Abstract
Introduction
Resource-constrained countries (RCCs) have the highest burden of cervical cancer (CC) in the world. Nonetheless, although CC can be prevented through screening for precancerous lesions, only a small proportion of women utilise screening services in RCCs. The objective of this study was to examine the magnitude of inequalities of women’s knowledge and utilisation of cervical cancer screening (CCS) services in RCCs.
Methods
A total of 1,802,413 sample observations from 18 RCC’s latest national-level Demographic and Health Surveys (2008 to 2017–18) were analysed to assess wealth-related inequalities in terms of women’s knowledge and utilisation of CCS services. Regression-based decomposition analyses were applied in order to compute the contribution to the inequality disparities of the explanatory variables for women’s knowledge and utilisation of CCS services.
Results
Overall, approximately 37% of women had knowledge regarding CCS services, of which, 25% belonged to the poorest quintile and approximately 49% from the richest. Twenty-nine percent of women utilised CCS services, ranging from 11% in Tajikistan, 15% in Cote d’Ivoire, 17% in Tanzania, 19% in Zimbabwe and 20% in Kenya to 96% in Colombia. Decomposition analyses determined that factors that reduced inequalities in women’s knowledge of CCS services were male-headed households (− 2.24%; 95% CI: − 3.10%, − 1.59%; P < 0.01), currently experiencing amenorrhea (− 1.37%; 95% CI: − 2.37%, − 1.05%; P < 0.05), having no problems accessing medical assistance (− 10.00%; 95% CI: − 12.65%, − 4.89%; P < 0.05), being insured (− 6.94%; 95% CI: − 9.58%, − 4.29%; P < 0.01) and having an urban place of residence (− 9.76%; 95% CI: − 12.59%, − 5.69%; P < 0.01). Similarly, factors that diminished inequality in the utilisation of CCS services were being married (− 8.23%;95% CI: − 12.46%, − 5.80%; P < 0.01), being unemployed (− 14.16%; 95% CI: − 19.23%, − 8.47%; P < 0.01) and living in urban communities (− 9.76%; 95% CI: − 15.62%, − 5.80%; P < 0.01).
Conclusions
Women’s knowledge and utilisation of CCS services in RCCs are unequally distributed. Significant inequalities were identified among socioeconomically deprived women in the majority of countries. There is an urgent need for culturally appropriate community-based awareness and access programs to improve the uptake of CCS services in RCCs.
Keywords: Cervical cancer screening services, Decomposition analyses, Resource-constrained countries, Knowledge, Utilisation
Background
Cervical cancer (CC) is the fourth leading cancer in women worldwide (570,000 new cases, accounting for 6.6% of all female cancers in 2018) and the eighth-most important cancer overall (contributing 3.3% of the total number of new cases diagnosed in 2018) [1, 2]. In 2018, there were 311,365 estimated deaths from CC worldwide, accounting for 7.5% of all cancer deaths in females, with approximately 90% of deaths occurring in resource-constrained countries (RCCs) [1–4], particularly African ones [5–7]. Infection with the human papillomavirus (HPV) is a key cause of CC and is associated with other anogenital (vulvar, vaginal, penile and anal) cancers [8, 9] as well as head and neck cancers [10]. The burden of CC is a growing public health concern in terms of high incidence and mortality rates worldwide, especially in RCCs [11]. However, the incidence and burden of CC have been drastically reduced in high-income countries in recent decades owing to women accessing CC screening services regularly [12]. Unfortunately, CC still remains the most common cancer among women in RCCs with high rates of associated mortality [12–15].
The high burden of CC in terms of incidence and mortality rates across the world could be decreased (by between one-third and one-half, respectively) with comprehensive primary prevention programs that incorporate early vaccination, early diagnosis, effective screening, adequate referral, and advanced treatment procedures [16–19]. Prevention mechanisms have become more pronounced in the developed world over the past couple of decades, and CC incidence rates have fallen there, largely because of primary prevention programs [19]. During the same period, however, rates in most developing countries have risen or remained unchanged, often based on limited access to health services, lack of awareness and absence of screening and treatment programmes [17, 20–24]. However, screening services are generally the most acceptable prevention strategy, detecting precancerous changes before they progress to the invasive cancer stage [25]. There is significant variation in women’s knowledge about CC and screening services available across countries. Whereas in high-income countries, knowledge about CC and related national screening programs have played a significant role in reducing the burden [22], the level of women’s knowledge about CC and related screening services remains a considerable challenge in RCCs.
The relationship between health care utilisation and socio-demographic characteristics has been widely addressed in the literature [26–30]. Low levels of knowledge and uptake of CC screening services are linked to low socio-economic status (SES) [23, 27, 31], poverty and poor economic development at the country level, and inadequate health services, such as limited health facilities, unaffordability of services, poor quality of cytology services and lack of culturally appropriate and acceptable screening methods [3, 8, 20–22, 26, 32–35].
The utilisation of CC screening services and the burden of CC is disproportionately distributed among poor women globally [36]. For example, just 19% of women utilised CC screening services in RCCs, whereas this figure was over 60% in high-income countries [22]. Further, the utilisation of CC screening services in RCCs ranged from 1.1% in Bangladesh to 57.6% in the Republic of Congo [37]. Substantial heterogeneity in the utilisation of CC screening services was also observed [36], with women from low and middle socioeconomic households receiving 43 and 33% less cervical cancer screening services, respectively than their wealthiest counterparts [37]. Although it is well-established that having an appropriate level of knowledge, awareness, vaccination and regular screening are the most effective ways for preventing CC [28, 35, 38, 39], few studies have attempted to assess the impact of wealth-related inequalities on women’s knowledge of CC and the uptake of CC screening in the context of RCCs.
Theoretical foundation
This work adopted the socio-ecological model (SEM) as the basis to explain individuals’ health behaviours [40, 41], such as women’s knowledge as well as CC screening participation, within the context of their environments. The SEM facilitates the exploration of the ecological niche (intrapersonal, interpersonal, organisational, community and policy levels) [42]. This framework is important because it allows for the investigation of all salient factors that are essential in policy formulation, which seeks to improve the knowledge and utilisation of CC screening services. At both the individual and micro levels factors like demographic characteristics and SES are considered. The interpersonal level factors include family influences, such as satisfaction in relationships and social support (e.g., support from a spouse and other family members along with relationship power and equity (gender equity)). In terms of the community-level factors, cultural and gender norms, and for the institutional/health systems, factors such as confidence in health care service providers, health insurance coverage and access to health facilities, are considered. Regional wealth inequalities, place of residence and ethnicity are categorised under structural factors, which are driven by the prevailing socio-cultural systems within a country. Even though different levels were distinguished, they were highly interactive because the structural factors function only with the cooperation of individual, interpersonal and institutional factors [43].
Methods
The aim of the study
The aim of this study was to examine the inequalities of women’s knowledge and utilisation of CC screening services in 18 RCCs. The point of departure of this study was to hypothesis that knowledge and screening practices of CC among women in RCCs are intricately linked to wealth. This study is the first of its kind that examines the impact of wealth on inequalities of CC screening knowledge and screening in economically poor countries. To achieve the research objective, the following three research questions (RQ) were posited:
RQ 1: What is the level of women’s knowledge about CC services and the level of utilisation in RCCs?
RQ 2: What are the potential factors associated with increased women’s knowledge of CC screening services and their utilisation?
RQ 3: What is the magnitude of wealth inequalities in terms of women’s knowledge about CC screening services and utilisation of screening services in RCCs?
Study design and settings
This study used data from the Demographic and Health Survey (DHS) conducted across the selected RCCs. As per the study objective(s), only the latest DHS conducted in 18 RCCs were utilised [44]. The DHS is a long-standing worldwide cross-sectional household survey performed in 90 developing countries [44]. Data collection is standardised but the explored health issues vary by country. Hence, data on CC are only available for 18 RCCs. Data captured by the DHS include information on various health indicators related to maternal and child health, maternal and child mortality, fertility, family planning, nutrition, and knowledge and awareness of health, health services and health care utilisation but they vary across countries based on important local health issues. The present study was restricted in 18 resource-constrained countries (RCCs), hence, data on cervical cancer-related information are only available in these countries (Fig. 1). The DHS program collects information on knowledge, awareness and utilisation of CC screening among women from 18 resource-constrained countries only (Fig. 1): Albania (2017–18), Bolivia (2008), Burkina Faso (2010), Colombia (2015–16), Cote d’Ivoire (2011–12), Dominican Republic (2013), Egypt (2015), Equatorial Guinea (2014–15), Honduras (2011–12), India (2015–16), Jordan (2012), Kenya (2014), Lesotho (2014), Namibia (2013), Philippines (2013), Tajikistan (2012), Tanzania (2011–12) and Zimbabwe (2015) (Fig. 1) [44].
