Abstract
Background:
Neutrophil-to-lymphocyte ratio (NLR) was introduced as a potential inflammatory marker in sickle cell disease (SCD). This study aimed to evaluate the impact of hydroxyurea (HU) treatment on the value of NLR and some inflammatory mediators in SCD.
Methods:
The hematological parameters and clinical events were analyzed in 35 children with SCD under HU treatment and followed up for 1 year and in 20 healthy controls. Enzyme-linked immunosorbent assay was performed for the evaluation of proinflammatory cytokines, including interleukin (IL) 6, IL-8, high-sensitivity C-reactive protein (hs-CRP), and tumor necrosis factor α (TNF-α).
Results:
Hydroxyurea significantly improves most of the hematological parameters in children with SCD. The percentages of hemoglobin fraction S, serum levels of TNF-α and IL-6 were significantly decreased when compared to baseline value but did not reach the value of the healthy control. The HU treatment led to a significant decrease in NLR compared to the baseline values and reached healthy control values. Neutrophil-to-lymphocyte ratio was positively correlated with hs-CRP, TNF-α, and IL-8 serum levels and negatively correlated with percentage of fetal hemoglobin and hematocrit values. The cutoff value of NLR to expect a response to HU among SCD was 3.0, with 76% specificity and 85% sensitivity (area under the curve: 0.85, P < .0001). In conclusion, hydroxyurea induced a decrease in NLR and inflammatory cytokines, which represent a biomarker of inflammation in SCD. The calculation of NLR is a straightforward and cheap method for SCD outcome prediction in young children.
Keywords: sickle cell disease, hydroxyurea, neutrophil-to-lymphocyte ratio
Introduction
Sickle cell disease (SCD) is an inherited genetic disorder of hemoglobin (Hb) resulted from β-globin gene mutation, which leads to alteration in the properties of the Hb tetramer, thus producing variable clinical manifestations, some of which include vaso-occlusive crises (VOCs), acute chest syndrome (ACS), hemolytic anemia, and recurrent infections with their attendant sequelae.1,2
Hydroxyurea (HU) is considered one of the best therapeutic agents for children with SCD. Hydroxyurea increases fetal Hb (HbF) in red blood cells (RBCs) by inhibiting ribonucleotide reductase enzyme. Besides, it blocks the formation of sickle erythrocytes with a decrease in the frequency of VOCs and other complications of SCD,3 and it has been widely used in pediatric sickle cell centers for these indications. Hydroxyurea plays an essential role in lowering total white blood cells (WBCs) and other immunological cells.1,3
Several studies have shown that at steady state, patients with SCD have increased levels of circulating proinflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor α (TNF-α), which lead to chronic endothelium activation, leukocyte aggregation, and adhesion of sickled RBCs which may lead to ischemia and tissue necrosis.4,5
Complete blood counts (CBCs), including leukocyte subtypes, are commonly screened during the admission of patients with SCD. The neutrophil-to-lymphocyte ratio (NLR) is a biomarker that can be calculated easily based on CBCs. It has significant prognostic implications in several disease states.6,7 Although NLR is considered one of the inflammatory biomarkers in SCD,8 there are limited data about the impact of HU on the changes in NLR among children with SCD. Our objectives were to evaluate the impact of HU on NLR and some inflammatory mediators. Also, to study the association between NLR and these proinflammatory cytokines among young children with SCD.
Patients and Methods
Study Participants
In a prospective study, 35 children with SCD recruited from Children’s Hospital, Assiut University, from May 2015 to August 2017, were treated and followed up for 1 year. All children with SCD were recruited from the pediatric hematology outpatient clinic during routine follow-up. We excluded any patient with significant acute complications in 1 month before investigations. Twenty age- and sex-matched children were enrolled from the vaccination clinics in our hospital as controls. All controls are healthy, with no history of anemia, chronic disease, or bleeding disorders. Patients with SCD had been taking HU 20 mg/kg for 12 months.
Sample Size
The sample size was calculated from the formula:
N is the sample size at 95% confidence level, Z 1 − X = 1.42 from the statistical table, P is the prevalence of SCD obtained, and D is the precision of accuracy which is 0.05. We found N is at least 30 patients.
