Key Points
Glycans that are present on glycoproteins often have a pivotal role in protein folding, trafficking and protection against degradation, the efficient operation of the innate immune system and, as in the case of pathogens such as HIV, escape by the immune system.
There exists a wide range of carbohydrate-binding agents (CBAs), including lectins and synthetic small-size non-peptidic agents, and their molecular interactions with carbohydrates are well described.
A number of CBAs, with a preference for mannose and/or N-acetylglucosamine (GlcNAc) recognition have been shown to inhibit HIV infections by blocking the viral entry process. They have also been shown to be active against other viruses such as human hepatitis C virus (HCV), coronavirus and influenza virus.
When HIV is exposed to CBAs, virus escape eventually occurs predominantly by deleting its glycans on the viral envelope glycoprotein gp120. It has been shown that such mutant virus strains have an increased sensitivity to neutralizing antibodies.
Numerous other pathogens, including HCV, bacteria such as Mycobacterium tuberculosis and Helicobacter pylori, parasites such as Leishmania spp. or fungi such as Aspergillus spp. and Candida spp., efficiently use the C-type lectin dendritic-cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN) present on DCs for transmission and/or immune suppression. These pathogens may represent prime candidate compounds that can be exposed to CBAs in an attempt to interrupt the infection or transmission process in the host.
CBA therapy of HIV (and possibly other pathogens) may represent a therapeutic approach with a dual mechanism of action: the direct inhibition of virus capture, transmission and entry into its target cells; and the recruitment of the immune system after glycan deletions occur under CBA pressure, uncovering previously hidden immunogenic epitopes on the viral envelope.
Carbohydrate-binding agents (CBAs) are a family of diverse molecules that can bind to specific glycan structures on viruses or target cells. Jan Balzarini describes a new antiviral mechanism that is based on the specific interaction of CBAs with the glycans that are present on viral-envelope glycoproteins.
Abstract
Several chronic viral infections (such as HIV and hepatitis C virus) are highly prevalent and are a serious health risk. The adaptation of animal viruses to the human host, as recently exemplified by influenza viruses and the severe acute respiratory syndrome coronavirus, is also a continuous threat. There is a high demand, therefore, for new antiviral lead compounds and novel therapeutic concepts. In this Review, an original therapeutic concept for suppressing enveloped viruses is presented that is based on a specific interaction of carbohydrate-binding agents (CBAs) with the glycans present on viral-envelope glycoproteins. This approach may also be extended to other pathogens, including parasites, bacteria and fungi.
Main
Any attempts to develop an efficient vaccine against chronic viruses, such as HIV and human hepatitis C virus (HCV), have so far failed. This is mainly due to the inventive immunological escape mechanisms of these viruses1,2, as well as a lack of efficient long-term protective vaccines that can be directed against conserved epitopes of viruses that are involved in acute infections (such as the influenza virus)3. Instead, a broad range of chemotherapeutic agents is available for the treatment of various viral infections, in particular for HIV4. However, the appearance of long-term side effects and, in particular, the eventual emergence of viral resistance under drug pressure, often weakens the therapy and makes the drugs useless and even harmful in the long run. The targets of the currently available antiviral agents are essential virus-encoded enzymes, virus-specific structural proteins or cellular proteins (that is, viral receptors)4, but the sometimes-abundant presence of glycans on viral-envelope glycoproteins has never been seriously envisaged as a therapeutic target.
Glycans on the viral envelope often have a crucial role in enabling an efficient transmission of the pathogen and/or entry into its susceptible target cells. Moreover, it has been shown that the presence of glycans on the envelope of viruses, such as HIV and HCV, is also of crucial importance for the evasion of the immunological surveillance of the host. Agents that interact with the viral-envelope glycans may, therefore, compromise the efficient entry of the virus into its susceptible target cells. Such agents do not interfere with the glycosylation enzymes from the cell, but rather act by directly binding to the intact glycans on the viral envelope. Perhaps more importantly, such carbohydrate-binding agents (CBAs) may force the virus to delete at least part of its glycan shield to escape drug pressure5; this might result in the initiation of an immune response against uncovered immunogenic envelope epitopes.
CBAs may become the first chemotherapeutics with a dual mechanism of antiviral action: first, through direct antiviral activity, by binding to the glycans of the viral envelope and subsequently blocking virus entry, and second, through indirect (additional) antiviral action resulting from the progressive creation of deletions in the envelope glycan shield, thereby triggering the immune system to act against previously hidden immunogenic epitopes of the viral envelope (Fig. 1). In the broader perspective, apart from viruses, other pathogens such as Mycobacterium tuberculosis, Helicobacter pylori and some parasites may also be susceptible to this novel therapeutic approach. This Review will focus on CBAs and the molecular mechanism of their antiviral activity (Fig. 1). The escape mechanisms of HIV in response to CBA pressure and how these escape mechanisms might involve the immune system to further combat the viral infection will also be discussed. The interference of CBAs with the dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing non-integrin (DC-SIGN)-directed capture and transmission of HIV and other pathogens will also be highlighted, and the unique features of the CBA therapeutic concept and its potential pitfalls will be discussed.
Figure 1. Overview of the particular antiviral activities of carbohydrate-binding agents (CBAs).
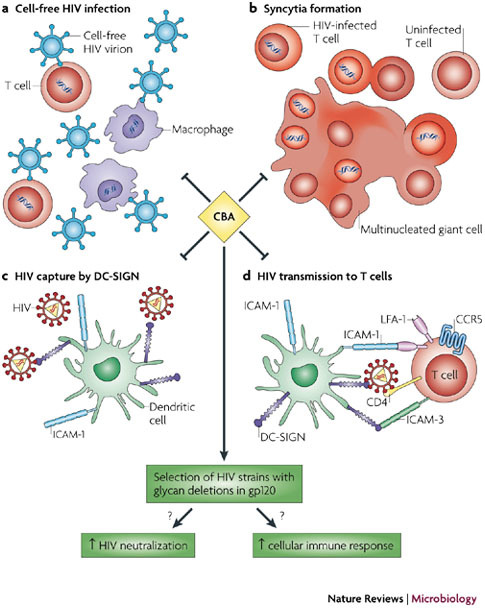
CBAs have been shown to efficiently inhibit the infection of T cells and macrophages by cell-free HIV particles (a), syncytia formation between HIV-infected cells and uninfected T cells (b), the capture of HIV particles by dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing non-integrin (DC-SIGN)-expressing cells such as DCs (c), and DC-SIGN-captured HIV transmission to T cells (d). Exposure of CBAs to the virus in cell culture has also been shown to force the virus to delete part of the protective glycan shield that is present on its envelope glycoprotein gp120. It is assumed that such glycan deletions trigger an enhanced neutralizing antibody response to the previously hidden immunogenic epitopes of gp120 and possibly also a cellular immune response. Parts c and d reproduced with permission from Ref. 122 © (2006) University of Amsterdam. CCR5, chemokine receptor 5; LFA-1, lymphocyte function-associated antigen 1.
Role of glycans on glycoproteins
Glycosylation is a highly diverse co- and post-translational protein-modification reaction that can be broadly divided into two categories; O-linked and N-linked glycosylation. In O-linked glycosylation, which probably occurs in the Golgi apparatus, the carbohydrate moiety is covalently linked to the hydroxyl oxygen of serine and threonine, but it can also be bound to tyrosine, or to 5-hydroxylysine and 4-hydroxyproline6. O-glycosylation has various functions, such as providing ligands for selectins, resistance to proteolysis of stem regions of membrane proteins and creating specific recognition phenomena6. It can also help to mask immunogenic epitopes on the protein. O-linked glycosylation usually has N-acetylgalactosamine (GalNAc) as the binding sugar but can also involve other sugars, such as fucose, glucose and N-acetylglucosamine (GlcNAc). Galactose and/or sialic acid are also often found in O-glycans.
The covalently N-linked glycans (Fig. 2) are added co-translationally to native polypeptides in the endoplasmic reticulum (ER) as blocks of fourteen sugars (Glc3Man9GlcNAc2). These glycans are then subject to extensive modification during their transport through the ER and the Golgi complex before reaching their final destinations inside or outside the cell7. In the ER and the early secretory pathway, the oligosaccharide repertoire is still small. In the Golgi complex, however, the glycans acquire complex and highly diverse structures by terminal glycosylation, which results in a tremendous heterogeneity. Such diversity differs between cell types, tissues and species, and helps to further increase microheterogeneity in the presence of an identical genetic polypeptide background. This results in the creation of new functionalities and specificities8,9. The N-glycans may also have an important role in proper protein folding and degradation10, and solubility, by avoiding the precipitation that is caused by lipophylic amino-acid stretches in the nascent polypeptide11. They also control proper peptidic oligomerization and the sorting of the peptides, as well as peptide transport and trafficking (by acting as universal 'tags' for specific cellular lectins and modifying enzymes)7,9,12. The presence of a glycan shield on the peptides also enables the efficient protection of the glycoproteins against degradation by proteases.
Figure 2. Structures of different N-glycan types.
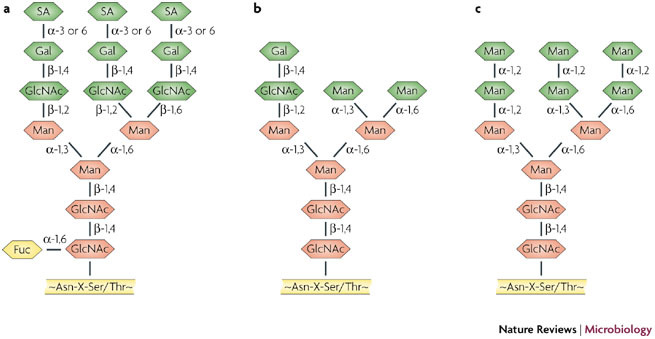
Examples of the structural composition of high-mannose-type N-glycans. a | Tri-antennary complex-type N-glycans. b | Hybrid-type N-glycans. c | High-mannose-type N-glycans that are abundantly present on the envelope glycoprotein gp120 of HIV, but are rare on mammalian glycoproteins. Besides high-mannose-type N-glycans, the complex-type and hybrid-type N-glycans are also present on gp120. Asn, asparagine; Fuc, fucose; Gal, Galactose; GlcNAc, N-acetylglucosamine; Man, mannose; SA, sialic acid; Ser, serine; Thr, threonine; X, any amino acid except proline.
The interactions of carbohydrates with cellular lectins are also of crucial importance for the efficient operation of the innate immune system. Examples include the mannose-binding lectin (MBL)13, DC-SIGN14, defensins15 and macrophage mannose receptors16. Leukocyte interactions with endothelial cells represent a well-characterized example of a cell-adhesion event that depends on glycan–receptor interactions17. Cell-surface glycoproteins can, therefore, mediate cell adhesion and signalling events, as well as intercellular communication.
As well as mammalian cells, many different pathogens, including viruses, bacteria, fungi and parasites, also use glycoproteins extensively for diverse functions, in part similar to eukaryotic cells. However, as the glycans on the pathogen (in particular, viral-derived glycoproteins) are produced by the cellular machinery, they are often recognized as 'self' by the immune system. Therefore, the glycans on pathogen glycoproteins in the viral envelope or bacterial cell wall help to escape recognition by the immune system, and the subsequent destruction or neutralization of the pathogen.
