Abstract
While it is generally accepted that structural and functional brain deficits underlie the behavioral deficits associated with fetal alcohol spectrum disorders (FASD), the degree to which these problems are expressed in sensory pathology is unknown. Electrophysiological measures indicate that neural processing is delayed in visual and auditory domains. Furthermore, multiple reports of white matter deficits due to prenatal alcohol exposure indicate altered cortical connectivity in individuals with FASD. Multisensory integration requires close coordination between disparate cortical areas leading us to hypothesize that individuals with FASD will have impaired multisensory integration relative to healthy control participants. Participants’ neurophysiological responses were recorded using magnetoencephalography (MEG) during passive unisensory or simultaneous, spatially congruent or incongruent multisensory auditory and somatosensory stimuli. Source timecourses from evoked responses were estimated using multi-dipole spatiotemporal modeling. Auditory M100 response latency was faster for the multisensory relative to the unisensory condition but no group differences were observed. M200 auditory latency to congruent stimuli was earlier and congruent amplitude was larger in participants with FASD relative to controls. Somatosensory M100 response latency was faster in right hemisphere for multisensory relative to unisensory stimulation in both groups. FASD participants’ somatosensory M200 responses were delayed by 13 ms, but only for the unisensory presentation of the somatosensory stimulus. M200 results indicate that unisensory and multisensory processing is altered in FASD; it remains to be seen if the multisensory response represents a normalization of the unisensory deficits.
Keywords: Fetal Alcohol Spectrum Disorders, Magnetoencephalography, Auditory, Somatosensory, Multisensory Integration
Introduction
Consumption of alcohol during pregnancy impacts fetal development and may lead to a variety of physical, cognitive, or behavioral abnormalities relative to non-exposed children. These neurobehavioral deficits are collectively known as fetal alcohol spectrum disorders (FASD). The FASD spectrum (with disabilities ranging from minor to severe) includes children with fetal alcohol syndrome (FAS), a term first coined in 1973 by Jones and Smith (Jones and Smith, 1973), partial fetal alcohol syndrome (pFAS), and alcohol-related neurodevelopmental disorder (ARND). At the severe end of the FASD spectrum (i.e. FAS), there are distinct craniofacial and growth abnormalities, microcephaly, delayed psychomotor maturation, and impaired intellectual development (Jones and Smith, 1973). Conversely, not all children with FASD will exhibit craniofacial abnormalities but manifest similar neurobehavioral disturbances implying that brain development is impacted by prenatal alcohol exposure across the FASD spectrum. With no visible structural abnormalities, identifying sensitive and specific functional markers in the sensory, cognitive, and behavioral domains is imperative (Mattson et al., 1997, Kodituwakku, 2007, Kodituwakku et al., 2011, Mattson et al., 2013). It has been suggested that both elementary and higher-order executive functioning must be investigated in children with FASD due to generalized deficits in the processing and integration of information (Kodituwakku, 2009, Kodituwakku and Kodituwakku, 2011).
While quality of life for individuals with FASD is primarily impacted by deficits in frontal lobe, such as executive function and impulsivity (Marshall et al., 2004, Kodituwakku, 2007), recent functional neuroimaging studies have provided considerable evidence that cognitive function is dependent on sensory processing (Brandwein et al., 2015). Our lab has shown that young children with FASD aged 3 to 6 years had significant delays in auditory M100 and M200 response latencies in a passive listening task (Stephen et al., 2012). Furthermore, we suggest that auditory responses may be a useful marker of alcohol-related damage given that this delay was found across the FASD spectrum; however, investigating other age groups may help to determine the diagnostic value of our previous results. In a follow-up study in an older FASD cohort, adolescents aged 12–21, we found that the visual M100 response latency was delayed in adolescents with FASD (Coffman et al., 2013). This visual M100 latency delay corresponded with increased gamma power over right central cortex in FASD subjects (Stephen et al. 2013). Delayed visual M100 in adolescents with FASD further supports the finding of basic sensory deficits following prenatal alcohol exposure. Other deficits related to FASD have also been described in visual perception (Bjuland et al., 2013), visual construction (Johnson, 2007), feature processing (Koenen et al., 2007), general sensory processing in each of the five senses (Ohls et al., 2016) and in performance of an auditory oddball task (Tesche et al., 2015).
At the next level of processing, multisensory integration requires interaction among multiple sensory areas to develop a coherent percept of the multisensory environment and this cross-modal communication has been established in both animal and human studies (Calvert et al., 2004). Multisensory integration is operationally defined as a response that cannot be explained by the unisensory responses alone. For example, certain neurons fire in response to both auditory and somatosensory stimulation, separately; when the stimuli are presented simultaneously these bimodal neurons may reveal either supra-additive or sub-additive firing rates relative to the unisensory firing rates representing a nonlinear multisensory response (Meredith and Stein, 1986, Wallace et al., 1993). Recent studies also indicate that primary and secondary sensory areas are largely involved in multisensory processing and exhibit both early and late responses to multisensory stimuli that cannot be explained by unisensory responses alone (Schroeder and Foxe, 2005, Lakatos et al., 2007, Stephen et al., 2010a). Multisensory integration occurs at different levels of processing depending on the timing of the response with early multisensory integration implicating impaired connectivity at either the subcortical or sensory level (Stehberg et al., 2014, Wu et al., 2015), whereas multisensory responses at later time windows (e.g. >200 ms) would likely include feedback from higher cognitive areas (Cui et al., 2017). Therefore, gaining an understanding of the spatiotemporal profile of multisensory deficits in FASD may help us better understand which cortical or subcortical areas are impacted by prenatal alcohol exposure. A number of different parameters influence the nonlinearity of the multisensory response including whether stimuli are high or low contrast (Stein and Meredith, 1993), temporally coincident (Colonius and Diederich, 2004), and spatially congruent (stimuli that are perceived as originating from the same side of the body) (Stein and Meredith, 1993). Therefore, manipulating the spatial congruency of sensory inputs should further influence the multisensory response and may directly target impaired hemispheric connectivity.
Deficits in multisensory integration have been reported in other developmental disorders with altered cortical connectivity (Wallace and Stevenson, 2014, Dionne-Dostie et al., 2015) such as autism spectrum disorders (Smith and Bennetto, 2007, Russo et al., 2010), dyslexia (Hayes et al., 2003) and schizophrenia (Williams et al., 2010). These deficits in multisensory processing have been attributed to sensory or cognitive overload in individuals with ASD (Russo et al., 2010, Noriega, 2015, Stevenson et al., 2018) with impaired multisensory processing leading to poor distillation of the external world into coherent objects from the natural multisensory environment. A more recent article has also demonstrated that multisensory processing deficits as opposed to sensory deficits alone are related to deficits in social interactions due to altered timing in the integration of auditory and visual information (Stevenson et al., 2018). Brett-Green et al. (2010) found differences in multisensory integration using auditory/somatosensory stimuli in children with sensory over-responsiveness providing additional evidence for the role of multisensory processing in sensory sensitivities which are now recognized as being present in children with PAE (Franklin et al., 2008). Therefore, impaired multisensory integration may similarly impair social and cognitive processing in children with FASD in direct and indirect ways.
Impaired cortical connectivity would be expected to impact the multisensory response through response delay and reduced multisensory nonlinearities based on reduced cortical connectivity that mediates these responses (Dionne-Dostie et al., 2015). Children with FASD have impaired cortico-cortical connectivity due to a variety of structural differences which include diminished corpus callosum density and thickness (Riley et al., 1995), along with microstructural abnormalities (Honey et al., 2012). Widespread decreases in white matter integrity were reported in children 5–13 years of age (Lebel et al., 2008, Sowell et al., 2008). Structure and function are closely linked in the brain such that structural abnormalities often translate directly into altered function as reported in fMRI resting-state functional connectivity studies in children with FASD (Wozniak et al., 2011). Taken together, the structural deficits found in children with FASD are expected to impede both intra- and inter-hemispheric transfer of information necessary for multisensory integration.