Fig. 1.
Mapping of the study settings across geographical distribution
The study adopted the World Bank’s definition of resource-constrained countries (RCC), a term used to refer to all countries economically classified as low- or middle-income [45]. The RCCs are typically attributed by a lack of funds to cover health care costs, on individual or societal perspectives, which leads to limited accessibility, affroadibility, accountability and availability of healthcare services in terms of limited infrastructure, poor health systems and delivery mechanisms, and trained personnel [46–48]. Indeed, for weak health care systems, it is plausible that effects beyond women cancer may be realised and may extend to cancer more generally or to women’s health. In addition, LRCs often lack the necessary infrastructure to ensure high-quality cancer screening services and subsequent follow-up care [48]. For example, RCCs often do not have the necessary infrastructure required for ensuring high-quality cancer screening services and associated follow-up care; which in turn may be compromised by the lack of a consistent supply of both electricity, x-ray films, and technicians (engineers, technicians, and radiologists) [46].
Sampling
A stratified two-stage cluster sampling is used in the most DHS surveys [49]. In the first stage, primary sampling units (PSUs) are selected from the main DHS sampling framework with probability proportional to a size measure; in the second stage, a fixed number of households (or residential dwellings) are selected from a list of households obtained in an updating operation in the selected PSUs using systematic random sampling. A PSU is usually a geographically constructed area, or a part of an area, called an enumeration area (EA), containing a number of households, created from the most recent population census. For simplicity, the DHS surveys captures two-stage surveys: the first stage is a systematic sampling with probability proportional to the EA size; the second stage is a systematic sampling of equal probability and fixed size across the EAs. This sampling procedure is usually more precise than simple random sampling at both stages. The detailed sample size calculation procedures are reported elsewhere [49], which depends on a function of the cost ratio and the intracluster correlation.
| 1 |
| 2 |
| 3 |
where, nopt is the number of required sample, C is the total cost of the survey, c1 is the unit cost per PSU for household lising and interview, c2 is the unit cost per individual interview, n is the total number of PSUs to be selected, m is the number of individuals to be selected in each PSU, and ρ is the intracluster correlation.
Data collection procedure
In this study, data from each country are nationally representative of each country’s eligible population. Eligible survey participants were surveyed through face-to-face interviews by a trained surveyor using the DHS model questionnaires. Data were collected by Measure DHS retrospectively using quantitative structural questionnaires which covered information on socio-demographic, reproductive health, access to services, and use of health services. Trained interviewers collected data via face-to-face interviews. All the data were collected at both household and individual levels of women still considered as reproductive (aged 15 to 49 years). The DHS dataset is publicly available; however, mailed consent was also taken as part of the Measure DHS protocol. Study participants were generated from the DHS as per the DHS protocol. Detailed information regarding survey sampling, quality control, management, and survey instruments are reported elsewhere [44]. Women were requested to provide information about CC screening knowledge along with awareness and utilisation of screening services. Written informed consent was taken from the respondents prior to conducting the survey. Rigorous data management was performed (e.g., data validity, reliability, quality control). This analysis considered the latest survey conducted by selected countries, and the data collection period was between 2008 and 2018. The survey response rate varied between 85 and 95%. The data set is publicly accessible after obtaining approval, which was received from the Measure DHS program.
A sample was drawn from the DHS database from each of the selected RCCs. After exclusion of non-responders and participants with missing data and unusual observations, data on 1,802,413 reproductive women living in these countries were included in the analysis (Table 1). India had the highest proportion of participants, followed by Burkina Faso and the Philippines. The average age ± Standard Deviation (SD) of the participants was 35.88 years (± 7.91 SD).
Table 1.
Distribution of study population
| Country | Type of survey | Survey years | Observation (N) |
|---|---|---|---|
| Albania | Standard DHS | 2017–18 | 15,306 |
| Bolivia | Standard DHS | 2008 | 40,479 |
| Burkina Faso | Standard DHS | 2010 | 112,661 |
| Colombia | Standard DHS | 2015–16 | 11,804 |
| Cote d’Ivoire | Standard DHS | 2011–12 | 26,939 |
| Dominican Republic | Standard DHS | 2013 | 17,480 |
| Egypt | Standard DHS | 2014 | 9209 |
| Equatorial Guinea | Standard DHS | 2014–15 | 2561 |
| Honduras | Standard DHS | 2011–12 | 46,592 |
| India | Standard DHS | 2015–16 | 1,289,652 |
| Jordan | Standard DHS | 2012 | 40,386 |
| Kenya | Standard DHS | 2014 | 36,540 |
| Lesotho | Standard DHS | 2014 | 11,575 |
| Namibia | Standard DHS | 2013 | 16,953 |
| Philippines | Standard DHS | 2013 | 71,280 |
| Tajikistan | Standard DHS | 2012 | 20,449 |
| Tanzania | Standard DHS | 2011–12 | 10,869 |
| Zimbabwe | Standard DHS | 2015 | 21,677 |
| Total | 2008–2018 | 1,801,987 |
Outcome variables
This study considered two outcome variables, namely ‘women’s knowledge and ‘utilisation of cervical cancer screening (CCS) services’. Participants were asked knowledge-specific questions related to CC screening services [50]. More specifically, questions such as ‘have you ever heard of a pap test’, ‘Do you know what a pap test is for?’, ‘Do you know what vaginal cytology is?’, ‘Have you ever heard of vaginal cytology?’, ‘How did you learn about vaginal cytology?’, ‘In the last 12 months, have you received educational information about cervical cancer screening?’ were asked to gather knowledge-related information on CC screening. The overall women’s knowledge surrounding CC screening services was measured as a dichotomous response (1 = ‘yes’ if the participant reported any positive response about CC screening services or 0 = ‘no’ otherwise). Further, participants were asked questions related to their CC screening service utilisation; for instance, questions associated with having a pap test, gynecologic examination or vaginal cytology examination, all of which depend on available services across countries [50]. Self-reported responses for CCS screening were considered and then categorised as ‘yes’ if the participant utilised any form of CCS or otherwise ‘no’ to measure the utilisation of CCS services.
Explanatory variables
Explanatory variables were selected based on the socio-ecological model for the women’s knowledge and utilisation of CCS services [40, 41], and these data were examined for potential confounders [42]. Participants’ characteristics, which included age, education, sex of the household head and age at the time of respondent’s first childbirth, were considered as the predisposing factors in the analysis. Age was grouped as follows: < 26 years, 26–35 years, 36–45 years or ≥ 46 years. Educational background was defined as no education, primary education, secondary education or higher education. Household size was classified as < 5 members, 5–7 members, and more than 8 members. Media exposure was assessed by means of access to radio and/or television, whereas health insurance coverage and wealth status were considered mediator factors. Women’s history of breastfeeding, having amenorrhea, abstaining, currently working, access to mass media exposure and having health insurance coverage were dichotomous variables (‘yes’ if present or ‘no’ otherwise). Access to medical help for the self was categorised into three groups (1 = no problem, 2 = some problem, 3 = extreme problem). SES was based on the ownership of durable assets [40]. This method has been used in previous studies employing DHS data from developing countries [39, 41, 42]. Each household’s characteristics (assets) were dichotomised (‘yes’ if present and ‘no’ if not) [51]. Country-specific principal components analysis (PCA) was performed using ownership of durable assets [40]. Weights were estimated by factor scores derived from the first principal component in the PCA. The constructed wealth index values were then assigned to individuals based on accessible variables. The wealth index was divided into five strata: poorest (Q1: lowest 20%), poorer (Q2), middle (Q3), richer (Q4) and richest (Q5: top 20%) [52, 53]. Location of residence was dichotomised as either urban or rural [52, 53].