Classes of carbohydrate-binding agents
Arbitrarily, two different categories of CBAs can be distinguished: lectins, which are proteins that specifically recognize carbohydrate (glycan) structures, and non-peptidic small-size agents that may have a good and often specific affinity for monosaccharide and/or oligosaccharide structures.
Peptidic carbohydrate-binding agents. Several CBAs of prokaryotic origin have been isolated and characterized (Table 1). The most well-studied CBA is undoubtedly cyanovirin-N (CV-N), an 11-kDa protein (composed of 101 amino acids consisting of two sequence repeats) originally purified from extracts of the cyanobacterium Nostoc ellipsosporum18. The elucidation of CV-N crystal structures revealed the existence of a domain-swapped dimer, with two primary carbohydrate-binding sites and two secondary carbohydrate-binding sites on opposite ends of the dimer19,20,21. The carbohydrate-recognition sites have a binding geometry of high-mannose glycans, in particular α(1,2)-linked mannose oligomers22,23. A monomeric 13-kDa protein isolated from the unicellular freshwater bloom-forming cyanobacterium Microcystis viridis NIES-102 strain (Microcystis viridis lectin (MVL))24 was also shown to be composed of two tandemly repeated homologous domains, with specificity for α(1,6)- and possibly α(1,3)-mannose oligomers. Its smallest target is a Man2GlcNAc2 tetrasaccharide core25. Scytovirin (SVN), a 9.7-kDa peptide, with 95 amino acids, has most recently been isolated from the cyanobacterium Scytonema varium26 and was shown to have a pronounced affinity for α(1,2)–α(1,6)-mannose trisaccharide units27. Both CV-N and SVN inhibit HIV infection in cell culture, at 50% effective concentrations of 0.1 and 0.3 nM, respectively. MVL, however, is less inhibitory against HIV28.
Table 1.
Carbohydrate-binding proteins of a non-mammalian origin
| Species | Lectin name | Abbreviation | Carbohydrate specificity |
|---|---|---|---|
| Cyanobacteria | |||
| Nostoc ellipsosporum | Cyanovirin-N | CV-N | α(1,2) Man |
| Scytonema varium | Scytovirin | SVN | α(1,2)-α(1-6)Man |
| Microcystis viridis | None | MVL | Manb(1,4)GlcNAc |
| Sea corals | |||
| Gerardia savaglia | None | GSL | d-Man |
| Algae | |||
| Griffithsia spp. | Griffithsin | GRFT | Man, Glc, GlcNAc |
| Fungae | |||
| Longispora albida | Actinohivin | AHA | Man |
| Annelida | |||
| Chaetopterus variopedatus | None | CVL | β-Gal |
| Laxus oneistus | Mermaid | None | Man |
| Plants | |||
| Orchidaceae | |||
| Listera ovata | Twayblade lectin | LOA | α(1,3)Man |
| Epipactis helleborine | Broad-leaved helleborine lectin | EHA | Man |
| Cymbidium hybrid | None | CHA | Man |
| Amaryllidaceae | |||
| Galanthus nivalis | Snowdrop lectin | GNA | α(1,3)Man |
| Hippeastrum hybrid | Amaryllis lectin | HHA | α(1,3)-α (1,6)Man |
| Narcissus pseudonarcissus | Daffodil lectin | NPA | α(1,6)Man |
| Alliaceae | |||
| Allium porrum | Leek lectin | APA | Man |
| Allium ursinum | Ramsons lectin | AUA | Man |
| Moraceae | |||
| Artocarpus integrifolia | Jacalin, jack fruit lectin | Jacalin | Galα(1,6) or Galβ(1,3)GalNAc |
| Fabaceae | |||
| Canavalia ensiformis | Jack bean lectin | ConA | Man>Glc>GlcNAc |
| Pisum sativum | Garden pea lectin | PSA | Man>Glc/GlcNAc |
| Lens culinaris | Lentil lectin | LCA | Man>Glc>GlcNAc |
| Vicia faba | Broad bean, faba bean lectin | VFA | Man>Glc/GlcNAc |
| Lathyrus odoratus | Sweet pea lectin | None | Man>Glc>GlcNAc |
| Urticaceae | |||
| Urtica dioica | Stinging nettle lectin | UDA | GlcNAc oligomers |
| Cecropiaceae | |||
| Myrianthus holstii | Myrianthin | MHA | GlcNAc |
| Euphorbiaceae | |||
| Hevea brasiliensis | Rubber tree lectin, hevein | HBA | GlcNAc |
| The > symbol indicates a higher preference. Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; Man, mannose. | |||
A CBA derived from the sea coral Gerardia savaglia (GSA) was one of the first lectins isolated from a primitive eukaryotic organism29. This D-mannose-specific CBA is a dimer, with each monomer being 14.8 kDa, and requiring calcium to preserve full carbohydrate-binding activity. Actinohivin30, derived from the actinomycete Longisporum alba (a 12.5-kDa protein, with 114 amino acids), and Griffithsin (GRFT)31, isolated from the red alga Griffithsin spp. (a 13-kDa protein, with 121 amino acids), were also recently shown to recognize mannose-type glycans. Interestingly, the calcium-independent GRFT, a dimeric protein with four α(1,2)-mannose carbohydrate-binding domains (CBDs) separated by short linker sequences, has no homology to any other primary amino-acid sequence that has been found so far31. GSA showed complete suppression of HIV-1 infection in the H9 cell line at a concentration of 0.2 μM. At the same concentration, syncytia formation between H9 and HIV-1-persistently infected Jurkat cells was blocked32. Actinohivin inhibits both T-cell and macrophage infection by HIV-1 at 60 to 700 nM concentrations in cell culture33; GRFT is exquisitely active against CXC-chemokine receptor 4 (CXCR4)-tropic HIV-1 (X4 HIV-1) and CC-chemokine receptor 5 (CCR5)-tropic HIV-1 (R5 HIV-1) isolates, with EC50s ranging from 0.04 to 0.63 nM31.
CBAs that have a broad array of carbohydrate specificities, including mannose, glucose, galactose, fucose, sialic acid, GlcNAc and GalNAc oligomers, are prevalent in many plant families. Monomer and dimer forms of plant lectins predominate, but trimer, tetramer and even octamer plant lectins exist that lead to quaternary protein complexes that have relatively high molecular weights (for an overview, see Refs 34,35). The crystal structures of a number of plant lectins in complex with carbohydrate oligomers have been determined, such as the mannose-specific lectin from Galanthus nivalis (GNA)36 and the GlcNAc-specific lectin from Urtica dioica (UDA)37. The anti-HIV activities of plant lectins vary greatly, depending on the nature of the lectin source. For GNA and UDA, EC50 values of approximately 0.01 and 0.1 μM, respectively, against HIV-1 in CEM cell cultures were reported38,39,40,41.
CBAs have also been isolated from invertebrates, such as CVL from Chaetopterus variapedatus42 or Mermaid from Laxus oneistus43. Whereas CVL has β-galactose specificity, Mermaid is a calcium-dependent mannose-specific CBA. Interestingly, Mermaid was reported to have a strong structural resemblance to mammalian DC-SIGN43. The antiviral activity of CVL against HIV is in the range of 0.004–0.06 μM42; the anti-HIV activity of Mermaid has not yet been reported.
Mammalians also have several types of CBA. MBL is a calcium-dependent multimeric CBA that is found in serum13,44 and contains subunits of approximately 31 kDa. Besides mannose, it also binds GlcNAc and fucose. MBL is part of the innate immune system and binds pathogens as the initiating step of the lectin pathway in order to opsonize the pathogen44,45. DC-SIGN is another example of a vertebrate mannose-specific lectin that is predominantly present on immature DCs46. It functions in DC recognition and the uptake of pathogens (such as HIV), leading to antigen presentation to T cells47. Various other CBAs, apart from MBL or DC-SIGN, are also part of the innate and/or adaptive immune system. Mammalian defensins (α, β and cyclic Φ), a family of soluble glycan-binding proteins, are probably the best-studied lectins of our immune system15. Galectins also have a role in cell–cell recognition and the triggering of intracellular signalling cascades that lead to apoptosis48.
Finally, the monoclonal antibody 2G12 is one of the few broadly neutralizing anti-HIV antibodies. It is directed against an epitope on the HIV envelope glycoprotein gp120, that lies around the C4–V4 region49,50. This epitope contains high-mannose-type glycans, which are present at several highly conserved N-glycosylation sites (specifically N295, N332 and N392 in gp120)49,51. The predominant interaction sites of 2G12 with gp120 are probably the terminal α(1,2)-mannose oligomers of the high-mannose glycans. It should be noted, however, that 2G12 specifically recognizes HIV gp120 glycans, but does not specifically interact with peptide moieties near the glycan structures on gp120 (Refs 49, 50, 51, 52, 53). The 2G12 antibody was found to be inhibitory to HIV-1(IIIB) in different cell types at an EC50 of 0.02 to 0.2 μg per ml. However, it should be noted that 2G12 activity can vary depending on the nature of the HIV-1 subtype isolates that are evaluated54.
Non-peptidic carbohydrate-binding agents. In the course of screening for new antibiotics that are active against fungi, the actinomycete strain Actinomadura hibisca was found to produce pradimicin A (PRM-A)55. This antibiotic has a unique non-peptidic structure that contains the amino acid D-Ala and the carbohydrates D-xylose and 4,6-dideoxy-4-methylamino-D-galactose attached to a substituted 5,6-dihydrobenzo[a]naphtacenequinone (Fig. 3a). PRM-A binds to terminal D-mannose pyranoside and calcium to yield a ternary complex that consists of two molecules of PRM-A, four molecules of mannose and one calcium atom56. Benanomicin A (BNM-A) (Fig. 3b), a closely related antibiotic with mannose specificity that is similar to PRM-A, has also been isolated from the actinomycete Actinomadura spadix and studied for antifungal activity57,58. PRM-A, BNM-A and semi-synthetic analogues of these compounds are the only antibiotics that are formally known to have well-defined carbohydrate-binding properties and for which the antiviral activity in cell culture has been reported (the 50% effective concentration against HIV-1 ranks in the lower micromolar range)58,59,60.
Figure 3. Low-size non-peptidic carbohydrate-binding antigens (CBAs).
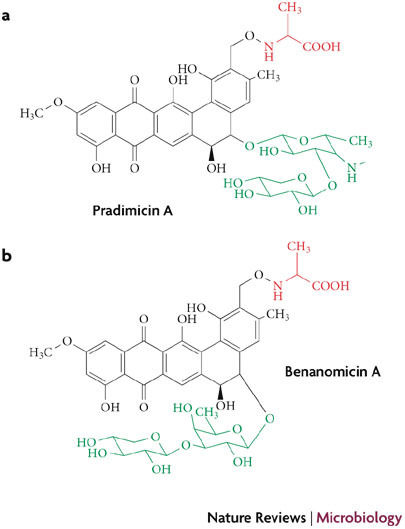
Structural formulae of the calcium-dependent mannose-binding pradimicin A (a) and benanomicin A (b) antibiotic CBAs that are produced by Actinomadura hibiscus and Actinomadura spadix, respectively. Red represents the D-alanine moiety; black represents the dihydrobenzonaphtacenequinone core; green represents the carbohydrate part of the molecule.