Auditory-somatosensory multisensory integration is particularly relevant to developmental research partly due to the ability to measure both auditory and somatosensory responses in passive conditions, negating the need for directed attention to the stimuli for the observed effects (Foxe et al., 2000). In multisensory interactions, when auditory and somatosensory stimuli are presented simultaneously, the amplified multisensory response is localized to the auditory association areas on the contralateral side of stimulation (Murray et al., 2005). In the current study, we examined the timing and amplitude of electrophysiological responses related to auditory-somatosensory multisensory integration in adolescents with FASD and normally developing age-matched healthy controls. To our knowledge, this is one of the first neurophysiological studies of auditory-somatosensory multisensory integration in FASD. We examined both latency and amplitude effects due to results indicating the time sensitive nature of multisensory integration and altered time perception in neurodevelopmental disorders (Wallace and Stevenson, 2014). Performing source analysis of the electrophysiological response provides additional insight into the timing and amplitude of the underlying sources that generate the multisensory responses (Stephen et al., 2010a). Based on previous unisensory results with FASD participants from our lab, we hypothesized that adolescents with FASD would be impaired (delayed latency and/or reduced amplitude) in the unisensory and multisensory responses involved in auditory-somatosensory multisensory integration relative to healthy controls. Given the considerable literature demonstrating thinning of the corpus callosum in FASD (Riley et al., 1995), we also examined effects of laterality and spatial congruence in the multisensory response, with the expectation that incongruent multisensory stimuli would reveal greater deficits than congruent multisensory stimuli in adolescents with FASD. The current MEG study was designed to further our understanding of how unisensory and multisensory responses in FASD may contribute to identifying a useful marker of alcohol-sensitive damage.
Materials and Methods
Participants
Forty-one participants participated in this study. Informed consent/assent was obtained from 41 participants and their parents. Individuals were excluded from the study if they had known peripheral visual or auditory deficits. We were unable to obtain reliable source localization results for one participant with FASD; therefore, results described here are based on analyses including 18 participants diagnosed with an FASD and 22 age- and gender-matched healthy controls, see Table 1. Healthy controls had IQ scores within the normal range and did not have known prenatal exposure to alcohol or other substances; nor did they have histories of developmental delays or neurological or psychological problems. Individuals with FASD were recruited from the FASD clinic located within the Center for Development and Disability, a Center of Excellence at the University of New Mexico Health Sciences Center. Participants were classified as having a prenatal alcohol-related diagnosis (fetal alcohol syndrome, partial fetal alcohol syndrome or alcohol related neurodevelopmental disorder) using the Institute of Medicine Criteria (Stratton et al., 1996). The FASD classification was made by consensus based on the assessment of a clinical psychologist (L. Kodituwakku), a neuropsychologist (P. Kodituwakku), and a pediatrician. Maternal alcohol consumption was confirmed either through direct confirmation by maternal interview, eyewitness reports of maternal drinking during pregnancy, or legal records confirming alcohol consumption during pregnancy (e.g. DWI arrest). Information on maternal alcohol consumption during pregnancy is collected as a part of the FASD clinical assessment; however, accurate estimates of quantity of alcohol consumption during pregnancy are often not available for adolescent children with FASD. The research protocol for this study was approved by the University of New Mexico Health Sciences Center Human Research Review Committee (UNM HSC HRRC). All procedures performed with human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Table I:
Participant Demographics
| HC (n=22) | FASD (n=18) | |
|---|---|---|
| Age Range in years | 12.1 – 20.3 | 12.0 – 21.0 |
| Mean Age in years (SD) | 16.2 (2.3) | 15.6 (2.6) |
| Male/Female (% Male) | 13/9 (59%) | 12/6 (67%) |
| Mean IQ (Range) | 107 (73–127) | 84 (54–101) |
| FASD Sub-diagnosis | 8 ARND/PAE, 8 FAS, 2 pFAS |
HC = Healthy Control; FASD = Fetal Alcohol Spectrum Disorder; ARND = Alcohol-Related Neurodevelopmental Disorder; PAE= Prenatal Alcohol Exposure; FAS= Fetal Alcohol Syndrome; pFAS= partial Fetal Alcohol Syndrome
Procedure
Data for this study were collected as part of a larger, multimodal neuroimaging study (Coffman et al., 2013). Participants’ brain responses were recorded using magnetoencephalography (MEG) during passive auditory and somatosensory stimulation. Unisensory and simultaneous spatially congruent or incongruent multisensory stimuli were presented while participants watched a silent cartoon (Disney’s Shrek, in most cases) that was unrelated to the stimuli presented. Participants’ hands rested in their laps after placing the stimulation devices on each index finger. Therefore, participants could see their hands if looking directly at them; however, their hands were outside of their field of view if gaze was directed to the movie as instructed. Auditory stimuli (1000 Hz tone) were presented for 50 ms, with volume adjusted to 72 dB above the participants’ individually determined hearing threshold (determined independently for each ear using the same equipment/software used to present auditory stimuli during MEG) to eliminate perceived volume differences across subjects due to poor ear insert placement or hearing deficits. To determine the hearing threshold, participants pressed a button whenever they heard a tone, 1000 Hz tones were randomly presented above and below threshold in a step-wise fashion. Information based on detected trials was used to calculate hearing threshold in decibels. If any participant had a hearing threshold greater than 37 dB, they were excluded from the study; however, no participants reached this exclusion criteria. Tactile pressure stimuli (40 psi) were delivered to the palmar aspect of the distal extremity of the index finger via a pneumatic stimulator positioned outside the magnetically shielded room (duration – 50 ms). The pneumatic stimulator was located outside the magnetically shielded room to ensure that participants could not hear the air puff stimulus. With the air regulator outside of the room, the balloons are silent when the balloon is filled. Somatosensory stimuli were initiated 30 ms earlier than auditory stimuli to account for stimulus equipment delays. This offset in the initiation of the auditory vs. somatosensory stimuli produced multisensory auditory/somatosensory (AS) stimuli with no delay between auditory & somatosensory relative to the participant. All stimuli were presented unilaterally. No responses were required from the participant; however, participants were instructed to attend the auditory and tactile stimuli during the silent cartoon. Therefore, eight conditions were presented to the participants including unilateral left and right auditory, unilateral left and right somatosensory, congruent AS, and incongruent AS. Stimuli from each condition were presented 140 times, and stimulus onset asynchrony varied between 800 and 1200 ms.
MEG Data Acquisition
MEG data were collected in a magnetically shielded room (Vacuumschmelze – Ak3B) at the Mind Research Network in Albuquerque, New Mexico using a 306-channel whole-head MEG system (Elekta Neuromag) with a sampling rate of 1000 Hz and an antialiasing filter with a passband of 0.1–330 Hz. Prior to data acquisition, four electromagnetic coils were placed on the participant’s left and right mastoid bone and upper forehead. The location of these coils was registered to the position of the nasion and preauricular points and to points representing the head shape/size using three-dimensional digitization equipment (Polhemus). Participants sat upright in the MEG during the task and were monitored at all times by an audio and video link between the magnetically shielded room and control room. Head position was tracked throughout the experiment.
Structural MRI Data Acquisition
Structural MRIs were obtained from most (95%) of the participants for use in mapping source locations. Source locations for the two participants for whom no structural MRIs were obtained were mapped onto MRIs obtained from participants with similar head size (circumference, length, & width) who had successfully completed the MRI scan. Sagittal T1-weighted anatomical MR images were obtained using a Siemens TIM Trio 3 Tesla MRI system with a multiecho 3D MPRAGE sequence [TR/TE/TI=2530/1.64, 3.5, 5.36, 7.22, 9.08/1200 ms, flip angle=7°, field of view=256 mm x 256 mm, matrix=256 x 256, 1 mm thick slice, 192 slices, GRAPPA acceleration factor=2].
Data analysis
Raw MEG data were first filtered for noise sources and corrected for head motion using the Neuromag Max-Filter software (Taulu and Kajola, 2005). Heartbeat and eye-blink artifacts were then removed by projecting electrocardiogram and electrooculogram data from MEG sensor waveforms using the signal-space projection method (Uusitalo and Ilmoniemi, 1997). Following filtering and projection of artifacts, the data obtained for each stimulus condition were signal-averaged from 100 ms prior to the onset of the stimulus to 700 ms following stimulus onset. Data were baseline-corrected and approximately 16 trials (10%) per condition were rejected in which the magnetic field at any sensor exceeded 5 pico-Tesla. To assess data quality, signal to noise ratio (SNR) was computed by dividing the peak amplitude in the post-stimulus evoked response (30–350 ms) by the standard deviation of the pre-stimulus (100 to 0 ms before stimulus onset) amplitude across MEG sensors for each stimulus condition. To ensure equivalent data quality between groups, Student’s t-tests were used to compare both SNR and number of trials included in signal averaging between HC and FASD groups for each condition.