Estimation strategies
Measuring and decomposing wealth-related inequalities
For the inequality analysis, comparisons of knowledge CC screening and utilisation of services were performed across wealth quintiles over the period specified. The standard measures of concentration index (Conc.I) were employed to examine the magnitude of household wealth-related inequality and the trends in CC screening knowledge and utilisation of services across 18 RCCs. The Conc. I was estimated as the covariance between knowledge and utilisation of CC screening services and the proportional rank in wealth score distribution [39] as follows:
where Conc. I is the concentration index, is the mean of knowledge and utilisation of CC screening services, ri is the cumulative proportion that each individual represents over the total population once the distribution of wealth score has ranked the latter. The values of Conc. I are bounded between and ; when y is dichotomous [41]. Conc. I acquires a negative value when the curve lies above the line of equality, which indicates a disproportionately lower prevalence of CC screening knowledge and utilisation of services among the poor (i.e., pro-poor). A positive value of Conc. I signifies a higher concentration of health indicators among the rich (i.e., pro-rich). There is no socioeconomic inequality in the distribution of CC screening knowledge and utilisation of services (y) when the value of Conc. I is zero and the concentration curve coincides with the 45° line. The dichotomous character of the knowledge and utilisation of CC screening services may result in unstable bounds in response to varying means; therefore, the normalised standard index was estimated to check the robustness of the estimation [42, 43]. In addition, when the outcome variable is dichotomous, the Conc. I has to be corrected in order to allow comparisons between groups of individuals from different time periods that may show different levels of use of health services [45]. In the context of a dichotomous outcome variable, the Erreygers’s Conc. I is the Conc. I multiplied by four times the mean health or outcome of interest [45]. Erreygers’ suggested corrected CI can be expressed as:
where ymax and ymin are the boundary of y (knowledge and utilisation of CC screening services). When the Erreygers’ corrected index is used, the decomposition of inequality is generally expressed as:
This estimate produces an index that satisfies various attractive axiomatic properties for an inequality index, including the sign condition, scale invariance and mirror properties [46, 47]. The adjusted Conc. I method allows for an examination of the causes of (and their corresponding contributions to) and levels of changes in inequalities in terms of knowledge and utilisation of CC screening services [40]. In addition, multiple logistic regression was applied to measure the likelihood of CC screening knowledge, awareness and utilisation of services. Adjusted odds ratios (AORs) with a 95% confidence interval (CI) were estimated for identifying influencing factors on CC screening knowledge and utilisation of services at a 5% or lower level of significance. All the estimates were considered by sampling weights according to the DHS guideline. According to the DHS guideline, sample weights are estimated to six decimals but are presented in the standard recode files without the decimal point. They need to be divided by 1,000,000 before use to approximate the number of cases. As part of complex sample parameters when standard errors, confidence intervals or significance testing is required for the indicator [54]. All statistical analyses were performed with Stata/SE-13 software (StataCorp, College Station, TX, USA).
Results
Background characteristics of study participants
Table 2 features the background characteristics of the study participants. Nearly 75.31% of total participants belonged to the 26–45-year age group. Approximately 41.05% of all participants had no formal education, whereas 31.15% of participants had completed secondary education, followed by primary education (21.79%). A wide gap existed in who led the household, with nearly 85.17% of households being male–headed, and two–thirds of the women’s family consisting of five or more members. A sizeable number of women had no current exposure to breastfeeding (78.97%), amenorrhea (91.70%) and abstaining (93.23%). Approximately half of the women were employed, with 60.06% of women’s households having access to mass media communications. Further, 74.23% of participants had reported moderate or extreme problems in accessing medical care from health centres or other sources. Overall, just 20.25% of the households had health insurance coverage. Approximately 66.70% of households lived in a rural community, with roughly 44.44% of women having a low SES.
Table 2.
Association of women’s knowledge of cervical cancer and utilisation of cervical cancer screening across participants characteristics
| Characteristics | Number of observation | Women’s knowledge of cervical cancer | Utilisation of cervical cancer screening services | ||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | 95% CI | P-value1 | n (%) | 95% CI | P-value1 | |
| Age group | |||||||
| < 26 years | 203,472 (11.75) | 63,232 (31.08) | (30.88–31.28) | 47,559 (26.02) | (25.82–26.22) | ||
| 26–35 years | 629,486 (36.36) | 217,696 (34.58) | (34.47–34.70) | < 0.001 | 175,494 (29.62) | (29.50–29.74) | < 0.001 |
| 36–45 years | 674,297 (38.95) | 241,054 (35.75) | (35.63–35.86) | 199,553 (31.06) | (30.95–31.17) | ||
| 46 or more | 223,878 (12.93) | 79,957 (35.71) | (35.52–35.91) | 67,803 (32.00) | (31.80–32.20) | ||
| Educational level | |||||||
| no education | 752,174 (41.05) | 203,970 (26.74) | (26.81–27.01) | 148,425 (22.19) | (22.09–22.29) | ||
| primary | 399,246 (21.79) | 140,437 (39.40) | (39.24–39.56) | < 0.001 | 116,728 (35.72) | (35.55–35.88) | < 0.001 |
| secondary | 570,867 (31.15) | 208,976 (39.93) | (39.80–40.06) | 182,532 (34.21) | (34.08–34.33) | ||
| higher | 110,019 (6.00) | 45,628 (47.35) | (47.05–47.65) | 42,724 (42.64) | (42.33–42.94) | ||
| Household head | |||||||
| male | 1,527,282 (85.17) | 558,890 (36.70) | (36.62–36.77) | < 0.001 | 414,864 (28.81) | (28.73–28.88) | < 0.001 |
| female | 265,922 (14.83) | 104,376 (39.75) | (39.56–39.94) | 79,670 (31.34) | (31.16–31.52) | ||
| Household size | |||||||