Several research groups focus on the synthesis and characterization of synthetic CBAs that bind specific oligosaccharide structures. Binuclear copper (II) complexes61, acyclic pyridine- and pyrimidine-based compounds62, and tetrapyrrole (porfyrin) derivatives have all been reported to have carbohydrate-binding properties. These compounds may have potential benefit as both antiviral and diagnostic agents. However, for these small-size CBAs, few, if any, antiviral data are available, and their potential for cytotoxicity has not been carefully addressed so far. It would be interesting, therefore, to explore the antiviral properties of such compounds to identify novel synthetic low-molecular-weight CBA lead compounds that have chemotherapeutic potential.
Interactions of CBAs with carbohydrates
A broad range of proteins bind high-mannose-type glycans of HIV gp120. Several binding modes can be distinguished63. One group of lectins interact as C-type lectins via a calcium ion. Two of the best-known examples of such calcium-dependent carbohydrate-binding lectins of the innate immune system are DC-SIGN and serum or liver MBLs. Another group of lectins interacts with single-terminal carbohydrates or have more intimate interactions with multiple sugar rings, without the need of a metal ion. For a third group of lectins, the interactions have not yet been resolved.
The carbohydrate specificity of MBL is broad. The MBLs recognize D-mannose, GlcNAc and L-fucose. A common motif among these sugars is defined by the presence of vicinal, equatorial hydroxyl groups in positions C-3 and C-4 of the sugar ring. Most binding affinity is derived from direct coordination of calcium with the C-3 and C-4 hydroxyls of the carbohydrate. In addition, the NH2 groups of two Asn residues further enable the efficient formation of the intimately linked ternary complex of protein, calcium and carbohydrate64 (Fig. 4a).
Figure 4. Molecular interactions of carbohydrate-binding agents (CBAs) with carbohydrate oligomers.
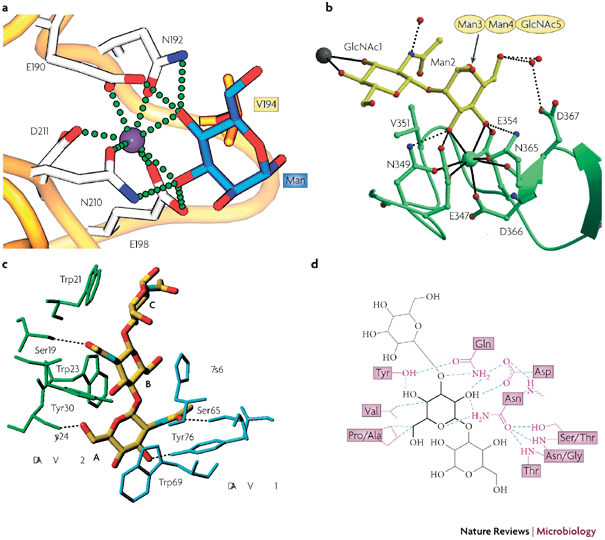
a | The terminal mannose (Man) of Manα1–3Man is complexed to liver MBP–C64 (Protein Data Bank (PDB) ID 1kza). Calcium is depicted as a purple sphere, with coordinating residues in stick representation. The amino-acid residue Val194 (in orange) participates in van der Waals interactions. The calcium coordination and hydrogen bonds between protein and carbohydrate (blue) are shown as green spheres. b | Ribbon diagram of dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing non-integrin (DC-SIGN) complexed with Man3 −(N-acetylglucosamine)2 (Man3GlcNAc2)65 (PDB ID 1k9i). Calcium is shown as a green sphere, whereas the carbohydrate is shown in a yellow stick representation. The calcium coordination is shown in detail, with the hydrogen bonds to important coordinating residues shown as dotted spheres. The gray sphere represents a calcium site of another carbohydrate-recognition domain. c | Hydrogen bonding, van der Waals interactions and aromatic ring stacking of GlcNAc3, with the GlcNAc-specific lectin from Urtica dioica (UDA)37 (PDB ID 1ehh). Two isolectin VI molecules are shown in blue and green. Dotted lines are hydrogen-bonding contacts between UDA and GlcNAc3. Sugar residues are labelled with A, B, and C from the non-reducing end. d | Hydrogen-bonding pattern and van der Waals contacts of the Narcissus psuedonarcissus lectin 7 (NPL7) carbohydrate-binding domain complexed to Manα(1– 3)Man66 (PDB ID 1npl). The mannose residue in the main binding pocket is shown in bold lines, with the hydrogen bonds depicted as dotted lines and van der Waals contacts as purple lines. MBP-C, mannose-binding protein C. Panel a reproduced with permission from Ref. 64 © (2005) Elsevier Science. Panel b reproduced with permission from Ref. 65 © (2001) American Association for the Advancement of Science. Panel c reproduced with permission from Ref. 37 © (2000) Elsevier Science. Panel d reproduced with permission from Ref. 66 © (1999) Elsevier Science.
Another example of a C-type lectin is DC-SIGN that has been crystallized in complex with Man3GlcNAc2(Ref. 65) (Protein Data Bank (PDB) ID 1k9i) (Fig. 4b). Interestingly, the internal α(1–3)–mannose (Man)-linked core carbohydrate binds to the principal calcium site. The equatorial C-3 and C-4 hydroxyls coordinate the calcium ion and form hydrogen bonds with the amino acids that coordinate the calcium.
UDA is an example of a calcium-independent CBA. A bound GlcNAc3 molecule is sandwiched between the binding sites of the high-affinity CBD of a first UDA molecule and the low-affinity CBD of a second UDA molecule. The high-affinity CBD is formed by the residues Ser19, Trp21, Trp23 and Tyr30. The low-affinity CBD is formed by the residues Ser65, His67, Trp69 and Tyr76. Of most importance for sugar recognition are the multiple hydrogen bonds, van der Waals interactions and aromatic-ring stacking of GlcNAc3 with the two UDA molecules37 (PDB ID 1ehh) (Fig. 4c). Indeed, at least five hydrogen bonds are formed in the carbohydrate–lectin complex, as well as several ring stackings between the individual GlcNAc entities and the Trp21, Trp23, Trp69 and His67 residues of the UDA molecules.
The molecular interaction of the Narcissus pseudonarcissus lectin with Manα1–3Man has also been revealed66 (PDB ID 1npl) (Fig. 4d). The Narcissus psuedonarcissus lectin 7 (NPL7) isolectin contains three CBDs in one monomer. Each CBD in NPL7 seems to have equal affinity for the particular carbohydrates and are equally occupied. In addition to the three conserved CBDs, a unique fourth (low-affinity) carbohydrate-binding site has been observed near the tryptophan cluster. Three residues (Asn30, Asp28 and Gln26) create a polar patch in the CBD pocket that restricts the carbohydate ligand to an axial hydroxyl group at C-2.
The multi-alignment of various monocot mannose-binding lectins revealed a striking sequence identity among Narcissus pneudonarcissus agglutinin (NPA), Narcissus hybrid cultivar agglutinin (NHA), Galanthus nivalis agglutinin (GNA), Lycoris radiata agglutinin (LRA), Lycoris aurea agglutinin (LAA) and Zephyranthus grandiflora agglutinin (ZGA), all of which contain three mannose-binding sites represented by the conserved QXDXNXVXY motif. This motif binds mannose through a network of four hydrogen bonds that interconnects the hydroxyls of C2, C3 and C4 of mannose to the four (QDNY) amino-acid residues36,66,67,68. A valine present within this motif also interacts with C3 and C4 of mannose through van der Waals interactions. Interestingly, as also described for NPA, a fourth mannose-binding site has been found in LAA near the tryptophane cluster68.
Investigation of the complex molecular interactions of CBAs with their carbohydrate ligands should enable the rational design of small-size molecules, including peptidomimetics. It is obvious that such low-molecular-weight CBAs will, by necessity, have fewer interactions with the individual carbohydrates of the gp120 glycans, and thus may display a lower affinity towards the carbohydrate oligomers. However, the non-peptidic small-size antibiotics PRM-A and BNM-A (Fig. 3) have shown that their affinity and specificity for mannose oligomers are sufficiently high to enable an efficient antiviral activity. Unfortunately, no crystal structures have yet been obtained for PRM-A that allow visualization of the molecular interactions of the antibiotic with its target α(1,2)-mannose glycan. If such information became available, further optimization of such CBAs may be possible.
Antiviral activity of CBAs
Numerous studies have shown that CBAs can prevent HIV infection of cell cultures. This has been shown for many virus strains (including laboratory HIV-1 and HIV-2 strains, members of Group O and different HIV-1 clades) that infect many different target cells (including laboratory cell lines, peripheral-blood mononuclear cells (PBMCs), macrophages and DCs). Inhibition of HIV infection by CBAs occurs at 50% effective concentrations that rank between the lower nanomolar and micromolar ranges. The antiviral activity will also depend on the nature of the CBA and the virus strains that are investigated18,26,30,31,32,38,39,40,41,58,60,63,69,72. Although most studies have been performed with laboratory virus strains that are adapted to growth in immortalized cell lines (such as MT-4, CEM and Molt), several other studies have shown the pronounced activity of CBAs against different virus clade isolates in PBMC cultures or against the R5-HIV-1 strain Ba-L in primary macrophage cell cultures41,60,69,71.
The size of the CBAs and their carbohydrate specificity seem to have an important role in their eventual potency and antiviral efficacy. In general, whereas most mannose-specific CBAs are inhibitory to HIV, UDA is the only example of an HIV-1-active GlcNAc-specific lectin, and poor, if any, anti-HIV activity has been reported for lectins with other carbohydrate specificities72. Interestingly, CBAs that are assumed to have a well-defined glycan specificity may still differ considerably in antiviral potency, although this phenomenon is not well understood. It is clear, however, that subtle differences in their three-dimensional conformation, as well as the proper steric availability of the glycans that efficiently interact with CBAs and the presence, or lack, of well-defined glycan conformations on the viral envelope, have an important role in the eventual antiviral efficacy of CBAs.
The formation of syncytial giant cells between cells persistently infected with HIV-1 and uninfected cells can also be efficiently prevented by CBAs40. Inhibition of giant-cell formation in HIV-1-infected and non-infected T-cell co-cultures usually requires CBA concentrations that are five to tenfold higher than those needed for inhibition of cell-free virus infection of the target cells. Some CBAs (such as the non-peptidic PRM-A), however, are equally effective in their inhibitory potential for both modes of viral transmission60.
