Abstract
Andexanet alfa is a recombinant factor Xa decoy protein, designed to reverse bleeding associated with oral anti-Xa agents. Andexanet alfa is also reported to neutralize the effects of heparin-related drugs. This study focused on the neutralization profiles of unfractionated heparin (UFH), enoxaparin, and, a chemically synthetic pentasaccharide, fondaparinux by andexanet alfa. Whole blood clotting studies were carried out using thromboelastography (TEG) and activated clotting time (ACT). The anticoagulant profile of UFH, enoxaparin, and fondaparinux was studied using the activated partial thromboplastin time (aPTT), thrombin time (TT), and amidolytic anti-Xa, and anti-IIa methods. Thrombin generation inhibition was studied using the calibrated automated thrombogram system. Reversal of each of these agents was studied by supplementing andexanet alfa at 100 µg/mL. In the TEG, andexanet alfa produced almost a complete reversal of the anticoagulant effects of UFH and enoxaparin; however, it augmented the effects of fondaparinux. In the ACT, aPTT, and TT, UFH produced strong anticoagulant effects that were almost completely neutralized by andexanet alfa. Enoxaparin produced milder anticoagulant responses that were partially neutralized, whereas fondaparinux did not produce any sizeable effects. In the anti-Xa and anti-IIa assays, UFH exhibited partial neutralization whereas enoxaparin and fondaparinux did not show any neutralization. All agents produced varying degrees of the inhibition of thrombin generation, which were differentially neutralized by andexanet alfa. These results indicate that andexanet alfa is capable of differentially neutralizing anticoagulant and antiprotease effects of UFH and enoxaparin in an assay-dependent manner. However, andexanet alfa is incapable of neutralizing the anti-Xa effects of fondaparinux.
Keywords: andexanet alfa, anticoagulants, unfractionated heparin, enoxaparin, pentasaccharide, fondaparinux
Introduction
Heparin and related drugs are widely used for the management of thrombotic and cardiovascular disorders. Unfractionated heparin (UFH) is the drug of choice for anticoagulation in surgical, interventional, and hemodialysis patients. Low-molecular weight heparins (LMWHs), such as enoxaparin, are widely used for the management of thrombosis. A chemically synthesized pentasaccharide, fondaparinux, is also used for various clinical indications.
Protamine sulfate is the only antidote available for the neutralization of UFH. Although protamine may also be used to control bleeding associated with enoxaparin, it does not effectively neutralize the anti-Xa effects of fondaparinux. Andexanet alfa has also been reported to neutralize the biologic activities of heparin by inhibiting its anti-Xa effect.1 Andexanet alfa is capable of neutralizing UFH and enoxaparin by binding to the respective antithrombin (AT) complexes. Newly developed broad-spectrum neutralizing agents such as ciraparantag are developed for the neutralization of not only the direct oral anticoagulants (DOACs) but also heparin and related drugs.2,3 Ciraparantag binds to pentasaccharide sequence weakly that are present in heparins. Binding of ciraparantag to heparin is comparable to that which has been reported for andexanet alfa. The studies reported on ciraparantag provide useful insights for the mechanisms involved in the binding of heparin–AT complexes with factor (F) Xa and related enzymes.1
More recently, oral anti-Xa agents such as apixaban, betrixaban, edoxaban, and rivaroxaban have become available for various clinical indications.4,5 Bleeding complications related to the use of these drugs have been reported.6–8 This has necessitated the development of a specific antidote for FXa inhibitors. This antidote is a FXa decoy known as andexanet alfa. Full study report of andexanet alfa for the control of bleeding associated with FXa inhibitors has reported that andexanet alfa markedly reduced the anti-Xa activity and over 80% of the patients showed good hemostatic efficacy at 12 hours.9 Previous studies reported from our laboratories have demonstrated a differential neutralization of these agents by andexanet alfa.10 Earlier reports have also shown that the anti-Xa effects of UFH, enoxaparin, and fondaparinux are neutralizable by andexanet alfa.1
The anticoagulant and antiprotease activities of UFH and related drugs are predominantly mediated by AT. The molecular weight and oligosaccharide composition of these agents are primary determinants of their interaction with AT and contribute to their anti-Xa and anti-IIa effects. The anticoagulant effects of these agents are usually proportional to the anti-IIa effects. Excessive anticoagulation with heparins can lead to bleeding. Such excess anticoagulation can be reversed by the administration of protamine, which binds to heparin in a charge-dependent manner and prevents the heparin from interacting with AT.
As discussed previously, andexanet alfa is a modified form of human FXa that was developed as an antagonist for the direct FXa inhibitors. Besides its high-affinity binding with direct FXa inhibitors, andexanet alfa has also been shown to bind AT-dependent FXa inhibitors such as UFH and LMWH.11,12 The high-binding affinity (Kd = 0.71 μM) of andexanet alfa to enoxaparin AT complex has been reported. Direct binding of andexanet alfa to heparin, enoxaparin, and fondaparinux in the absence of AT has been shown to be of low affinity, (Kd = 34-110 μM), suggesting that this interaction is not the physiological mechanism of action for the reversal of AT-dependent anticoagulation by andexanet alfa.1
The anti-Xa effects of direct FXa inhibitors, UFH, enoxaparin, and fondaparinux are reported to be neutralized to varying degrees. A systemic study to investigate the neutralization of UFH, enoxaparin, and fondaparinux using various clot-based, antiprotease, and thrombin generation-based assays is not available. Assay-based variations in the biological activities of various anti-Xa agents and their neutralization by andexanet alfa have been recently reported.10
Although the FXa inhibitors are usually potency evaluated in the chromogenic anti-Xa assays, these agents are capable of producing assay-dependent effects in the whole blood analysis, including thromboelastographic (TEG) analysis, activated clotting time (ACT) assays, plasma and whole blood-based prothrombin time, activated partial thromboplastin time (aPTT), and thrombin generation inhibition assays. These assays have been used previously in the evaluation of biologic effects of FXa inhibitors.13 The current study was undertaken to compare the efficacy of andexanet alfa in neutralizing the effects of UFH, enoxaparin, and fondaparinux in various laboratory assays.
Materials and Method
Whole Blood Analysis
Thromboelastographic studies were carried out using a TEG 5000 Hemostasis System (Haemonetics Corp, Massachusetts). Freshly drawn blood samples from healthy individual donors (n = 3) were collected in 3.2% sodium citrate tubes. To the TEG cup, 0.025 M CaCl2, heparin, enoxaparin, and fondaparinux at a final concentration of 1.25 µg/mL and citrated human whole blood were added in the individual studies performed on 3 donors. For reversal studies, heparins and fondaparinux at 1.25 µg/mL and andexanet alfa at 100 µg/mL were mixed with the same whole blood samples. The TEG profile was monitored for 45 minutes. The clotting profile was characterized in terms of R-time, K-time, maximum amplitude (MA), and angle.
