Abstract
This study was conducted to assess the levels of inflammatory factors and angiogenic factors in patients with severe hemophilia A and evaluate their diagnostic values for acute joint bleeding. This study included a total of 144 patients with severe hemophilia A. Of them, 66 had acute joint bleeding. Ninety healthy volunteers were recruited as control. The levels of leukocytes, monocytes, platelets, hemoglobin, phagocyte migration inhibitory factor (MIF), plasminogen, fibrin/fibrinogen degradation products, d-dimer, and α2 antifibrinolytic enzyme were measured using hematology analyzer. Thrombomodulin, endostatin, intercellular adhesion molecule 1, and vascular endothelial growth factor (VEGF) were assessed using enzyme-linked immunosorbent assay. Logistic regression analysis was performed to analyze the factors affecting acute joint bleeding. Compared with healthy volunteers, the levels of leukocytes, C-reactive protein (CRP), MIF, and VEGF were significantly (P < .05) elevated in the patients with severe hemophilia A and were significantly higher in patients with joint bleeding than in patients with nonbleeding (P < .05). Multivariate analysis showed that CRP and VEGF were independent risk factors for acute joint bleeding (P < .05). The area under the curve, sensitivity, and specificity of CRP for the diagnosis of acute joint bleeding were 0.829, 88.43%, and 67.87%, respectively, and those of VEGF were 0.758, 82.8%, and 68.3%, respectively. The levels of inflammatory factors and angiogenesis factors are elevated in patients with severe hemophilia A and both CRP and VEGF are closely related to acute joint bleeding and may be used as potential biomarkers for predicting acute joint bleeding in patients with severe hemophilia A.
Keywords: severe hemophilia A, joint bleeding, inflammatory factors, angiogenic factors, predictive value
Introduction
Hemophilia A is a common hereditary hematological disease. Patients with hemophilia A have spontaneous and repeated bleeding, especially in the muscles and joints. Severe hemophilia A accounts for 60% of patients with hemophilia and has high frequent and serious joint bleeding1 and neoangiogenesis contributes to the development of hemophilic synovitis.2 At present, blood coagulation factors are used to measure and predict the bleeding risk for patients with hemophilia.3 Also, injections of mesenchymal stem cells appear to be efficacious for decreasing pain in patients with hemophilic arthropathy.4 However, due to the influence of other factors, the accuracy of prediction is not satisfactory. Although hemophilia is a hereditary disorder, its phenotype is affected by environmental factors. Despite intensive studies on severe hemophilia A, reliable predictor for joint bleeding is still unavailable. Clinical studies have shown that in patients with hemophilia, blood coagulation factors play an important role and their elevation is often associated with disorder of joint function.5 In addition, some promising cartilage and synovium biomarkers are at various stages of development and awaiting further validation in larger patient populations with osteoarthritis and these markers may be potentially used for the diagnosis and prognosis of hemophilic arthropathy.6 Furthermore, proangiogenic factors such as vascular endothelial growth factor A (VEGF-A), stromal cell-derived factor 1, and matrix metalloproteinase 9 were found to be elevated in proangiogenic macrophage/monocyte cells (VEGF+/CD68+ and VEGFR1+/CD11b+) in the pathogenesis of hemophilic joint disease, suggesting that angiogenesis is involved in the disease.2
Since it is important to predict joint bleeding for prevention and treatment, we investigated the levels of inflammatory and angiogenic factors in patients with severe hemophilia A to identify possible diagnostic biomarkers. The findings would help better management and treatment of the disease.
Materials and Methods
Patients
A total of 144 patients with severe hemophilia A and 90 healthy volunteers were recruited between October 2015 and August 2018 for this study. Among the patients with hemophilia, 78 were nonbleeding and 66 had acute joint bleeding. All patients were male and aged between 11 and 55 years, with an average age of 21.80 ± 7.73 years. The healthy volunteers were also male and aged 11 to 56 years, with an average age of 22.11 ± 7.93 years. Patients were included if they had baseline factor VIII (FVIII) activity ≤1% and were diagnosed using X-rays and magnetic resonance imaging. They were excluded if they were continuously treated for more than 3 months within 1 year, had chronic joint bleeding or combined bleeding with other organs, recently infused with red blood cells or whole blood, and had hepatitis B virus and HIV infection and severe impairment of liver and kidney function or underlying diseases. Patients with incomplete clinical data were also excluded. Informed consent was obtained from all participants and the study was approved by the research ethics committee of Qingdao University.
