Abstract
Frailty, a syndrome characterized by an exaggerated decline in function and reserve of multiple physiological systems, is common in older patients with heart failure (HF) and is associated with worse clinical and patient-reported outcomes. Although several detailed assessment tools have been developed and validated in the geriatric population, they are cumbersome, not validated in patients with HF, and not commonly used in routine management of patients with HF. More recently, there has been an increasing interest in developing simple frailty screening tools that could efficiently and quickly identify frail patients with HF in routine clinical settings. As the burden and recognition of frailty in older patients with HF increase, a more comprehensive approach to management is needed that targets deficits across multiple domains, including physical function and medical, cognitive, and social domains. Such a multidomain approach is critical to address the unique, multidimensional challenges to the care of these high-risk patients and to improve their functional status, quality of life, and long-term clinical outcomes. This review discusses the burden of frailty, the conceptual underpinnings of frailty in older patients with HF, and potential strategies for the assessment, screening, and management of frailty in this vulnerable patient population.
Keywords: aging, frailty, Fried phenotype, heart failure, physical function, quality of life
Frailty is a syndrome characterized by an exaggerated decline in function and reserve of multiple physiological systems, resulting in a lower homeostatic tolerance of stressors and increased sensitivity and vulnerability to a wide range of adverse outcomes (1). Frailty has long been considered as a proxy for accelerated aging with cumulative manifestation of age-related impairment in multiple physiological systems that predispose to adverse outcomes (2). However, there is substantial variability in the rate of aging-related functional decline, and frailty is recognized as a distinct biologic syndrome that underlies this heterogeneity (3,4).
Frailty is of particular relevance to HF. As with frailty, HF is strongly associated with age such that older individuals have a significantly higher incidence and prevalence of HF, worse clinical outcomes with high burden of HF hospitalization, and associated health care costs (5). Even with evidence-based therapies to improve symptoms and long-term outcomes in patients with HF and reduced ejection fraction (HFrEF) (6), prognosis and quality of life of older patients with HF continue to be poor (7). This scenario may be especially true for patients with HF and preserved ejection fraction (HFpEF), the most common type of HF in the elderly, who report worse quality of life after an HF hospitalization compared with patients with HFrEF (8).
Frailty commonly coexists with HF, as both conditions share predisposing pathophysiological abnormalities, including high comorbidity burden, aging, and hospitalizations, contributing to accelerated functional decline and sarcopenia. When presenting together, frailty and HF are associated with worse patient-reported outcomes as well as clinical outcomes (9,10). Accordingly, there is a greater emphasis on incorporating frailty assessments into the prognostic and treatment models for HF to promote a more comprehensive approach to management (11,12).
Despite the importance of frailty, several challenges exist with implementation of frailty assessment into the routine clinical management of patients with HF. Currently, there is no consensus on how to best define frailty in HF. Although several frailty assessment tools have been described (13), the most well-validated tools can be too cumbersome and labor-intensive for routine clinical practice. Moreover, there is limited understanding regarding how the presence of frailty affects clinical management, including interventions directly targeting frailty, adaptive treatment strategies based on frailty status, and suitability for therapies.
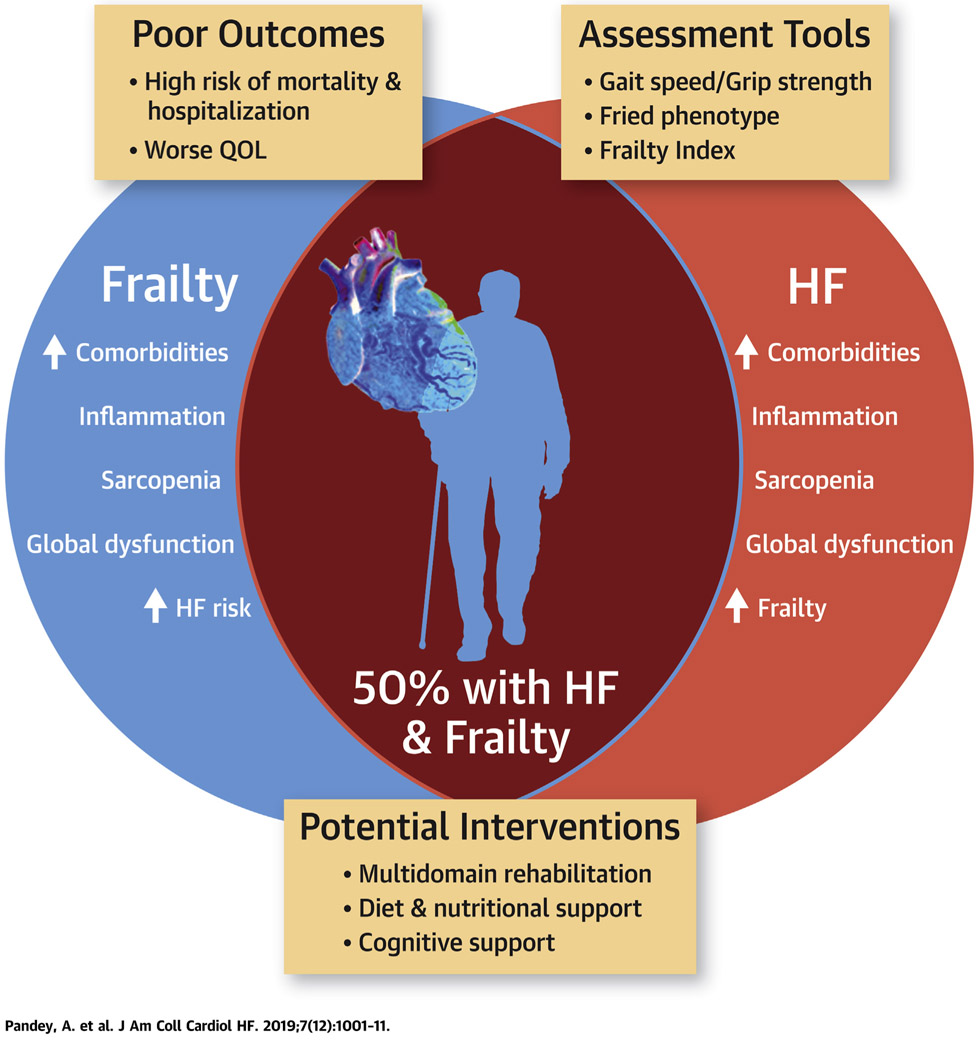
The current narrative review discusses the various frailty definitions and related conceptual models, assessment techniques and operationalization, and implications of frailty for the development and progression of HF, including prognostic implications, and emerging therapeutic strategies to improve clinical and patient-centered outcomes among the growing population of frail patients with HF (Central Illustration). Although an extensive review of the relevant published reports was undertaken, a comprehensive, systematic search of the published reports was not performed, and some studies relevant to this field may have been missed in our published reports review.