Sources were localized independently for each subject using cortical start spatiotemporal (CSST) multidipole analysis (Ranken et al., 2002) with integrated Multiple Signal Classification (MUSIC) (Tang et al., 2014). Gradiometer and magnetometer channels were used in source modeling. Dipole starting locations were randomly selected from within a predefined cortical volume, as determined by co-registered structural MR images. CSST source localization was calculated for four separate models (modeling 3, 4, 5, or 6 dipoles) based on the averaged evoked responses occurring between 30 ms and 350 ms after the onset of the stimulus. The Nelder–Meade minimization procedure employed by CSST was carried out with randomly selected starting locations each of 1500 to 6000 times, depending on the number of dipoles in the model. The 10 best fits (based on the reduced chi-square value) were then displayed and the dipole model that best explained the data was selected for each subject individually for source timecourse analysis. Models were selected based on the residual activity (error) after subtracting empirical and modeled waveforms and the general acceptability (based on previous research in auditory and somatosensory evoked responses) of the dipole locations displayed in the results (for a more complete description of CSST see Stephen et al. (2006a)). Following model selection, timecourses associated with sources localized to the primary auditory or somatosensory cortices were processed further to determine group differences in peak amplitudes and latencies. Presentation of auditory stimuli to either ear often results in bilateral activation of primary auditory cortex while somatosensory stimuli only reliably elicit contralateral primary sensory activation (Fevang et al., 2016); therefore, response latencies to auditory stimuli were averaged across left and right auditory stimulus presentations except in the case where hemispheric differences were tested. Other commonly identified source locations included secondary somatosensory cortex (SII), superior temporal sulcus, and intraparietal sulcus, however these activations were not examined further, as these dipole locations were inconsistent across subjects leading to low subject N for any individual source location.
Missing peak amplitude or latency data (11.4%) were replaced with the mean for that variable and subject group (HC or FASD). For each subject group, 11.8% of the data were replaced with the mean for HC, and 10.9% of the data were replaced with the mean for FASD. Normality and equivalent variances of the dependent variables were confirmed prior to statistical comparisons. Differences in response latency were compared separately for auditory and somatosensory responses and for each response window. Response latencies were analyzed using split-plot analysis of variance where participant group (HC or FASD) was entered as a between-subject variable, and stimulus condition (Unisensory, Congruent, or Incongruent) and cortical hemisphere (left or right primary auditory/somatosensory cortex) were entered as within-subject variables. All statistical tests were corrected for multiple comparisons using Bonferroni-corrected alpha, where appropriate.
For display purposes only, the raw MEG data were also transformed to an average head position across subjects relative to the helmet array using Maxfilter 2.1 MaxMove option. This procedure transforms the MEG data such that each sensor is in an equivalent location relative to the head coordinate system across subjects, similar to EEG, to generate sensor-level, group-averaged, event-related field (ERF) waveforms. Artifact rejection was also applied to obtain the averaged evoked responses of the spatially transformed MEG data. The averaged evoked responses were then averaged across subjects to provide sensor-level group ERF waveforms (Figure 1). No group comparisons were performed on the sensor-level ERF waveforms since the primary goal of the study was to determine group differences at the source level.
Figure 1:
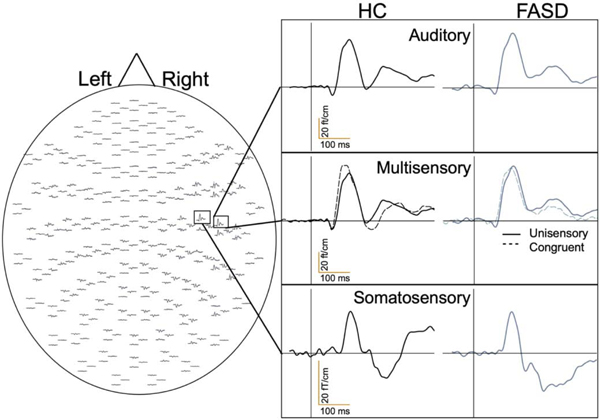
Representative MEG Sensor-Based Group Averaged Timecourses. The MEG sensor array and group-averaged sensor-based timecourses are shown to demonstrate average auditory responses, unisensory auditory with congruent multisensory responses, and somatosensory responses within the right hemisphere for healthy controls (HC) and participants with fetal alcohol spectrum disorder (FASD).
Results
MEG Data Quality
There were no significant differences in SNR between FASD and HC groups with α=0.05; however, marginally greater SNR was found for FASD participants in response to unisensory auditory stimuli presented to the right ear (mean ± SEM; FASD=26.7±2.5, HC=20.9±1.6; p>0.05). The high SNR across both groups is evident in the example sensor-level, group-averaged responses for auditory, somatosensory, and multisensory conditions (Figure 1). Source localization provided reliable contralateral primary sensory cortex activation (primary auditory and somatosensory) with example source locations displayed (Figure 2). Dipole source localization success was equivalent between groups (X2max=2.037, pmin > 0.1). Averaged source timecourses for the auditory and somatosensory sources are shown in Figs. 3 & 4, respectively. While clear M100 and M200 peaks are not visible in the averaged source timecourses, reliable M100 and M200 peaks were identifiable in the individual subject data. All results reported are based on peak latencies identified from the individual source timecourses. The dependent variables were confirmed to be normally distributed, with maximum observed skewness of 1.2 and kurtosis of 1.9.
Figure 2:
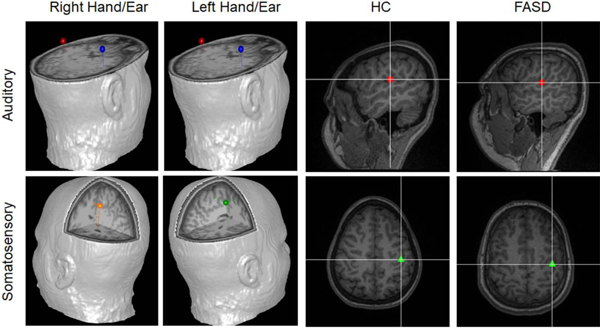
Source Locations. Example source locations are shown for auditory (upper panels) and somatosensory (lower panels) responses to stimuli presented to (from left to right) the right sensory hemifield, left sensory hemifield, a HC participant, and a FASD participant. HC and FASD examples are in response to stimuli presented to the left sensory hemifield. Dipole locations corresponding for left hemisphere auditory responses are shown in red, right hemisphere auditory responses are shown in blue, left hemisphere somatosensory responses are shown in orange and right hemisphere somatosensory responses are shown in red.
Figure 3:
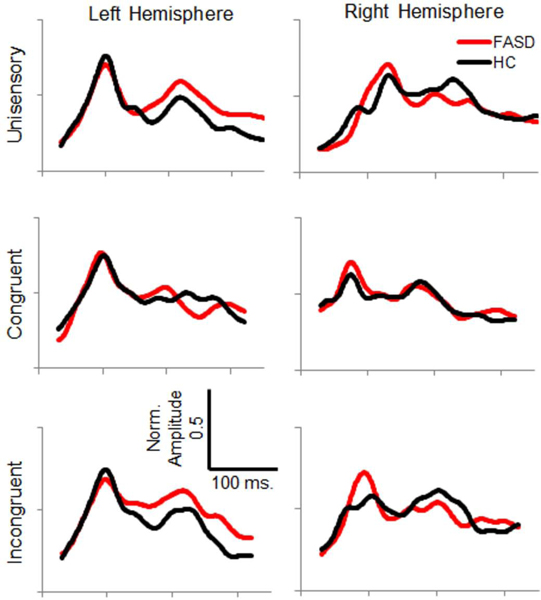
Average Normalized Auditory Source Timecourses – Timecourse of primary auditory cortex source activity is shown for healthy controls (HC, black) and participants with FASD (red) for each stimulation condition and hemisphere.
