Abstract
Pediatric Philadelphia chromosome-like (Ph-like) acute B-lymphoblastic leukemia (B-ALL), a high-risk subset of B-ALL characterized by a gene expression profile similar to that of Ph-positive ALL, has extremely poor outcome after a relapse following autologous chimeric antigen receptor (CAR)-T and haploidentical (haplo) hematopoietic stem cell transplantation(HSCT)therapy. with very limited treatment options. Donor-derived CAR T-cell therapy, the most vital advanced anticancer technology, may be a promising salvage strategy for patients with Ph-like B-ALL. Here, we presented a relapsed and refractory case of a child with Ph-like B-ALL after autologous anti-CD19 CAR T-cell therapy followed by haplo-HSCT. She successfully achieved the fourth complete remission (CR4) and maintained CR for five months after the sequential infusion of donor-derived anti-CD22 and anti-CD19 CAR T cells, with mild CRS side effects and no obvious graft-versus-host disease. A donor-derived anti-CD22 and -CD19 CAR T-cell therapy combined with a sequential infusion strategy may provide a promising alternative treatment strategy as effective and safe salvage therapy for children with recurrent and refractory Ph-like B-ALL after autologous CD19-directed CAR T-cell therapy followed by haplo-HSCT.
Keywords: Philadelphia-chromosome-like, acute lymphoblastic leukemia, chimeric antigen receptor, CD19, CD22
Background
In recent years, the best therapy outcomes have been achieved in children with acute B-lymphoblastic leukemia(B-ALL). However, recurrence still occurs in 15–20% of patients with B-ALL.1 Allogeneic hematopoietic stem cell transplantation(HSCT) is considered the best treatment option in these high-risk for relapse and refractory groups of pediatric patients.2 Unfortunately, some patients have disease relapse and progression, and their prognosis remains poor. Adverse genetic alterations and multiple gene mutations are considered as the main causes. Recently, Philadelphia chromosome-like (Ph-like) B-ALL was identified in children and becomes concerning. These patients account for approximately 15% of pediatric B-ALL, and have a very high rate of disease relapse and significantly poor outcomes.3,4 Data on how to treat pediatric patients with Ph-like B-ALL that relapsed after haploidentical (haplo)-HSCT are limited. In recent years, both autologous and allogeneic chimeric antigen receptor(CAR) T-cell technologies have yielded astonishing results in relapsed and refractory B-ALL.5–7 Here, we report on a child with Ph-like B-ALL that relapsed after autologous CAR T-cell therapy and haplo-HSCT and attained the fourth complete remission (CR4) and negative minimal residual disease (MRD) status through sequential infusion of allogeneic anti-CD22 and -CD19 CAR T cells.
Case Presentation
The patient was a 10-year-old female child admitted to the Department of Hematology of Nanjing Children’s Hospital due to dizziness, fatigue and mucosal bleeding on July 20, 2016. No special disease history was reported. The morphology of the bone marrow indicated acute lymphoblastic leukemia. Flow cytometry(FCM) of bone marrow cells revealed a B-cell phenotype. Karyotype analysis was 49, xx,+x,+8,+21[18]/46, xx[2]. Results of the fusion gene analysis by fluorescence in situ hybridization (FISH) were negative. Therefore, the diagnosis of B-ALL was confirmed, CR(defined as <5% lymphoblasts in bone marrow aspirate) was achieved through the standard risk protocol of CCCG-ALL-2015 (upfront window therapy with dexamethasone for 4 days; subsequently, remission induction with prednisone, vincristine, daunorubicin and PEG-asparaginase from day 5 to day 28, and cyclophosphamide, cytarabine and mercaptopurine from day 29 to day 35),8 followed by consolidated combination chemotherapy in next 10 months. However, fever and bleeding occurred. Bone marrow smear showed relapse in May 2017. She then received a reinduction therapy regimen of mitoxantrone, vincristine, L-asparaginase, and prednisone. However, intracranial hemorrhage occurred during the course of the treatment. Several weeks later, the blood in the brain was gradually absorbed by management with hemostasis, dehydration, hypotension, lumina and oxcarbazepine. Subsequently, she received chemotherapy consisting of high-dose cytarabine and vincristine, and achieved complete CR2 at 30 days. Nevertheless, two weeks later, she relapsed again with 50% prolymphocyte cells.