Hemochron whole blood coagulation system (Accriva Diagnostics, Inc, California) was used to measure whole blood ACT. Freshly drawn whole blood (n = 3) was supplemented with the each of the drugs at a final concentration of 10 µg/mL and transferred into individual celite ACT tubes from each of the 3 donors. For reversal studies, blood was supplemented with each agent at a final concentration of 10 µg/mL with andexanet alfa at a final concentration of 100 µg/mL and transferred into celite ACT tubes. Results were recorded in seconds and compiled as means ± standard deviation (SD).
Clot-Based Assays
Unfractionated heparin, enoxaparin, and fondaparinux were supplemented into citrated plasma over a concentration range of 0.62 to 10.0 µg/mL. Either saline as a control or andexanet alfa at a final concentration of 100 µg/mL was added to individual aliquots of plasma supplemented of each anticoagulant. Samples were analyzed using the TriniCLOT aPTT reagent (Diagnostica Stago, Parsippany, New Jersey). Human thrombin (Enzyme Research Laboratories, South Bend, Indiana) reconstituted with 0.025 M CaCl2 at a concentration of 5 U/mL was used to measure thrombin time (TT). All reagents were reconstituted according to the manufacturer’s instructions. Both aPTT and TT were measured using an ACL-Elite (Instrumentation Laboratory, Bedford, Massachusetts). Results were compiled as mean ± SD.
Chromogenic Assay
Anti-FXa and anti-FIIa activities were measured using kinetic amidolytic methods on the ACL-Elite instrument. All drugs were supplemented into citrated plasma over a concentration range of 0.62 to 10 µg/mL. Either saline or andexanet alfa at a final concentration of 100 µg/mL was added to individual aliquots of plasma supplemented with each anticoagulant. Bovine FXa (Enzyme Research Laboratories) was diluted in 5.0 nM Tris buffer (pH = 8.4) to a concentration of 1.25 IU/mL and FXa substrate (BioMedica Diagnostics, Connecticut) reconstituted in sterile water to a concentration of 2.5 µM were used in the anti-Xa assay. Human thrombin (Enzyme Research Laboratories) diluted in 5.0 nM Tris buffer (pH = 8.4) to a concentration of 1.25 IU/mL and FIIa substrate (BioMedica Diagnostics) reconstituted in sterile water to a concentration of 1 µM were used in the anti-IIa assay.
Inhibition of Thrombin Generation
Inhibition of thrombin generation was measured using the calibrated automated thrombogram assay (Diagnostica Stago) on a Fluoroskan Ascent fluorimeter. All drugs were supplemented in normal pooled plasma to obtain concentration ranges from 0.62 to 10 µg/mL. Saline or andexanet alfa at a final concentration of 100 µg/mL was added to individual aliquots of plasma supplemented with each of the agents. Reagents used in this assay included the fluo-substrate, fluo-buffer, tissue factor high reagent (mixture of tissue factor and phospholipids), and a thrombin calibrator. The thrombin generation assay was carried out in 96-well Immulon 2HB transparent round bottom plates. The thrombin generation potential was measured in terms of the peak thrombin concentration, lag time, and endogenous thrombin potential (ETP) /area under the curve (AUC). Results were compiled in terms of mean ± SD.
Results
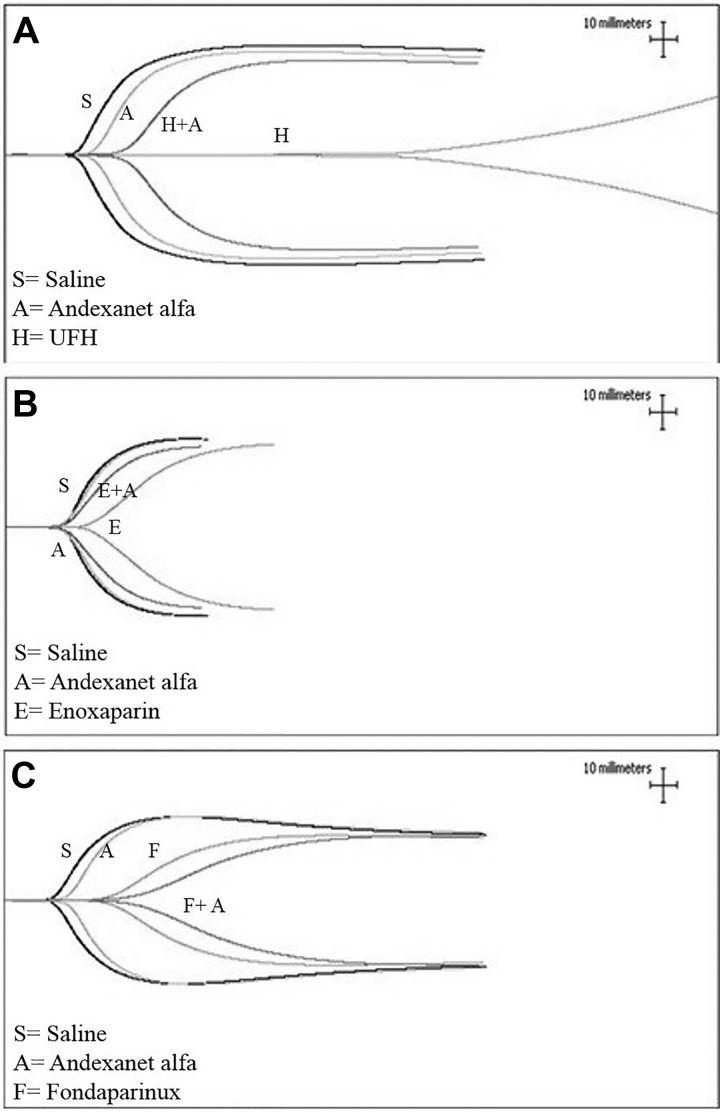
A composite of the representative TEG from one donor demonstrating the anticoagulant effects of UFH, enoxaparin, and fondaparinux and their relative neutralization by andexanet alfa is shown in Figure 1. Unfractionated heparin exhibited the strongest anticoagulant effects, producing marked alteration of the TEG profile. Andexanet alfa produced an almost complete neutralization of the anticoagulant effects of UFH and reversed the TEG profile to near normal. Andexanet alfa itself did not produce any alteration of the TEG profile. Enoxaparin produced relatively weaker anticoagulant effects, which were apparently neutralized by andexanet alfa. Fondaparinux produced stronger anticoagulant effect in comparison to enoxaparin; however, these effects were weaker than those observed with UFH. Addition of andexanet alfa to fondaparinux supplemented whole blood did not result in any neutralization of the anticoagulant effect of this agent, but further enhanced its anticoagulant effects.