CENTRAL ILLUSTRATION. The Inter-Relationship Between Frailty and Heart Failure.
Frailty and heart failure share common pathological mechanisms, often coexist and associated with worse clinical and patient-oriented outcomes. Screening for frailty using simple, easy to use tests followed by detailed assessments is important to identify and target frail HF patients with multi domain interventions to improve outcomes.
BIOLOGICAL MECHANISMS UNDERLYING FRAILTY IN HF
The high burden of frailty in patients with chronic HF is likely related to a coordinated multisystem dysfunction that is precipitated by the systemic nature of HF, including systemic inflammation, high comorbidity burden, older age, and chronic skeletal muscle abnormalities (Online Ref. 1). Chronic HF accelerates the aging-associated decline in muscle mass with relative preservation or accumulation of adipose, leading to higher rates of sarcopenic obesity than with aging alone (Online Refs. 2-4). Chronic HF is also associated with abnormal muscle composition (i.e., high levels of intermuscular adipose tissue, shift in fiber type, reduced capillary density) that contributes to impaired mitochondrial function in skeletal muscle, reduced exercise capacity, and physical frailty (Online Refs. 5-7). The accelerated changes in muscle composition and associated physical frailty in chronic HF are likely the result of an upregulation of a proinflammatory state causing metabolic impairment, especially insulin resistance (Online Refs. 8-13).
Comorbidities common in older patients with chronic HF are also pro-inflammatory and associated with insulin resistance, further accelerating adverse changes in muscle composition, size, and performance (Online Refs. 13-17). Furthermore, hemodynamic abnormalities associated with HF can lead to tissue hypoxia, cellular apoptosis, and inflammation. Chronic congestion, volume overload, and hypoperfusion can also contribute to gut ischemia, translocation of gut microbiome, and upregulation of inflammatory pathways. Moreover, activation of neurohormonal pathways in chronic HF can also contribute to the pro-inflammatory state (Online Ref. 13). The pro-inflammatory state and associated metabolic impairment, coupled with chronic hypoperfusion in HF, lead to structural and functional abnormalities in other organ systems and contribute to global decreases in physiological reserve and a state of heightened vulnerability (Online Refs. 1,18,19).
The relation between frailty and HF is bidirectional: higher frailty contributes to worse physical functional status, cognitive impairment, and quality of life in patients with HF through upregulation of pro-inflammatory pathways and lower tolerance to physiological stressors (Online Refs. 12,20-22). Furthermore, these chronic processes may be exacerbated by an acute rise in inflammatory cytokines and worsened insulin resistance and further compounded by profound hospital-associated inactivity (Online Refs. 13,23-26). These acute factors promote muscle loss as well as adipocyte proliferation and lipid accumulation, which may further impair muscle function and recovery and contribute to sustained, prolonged global decline in functional status through local and systemic inflammatory and metabolic pathways (Online Refs. 27-32). This may contribute to hospital-associated functional decline and a “posthospital syndrome” such that even after resolution of decompensated HF, patients continue to have marked impairments in physical function and a higher burden of frailty (Online Refs. 20,30-34).
PREVALENCE AND PROGNOSTIC IMPLICATIONS OF FRAILTY IN PATIENTS WITH HF
Frailty is common among patients with HF, and its prevalence varies according to the frailty assessment method used and HF population assessed (e.g., ambulatory vs. hospitalized). The prevalence of frailty among outpatients with HF ranges from 19% to 52% according to the Fried frailty phenotype, the most well-validated and commonly used measure for frailty assessment (13-15). This rate is much higher than frailty rates in community-dwelling elderly subjects without HF, which is as low as 3% in the group aged 65 to 70 years, to 23% among those $90 years of age using similar frailty criteria (1). Among HF subtypes, the prevalence of frailty is higher in patients with chronic stable HFpEF versus HFrEF, with up to 60% to 90% of patients with HFpEF identified as frail. This finding may be related to the older age and higher comorbidity burden among HFpEF versus HFrEF patients (15,16). Among hospitalized patients with HF, the burden of frailty is even higher (56% to 76%) and similar in HFpEF versus HFrEF. Similarly, the prevalence of frailty is also noted to be higher among patients with advanced HF (50% to 65%) in small single-center studies (17,18).