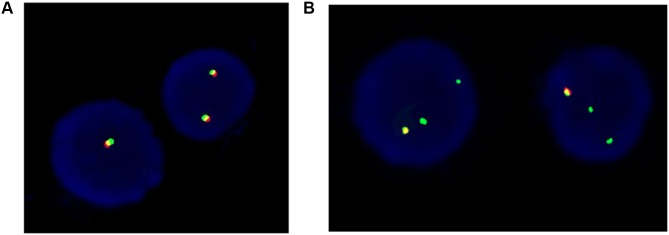
Due to the serious relapse and refractory leukemia, the patient was transferred to our hospital. Gene check analysis showed that the patient had Wilms tumor 1 and ecotropic viral integration site 1 gene mutations. Next generation sequence analysis of the patient’s bone marrow and oral mucosa revealed positive CSF1R gene (germline mutation), and cytokine receptor-like factor 2(CRLF2) gene rearrangement showed a 65% positive rate with FISH (Figure 1). All genetic analysis data supported the diagnosis of Ph-like B-ALL. Chemotherapy with hyper-CVAD(A) regimen was then initiated in our hospital with good therapeutic response. Three weeks later, the bone marrow smear showed primitive cells accounting for 4%, and the MRD examined by FCM was 1 × 10−4. All the examination data indicated that CR3 was achieved. The peripheral blood count results were as follows: white blood cell count,2.73×109/L; red blood cell count,2.38×109/L; hemoglobin 69g/L; and platelet count,60×109/L.
Figure 1.
FISH analysis of the bone marrow. (A) yellow signal indicates negative CRLF2 rearrangement; (B) two signals of red and green indicate CRLF2 abnormal rearrangement.
To achieve deep remission and remove leukemia residues, an autologous CAR T-cell therapy strategy was considered because of its remarkable therapeutic results. After a detailed conversation with her father and after obtaining written consent, peripheral blood mononuclear cells were collected from the patient for preparation of anti-CD19 CAR T cells by the Shanghai UniCAR Technology Co., Ltd. (UCT, Shanghai, China). On September 12, 2017, preconditioning lymphodepleting chemotherapy with the FC regimen (Cy30 mg/m2, day-5 to −3, fludarabine 30 mg/m2, day-5 to −3) was given 4 days before the infusion of anti-CD19 CAR T cells for a total T cell dose of 5×105/kg. Seven days later, the patient experienced moderate CRS with high fever (39.3 °C), elevated interleukin (IL)-6 (79.8pg/mL), and increased C-reactive protein (129 mg/L). Two weeks later, the bone marrow analysis showed an astonishing therapeutic efficacy with 1% prolymphocyte cells in the bone marrow and MRD of 1.1×10−4. More surprising, data showed that the CRLF2 gene rearrangement was negative.
To further control leukemia,8 weeks after CAR T-cell therapy, the patient received haplo-HSCT with granulocyte colony-stimulating factor that mobilized bone marrow cells and peripheral blood hematopoietic stem cells from her father. The preconditioning regimen was myeloablative and modified BUCY. During the course of HSCT, the patient first received umbilical cord blood infusions and then the bone marrow (mononuclear cells 8.58×108/kg;CD34+ cells 3.68×106/kg;CD3+cells 0.44×108/kg) and peripheral blood stem cells (mononuclear cells 2.2×108/kg; CD34+ cells 1.25×106/kg; CD3+cells 0.47×108/kg). In addition, on November 24, umbilical cord blood was infused.9 Neutrophil and platelet engraftments were observed on days +12 and +17, respectively. Acute graft-versus-host disease (GVHD) was not observed. The patient regularly returned to our outpatient department for follow-up. Her bone marrow remained CR3 status for approximately 240 days.
However, the patient was admitted to the Department of Hematology eight months after HSCT with symptoms of fatigue, abdominal pain, and marked emaciation. She was too weak to walk. Physical examination revealed a body weight of 25 kg, severe anemia, extreme emaciation, no skin rash and yellow stain, multiple ulcers in the oral mucosa, no swelling of superficial lymph nodes, and no tenderness of the sternum. Peripheral blood analysis revealed the following results: white blood cell count, 5.37×109/L; hemoglobin, 67 g/L; and platelet count, 74 ×109/L. Bone marrow examination showed the patient had relapsed for the fourth time with 79% primary and immature cells.