Figure 1.
A comparison of unfractionated heparin (UFH), enoxaparin, and fondaparinux and their neutralization in whole blood thromboelastographic (TEG) analysis. A, TEG for UFH, (B) TEG for enoxaparin, and (C) TEG for fondaparinux.
The detailed analysis of the TEG studies with the 3 agents and their neutralization by andexanet alfa is given in Table 1. The results shown in this table represent the mean obtained with 3 blood donors. R-time was markedly increased by heparin (90.37 ± 29.22) in comparison to saline (10.1 ± 1.06). The prolongation of R-time by enoxaparin (14.43 ± 1.87) and fondaparinux (16.33 ± 2.92) were relatively weaker. K-time was markedly higher in the case of UFH (43.35 ± 6.29), followed by fondaparinux (8.17 ± 1.94) and enoxaparin (6.03 ± 2.21), in comparison to saline (3.53 ± 0.86). The angle values were markedly higher in the case of enoxaparin (36.4 ± 10.08) and fondaparinux (26.33 ± 5.78) compared to UFH (5.8 ± 1.84). The MA was highest with enoxaparin (57.83 ± 9.32) followed by fondaparinux (48.43 ± 10.95), whereas UFH (41.3 ± 19.94) showed the relatively stronger effect in comparison to saline (60.53 ± 8.65). Although andexanet alfa on its own did not impact the values for R-time, K-time, angle, or MA, it completely reversed the anticoagulant effects of UFH.
Table 1.
A Comparison of UFH, Enoxaparin, and Fondaparinux and Their Neutralization in Thromboelastography.
| Drug | R-Time (Minutes) | K-Time (Minutes) | Angle (Degrees) | MA (mm) |
|---|---|---|---|---|
| Normal saline | 10.1 ± 1.06 | 3.53 ± 0.86 | 47.43 ± 8.57 | 60.53 ± 8.65 |
| Andexanet FC = 100 μg/mL | 10.8 ± 2.65 | 4.07 ± 1.1 | 45.1 ± 6.27 | 58.73 ± 8.86 |
| Heparin FC = 1.25 μg/mL | 90.37 ± 29.22 | 43.35 ± 6.29 | 5.8 ± 1.84 | 41.3 ± 19.94 |
| Heparin FC = 1.25 μg/mL and andexanet FC = 100 μg/mL | 16.53 ± 4.61 | 5.73 ± 0.6 | 28.4 ± 9.83 | 56.07 ± 5.59 |
| Enoxaparin FC = 1.25 μg/mL | 14.43 ± 1.87 | 6.03 ± 2.21 | 36.4 ± 10.08 | 57.83 ± 9.32 |
| Enoxaparin FC = 1.25 μg/mL and andexanet FC = 100 μg/mL | 11.5 ± 0.53 | 3.97 ± 1.52 | 37.37 ± 8.72 | 57.77 ± 10.38 |
| Fondaparinux FC = 1.25 μg/mL | 16.33 ± 2.92 | 8.17 ± 1.94 | 26.33 ± 5.78 | 48.43 ± 10.95 |
| Fondaparinux FC = 1.25 μg/mL and andexanet FC = 100 μg/mL | 18.87 ± 4.13 | 12.83 ± 5.52 | 18.9 ± 10.96 | 46.13 ± 9.46 |
Abbreviations: FC, fold change; MA, maximum amplitude.
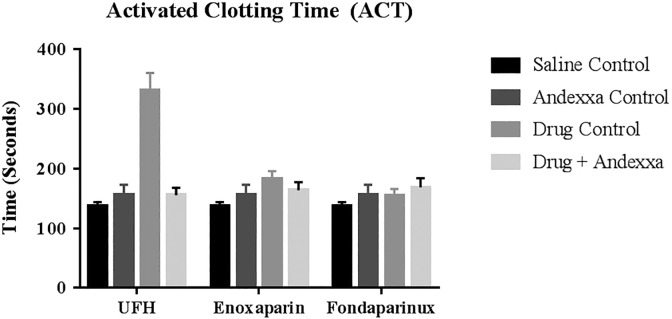
The effects of UFH, enoxaparin, and fondaparinux and their reversal by andexanet alfa in whole blood ACT performed in 5 individual donors is depicted as mean ± SD as composite bar graphs in Figure 2. In ACT assay, UFH produced a stronger response in comparison to enoxaparin that produced minimal response, and fondaparinux did not produce any prolongation of clotting time. Andexanet alfa at a concentration of 100 µg/mL completely reversed the anticoagulant effects of UFH and partially reversed the effects of enoxaparin.
Figure 2.
A comparison of unfractionated heparin (UFH), enoxaparin, and fondaparinux and their neutralization in whole blood activated clotting time (ACT) assay. All results shown represent a mean of 5 individual donor ACT values 1 standard deviation.
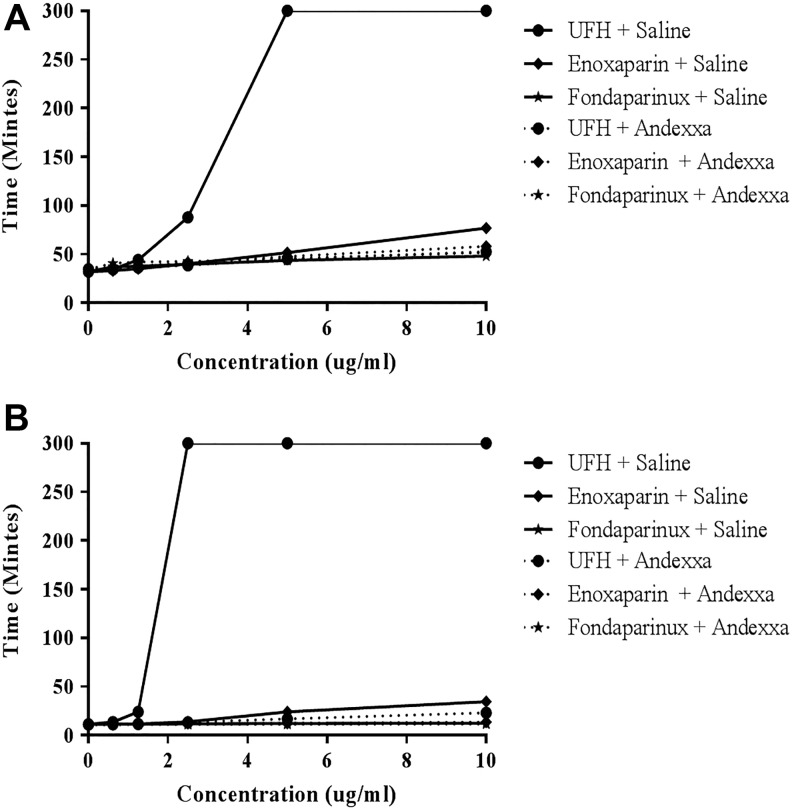
Figure 3A and B shows a comparison of the effects of UFH, enoxaparin, and fondaparinux when supplemented in citrated plasma as measured by aPTT and TT assays. Unfractionated heparin produced concentration-dependent anticoagulant effects in these assays. In the aPTT assay, UFH produced maximum response while enoxaparin and fondaparinux produced weaker anticoagulant activities (rank order UFH > enoxaparin > fondaparinux). The same trend was observed with the TT assay. At a final concentration of 100 μg/mL, andexanet alfa neutralized the prolongation of the aPTT and TT induced by heparin or enoxaparin supplementation.