Frail patients with HF have higher symptom burden, with twice as much dyspnea and 75% worse sleep disturbances and depressive symptoms, compared with the nonfrail patients (19). Quality of life is also significantly worse in frail versus nonfrail patients with chronic and acute HF (20). Among clinical outcomes, a recent meta-analysis showed that patients with HF and frailty, determined by using the Fried phenotype, had a 57% higher risk of hospitalization and 80% higher risk of mortality compared with nonfrail patients (14). Among HF subtypes, frailty is associated with a higher risk of adverse outcomes in both HFpEF and HFrEF. In the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial, cohort patients with HFpEF and a high Frailty Index (FI) versus a low FI (>0.5 vs. <0.3) had markedly higher risk of HF hospitalization and all-cause mortality (16).
The prognostic value of frailty has also been shown in hospitalized patients with acute decompensated HF. Volpato et al. (21) reported that among patients with acute HF, lower Short Physical Performance Battery (SPPB) score at admission was associated with longer stay, and a lower SPPB score at discharge was associated with a higher burden of disability in activities of daily living (ADL), readmission, and mortality. Similarly, in the FRAIL-HF cohort, among patients hospitalized with HF, frailty was associated with a higher risk of 1-year readmission and mortality (22). Taken together, frailty assessment may identify patients with HF who are at higher risk of disability and adverse clinical outcomes at each stage of the disease manifestation, and it may facilitate targeted interventions that reduce frailty burden and improve outcomes.
FRAILTY ASSESSMENT MODELS
Although there is consensus regarding the conceptual definition of frailty, achieving consensus for an operationalized definition providing objective, measurable assessment of frailty has proved much more challenging. Currently, there are several approaches to the assessment of frailty; the 2 most common are the Fried phenotype model and the FI or deficit index (Rockwood model).
FRIED PHENOTYPE METHOD.
It has been >20 years since Fried et al. first described the frailty phenotype in the landmark Cardiovascular Health Study, which was subsequently validated in the Women’s Health and Aging Study (1,23). Since then, the Fried model has become the most widely adopted and is generally regarded as the standard tool for assessment of frailty (1). According to this conceptual model, decline in physiological reserve is reflected across 5 domains: weight loss, weakness, poor endurance, slowness, and low physical activity level. Frailty is identified by fulfilling criteria for at least 3 of the 5 domains. Those who meet only 1 to 2 domains are generally referred to as “pre-frail.” The presence of frailty based on the Fried phenotype has been consistently associated with worse clinical outcomes, greater functional impairment, and poor quality of life in older, community-dwelling individuals, as well as those with HF (14,24).
Although the Fried phenotype is the most commonly used tool to assess frailty, there are several challenges to its utility in patients with HF. First, because of the high burden of frailty in patients with HF, measurement cutoffs for diagnosing frailty, derived in a general community-dwelling population, may lose discriminatory power. Second, the substantial overlap in the clinical manifestations of HF and the frailty phenotype makes it difficult to distinguish to what extent measured frailty may be HF dependent versus HF independent (25). Third, measuring the Fried phenotype can be cumbersome and relatively time-intensive in the clinical setting because it involves performing and scoring selfreported assessments combined with objective physical function tests. Finally, the Fried phenotype predominantly focuses on physical impairments and does not account for other domains such as cognitive dysfunction, which are common in older patients with HF and contribute independently to poor functional status and quality of life (26).
Despite these limitations, the operationalized Fried definition of frailty has been instrumental to the study of frailty, contributing significantly to a growing appreciation of its importance and stimulating further frailty-related research.
FI OR DEFICIT INDEX.
An alternative method for assessing frailty is the FI developed by Rockwood et al. (27) in the CSHA (Canadian Study of Health and Aging). The Rockwood scale is based on a “multiple hit” model and characterizes frailty as an accumulation of health deficits across multiple domains. The FI uses a multidisciplinary list of variables that consists of 20 to 130 items encompassing information on signs, symptoms, comorbidity burden, laboratory results, and ADL. The FI is calculated as the proportion of the total number of deficits present to the number of deficits assessed, such that those with more deficits are scored as frailer than those with fewer deficits. However, the number of deficits assessed to determine the FI are not standardized and vary widely based on the clinical setting, available data, and/or population characteristics.
There are several advantages to the use of the FI for identifying frail patients. It provides a continuous estimate of frailty, with a wide range of distribution, allowing for a more granular assessment of subtle differences in frailty among individuals or across time. Furthermore, the frailty assessment can be performed by using data from medical records compared with use of the Fried phenotype, which relies on real-time measurements. Also, its quantitative nature allows establishment of cutoffs tailored to specific populations or clinical scenarios (28).
Recent studies using this tool for assessment of frailty in patients with HF have reported high prognostic value of the FI in predicting long-term outcomes among patients with chronic HFpEF and HFrEF (14,16). Furthermore, among patients with advanced HF, frailty before implantation of a left ventricular assist device, as assessed by using the FI, has also been associated with increased risk of death (29).