Recently, it was shown that CBAs inhibit HIV-1 infection of DCs and DC-directed HIV-1 transfer71. It has also been demonstrated that CBAs have a pronounced inhibitory effect on virus capture by cells that express DC-SIGN and on the subsequent transmission of the virus to uninfected T cells73. DC-SIGN is a natural C-type lectin that has a pivotal role in innate immune defence46 (Box 1; Fig. 1). To eliminate the pathogen from the infected host, DC-SIGN captures pathogens such as HIV through its envelope glycans, and subsequently presents the pathogen or pathogen fragments to appropriate T cells74. These observations are potentially important from a microbicide viewpoint, as microbicides aim to prevent virus infection that may occur after the exposure of an individual to the virus through sexual intercourse. Indeed, DC-SIGN-mediated virus capture and transmission to T cells is believed to be among the first events that occur during the viral infection process through sexual contact75,76 (Box 1). However, its exact role in HIV transmission remains controversial — Boggiano et al.77 and Wang et al.78 recently provided evidence showing the enhancement of DC-SIGN-independent trans infection by DCs, and demonstrated the regulatory role of CD4 during DC-directed HIV-1 transmission.
Agents that efficiently prevent the first steps of virus propagation may qualify as promising microbicidal agents and have the potential to prevent persistent establishment of infection. The observation that CBAs might compete directly with DC-SIGN to capture HIV deserves further investigation in terms of their impact on the efficiency of virus capture and the subsequent transmission of the virus to T cells. Interestingly, it was recently reported that langerin, a C-type lectin that is present on epithelial Langerhans cells (LCs), prevents HIV-1 transmission by LCs by internalization of captured HIV particles and subsequent intracellular degradation79. Although such a mechanism may be efficient for a first-line inactivation of HIV, CBA-exposed HIV strains may decrease the efficiency of LCs to eliminate HIV, but at the same time may compromise the ability of the virus to be efficiently transmitted by DCs.
Mechanism of the antiviral action of CBAs
The interactions of several CBAs have been extensively investigated, including: the prokaryotic CV-N and actinohivin; a variety of plant lectins, including Hippeastrum hybrid agglutinin (HHA) and UDA; the non-peptidic low-molecular-weight antibiotic PRM-A; and the monoclonal antibody 2G12 with the HIV-envelope gp120 and/or several glycan structures. Interestingly, deletion of the N-glycans at amino-acid positions 295, 392 and/or 406 rendered the 2G12 monoclonal antibody completely incapable of efficiently neutralizing the virus particle54. The asparagines at positions 295, 332 and 392 were previously shown in crystallographic gp120–2G12 complexes to be indispensable for an efficient interaction with the 2G12 monoclonal antibody80. The interaction of the monoclonal antibody 2G12 with the glycans on a well-defined gp120 epitope has also been revealed in several studies49,51,52.
Alteration of the glycosylation pattern of HIV gp120 in the ER by inhibiting the glycosidases I and II prevents proper folding of gp120. Consequently, the interaction of gp120 with the ER lectin calnexin is inhibited81 and, as a result, fewer infectious virus particles are released by the drug-treated cells. In addition, virus particles from which the glycans had been stripped became markedly less infectious. Interestingly, combining CBAs with the mannosidase inhibitor 1-deoxymannojirimycin (DMJ) led to the expression of synergistic antiviral activity82. Exposure of glycan-deleted mutant HIV-1 strains to DMJ results in an enhanced suppression of mutant virus-induced cytopathicity in cell culture82. Therefore, the presence of an intact glycan shield on gp120 has also been proven to be indispensable for optimal infectivity and for an efficient interaction of HIV with its target cells.
Studies determining the direct interaction of CBAs with fixed gp120 molecules have revealed the strong binding affinity of CV-N, HHA, PRM-A and actinohivin with gp120. However, for certain CBAs (such as CV-N and HHA), a low dissociation rate was observed22,60. These results indicate that some of the CBAs exert a strong and virtually irreversible binding to gp120. This is in agreement with cellular experiments, which show that a short pre-incubation of cell-free virus particles with CBAs before infection of T cells markedly increases the antiviral activity of the CBAs18,40.
The binding of the CBA to gp120 does not prevent the initial virus–cell interaction. Indeed, it was found that, in the presence of CBAs, the virus could still efficiently bind CD4+ T cells38. However, one of the next steps during the entry process (which is binding to the co-receptor(s) and/or the subsequent exposure of gp41 to the target-cell membrane) becomes blocked in the presence of the CBA. The exact molecular mechanism of the blockade of virus entry and/or fusion has not yet been determined. However, it can be assumed that the attachment of the CBA to the glycans of gp120 hinders or prevents the conformational changes and flexibility of gp120 that are required to properly interact with the cell-membrane receptor, before or during the fusion process. In a number of cases, it has even been observed that the binding of virus particles to their target cells can be enhanced in the presence of some CBAs60. This phenomenon may indicate that some sort of crosslinking between the virus particles and the cell-membrane glycoproteins occurs. Alternatively, CBA-induced conformational changes in gp120 may allow a more optimal interaction of the CD4-binding site on gp120 with the cellular receptor. It should be mentioned that the CD4-binding site on gp120 does not contain N-glycans, and deletion of N-glycans in the vicinity of the CD4-binding site on gp120 (of simian immunodeficiency virus strain SIV mac239) has been reported to increase the binding efficiency of the mutant gp120 with CD4; it was concluded that certain glycans might be particularly important for the shielding of the CD4-binding site from antibody recognition83.
Effect of CBA pressure on viral evolution
The exposure of HIV to any antiviral drug will eventually result in the appearance of phenotypic resistance, both in cell culture and in HIV-infected individuals. Antiviral drug resistance is usually due to amino-acid mutations and/or deletions in the viral target with which the particular drug directly or indirectly interacts. In many cases, one amino-acid change is sufficient to provoke a marked degree of drug resistance, although sometimes several mutations are required before pronounced phenotypic resistance becomes evident. Viral fitness or infectivity is often compromised in the presence of such resistance mutations. However, compensatory mutations may also occur. These amino-acid mutations do not contribute to drug resistance, but rather are meant to restore the fitness of the virus. For HIV, exposure to CBAs invariably results in the appearance and accumulation of amino-acid mutations, predominantly in the putative N-glycosylation motifs of gp120 (Refs 41,54,60,69,84,85,86). Either an asparagine, or a serine or threonine, are mutated, leading to the annihilation of the glycosylation site. No glycan deletions were observed, however, in the HIV-1 glycoprotein gp41 of such mutant virus strains.
The HIV-1 gp120 consists of approximately 30–40% high-mannose-type glycans (approximately 10 out of 24 glycans in HIV-1/IIIB)87,88 (Fig. 5a), which are the preferential sites for glycan deletions that occur under CBA pressure (Fig. 5b). Indeed, up to 80% of high-mannose-type glycan sites are affected in HIV-1 gp120 under prolonged CBA pressure. Interestingly, such a phenomenon consistently occurs regardless of the nature of the CBA, and has been observed in the presence of the α(1,2)-Man-specific CV-N69 and PRM-A60, the α(1,3)- and/or α(1,6)-Man-specific plant lectins (HHA and GNA)84,85, the GlcNAc-specific plant lectin UDA41 and the N-glycan-recognizing monoclonal antibody 2G12 (Ref. 54).
Figure 5. The HIV envelope glycoprotein gp120.
Ribbon diagrams showing the 24 putative N-glycosylation sites (coloured circles) in the HIV-1(IIIB) envelope glycoprotein gp120 according to Kwong et al.123 and Leonard et al.87 a | High-mannose-type (green) and complex or hybrid-type (yellow) glycans. b | The red circles indicate the deleted N-glycosylation sites that appear under pressure from carbohydrate-binding agents (CBAs) (for example, Galanthus nivalis agglutinin (GNA), Hyppeastrum hybrid agglutinin (HHA), Urtica dioica agglutinin (UDA), cyanovirin-N (CV-N), pradimicin A (PRM-A) and the monoclonal antibody 2G12) in more than 30 different mutant virus isolates. The green circles represent glycosylation sites that have not yet been found to be deleted under CBA pressure123. Images courtesy of Ir. K. François and M. Froeyen, Rega Institute, Leuven, Belgium.
There is a close correlation between the number of glycan deletions in the envelope of a particular HIV strain and the degree of phenotypic drug resistance and, in general, the greater the number of glycan deletions in HIV gp120, the greater the degree of CBA resistance41,84,85. However, when the first glycosylation-site deletions occur under CBA pressure in HIV-infected cell cultures, phenotypic resistance is rarely observed. For example, mutant virus strains have been isolated that contain at least three or more glycosylation-site mutations in gp120 without the appearance of visible phenotypic resistance to the GlcNAc-specific UDA41,85. Therefore, there seems to be a threshold for the number of glycan deletions below which no significant phenotypic CBA resistance is evident.
So far, many mutant HIV-1 strains containing up to nine glycan deletions in gp120 have been isolated under escalating CBA pressure41 and such mutant virus strains can be up to 100-fold less sensitive to some CBAs. Interestingly, several of these mutant virus strains are less infective, resulting in a lower viral fitness compared with the wild-type virus60. However, in a few cases, mutant virus strains have been isolated that contain 4 to 5 glycan deletions in gp120, yet have an increased infectivity and fitness85. It is unclear what structural requirements the mutant gp120 must fulfill to have an increased infectivity. However, such virus strains have never been observed to emerge when more than five glycans were concomitantly affected in gp120. In fact, among more than fifty independent mutant virus isolates that emerged under CBA pressure, only three were demonstrated to have an increased infectivity.
Mutant gp120 antibody susceptibility
There is much evidence indicating that the glycan shield of HIV-1 prevents the immune system from efficiently neutralizing the virus. HIV-1 strains lacking the highly conserved N-linked glycan at position 306 (designated as 301 in Fig. 5 owing to a different numbering of the amino acids) within the V3 loop of gp120 are highly sensitive to neutralization. Bolmstedt and co-workers89 also showed that glycosylation at this amino-acid position shields HIV-1 from neutralizing antibodies. Kang and colleagues90 recently reported that HIV env-encoded proteins, with deleted glycans in the gp120 domains surrounding the CD4 binding site, or in the gp120 variable loop, expose immunogenic epitopes at much higher levels than wild-type virus does, which may provide a tool for novel vaccine immunogens. Specific N-linked glycosylation modifications in the envelope V1 domain of SIV or in a SIV–HIV hybrid variant have also been shown to evolve in the host and alter recognition by neutralizing antibodies91,92.
Studies with SIVmac239, which is highly resistant to neutralization by polyclonal antisera or monoclonal antibodies, have shown that elimination of N-glycan attachment sites in the envelope gp120 results in a dramatically increased sensitivity to neutralization by monoclonal antibodies93. Importantly, removal of specific N-glycans from V1 and V2 led to an increase in sensitivity to neutralization by antibodies recognizing epitopes from both within and outside the V1–V2 sequence. Indeed, mutations in V1 not only resulted in an increased antibody recognition to epitopes in V1, but also in a redirection of antibody responses to the V3 loop, which is distant in the linear polypeptide sequence94. When Rhesus monkeys were infected with mutant SIV strains that were lacking in combinations of the two N-glycosylation sites in gp120, a marked increase in antibody binding to specific peptides derived from the glycan-deleted regions was observed, which resulted in an increased neutralizing activity. These results convincingly demonstrated that the presence of N-glycans limits the neutralizing antibody response to SIV, and helps shield the virus from immune recognition95. These results also illustrate that deletion of as few as two glycosylation sites in the viral env gene is sufficient to trigger a significant neutralizing antibody response.