Approval was obtained from the ethical committee of Assiut University. Informed consent was taken from parents of all participants.
Hematological Parameters
Morning fasting blood samples were collected for CBCs, Hb fraction S (HbS), and HbF before and after HU therapy for 1 year.
Proinflammatory Cytokines Measurements
Serum levels of IL-6, IL-8, and TNF-α were measured using ELISA kits (R&D Systems, Minneapolis, Minnesota, United States). High-sensitivity C-reactive protein (hs-CRP) concentrations were measured by competitive immunoassay (BioCheck, Burlingame, California).
Neutrophil-to-Lymphocytes Ratio
We calculated NLR from the ratio of the neutrophils and lymphocytes from the same automated CBCs.
Statistical Analysis
The statistical package for social sciences, version 22, was used for data collection and analysis. All data were expressed as mean ± standard deviation (SD) of the mean. Differences between the groups were examined for statistical significance using the Mann-Whitney test. Correlations were calculated using Spearman linear regression analysis. Receiver operating characteristic (ROC) analysis was used to measure the ability of the NLR to predict the presence of SCD. A P value of ≤ .05 denoted a significant difference.
Results
Baseline Clinical and Hematologic Parameters of Patients With SCD
In all, 35 children with SCD and 20 control children were enrolled. The age of patients ranged from 4 to 13 years, with mean ± SD of 10.73 years ± 2.1. No significant difference was found in the mean age and sex distribution between patients with SCD and the healthy group. Red blood cells and hematocrit count were significantly lower than the healthy control group (P = .0001 for both). White blood cells, neutrophils, monocytes, and total lymphocytes counts were significantly higher than the healthy controls (Table 1).
Table 1.
Hematological and Clinical Parameters of Patients With SCD and Controls.a
| Before HU Therapy | After 1 Year | Percentage of Changes | Control Healthy | P Valueb | P Valuec | P Valued | |
|---|---|---|---|---|---|---|---|
| N = 35 | N = 35 | N = 20 | |||||
| Laboratory and hematological parameters | |||||||
| Hb (g/dL) | 7.06 ± 0.87 | 10.04 ± 1.35 | 29.70% | 11.91 ± 0.64 | .0001 | .0001 | .0001 |
| %Hb F | 7.11 ± 1.33 | 13.89 ± 3.57 | 48.80% | – | <.0001 | – | – |
| %HbS | 80·9 | 74·6 | 7.80% | – | <.0001 | – | – |
| RBCs (1012/L) | 2.45 ± 0.5 | 3.33 ± 0.73 | 26.40% | 4.43 ± 0.62 | .002 | .0001 | .0001 |
| Hematocrit | 22.79 ± 4.66 | 33.13 ± 6.91 | 31.20% | 38.99 ± 5.8 | .0001 | .0001 | .011 |
| Platelets (109/L) | 315.2 ± 91.6 | 290.9 ± 65.9 | 7.70% | 271.2 ± 65.2 | .649 | .243 | .669 |
| WBCs (109/L) | 11.35 ± 2.72 | 8.92 ± 2.3 | 21.40% | 6.4 ± 2.34 | .028 | .0001 | .006 |
| Monocytes (109/L) | 0.284 ± 0.082 | 0.234 ± 0.085 | 17.60% | 0.202 ± 0.099 | .313 | .049 | .514 |
| Neutrophils (109/L) | 5.95 ± 2.78 | 4.44 ± 2.27 | 25.30% | 3.38 ± 1.67 | .171 | .008 | .293 |
| Total lymphocytes (109/L) | 4.16 ± 0.18 | 3.16 ± 0.35 | 24% | 3.06 ± 0.29 | .0001 | .0001 | .570 |
| Proinflammatory cytokines | |||||||
| hs-CRP (mg/L) | 4.14 ± 1.29 | 3.61 ± 1.25 | 12.80% | 1.65 ± 0.82 | .15 | <.0001 | <.0001 |
| IL-6 (pg/mL) | 14.45 ± 3.94 | 11.42 ± 5.03 | 21% | 6.67 ± 2.93 | .001 | <.0001 | .001 |
| IL-8 (Pg/mL) | 18.43 ± 5.86 | 16.70 ± 4.52 | 9.30% | 6.24 ± 3.07 | .11 | <.0001 | <.0001 |
| TNF-α (pg/mL) | 12.71 ± 4.22 | 9.20 ± 1.66 | 27.5% | 4.75 ± 2.53 | <.0001 | <.0001 | <.0001 |
| Clinical variables | |||||||
| VOC event/year | 1.84 ± 1.58 | 0.91 ± 1.47 | 50.5% | – | .0014 | – | – |
| ACS event/year | 0.35 ± 0.48 | 0.08 ± 0.28 | 77% | – | .004 | – | – |
Abbreviations: ACS, acute chest syndrome; Hb, hemoglobin; HbF, fetal hemoglobin; HbS, hemoglobin S; hs-CRP, high-sensitivity C reactive protein; HU, hydroxyurea; RBCs, red blood cells; SCD, sickle cell disease; TNF-α, tumor necrosis factor α; VOC, vaso-occlusive crisis; WBCs, white blood cells.