However, certain limitations to using the FI, particularly in patients with HF, are noteworthy. First, the number of deficits assessed to determine the FI are not standardized and vary widely based on the clinical setting, available data, and/or population characteristics (27). Furthermore, some deficits are nonmodifiable and are not expected to improve but only accumulate over time (e.g., chronic disease diagnoses). Consequently, the responsiveness of the FI to an intervention may vary based on the composition of deficit items. Second, the FI relies more on the number of deficits (as opposed to the nature of the deficit), and the clinical parameters contributing to the FI derivation are not weighted. Third, the FI may not distinguish between clinical deficits that are related to frailty versus those driven by transient deficits (e.g., related to acute illness), and it may overestimate the frailty burden in certain clinical settings. Finally, the FI depends on a large number of variables being accurately recorded and accessible in a large population. Although this approach is feasible with modern era electronic medical records, widespread use of this tool to assess frailty would require standardization of variables used in the FI. In addition, substantial resources and infrastructure would be needed to design and program this model across electronic medical records and health systems to systematically collect and input the necessary data.
PHYSICAL FUNCTION: A COMMON THEME IN ASSESSMENT OF FRAILTY
Although distinct in their conceptual underpinnings and methodology, both the Fried criteria and the FI rely heavily on assessment of physical function, whether through objective measures of physical performance such as gait speed or grip strength, or patient-reported performance such as assessment of ADLs. Accordingly, there has been considerable interest in evaluating functional performance with easy-to-administer, less time-intensive assessments that can be more easily integrated into clinical workflows. Although abnormal performance on these abbreviated tools does not conclusively identify frailty, it may help identify individuals who warrant more detailed frailty assessment (15).
We provide a brief review of key objective physical function screening assessments; Table 1 also describes additional abbreviated frailty screening tools. More extensive discussion of this topic is provided elsewhere (2,30).
TABLE 1.
Performance of Different Screening Tests to Identify Frailty Among Patients With HF
| Test Performance | Strengths | Weaknesses | Cutoffs | Outcomes | |
|---|---|---|---|---|---|
| Gait speed (58) | Patients are instructed to walk at a normal pace for a short distance (4 to 5 m). The time taken from the word “go” to reaching the stopping point. Some protocols have patients walk 1 m beyond stopping point to avoid slowing down near the finish | Quick, precise, objective measurement; simple; considered the “sixth vital sign”; feasible in multiple settings (clinic, home, hospital); well validated | Sensitive but not specific for frailty by most common cutoffs Limited to ambulatory patients | Slower speed indicates higher risk. Range from 0.4 to 1.0 m/s; 0.8 m/s common. Sometimes adjusted for sex and height | Slower gait speed is independently associated with increased risk of mortality and adverse outcomes in HF |
| Timed Up and Go Test (59,60) | The patient is instructed as follows: “Sit with your back against the chair and your arms on the arm rests. On the word ‘go,’ stand upright, then walk at your normal pace to the line on the floor, turn around, return to the chair, and sit down.” The time required to complete the test was time from the word “go” to time when the subject returned to the starting position | Quick, precise, objective measurement; highly correlated with other functional tests in HF | Score may be affected by type of chair and footgear/assistive devices. Limited to ambulatory patients | No specific cutoffs reported in patients with HF. Slower time recorded in frail vs. nonfrail HF patients (15 to 28 s vs. 9 to 18 s) (59,60) | Faster time associated with better QOL, fewer falls |
| Hand grip strength (58) | Obtained with hand grip dynamometer. Has been done in seated or standing position. Usually multiple attempts allowed with scoring based on best performance or average performance | Rapid, objective measurement; no ambulation required | Heterogeneity in testing protocols; measurement tools not universally available | Range from approximately <28 to 30 kg in men and <18 to 20 kg in women. Can also be indexed to body mass with <0.25 indicating higher risk. Data-derived thresholds also used | Higher grip strength associated with lower risk of mortality and hospitalization |
| Short Physical Performance Battery | Combination of 3 tests: standing balance, gait speed, and chair rise. The patient is instructed to stand for 10 s in 3 positions (feet together side by side, semitandem, tandem); then perform 4-m gait speed test; and then stand from a chair with the arms across the chest 5 times. Each section is scored 0 to 4 points | Highly correlated with frailty by Fried criteria in community population; objective; well validated | Ceiling effect in highfunctioning individuals; <10 min but more time-intensive than some single-item tests | Lower score indicates greater risk; score <10 indicates mild increased risk; score ≤6 often used for frailty | Lower score associated with mortality, rehospitalization, length of stay, disability |
| Clinical frailty scale (15,61) | Measures between 1 (very fit) and 9 (terminally ill); patients are scored according to their functional capacity, level of dependence, and comorbidities; patients are identified as frail if score is >4 | Rapid; no physical testing required; score associated with 5-yr mortality in a graded fashion | Limited data on HF outcomes; scoring somewhat subjective | Patients are identified as frail if score >4; 53% of patients with ADHF and 47% with chronic HF identified as frail | Higher score associated with higher risk of mortality |
| Derby frailty index (15,61) | One of the following criteria is met: ≥65 yrs of age and a care home resident; ≥75 yrs of age with confusion, falls, or reduced mobility; ≥85 yrs of age with >4 comorbidities | Rapid, no physical testing required, objective criteria | Limited data on HF outcomes | Frail if 1 of 3 criteria are met; 50% of patients with ADHF and 48% with chronic HF identified as frail | Higher score associated with higher risk of mortality |
| Acute frailty network criteria (15,61) | Age ≥85 yrs or age ≥65 yrs with 1 or more of the following: cognitive impairment; resident in a care home; history of fragility fractures; Parkinson disease; recurrent falls | Rapid; no physical testing required; objective criteria, focuses on acute care needs | Limited data on HF outcomes | Frail vs. nonfrail if 1 of the 2 criteria are met; 53% of patients with ADHF and 44% with chronic HF identified as frail | Higher score associated with higher risk of mortality |
ADHF = acute decompensated heart failure; AUC = area under the curve; HF = heart failure; HR = hazard ratio; SBBP = Short Physical Performance Battery.