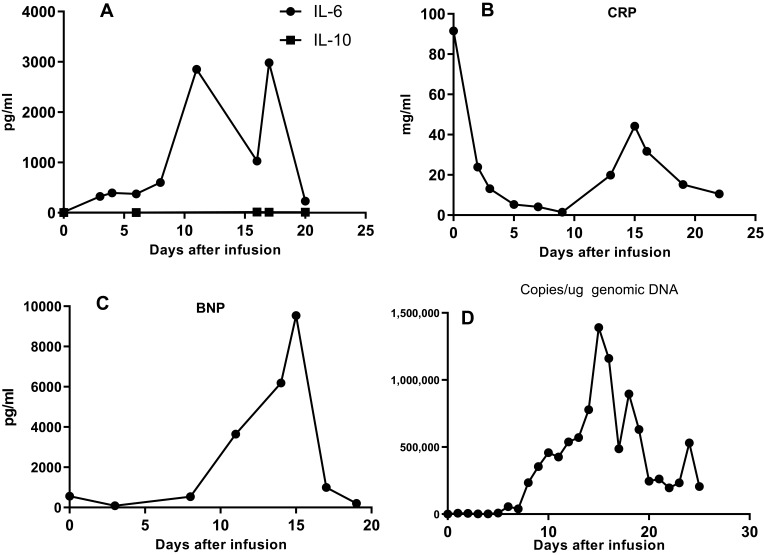
FCM of the bone marrow cells detected positive expression of CD10, CD19, CD20, CD38, and CD22, and, most importantly, the CD19-and CD22-positive rates were 99.6% and 84.9%, respectively. The CRLF2 gene rearrangement showed 90% a positive rate in FISH analysis. Nuclear magnetic resonance imaging of the abdomen showed bilateral kidney enlargement with abnormal signals, abnormal signals in the pancreas, gallbladder effusion, and decreased diffuse signal in the liver and spleen in the diffusion-weighted imaging sequence. These results confirmed that the patient was on the fourth relapse. The patient was at very late stage of the disease with poor physical tolerance and an Eastern Cooperative Oncology Group score of 2–3. Any traditional treatment strategies are high-risk at this point. The patient’s parents were informed of the serious situation of their daughter and pressed for further treatment. Written informed consent was obtained. After carefully discussing this issue, a salvaged therapeutic regimen was performed, in which donor-derived anti-CD19 and -CD22 CAR T-cell therapy was considered due to the highly expressed CD19 and CD22 on the surface of the leukemia cells. CAR T cells against different targets were successfully engineered by UCT. Preconditioning chemotherapy (CY 20 mg/m2, day−5 to −3, fludarabine 20 mg/m2, day−5 to −3)was administered at lower doses because of the patient’s poor condition. Then anti-CD22 CAR T cells were infused at a total dose of 1×107 cells/kg (transduction efficiency of 50.3%) on days 1 to 2, followed by anti-CD19 CAR T cells at a total dose of 1×107 cells/kg (transduction efficiency of 53.61%) on days 3 to 5. No immediate infusion-related toxic effects were observed. In addition, tocilizumab was administered to prevent CRS. The patient was strictly monitored for body temperature, cardiovascular, respiratory function, C-reactive protein, cytokines (IL-2, IL-4, IL-6, IL-10, etc.), and B-type natriuretic peptide precursor (PRO-BNP). Three days after the infusion of anti-CD22 CAR T cells, the levels of IL-6 and PRO-BNP increased rapidly (Figure 2) with peak levels on days 10 and 12, respectively. The CRS response level was ranked grade 2 based on the University of Pennsylvania grading system. Other indicators, such as body temperature, lactic acid dehydrogenase, and ferritin, mildly fluctuated. After anti–infection, supportive transfusions of red blood cells and platelets, and nutritional support were administered, the patient’s condition quickly improved. The levels of IL-6 and PRO-BNP returned to near normal and the symptoms were gradually alleviated. Thirteen days later, 79% of primary and immature cells were found in the bone marrow smear, and the MRD was 37.3%. Copies of anti-CD22 and anti-CD19 CAR DNA quickly increased in the blood (Figure 2). STR examination of bone marrow cells revealed complete donor chimerism (98.9% of donor origin).