Figure 4:
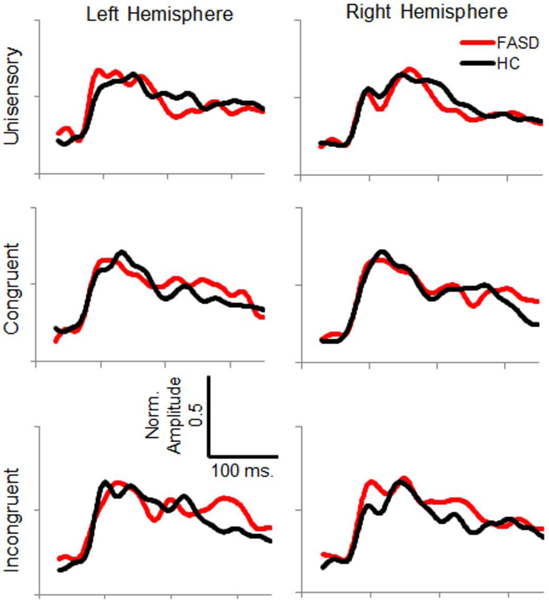
Average Normalized Somatosensory Source Timecourses – Timecourse of primary somatosensory cortex source activity is shown for healthy controls (HC, black) and participants with FASD (red) for each stimulation condition and hemisphere.
Auditory Response Latency and Amplitude
Comparison of auditory M100 peak latency revealed an earlier M100 in response to multisensory stimuli compared to unisensory stimuli, regardless of spatial congruence (approximately 14 ms difference, Figure 5). Auditory M100 peak amplitude was not different between conditions or groups, but left hemisphere response amplitudes were 10 nAm larger than responses in right auditory cortex. Comparison of auditory M200 peak latency also revealed earlier responses to multisensory stimuli than unisensory stimuli, regardless of spatial congruence (approximately 30 ms difference, Figure 6). Differences in M200 amplitude did not reach statistical significance. See Table 2 for inferential statistics.
Figure 5:
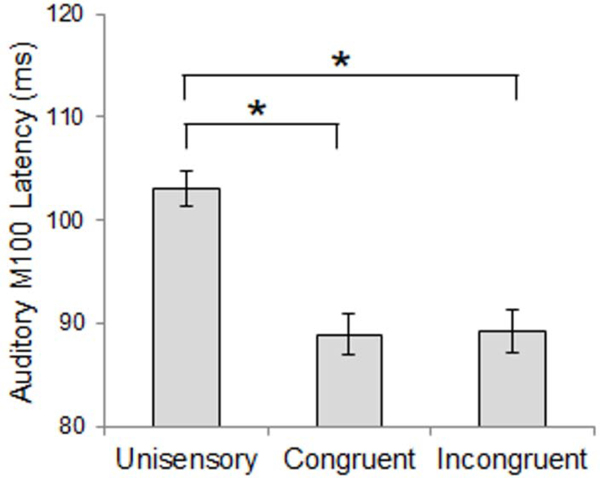
Auditory M100 Latency – Stimulus Condition Effect. M100 responses were earlier for multisensory stimuli compared to unisensory stimuli. Data shown are collapsed across participant groups. Asterisks represent significant differences (p<0.05).
Figure 6:
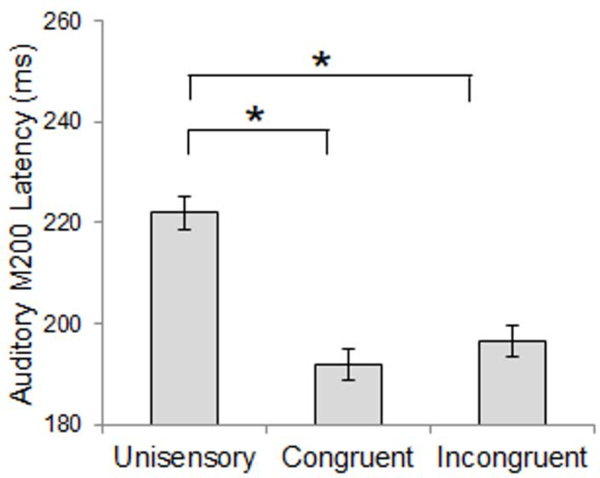
Auditory M200 Latency – Stimulus Condition Effect. M200 responses were earlier for multisensory stimuli compared to unisensory stimuli. Data shown are collapsed across participant groups. Asterisks represent significant differences (p<0.05).
Table II:
Auditory Response Latency and Amplitude
| Effect | F | p | η2p |
|---|---|---|---|
| M100 Latency | |||
| Condition | 21.63 | <0.001* | 0.54 |
| Unisensory vs. Congruent b | 30.19 | <0.001* | 0.44 |
| Unisensory vs. Incongruent b | 32.86 | <0.001* | 0.46 |
| M100 Amplitude | |||
| Hemisphere | 11.08 | 0.002* | 0.23 |
| M200 Latency | |||
| Condition | 58.49 | <0.001* | 0.61 |
| Unisensory vs. Congruent b | 82.43 | <0.001* | 0.68 |
| Unisensory vs. Incongruent b | 98.95 | <0.001* | 0.72 |
L = Left; R = Right; HC = Healthy Control subject; FASD = Fetal Alcohol Spectrum Disorder subject
Simple effects comparison
Pairwise comparison
Significant effect (p < 0.05, with Bonferroni correction)
Trend-level effect (p < 0.1, uncorrected)
Somatosensory Response Latency and Amplitude
Analysis of somatosensory M100 response latency revealed a significant interaction of stimulus condition and hemisphere, where congruent multisensory responses were significantly earlier than unisensory or incongruent multisensory responses within the right hemisphere (Figure 7). No significant differences were found between FASD and HC groups in M100 response latency. However, group differences in M100 amplitude were identified. Although a significant main effect of group was present (HC > FASD), the effect of group interacted with hemisphere and stimulus condition, with significant group differences only within left hemisphere responses to unisensory stimuli. FASD participants’ left hemisphere M100 response amplitude was less than half that of healthy controls (Figure 8). This effect is further evidenced by an interaction of hemisphere and diagnosis within responses to unisensory stimuli. Further, overall M100 amplitude was greater in response to congruent stimuli than unisensory and incongruent multisensory stimuli, and left hemisphere responses were of greater amplitude than right hemisphere responses.
Figure 7:
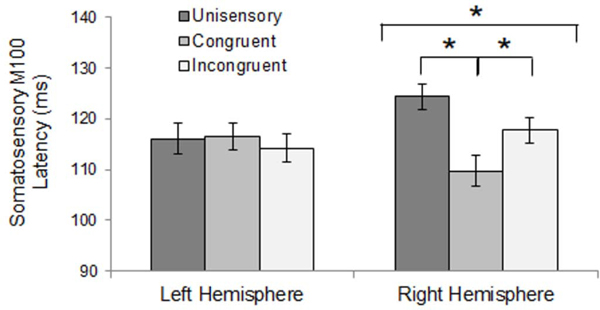
Somatosensory M100 Latency – Hemisphere x Stimulus Condition Interaction. Right hemisphere M100 response latency was earlier for congruent multisensory stimuli than unisensory or incongruent stimuli. No effect of stimulus condition was found for left hemisphere. Asterisks represent significant differences (p<0.05).
Figure 8:
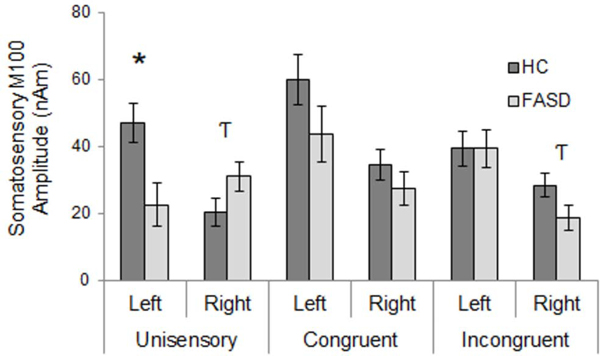
Somatosensory M100 Amplitude – Group x Stimulus Condition x Hemisphere Interaction. Responses were generally reduced for FASD compared to HC, with the exception of right hemisphere responses to unisensory stimuli which were greater amplitude for FASD compared to HC. Asterisks represent significant differences (p<0.05, corrected). Hooked T’s represent trend-level effects (p<0.1, uncorrected).
FASD participants’ unisensory M200 response latencies were delayed by 13 ms relative to HC, slightly faster than HC for congruent multisensory responses, and unaffected for incongruent multisensory responses, though these mean differences were not significant in post-hoc tests of simple effects (Figure 9). No main or interaction effects of cortical hemisphere were found for somatosensory M200 response latency. Similar to the somatosensory M100 response amplitude, a group by hemisphere interaction was observed only for responses to unisensory stimuli with reduced left hemisphere amplitude in FASD relative to HC, and the opposite pattern in right hemisphere (Figure 10). See Table 3 for inferential statistics.