GAIT SPEED.
Gait speed, included as 1 of the 5 components of the Fried phenotype, is the most extensively studied single-item frailty assessment (Online Ref. 35). Typically measured as usual walking speed over a short distance (4), it is a simple, quick, and easy test to administer and can be performed in a reliable manner by clinic staff and requires no special equipment. It is also highly clinically relevant. Independent ambulation is fundamental to functional independence for most adults. Furthermore, gait coordination requires rapid and precise integration of multiple organ systems (e.g., neuromuscular, neurosensory, musculoskeletal), providing a global assessment of impairment (31). Finally, test performance is generally less dependent on cardiorespiratory fitness due to its short duration, capturing a different domain of functional performance compared with more sustained walking tests such as the 6-min walk test or exercise treadmill tests.
Gait speed has been consistently shown to be an independent predictor of adverse clinical events as well as patient-reported outcomes (Online Refs. 36,37). In a study with 34,485 older community-dwelling individuals, Studenski et al. (Online Ref. 38) reported that each 0.1 m/s increment in gait speed was associated with a 12% lower risk of death. Similarly, among patients with chronic HF, slow gait speed is associated with a 4-fold higher risk of mortality and 2-fold higher risk of hospitalization (Online Ref. 39). The feasibility and prognostic utility of gait speed assessment have also been reported in older, hospitalized patients with acute decompensated HF (Online Refs. 20,22).
GRIP STRENGTH.
Hand grip strength, as measured by using a dynamometer, is another simple, single-item measurement that has been used to assess frailty in the older population. Hand grip strength, like gait speed, is one of the components of the Fried phenotype and when used alone, it is an independent predictor of clinical outcomes in older individuals (Online Refs. 40,41). Grip strength is particularly well suited for use in nonambulatory or hospitalized patients and in those with more advanced disease. Prevalence of weak grip strength, defined by using age- and sex-specific cutoffs from community-dwelling adults, was 42% among ambulatory, newly diagnosed patients with HF and 60% among those hospitalized for HF (Online Refs. 42,43). Weak grip strength is associated with higher risk adverse clinical outcomes, independent of other risk factors, across the spectrum of patients with HF, including those with advanced HF undergoing left ventricular assist device implantation or cardiac transplantation (Online Refs. 42-44).
SHORT PHYSICAL PERFORMANCE BATTERY.
The SPPB is a simple, lower-extremity functional test that is a highly effective tool for frailty assessment (32-34). It is a 3-part test that incorporates balance, strength (repeated chair raise), and mobility (gait speed) assessment. Each component of the SPPB is scored from 0 to 4, for a total score of 0 to 12. A score <10 indicates at least mildly elevated global risk (35), and a score ≤6 is a marker of severe frailty (2). The SPPB can be administered easily and cost-effectively in various clinical settings. The SPPB score is sensitive to longitudinal changes in physical performance observed on serial testing, with a 1-point change representing a substantial change in functional status (36). Low baseline score as well as longitudinal decline in the SPPB score are strong predictors of worse outcomes, including all-cause mortality (36-38). Each component of the SPPB is also of independent prognostic importance (38).
ADL ASSESSMENT.
Basic and instrumental assessments of ADL are of inherent importance to older adults because maintaining functional independence is often a primary goal for this population. Performance with ADL may also be independently predictive of other important clinical outcomes (39). Assessment of ADL is central to the FI model, as previously discussed, contributing a large portion of the criteria not typically considered in conventional risk models.
Deficits in ADL were considered conceptually distinct from the original Fried frailty phenotype, with frailty often serving as a marker of risk for disability in functionally independent, community-dwelling elders (40,41). However, assessment of ADL has been successfully incorporated into several frailty phenotype models. These are particularly relevant in older and sicker patients with cardiovascular disease.
IMPLICATIONS OF FRAILTY IN THE MANAGEMENT OF OLDER PATIENTS WITH HF
The hemodynamic and perfusion consequences associated with HF may be uniquely relevant to several key pathophysiological mechanisms underlying frailty in HF, as discussed earlier. These observations suggest that the disease-specific therapies may play an important role in modifying frailty burden in patients with HF. Along these lines, advanced HF therapies such as left ventricular assist device and cardiac transplantation have been shown to improve frailty burden in patients with severe HF (42). However, use of guideline-directed HF therapies such as angiotensin-converting enzyme inhibitors, beta-blockers, and mineralocorticoid antagonists can be challenging in older, frail patients with HF due to their increased vulnerability to the adverse effects of these medications. Thus, the management of these vulnerable patients with HF may require a shift from the current paradigm of disease-specific management to a more comprehensive approach with management that addresses the systemic impact and global risk associated with frailty in HF. Along these lines, Gorodeski et al. (43) have proposed a “domain management approach” targeting deficits in the medical, physical function, emotion and cognition, and social environmental domains stemming from the cumulative systemic effects of HF, aging, multimorbidity, and recurrent illness. Comprehensive approaches such as this suggest novel, systemic interventions (e.g., exercise and physical rehabilitation, diet, nutritional support) to improve clinical, functional, and patient-reported outcomes (discussed later and summarized in Table 2). Furthermore, newer approaches targeting systemic inflammation with anti-inflammatory therapies have been tested in small studies with limited success in improving the quality of life and functional status in older patients with HF (44,45).