Blay et al.96 showed that a significant divergence in the Env proteins occurs over time in macaques infected with the SIV strain SHIV-89.6P (containing HIV env subtype B in a SIV background). Importantly, the total number of potential N-glycosylation sites did not increase over time, and there was a remarkable degree of conservation in patterns of change in Env glycans. These findings suggest that the configuration of the glycan shield is under considerable constraints, which is in agreement with the findings of Poon et al.97, who showed that negative (exclusive) interactions occur more often between co-localized glycans, whereas positive (inclusive) interactions are restricted to more distant glycans. These data imply that the adaptive repertoire of alternative configurations in the HIV-1 glycan shield is limited by functional interactions between the N-glycans97. Therefore, it seems likely that CBA exposure to SIV or HIV strains would seriously compromise these constraints, by forcing the virus to progressively delete envelope glycans and allowing the immune system to become actively involved in inhibiting the virus infection. In conclusion, much data are currently available to show that glycan deletions in the viral envelope uncover immunogenic epitopes that result in an increased neutralization of the mutant virus.
Pathogen susceptibility to CBA therapy
It has been shown that DC-SIGN can recognize and internalize numerous other viruses, bacteria and protozoa in addition to HIV98 (Table 2). In this way, DC-SIGN can be considered to be a universal pathogen receptor. Indeed, the recent identification of the carbohydrate specificity of the SIGN molecules for high-mannose- and/or fucose-containing glycans has led to the identification of various pathogens that are recognized by these receptors (Table 1). DC-SIGN binds and internalizes Dengue virus99, human cytomegalovirus100, HCV101 and Ebola virus102 to allow efficient trans infection of the target cells. It is also well documented that HCV closely interacts with both DC-SIGN and liver/lymph node (L)-SIGN, a close homologue of DC-SIGN that is expressed on specialized liver and lymph-node endothelial cells that have antigen-presenting capacity98. The capture of HCV by the SIGN-positive cells, through the highly glycosylated (high-mannose type) envelope glycoprotein E2, facilitates HCV transmission to proximal hepatocytes102. DC-SIGN and L-SIGN were also shown to enhance infection mediated by the Marburg virus glycoprotein GP and the S protein of severe acute respiratory syndrome coronavirus (SARS-CoV) through pH-dependent endocytosis, and might promote virus dissemination103.
Table 2.
Pathogens that interact with DC- or L-SIGN for transmission or immune suppression
| Pathogens | Associated disease | References |
|---|---|---|
| Viruses | ||
| HIV | AIDS | 47 |
| HCV | Hepatitis | 101,124 |
| Dengue virus | Haemorrhagic fever | 99,125 |
| Marburg virus | Haemorrhagic fever | 103 |
| Ebola virus | Haemorrhagic fever | 126 |
| West Nile encephalitis virus | Encephalitis | 125 |
| Cytomegalovirus | Congenital infection; general infection (for example, retinitis, hepatitis, colitis) | 100 |
| Herpes virus type 8 | Kaposi's sarcoma | 127 |
| Corona (SARS) virus | SARS | 103 |
| Measles virus | Measles | 128 |
| Bacteria | ||
| Mycobacterium tuberculosis | Tuberculosis | 104 |
| Mycobacterium leprae | Lepra | 129 |
| Mycobacterium bovis | Tuberculosis | 104 |
| Streptococcus pneumoniae serotype 3 and 14 | Pneumonia | 107 |
| Helicobacter pilori | Gastric pathogen (ulcers) | 110 |
| Lactobacillus spp. | Vaginal commensals | 106 |
| Parasites | ||
| Leishmania infantum | Visceral leishmaniasis | 130 |
| Leishmania pifanoi | Cutaneous leishmaniasis | 1 |
| Leishmania mexicana | Leishmaniasis | 1 |
| Schistosoma mansoni parasite | Bilharziosis | 111 |
| Fungi | ||
| Aspergillus fumigatus | Aspergillosis | 131 |
| Candida albicans | Fungal infection (candidiasis) | 132 |
| AIDS, acquired immunodeficiency syndrome; DC-SIGN, dendritic-cell-specific intercellular adhesion molecule 3-grabbing non-integrin; HCV, human hepatitis C virus; L-SIGN, liver/lymph node-SIGN ; SARS, severe acute respiratory syndrome. | ||
Although M. tuberculosis primarily infects macrophages, it also binds to DCs through the interaction of its cell-wall component ManLAM (mannosylated lipoarabinomannan) with DC-SIGN104,105. The binding of ManLAM to DC-SIGN on DCs blocks DC maturation and induces the expression of immunosuppressive interleukin-10 (IL-10)104. As a result, M. tuberculosis enables the suppression of immune activation signals, which allows immune escape. Probiotic bacteria, such as Lactobacillus spp., also exert immune suppression through DC-SIGN by the induction of IL-10-producing regulatory T cells106. It has recently been shown that serotypes 3 and 14 of Streptococcus pneumoniae specifically interact with DC-SIGN through the capsular polysaccharide, but the immunological consequences are unclear107. H. pylori and the parasite (protozoa) Schistosoma mansoni were shown to bind to DC-SIGN through non-sialylated Lewis antigens that are expressed on lipopolysaccharides of the bacterium and the cell-wall glycolipids of the parasite, respectively108,109. This results in immune regulation that is to the advantage of the pathogen110,111. Leishmania mexicana has also been shown to express glycoconjugates that are recognized by DC-SIGN108.
It is clear that the often-indispensable interactions of various pathogens with DC-SIGN are required to allow efficient pathogen transmission to its eventual target cells and/or immune escape and successful persistence in the host. It is therefore likely that CBAs, by binding to the pathogens' glycoconjugates, directly compete with the lectins of the innate immune system. The CBA may prevent efficient capture and transmission of the pathogen and/or suppression of an efficient immune response against the pathogen. Therefore, it can be predicted that CBAs may have a more general role in abrogating successful pathogen infection and persistence. CBAs should, therefore, be put in a broader microbial therapeutic context, and not be explored solely for HIV therapy.
Unique features of the CBA concept
It has been unambiguously shown that CBAs efficiently inhibit virus entry by inhibiting the fusion of cell-free HIV particles with susceptible cells, and forming syncytia between persistently infected and uninfected cells38. CBAs also prevent the capture of virus particles by DC-SIGN, and the subsequent transmission of the virus to T cells73. CBA treatment of virus-infected cells provokes drug pressure on the virus, resulting in predominant deletions of N-glycans in the HIV gp120 envelope121. Such glycan deletions uncover previously hidden immunogenic epitopes on gp120, which may give the immune system the opportunity to produce a humoral and/or cellular response (Fig. 1). What are the unique features of the CBA concept that differentiate this therapeutic approach from those that are currently available (Box 2)?
In contrast to the existing drugs for the treatment of HIV, which interact with specific amino-acid configurations on their target proteins, CBAs directly interact with the glycans that are present on the envelope gp120 of HIV. It is important to realize that CBAs do not need to be taken up by the virus-infected cell in order to exert their antiviral activity and do not interfere with the synthesis of the glycans on glycoproteins per se. In this respect, this concept differs entirely from inhibitors of cellular glycosylation, such as DMJ and castanospermine, which aim to disturb the glycan formation in viral glycoproteins, but at the same time may also disturb the formation of glycans on cellular glycoproteins.
CBA pressure progressively forces the virus to delete N-glycans in gp120. This mutational pattern is unique and does not consistently occur in the presence of other anti-HIV drugs, nor in any other known antiviral. A relatively high number of N-glycans are present on each HIV-1 gp120 molecule (approximately 20 to 30 glycans, depending on the nature of the virus clade and the individual virus strain)87. It can be reasonably assumed that multiple individual CBAs simultaneously bind to every HIV-1 gp120 molecule. By contrast, HIVHIV-1 inhibitors other than CBAs stoichiometrically bind to their target — one drug molecule interacts with one target protein molecule.
The multiple bindings of CBAs to single gp120 molecules results in the CBAs having a high genetic barrier. This means that several mutations (owing to glycan deletions) need to accumulate in gp120 before significant phenotypic drug resistance becomes evident. With the 'traditional' current drugs, the appearance of a single mutation, or at least two or three mutations in the target protein, is usually sufficient to produce a significant drop in sensitivity of the virus to the particular drug.
As CBAs selectively target N-glycans on gp120, CBA-mutated virus strains are not likely to show cross-resistance to drugs that act against other targets (such as protease, integrase and reverse transcriptase). The exception to this rule may be drugs that target the HIV-1 transmembrane gp41. Indeed, preliminary findings indicate that some mutant virus strains that contain various N-glycan deletions in gp120 show some diminished sensitivity to the fusion gp41 inhibitor enfuvirtide (also known as T20 or Fuzeon). It remains to be seen, however, whether this is a consistent behaviour of these mutant virus strains or whether the particular glycan-deleted virus strains have an intrinsically low sensitivity to enfuvirtide that is unrelated to the absence of some of the N-glycans of gp120.
Certain glycans may have an instrumental role in the correct folding of the protein immediately after the native peptide has been formed on the ribosomes of the ER7. Therefore, it can be expected that correct folding can become hampered if glycans are lacking on the viral envelope after amino-acid mutation of the glycosylation motif. Such compromised (or altered) gp120 folding may affect the efficient interaction of the mutated gp120 envelope with the co-receptor molecules, virus fusion efficacy and the eventual fitness of the mutant virus strains. As the transmission of HIV is believed to be mediated by carbohydrate-recognizing DC-SIGN-expressing cells (that is, immature DCs) that are present in the vaginal–uterine mucosa–epithelial border46, it can be assumed that the mode of interaction of cells with mutated glycan-deficient HIV particles is altered owing to a changed glycan landscape on the gp120 envelope.
The protective role of the glycan shield in hiding immunogenic epitopes on gp120 from the immune system may get lost, or at least compromised, on deletion of the particular glycans112. Such glycan deletions may trigger the production of neutralizing antibodies that are specific for those gp120 peptide epitopes that were previously shielded by the glycans. Whether this phenomenon will occur in CBA-treated HIV-1-infected individuals remains to be determined, but it seems likely. It is also currently unknown how strong and efficient the neutralizing antibody response will be on the mutant virus strains that emerge under CBA pressure and whether the virus can use other immunological subversions to circumvent CBA drug pressure. It would also be interesting to see whether, and to what extent, a cellular immune response would be triggered under such conditions, and what contribution a provoked cellular immune response might make in the eventual inhibition of the virus infection in CBA-treated individuals.
None of the existing antiviral chemotherapeutics has been shown to have the potential to act in concert with the immune system to further increase the therapeutic pressure on the mutated virus. Therefore, treatment of HIV with CBAs may become the first strategy to combine drug-mediated virus suppression and induction of a specific antiviral immunological response5. Such a phenomenon may result in 'self-vaccination' of the CBA-exposed HIV-infected individuals by means of a chemotherapeutic agent. If this principle of a combined concerted action between chemotherapy and the immune response proves valid, it may also be applied to other chronic infections by viruses with a highly glycosylated envelope, such as HCV113,114. Also, more acute virus infections (for example, influenza virus, SARS-CoV and Ebola virus) may be highly sensitive to the inhibitory action of CBAs, as has been shown for certain CBAs against feline and human coronaviruses115,116 and for CV-N against Ebola virus117 in cell culture.