Reagents and Instruments
Hematology analyzer (DxH600) was from Beckman, Brea, California; enzyme-linked immunosorbent assay (ELISA) plate reader was purchased from Thermo Scientific Company, Waltham, Massachusetts; ELISA kits were purchased from Roche, Switzerland; and AU400 automatic biochemical analyzer was a product of Olympus, Japan.
Blood Samples
Peripheral blood samples were collected on an empty stomach in the morning, added immediately with anticoagulant sodium citrate, and mixed by inverting the tube several times. The serum was collected after centrifugation at 3000 g for 20 minutes and stored at −80°C for subsequent use.
Blood Analysis
Blood samples were counted for leukocyte, monocyte, and platelet and analyzed for hemoglobin, macrophage migration inhibitor (MIF), plasminogen (PLG), fibrin/fibrinogen degradation products (FDP), d-dimer (D-D), and α2-antiplasmin (α2-AP) using a hematology analyzer. Immunoturbidimetry was used to determine the levels of serum C-reactive protein (CRP) and chemiluminescence was used to measure the levels of ferritin.
Enzyme-Linked Immunosorbent Assay
Thrombomodulin (TM), endostatin (ES), intercellular adhesion molecule-1 (ICAM-1), and vascular endothelial growth factor (VEGF) were analyzed using ELISA with standards purchased from Roche, Switzerland.
Statistical Analysis
The data were analyzed using SPSS version 21.0 software. Enumeration data were expressed in percentage and tested using χ2 test; measurement data were expressed as means ± standard deviation. Multiple comparisons were performed using analysis of variance and paired comparisons were performed using q test. Logistic regression analysis was performed to analyze the factors affecting acute joint bleeding. The predictive performance was evaluated with the receiver operating characteristic (ROC) curve and the area under the curve (AUC). P value < .05 was considered statistically significant.
Results
Levels of PLG, FDP, and d-D
The clinical data of participants are given in Table 1. The 3 groups had no difference in averaged age and body mass index. Compared with healthy people, the levels of PLG, FDP, and d-D in the patients with hemophilia were significantly higher (P < .05), and compared with patients with nonjoint bleeding, patients with joint bleeding had higher levels of PLG, FDP, and d-D (P < .05). On the other hand, the levels of α2-AP were similar among the groups (Table 1; P > .05).
Table 1.
Comparison of Clinical Data in Patients With Hemophilia.a
| Group | Number of Case | Average Age (Years) | BMI | PLG (%) | FDP (μg/mL) | D-D (μg/L) | α2-AP (%) |
|---|---|---|---|---|---|---|---|
| Healthy people | 90 | 16 | 16.62 ± 3.12 | 66.28 ± 11.43b | 0.48 ± 0.09b | 71.33 ± 18.64b | 149.43 ± 13.57 |
| Nonbleeding | 78 | 15 | 16.59 ± 2.93 | 86.42 ± 17.26c | 0.67 ± 0.12c | 91.66 ± 17.43c | 152.64 ± 16.69 |
| Bleeding | 66 | 18 | 17.76 ± 4.23 | 103.57 ± 13.29d | 0.83 ± 0.19d | 112.68 ± 24.19d | 152.37 ± 18.24 |
Abbreviations: α2-AP, α2-antiplasmin; BMI, body mass index; d-D, d-dimer; FDP, fibrin/fibrinogen degradation products; PLG, plasminogen.
a Figures in the same column labeled with different superscript letters (b to d) are different significantly (P < .05).
Serum Inflammatory Factors
Compared with the control group, patients with severe hemophilia A had significantly higher levels of leukocyte, CRP, ferritin, and MIF (P < .05). Furthermore, these factors were even higher in the bleeding group than in the nonbleeding group (P < .05, Table 2). On the other hand, other indexes such as the levels of monocyte, hemoglobin, platelet, and lactic acid were similar between the groups (Table 2).