Table 2.
Efficacy of Different Interventions to Improve Quality of Life and Physical Performance or Exercise Capacity Among Older Patients With HF
| Design | Details About the Intervention | Comparator Group | QOL | Physical Function Exercise Capacity |
|
|---|---|---|---|---|---|
| Dietary intervention (56) | RCT | DASH + sodium-restricted diet in patients after discharge from HF hospitaLization (mean age, 71 yrs) | Usual care | Modest statistically insignificant improvement in KCCQ clinical score | Not assessed |
| Weight loss + exercise (57) | RCT (factorial design trial of diet and exercise intervention) | Caloric restriction with a calorie deficit of ~400 kcal/day in patients with chronic stable HFpEF (mean age, 66 yrs) + 1 h supervised exercise 3 times a week for 20 weeks | Diet and/or exercise vs. usual care | Better QOL according to the KCCQ and SF-36 by the diet intervention | Improvement in exercise capacity by both diet (peak VO2 +0.7 MET) and exercise interventions (peak VO2 +0.8 MET) |
| Multidomain rehabilitation (32) | Pilot RCT | Multidomain physical rehabilitation intervention beginning in the hospital for patients with ADHF (mean age, 72 yrs) | Usual care | Better QOL by KCCQ (+5.4 U) | Trends in improvement in SPPB performance (+ 1.1 U) and 6MWD (+23 m) |
| Supervised exercise training (49) | Meta-analysis of RCTs | Supervised moderate-intensity exercise in patients with chronic stable HFpEF | Usual care | Better QOL by MLWHF (−4 U) score | Improvement in exercise capacity (peak VO2 +0.8 MET) |
| Home-based exercise training (51) | Meta-analysis of RCTs | Mild to moderate intensity walking (40%-75% of peak heart rate); strength training and stretch exercises | Usual care | Better QOL (moderate improvement by pooled effect size across different QOL instruments) | Improvement in exercise capacity (peak VO2 +1 MET) |
| Anti-inflammatory agents (IL-1) (44,45) | RCT | Anakinra in patients with chronic stabLe HFpEF (age range, 45 to 46 yrs) and HFrEF (age range, 49 to 68 yrs) | Usual care | No improvement in QOL (DASI and MLWHF scores) | No improvement in peak VO2 |
6MWD = 6-min walk distance; ADHF = acute decompensated heart failure; DASH = Dietary Approaches to Stop Hypertension; DASI = Duke Activity Status Index; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; IL = interlukin; KCCQ = Kansas City Cardiomyopathy Questionnaire; MET = Metabolic equivalents; MLWHF = Minnesota Living With Heart Failure; QOL = quality of life; peak VO2 = peak exercise oxygen uptake; RCT = randomized controlled trial; SF-36 = 36-item Short-Form Health Survey.
EXERCISE AND PHYSICAL REHABILITATION FOR MANAGEMENT OF FRAIL PATIENTS WITH HF.
Given the contribution of sarcopenia and functional impairments to frailty in patients with HF, recent studies have evaluated targeted interventions such as supervised exercise training and multidomain physical rehabilitation to reduce the frailty burden and improve patient-reported and clinical outcomes (32,46). Supervised exercise training has been associated with improvement in exercise capacity and quality of life in patients with HF (47-49). However, older patients with HF and a high frailty burden from recent hospitalization, high comorbidity burden, immobility, or cognitive impairment were grossly underrepresented in the exercise training trials (48). Furthermore, supervised exercise training largely focused on endurance and does not address other physical function domains that are common in frail patients with HF. To address these knowledge gaps, the ongoing REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial is evaluating the efficacy of a tailored, progressive, physical rehabilitation intervention that begins during hospitalization, continues for 3 months after discharge, and addresses deficits in balance, mobility, strength, and endurance (46). The primary outcomes in REHAB-HF are the SPPB score and 6-month rehospitalization rates. The REHAB-HF pilot study results were encouraging, and the completed REHAB-HF trial will determine the role of multidomain physical rehabilitation interventions in older, frail patients with acute HF (32).
The initiation and maintenance of supervised exercise training regimens in older patients with HF and frailty may be challenging as reflected by the overall low participation rates in the current cardiac rehabilitation programs (50). This factor highlights the need for future research evaluating alternative cardiac rehabilitation strategies such as home-based exercise with formats specifically designed to optimize adherence and successful participation despite the challenges of frailty (51).
DIET AND NUTRITIONAL STRATEGIES FOR MANAGEMENT OF FRAIL PATIENTS WITH HF.