Figure 3.
A comparison of unfractionated heparin, enoxaparin, and fondaparinux and their neutralization in clotting assays. A, Activated partial thromboplastin time and (B) thrombin time.
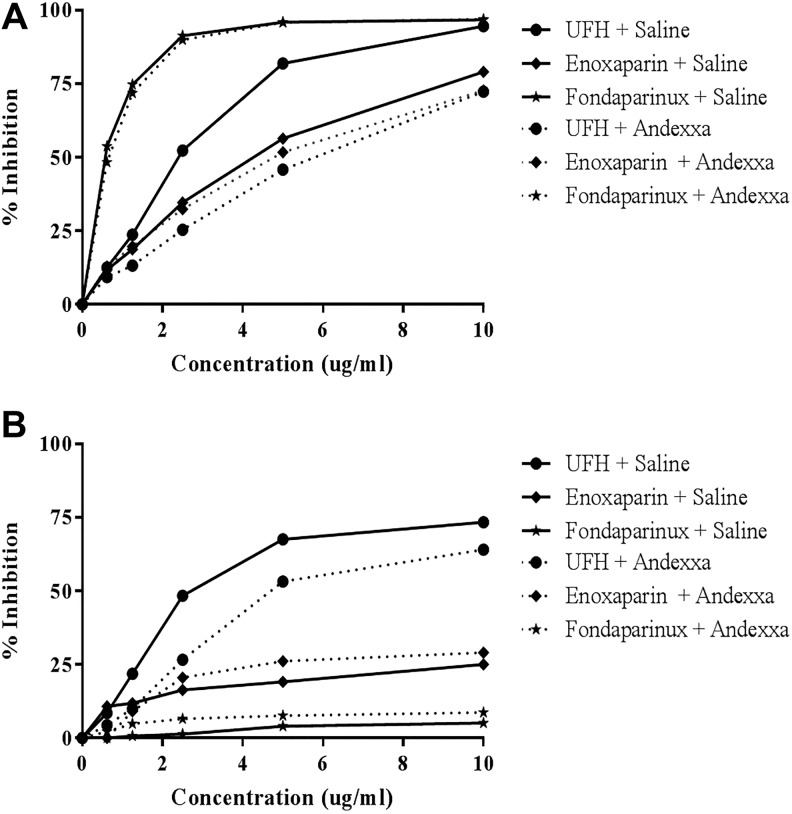
Figure 4A and B depicts the amidolytic anti-Xa and anti-IIa activity in normal human plasma supplemented with heparin, enoxaparin, or fondaparinux. In anti-Xa assay, fondaparinux produced stronger response followed by UFH and enoxaparin. Although UFH was partially neutralized by andexanet alfa at final concentration of 100 μg/mL, no effect was observed on the anti-Xa activity of enoxaparin and fondaparinux. In the anti-IIa assay, UFH produced strongest effects while enoxaparin produced relatively weaker anti-IIa effects, fondaparinux didn’t have any measurable anti-IIa effects. Supplementation of andexanet alfa had minimal effect on the anti-IIa activity of UFH.
Figure 4.
A comparison of unfractionated heparin, enoxaparin, and fondaparinux and their neutralization in amidolytic assays. A, Anti-Xa activity and (B) anti-IIa activity.
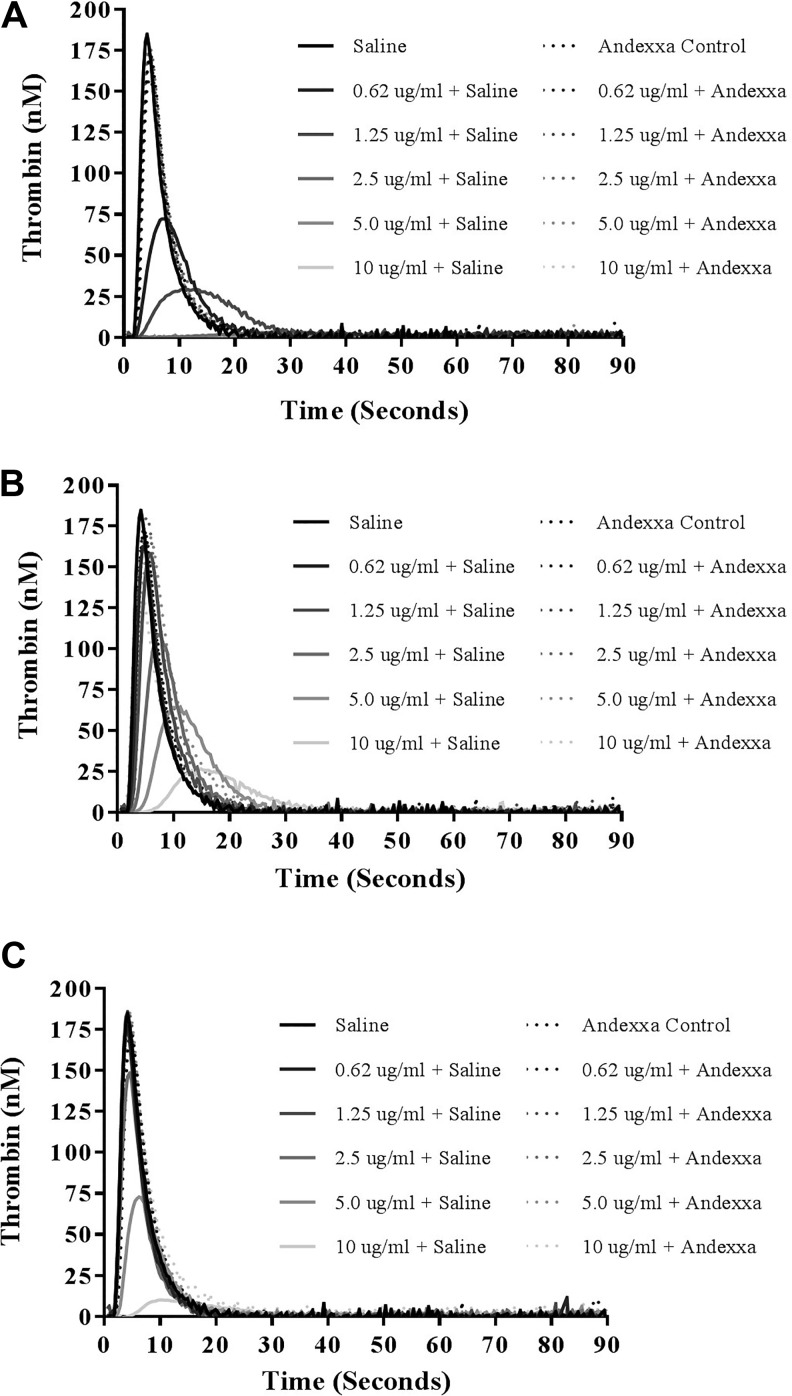
The effect of UFH, enoxaparin, and fondaparinux on inhibition of thrombin generation and their relative neutralization by andexanet alfa is shown in Figure 5A to C. In thrombin generation assay, UFH produced stronger inhibition of thrombin formation and thrombin generation was completely inhibited at a concentration of 2.5 µg/mL. Fondaparinux and enoxaparin produced weaker inhibition. In this assay, andexanet alfa at final concentration of 100 μg/mL completely neutralized UFH, enoxaparin, and fondaparinux in a comparable fashion.
