 to 25 min, 95% A–5% B to 70% A–30% B; from 25 to 50 min, to 65% A–35% B; from 50 to 65 min, to 30% A–70% B; from 65 to 70 min, to 100% B; the gradient was then brought back to 95% A–5% B in 5 min. The diode array detection (DAD) was performed at 280 and 340 nm. The quantification was determined by using the concentration relative to the concentration of IS.
Statistical Analysis
A factor-factorial experimental design considering olive cultivar (Arbequina and Koroneiki), maturity (low and high maturity index), hammer mill screen size (4.5 and 8.5 mm), and addition of cell wall-degrading enzymes during malaxation (control and 500 ppm) was used for this experiment. Factorial ANOVA was carried out to assess the effect of each of the factors on oil recovery, water recovery, total water-soluble carbohydrates, total phenol content in the olive paste, and total and individual phenol content on olive oil. Data normality and homoscedasticity assumptions were tested using the Shapiro–Wilk test and Breusch–Pagan test, respectively. Fisher’s least significant difference (LSD) tests were applied to establish the differences between each treatment. Data was analyzed using Minitab v. 19.2.
Acknowledgments
The authors would like to thank the California Olive Ranch for providing the olive samples used for this experiment. J.J.P. is deeply grateful to Firmin Berta for supporting his graduate studies at UC Davis.
The authors declare no competing financial interest.
References
- Polari J. J.; Wang S. C. Hammer Mill Sieve Design Impacts Olive Oil Minor Component Composition. Eur. J. Lipid Sci. Technol. 2019, 121, 1900168 10.1002/ejlt.201900168. [DOI] [Google Scholar]
- Inarejos-García A. M.; Fregapane G.; Salvador M. D. Effect of crushing on olive paste and virgin olive oil minor components. Eur. Food Res. Technol. 2011, 232, 441–451. 10.1007/s00217-010-1406-4. [DOI] [Google Scholar]
- Leone A.; Romaniello R.; Zagaria R.; Sabella E.; De Bellis L.; Tamborrino A. Machining effects of different mechanical crushers on pit particle size and oil drop distribution in olive paste. Eur. J. Lipid Sci. Technol. 2015, 117, 1271–1279. 10.1002/ejlt.201400485. [DOI] [Google Scholar]
- Preziuso S. M.; Di Serio M. G.; Biasone A.; Vito R.; Mucciarella M. R.; Di Giovacchino L. Influence of olive crushing methods on the yields and oil characteristics. Eur. J. Lipid Sci. Technol. 2010, 112, 1345–1355. 10.1002/ejlt.201000303. [DOI] [Google Scholar]
- Peres F.; Martins L. L.; Ferreira-Dias S. Influence of enzymes and technology on virgin olive oil composition. Crit. Rev. Food Sci. Nutr. 2017, 57, 3104–3126. 10.1080/10408398.2015.1092107. [DOI] [PubMed] [Google Scholar]
- Vierhuis E.; Servili M.; Baldioli M.; Schols H. A.; Voragen A. G. J.; Montedoro G. Effect of enzyme treatment during mechanical extraction of olive oil on phenolic compounds and polysaccharides. J. Agric. Food Chem. 2001, 49, 1218–1223. 10.1021/jf000578s. [DOI] [PubMed] [Google Scholar]
- Ranalli A.; Pollastri L.; Contento S.; Iannucci E. The glyceridic and nonglyceridic constituents of virgin olive oil after use of a novel method of enzyme extraction. Int. J. Food Sci. Technol. 2003, 38, 17–27. 10.1046/j.1365-2621.2003.00632.x. [DOI] [Google Scholar]
- De Faveri D.; Aliakbarian B.; Avogadro M.; Perego P.; Converti A. Improvement of olive oil phenolics content by means of enzyme formulations: Effect of different enzyme activities and levels. Biochem. Eng. J. 2008, 41, 149–156. 10.1016/j.bej.2008.04.007. [DOI] [Google Scholar]
- Iconomou D.; Arapoglou D.; Israilides C. Improvement of phenolic antioxidants and quality characteristics of virgin olive oil with the addition of enzymes and nitrogen during olive paste processing. Grasas Aceites 2010, 61, 303–311. 10.3989/gya.064809. [DOI] [Google Scholar]
- García A.; Brenes M.; Moyano M. J.; Alba J.; García P.; Garrido A. Improvement of phenolic compound content in virgin olive oils by using enzymes during malaxation. J. Food Eng. 2001, 48, 189–194. 10.1016/S0260-8774(00)00157-6. [DOI] [Google Scholar]
- Najafian L.; Ghodsvali A.; Khodaparast M. H. H.; Diosady L. L. Aqueous extraction of virgin olive oil using industrial enzymes. Food Res. Int. 2009, 42, 171–175. 10.1016/j.foodres.2008.10.002. [DOI] [Google Scholar]
- Canamasas P.; Ravetti L. M. Evaluation of traditional and new processing aids for olive oil extraction. Acta Hortic. 2014, 1057, 677–684. 10.17660/ActaHortic.2014.1057.86. [DOI] [Google Scholar]
- Ben-David E.; Kerem Z.; Zipori I.; Weissbein S.; Basheer L.; Bustan A.; Dag A. Optimization of the Abencor system to extract olive oil from irrigated orchards. Eur. J. Lipid Sci. Technol. 2010, 112, 1158–1165. 10.1002/ejlt.201000056. [DOI] [Google Scholar]
- Canamasas P.; Ravetti L.. Evaluation of Processing Aids for Olive Oil Extraction and Quality Improvement, Rural Industries Research and Development Corporation, October 2011.