| < 5 members | 534,170 (29.64) | 189,376 (38.17) | (38.04–38.30) | 16,4587 (32.61) | (32.48–32.74) | ||
| 5–7 members | 858,427 (47.63) | 318,362 (36.53) | (36.43–36.63) | < 0.001 | 239,802 (29.20) | (29.10–29.30) | < 0.001 |
| ≥ 8 members | 409,815 (22.74) | 156,481 (36.45) | (36.30–36.59) | 90,147 (24.46) | (24.32–24.60) | ||
| Currently breastfeeding | |||||||
| no | 1,341,835 (78.97) | 463,050 (35.19) | (35.11–35.27) | < 0.001 | 403,083 (31.13) | (31.05–31.21) | < 0.001 |
| yes | 357,415 (21.03) | 119,168 (31.63) | (31.47–31.78) | 77,186 (23.82) | (23.67–23.96) | ||
| Currently amenorrheic | |||||||
| no | 1,569,088 (91.70) | 539,226 (34.69) | (34.61–34.76) | 460,973 (30.57) | (30.49–30.64) | < 0.001 | |
| yes | 141,967 (8.30) | 51,881 (35.14) | (34.89–35.39) | 0.312 | 29,434 (24.23) | (23.99–24.47) | |
| Currently abstaining | |||||||
| no | 1,595,179 (93.23) | 553,764 (34.71) | (34.64–34.79) | < 0.001 | 465,436 (30.40) | (30.32–30.47) | < 0.001 |
| yes | 115,876 (6.77) | 40,415 (34.88) | (34.60–35.15) | 24,971 (25.40) | (25.13–25.67) | ||
| Marital status | |||||||
| married | 1,529,410 (88.82) | 517,451 (33.83) | (33.76–33.91) | < 0.001 | 408,194 (28.19) | (45.00–45.46) | < 0.001 |
| others | 192,514 (11.18) | 83,854 (43.56) | (43.34–43.78) | 82,213 (45.23) | (28.12–28.27) | ||
| Employment status | |||||||
| no | 333,605 (51.07) | 149,712 (44.88) | (44.71–45.05) | < 0.001 | 110,798 (36.11) | (35.94–36.28) | 0.174 |
| yes | 319,666 (48.93) | 165,495 (51.77) | (51.60–51.94) | 93,590 (36.83) | (36.64–37.01) | ||
| Access to health facility | |||||||
| no problem | 437,869 (25.77) | 136,530 (31.18) | (31.04–31.32) | 148,406 (32.60) | (32.46–32.73) | < 0.001 | |
| some problem | 672,322 (39.57) | 262,319 (39.02) | (38.90–39.13) | < 0.001 | 179,888 (29.45) | (29.33–29.56) | |
| extreme problem | 588,996 (34.66) | 186,316 (31.63) | (31.51–31.75) | 151,967 (27.49) | (27.37–27.61) | ||
| Health insurance coverage | |||||||
| no | 1,372,070 (79.75) | 467,290 (34.06) | (33.98–34.14) | < 0.001 | 359,259 (27.86) | (27.78–27.94) | < 0.001 |
| yes | 348,436 (20.25) | 147,864 (42.44) | (42.27–42.60) | 114,053 (33.28) | (33.12–33.44) | ||
| Mass media exposure | |||||||
| no | 704,804 (39.94) | 214,934 (30.50) | (30.39–30.60) | < 0.001 | 120,414 (19.33) | (19.24–19.43) | < 0.001 |
| yes | 1,059,673 (60.06) | 439,673 (41.49) | (41.40–41.59) | 363,979 (35.13) | (35.04–35.23) | ||
| Wealth Index | |||||||
| poorest (Q1) | 410,984 (22.92) | 104,745 (25.49) | (25.35–25.62) | < 0.001 | 70,699 (18.76) | (18.64–18.89) | |
| poorer (Q2) | 385,984 (21.52) | 123,850 (32.09) | (31.94–32.23) | 88,121 (24.39) | (24.25–24.53) | < 0.001 | |
| middle (Q3) | 364,113 (20.31) | 139,510 (38.32) | (38.16–38.47) | 104,965 (30.52) | (30.36–30.67) | ||
| richer (Q4) | 341,052 (19.02) | 149,721 (43.90) | (43.73–44.07) | 114,233 (35.07) | (34.90–35.23) | ||
| richest (Q5) | 299,854 (16.64) | 148,328 (48.68) | (44.52–55.29) | 116,516 (40.19) | (35.16–42.92) | ||
| Place of residence | |||||||
| urban | 600,094 (33.30) | 271,061 (45.17) | (45.04–45.30) | < 0.001 | 206,979 (36.11) | (35.99–36.23) | < 0.001 |
| rural | 1,201,893 (66.70) | 395,695 (32.92) | (32.84–33.01) | 287,558 (25.65) | (25.56–25.73) | ||
| Total | 1,801,987 (100.00) | 666,789 (36.99) | (36.92–37.06) | 494,537 (29.19) | (29.12–29.25) | ||
1P-values were derived using chi-square test, CI confidence interval
Distribution of participants’ knowledge and utilisation of CCS services (for RQ 1)
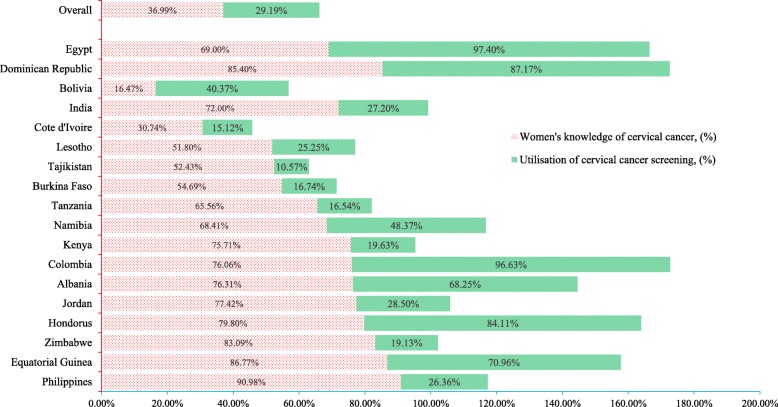
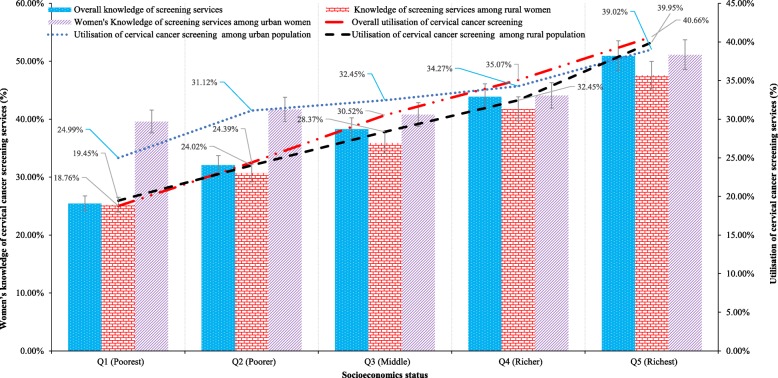
Approximately 37% of women had adequate knowledge about CCS services, with 29.19% of women having utilised the services, ranging from 10.57% in Tajikistan to 96.63% in Colombia (Fig. 2). The participants’ characteristics included in the analyses are predisposing factors (e.g., age, educational background, sex of household head, number of family members, marital status), enabling factors (e.g., employment status, access to medical help, health insurance coverage, access to mass media communications), and community and economic factors, all of which were significantly associated to women’s knowledge of screening services and utilisation (Table 2). Overall, women’s knowledge about screening services were significantly increased (P < 0.001) with a higher level of education (i.e., 26.74% for no formal education, 39.40% for primary school, 39.93% for secondary school and 47.35% for higher education). Female-headed households (39.75%) had slightly more knowledge about cervical cancer than male-headed ones (36.70%). Further, 42.44% of women’s households were insured, and 41.49% exposed to mass media had knowledge of CCS. Participants living in urban settings (45.17%) had more knowledge of screening services than rural women (32.92%). In addition, women’s level of knowledge and utilisation of CCS services were disproportionately greater for those of higher SES. For example, only 24.99% of the poorest women had knowledge of CC, whereas 39.95% of women in the richest strata did (Fig. 3). Similarly, 18.76% of the poorest women utilised CCS services, whereas 40.19% of women utilised in the richest quintile.
Fig. 2.
Distribution of women’s knowledge and utilisation of cervical cancer screening services across countries
Fig. 3.
Unequal distribution of women’s knowledge surrounding cervical cancer (CC) screening services and utilisation of CC screening services by socioeconomic status
The magnitude of inequality in terms of utilisation of CCS services (rich-poor ratio, RPR = 2.14 times, concentration index, Conc. I = 0.298) were disproportionately concentrated among the most socioeconomic advantaged households (Table 3). A similar distribution was found across participants’ characteristics for utilising screening services in RCCs. Compared to the existing study’s findings that conducted in high income countries, the magnitude of inequality in terms of utilisation of CCS services were disproportionately also concentrated among women in the most socioeconomic advantaged households, including Austria (RPR = 1.34 times, Conc. I = 0.315), Australia (RPR = 1.23 times, Conc. I = 0.126), Brazil (RPR = 1.60 times, Conc. I = 0.296), France (RPR = 1.88, Conc. I = 0.269), Greece (RPR = 1.99 times, Conc. I = 0.269), Hungary (RPR = 2.03 times, Conc. I = 0.285), Ireland (RPR = 2.29 times, Conc. I = 0.220), Luxembourg (RPR = 1.12 times, Conc. I = 0.243) and UK (RPR = 1.12, Conc. I = 0.069).
Table 3.