a P values were calculated using the Mann-Whitney test.
b Baseline versus treated patients with SCD.
c Baseline versus healthy control
d Treated versus healthy control.
Effect of HU 1-Year Treatment on the Clinical and Hematological Characterization of Patients With SCD
After a 1-year treatment and follow-up, Hb levels were significantly increased (by 29.7%) in patients who received HU when compared to the baseline levels. However, their levels were still significantly lower than the healthy controls. The percentage of HbS was significantly lowered in children with SCD after treatment with HU, whereas the percentage of HbF was significantly higher after HU therapy than the baseline values (Table 1).
Red blood cells and hematocrit count were significantly decreased (P = .002) and (P = .0001), respectively, but did not reach the value of the healthy children (P = .0001) and (P = .01), respectively. Total WBC counts were significantly decreased in patients with SCD after HU therapy when compared to the baseline values and still significantly higher than normal values (Table 1). Platelet counts were comparable between baseline values and normal values. Total lymphocyte counts were significantly decreased in patients with SCD after treatment, and the difference was not statistically significant from the value of healthy children. Monocyte counts were decreased but with a nonsignificant difference from the baseline and healthy control value. Also, at the end of the study, we found a significant improvement in the rates of VOCs and ACS among patients with SCD (Table 1).
Proinflammatory Cytokines Before and After HU Treatment
The serum levels of hs-CRP, IL-6, IL-8, and TNF-α were significantly higher at baseline when compared to the control group (P < .0001 for each). After 1 year of initiating HU treatment, there were significant decreases in IL-6 and TNF-α serum levels when compared to the baseline values (P = .001) and (P < .0001), respectively. However, serum levels of IL-8 and hs-CRP did not change after receiving HU treatment. Moreover, none of these parameters reached the values of the healthy group.
Effect of HU on NLR
Before initiating HU treatment, the NLR values in patients with SCD were significantly higher than healthy children (P < .0001). After 1 year of receiving HU treatment, NLR values were significantly decreased when compared to the baseline value (P = .04) and did not differ from the value of the healthy group (Figure 1). With ROC analysis, the cutoff value of NLR to predict the response of HU was 3.0 with 85% sensitivity and 76% specificity (area under the curve: 0.85, P < .0001; Figure 2).
Figure 1.
Difference in the values of NLR between patients with SCD at baseline (black bars) and after 1 year of HU treatment (plotted bars) and healthy control group (grey bars). HU indicates hydroxyurea; NLR, neutrophil-to-lymphocyte ratio; SCD, sickle cell disease.
Figure 2.
Receiver Operating Characteristic (ROC) curve analysis of NLR to predict the presence of sickle cell disease (SCD). NLR indicates neutrophil-to-lymphocyte ratio.