Besides viruses, other pathogens, such as the DC-SIGN-recognizing M. tuberculosis and H. pylori or fungi that contain a glycan-rich cell wall (such as Aspergillus spp., Cryptococcus spp. and Candida spp.), and even parasites, may become ideal candidate microorganisms to explore their susceptibility to the inhibitory action of CBAs. Therefore, the potential of CBAs to selectively target some enveloped viruses may also be extended to other pathogens of an entirely different nature. If the glycans on these pathogens are sufficiently different from those of the host, an acceptable therapeutic window may be achieved. Considering CBAs in the larger context, beyond that of the therapeutic field of virus infections, may reveal an unprecedented therapeutic potential, and should trigger extensive efforts by both chemists and microbiologists to explore this novel therapeutic avenue in the broadest possible sense.
Potential pitfalls of the CBA concept
Virtually all known CBAs that are inhibitory to HIV infection (with the exception of the low-molecular-weight non-peptidic antibiotic PRM-A and BMN-A analogues (Fig. 3)) are proteins. Such agents are expensive to produce, scale-up and purify. There may also be storage and stability problems, although some plant lectins are remarkably temperature- and pH-stable34,40. Bioavailability is also expected to be low for peptidic CBAs and, therefore, their pharmacokinetics and pharmacodynamics could be unfavourable, particularly for chronic therapeutic administration. Besides a sometimes pronounced mitogenic and red-blood-cell-agglutinating activity, lectins might also be endowed with inflammatory activity and cellular toxicity34. Also, lectins such as CV-N have the capacity to stimulate various differentiation markers (such as CD25, CD69 and human leukocyte antigen (HLA-DR))69.
Again, it must be emphasized that, although these properties are unfavourable and undesirable from a therapeutic viewpoint, the number and intensity of biological side effects is highly dependent on the nature of the CBA. For example, whereas CV-N was shown to display a broad variety of side effects69, other CBAs such as GNA and HHA showed much fewer, if any, side effects118. Moreover, some of the side effects that were observed for CV-N were shown to be independent of its carbohydrate-binding properties69. Therefore, the proper selection of CBAs that have a high selectivity for viral glycans and minimal cellular side effects must be an achievable goal.
Given the proteinaceous nature of lectins, it could be assumed that repeated systemic administration of CBAs will eventually elicit a specific antibody response. Such a reaction by the immune system may hamper and attenuate the activity of the CBA against the viral carbohydrates (making them less antivirally active). It may also provoke hyperreactivity of the immune system, which would necessitate premature abrogation of the continued administration of the CBA. Obviously, low-molecular-weight non-peptidic CBAs will not suffer from this potential drawback.
The greatest concern, for both protein and non-peptidic CBAs, is the degree of selectivity they may eventually show for the viral glycoproteins — that is, their potential to discriminate between pathogen (non-self) glycoproteins and cellular (self) glycoproteins. However, the HIV envelope gp120 carries a higher proportion of high-mannose-type glycans than do mammalian glycoproteins87. The three-dimensional configuration of the glycans that are displayed on the glycoproteins of the pathogen has been shown to be important, which may help the CBA to distinguish between 'non-self' glycans of the pathogen and 'self' glycans of the host. High-mannose-type glycans contain terminal α(1,2)-mannose oligomers that are rare on glycans of mammalian glycoproteins. In fact, MBL and DC-SIGN can distinguish between pathogen-derived glycoproteins and cellular glycoproteins, enabling a selective elimination of the pathogen through specific interaction with its carbohydrate configuration65,119. It is also important that the CBA can discriminate between the glycans present on commensal bacteria and the glycans that must be targeted on the viral-envelope glycoproteins. It will be a challenging goal, but one, I believe, which is achievable, to discover or design CBAs that show a marked degree of discriminating selectivity between pathogen glycans and the glycans of the host, including the glycans of commensal bacteria.
Future perspectives
Although most CBAs are proteins (such as prokaryotic and plant-lectin CBAs), the demonstration that small-size non-peptidic CBAs (that is, those with a molecular weight of less than 1–2 kDa) (Fig. 3) can efficiently suppress viral and fungal infections makes this class of compounds a feasible and realistic tool for pathogen inhibition in the clinical setting. However, much work still has to be done, and more synthetic low-molecular-weight compounds need to be designed or discovered to enable the efficient exploration of this novel functional class of antivirals. This is the only way to enable a careful and rational selection of CBAs that have a high specificity and selectivity for the pathogen and few, if any, side effects in the host, especially when included in long-term treatment modalities. Several synthetic CBA lead compounds are already available61,62, which should trigger the synthesis of structurally related compounds, by organic and medicinal chemists, to allow extensive structure–activity relationship studies. Such investigations could indicate which CBAs are likely to be the most potent and selective candidates for further pre-clinical investigations.
Glycans on the envelope or cell wall of pathogens often seem to have similar functional roles, such as escape from recognition by the immune system or recognition by lectins from the innate immune system, that allow efficient transmission of the pathogen. The concept of interfering with and/or abrogating these protective mechanisms should, therefore, be put into a broader context than solely antiviral therapeutic intervention. Many different microorganisms other than viruses (such as certain bacteria, fungi, yeasts and parasites) should be thoroughly investigated for their potential interactions with CBAs. However, care should be taken to ensure that those microorganisms that have a pivotal role in maintaining the homeostasis of human functions, such as non-pathogenic lactobacilli in the vaginal environment or commensal bacteria in the intestine, are not negatively affected by the CBA. Including such bacterial strains in the screening of CBAs may allow selection of the highest-possible pathogen-selective and specific CBAs in the early stages of drug development.
CBAs should be considered to be valuable agents in their own right, directly suppressing or preventing pathogen infection in the host (Fig. 1). However, as they can force the pathogen to mutate (or delete) its protective glycan shield to escape CBA pressure this adds an exciting new dimension to CBAs as a novel conceptual class of antimicrobial agents. They may indeed represent the first agents that combine direct chemotherapeutic activity and an indirect, active involvement of the immune system by triggering a humoral response (by producing neutralizing antibodies) and/or a cellular response (T-cell-based immunity). Although the triggering of a cellular immune response by CBAs is still to be confirmed in vivo, indirect information suggests that an immune response is to be expected after prolonged treatment of the pathogen with CBAs.
Box 1 | Dendritic cells, innate immune defence and HIV transmission.
Most pathogens that bind to DC-SIGN (dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing non-integrin) cause long-lasting and chronic infections, and often induce tolerance or immune evasion74. DC-SIGN is a C-type (calcium-dependent) lectin that is predominantly expressed on DCs, but also to some extent on macrophages, activated B cells, lymphoid tissues, skin dermis, placenta and the intestinal and genital mucosa. It functions as a tetramer, and consists of a cytoplasmic domain, a transmembrane domain, an exogenous (hepta) repeat domain that allows multimerization, and a terminal carbohydrate (high-mannose)-recognition domain120. DCs that are present at the sites of pathogen entry (for example, at the mucosal barrier underneath the vaginal epithelia) recognize and trap the pathogen. The activated DCs then migrate to draining lymph nodes, where naive T cells are primed to eradicate the pathogen (Fig. 1). One of the roles of DC-SIGN is to grab ICAM-3 that is present on T cells to enable close contact and allow antigen presentation to the T cells.
DCs can efficiently capture HIV-1 particles through their C-type lectin (DC-SIGN) receptors. Following DC contact with T cells, virus particles can be observed at the cell–cell junctions, which possibly creates an infectious synapse in which the passage of virions between the two cell types is facilitated. Such a synapse depends on DC-SIGN expression and strong cell–cell adhesion mediated by the ICAM-1–LFA-1 (lymphocyte function-associated antigen 1) interaction. It has been shown that, in the case of HIV, the infectious synapse leads to an efficient transfer of the pathogen to T cells120.
Box 2 | Unique features of carbohydrate-binding agents as therapeutic agents.
The direct interaction of carbohydrate-binding agents (CBAs) with the glycans of the viral envelope glycoproteins.
CBA pressure forces HIV to delete N-glycans in the envelope glycoprotein gp120.
Multiple CBAs bind to one envelope gp120 target molecule.
A high genetic barrier.
No cross-resistance of CBA-resistant mutant virus strains to other antivirals.
Altered interaction of mutant (glycan-deleted) viruses with target cells.
The CBA-induced glycan deletions compromise the protective role of the intact glycan shield on HIV gp120.
The eventual antiviral CBA activity may combine a direct drug-mediated virus suppression and an indirect (delayed) induction of a specific antiviral immune response against the mutant gp120 envelope.
The CBA concept may also apply to other chronic enveloped virus infections, such as the human hepatitis C virus.
Other pathogens such as dendritic-cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-recognizing bacteria, fungi or parasites may also be susceptible to the CBA approach.
Acknowledgements
The research of the author has been supported by grants from the Rega Center of Excellence at the Katholieke Universiteit Leuven, the Geconcerteerde Onderzoeksacties and the European Commission. D. Schols and S. Liekens, Rega Institute, Leuven, Belgium, are acknowledged for the critical reading of the manuscript and valuable discussions. I thank C. Callebaut and C. Biernaux for their dedicated editorial help.
Glossary
- Mannose-binding lectins
(MBLs). A superfamily of strictly mannose-specific lectins, all of which consist of subunits with a similar sequence and overall three-dimensional structure.
- Syncytium
A multinucleated giant cell that is formed following the fusion of infected cells expressing HIV-encoded envelope glycoproteins and uninfected cells expressing the CD4 co-receptor. The resulting syncytium subsequently undergoes apoptosis.
- Peptidomimetic
A compound containing non-peptidic structural elements that is capable of mimicking or antagonizing the biological action(s) of a natural parent peptide.
Biography
Jan Balzarini is Professor at the Rega Institute for Medical Research in the Faculty of Medicine at the Katholieke Universiteit Leuven, Belgium, and Head of the Laboratory of Virology and Chemotherapy there. His research is focused on the discovery of novel drug lead compounds and therapeutic targets for antiviral and anticancer intervention. Special emphasis is given to the investigation of drug resistance and molecular mechanisms of action of new lead compounds.
Related links
DATABASES
Entrez Genome Project
Entrez Protein
Protein Data Bank
Entrez Gene
Accession codes
Accessions
Protein Data Bank
Competing interests
The author declares no competing financial interests.