Figure 2.
Dynamic changes following the infusion of anti-CD22 and -CD19 cells within 25 days: (A) IL-6 reached peak level on day 17 and returned to normal on day 20 after infusion. IL-10 level was basically normal in the whole course; (B) C-reactive protein rapidly decreased from 92mg/mL to normal, then increased to 44.2 mg/mL on day 15; (C) During the first 15 days, BNP gradually increased to peak level; (D) Expansion of anti-CD22 and anti-CD19 CAR T cells in the peripheral blood after infusion. Genomic DNA copies increased to peak level on day 15.
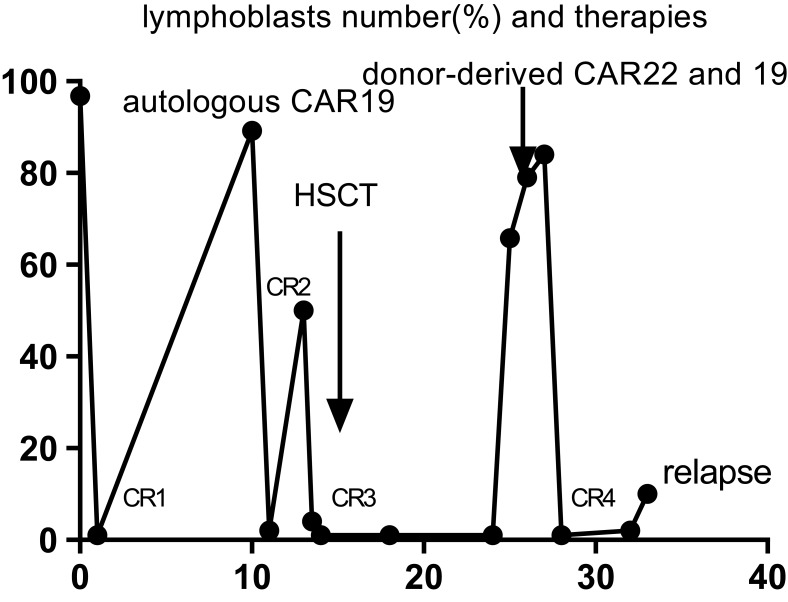
One month after the infusion, the patient was re-examined and the bone marrow was CR4 and MRD was negative (Figure 3). She gained 14 kg of body weight in a month and her general condition improved. Ruxolitinib was given as a maintenance therapy. Over the following five months, her peripheral blood examination and bone marrow cell investigation still showed CR10 without GVHD. At six months after the last infusion, the disease relapsed.
Figure 3.
Morphological CR status and number of lymphoblasts in the bone marrow at different times. Autologous anti-CD19 CAR T cells bridging with allo-HSCT post CR3. CR4 was obtained followed by sequential of infusion donor-derived anti-CD22 and -CD19 CAR T cells.
Discussion and Conclusions
We reported a child with highly refractory Ph-like B-ALL and multiple relapses who underwent multiple chemotherapies, and autologous anti-CD19 CAR T-cell therapy and haplo-HSCT. CR4 was achieved through sequential infusions of donor-derived anti-CD22 and-CD19 CAR T cells. Presently, morphological CR has been maintained for five months. Ph-like B-ALL was first reported in 2009. Den Boer et al11 found that approximately 15–19% of the gene expression profile was undetermined in B-ALL. The GEP is very similar to that of Ph- positive B-ALL, and both have similar patient prognosis. In recent years, with further in-depth research, more comprehensive understanding has been acquired about Ph-like B-ALL.12–14 The majority of Ph-like B- ALL genetic alterations are divided into the following two main groups: 1) is CRLF2 rearrangements along with JAK-STAT pathway aberrations, and 2) fusions involving JAK2, ABL1, ABL2, and CSF1R.15 In this case, positive CRLF2 gene rearrangement was revealed through an additional FISH analysis. The most prevalent molecular genetic alteration in children with Ph-like B-ALL is the abnormal expression of CRLF2.16,17 Therefore, the patient was diagnosed with pediatric Ph-like B-ALL. Diagnosing pediatric Ph-like B-ALL is challenging for clinical doctors due to highly heterogeneous changes in molecular biology.18 At present, no unified diagnostic standard is available. Therefore, children with Ph-like B-ALL with poor therapeutic effects or multiple recurrences should be carefully monitored. Currently, screening of potential kinase-activating alterations at the initial workup by an increasing number of hematology doctors is necessary. Previous studies18–20 reported that pediatric patients with Ph-like B-ALL often have high relapse rates and poor clinical outcomes. High-risk patients may be advised to receive allogeneic stem cell transplantation;21 however, some patients still relapse after HSCT. The patient in our case experienced three relapses after receiving standard and intensive combination chemotherapy regimens and CAR T-cell therapy plus haplo-HSCT in less than 2 years. Her clinical outcome is consistent with Ph-like B-ALL, a characteristic of treatment failure and a high relapse rate.