Figure 5.
A comparison of unfractionated heparin (UFH), enoxaparin, and fondaparinux and their neutralization in thrombin generation analysis. Thrombokinetograms for (A) UFH, (B) enoxaparin, and (C) fondaparinux.
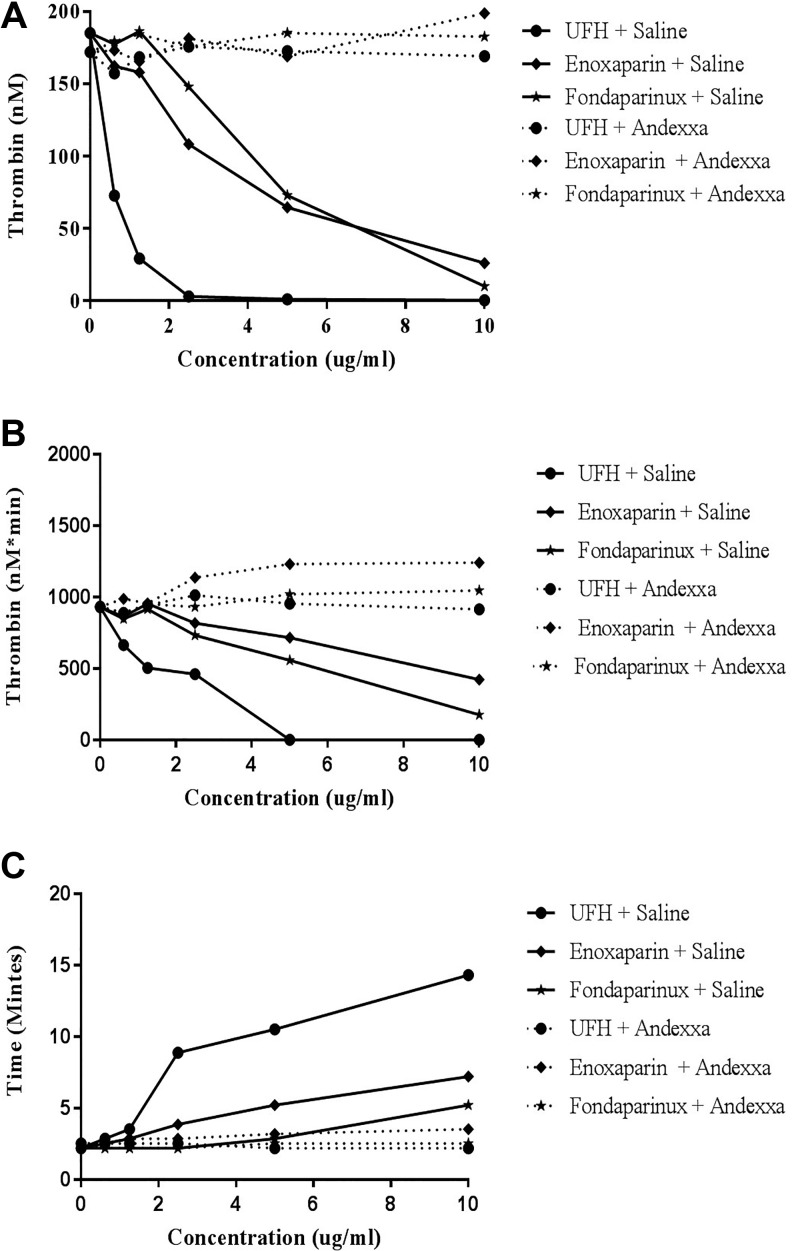
The effect of UFH, enoxaparin, and fondaparinux on thrombin generation was measured in terms of peak thrombin, ETP (AUC), and lag time. A comparison of the individual thrombin generation parameters for all of these drugs is shown in composite form in Table 2 and Figure 6A to C. Unfractionated heparin was found to be most effective inhibitor of thrombin generation over a concentration range of 0 to 10 µg/mL. At a concentrations less than 2.5 µg/mL, UFH produces a complete inhibition of peak thrombin and andexanet alfa effectively neutralized UFH. Enoxaparin and fondaparinux produced weaker inhibition of thrombin generation and required much higher concentrations for the complete inhibition of thrombin generation. At 10 µg/mL, both of these agents exhibited residual thrombin generation ranging from 10 to 26 nM. Andexanet alfa also neutralized both the enoxaparin and fondaparinux as measured by this parameter. The neutralization effects of andexanet alfa were also evident in term of other parameters such as the AUC and the lag time. Lag time was markedly increased in a dose-dependent manner with UFH. Enoxaparin and fondaparinux produced mild increase in the lag time. Andexanet alfa supplementation resulted in the restoration in the lag time to near-normal level for all of these agents.
Table 2.