Association Between NLR and Hematological Parameters and Proinflammatory Mediators
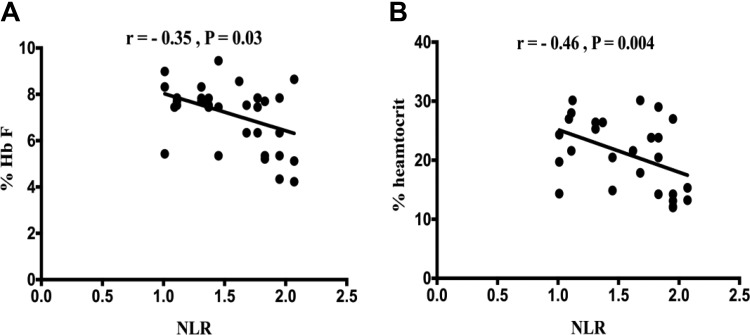
We analyzed the association between NLR and some hematological parameters among children with SCD. Our results showed significant negative correlations between NLR and the percentage of HbF (r = −0.35, P = .03) and with hematocrit (r = −0.46, P = .004; Figure 3a and b). Although a significant positive correlation was found between NLR and hs-CRP serum levels (r = .41, P = .01), TNF-α (r = .38, P = .02), and IL-8 (r = 0.5, P = .002; Figure 4a-c), no correlation was found between NLR, and IL-6 serum, and Hb levels.
Figure 3.
Association between NLR and (a) the percentage of fetal hemoglobin (HbF) and hematocrit (b). NLR indicates neutrophil-to-lymphocyte ratio.
Figure 4.
Association between NLR and serum levels of (a) hs-CRP, (b) TNF-α, and (c) IL-8. hs-CRP indicates high-sensitivity C-reactive protein; IL-8, interleukin 8; NLR, neutrophil-to-lymphocyte ratio; TNF-α, tumor necrosis factor α.
Discussion
Neutrophil-to-lymphocyte ratio is a readily available biomarker of inflammation, which is very common in SCD. What’s more important than most studies have pointed out the valuable effects of HU in the management of SCD in young children. There is a restricted number of researches investigating the relationship between NLR and inflammatory mediators in SCD. Also, due to the limited data on the impact of HU on the change of NLR, it was essential to study the effect of HU on NLR and proinflammatory cytokines at baseline and after 1 year from initiation of the treatment among children with SCD. Also, we aimed to investigate the relationship between NLR and these inflammatory mediators in children with SCD.
Concerning the hematological and clinical effects of HU on children with SCD followed up at our hospital, our patients had substantial hematological and clinical improvements, which came in line with other studies.9,10 At the end of the study, our patients had a significant rise in Hb and hematocrit values and reductions in platelet and WBCs. Our results agreed with other studies.10,11
Moreover, we found that HU therapy significantly reduces the percentage of HbS levels, with increased HbF levels, when compared to the baseline values, which was consistent with previous reports, which confirmed the eficacy of HU in reducing HbS and inducing HbF synthesis.12–14
Although changes in the number of inflammatory biomarkers and cytokines have been linked previously,15 reports were conflicting, and the precise role of cytokines in SCD remains to be elucidated. Data presented herein documented an increased in serum levels of proinflammatory mediators, including TNF-α, IL-8, IL-6, and hs-CRP before initiating HU treatment. Our results came in accordance with earlier researches, which established high levels of TNF-α in patients with SCD.16 On the other hand, IL-8 has been detected in patients with SCD having ACS and VOC.17 Also, previous studies have reported increased levels of IL-6, IL-8, and CRP in individuals with SCD.18,19
After 1 year of HU treatment, serum levels of TNF-α and IL-6 decreased significantly when compared to baseline values. Our results came in line with Lanaro et al study, which documented that HU treatment leads to a significant decrease in serum TNF-α among patients with SCD.20 Contrary to our results, Bandeira et al reported that HU treatment does not affect the serum level of IL-6 in SCD.21 Our results did not show any significant difference in CRP and IL-8 levels when compared to the baseline value and did not reach the standard values. The failure of HU treatment to normalize hs-CRP and IL-8 has been previously reported.22 Recently, there is considerable attention to the vital role of hs-CRP as a good biomarker for chronic systemic inflammations, which are very common in SCD.23 The availability of more sophisticated, sensitive tools may explain this discrepancy in the results of different studies.