Anti-CD19 CAR T-cell therapy has a higher CR rates as compared to chemotherapy regimens alone in children with relapsed or refractory B-ALL,22,23 with an overall remission rate of approximately 81% within three months. In our case, the donor-derived CD19-directed CAR T-cell therapy showed very good safety outcomes with mild CRS and promising therapeutic results with CR4 and subsequent CRLF2 negative rearrangement. However, when facing recurrence and progress of Ph-like B-ALL after autologous anti-CD19 CAR T-cell therapy and/or allo-HSCT, the viable options are limited. Studies have reported that infusion of allogeneic anti-CD19 CAR T-cell therapy can result in regression of B-cell malignancies.5,24 CD22-targeted CAR T-cell treatments were reported to have a significant efficacy on refractory/relapsed B-ALL.25,26 Preclinical models may cause sequential infusion of CAR T cells therapy synergistic responses as compared to targeting a single antigen, improving the response rates.27,28 Based on the above theoretical data and the patient’s leukemia cells that highly expressed CD19 and CD22, the patient received donor-derived anti-CD22 and anti-CD19 CAR T cells. In our case, CD22-directed CAR T cells were infused first, followed by CD19-directed CAR T cells, because of the patient’s high leukemia cell burden and high CD22 expression rate. Moreover, anti-CD22 CAR T cells may cause less severe CRS as compared to CD19-directed CAR T cells. Surprisingly, the patient achieved CR with negative MRD one month after the CAR T-cell infusion, and the CRS showed a grade II level with the main side effects of rapidly increased IL-6 and PRO-BNP levels, reaching their peak on the 13–15th day after the sequential infusion anti-CD22 and anti-CD19 CAR T cells, without producing serious acute GVHD, tumor lysis syndrome, and later neurotoxicity. The Side effects were relatively mild and easily controlled.
This is the first report that utilized allogeneic donor-derived anti-CD22 and -CD19 CAR T cells to successfully treat a patient with relapsed and refractory pediatric Ph-like B-ALL after autologous anti-CD19 CAR T-cell therapy and haplo-HSCT. Other major issues to consider include the following: (1) How to maintain the CR status? (2) What is the protocol of maintenance treatment? (3) Are other immunotherapeutic methods effective and safe? And (4) The patient had five relapses, is there any available salvage therapeutic method?
Our case report showed the excellent efficacy and safe tolerance of allogeneic anti-CD22and-CD19 CAR T-cell therapy. Donor-derived dual CAR T-cell infusion may be a promising and novel strategy for the treatment of pediatric Ph-like B-ALL with recurrence following autologous CAR T-cell therapy and haplo-HSCT. Due to high relapse rates and poor prognosis, novel highly effective strategies are needed to improve the treatment outcome of these patients. To our knowledge, this is the first reported case worldwide where a patient with pediatric Ph-like B-ALL that relapsed after donor CAR T-cell treatment and HSCT successfully obtained CR4 with donor-derived CAR T-cell (anti-CD22 and anti-CD19) technology and regimen.
Acknowledgments
The authors would like to thank all members of the study team, the patient and her family, and Shanghai Uni-CAR Technology Co., Ltd. Thank Xue bing Bao, Liqing Kang, and Depei Wu for providing writing advices.
Consent for Publication
The patient’s parents provided written informed consent for the case details to be published.
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Soochow University (IRB ID:2015070). The patient and donor provided their written informed consent according to the Declaration of Helsinki. The study was registered at https://www.clinicaltrials.gov as NCT03614858.