Figure 9:
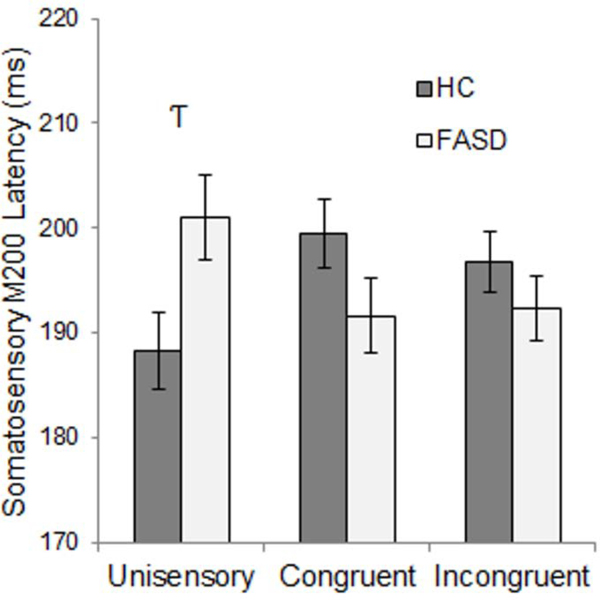
Somatosensory M200 Latency – Group x Stimulus Condition Interaction. Response latency differences between healthy controls (HC) and participants with FASD were greater for unisensory and congruent multisensory stimuli, however, these differences were trend-level effects. Hooked T’s represent trend-level effects (p<0.1, uncorrected).
Figure 10:
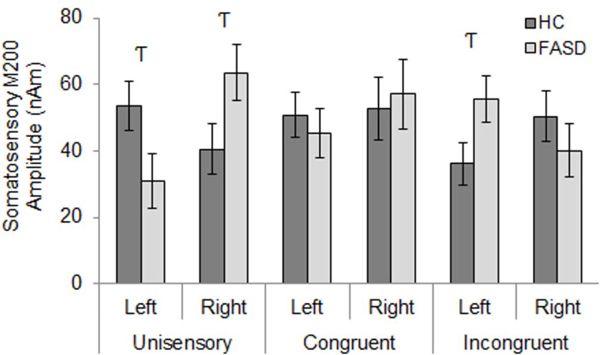
Somatosensory M200 Amplitude – Group x Stimulus Condition x Hemisphere Interaction. Left hemisphere responses to unisensory stimuli were reduced in FASD compared to HC, while right hemisphere responses to unisensory stimuli and left hemisphere responses to incongruent multisensory stimuli were increased in FASD relative to HC. Hooked T’s represent trend-level effects (p<0.1, uncorrected).
Table III:
Somatosensory Response Latency and Amplitude
| Effect | F | Sig. | η2p |
|---|---|---|---|
| M100 Latency | |||
| Hemisphere x Condition | 5.32 | 0.009* | 0.12 |
| Hemisphere @ Unisensorya | 5.39 | 0.026Ƭ | 0.12 |
| Hemisphere @ Congruenta | 3.29 | 0.078Ƭ | 0.08 |
| Condition @ R Hemispherea | 10.22 | <0.001* | 0.21 |
| Unisensory vs. Congruentb | 14.04 | <0.001* | 0.27 |
| Unisensory vs. Incongruentb | 4.37 | 0.043Ƭ | 0.10 |
| Congruent vs. Incongruentb | 9.71 | 0.003* | 0.20 |
| M100 Amplitude | |||
| Group | 5.04 | 0.031* | 0.12 |
| Hemisphere | 17.26 | <0.001* | 0.31 |
| Condition | 4.88 | 0.010* | 0.11 |
| Group x Condition x Hemisphere | 5.10 | 0.011* | 0.12 |
| Group x Condition @ R Hemi a | 4.03 | 0.024* | 0.10 |
| Group @ R Hemi, Unisensory a | 3.21 | 0.081Ƭ | 0.08 |
| Group @ R Hemi, Incongruent a | 3.47 | 0.070Ƭ | 0.08 |
| Group @ L Hemi a | 5.31 | 0.027* | 0.12 |
| Group x Hemisphere @ Unisensory a | 10.81 | 0.002* | 0.22 |
| Group @ R Hemi, Unisensory a | 3.21 | 0.081Ƭ | 0.08 |
| Group @ L Hemi, Unisensory a | 7.56 | 0.009* | 0.17 |
| M200 Latency | |||
| Group x Condition | 5.65 | 0.006* | 0.13 |
| Group @ Unisensory a | 5.25 | 0.028Ƭ | 0.12 |
| Condition @ HC a | 3.09 | 0.058Ƭ | 0.13 |
| Condition @ FASD a | 2.77 | 0.086Ƭ | 0.21 |
| M200 Amplitude | |||
| Group x Condition x Hemisphere | 7.22 | 0.002* | 0.16 |
| Group @ L Hemi, Unisensory a | 4.02 | 0.052Ƭ | 0.10 |
| Group @ L Hemi, Incongruent a | 4.22 | 0.047Ƭ | 0.10 |
| Group x Hemi @ Unisensory a | 9.14 | 0.004* | 0.19 |
| Group @ R Hemi, Unisensory a | 4.12 | 0.049Ƭ | 0.10 |
| Group @ L Hemi, Unisensory a | 4.02 | 0.052Ƭ | 0.10 |
| Group x Hemi @ Incongruent a | 4.78 | 0.035Ƭ | 0.11 |
L = Left; R = Right; HC = Healthy Control subject; FASD = Fetal Alcohol Spectrum Disorder subject
Simple effects comparison
Pairwise comparison
Significant effect (p < 0.05, with Bonferroni correction)
Trend-level effect (p < 0.1, uncorrected)
Finally, the MEG latency and amplitude measures (primary somatosensory M100 amplitude and M200 latency in response to unisensory stimuli) were moderately correlated with IQ, however no significant correlations were observed after correcting for multiple comparisons (all |r|’s<0.35).
Discussion
Prior results examining spatial congruency within an AS integration task have been mixed with some studies indicating dramatic differences in the multisensory interaction response between spatially congruent and spatially incongruent stimuli and others revealing little difference (Wu et al., 2015). Based primarily on auditory/visual multisensory integration studies focused on responses in the superior colliculus, results initially indicated that stimuli must be spatially congruent to elicit multisensory responses (Stein and Meredith, 1993). However, both Murray et al. (2005) and Geischeider and Niblette (1967) demonstrated that auditory/somatosensory integration is less sensitive to spatial congruence at the cortical level. Our results reveal some differences between congruent and incongruent stimuli with faster somatosensory M100 latency for the congruent condition in right hemisphere and larger somatosensory amplitude to congruent versus incongruent or unisensory stimuli, but no effect of congruency in auditory cortex. A recent study by Spence (2013), questions the generalization of spatial congruency in multisensory integration with the evidence now indicating that spatial congruency depends on task demands.
Our current results in healthy children are consistent with the landmark pediatric study of Brett-Green et al. (2008) who revealed multisensory integration responses in children 6–13 years of age in the following time windows: 60–80 ms (bilateral), 110–150 ms (ipsilateral) and 180–220 ms (contralateral). Based on the timing differences between median nerve stimulation, used by Brett-Green and colleagues, and the more delayed neural response to a tactile (airpuff) stimulus used here, we assume that the 60–80 ms and the 180–220 ms responses correspond best with our M100 and M200 responses, revealing multisensory effects in similar time windows. However, other differences between the Brett Green study and the current study limit the interpretation including their focus on the subtracted multisensory interaction response rather than the sensory response peak and the contrast between scalp recordings of ERPs and source analysis of MEG recordings applied here. That said, Brett-Green et al. (2008) confirmed that children exhibited multisensory interaction responses similar to adults allowing us to compare our results in 12–21 year olds to the more prevalent adult literature (Lutkenhoner et al., 2002, Murray et al., 2005, Wu et al., 2015).