Nutritional intake can be limited in patients with HF due to early satiety, impaired sense of smell and taste, chronic dyspnea and nausea, comorbid conditions such as depression, and disease-specific dietary restrictions related to HF and comorbidities (43,52). As a result, patients with HF are at an increased risk for nutritional deficiency and malnourishment and require careful optimization of their dietary regimen. Nutritional deficits may contribute to weight loss cardiac cachexia and frailty in patients with HF (53). Several studies have evaluated the efficacy of nutritional supplementation in improving functional status among older, frail individuals. In a meta-analysis, multinutrient and protein supplementation was associated with improved physical function (54).
The PICNIC trial, a 6-month nutritional support program, found that individualized nutritional counseling significantly lowered 1-year mortality and HF readmission rate among malnourished patients with HF (55). GOURMET-HF (Geriatric Out-of-Hospital Randomized Meal Trial in Heart Failure) evaluated home-delivered, nutritionally complete, low-sodium meals versus usual care in patients with HF being discharged post-hospitalization (56). Although the primary trial was negative for any differences in quality of life across the 2 study arms, a secondary analysis showed trends toward potential benefits of the dietary intervention in improving HF symptoms, physical limitations, and readmission rates.
At the other end of the spectrum, recent studies have also evaluated the role of caloric restriction and weight loss in improving functional status in patients with chronic HFpEF. Kitzman et al. (57) showed that among older, obese individuals with HFpEF, weight loss via caloric restriction resulted in improved exercise capacity, quality of life, body composition, and systemic inflammation. Future studies are needed to determine if weight loss and its associated favorable effects can be maintained in the long term and translate into lower risk of adverse clinical events.
CONCLUSIONS
Frailty is a multidimensional, multisystem syndrome that is highly prevalent in older patients with HF and contributes to poor functional status and worse clinical outcomes. Integration of routine frailty screening into outpatient and inpatient clinical practice can identify older patients with HF and frailty, enhance risk stratification, and facilitate novel management strategies to improve outcomes and reduce the burden of frailty in this high-risk, vulnerable population.
HIGHLIGHTS.
Frailty is common in older patients with heart failure, and both frailty and heart failure share common mechanistic features, including strong relations with a high burden of comorbidities, inflammation, and sarcopenia.
Frailty is associated with worse clinical, functional, and quality of life outcomes in older patients with heart failure.
Frailty should be considered for routine assessment by using well-validated assessment tools to better inform prognosis.
In older patients with heart failure and frailty, novel management strategies, such as those addressing multiple domains through multidisciplinary assessment and intervention, should be investigated further.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the critical input of Pamela Duncan, PhD, and Vijay Agusala during manuscript preparation.
Dr. Kitzman is supported in part by the National Institutes of Health (NIH) research grants R01AG045551 and R01AG18915, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine, the Claude D. Pepper Older Americans Independence Center NIH Grants P30AG021332 and P30AG028716, the OAIC Pepper National Coordinating Center NIH Grant U24 AG05964, and the Wake Forest Clinical and Translational Science Award, NIH Grant UL1TR001420. Dr. Pandey is supported by the Texas Health Resources Clinical Research Scholarship. Dr. Kitzman has been a consultant for AbbVie, AstraZeneca, Merck, Novartis, Corvia Medical, Bayer, CinRx, Boehringer Ingelheim, and St. Luke’s Medical Center; received grant support from Novartis, Bayer, AstraZeneca, and St. Luke’s Medical Center; and owns stock in Gilead Sciences. Dr. Reeves has reported that he has no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ADL
activities of daily living
- FI
Frailty Index
- HF
heart failure
- HFpEF
heart failure and preserved ejection fraction
- HFrEF
heart failure and reduced ejection fraction
- SPPB
Short Physical Performance Battery
Footnotes
APPENDIX For supplemental references, please see the online version of this paper.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 2.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 Update: a report from the American Heart Association. Circulation 2018;137:e67–492. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 7.Khera R, Pandey A, Ayers CR, et al. Contemporary epidemiology of heart failure in fee-for-service Medicare beneficiaries across healthcare settings. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warraich HJ, Kitzman DW, Whellan DJ, et al. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart FaiL 2018;11:e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph SM, Rich MW. Targeting frailty in heart failure. Curr Treat Options Cardiovasc Med 2017;19:31. [DOI] [PubMed] [Google Scholar]
- 10.Vitale C, Spoletini I, Rosano GM. Frailty in heart failure: implications for management. Card Fail Rev 2018;4:104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman DE, Santanasto AJ, Boudreau R, et al. Impact of incident heart failure on body composition over time in the health, aging, and body composition study population. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich MW, Chyun DA, Skolnick AH, et al. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016;133:2103–22. [DOI] [PubMed] [Google Scholar]
- 13.McDonagh J, Martin L, Ferguson C, et al. Frailty assessment instruments in heart failure: a systematic review. Eur J Cardiovasc Nurs 2018;17:23–35. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Lupon J, Vidan MT, et al. Impact of frailty on mortality and hospitalization in chronic heart failure: a systematic review and meta-analysis. J Am Heart Assoc 2018;7:e008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of frailty in chronic heart failure. J Am Coll Cardiol HF 2019;7:291–302. [DOI] [PubMed] [Google Scholar]
- 16.Sanders NA, Supiano MA, Lewis EF, et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 2018;20:1570–7. [DOI] [PubMed] [Google Scholar]