Relative Neutralization of UFH, Enoxaparin, and Fondaparinux by Andexanet Alfa on Thrombin Generation and Their Parameters.
| Concentration | UFH | Enoxaparin | Fondaparinux | |||
|---|---|---|---|---|---|---|
| Saline | Andexanet | Saline | Andexanet | Saline | Andexanet | |
| Peak thrombin | ||||||
| 0 | 185.02 | 171.93 | 185.02 | 171.93 | 185.02 | 171.93 |
| 0.62 | 72.76 | 157.19 | 162.22 | 173.13 | 177.46 | 179.24 |
| 1.25 | 29.22 | 168.47 | 157.98 | 165.4 | 186.21 | 184.29 |
| 2.5 | 3.05 | 175.75 | 108.27 | 181.49 | 147.92 | 175.54 |
| 5.5 | 1.04 | 172.6 | 64.61 | 168.89 | 72.84 | 185.13 |
| 10 | 0.37 | 169.05 | 26.06 | 198.76 | 10.06 | 182.56 |
| Area under the curve | ||||||
| 0 | 929.57 | 932.92 | 929.57 | 932.92 | 929.57 | 932.92 |
| 0.62 | 664.05 | 888.73 | 862.77 | 988.18 | 848.01 | 888.34 |
| 1.25 | 504 | 941.83 | 953.48 | 955.84 | 915.43 | 950.31 |
| 2.5 | 460 | 1012.01 | 816.99 | 1136.44 | 733.32 | 931.19 |
| 5.5 | 0.99 | 953.85 | 716.45 | 1231.1 | 557.42 | 1019.23 |
| 10 | 0 | 913.57 | 422.83 | 1240.47 | 175.42 | 1045.20 |
| Lag time | ||||||
| 0 | 2.19 | 2.52 | 2.19 | 2.52 | 2.19 | 2.52 |
| 0.62 | 2.86 | 2.52 | 2.52 | 2.52 | 2.19 | 2.19 |
| 1.25 | 3.52 | 2.52 | 2.86 | 2.86 | 2.19 | 2.19 |
| 2.5 | 8.87 | 2.52 | 3.86 | 2.86 | 2.19 | 2.19 |
| 5.5 | 10.5 | 2.19 | 5.2 | 3.19 | 2.86 | 2.52 |
| 10 | 14.3 | 2.19 | 7.2 | 3.52 | 5.2 | 2.52 |
All drugs were tested in a concentration range of 10-0 ug/ml.
Abbreviation: UFH, unfractionated heparin.
Figure 6.
A comparison of unfractionated heparin, enoxaparin, and fondaparinux and their neutralization in thrombin generation and their parameters. A, Peak thrombin, (B) area under the curve, and (C) lag time.
The AUC for total thrombin generation followed the similar pattern as the one for peak thrombin. Unfractionated heparin reduced the AUC in a concentration-dependent manner and at concentrations >5 µg/mL totally inhibited the generation of thrombin. In contrast, enoxaparin and fondaparinux produced a more progressive decrease in the AUC and even at concentrations of 10 µg/mL exhibited measurable AUC values. Andexanet alfa supplementation to UFH, enoxaparin, and fondaparinux resulted in a total restoration of AUC at all concentrations. These results are consistent with the neutralization profile observed in the clot-based assays.
Discussion
The neutralization of the bleeding effects of enoxaparin and fondaparinux by andexanet alfa has been reported previously.14 In these reported studies, andexanet alfa effectively neutralized the anti-Xa effects of both drugs, reversed the thrombin generation inhibitory effects, and corrected blood loss due to these drugs. Similarly, a high dosage of andexanet alfa has been shown to reverse the anti-Xa effects in rats treated with fondaparinux.15 Fondaparinux, on the other hand, has a very high affinity to AT, and this may be the reason that andexanet alfa fails to neutralize the anti-Xa effect of this high-affinity heparin oligosaccharide.5
In circulating blood, andexanet alfa competes with FXa to bind with direct inhibitors of FXa and the pentasaccharide consensus binding oligosaccharide sequence in heparin-AT, enoxaparin-AT, and fondaparinux-AT activated complexes, thus restoring the capacity of prothrombinase to generate thrombin. Therefore, in the case of UFH, it restores the anti-Xa activity. Since andexanet alfa does not reverse anti-IIa activity, it is considered to be a partial antidote of heparin.16 These findings are in contrast to our results in the whole blood clotting assays such as the TEG and ACT, where the anticoagulant actions of heparin are completely neutralized due to inhibition of thrombin generation. Similarly, in the clot-based TT and aPTT that are thrombin-based assays, andexanet alfa almost totally neutralized the anticoagulant effects of heparin and enoxaparin. It is interesting to note that while andexanet alfa neutralizes the anticoagulant effects of heparins in whole blood and plasma clot-based assays, it does not neutralize the anti-Xa and anti-IIa effects with the same efficiency as measured by amidolytic methods.
In the TEG analysis at equigravimetric levels, heparin was found to be a stronger anticoagulant agent as measured by various parameters. Enoxaparin showed a relatively weaker anticoagulant effect, which was comparable to fondaparinux. Andexanet alfa itself did not have any effect on the TEG profile as compared to saline at concentration of up to 100 μg/mL. In these studies, heparin was most efficiently neutralized by andexanet alfa in terms of all of the parameters of the TEG profile. Although the effect of enoxaparin was much weaker, andexanet alfa also neutralized these effects. Contrary to both the heparin and enoxaparin, andexanet alfa supplementation to fondaparinux only produced mild anticoagulant response. Addition of andexanet alfa to fondaparinux-supplemented whole blood resulted in the modest prolongation of the R value and an increase in K-time. The angle and MA values were consistent to mild anticoagulation. The differential inhibitory profile of enoxaparin and fondaparinux by andexanet alfa may be due to the binding of these agents to AT and other mechanistic factors, which have been discussed previously.1,12
In the ACT assays, supplementation of heparin produced a strong anticoagulant effect. This anticoagulant effect was almost entirely neutralized by andexanet alfa. This observation is consistent to the previously reported neutralizing effects of andexanet alfa.14 Enoxaparin only produced a modest anticoagulant effect in the ACT, which was partially neutralized by andexanet alfa. Fondaparinux produced a weaker anticoagulant effect, which was not affected by andexanet alfa. Interestingly, only a slight increase in the ACT was noted with andexanet alfa.
In the plasma-based clotting assays such as the aPTT and TT, UFH produced a pronounced concentration-dependent anticoagulant effect in both assays, which was completely neutralized by andexanet alfa at 100 μg/mL. In comparison to UFH, enoxaparin and fondaparinux produced minimal anticoagulant effects, which were blunted by andexanet alfa. In the anti-Xa study, andexanet alfa partially neutralized the anti-Xa activity of UFH; however, the anti-Xa activity of enoxaparin and fondaparinux was not neutralized. In the anti-IIa assays, UFH had significant anti-IIa activity, which was only partially neutralized. Enoxaparin had relatively lower anti-IIa activity, which was not neutralized. Fondaparinux did not exhibit any anti-IIa activity and supplementation of andexanet alfa had no effect. These results show a differential neutralization of the anticoagulant and antiprotease effects of heparin and related drugs in the clot-based and amidolytic assays.
Thrombin generation assay provides a global approach to the activation of coagulation as measured by various parameters such as the peak thrombin, total amount of thrombin generated as measured by AUC, and the lag time for the initiation of the thrombin formation. Various direct anti-Xa agents were previously reported to produce varying degrees of the inhibition of this process.13 In a subsequent publication, the reversal of thrombin generation inhibitory effects of the direct anti-Xa drugs was discussed in terms of their relative neutralization by andexanet alfa (Table 3).10
Table 3.