Data Sharing Statement
The material supporting the conclusion of this study has been included within the manuscript.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Jingsheng Hua, Weiqing Qian, Xiaoxia Wu contributed equally as first co-author, Jian Zhang, and Huiying Qiu equally as co-corresponding Author.
Funding
This work was supported by the National Natural Science Foundation of Jiangsu Province (BE2018652).
Disclosure
All authors declare no conflicts of interest in this work.
References
- 1.Hunger SP, Mullighan CG, Longo DL. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–1552. doi: 10.1056/NEJMra1400972 [DOI] [PubMed] [Google Scholar]
- 2.Peters C, Schrappe M, von Stackelberg A, et al. Stem cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors the ALL-SCTBFM-2003 trial. J Clin Oncol. 2015;33:126574. doi: 10.1200/JCO.2014.58.9747 [DOI] [PubMed] [Google Scholar]
- 3.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371:1005–15. doi: 10.1056/NEJMoa1403088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey RC, Mullighan CG, Wang X, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010;116(23):4874–4884. doi: 10.1182/blood-2009-08-239681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brudno JN, Somerville RPT, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-Cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–1121. doi: 10.1200/JCO.2015.64.5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davila ML, Brentjens RJ. CD19-targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14:802–808. [PMC free article] [PubMed] [Google Scholar]
- 7.Kebriaei P, Singh H, Huls MH, et al. Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest. 2016;126:3363–3376. doi: 10.1172/JCI86721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai J, Yu J, Zhu X, et al. Treatment abandonment in childhood acute lymphoblastic leukaemia in China: a retrospective cohort study of the Chinese Children’s Cancer Group. Arch Dis Child. 2019:104(6);1–8. Web of science. [DOI] [PubMed] [Google Scholar]
- 9.Tao T, Li Z, Chu X, et al. Clinical features of chronic graft-versus-host disease following haploidentical transplantation combined with infusion of a cord blood. Stem Cells Dev. 2019;28(11):745–753. doi: 10.1089/scd.2018.0259 [DOI] [PubMed] [Google Scholar]
- 10.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28. doi: 10.1038/nm.4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–134. doi: 10.1016/S1470-2045(08)70339-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain N, Roberts KG, Jabbour E, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129(5):572–581. doi: 10.1182/blood-2016-07-726588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts KG, Gu Z, Prayne-Tuner D, et al. High frequency and poor outcome of philadelphia chromosome-like acute lymphoblastic leukemia in adults. J Clin Oncol. 2017;35(4):394–401. doi: 10.1200/JCO.2016.69.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 15.Yap KL, Furtado LV, Kiyotani K, et al. Diagnostic evaluation of RNA sequencing for the detection of genetic abnor malities associated with Ph-like acute lymphoblastic leukemia (ALL). Leuk Lymphoma. 2017;58(4):950–958. doi: 10.1080/10428194.2016.1219902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephen P, Hunger MD, Charles G, et al. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972 [DOI] [PubMed] [Google Scholar]
- 17.Perez-Andreu V 1, Roberts KG, Harvey RC, et al. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat Genet. 2013;45(12):1494–1498. doi: 10.1038/ng.2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boer JM, Marchante JR, Evans WE, et al. BCR-ABL1-like cases in pediatric acute lymphoblastic leukemia: a comparison between DCOG/erasmus MC and COG/St. Jude signatures. Haematologica. 2015;100:354–357. doi: 10.3324/haematol.2015.124941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, hispanic/latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–5321. doi: 10.1182/blood-2009-09-245944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cario G, Zimmermann M, Romey R, et al. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prog nosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood. 2010;115(26):5393–5397. doi: 10.1182/blood-2009-11-256131 [DOI] [PubMed] [Google Scholar]
- 21.Roberts KG, Pei D, Campana D, et al. Outcomes of children with BCR-ABL1-like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol. 2014;32(27):3012–3020. doi: 10.1200/JCO.2014.55.4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–4139. doi: 10.1182/blood-2013-08-519413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2017;24:20–28. doi: 10.1038/nm.4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegde M, Corder A, Chow KK, et al. Combi national targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther.2013;21:2087–2101. doi: 10.1038/mt.2013.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grada Z, Hegde M, Byrd T, et al. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids.2013;2:e105. doi: 10.1038/mtna.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]