Comparison of the utilisation of CCS services between the present study findings (RCCs) and previous studies in HICs
| Countries | Utilisation of CCS services, % | Degree of inequalities | Sources | ||||
|---|---|---|---|---|---|---|---|
| Poorest (Q1) | Richest (Q5) | RPD | RPR | (Q5-Q1) / Q5 | Conc.I | ||
| Present study findings (18 RCCs) | 18.76 | 40.19 | 21.43 | 2.14 | 0.53 | 0.298 | Present study |
| Austria | 68.40 | 91.80 | 23.40 | 1.34 | 0.25 | 0.315 | [36] |
| Australia | 50.40 | 62.10 | 11.70 | 1.23 | 0.19 | 0.126 | [55] |
| Brazil | 55.40 | 88.40 | 33.00 | 1.60 | 0.37 | 0.296 | [36] |
| Denmark | 45.00 | 78.00 | 33.00 | 1.73 | 0.42 | 0.098 | [56] |
| France | 42.50 | 80.10 | 37.60 | 1.88 | 0.47 | 0.269 | [36] |
| Finland | 49.90 | 74.10 | 24.20 | 1.48 | 0.33 | 0.122 | [36] |
| Germany | 68.30 | 80.50 | 12.20 | 1.18 | 0.15 | 0.139 | [36] |
| Greece | 31.40 | 62.40 | 31.00 | 1.99 | 0.50 | 0.266 | [36] |
| Hungary | 38.60 | 78.30 | 39.70 | 2.03 | 0.51 | 0.285 | [36] |
| Italy | 72.10 | 69.90 | −2.20 | 0.97 | −0.03 | 0.061 | [36] |
| Ireland | 19.90 | 45.50 | 25.60 | 2.29 | 0.56 | 0.220 | [36] |
| Luxembourg | 79.20 | 88.90 | 9.70 | 1.12 | 0.11 | 0.243 | [36] |
| Mexico | 57.30 | 69.60 | 12.30 | 1.21 | 0.18 | 0.119 | [36] |
| Netherlands | 46.40 | 65.90 | 19.50 | 1.42 | 0.30 | 0.151 | [36] |
| Paraguay | 32.40 | 71.00 | 38.60 | 2.19 | 0.54 | 0.314 | [36] |
| Portugal | 18.70 | 71.30 | 52.60 | 3.81 | 0.74 | 0.318 | [36] |
| Russia | 60.50 | 77.00 | 16.50 | 1.27 | 0.21 | 0.142 | [36] |
| Spain | 44.00 | 72.30 | 28.30 | 1.64 | 0.39 | 0.207 | [36] |
| Slovenia | 62.50 | 78.40 | 15.90 | 1.25 | 0.20 | 0.273 | [36] |
| Sweden | 62.50 | 75.80 | 13.30 | 1.21 | 0.18 | 0.177 | [36] |
| Slovakia | 47.00 | 70.10 | 23.10 | 1.49 | 0.33 | 0.208 | [36] |
| Uruguay | 48.10 | 75.50 | 27.40 | 1.57 | 0.36 | 0.246 | [36] |
| UK | 56.80 | 63.40 | 6.60 | 1.12 | 0.10 | 0.069 | [36] |
Note: CCS cervical cancer screening, RCCs resource-constrained countries, HICs high-income countries, Q1 poorest socio-economic status, Q5 richest socio-economic status, RPR rich-poor ratio (Q5/Q1), RPD rich-poor difference (Q5-Q1), Conc. I Concentration Index
Factors influencing women’s knowledge and utilisation of CC screening services (for RQ 2)
Several factors influence women’s knowledge of screening and utilisation of CC screening services (Table 4). For example, age (OR = 1.03; 95% CI:1.01, 1.05; P < 0.01), year of schooling (OR = 1.08; 95% CI: 1.05, 1.11; P < 0.001), breastfeeding practices (OR = 1.03; 95% CI: 1.01, 1.05; P < 0.05), having amenorrhea (OR = 1.23; 95% CI: 1.19, 1.27; P < 0.01), employed women (OR = 1.39; 95% CI: 1.39, 1.41; P < 0.01), problems accessing medical help (OR = 1.55; 95% CI: 1.52, 1.58; P < 0.01), living in urban locations (OR = 1.13; 95% CI: 1.13, 1.15; P < 0.01) and higher wealth (OR = 1.26; 95% CI: 1.26, 1.27; P < 0.001) had a significant impact on possessing increased knowledge of CC compared with their counterparts. However, women from male-headed households (24.00%, OR = 0.76; 95% CI: 0.74, 0.77; P < 0.05) and having no mass media exposure (16.00%, OR = 0.84; 95% CI: 0.83, 0.85; P < 0.001) had low-level knowledge of screening services. Similarly, a number of factors significantly drove higher rates of utilisation of CCS services, including being married (OR = 2.11; 95% CI: 2.07, 2.15; P < 0.05), insured (OR = 1.58; 95% CI: 1.55, 1.61; P < 0.01) and being a woman in the richest households (OR = 2.00; 95% CI: 1.09, 2.11; P < 0.01). Participants’ with a current practice of abstaining (OR = 0.79; 95% CI: 0.76, 0.82; P < 0.01), not having access to mass media communications (OR = 0.43; 95% CI: 0.39, 0.47; P < 0.01) and living in a rural community (OR = 0.91; 95% CI: 0.89, 0.93; P < 0.01) were less likely to utilise CCSs.
Table 4.
Inequality decompositions of the Erreygers’s concentration index for women’s knowledge of cervical cancer and utilisation of cervical cancer screening
| Variables |
1OR (95% CI) |
Elast. | Erreygers’sConc.I | RC to the Erreygers’s Conc.I, % (95% CI) |
|---|---|---|---|---|
| Knowledge of cervical cancer | ||||
| Age (years) | 1.03** (1.01, 1.05) | 0.49 | 0.71 | 25.62*** (10.12, 30.59) |
| Schooling (years) | 1.08* (1.05, 1.11) | 0.32 | 0.36 | 15.07*** (10.26, 19.57) |
| Household head (= male) | 0.76*** (0.74, 0.77) | − 0.24 | 0.01 | −2.24*** (−3.10, −1.59) |
| Household size | 0.99 (0.99, 1.00) | − 0.01 | − 0.25 | 8.59 (− 2.36, 25.65) |
| Currently breastfeeding (= yes) | 1.03*** (1.01, 1.05) | 0.02 | 0.11 | 6.51 (− 0.25, 12.69) |
| Currently amenorrhea (= yes) | 1.23*** (1.19, 1.27) | 0.02 | − 0.06 | − 1.37*** (− 2.37, − 1.05) |
| Currently abstaining (= yes) | 0.93** (0.90, 0.96) | − 0.01 | − 0.03 | 0.32 (− 0.24, 2.38) |
| Marital status (= married) | 1.02* (1.00, 1.04) | 0.01 | − 0.01 | − 0.08 (− 1.21, 0.09) |
| Currently working status (= yes) | 1.39*** (1.37, 1.41) | 0.15 | 0.06 | 15.21*** (11.23, 59.45) |
| Access to health facility (= yes) | 1.55*** (1.52, 1.58) | 0.33 | −0.24 | − 10.00** (− 12.65, −4.89) |
| Health insurance coverage (= yes) | 0.66*** (0.65, 0.67) | − 0.08 | 0.04 | −6.94*** (− 9.58, − 4.29) |
| Mass media exposure (= no) | 0.84*** (0.83, 0.85) | −0.15 | − 0.26 | 15.26** (5.16, 23.36) |
| Place of residence (= urban) | 1.13*** (1.11, 1.15) | 0.09 | −0.49 | −9.76** (− 12.59, −5.69) |
| Wealth score | 1.26*** (1.25, 1.27) | 0.64 | 0.61 | 24.00* (3.68, 55.23) |
| Total | 80.20** (60.55, 89.65) | |||
| Utilisation of cervical cancer screening | ||||
| Age (years) | 1.03** (1.02, 1.05) | 0.79 | 0.51 | 29.00*** (10.20, 39.51) |
| Schooling (years) | 0.99* (0.98, 0.99) | −0.08 | 0.36 | 17.00** (12.59, 51.16) |
| Household head (= male) | 1.12*** (1.10, 1.15) | 0.12 | 0.01 | 3.33 (−2.36, 6.23) |
| Household size | 0.98* (0.97, 0.98) | −0.11 | −0.25 | 1.29 (−1.20, 2.31) |
| Currently breastfeeding (= yes) | 1.16*** (1.14, 1.19) | −0.03 | − 0.12 | 9.51*** (2.59, 12.59) |
| Currently amenorrhea (= yes) | 0.96** (0.93, 0.99) | −0.01 | − 0.06 | 0.63 (−2.16, 2.13) |
| Currently abstaining (= yes) | 0.79*** (0.76, 0.82) | −0.01 | − 0.03 | 2.04* (0.59, 4.57) |
| Marital status (= married) | 2.11*** (2.07, 2.15) | 0.09 | −0.02 | −8.23*** (−12.46, −5.80) |
| Currently working status (= no) | 0.86*** (0.85, 0.88) | − 0.05 | 0.061 | − 14.16*** (−19.23, − 8.47) |
| Access to health facility (= no) | 0.73*** (0.72, 0.74) | −0.18 | − 0.24 | 11.22* (2.36, 19.49) |
| Health insurance coverage (= yes) | 1.58*** (1.55, 1.61) | 0.07 | 0.04 | 8.08* (1.26, 9.45) |
| Mass media exposure (= no) | 0.43*** (0.39, 0.47) | −0.70 | −0.26 | 6.72** (1.56, 16.81) |
| Place of residence (= urban) | 0.91** (0.89, 0.93) | 0.09 | −0.49 | −9.76*** (−15.62, −6.85) |
| Wealth score | 1.99*** (1.09, 2.11) | 0.14 | 0.62 | 27.00** (14.56, 45.65) |
| Total | 83.69*** (75.85, 95.54) | |||
OR odds ratio, CI confidence interval, Elast elasticity, Conc.I concentration index, RC relative contribution, 1ORs were derived using logit regression model, ***P < 0.001, **P < 0.01, *P < 0.05
Decomposition of women’s knowledge and utilisation of CCS services (for RQ 3)