Neutrophil-to-lymphocyte ratio is one of the most common indicators of inflammatory processes in SCD.8 The values of NLR are usually increased in patients with SCD. In our study, NLR was significantly higher before starting HU therapy than the controls, and their level decreased after receiving HU therapy but did not reach the level of healthy controls. Our results are consistent with recent studies, which evaluated the leukocyte ratios in patients with various disease conditions other than SCD.24 Another study showed that the increase in proinflammatory cytokines was significantly linked with high NLR.25 The decrease in NLR can provide another explanation for the improvement of the clinical status of SCD in our study.
There is a great interest in using leukocyte ratios in assessing inflammatory status in SCD because the ratios are relatively stable and may not be easily affected by physiological, pathological, and physical events.24 Neutrophil represents the active nonspecific inflammatory mediator of cellular immunity (innate), while lymphocytes mediate the adaptive or protective aspect of inflammation. Lymphocytes play an essential role in the regulation of a proper inflammatory response and decrease in population either due to downregulation, apoptosis, or damage of the cellular DNA, which could impair adverse inflammatory conditions.26 On the other hand, lowering the WBC count, including neutrophil in SCD, is itself potentially therapeutic. Because neutrophils may promote VOCs through vascular adhesion, HU depresses their absolute numbers and decreases the surface expression of adhesion receptors.27
Importantly, we found that NLR was positively correlated with serum levels of proinflammatory cytokines including hs-CRP, TNF-α, and IL-8, which could explain the high value of NLR before initiating HU therapy in our study and that the increase in NLR was associated with the increase in the proinflammatory cytokines which came in accordance with previous studies.25 Interlukin-8 is considered as a mediator in neutrophil-mediated acute inflammation, with a critical role in some chronic inflammatory diseases.28 Previously, Lanaro et al have confirmed that the gene expressions of TNF-α and IL-8 genes were increased in SCD mononuclear cells, and levels of IL-8 messenger RNA were higher in the neutrophils of patients with SCD. They concluded that that mononuclear cells and neutrophils are essential for inflammatory molecule production, such as TNF-α and IL-8 in patients with SCD, which may explain our findings.20
In conclusion, HU induced a decrease in NLR and inflammatory cytokines, which represent a biomarker of inflammation in SCD. The calculation of NLR is a straightforward and cheap method for prediction of SCD outcome in young children.
Recommendations: further prospective studies with a larger number of patients are necessary for the identification of other factors responsible for increase NLR.
Footnotes
Authors’ Note: Amira Elhoufey is also affiliated with Department of Community Health Nursing, Sabia University College, Jazan University, Jazan, Kingdom of Saudi Arabia.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Khaled Saad  https://orcid.org/0000-0002-8473-6116
https://orcid.org/0000-0002-8473-6116
References
- 1. Zahran AM, Elsayh KI, Saad K, et al. Circulating microparticles in children with sickle cell anemia in a tertiary center in upper Egypt. Clin Appl Thromb Hemost. 2019;25:1076029619828839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zahran AM, Elsayh KI, Saad K, Embaby M, Ali AM. Regulatory B cells (CD19+ CD38hiCD24hi) in alloimmunized and non-alloimmunized children with β-thalassemia major. Blood Cells Mol Dis. 2016;57:91–96. [DOI] [PubMed] [Google Scholar]
- 3. Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008;122(6):1332–1342. [DOI] [PubMed] [Google Scholar]
- 4. Akohoue SA, Shankar S, Milne GL, et al. Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediat Res. 2007;61(2):233–238. [DOI] [PubMed] [Google Scholar]
- 5. Makis AC, Hatzimichael EC, Bourantas KL. The role of cytokines in sickle cell disease. Ann Hematol. 2000;79(8):407–413. [DOI] [PubMed] [Google Scholar]