Multisensory responses in healthy controls were consistent with previous findings indicating that multisensory stimuli generally lead to faster processing or a nonlinear increase in amplitude relative to unisensory stimuli (Lutkenhoner et al., 2002, Murray et al., 2005, Brett-Green et al., 2008) with our M100 and M200 peaks consistent with Lutkenhoner’s prior MEG study. However, Lutkenhoner and colleagues did not examine integration effects in primary sensory areas but performed source analysis on the difference waveform (summed unisensory minus multisensory responses). While they determined that the interaction field arose from secondary somatosensory cortex (SII), they also acknowledged that the location of this source was not consistent with the unisensory SII location and suggested that contributions from both primary somatosensory and auditory cortex may have contributed to the interaction field. Our focus on primary sensory cortex was driven by the reliability of these source locations across individuals and does not preclude the presence of interaction effects in SII. SII was localized in >50% of participants, however, it was not identified reliably enough across conditions to perform statistical analysis within this cohort. A smaller contribution of SII in this study may be partially attributed to the focus on children rather than adults. Also, inter-stimulus interval (ISI) has been shown to be a large moderator of the amplitude of later somatosensory components that are dominated by SII activity in both adults (Hari and Forss, 1999) and children (Uppal et al., 2016). The ISI of the current study of 1 second differed slightly from Lutkenhoner and colleagues’ ISI of 1.3 seconds. Differences in results have also been attributed to differences in task design across prior multisensory auditory/somatosensory studies (Soto-Faraco and Deco, 2009, Wu et al., 2015). The current design with a focus on viewing a silent movie and short ISI will inhibit SII activation based on previous studies (Mima et al., 1998, Hari and Forss, 1999). Our results are consistent with Hoefer et al. (2013) who also found multisensory integration effects in auditory and somatosensory cortex.
An important distinction from previous EEG multisensory studies is that the current analysis was performed on the MEG source-level timecourses rather than the sensor waveforms. The objective, multi-dipole source analysis (Aine et al., 2000, Ranken et al., 2002) reliably identified primary sensory sources in all participants. With the analysis of primary sensory sources a direct comparison of unisensory versus multisensory responses (A vs. AS) is performed (Stephen et al., 2010a) because unisensory stimulation is not expected to activate other primary sensory cortices (e.g. a unisensory somatosensory stimulus will not activate auditory cortex (Koehler et al., 2011, King and Walker, 2012)). Therefore, we did not compare multisensory (AS) versus summed (A+S) responses as is done in multisensory studies performed at the sensor level (Murray et al., 2005). However, the close spatial proximity of primary and secondary auditory cortex likely means that contributions from both regions contributed to the single auditory source timecourse (Stephen et al., 2010a). We do not expect this to be the case for somatosensory cortex based on our ability to reliably localize secondary somatosenosry cortex independent of the primary somatosensory source (Stephen et al., 2006a, Stephen et al., 2010b).
In contrast to our previous findings reporting auditory delays in preschool children with FASD (Stephen et al., 2012), this study in adolescents did not find delays in auditory processing in the FASD group. This is consistent with reports in a rat study indicating decreasing auditory delays with increasing age (Church, 1987), as well as the recent findings by Tesche et al. (2015), who reported no delays in simple auditory processing in children 12–22 years of age with FASD relative to healthy controls. The only measurable group delay for FASD was a delayed M200 response for the unisensory somatosensory condition, indicating some persistent differences in somatosensory response in FASD relative to HC in adolescence. Therefore, our results revealing no deficits in auditory processing are in contrast to our hypothesized group differences but are consistent with the existing literature in this older adolescent age range.
The somatosensory results also indicated group differences by hemisphere. Results from our lab and others have found similar hemisphere specific deficits in FASD, ranging from reduced gamma-band oscillations with differences primarily identified in right hemisphere (Stephen et al., 2013) to decreased white matter integrity in right hemisphere (Green et al., 2013). However, most FASD studies show bilateral deficits within the FASD spectrum. Interestingly, in some studies multisensory integration has been attributed to right hemisphere function (Vercillo and Gori, 2015) consistent with the results of Hoefer and colleagues (2013). In the case of the M100 latency results, significant differences by condition (both groups) were observed only in right hemisphere with the congruent condition revealing the shortest peak latency followed by incongruent and finally by unisensory conditions, consistent with right hemisphere multisensory function. However, our results revealed unisensory deficits in the left hemisphere in FASD with equivalent activity as HC in multisensory conditions. This may provide additional evidence that multisensory integration helps compensate for right hemisphere deficits in children with FASD.
Our results are also different from our hypothesized multisensory group effects where we expected multisensory responses to be delayed or of lower amplitude in FASD relative to HC. Specifically, in somatosensory cortex, where unisensory deficits were found in FASD, multisensory responses did not differ by group. These results, similar to our multisensory studies in schizophrenia (Stone et al., 2011, Stone et al., 2014), indicate that the recruitment of multiple sensory modalities may help to compensate for unisensory deficits in individuals with FASD. One possible explanation for improved multisensory integration response in FASD (earlier latency than HC in the congruent multisensory condition and equivalent latency to HC for the incongruent condition in children with FASD despite slower latency in FASD in the unisensory condition) is that the sensory stimuli may be perceived at lower contrast relative to the control group – due to either peripheral or central nervous system deficits. The well-accepted inverse effectiveness rule established by Stein and Meredith (1993) suggests that greater multisensory gains are obtained in cases where stimuli are more difficult to perceive or are at lower contrast relative to other environmental stimuli. In the current study, we matched on auditory volume by setting the volume relative to each participant’s measured auditory threshold; however, we are not aware of an established testing method to equilibrate tactile airpuff stimuli so we did not control for level of tactile stimulation beyond using a well-calibrated pressure device. Therefore, we cannot rule out the possibility that FASD participants experienced greater multisensory facilitation due to different perceived contrast levels of the tactile stimulus. To better understand whether multisensory integration responses are driven by peripheral sensory deficits, it would be ideal to match contrast levels for both sensory modalities on an individual basis. One advantage of median nerve stimulation (as employed by Brett-Green et al. (2008)) is that median nerve stimulation is calibrated to the onset of a current-induced motor twitch. It is perhaps relevant that fewer group differences were observed in auditory cortex relative to somatosensory cortex in the current study. However, the results from the auditory and somatosensory M100 peaks do not strongly argue for simple peripheral deficits in FASD beyond a reduced amplitude response in left hemisphere relative to HC. Therefore, it is also possible that the inverse effectiveness rule applies more broadly in the sense that impairments in sensory processing at the cortical level may benefit from the increased salience of multisensory stimuli.
However, our multisensory studies in schizophrenia also demonstrated a behavioral improvement (speeded reaction time), which was not measured in this passive task. Therefore, our current study does not provide behavioral evidence for a multisensory benefit for children with FASD. That is, the normalization of the multisensory response may still represent altered brain function in these children and will need to be further tested in future studies. The “normalized” somatosensory responses to multisensory stimuli observed here may indicate maladaptive sensory modulation in FASD. In other words, greater responses to multisensory stimuli relative to lower amplitude/delayed unisensory response may indicate a general dysfunction of the ability to modulate neurophysiological responses to incoming stimuli. The central nervous system is constantly regulating sensory input by adjusting thresholds for activation through inhibition and/or facilitation, such as during adaptation to quiet or loud environments while listening to and engaging in conversation (Wark et al., 2007, Todorovic et al., 2011). Some have suggested that this process of sensory modulation goes awry in FASD, particularly for somatosensation. Individuals with FASD have been characterized as having oversensitivity to stimulation, or “sensory overload”, particularly for the somatosensory system (Jirikowic et al., 2008) which is in turn related to poor adaptive behavior. Our data partially support this theory, as simple effects were restricted to (reduced) amplitudes and (delayed) latencies of somatosensory M100/M200 responses. Moreover, although group differences in mean multisensory responses were not significant when reduced to simple effects within conditions, significant interactions between group and stimulus condition observed here indicate increased differential (multisensory minus unisensory) amplitudes/latencies in FASD compared to healthy controls. This is in-line with studies in children with sensory processing disorders indicating reduced unisensory responses with intact multisensory integration (Davies and Gavin, 2007, Brett-Green et al., 2010).
It should also be noted that attention may also play a role in the reported results. Individuals with FASD often have co-morbid attention deficits as a core feature of the disorder (Fryer et al., 2007). The instruction to view a movie while also acknowledging the incoming stimuli may have led to a more difficult dual-task design. However, in our experience viewing a movie during a simple passive multisensory task helps to maintain attention and reduces the likelihood that children become bored. However, without behavioral data we cannot confirm that both groups attended to the stimuli to the same degree. Results from prior studies have established that early multisensory effects occur regardless of attentional focus (Brett-Green et al., 2008). Furthermore, group effects were not observed in auditory cortex despite the fully randomized design. Also, the effects are in the opposite direction than expected, since the FASD group revealed multisensory benefit rather than multisensory impairment. The results instead argue that multisensory stimuli may help individuals with FASD attend to stimuli similar to Talsma et al. (2010) where multisensory stimuli helped capture attention.