Table 2.
Comparison of Serum Inflammatory Factors in Patients With Hemophilia.a
| Group | Number of Case | Leukocyte (109/L) | Monocyte (106/L) | Hemoglobin (g/dL) | Platelet (1011/L) | CRP (109/L) | Ferritin (mg/dL) | Lactic Acid (ng/mL) | MIF (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| Healthy people | 90 | 6.52 ± 0.86 | 483.18 ± 31.41 | 12.75 ± 2.27 | 2.49 ± 0.31 | 2.56 ± 0.67 | 31.64 ± 6.87 | 1.19 ± 0.23 | 0.19 ± 0.03 |
| Nonbleeding | 78 | 7.76 ± 1.05b | 491.63 ± 33.74 | 13.18 ± 2.83 | 2.56 ± 0.33 | 6.79 ± 1.23b | 77.68 ± 9.46b | 1.2 ± 0.27 | 0.51 ± 0.07b |
| Bleeding | 66 | 8.58 ± 1.33c | 503.46 ± 45.17 | 13.54 ± 2.67 | 2.62 ± 0.39 | 16.88 ± 3.27c | 86.85 ± 11.34c | 1.33 ± 0.29 | 0.67 ± 0.09c |
Abbreviations: CRP, C-reactive protein; MIF, migration inhibitory factor.
a Figures in the same column labeled with different superscript letters (b to c) are different significantly (P < .05).
Serum Angiogenic Factors
Compared with healthy participants, the patients with hemophilia had significantly elevated VEGF levels, which were even higher in the joint bleeding group (P < .05, Table 3). Other angiogenic factors analyzed such as TM, ICAM-1, and ES did not differ between the groups (P > .05, Table 3).
Table 3.
Comparison of Serum Angiogenic Factors in Patients With Hemophilia.a
| Group | Number of Case | VEGF (pg/mL) | TM (ng/mL) | ICAM-1 (ng/mL) | ES (ng/mL) |
|---|---|---|---|---|---|
| Healthy people | 90 | 54.38 ± 9.67 | 0.68 ± 0.14 | 0.36 ± 0.06 | 60.55 ± 8.11 |
| Nonbleeding | 78 | 70.63 ± 11.24b | 0.72 ± 0.18 | 0.35 ± 0.05 | 62.15 ± 7.01 |
| Bleeding | 66 | 113.26 ± 15.49c | 0.73 ± 0.116 | 0.37 ± 0.06 | 61.51 ± 7.41 |
Abbreviations: ES, endostatin; ICAM-1, intercellular adhesion molecule 1; TM, thrombomodulin; VEGF, vascular endothelial growth factor.
a Figures in the same column labeled with different superscript letters (b and c) are different significantly (P < .05).
Logistic Analysis of Factors Affecting Joint Bleeding
Univariate analysis showed that serum CRP, FDP, VEGF, and ferritin were associated with acute joint bleeding in the patients with hemophilia (P < .05). Multivariate analysis showed that CRP and VEGF were independent risk factors for the incidence of the joint bleeding (P < .05, Table 4).
Table 4.
Logistic Regression Analysis of Acute Joint Bleeding in Patients With Severe Hemophilia A.
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| CRP | 6.363 | 3.546-11.418 | .021 | 2.234 | 1.269-3.933 | .026 |
| FDP | 2.366 | 1.556-3.575 | .018 | 1.053 | 0.948-1.170 | .516 |
| VEGF | 4.515 | 2.649-7.695 | .001 | 2.826 | 2.065-3.867 | .007 |
| Ferritin | 3.768 | 2.161-6.570 | .006 | 2.836 | 0.925-4.938 | .053 |
| MIF | 1.894 | 1.507-2.381 | .117 | 0.736 | 0.677-0.801 | .663 |
| BMI | 1.042 | 0.682-1.590 | .196 | 0.852 | 0.392-1.852 | .769 |
| d-D | 2.311 | 0.776-6.882 | .071 | 1.622 | 0.929-2.832 | .151 |
| Leukocyte | 1.591 | 1.456-1.739 | .064 | 1.723 | 1.319-2.251 | .106 |
Abbreviations: BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; d -D, d-dimer; FDP, fibrin/fibrinogen degradation products; MIF, migration inhibitory factor; OR, odds ratio; VEGF, vascular endothelial growth factor.