- 6. Binnetoglu E, Sengul E, Halhalli G, Dindar S, Sen H. Is neutrophil-lymphocyte ratio an indicator for proteinuria in chronic kidney disease? J Clin Lab Analys. 2014;28(6):487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uslu AU, Kucuk A, Sahin A, et al. Two new inflammatory markers associated with disease activity score-28 in patients with rheumatoid arthritis: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int J Rheum Dis. 2015;18(7):731–735. [DOI] [PubMed] [Google Scholar]
- 8. Emokpae MA, Aruomaren A, Osime E. Relationship between neutrophil-to-lymphocyte ratio and inflammatory markers in sickle cell anaemia patients with proteinuria. Med Sci (Basel). 2016;4(3):E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferster A, Tahriri P, Vermylen C, et al. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97(11):3628–3632. [DOI] [PubMed] [Google Scholar]
- 10. Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS). Blood. 2010;115(12):2354–2363. [DOI] [PubMed] [Google Scholar]
- 11. Kinney TR, Helms RW, O’Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Blood. 1999;94(5):1550–1554. [PubMed] [Google Scholar]
- 12. Shome DK, Al Ajmi A, Radhi AA, Mansoor EJ, Majed KS. The effect of hydroxyurea therapy in Bahraini sickle cell disease patients. Indian J Hematol Blood Transfus. 2016;32(1):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torres LS, da Silva DGH, Belini Junior E, et al. The influence of hydroxyurea on oxidative stress in sickle cell anemia. Rev Bras Hematol Hemoter. 2012;34(6):421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pallis FR, Conran N, Fertrin KY, Olalla Saad ST, Costa FF, Franco-Penteado CF. Hydroxycarbamide reduces eosinophil adhesion and degranulation in sickle cell anaemia patients. Brit J Haematol. 2014;164(2):286–295. [DOI] [PubMed] [Google Scholar]
- 15. Pathare A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D. Cytokine profile of sickle cell disease in Oman. Ame J Hematol. 2004;77(4):323–328. [DOI] [PubMed] [Google Scholar]
- 16. Tavakkoli F, Nahavandi M, Wyche MQ, Perlin E. Plasma levels of TNF-α in sickle cell patients receiving hydroxyurea. Hematology. 2004;9(1):61–64. [DOI] [PubMed] [Google Scholar]
- 17. Duits AJ, Schnog JB, Lard LR, Saleh AW, Rojer RA. Elevated IL-8 levels during sickle cell crisis. Europ J Haematol. 1998;61(5):302–305. [DOI] [PubMed] [Google Scholar]
- 18. Schnog JB, Mac Gillavry MR, van Zanten AP, et al. Protein C and S and inflammation in sickle cell disease. Amer J Hematol. 2004;76(1):26–32. [DOI] [PubMed] [Google Scholar]
- 19. Mohammed FA, Mahdi N, Sater MA, Al-Ola K, Almawi WY. The relation of C-reactive protein to vaso-occlusive crisis in children with sickle cell disease. Blood Cells Mol Dis. 2010;45(4):293–296. [DOI] [PubMed] [Google Scholar]
- 20. Lanaro C, Franco Penteado C, Albuqueque D, Saad S, Conran N, Costa F. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukocy Biol. 2009;85(2):235–242. [DOI] [PubMed] [Google Scholar]
- 21. Bandeira ICJ, Rocha LBS, Barbosa MC, et al. Chronic inflammatory state in sickle cell anemia patients is associated with HBB*S haplotype. Cytokine. 2014;65(2):217–221. [DOI] [PubMed] [Google Scholar]
- 22. Saleh A, Hillen H, Duits A. Levels of endothelial, neutrophil and platelet-specific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematol. 1999;102(1):31–37. [DOI] [PubMed] [Google Scholar]
- 23. Kamath DY, Xavier D, Sigamani A, Pais P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: an Indian perspective. Indian J Med Res. 2015;142(3):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lou M, Luo P, Tang R, Peng Y, Yu S, Huang W, et al. Relationship between neutrophil-lymphocyte ratio and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMC Endocri Disord. 2015;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9(11):e112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heffernan DS, Monaghan SF, Thakkar RK, Machan JT, Cioffi WG, Ayala A. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Critical Care (London, England). 2012;16(1):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agrawal RK, Patel RK, shah V, Nainiwal L, Trivedi B. Hydroxyurea in sickle cell disease: drug review. Ind J Hematol Blood Transfus. 2014;30(2):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Ame J Physiol Lung Cell Mole Physiol. 2003;284(4):L566–L577. [DOI] [PubMed] [Google Scholar]