The results of the analysis comprising the elasticity of odds of knowledge and utilisation of CC screening with respect to each factor are shown in Table 4. Higher elasticity values were determined for women’s age, year of schooling, sex of household head, employment status, access to medical assistance, mass media exposure, and wealth score determinants of women’s knowledge and utilisation of CCS services. The higher values of elasticity signified that these factors had a significant impact on women’s knowledge of screening as well as utilisation of screening services. Negative values for CI of the determining factors for both knowledge and utilisation of CC screening include women currently experiencing amenorrhea, abstaining, experiencing problems in accessing medical assistance and not having mass media exposure. These factors were significantly concentrated on economically disadvantaged households. In addition, this study also found that male-headed households (− 2.24%; 95% CI: − 3.10%, − 1.59%; P < 0.01), currently experiencing amenorrhea (− 1.37%; 95% CI: − 2.37%, − 1.05%; P < 0.05), having no problem accessing medical assistance (− 10.00%; 95% CI: − 12.65%, − 4.89%; P < 0.05), being insured (− 6.94%; 95% CI: − 9.58%, − 4.29%; P < 0.01) and place of residence (− 9.76%; 95% CI: − 12.59%, − 5.69%; P < 0.05) contributed to reducing inequality in women’s knowledge of screening services. Similarly, marital status (− 8.23%; 95% CI: − 12.46%, − 5.80%; P < 0.01), current work status (− 14.16%; 95% CI: − 19.23%, − 8.47%; P < 0.05) and place of residence (− 9.76%; 95% CI: − 15.62%, − 5.80%; P < 0.01) were observed to be significant factors that contributed to reducing inequality in the utilisation of CCS. However, factors that have the most positive contributions to the inequality in knowledge and utilisation of CC services include age (P < 0.01), years of schooling (P < 0.01), currently breastfeeding (P < 0.01), mass media exposure (P < 0.01) and wealth score (P < 0.01).
Discussion
RCCs, over the past couple of decades, have witnessed remarkable progress in population health improvements by introducing a range of health-related interventions. However, socio-economic inequality is still a leading contributor to the lack of access to health promotion activities and utilisation of healthcare services. Our findings in this study suggested that socio-economic inequality was the most dominant predictor driving inequalities in women’s knowledge and utilisation of CCS services in RCCs. This study showed that knowledge of CCS and service utilisation is relatively poor among women, but that this knowledge did, however, vary widely across the relevant countries. The level of knowledge and utilisation of CCS were disproportionately distributed the higher proportion was in the richest wealth quintile at a level that was significantly higher than that of the poorest quintile. The analysis demonstrated a range of characteristics in the trends. Wealth score is one of the main factors that positively contributed to inequality levels in women’s knowledge of screening services. Factors that also contributed significantly to improvements include mass media exposure, current working status and schooling. On the contrary, factors that played a vital role in reducing inequality in women’s knowledge of CC included access to medical help, urban residence and health insurance coverage.
Women from disadvantaged socioeconomic households were found to have suboptimal knowledge of CC and underutilised CC screening services compared to their counterparts from more advantaged households, confirming findings reported by others [22, 23, 29, 36, 37]. Unfortunately, opportunistic CCS services are usually practised in RCCs [4, 21, 30, 57]. This method of provision of screening services is less effective because it primarily targets a small proportion of women who have the chance to come in contact with health care providers either in a health facility or within the community [31, 35, 57]. These opportunistic screening services are not widely available; where they are available, the service is grossly underutilised [20, 30, 31]. However, a wider variation was observed between awareness about CC, knowledge of the disease and utilisation of CC screening services [4, 20, 30, 31, 35, 39, 58, 59]. Community mobilisation, peer-to-peer engagement and organising health systems to track and follow-up with targeted women should play a substantial role part mitigating barriers and ensuring increased utilisation of screening services.
The decomposition analysis also revealed that the disparities in women’s knowledge of CC and utilisation of CCS were demonstrated by socio-economic inequalities via factors such as demographic characteristics (e.g., age, educational attainment, married), access to health facilities, currently breastfeeding, living in an urban community and mass media communications. Certain studies have shown that women living in deprived households with low levels of schooling and with a disproportionately lower level of knowledge experienced the lowest utilisation of screening services [22, 36]. These women have limited knowledge and practice in terms of use/non-use of sanitary pads, access to hygiene facilities, improper personal hygiene, health communication, health services and medical complications [4, 21, 60, 61]. Further, a number of factors were also highly related to these women such as a feeling of embarrassment, perceived pain during screening and unsupportive family members (e.g., husband, mother-in-law) owing to a lack of adequate knowledge of CC [62] and screening services [35, 60, 61]. Although differences in the assessment of knowledge about cancer make comparisons difficult, the level of knowledge about CCS is, however, suboptimal and of concern because women’s knowledge plays a primary role in increasing the rate of screening uptake [28, 63]. To facilitate acceptance of CC screening services, sensitisations should be carried out to increase awareness of the disease and the significance of screening [60]. Further, efforts should be focused on reducing identified barriers (such as fear of testing, outcome and consequences, financial constraints), strengthening health systems’ capacity and making use of female health workers to carry out screening services [60]. To diminish the existing inequalities in women’s knowledge and utilisation of CC screening in RCCs, establishment of the contributors of inequalities along with formulation and implementation of effective policies are necessary. Further, behavioural change through health promotion interventions could be an effective strategy for reducing the disparity of women’s knowledge and utilisation of screening services within poorer households and for women with low levels of schooling. In addition, community awareness and mass media communication (e.g., radios, newspapers) can also increase the utilisation rate of CC screening services [60].
There were several strengths of this study. Firstly, this research attempted to decompose wealth-related inequalities of both women’s knowledge and utilisation of CCS services by considering a number of countries. Secondly, the study reported determinants and decomposed wealth-related inequalities separately for both outcomes. Furthermore, the current study has analysed data on 1,802,413 reproductive women to obtain precise estimates. Hence, it is expected that the main findings of the work are likely to be similar for most other developing countries and therefore can assist policymakers in other nations.
Some limitations of this study also exist. All information collected from the reproductive age women was self-reported, representing an issue in terms of recall and social desirability biases. Recall bias and under-reporting of knowledge and utilisation of CC screening services may affect subsequently inferences. Further, the collected data may be less accurate than medical records that may have slight effect on the precision of the study outcome. Future studies might confirm these results using better quality data. Moreover, the data collection period was different for individual countries. To overcome this limitation, data were pooled from the DHS’ from multiple countries for the analysis, ignoring data collection time and country setting, an approach that may lead to biased estimates and weaken the generalisability of the study findings. Finally, the nature of the cross-sectional study does not allow for exploring the causal inference of knowledge and utilisation of CCS services [52, 53].