Predictive Value of CRP and VEGF for Joint Bleeding
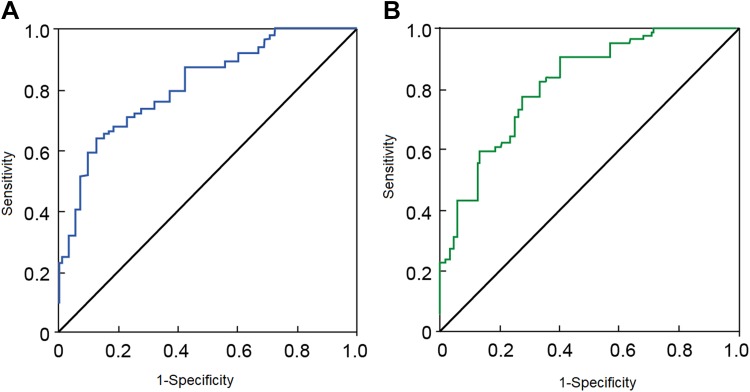
The ROC curve analysis showed that CRP and VEGF had predictive value for joint bleeding in patient with severe hemophilia A (Figure 1). The AUC of CRP in the diagnosis of acute joint bleeding was 0.829 (P < .05), the sensitivity was 88.43%, and the specificity was 67.87% (Figure 1A); and the AUC of VEGF was 0.758 (P < .05), the sensitivity was 82.8%, and the specificity was 68.3% (Figure 1B).
Figure 1.
Receiver operating characteristic curve of CRP (A) and VEGF (B) for predicting acute joint bleeding in patients with severe hemophilia. CRP indicates C-reactive protein; VEGF, vascular endothelial growth factor.
Discussion
Hemophilia A is a hereditary bleeding disorder caused by the lack of blood clotting factor VIII. The incidence is about 1 in 5000. Although some progresses have been made in the treatment of the disease, arthropathy caused by joint bleeding is still a challenge to prevent and treat, which often results in joint deformity and disability.7 Therefore, a better understanding of the mechanisms underlying intra-articular bleeding (hemarthrosis) in patients with hemophilia A is urgently needed to prevent and treat the disease. The severity of hemarthrosis is related to the number of bleeding, but studies also show that inflammatory factors in the joint and angiogenesis factors also play an important role in joint bleeding.8 Therefore, we set to investigate the relationship between these factors and joint bleeding for the prevention and treatment of joint bleeding.
In the animal model of hemophilia arthropathy, it was found that inflammation may last long time after joint bleeding and that leukocyte, interleukin 6, murine double min 2 (mdm2), the p53-binding protein,21 and CRP play an important role.9–11 Forsyth et al12 showed that after bleeding, the bled blood could damage the joint directly and the released inflammatory factors could stimulate the proliferation of macrophage-like synovial cells, causing further damage to the cartilage and synovium and sustained joint damage. Our results showed that CRP, MIF, and leukocyte levels in the patients with joint bleeding are significantly higher than in the patients with nonbleeding, suggesting that the elevation of inflammatory factors is associated with the possibility of joint bleeding. This is consistent with an earlier study, which showed that increased serum CPR level is associated with increased ferritin level and occurrence of arthropathy in patients with hemophilia A.13 The deposition of ferritin in the synovium may cause inflammation and cartilage damage. Our data show that the level of ferritin in the bleeding group is significantly higher than in nonbleeding group, indicating that high ferritin level is associated with bleeding. The above findings indicate that serum CRP, MIF, ferritin, and leukocyte are related to articular bleeding, suggesting that anti-inflammation therapy may be a direction to treat bleeding in severe hemophilia A. These results are similar to a recent study with relatively small patient cohorts.14 Our study, on the other hand, was conducted with relatively large sample size and is therefore more statistically accurate.