Relative Neutralization of UFH, Enoxaparin, and Fondaparinux by Andexanet Alfa.
| Test | UFH | Enoxaparin | Fondaparinux | |||
|---|---|---|---|---|---|---|
| Saline | Andexanet | Saline | Andexanet | Saline | Andexanet | |
| ACT (seconds) | 330.4 | 155.6 | 182.6 | 163.8 | 154.4 | 168.4 |
| Thrombin generation (nM) | 0.37 | 169.1 | 26.06 | 198.8 | 10.6 | 182.6 |
| Anti-Xa activity (% inhibition) | 94.5 | 72.3 | 79 | 72 | 96.7 | 96.9 |
| Anti-IIa activity (% inhibition) | 73.4 | 64 | 25 | 29.0 | 5.1 | 8.7 |
| aPTT (seconds) | 300 | 52.5 | 76.9 | 58.2 | 48.2 | 51.7 |
| TT (seconds) | 300 | 23.1 | 34.5 | 13.5 | 12.3 | 11.6 |
Abbreviations: ACT, activated clotting time; aPTT, activated partial thromboplastin time; UFH, unfractionated heparin.
In this study, indirect acting anti-Xa drugs that mediate their effects via AT were used. Unfractionated heparin strongly inhibited peak thrombin, followed by fondaparinux and enoxaparin. The effects of all 3 agents were completely reversed by andexanet alfa at a final concentration of 100 μg/mL. Unfractionated heparin produced stronger inhibition of thrombin generation and fondaparinux produced moderate response followed by enoxaparin. All agents were completely neutralized by andexanet alfa at a concentration of 100 µg/mL. Unfractionated heparin showed an increase in lag time, followed by enoxaparin and fondaparinux.
The neutralization studies in different assays were carried out with andexanet alfa for heparin and related drugs utilized a fixed concentration of this agent at a 100 μg/mL. This concentration was chosen since it approximates the averaged circulating level of this antidote after intravenous administration. The dosing regimen of andexanet alfa ranges from 400 to 800 mg bolus, followed by 4 to 8 mg/min for up to 2 hours. This dosing regimen is projected to result in a circulating concentration of 75 to 150 μg/mL, this approximates to be 2 to 4 μM. In the current studies, heparin and its derivatives were used in a concentration range on 1 to 10 μg/mL in the plasma-based anticoagulant assays, anti-Xa, anti-IIa, and thrombin generation studies, which approximates to be 0.07 to 0.7 μM for UFH, 0.02 to 0.2 μM for enoxaparin, and 0.01 to 0.1 μM for fondaparinux. The whole blood assays, such as TEG and ACT, were performed at fixed concentrations on 1.25 and 10 μg/mL, respectively. These concentrations were selected in an assay range sensitive to the drugs and their neutralization by andexanet alfa. Additionally, the fixed concentrations were chosen due to the limitation for the maximum amount of allowable blood volume drawn from each donor. The andexanet alfa concentration used was always in molar access in comparison to heparin and its derivatives in all of these assays.
Andexanet alfa was mainly developed for the neutralization of DOACs, such as apixaban and rivaroxaban.17 However, because of the structure similarity of the FXa inhibitors, betrixaban and edoxaban, it also neutralizes these agents in the same manner as apixaban and rivaroxaban. It is likely that any additional FXa inhibitors with similar structure will also be neutralized by this agent.
Although conventional methods have been used for the evaluation of the neutralizing effects of antidotes such as andexanet alfa, other methods used for measuring heparin’s biologic actions can also be used to determine the relative potential of andexanet alfa as an antidote. Prothrombinase-induced clotting time (PiCT test, PentaPharm, Basel, Switzerland) is a clot-based test that can also be used for the measurement of the anticoagulant effects of heparin and related drugs and their relative neutralization by andexanet alfa. As the mechanism of this test is different from aPTT, it may provide some additional insight on the mechanism of interaction of heparins and their relative neutralization by andexanet alfa. ST Genesia (Diagnostica Stago) is automated of thrombin generation testing analyzer that may be useful in the assessment of low level of heparins and DOACs and their relative neutralization by such antidotes as andexanet alfa.18
Our results are consistent with other reports with the exception of the nonreversibility of the anti-Xa effects of enoxaparin and fondaparinux as measured by chromogenic substrate method.14 Our results are contrary to the reported reversal of the anti-Xa effects of fondaparinux in rats administered with 0.5 mg/kg fondaparinux, where the circulating anti-Xa effects were completely neutralized by high dosage of andexanet alfa.15 These studies indicate that the anticoagulant effects of FXa inhibitors are not proportional to their anti-Xa effects as reported previously; therefore, FXa inhibition is not a true measure of the biological effects of these drugs. This may be due to the differences in the mechanisms involved in the design of the assay methods. The clot-based methods are usually performed in blood or plasma in the presence of phospholipids, whereas the amidolytic methods are performed using synthetic substrates in diluted buffer systems. Thus, the andexanet alfa complexed with the FXa inhibitors is partially capable of neutralizing the anti-Xa activities in the nonphospholipid-based assays.
Although andexanet alfa may be useful in the neutralization of the anticoagulant effects of DOACs such as the oral FXa inhibitors in qualified and risk assessed patient populations, it’s unlikely to be used as antidote to heparin and related drugs. Protamine sulfate is an effective antidote for heparin; however, it has relatively limited neutralization capacity for enoxaparin and fondaparinux. It is unclear whether andexanet alfa can be used as an antidote for enoxaparin and fondaparinux, despite the reported studies where it has been shown to neutralize these agents in experimental animal model studies.14 Since both enoxaparin and fondaparinux have much longer half-life, andexanet alfa is unlikely to be effective antidote for these agents. It’s also interesting to note that assay-dependent differences in the neutralization profile of heparin and related drugs with andexanet alfa are evident, in particular while the anticoagulant effects in the clot-based assays for these agents are readily neutralized, the amidolytic anti-Xa and anti-IIa effects are not proportionately effected by andexanet alfa.
As reported recently, andexanet alfa is capable of neutralizing not only heparins and fondaparinux but other glycosaminoglycans (GAGs) such as sulodexide.19,20 Therefore, intravenously administered andexanet alfa may also bind to endothelium-bound GAGs such as heparan sulfate and their complexes with AT, this particular interaction may also modulate the EAP of vasculature and facilitate thrombogenesis. The experimental studies reported in this investigation provide a partial explanation of the thrombogenic complications related to andexanet alfa. Additional in vivo studies to demonstrate the endogenous interactions of andexanet alfa with endothelium and other cells may be helpful in providing useful information on the mechanisms of thrombogenesis associated with andexanet alfa. As fixed dosage of this agent is used, irrespective of the weight and renal function, such thrombogenic complications can be minimized by taken into account some of these factors while using andexanet alfa as an antidote for the anti-Xa agents.