Conclusions
In conclusion, the result of the present study revealed that a pro-rich inequality exists in women’s knowledge and utilisation of CCS services in RCCs. Wealth score, followed by mass media exposure, current working status and schooling explained a high proportion of inequality in women’s knowledge of screening services, whereas access to medical assistance, urban residence and health insurance coverage played a role in reducing these issues. As part of the present country-wise national priorities, initiatives should, therefore, be considered to address inequality and service utilisation. This aim will necessitate targeting underprivileged women with specific health interventions, with the object of ensuring accessibility to and affordability of adequate health care services in relation to the protection and promotion of women’s health. In addition, taking into account the societal perspective, policy efforts should be explored that mitigate structural factors, such as unequal wealth distribution, by focusing on effective financial mechanisms, social safety net programs and the creation of employment opportunities, all of which might contribute to reducing inequalities in health outcomes. Thus, to lower the socioeconomic inequalities that prevail in women’s knowledge and utilisation of CCS services in developing countries, interventions should be concentrated on these factors. In addition, an effective policy strategy should be developed through active collaboration among the different health systems along with the social and economic sectors to diminish wealth-related inequalities in women’s knowledge and utilisation of CCS services in low-resource settings.
Acknowledgements
This research paper was completed during the first author’s postdoctorial research work, under the School of Social Sciences and Psychology and Translational Health Research Institute, Western Sydney University, Kingswood Campus, Australia. We would also like to thank the Measure DHS program for providing access to the data used in the research. Finally, we would like to gratefully acknowledge the study’s participants, reviewers and the academic editors of our manuscript.
Abbreviations
- RCCs
Resource-constrained countries
- CC
Cervical cancer
- CCS
Cervical cancer services
- HPV
Human papillomavirus
- SES
Socio-economic status
- SEM
Socio-ecological model
- DHS
Demographic and health survey
- PCA
Principal components analysis
- Conc.I
Concentration index
Authors’ contributions
Conceptualized the study: RAM, KA, JG and AR; Contributed data extraction and analyses: RAM, under the guidance of KA, JG and AR. Result interpretation: RAM and SAK, under the guidance of KA, JG and AR. Prepared the first draft: RAM, SAK, GO, MS, LR, KA, JG and AR. Contributed during the conceptualization and interpretation of results and substantial revision: RAM, SAK, GO, MS, LR, KA, JG and AR. Revised and finalized the final draft manuscript: RAM, SAK, GO, MS, LR, KA, JG and AR. All authors read and approved the final version of the manuscript.
Funding
This study was conducted without any financial support from the external funding body. No funding grant was received.
Availability of data and materials
The DHS data are publicly accessible and were made available to us upon request by Measure DHS (https://dhsprogram.com/data/available-datasets.cfm).
Ethics approval and consent to participate
Ethical approval to conduct the DHS survey was obtained from Measure DHS and the Ethics Committee of ICF Macro (Calverton, MD, USA). The survey followed standardised data collection procedures and received ethics approval from the National Research Ethics Committee (NREC) of the studied countries. According to the DHS research protocol, written informed consent was obtained from all individual participants included in the study before they enrolled in the original survey. We analysed the dataset after receiving approval from the Measure DHS program office.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) Human papillomavirus (HPV) and cervical cancer. 2019. [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I. Global Cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.de Sanjose S, Tsu V. Prevention of cervical and breast cancer mortality in low- and middle-income countries: a window of opportunity. Int J Women's Health. 2019;11:381–386. doi: 10.2147/IJWH.S197115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tapera O, Dreyer G, Kadzatsa W, Nyakabau AM, Stray-Pedersen B, Sjh H. Cervical cancer knowledge, attitudes, beliefs and practices of women aged at least 25 years in Harare, Zimbabwe. BMC Womens Health. 2019;19:1–10. doi: 10.1186/s12905-019-0790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regional Committee for Africa. Cancer of the cervix in the African Region: Current situation and way forward. World Health Organization (WHO); 2011. https://apps.who.int/iris/handle/10665/1684.
- 6.Anorlu RI. Cervical cancer: the sub-Saharan African perspective. Reprod Health Matters. 2008;16:41–49. doi: 10.1016/S0968-8080(08)32415-X. [DOI] [PubMed] [Google Scholar]
- 7.Teteh DK, Dawkins-Moultin L, Robinson C, LaGroon V, Hooker S, Alexander K, et al. Use of community forums to increase knowledge of HPV and cervical cancer in African American communities. J Community Health. 2019;44:492–499. doi: 10.1007/s10900-019-00665-2. [DOI] [PubMed] [Google Scholar]
- 8.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 9.Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449–457. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- 10.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systemic review. Cancer Epidemiol Biomark Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) Cervical cancer. Cancer, Cancer prevention, Early Diagnosis and Screening. 2019. [Google Scholar]
- 12.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 13.AICR. Cervical cancer statistics. American Institute for Cancer Research (AICR). 2019. https://www.wcrf.org/dietandcancer/cancer-trends/cervical-cancer-statistics. Accessed 25 Nov 2019.
- 14.Vorn R, Ryu E, Srun S, Chang S, Suh I, Kim W. Breast and cervical cancer screening for risk assessment in Cambodian women. J Obstet Gynaecol. 2020;40:1–6. [DOI] [PubMed]
- 15.Palència L, Espelt A, Rodríguez-Sanz M, Puigpinós R, Pons-Vigués M, Pasarín MI, et al. Socio-economic inequalities in breast and cervical cancer screening practices in Europe: influence of the type of screening program. Int J Epidemiol. 2010;39:757–765. doi: 10.1093/ije/dyq003. [DOI] [PubMed] [Google Scholar]
- 16.International Agency for Research on Cancer. World Cancer report 2014. Lyon: International Agency for Research on Cancer. 2014. https://shop.iarc.fr/%0Aproducts/world-cancer-report-2014. Accessed 10 Sep 2019.
- 17.Finocchario-Kessler S, Wexler C, Maloba M, Mabachi N, Ndikum-Moffor F, Bukusi E. Cervical cancer prevention and treatment research in Africa: a systematic review from a public health perspective. BMC Womens Health. 2016;16. 10.1186/s12905-016-0306-6. [DOI] [PMC free article] [PubMed]
- 18.Arrossi S, Temin S, Garland S, Eckert LO, Bhatla N, Castellsagué X, et al. Primary prevention of cervical cancer: American Society of Clinical Oncology resource-stratified guideline. J Glob Oncol. 2017;3:611–634. doi: 10.1200/JGO.2016.008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). New WHO guide to prevent and control cervical cancer. Comprehensive cervical cancer control: A guide to essential practice. 2019.