Angiogenesis is an early manifestation of joint lesions, which provides synovial cells with more nutrients and inflammatory factors to infiltrate the synovium. Therefore, it plays important role in the occurrence and development of joint lesions.12,15 In arthritis, angiogenesis is a complex process and involves many factors such as ICAM-1, ES, VEGF, and protease. Vascular endothelial growth factor is the most studied and its elevation promotes the division and proliferation of vascular endothelial cells and cell migration and plays an important regulatory role in angiogenesis.16,17 Intercellular adhesion molecule 1 is strongly expressed in proliferated vascular endothelial cells, promoting adhesion between leukocytes and endothelial cells that induces angiogenesis.18,19 Endostatin is an inhibitor of angiogenesis. Once injected into immunodeficient rats, although it inhibits neovascularization and alleviates inflammation, the joint deformity is serious.20,21 Thrombomodulin is a transmembrane glycoprotein, which mainly exists in vascular endothelial cells. Recently, TM protein fragments have been found to promote angiogenesis and stimulate the migration and proliferation of endothelial cells and formation of blood vessels.22 However, our study shows that the levels of ICAM-1, ES, and TM are similar between bleeding group and nonbleeding group, but the level of VEGF is significantly higher in the bleeding group than in the nonbleeding group, suggesting that only VEGF is related to joint bleeding and may be an important cause of bleeding. On other hand, ICAM-1, ES, and TM may not be direct risk factors. It was found that serum VEGF alone can induce ovascularization,23 supporting that there is a correlation between VEGF and joint bleeding.
The frequency of joint bleeding in patients with severe hemophilia A is high, and only 15% of the patients in Chinese population has mild bleeding.24 It is important to deliberate the causes of the bleeding for better prognosis of patients. Our logistic regression analysis showed that CRP and VEGF are independent risk factors for acute joint bleeding in patients with severe hemophilia A. The ROC analysis showed that the serum levels of CRP and VEGF could be potentially used for predicting the risk of joint bleeding. The AUC, sensitivity, and specificity of CRP to diagnose joint bleeding are 0.829, 88.43%, and 67.87%, respectively, and those of VEGF were 0.758, 82.8%, and 68.3%, respectively, suggesting that these 2 factors are useful and reliable biomarkers to predict joint bleeding.
Conclusions
Our analysis demonstrates that the levels of inflammatory and angiogenesis factors increase in patients with severe hemophilia A. Both CRP and VEGF levels are associated with joint bleeding in patients with severe hemophilia and may be used as diagnostic markers to predict joint bleeding. However, due to the heredity nature of the disease, the number of patients included in the study is limited and were not followed up for long time. Further studies with long follow-up and large number, particularly with patents before hemarthrosis, are needed to further validate our conclusions.
Footnotes
Authors’ Note: HX and LS contributed to project conceptualization, investigation, and data analysis. ZR, KW, YZ, and XL contributed to data collection, analysis, and methodology development. ZR, KW, JJ, and SS did investigation and methodology development. Project conceptualization and manuscript writing were done by SW. SS and LS contributed to project conceptualization, investigation, and manuscript writing. The manuscript was read and approved by all authors.
Availability of Data and Material: The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lirong Sun  https://orcid.org/0000-0002-1403-328X
https://orcid.org/0000-0002-1403-328X
Ethics Approval and Consent to Participate: The study was approved by the research ethics committee of Qingdao University and written consent was received from every participant.