This study was designed to compare the neutralization profile of andexanet alfa, where UFH, enoxaparin, and fondaparinux were used as indirect anti-Xa agents as their actions are mediated by AT. Another approach to compare the neutralization profile of high AT affinity and low AT affinity heparins, additionally, the effects of molecular weight and charged density on heparins should be taken into considerations for such studies. For better understanding of the mechanisms involved, andexanet alfa should be used at different concentrations. Antithrombin levels vary among individuals, which may also alter the responses of heparin and related drugs. In the current studies, pooled plasma was used and the AT levels in all studies remain constant. Individual variations, gender differences, and other plasmatic factors and the contribution of blood cells including platelets need to be taken into account; most of the critically ill patients may have hemodilution and other fluid imbalance, which can contribute to the altered responses to andexanet alfa. Hopefully, such issues can be addressed in additional clinical trials and observational outcomes in patients treated with this antidote.
Conclusion
The availability of antidote for the neutralization of direct FXa inhibitors provides a clinically useful approach to neutralize the potential bleeding complications associated with their use. Heparin and related drugs produce their anti-Xa and anti-IIa effects, which are mediated through complexing with AT. These studies demonstrate that andexanet alfa originally designed as an antidote for apixaban and rivaroxaban is also capable of neutralizing the in vitro anticoagulant effects of UFH. Additionally, it has differential assay-dependent neutralization profile for the LMWHs such as enoxaparin and the synthetic high-affinity pentasaccharide fondaparinux. These studies further suggest that the mechanisms by which andexanet alfa neutralizes the biologic effects of heparin related drugs may differ in comparison to the direct anti-Xa agents, which may impact on their pharmacokinetic and pharmacodynamic parameters. Thus, additional experimental and clinical studies to validate their potential use as an antidote for heparin and related drugs are warranted.
Acknowledgments
The authors are thankful to the staff of the Hemostasis Research Laboratory for their skillful assistance in completing this study. Special thanks to Dr Paul Riley of Diagnostica Stago (Paris, France) for facilitating the thrombin generation inhibition studies by providing the instrument and reagents.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by internal research funds and the Hemostasis Research Laboratory and Cardiovascular Institute, Health Science Division, Loyola University Chicago.
ORCID iDs: Fakiha Siddiqui  https://orcid.org/0000-0002-2219-7049
https://orcid.org/0000-0002-2219-7049
Debra Hoppensteadt  https://orcid.org/0000-0001-8235-3624
https://orcid.org/0000-0001-8235-3624
Eduardo Ramacciotti  https://orcid.org/0000-0002-5735-1333
https://orcid.org/0000-0002-5735-1333
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Kalathottukaren MT, Creagh AL, Abbina S, et al. Comparison of reversal activity and mechanism of action of UHRA, andexanet alfa, and PER977 on heparin and oral FXa inhibitors. Blood Adv. 2018;2(16):2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu G, Kotha J, Cardenas J, et al. In vitro characterization of ANDEXANET ALFA (PRT064445), a specific fXa inhibitor antidote versus aripazine (PER977), a non-specific reversal agent. Circulation. 2014;130:A18218. [Google Scholar]
- 3. Hu TY, Vaidya VR, Asirvatham SJ. Reversing anticoagulant effects of novel oral anticoagulants: role of ciraparantag, andexanet alfa, and idarucizumab. Vasc Health Risk Manag. 2016;12:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weitz JI, Jaffer IH, Fredenburgh JC. Recent advances in the treatment of venous thromboembolism in the era of the direct oral anticoagulants. F1000Res. 2017;23(6):985 doi:10.12688/f1000research.11174.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fareed J, Thethi I, Hoppensteadt D. Old versus new oral anticoagulants: focus on pharmacology. Annu Rev Pharmacol Toxicol. 2012;52:79–99. doi:10.1146/annurev-pharmtox-010611-134633. [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi:10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 7. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation [published online August 30, 2009]. N Engl J Med. 2009;361(12):1139–1151. doi:10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 8. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi:10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 9. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019. doi:10.1056/NEJMoa1814051 ANNEXA-4 ClinicalTrials.gov number, NCT02329327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siddiqui F, Tafur A, Ramacciotti LS, et al. Reversal of factor Xa inhibitors by andexanet alfa may increase thrombogenesis compared to pretreatment values. Clin Appl Thromb Hemost. 2019;25.doi:10.1177/1076029619863493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446–451. [DOI] [PubMed] [Google Scholar]
- 12. Lu G, Lin J, Curnutte JT, Conley PB. Reversal of heparin-induced anticoagulation by andexanet alfa, a universal antidote for factor Xa inhibitors. Blood. 2015;126(23):2329.26359437 [Google Scholar]
- 13. Siddiqui F, Hoppensteadt D, Jeske W, et al. Factor Xa inhibitory profile of apixaban, betrixaban, edoxaban, and rivaroxaban does not fully reflect their biologic spectrum. Clin Appl Thromb/Hemosts. 2019;25(1). doi:10.1177/107602961984752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu G, DeGuzman FR, Hollenbach SJ, et al. Reversal of low molecular weight heparin and fondaparinux by a recombinant antidote (r-Antidote, PRT064445). Circulation. 2010;122(suppl 21):A12420. [Google Scholar]
- 15. Portola Pharmaceuticals. Andexxa (coagulation factor Xa (recombinant), inactivated-zhzo): US Prescribing Information. 2018. http://www.fda.gov/. Accessed September 15, 2019.
- 16. Greinacher A, Thiele T, Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113(5):931–942. [DOI] [PubMed] [Google Scholar]
- 17. Kandexanet S, Alfatz H, Bhansali J, et al. Reversing factor Xa inhibitors – clinical utility of andexanet alfa. J Blood Medicine. 2017;8:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gosselin RC, Adcock DM, Douxfill J. An update on laboratory assessment for direct oral anticoagulants (DOACs). Int J Lab Hematol. 2019;41(suppl 1):33–39. [DOI] [PubMed] [Google Scholar]
- 19. Hoppensteadt D, Siddiqui F, Jeske W, et al. Relative neutralization of the anticoagulant, antiprotease and thrombin generation effects of sulodexide by a recombinant Xa decoy antidote (Andexxa). Phlebol Rev. 2019(suppl 1):41. [Google Scholar]
- 20. Siddiqui F, Walenga JM, Tafur A, et al. A factor Xa inhibitor antidote (andexanet alfa) is capable of neutralizing the anticoagulant effects of unfractionated heparin of bovine, ovine and porcine origin in a comparable manner as protamine sulfate. Phlebol Rev. 2019(suppl 1):43. [Google Scholar]