References
- 1.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature Rev. Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 2.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nature Rev. Immunol. 2006;6:930–939. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 3.Price GE, Ou R, Jiang H, Huang L, Moskophidis D. Viral escape by selection of cytotoxic T-cell-resistant variants in influenza A virus pneumonia. J. Exp. Med. 2000;191:1853–1867. doi: 10.1084/jem.191.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Clercq E. Antivirals and antiviral strategies. Nature Rev. Microbiol. 2004;2:704–720. doi: 10.1038/nrmicro975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini J. Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect. Dis. 2005;5:726–731. doi: 10.1016/S1473-3099(05)70271-1. [DOI] [PubMed] [Google Scholar]
- 6.Christlet THT, Veluraja K. Database analysis of O-glycosylation sites in proteins. Biophys. J. 2001;80:952–960. doi: 10.1016/S0006-3495(01)76074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 8.Rudd PM, Dwek RA. Glycosylation: heterogeneity and the 3D structure of proteins. Crit. Rev. Biochem. Mol. Biol. 1997;32:1–100. doi: 10.3109/10409239709085144. [DOI] [PubMed] [Google Scholar]
- 9.Dwek RA. Biological importance of glycosylation. Dev. Biol. Stand. 1998;96:43–47. [PubMed] [Google Scholar]
- 10.Parodi AJ. Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation. Biochem. J. 2000;348:1–13. doi: 10.1042/bj3480001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagger HL, Fuglsang CC, Westh P. Hydration of a glycoprotein: relative water affinity of peptide and glycan moieties. Eur. Biophys. J. 2006;35:367–371. doi: 10.1007/s00249-005-0035-5. [DOI] [PubMed] [Google Scholar]
- 12.Ruddock LW, Molinari M. N-glycan processing in ER quality control. J. Cell Sci. 2006;119:4373–4380. doi: 10.1242/jcs.03225. [DOI] [PubMed] [Google Scholar]
- 13.Ezekowitz RA. Role of the mannose-binding lectin in innate immunity. J. Infect. Dis. 2003;187:S335–S339. doi: 10.1086/374746. [DOI] [PubMed] [Google Scholar]
- 14.van Liempt E, et al. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Chang TL, Klotman ME. Defensins: natural anti-HIV peptides. AIDS Res. 2004;6:161–168. [PubMed] [Google Scholar]
- 16.Buzas EI, et al. Carbohydrate recognition systems in autoimmunity. Autoimmunity. 2006;39:691–704. doi: 10.1080/08916930601061470. [DOI] [PubMed] [Google Scholar]
- 17.Etzioni A. Adhesion molecules in leukocyte endothelial interaction. Adv. Exp. Med. Biol. 1996;408:151–157. doi: 10.1007/978-1-4613-0415-9_17. [DOI] [PubMed] [Google Scholar]
- 18.Boyd MR, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 1997;41:1521–1530. doi: 10.1128/AAC.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bewley CA, et al. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nature Struct. Biol. 1998;5:571–578. doi: 10.1038/828. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, et al. Crystal structure of cyanovirin-N, a potent HIV-inactivating protein, shows unexpected domain swapping. J. Mol. Biol. 1999;288:403–412. doi: 10.1006/jmbi.1999.2693. [DOI] [PubMed] [Google Scholar]
- 21.Botos I, et al. Structures of the complexes of a potent anti-HIV protein cyanovirin-N and high mannose oligosaccharides. J. Biol. Chem. 2002;277:34336–34342. doi: 10.1074/jbc.M205909200. [DOI] [PubMed] [Google Scholar]
- 22.Bewley CA. Solution structure of a cyanovirin-N:Man α1–2Manα complex: structural basis for high-affinity carbohydrate-mediated binding to gp120. Structure. 2001;9:931–940. doi: 10.1016/S0969-2126(01)00653-0. [DOI] [PubMed] [Google Scholar]
- 23.Bolmstedt AJ, O'Keefe BR, Shenoy SR, McMahon J, Boyd MR. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol. Pharmacol. 2001;59:949–954. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, et al. Isolation and characterization of a mannan-binding lectin from the freshwater Cyanobacterium (blue-green algae) Microcystis viridis. Biochem. Biophys. Res. Commun. 1999;265:703–708. doi: 10.1006/bbrc.1999.1749. [DOI] [PubMed] [Google Scholar]
- 25.Williams DC, Jr, Lee JY, Cai M, Bewley CA, Clore GM. Crystal structures of the HIV-1 inhibitory cyanobacterial protein MVL free and bound to Man3GlcNAc2: structural basis for specificity and high-affinity binding to the core pentasaccharide from N-linked oligomannoside. J. Biol. Chem. 2005;280:29269–29276. doi: 10.1074/jbc.M504642200. [DOI] [PubMed] [Google Scholar]
- 26.Bokesch HR, et al. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42:2578–2584. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- 27.Adams EW, et al. Oligosaccharide and glycoprotein microassays as tools in HIV glycobiology: glycan-dependent gp120/protein interactions. Chem. Biol. 2004;11:875–881. doi: 10.1016/j.chembiol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Ziolkowska NE, Wlodawer A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim. Pol. 2006;53:617–626. [PubMed] [Google Scholar]
- 29.Kljajic Z, et al. A D-mannose-specific lectin from Gerardia savaglia that inhibits nucleocytoplasmic transport of mRNA. Eur. J. Biochem. 1987;169:97–104. doi: 10.1111/j.1432-1033.1987.tb13585.x. [DOI] [PubMed] [Google Scholar]
- 30.Inokoshi J, et al. Molecular cloning of actinohivin, a novel anti-HIV protein from an actinomycete, and its expression in Escherichia coli. Biochem. Biophys. Res. Commun. 2001;281:1261–1265. doi: 10.1006/bbrc.2001.4496. [DOI] [PubMed] [Google Scholar]
- 31.Mori T, et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia spp. J. Biol. Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 32.Muller WE, Renneisen K, Kreute MH, Schroder HC, Winkler I. The D-mannose-specific lectin from Gerardia savaglia blocks binding of human immunodeficiency virus type 1 to H9 cells and human lymphocytes in vitro. J. Acquir. Immune Defic. Syndr. 1988;1:453–458. [PubMed] [Google Scholar]
- 33.Chiba H, et al. Actinohivin, a novel anti-HIV protein from an actinomycete that inhibits syncytium formation: isolation, characterization, and biological activities. Biochem. Biophys. Res. Commun. 2001;282:595–601. doi: 10.1006/bbrc.2001.4495. [DOI] [PubMed] [Google Scholar]
- 34.Van Damme EJM, Peumans WJ, Pusztai A, Barocz S. Handbook of Plant Lectins: Properties and Biomedical Applications. 1998. [Google Scholar]
- 35.Sharon N, Lis H. Lectins. 2003. [Google Scholar]
- 36.Hester G, Wright CS. The mannose-specific bulb lectin from Galanthus nivalis (snowdrop) binds mono- and dimannosides at distinct sites. Structure analysis of refined complexes at 2.3 Å and 3.0 Å resolution. J. Mol. Biol. 1996;262:516–531. doi: 10.1006/jmbi.1996.0532. [DOI] [PubMed] [Google Scholar]
- 37.Harata K, Muraki M. Crystal structures of Urtica dioica agglutinin and its complex with tri-acetylchitotriose. J. Mol. Biol. 2000;297:673–681. doi: 10.1006/jmbi.2000.3594. [DOI] [PubMed] [Google Scholar]
- 38.Balzarini J, et al. α-(1–3)- and α-(1–6)-D-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob. Agents Chemother. 1991;35:410–416. doi: 10.1128/AAC.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balzarini, et al. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 1992;18:191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 40.Balzarini J, et al. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2004;48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balzarini J, et al. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV gp120. A new therapeutic concept to hit the Achilles heel of HIV. J. Biol. Chem. 2005;280:41005–41014. doi: 10.1074/jbc.M508801200. [DOI] [PubMed] [Google Scholar]
- 42.Wang JH, et al. A β-galactose-specific lectin isolated from the marine worm Chaetopterus variopedatus possesses anti-HIV-1 activity. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2005;142:111–117. doi: 10.1016/j.cbpc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Bulgheresi S, et al. A new C-type lectin similar to the human immunoreceptor DC-SIGN mediates symbiont acquisition by a marine nematode. Appl. Environ. Microbiol. 2006;72:2950–2956. doi: 10.1128/AEM.72.4.2950-2956.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji X, Gewurz H, Spear GT. Mannose-binding lectin (MBL) and HIV. Mol. Immunol. 2005;42:145–152. doi: 10.1016/j.molimm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Klein NJ. Mannose-binding lectin: do we need it? Mol. Immunol. 2005;42:919–924. doi: 10.1016/j.molimm.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Geijtenbeek TB, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 47.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 48.Drickamer K, Taylor ME. Targeting diversity. Nature Struct. Mol. Biol. 2006;12:830–831. doi: 10.1038/nsmb1005-830. [DOI] [PubMed] [Google Scholar]
- 49.Scanlan CN, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trkola A, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calarese DA, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIVHIV-1 antibody 2G12. Proc. Natl Acad. Sci. USA. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanders RW, et al. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 54.Huskens D, Van Laethem K, Vermeire K, Balzarini J, Schols D. Resistance of HIV-1 to the broadly HIV-1-neutralizing, anti-carbohydrate antibody 2G12. Virology. 2007;360:294–304. doi: 10.1016/j.virol.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 55.Oki T, et al. Pradimicin, a novel class of potent antifungal antibiotics. J. Antibiot. 1988;41:1701–1704. doi: 10.7164/antibiotics.41.1701. [DOI] [PubMed] [Google Scholar]
- 56.Ueki T, Oka M, Fukagawa Y, Oki T. Studies on the mode of antifungal action of pradimicin antibiotics. III. Spectrophotometric sequence analysis of the ternary complex formation of BMY-28864 with D-mannopyranoside and calcium. J. Antibiot. (Tokyo) 1993;46:465–477. doi: 10.7164/antibiotics.46.465. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi T, et al. New antifungal antibiotics, benanomicins A and B from an actinomycete. J. Antibiot. (Tokyo) 1988;41:807–811. doi: 10.7164/antibiotics.41.807. [DOI] [PubMed] [Google Scholar]
- 58.Hoshino H, Seki J, Takeuchi T. New antifungal antibiotics, benanomicins A and B inhibit infection of T-cell with human immunodeficiency virus (HIV) and syncytium formation by HIV. J. Antibiot. (Tokyo) 1989;42:344–346. doi: 10.7164/antibiotics.42.344. [DOI] [PubMed] [Google Scholar]
- 59.Tanabe-Tochikura A, Tochikura T, Yoshida O, Oki T, Yamamoto N. Pradimicin A inhibition of human immunodeficiency virus: attenuation by mannan. Virology. 1990;176:467–473. doi: 10.1016/0042-6822(90)90016-K. [DOI] [PubMed] [Google Scholar]
- 60.Balzarini J, et al. Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human immunodeficiency virus. J. Virol. 2007;81:362–373. doi: 10.1128/JVI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Striegler S, Dittel M. A sugar discriminating binuclear copper (II) complex. J. Am. Chem. Soc. 2003;125:11518–11524. doi: 10.1021/ja035561f. [DOI] [PubMed] [Google Scholar]
- 62.Mazik M, Cavqa H, Jones PG. Molecular recognition of carbohydrates with artificial receptors: mimicking the binding motifs found in the crystal structures of protein-carbohydrate complexes. J. Am. Chem. Soc. 2005;127:9045–9052. doi: 10.1021/ja043037i. [DOI] [PubMed] [Google Scholar]
- 63.Ng KK, et al. Orientation of bound ligands in mannose-binding proteins. Implications for multivalent ligand recognition. J. Biol. Chem. 2002;277:16088–16095. doi: 10.1074/jbc.M200493200. [DOI] [PubMed] [Google Scholar]
- 64.Botos I, Wlodawer A. Proteins that bind high-mannose sugars of the HIV-1 envelope. Prog. Biophys. Mol. Biol. 2005;88:233–282. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 66.Sauerborn MK, Wright LM, Reynolds CD, Grossmann JG, Rizkallah PJ. Insights into carbohydrate recognition by Narcissus pseudonarcissus lectin: the crystal structure at 2 Å resolution in complex with α1–3 mannobiose. J. Mol. Biol. 1999;290:185–199. doi: 10.1006/jmbi.1999.2862. [DOI] [PubMed] [Google Scholar]
- 67.Hester G, Kaku H, Goldstein IJ, Wright CS. Strucure of mannose-specific snowdrop (Galanthus nivalis) lectin is representative of a new plant lectin family. Nature Struct. Biol. 1995;2:472–479. doi: 10.1038/nsb0695-472. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, et al. A novel tetrameric lectin from Lycoris aurea with four mannose binding sites per monomer. Acta Biochim. Pol. 2007;54:159–166. [PubMed] [Google Scholar]
- 69.Balzarini J, et al. Mutational pathways, resistance profile, and side effects of Cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J. Virol. 2006;80:8411–8421. doi: 10.1128/JVI.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lifson J, Coutre S, Huang E, Engleman E. Role of envelope glycoprotein carbohydrate in human immunodeficiency virus (HIV-1) infectivity and virus-induced cell fusion. J. Exp. Med. 1986;164:2101–2106. doi: 10.1084/jem.164.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turville SG, Vermeire K, Balzarini J, Schols D. Sugar-binding proteins potently inhibit dendritic cell human immunodeficiency virus type 1 (HIV-1) infection and dendritic-cell-directed HIV-1 transfer. J. Virol. 2005;79:13519–13527. doi: 10.1128/JVI.79.21.13519-13527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. Antiviral Res. 2006;71:237–247. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Balzarini J, Van Herrewege Y, Vermeire K, Vanham G, Schols D. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIVHIV-1-1 transmission to T-lymphocytes. Mol. Pharmacol. 2007;71:3–11. doi: 10.1124/mol.106.030155. [DOI] [PubMed] [Google Scholar]
- 74.Van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nature Rev. Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 75.Balzarini J, Van Damme L. Anti-human immunodeficiency virus (HIV-1) microbicide drug candidates to prevent HIV infection. Lancet. 2007;5:726–731. doi: 10.1016/S1473-3099(05)70271-1. [DOI] [PubMed] [Google Scholar]
- 76.Izquierdo-Useros N, et al. Maturation of blood derived dendritic cells enhances HIV-1 capture and transmission. J. Virol. 2007;81:7559–7570. doi: 10.1128/JVI.02572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boggiano C, Manel N, Littman DR. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J. Virol. 2007;81:2519–2523. doi: 10.1128/JVI.01661-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang JH, Janas AM, Olson WJ, KewalRamani VN, Wu L. CD4 co-expression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1. J. Virol. 2007;81:2497–2507. doi: 10.1128/JVI.01970-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Witte L, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 80.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 81.Dwek, R. A. Glycobiology at Oxford. A personal view. The Biochemist 4–6 (2006).