References
- 1. Rendo P, Shafer F, Korth-Bradley JM, Sivamurthy K, Korin J. Factors that influence the bleeding phenotype in severe hemophilic patients. Blood Coagul Fibrinol. 2013;24(7):683–690. [DOI] [PubMed] [Google Scholar]
- 2. Acharya SS, Kaplan RN, Macdonald D, Fabiyi OT, DiMichele D, Lyden D. Neoangiogenesis contributes to the development of hemophilic synovitis. Blood. 2011;117(8):2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chelle P, Montmartin A, Piot M, et al. Prediction of individual factor VIII or IX level for the correction of thrombin generation in haemophilic patients. Haemophilia. 2018;24(6):995–1001. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez-Merchan EC. Intra-articular injections of mesenchymal stem cells (MSCs) as a treatment for hemophilic arthropathy. Expert Rev Hematol. 2016;9(8):737–741. [DOI] [PubMed] [Google Scholar]
- 5. Keshava S, Sundaram J, Rajulapati A, Esmon C, Pendurthi U, Rao LVM. Factor VIIa interaction with EPCR modulates the hemostatic effect of rFVIIa in hemophilia therapy: mode of its action. Blood Adv. 2017;1(15):1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez-Merchan EC. Synovium and cartilage biomarkers in hemophilic arthropathy. Expert Rev Hematol. 2016;9(4):409–414. [DOI] [PubMed] [Google Scholar]
- 7. Valentino LA. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. J Thromb Haemost. 2010;8(9):1895–1902. [DOI] [PubMed] [Google Scholar]
- 8. Angelini D, Konkle BA, Sood SL. Aging among persons with hemophilia: contemporary concerns . Semin Hematol. 2016;53(1):35–39. [DOI] [PubMed] [Google Scholar]
- 9. Lozier JN, Nichols TC. Animal models of hemophilia and related bleeding disorders. Semin Hematol. 2013;50(2):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hakobyan N, Kazarian T, Jabbar AA, Jabbar KJ, Valentino LA. Pathobiology of hemophilic synovitis. Part I: overexpression of mdm2 oncogene. Blood. 2004;104(7):2060–2064. [DOI] [PubMed] [Google Scholar]
- 11. Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forsyth AL, Rivard GE, Valentino LA, et al. Consequences of intra-articular bleeding in haemophilia: science to clinical practice and beyond. Haemophilia. 2012;18(suppl 4):112–119. [DOI] [PubMed] [Google Scholar]
- 13. Guo D, Wang Y, Meng D. The relationship between serum ferritin and rheumatoid arthritis severity. J Mod Lab Med. 2013;31(2):123–127. [Google Scholar]
- 14. Karapnar TH, Karadas N, Ozek G, et al. The investigation of relationship between joint findings and serum angiogenic and inflammatory factor levels in severe hemophilia A patients. Blood Coagul Fibrinol. 2014;25(7):703–708. [DOI] [PubMed] [Google Scholar]
- 15. Mathieu C, Chevrier A, Lascau-Coman V, Rivard GE, Hoemann CD. Stereological analysis of subchondral angiogenesis induced by chitosan and coagulation factors in microdrilled articular cartilage defects. Osteoarthr Cartil. 2013;21(6):849–859. [DOI] [PubMed] [Google Scholar]
- 16. Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J Bone Miner Res. 2016;31(5):911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan Q, Sun L, Li JJ, An CH. Elevated VEGF levels contribute to the pathogenesis of osteoarthritis. BMC Musculoskelet Disord. 2014;15:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amin MA, Campbell PL, Ruth JH, et al. A key role for Fut1-regulated angiogenesis and ICAM-1 expression in K/BxN arthritis. Ann Rheum Dis. 2015;74(7):1459–1466. [DOI] [PubMed] [Google Scholar]
- 19. Langston W, Chidlow JH, Jr, Booth BA, et al. Regulation of endothelial glutathione by ICAM-1 governs VEGF-A-mediated eNOS activity and angiogenesis. Free Radic Biol Med. 2007;42(5):720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walia A, Yang JF, Huang YH, Rosenblatt MI, Chang JH, Azar DT. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta. 2015;1850(12):2422–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Chen Y, Lu XA, Liu G, Fu Y, Luo Y. Endostatin prevents dietary-induced obesity by inhibiting adipogenesis and angiogenesis. Diabetes. 2015;64(7):2442–2456. [DOI] [PubMed] [Google Scholar]
- 22. Sun J, Xia Z, Tan D. Study on the protective effect of ulinastatin on vascular endothelial cells in patients with capillary leak syndrome. Pract Med. 2015;32(4):12–17. [Google Scholar]
- 23. Kang Y, Han M, Zhao P. Expression of VEGF mRNA and protein in oxygen-induced retinopathy in mouse. Chin Ophthalmol Res. 2015;22(3):22–27. [Google Scholar]
- 24. Li H, Sun J, Zhou X, Liu Y, Song X, Ma Q. Clinical predictors for the phenotypic heterogeneity of severe hemophilia A in China. J South Med Univ. 2013;33(3): 424–427. [PubMed] [Google Scholar]