- 17.Madan SA, Fida N, Barman P, et al. Frailty assessment in advanced heart failure. J Card Fail 2016;22:840–4. [DOI] [PubMed] [Google Scholar]
- 18.Joyce E Frailty in advanced heart failure. Heart Fail Clin 2016;12:363–74. [DOI] [PubMed] [Google Scholar]
- 19.Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO, Lee CS. Identifying a relationship between physical frailty and heart failure symptoms. J Cardiovasc Nurs 2018;33:E1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol 2017;236:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volpato S, Cavalieri M, Guerra G, et al. Performance-based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci 2008;63: 1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail 2016;18:869–75. [DOI] [PubMed] [Google Scholar]
- 23.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci 2006;61:262–6. [DOI] [PubMed] [Google Scholar]
- 24.Vermeiren S, Vella-Azzopardi R, Beckwee D, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 2016;17:1163. e1–.e17. [DOI] [PubMed] [Google Scholar]
- 25.Flint KM, Matlock DD, Sundareswaran KS, et al. Pre-operative health status and outcomes after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2013;32:1249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med 2013;126:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunlay SM Park SJ, Joyce LD, et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant 2014;33:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forman DE, Arena R, Boxer R, et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e894–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology 2013;80:S65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves GR, Whellan DJ, O’Connor CM, et al. A novel rehabilitation intervention for older patients with acute decompensated heart failure: the REHAB-HF Pilot Study. J Am Coll Cardiol HF 2017;5:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves GR, Whellan DJ, Patel MJ, et al. Comparison of frequency of frailty and severely impaired physical function in patients ≥60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol 2016;117:1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sayers SP, Guralnik JM, Newman AB, Brach JS, Fielding RA. Concordance and discordance between two measures of lower extremity function: 400 meter self-paced walk and SPPB. Aging Clin Exp Res 2006;18:100–6. [DOI] [PubMed] [Google Scholar]
- 35.Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016;14:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54: 743–9. [DOI] [PubMed] [Google Scholar]
- 37.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011;66:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gastelurrutia P, Lupon J, Altimir S, et al. Fragility is a key determinant of survival in heart failure patients. Int J Cardiol 2014;175:62–6. [DOI] [PubMed] [Google Scholar]
- 40.Quick Kojima G. and simple FRAIL scale predicts incident activities of daily living (ADL) and instrumental ADL (IADL) disabilities: a systematic review and meta-analysis. J Am Med Dir Assoc 2018;19:1063–8. [DOI] [PubMed] [Google Scholar]
- 41.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–63. [DOI] [PubMed] [Google Scholar]
- 42.Maurer MS, Horn E, Reyentovich A, et al. Can a left ventricular assist device in individuals with advanced systolic heart failure improve or reverse frailty? J Am Geriatr Soc 2017;65:2383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorodeski EZ, Goyal P, Hummel SL, et al. Domain management approach to heart failure in the geriatric patient: present and future. J Am Coll Cardiol 2018;71:1921–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Tassell BW, Canada J, Carbone S, et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Tassell BW, Trankle CR, Canada JM, et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail 2018; 11:e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reeves GR, Whellan DJ, Duncan P, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: design and rationale. Am Heart J 2017;185:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 2015;8: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golwala H, Pandey A, Ju C, et al. Temporal trends and factors associated with cardiac rehabilitation referral among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure Registry. J Am Coll Cardiol 2015;66:917–26. [DOI] [PubMed] [Google Scholar]
- 51.Imran HM, Baig M, Erqou S, et al. Home-based cardiac rehabilitation alone and hybrid with center-based cardiac rehabilitation in heart failure: a systematic review and meta-analysis. J Am Heart Assoc 2019;8:e012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vest AR, Chan M, Deswal A, et al. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail 2019;25:380–400. [DOI] [PubMed] [Google Scholar]
- 53.Bellumkonda L, Tyrrell D, Hummel SL, Goldstein DR. Pathophysiology of heart failure and frailty: a common inflammatory origin? Aging Cell 2017;16:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veronese N, Stubbs B, Punzi L, et al. Effect of nutritional supplementations on physical performance and muscle strength parameters in older people: a systematic review and meta-analysis. Ageing Res Rev 2019;51:48–54. [DOI] [PubMed] [Google Scholar]
- 55.Bonilla-Palomas JL, Gamez-Lopez AL, Castillo-Dominguez JC, et al. Nutritional intervention in malnourished hospitalized patients with heart failure. Arch Med Res 2016;47:535–40. [DOI] [PubMed] [Google Scholar]
- 56.Hummel SL, Karmally W, Gillespie BW, et al. Home-delivered meals postdischarge from heart failure hospitalization. Circ Heart Fail 2018;11:e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaudhry SI, McAvay G, Chen S, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure findings from the Cardiovascular Health Study. J Am Coll Cardiol 2013;61:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang R, Morris NR, Mandrusiak A, et al. Timed Up and Go Test: a reliable and valid test in patients with chronic heart failure. J Card Fail 2016;22:646–50. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Pascual C, Paredes-Galan E, Ferrero-Martinez AI, et al. The frailty syndrome is associated with adverse health outcomes in very old patients with stable heart failure: a prospective study in six Spanish hospitals. Int J Cardiol 2017;236:296–303. [DOI] [PubMed] [Google Scholar]
- 61.Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol 2017;106:533–41. [DOI] [PubMed] [Google Scholar]