- 82.Balzarini J. The α(1, 2)-mannosidase I inhibitor 1-deoxymannojirimycin potentiates the antiviral activity of carbohydrate-binding agents against wild-type and mutant HIV-1 strains containing glycan deletions in gp120. FEBS Lett. 2007;581:2060–2064. doi: 10.1016/j.febslet.2007.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pikora C, Wittish C, Desrosiers RC. Identification of two N-linked glycosylation sites within the core of the simian immunodeficiency virus glycoprotein whose removal enhances sensitivity to soluble CD4. J. Virol. 2005;79:12575–12583. doi: 10.1128/JVI.79.19.12575-12583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balzarini J, et al. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J. Virol. 2004;78:10617–10626. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balzarini J, et al. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and. Galanthus nivalis. Mol. Pharmacol. 2005;67:1556–1565. doi: 10.1124/mol.104.005082. [DOI] [PubMed] [Google Scholar]
- 86.Witvrouw M, et al. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents Cyanovirin N and Concanavalin A. J. Virol. 2005;79:7777–7784. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leonard CK, et al. Assignment of intrachain disulphide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 88.Gallaher WR, et al. A general model for the surface glycoproteins of HIV-1 and other retroviruses. AIDS Res. Hum. Retroviruses. 1995;11:191–202. doi: 10.1089/aid.1995.11.191. [DOI] [PubMed] [Google Scholar]
- 89.Bolmstedt A, et al. Enhanced immunogenicity of a human immunodeficiency virus type 1 env DNA vaccine by manipulating N-glycosylation signals. Effects of elimination of the V3 306 glycan. Vaccine. 2001;20:397–405. doi: 10.1016/S0264-410X(01)00358-9. [DOI] [PubMed] [Google Scholar]
- 90.Kang SM, et al. Modified HIV envelope proteins with enhanced binding to neutralizing monoclonal antibodies. Virology. 2005;331:20–32. doi: 10.1016/j.virol.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 91.Chackerian B, Rundensey LM, Overbaugh J. Specific N-linked and O-linked glycosylation modifications in the envelope VI domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 1997;71:7719–7727. doi: 10.1128/jvi.71.10.7719-7727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng-Mayer C, Brown A, Harouse J, Luciw PA, Mayer AJ. Selection for neutralization resistance of the simian human immunodeficiency virus sHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 1999;73:5294–5300. doi: 10.1128/jvi.73.7.5294-5300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson WE, et al. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J. Virol. 2003;77:9993–10003. doi: 10.1128/JVI.77.18.9993-10003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cole KS, Steckbeck JD, Rowles JL, Desrosiers RC, Montelaro RC. Removal of N-linked glycosylation sites in the V1 region of simian immunodeficiency virus gp120 results in redirection of B-cell responses to V3. J. Virol. 2004;78:1525–1539. doi: 10.1128/JVI.78.3.1525-1539.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reitter JN, Means RE, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nature Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 96.Blay WM, et al. Consistent patterns of change during the divergence of human immunodeficiency virus type 1 envelope from that of the inoculated virus in simian/human immunodeficiency virus-infected macaques. J. Virol. 2006;80:999–1014. doi: 10.1128/JVI.80.2.999-1014.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poon AFY, Lewis FI, Kosakovsky Pond SL, Frost SDW. Evolutionary interactions between N-linked glycosylation sites in the HIV-1 envelope. PLoS Comput. Biol. 2007;3:e11. doi: 10.1371/journal.pcbi.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koppel EA, van Gisbergen KPJM, Geijtenbeek TBH, van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 2005;7:157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 99.Tassaneetrithep B, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Halary F, et al. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653–654. doi: 10.1016/S1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- 101.Lozach PY, et al. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J. Biol. Chem. 2004;279:32035–32045. doi: 10.1074/jbc.M402296200. [DOI] [PubMed] [Google Scholar]
- 102.Alvarez CP, et al. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marzi A, et al. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 2004;78:12090–12095. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tailleux L, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smits HH, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 107.Koppel EA, Saeland E, de Cooker DJ, van Kooyk Y, Geijtenbeek TB. DC-SIGN specifically recognizes Streptococcus pneumoniae serotypes 3 and 14. Immunobiology. 2005;210:203–210. doi: 10.1016/j.imbio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 108.Appelmelk BJ, et al. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 109.van Die I, et al. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- 110.Bergman MP, et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 2004;200:979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meyer S, et al. DC-SIGN mediates binding of dendritic cells to authentic pseudo-LewisY glycolipids of Schistosoma mansoni cercariae, the first parasite-specific ligand of DC-SIGN. J. Biol. Chem. 2005;280:37349–37359. doi: 10.1074/jbc.M507100200. [DOI] [PubMed] [Google Scholar]
- 112.Wei X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 113.Helle F, et al. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J. Biol. Chem. 2006;281:25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 114.Bertaux, C. et al. The inhibition of infection and entry of the human immunodeficiency virus (HIVHIV-1) and hepatitis C virus (HCV) closely correlates for carbohydrate-binding agents (CBA), but not for polyanions. Virology (in the press). Together with reference 113, this reference gives the first demonstration that a CBA can inhibit human hepatitis C virus.
- 115.Keyaerts E, et al. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.VANDERMEER F, DEHAAN C, SCHUURMAN N, HAIJEMA B, PEUMANS W, VANDAMME E, DELPUTTE P, BALZARINI J, EGBERINK H. Antiviral activity of carbohydrate-binding agents against Nidovirales in cell culture. Antiviral Research. 2007;76(1):21–29. doi: 10.1016/j.antiviral.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barrientos LG, et al. Cyanovirin-N binds to the viral surface glycoprotein, GP1, 2 and inhibits infectivity of Ebola virus. Antiviral Res. 2003;58:47–56. doi: 10.1016/S0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 118.Huskens D, Vandemeulebroucke E, Willaert X, Balzarini J, Schols D. Abstracts of the 14th Conference on Retroviruses and Opportunistic Infections. 2007. Safety concerns for the potential use of cyanovirin as a microbicidal anti-HIV agent; pp. 25–28. [Google Scholar]
- 119.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 120.Wu L, KewalRamani N. Dendritic-cell interactions with HIV: infection and viral dissemination. Nature Rev. Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balzarini J. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antivir. Chem. Chemother. 2007;18:1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- 122.Groot F. Dendritic cell-mediated HIV-1 transmission. 2006. [Google Scholar]
- 123.Kwong PD, et al. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ludwig JS, et al. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. J. Virol. 2004;78:8322–8332. doi: 10.1128/JVI.78.15.8322-8332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Davis CW, et al. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baribaud F, Doms RW, Pohlmann S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets. 2002;6:423–431. doi: 10.1517/14728222.6.4.423. [DOI] [PubMed] [Google Scholar]
- 127.Rappocciolo G, et al. DC-SIGN is a receptor for human herpes virus 8 on dendritic cells and macrophages. J. Immunol. 2006;176:1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- 128.de Witte L, Abt M, Schneider-Schaulies S, von Kooyk Y, Geijtenbeek TB. Measles virus targets DC-SIGN to enhance dendritic cell infection. J. Virol. 2006;80:3477–3486. doi: 10.1128/JVI.80.7.3477-3486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barreiro LB, et al. DC-SIGN interacts with Mycobacterium leprae but sequence variation in this lectin is not associated with leprosy in the Pakistani population. Hum. Immunol. 2006;67:102–107. doi: 10.1016/j.humimm.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Caparros E, et al. Role of the C-type lectins DC-SIGN and L-SIGN in Leishmania interaction with host phagocytes. Immunobiology. 2005;210:185–193. doi: 10.1016/j.imbio.2005.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Serrano-Gomez D, Leal JA, Corbi AL. DC-SIGN mediates the binding of Aspergillus fumigatus and keratinophylic fungi by human dendritic cells. Immunobiology. 2005;210:175–183. doi: 10.1016/j.imbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 132.Cambi A, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 2003;33:532–538. doi: 10.1002/immu.200310029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