There are acknowledged limitations with respect to the current study. First, the analysis of primary sensory cortices limits our ability to interpret the results relative to higher order multisensory areas. It is important to note however, that the auditory source likely encompasses responses from primary/secondary auditory cortex based on the close vicinity of these regions. A larger study that actively engages the participant in a multisensory task may more reliably activate these higher order areas to examine the broader multisensory network. A second limitation of the study is that we did not analyze the subcategories of FASD separately and do not have estimates of the amount of alcohol consumed during pregnancy in the FASD children. Prospective studies of children with prenatal alcohol exposure are required to obtain reliable estimates of alcohol consumption during pregnancy due to the difficulty in reliably recalling this information using a retrospective design (Bhuvaneswar et al., 2007). Finally, recent results indicate the importance of considering sex when interpreting results for individuals with FASD (Paolozza et al., 2015, Tesche et al., 2015). Due to the limited sample size, the current analysis did not explore differences in sensory responses separately for male/female participants. Future studies with a larger sample size are needed to fully understand developmental differences in sensory and cognitive processing in males versus females.
The results from this study indicate that multisensory processing in adolescents with FASD is primarily different from HC at the M200 processing stage. The current literature supports the integral role that sensory processing plays in higher order cognition; examining multisensory integration may provide additional information for how sensory processing influences cognitive function. Further studies are needed to determine if the normalization of the multisensory M200 response is adaptive (conferring a benefit in children with FASD) or maladaptive contributing to sensory processing deficits that have previously been associated with poor social interaction and adaptive behaviors in children with developmental disorders more broadly.
Highlights.
Response latencies were facilitated with multisensory versus unisensory stimuli.
Somatosensory responses revealed larger amplitudes in M100 peaks in HC than FASD.
Despite M200 somatosensory delays, there were no multisensory delays in FASD.
Response latencies were facilitated with multisensory versus unisensory stimuli.
Somatosensory responses revealed larger amplitudes in M100 peaks in HC than FASD.
Despite M200 somatosensory delays, there were no multisensory delays in FASD.
Acknowledgements
We thank the parents and participants who graciously offered their time for this study. We also thank Daniel Savage, Director of the New Mexico Alcohol Research Center, for his full support of this study. This work was supported by the National Institutes of Health [3P20AA017068–03S1, P20 AA017068, 5P20 GM103472, P50 AA022534, R01 AA021771, T32 AA014127]; and the National Science Foundation [1539067]. The study sponsors had no role in collection, analysis, interpretation of the data or writing of the manuscript.
Abbreviations:
- FASD
Fetal Alcohol Spectrum Disorder
- HC
Healthy Control
- FAS
Fetal Alcohol Syndrome
- ISI
Interstimulus interval
- SII
secondary somatosensory cortex
- AS
auditory/somatosensory
- CSST
cortical start spatiotemporal
- SNR
signal to noise ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have potential conflicts of interest to be disclosed.
References
- Aine C, Huang M, Stephen J, Christner R (2000) Multistart algorithms for MEG empirical data analysis reliably characterize locations and time courses of multiple sources. NeuroImage 12:159–172. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswar C, Chang G, Epstein L, Stern T (2007) Alcohol use during pregnancy: Prevalence and Impact. Prim Care Companion J Clin Psychiatry 9:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjuland KJ, Lohaugen GC, Martinussen M, Skranes J (2013) Cortical thickness and cognition in very-low-birth-weight late teenagers. Early Hum Dev 89:371–380. [DOI] [PubMed] [Google Scholar]
- Brandwein AB, Foxe JJ, Butler JS, Frey HP, Bates JC, Shulman LH, Molholm S (2015) Neurophysiological indices of atypical auditory processing and multisensory integration are associated with symptom severity in autism. J Autism Dev Disord 45:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett-Green BA, Miller LJ, Gavin WJ, Davies PL (2008) Multisensory integration in children: a preliminary ERP study. Brain Res 1242:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett-Green BA, Miller LJ, Schoen SA, Nielsen DM (2010) An exploratory event-related potential study of multisensory integration in sensory over-responsive children. Brain Res 1321:67–77. [DOI] [PubMed] [Google Scholar]
- Calvert G, Spence C, Stein BE (2004) The handbook of multisensory processes. Cambridge, Mass: MIT Press. [Google Scholar]
- Church MW (1987) Chronic in utero alcohol exposure affects auditory function in rats and in humans. Alcohol 4:231–239. [DOI] [PubMed] [Google Scholar]
- Coffman BA, Kodituwakku P, Kodituwakku EL, Romero L, Sharadamma NM, Stone D, Stephen JM (2013) Primary visual response (M100) delays in adolescents with FASD as measured with MEG. Hum Brain Mapp 34:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonius H, Diederich A (2004) Multisensory interaction in saccadic reaction time: a time-window-of-integration model. J Cogn Neurosci 16:1000–1009. [DOI] [PubMed] [Google Scholar]
- Cui Z, Wang Q, Gao Y, Wang J, Wang M, Teng P, Guan Y, Zhou J, Li T, Luan G, Li L (2017) Dynamic Correlations between Intrinsic Connectivity and Extrinsic Connectivity of the Auditory Cortex in Humans. Frontiers in Human Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Gavin WJ (2007) Validating the diagnosis of sensory processing disorders using EEG technology. Am J Occup Ther 61:176–189. [DOI] [PubMed] [Google Scholar]
- Dionne-Dostie E, Paquette N, Lassonde M, Gallagher A (2015) Multisensory integration and child neurodevelopment. Brain sciences 5:32–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevang SK, Hysing M, Markestad T, Sommerfelt K (2016) Mental Health in Children Born Extremely Preterm Without Severe Neurodevelopmental Disabilities. Pediatrics. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Morocz IA, Murray MM, Higgins BA, Javitt DC, Schroeder CE (2000) Multisensory auditory-somatosensory interactions in early cortical processing revealed by high-density electrical mapping. Brain Res Cogn Brain Res 10:77–83. [DOI] [PubMed] [Google Scholar]
- Franklin L, Deitz J, Jirikowic T, Astley S (2008) Children with fetal alcohol spectrum disorders: problem behaviors and sensory processing. Am J Occup Ther 62:265–273. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN (2007) Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics 119:e733–741. [DOI] [PubMed] [Google Scholar]
- Gescheider GA, Niblette RK (1967) Cross-modality masking for touch and hearing. J Exp Psychol 74:313–320. [DOI] [PubMed] [Google Scholar]
- Green CR, Lebel C, Rasmussen C, Beaulieu C, Reynolds JN (2013) Diffusion Tensor Imaging Correlates of Saccadic Reaction Time in Children with Fetal Alcohol Spectrum Disorder. Alcohol Clin Exp Res. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N (1999) Magnetoencephalography in the study of human somatosensory cortical processing. Philos Trans R Soc Lond B Biol Sci 354:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EA, Tiippana K, Nicol TG, Sams M, Kraus N (2003) Integration of heard and seen speech: a factor in learning disabilities in children. Neurosci Lett 351:46–50. [DOI] [PubMed] [Google Scholar]
- Hoefer M, Tyll S, Kanowski M, Brosch M, Schoenfeld MA, Heinze HJ, Noesselt T (2013) Tactile stimulation and hemispheric asymmetries modulate auditory perception and neural responses in primary auditory cortex. Neuroimage 79:371–382. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Thesen T, Donner TH, Silbert LJ, Carlson CE, Devinsky O, Doyle WK, Rubin N, Heeger DJ, Hasson U (2012) Slow cortical dynamics and the accumulation of information over long timescales. Neuron 76:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirikowic T, Olson HC, Kartin D (2008) Sensory processing, school performance, and adaptive behavior of young school-age children with fetal alcohol spectrum disorders. Phys Occup Ther Pediatr 28:117–136. [DOI] [PubMed] [Google Scholar]
- Johnson S (2007) Cognitive and behavioural outcomes following very preterm birth. Semin Fetal Neonatal Med 12:363–373. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet 302:999–1001. [DOI] [PubMed] [Google Scholar]
- King AJ, Walker KM (2012) Integrating information from different senses in the auditory cortex. Biol Cybern 106:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku P, Segall JM, Beatty GK (2011) Cognitive and behavioral effects of prenatal alcohol exposure. Future Neurology 6:237–259. [Google Scholar]
- Kodituwakku PW (2007) Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev 31:192–201. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW (2009) Neurocognitive Profile In Children With Fetal Alcohol Spectrum Disorders. Developmental disabilities research reviews 15:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku PW, Kodituwakku EL (2011) From research to practice: an integrative framework for the development of interventions for children with fetal alcohol spectrum disorders. Neuropsychol Rev 21:204–223. [DOI] [PubMed] [Google Scholar]