- 20.Udigwe G. Knowledge, attitude and practice of cervical cancer screening (pap smear) among female nurses in Nnewi, south eastern Nigeria. Niger J Clin Pr. 2006;9:40–43. [PubMed] [Google Scholar]
- 21.Black E, Hyslop F, Richmond R. Barriers and facilitators to uptake of cervical cancer screening among women in Uganda: a systematic review. BMC Womens Health. 2019;19:108. doi: 10.1186/s12905-019-0809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5:0863–0868. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kangmennaang J, Onyango EO, Luginaah I, Elliott SJ. The next sub Saharan African epidemic? A case study of the determinants of cervical cancer knowledge and screening in Kenya. Soc Sci Med. 2018;197:203–212. doi: 10.1016/j.socscimed.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 24.The Lancet Editorial Breast cancer in developing countries: The Breast Health Global Initiative’s guidelines on breast health care in low-income and middle-income countries. Lancet Oncol. 2009;10:1077–1085. [Google Scholar]
- 25.World Health Organization (WHO) Screening for cervical cancer. Cancer, Early detection of cancer. 2019. [Google Scholar]
- 26.Tapera O, Kadzatsa W, Nyakabau AM, Mavhu W, Dreyer G, Stray-Pedersen B, et al. Sociodemographic inequities in cervical cancer screening, treatment and care amongst women aged at least 25 years: evidence from surveys in Harare, Zimbabwe. BMC Public Health. 2019;19:1–12. doi: 10.1186/s12889-019-6749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merino-Ventosa M, Urbanos-Garrido RM. Changes in income-related inequalities in cervical cancer screening during the Spanish economic crisis: a decomposition analysis. Int J Equity Health. 2018;17:1–12. doi: 10.1186/s12939-018-0894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitiku I, Tefera F. Knowledge about cervical cancer and associated factors among 15-49 year old women in Dessie town, Northeast Ethiopia. PLoS One. 2016;11:1–10. doi: 10.1371/journal.pone.0163136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Hwang J. Assessment of trends in socioeconomic inequalities in cancer screening services in Korea , 1998–2012. Int J Equity Health. 2016;15:1–11. doi: 10.1186/s12939-016-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chidyaonga-Maseko F, Chirwa ML, Muula AS. Underutilization of cervical cancer prevention services in low and middle income countries: a review of contributing factors. Pan Afr Med J. 2015;21:1–9. doi: 10.11604/pamj.2015.21.231.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okunowo AA, Daramola ES, Soibi-Harry AP, Ezenwankwo FC, Kuku JO, Okunade KS, et al. Women’s knowledge of cervical cancer and uptake of pap smear testing and the factors influencing it in a Nigerian tertiary hospital. J Cancer Res Pract. 2018;5:105–111. [Google Scholar]
- 32.Litaker D, Tomolo A. Association of contextual factors and breast cancer screening: finding new targets to promote early detection. J Women’s Heal. 2007;16:36–45. doi: 10.1089/jwh.2006.0090. [DOI] [PubMed] [Google Scholar]
- 33.Abamecha F, Tena A, Kiros G. Psychographic predictors of intention to use cervical cancer screening services among women attending maternal and child health services in southern Ethiopia: the theory of planned behavior (TPB) perspective. BMC Public Health. 2019;19:1–9. doi: 10.1186/s12889-019-6745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venturelli F, Sampaolo L, Carrozzi G, Zappa M, Giorgi RP. Associations between cervical, breast and colorectal cancer screening uptake, chronic diseases and health-related behaviours: data from the Italian PASSI nationwide surveillance. Prev Med (Baltim) 2018;2019(120):60–70. doi: 10.1016/j.ypmed.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Gichangi P, Estambale B, Bwayo J, Rogo K, Ojwang S, Opiyo A, et al. Knowledge and practice about cervical cancer and pap smear testing among patients at Kenyatta National Hospital, Nairobi. Kenya Int J Gynecol Cancer. 2003;13:827–833. doi: 10.1111/j.1525-1438.2003.13612.x. [DOI] [PubMed] [Google Scholar]
- 36.McKinnon B, Harper S, Moore S. Decomposing income-related inequality in cervical screening in 67 countries. Int J Public Heal. 2011;56:139–152. doi: 10.1007/s00038-010-0224-6. [DOI] [PubMed] [Google Scholar]
- 37.Akinyemiju TF. Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries : analysis of the world health survey. PLoS One. 2012;7:e48834. doi: 10.1371/journal.pone.0048834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pons-Vigués M, Puigpinós-Riera R, Rodríguez-Sanz M, Serral G, Palència L, Borrell C. Preventive control of breast and cervical cancer in immigrant and native women in Spain: the role of country of origin and social class. Int J Health Serv. 2011;41:483–499. doi: 10.2190/HS.41.3.e. [DOI] [PubMed] [Google Scholar]
- 39.Eaker S, Adami HO, Sparén P. Attitudes to screening for cervical cancer: a population-based study in Sweden. Cancer Causes Control. 2001;12:519–528. doi: 10.1023/a:1011233007132. [DOI] [PubMed] [Google Scholar]
- 40.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Heal Educ Q. 1988;15:351–77. [DOI] [PubMed]
- 41.Glanz K, Rimer BK. Theory at a glance: A guide for health promotion practice. 1995. [Google Scholar]
- 42.Reifsnider E, Gallagher M, Forgione B. Using ecological models in research on health disparities. J Prof Nurs. 2005;21:216–222. doi: 10.1016/j.profnurs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Kauffman RP, Griffin SJ, Lund JD, Tullar PE. Current recommendations for cervical cancer screening: do they render the annual pelvic examination obsolete? Med Princ Pract. 2013;22:313–322. doi: 10.1159/000346137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ICF International. The demographic and health survey (DHS) program. The DHS Program website. Funded by USAID; 2018. http://www.dhsprogram.com.
- 45.World Bank . Low & middle income. The World Bank. 2019. [Google Scholar]
- 46.Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited settings. Afr Health Sci. 2010;10:89–98. [PMC free article] [PubMed] [Google Scholar]
- 47.Nambiar B, Hargreaves DS, Morroni C, Heys M, Crowe S, Pagel C, et al. Improving health-care quality in resource-poor settings. Bull World Health Organ. 2017;95:76–78. doi: 10.2471/BLT.16.170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harford JB. Breast-cancer early detection in low-income and middle-income countries: do what you can versus one size fits all. Lancet Oncol. 2011;12:306–312. doi: 10.1016/S1470-2045(10)70273-4. [DOI] [PubMed] [Google Scholar]
- 49.Ren A, Ruilin A. The optimal sample sizes for Two-stage cluster sampling in demographic and health surveys. ORC Macro, Demographic and Health Research Division, 11785 Beltsville Drive, Suite 300, Calverton, MD 20705; 2006. https://www.dhsprogram.com/pubs/pdf/WP30/WP30.pdf.
- 50.Viens L, Perin D, Senkomago V, Neri A, Saraiya M. Questions about cervical and breast cancer screening knowledge, practice, and outcomes: A review of demographic and health surveys. J Womens Heal. 2017;26:403–412. doi: 10.1089/jwh.2017.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolenikov S, Angeles G. Socioeconomic status measurement with discrete proxy variables: is principal component analysis a reliable answer? Rev Income Wealth. 2009;55:128–65. [Google Scholar]
- 52.Sheikh N, Sultana M, Ali N, Akram R. Coverage , Timelines , and Determinants of Incomplete Immunization in Bangladesh. Trop Med Infect Dis. 2018;3:1–14. doi: 10.3390/tropicalmed3030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahumud RA, Sultana M, Sarker AR. Distribution and determinants of low birth weight in developing countries. J Prev Med Public Heal. 2017;50:18–28. doi: 10.3961/jpmph.16.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Croft TN, Marshall AMJ, Allen CK. Guide to DHS statistics. Rockville, Maryland, USA: ICF; 2018. [Google Scholar]
- 55.Australian Institute of Health and Welfare . Cervical screening in Australia 2019. Canberra: Australia Australian Government; 2019. [Google Scholar]
- 56.Hertzum-Larsen R, Kjær SK, Frederiksen K, Thomsen LT. Participation in cervical cancer screening among immigrants and Danish-born women in Denmark. Prev Med (Baltim). 2019;123:55–64. doi: 10.1016/j.ypmed.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 57.Ngoma TA. World Health Organization cancer priorities in developing countries. Ann Oncol. 2006;17(Suppl.8):9–14. doi: 10.1093/annonc/mdl982. [DOI] [PubMed] [Google Scholar]
- 58.Gustafsson L, Sparén P, Gustafsson M, Wilander E, Bergström R, Adami HO. Efficiency of organised and opportunistic cytological screening for cancer in situ of the cervix. Br J Cancer. 1995;72:498–505. doi: 10.1038/bjc.1995.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han MA, Choi KS, Lee HY, Jun JK, Jung KW, Kang S, et al. Performance of papanicolaou testing and detection of cervical carcinoma in situ in participants of organized cervical cancer screening in South Korea. PLoS ONEne. 2012;7:1–8. doi: 10.1371/journal.pone.0035469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ndejjo R, Mukama T, Kiguli J, Musoke D. Knowledge, facilitators and barriers to cervical cancer screening among women in Uganda: a qualitative study. BMJ Open. 2017;7:1–8. doi: 10.1136/bmjopen-2017-016282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gönenç İM, Abbas MN, Çalbayram N, Yılmaz S. A review of knowledge and attitudes of young people on cervical cancer and HPV vaccination. J Public Health. 2020;28:97–103.
- 62.Ally M, John T, Morgan W, Hutubessy R, Broutet N, Levin A, et al. A case study using the United Republic of Tanzania: costing nationwide HPV vaccine delivery using the WHO cervical Cancer prevention and control costing tool. BMC Med. 2012;10:1–10. doi: 10.1186/1741-7015-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kangmennaang J, Mkandawire P, Luginaah I. Breast cancer screening among women in Namibia : explaining the effect of health insurance coverage and access to information on screening behaviours. Glob Health Promot. 2019;26:50–61. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The DHS data are publicly accessible and were made available to us upon request by Measure DHS (https://dhsprogram.com/data/available-datasets.cfm).