- Aliakbarian B.; De Faveri D.; Converti A.; Perego P. Optimisation of olive oil extraction by means of enzyme processing aids using response surface methodology. Biochem. Eng. J. 2008, 42, 34–40. 10.1016/j.bej.2008.05.006. [DOI] [Google Scholar]
- Meyer A. S.; Jepsen S. M.; Sørensen N. S. Enzymatic Release of Antioxidants for Human Low-Density Lipoprotein from Grape Pomace. J. Agric. Food Chem. 1998, 46, 2439–2446. 10.1021/jf971012f. [DOI] [Google Scholar]
- Vierhuis E.; Servili M.; Baldioli M.; Schols H. A.; Voragen A. G. J.; Montedoro G. Effect of enzyme treatment during mechanical extraction of olive oil on phenolic compounds and polysaccharides. J. Agric. Food Chem. 2001, 49, 1218–1223. 10.1021/jf000578s. [DOI] [PubMed] [Google Scholar]
- Jiménez A.; Rodríguez R.; Ferriáhdez-Caro I.; Guillén R.; Fernández-Bolaños J.; Heredia A. Olive fruit cell wall: Degradation of peptic polysaccharides during ripening. J. Agric. Food Chem. 2001, 49, 409–415. 10.1021/jf000235u. [DOI] [PubMed] [Google Scholar]
- Vierhuis E.; Schols H. A.; Beldman G.; Voragen A. G. J. Isolation and characterization of cell wall material from olive fruit (Olea europaea cv koroneiki) at different ripening stages. Carbohydr. Polym. 2000, 43, 11–21. 10.1016/S0144-8617(99)00204-0. [DOI] [Google Scholar]
- Mafra I.; Lanza B.; Reis A.; Marsilio V.; Campestre C.; De Angelis M.; Coimbra M. A. Effect of ripening on texture, microstructure and cell wall polysaccharide composition of olive fruit (Olea europaea). Physiol. Plant 2001, 111, 439–447. 10.1034/j.1399-3054.2001.1110403.x. [DOI] [PubMed] [Google Scholar]
- USDA. United States Standards for Grades of Olive Oil and Olive-Pomace Oil, 2010; p 21.
- International Olive Council. Trade Standard Applying to Olive Oils and Olive Pomace Oils (COI/T.15/NC No 3/Rev. 14), 2019; pp 7–8.
- Salvador M. D.; Aranda F.; Fregapane G. Influence of fruit ripening on “Cornicabra” virgin olive oil quality: A study of four successive crop seasons. Food Chem. 2001, 73, 45–53. 10.1016/S0308-8146(00)00276-4. [DOI] [Google Scholar]
- Yousfi K.; Cert R. M.; García J. M. Changes in quality and phenolic compounds of virgin olive oils during objectively described fruit maturation. Eur. Food Res. Technol. 2006, 223, 117–124. 10.1007/s00217-005-0160-5. [DOI] [Google Scholar]
- Polari J. J.; Garcí-Aguirre D.; Olmo-García L.; Carrasco-Pancorbo A.; Wang S. C. Interactions Between Hammer Mill Crushing Variables and Malaxation Time During Continuous Olive Oil Extraction. Eur. J. Lipid Sci. Technol. 2018, 120, 1800097 10.1002/ejlt.201800097. [DOI] [Google Scholar]
- Dabbou S.; Dabbou S.; Chehab H.; Brahmi F.; Taticchi A.; Servili M.; Hammami M. Chemical composition of virgin olive oils from Koroneiki cultivar grown in Tunisia with regard to fruit ripening and irrigation regimes. Int. J. Food Sci. Technol. 2011, 46, 577–585. 10.1111/j.1365-2621.2010.02520.x. [DOI] [Google Scholar]
- Uceda M.; Frías L. In Épocas de recolección. Evolución del contenido graso del fruto y de la composición y calidad del aceite. IOOC Proceedings of II Seminario Oleícola International, Córdoba, Spain, 1975; pp 25–46.
- Slominski B. A.; Guenter W.; Campbell L. D. New Approach to Water-Soluble Carbohydrate Determination as a Tool for Evaluation of Plant Cell Wall Degrading Enzymes. J. Agric. Food Chem. 1993, 41, 2304–2308. 10.1021/jf00036a016. [DOI] [Google Scholar]
- Brink R. H.; Dubach P.; Lynch D. L. Measurement of carbohydrates in soil hydrolyzates with anthrone. Soil Sci. 1960, 89, 157–166. 10.1097/00010694-196003000-00006. [DOI] [Google Scholar]
- Gómez-Rico A.; Inarejos-García A. M.; Salvador M. D.; Fregapane G. Effect of malaxation conditions on phenol and volatile profiles in olive paste and the corresponding virgin olive oils (Olea europaea L. Cv. cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. 10.1021/jf803505w. [DOI] [PubMed] [Google Scholar]