- Koehler SD, Pradhan S, Manis PB, Shore SE (2011) Somatosensory inputs modify auditory spike timing in dorsal cochlear nucleus principal cells. Eur J Neurosci 33:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A (2007) Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychological medicine 37:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O’Connell MN, Mills A, Schroeder CE (2007) Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron 53:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C (2008) Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 32:1732–1740. [DOI] [PubMed] [Google Scholar]
- Lutkenhoner B, Lammertmann C, Simoes C, Hari R (2002) Magnetoencephalographic correlates of audiotactile interaction. Neuroimage 15:509–522. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA, Bucharest Early Intervention Project Core G (2004) A comparison of the electroencephalogram between institutionalized and community children in Romania. J Cogn Neurosci 16:1327–1338. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL (1997) Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr 131:718–721. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP, Cifasd (2013) Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 37:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Stein BE (1986) Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol 56:640–662. [DOI] [PubMed] [Google Scholar]
- Mima T, Nagamine T, Nakamura K, Shibasaki H (1998) Attention modulates both primary and second somatosensory cortical activities in humans: a magnetoencephalographic study. J Neurophysiol 80:2215–2221. [DOI] [PubMed] [Google Scholar]
- Murray MM, Molholm S, Michel CM, Heslenfeld DJ, Ritter W, Javitt DC, Schroeder CE, Foxe JJ (2005) Grabbing your ear: rapid auditory-somatosensory multisensory interactions in low-level sensory cortices are not constrained by stimulus alignment. Cereb Cortex 15:963–974. [DOI] [PubMed] [Google Scholar]
- Noriega G (2015) A neural model to study sensory abnormalities and multisensory effects in autism. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society 23:199–209. [DOI] [PubMed] [Google Scholar]
- Ohls RK, Cannon DC, Phillips J, Caprihan A, Patel S, Winter S, Steffen M, Yeo RA, Campbell R, Wiedmeier S, Baker S, Gonzales S, Lowe J (2016) Preschool Assessment of Preterm Infants Treated With Darbepoetin and Erythropoietin. Pediatrics 137:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolozza A, Munn R, Munoz DP, Reynolds JN (2015) Eye movements reveal sexually dimorphic deficits in children with fetal alcohol spectrum disorder. Frontiers in neuroscience 9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranken D, Best E, Stephen J, Schmidt D, George J, Wood C, Huang M (2002) MEG/EEG forward and inverse modeling using MRIVIEW. In: Proceedings of the 13th International Conference on Biomagnetism (Nowak H et al., eds), pp 785–787 Berlin: VDE Verlag. [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL (1995) Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res 19:1198–1202. [DOI] [PubMed] [Google Scholar]
- Russo N, Foxe JJ, Brandwein AB, Altschuler T, Gomes H, Molholm S (2010) Multisensory processing in children with autism: high-density electrical mapping of auditory-somatosensory integration. Autism Res. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Foxe J (2005) Multisensory contributions to low-level, ‘unisensory’ processing. Current Opinion in Neurobiology 15:454–458. [DOI] [PubMed] [Google Scholar]
- Smith EG, Bennetto L (2007) Audiovisual speech integration and lipreading in autism. Journal of Child Psychology and Psychiatry 48:813–821. [DOI] [PubMed] [Google Scholar]
- Soto-Faraco S, Deco G (2009) Multisensory contributions to the perception of vibrotactile events. Behav Brain Res 196:145–154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O’Connor MJ, Bookheimer SY (2008) Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci 28:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C (2013) Just how important is spatial coincidence to multisensory integration? Evaluating the spatial rule. Ann N Y Acad Sci 1296:31–49. [DOI] [PubMed] [Google Scholar]
- Stehberg J, Dang PT, Frostig RD (2014) Unimodal primary sensory cortices are directly connected by long-range horizontal projections in the rat sensory cortex. Front Neuroanat 8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Meredith MA (1993) The merging of the senses. Cambridge, MA: MIT Press. [Google Scholar]
- Stephen J, Kodituwakku P, Kodituwakku EL, Romero L, Peters AM, Sharadamma NM, Caprihan A, Coffman BA (2012) Delays in auditory processing identified in preschool children with FASD. Alcoholism: Clinical and Experimental Research 36:1720–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Coffman BA, Stone DB, Kodituwakku P (2013) Differences in MEG gamma oscillatory power during performance of a prosaccade task in adolescents with FASD. Front Hum Neurosci 7:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Knoefel JE, Adair J, Hart B, Aine CJ (2010a) Aging-related changes in auditory and visual integration measured with MEG. Neurosci Lett 484:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Montano R, Donahue CH, Adair JC, Knoefel J, Qualls C, Hart B, Ranken D, Aine CJ (2010b) Somatosensory responses in normal aging, mild cognitive impairment, and Alzheimer’s disease. J Neural Transm 117:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Ranken D, Best E, Adair J, Knoefel J, Kovacevic S, Padilla D, Hart B, Aine CJ (2006a) Aging changes and gender differences in response to median nerve stimulation measured with MEG. Clin Neurophysiol 117:131–143. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Segers M, Ncube BL, Black KR, Bebko JM, Ferber S, Barense MD (2018) The cascading influence of multisensory processing on speech perception in autism. Autism 22:609–624. [DOI] [PubMed] [Google Scholar]
- Stone DB, Coffman BA, Bustillo JR, Aine CJ, Stephen JM (2014) Multisensory stimuli elicit altered oscillatory brain responses at gamma frequencies in patients with schizophrenia. Front Hum Neurosci 8:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DB, Urrea LJ, Aine CJ, Bustillo JR, Clark VP, Stephen JM (2011) Unisensory processing and multisensory integration in schizophrenia: a high-density electrical mapping study. Neuropsychologia 49:3178–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia FP (eds.) (1996) Institute of Medicine. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. Washington, D.C: National Academy Press. [Google Scholar]
- Talsma D, Senkowski D, Soto-Faraco S, Woldorff MG (2010) The multifaceted interplay between attention and multisensory integration. Trends Cogn Sci 14:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS (2014) On the causes of early life experience effects: evaluating the role of mom. Frontiers in neuroendocrinology 35:245–251. [DOI] [PubMed] [Google Scholar]
- Taulu S, Kajola M (2005) Presentation of electromagnetic multichannel data: The signal space separation method. Journal of Applied Physics 97. [Google Scholar]
- Tesche CD, Kodituwakku PW, Garcia CM, Houck JM (2015) Sex-related differences in auditory processing in adolescents with fetal alcohol spectrum disorder: A magnetoencephalographic study. NeuroImage Clinical 7:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic A, van Ede F, Maris E, de Lange FP (2011) Prior expectation mediates neural adaptation to repeated sounds in the auditory cortex: an MEG study. J Neurosci 31:9118–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal N, Foxe JJ, Butler JS, Acluche F, Molholm S (2016) The neural dynamics of somatosensory processing and adaptation across childhood: a high-density electrical mapping study. J Neurophysiol 115:1605–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ (1997) Signal-space projection method for separating MEG or EEG into components. Medical & Biological Engineering & Computing 35:135140. [DOI] [PubMed] [Google Scholar]
- Vercillo T, Gori M (2015) Attention to sound improves auditory reliability in audio-tactile spatial optimal integration. Front Integr Neurosc 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE (1993) Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol 69:1797–1809. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stevenson RA (2014) The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia 64:105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark B, Lundstrom BN, Fairhall A (2007) Sensory adaptation. Curr Opin Neurobiol 17:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Light GA, Braff DL, Ramachandran VS (2010) Reduced multisensory integration in patients with schizophrenia on a target detection task. Neuropsychologia 48:3128–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Muetzel RL, Bell CJ, Hoecker HL, Nelson ML, Chang PN, Lim KO (2011) Inter-Hemispheric Functional Connectivity Disruption in Children With Prenatal Alcohol Exposure. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Stefanescu RA, Martel DT, Shore SE (2015) Listening to another sense: somatosensory integration in the auditory system. Cell and tissue research 361:233–250. [DOI] [PMC free article] [PubMed] [Google Scholar]


