Abstract
The aim of this study was to investigate the utility of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) to predict all-cause mortality in patients presenting with acute pulmonary embolism (PE). Three hundred consecutive patients with acute PE between March 2016 and December 2018 were retrospectively analyzed. We identified 191 patients who met the study inclusion criteria. Twenty-eight patients died during the study period. There was a significant difference in PLR, but not NLR, between patients with low risk, submassive, and massive risk PE (P = .02 and P = .58, respectively, by the Kruskal-Wallis test). Elevated NLR and PLR were associated with all-cause mortality (P < .01 and P < .01, respectively). Neutrophil-to-lymphocyte ratio of 5.46 was associated with all-cause mortality with sensitivity of 75.0% and specificity of 66.9% (area under the curve [AUC]: 0.692 [95% confidence interval, CI]: 0.568-0.816); P < .01). Platelet-to-lymphocyte ratio of 256.6 was associated with all-cause mortality with sensitivity of 53.6% and specificity of 82.2% (AUC: 0.693 [95% CI: 0.580-0.805]; P < .01). Neutrophil-to-lymphocyte ratio and PLR are simple biomarkers that are readily available from routine laboratory values and may be useful components of PE risk prediction models.
Keywords: acute pulmonary embolism, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, NLR, PLR, inflammation
Introduction
Acute pulmonary embolism (PE) is a heterogeneous disease process with varied presentation. Pulmonary embolism is a leading cause of cardiovascular mortality accounting for approximately 100 000 to 200 000 annual deaths in the United States.1 With a diverse range of clinical presentations, diagnosis and timely risk stratification is important. Patients are commonly risk stratified based on blood pressure, the Pulmonary Embolism Severity Index (PESI), echocardiographic evidence of right ventricular (RV) dysfunction, lower extremity venous Doppler ultrasonography, computed tomography (CT) angiography, and biomarker evidence of RV ischemia.2,3 These markers, however, lack positive-predictive power (21% PPV) and are cumbersome or costly.4
Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are novel biomarkers that may improve risk stratification of patients presenting with acute PE. These markers are readily available from routine laboratory studies and provide important information about systemic inflammation status. Complex interactions between inflammatory and coagulation factors play a role in the pathophysiology of vascular disease and can lead to thromboembolic complications.5 In acute PE, there is evidence of neutrophil and macrophage infiltration of the pulmonary arterial wall and the RV, with the latter contributing to RV dysfunction.6,7 Additionally, serum levels of pro-coagulatory and pro-inflammatory microparticles originating from platelets, leukocytes, and endothelial cells are increased in the setting of pulmonary hypertension.8 This acute inflammatory response leads to an increase in platelet activation and neutrophil recruitment and has been associated with poor prognosis and short-term mortality in patients presenting with PE.9,10 Lymphocyte count may decrease in response to adrenaline and glucocorticoids released during a sympathetic response.11 Neutrophil-to-lymphocyte ratio and PLR integrate information from these multiple cell lines and thus offer a convenient way to rapidly assess inflammation status.
Neutrophil-to-lymphocyte ratio and PLR are promising biomarkers that may enhance existing risk prediction tools and help guide management in acute PE. Currently, the prognostic value of NLR and PLR in acute PE is not fully understood. The aim of this study is to investigate the utility of NLR and PLR to predict all-cause mortality in patients with PE.
Materials and Methods
Study Population
We retrospectively analyzed 300 consecutive patients with acute PE from the Pulmonary Embolism Response Team registry at Loyola University Medical Center in Maywood, Illinois, and Gottlieb Memorial Hospital in Melrose Park, Illinois, between March 2016 and December 2018. The diagnosis of PE was determined using CT pulmonary angiography or ventilation–perfusion scan. Demographic information, vitals, comorbidities, echocardiographic findings, and clinical laboratory parameters were gathered from the electronic medical record. Complete blood count with differential was obtained at the time of PE diagnosis. Patients were excluded from further analysis if they had an active condition at the time of PE diagnosis that could significantly influence blood cell count, including sepsis (n = 14), infection (n = 41), chronic inflammatory conditions (n = 8), cancer (n = 14), and use of immunosuppressive therapy (n = 10). Furthermore, patients who did not have a complete blood count with differential at the time of PE diagnosis were also excluded (n = 22). This study was approved by the institutional review board. Informed consent for patient information to be used in this study was not obtained because the institutional review board provided a waiver of informed consent to conduct this research.
Clinical Parameters
Neutrophil-to-lymphocyte ratio and PLR were calculated by dividing absolute granulocyte count and absolute platelet count by absolute lymphocyte count, respectively. All-cause mortality was identified through the electronic medical record. The PESI score was calculated based on age, male sex, history of cancer, history of heart failure, history of chronic pulmonary disease, heart rate ≥ 110/minute, systolic blood pressure < 100 mm Hg, respiratory rate ≥ 30/minute, temperature <36°C, altered mental status, and arterial oxygen saturation <90%.12 The Simplified PESI (sPESI) score was calculated based on age >80 years, history of cancer, history of chronic cardiopulmonary disease, heart rate ≥110/minute, systolic blood pressure <100 mm Hg, and arterial oxygen saturation <90%.13
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics version 25.0 (Released 2017; IBM SPSS Statistics for Macintosh, Armonk, New York). The Kolmogorov-Smirnov test was used to evaluate the data for normality of distribution. Normally distributed data, including patient age and heart rate, were expressed as mean (standard deviation). Non-normally distributed data were expressed as median (interquartile range). Comparisons between groups were evaluated using the nonparametric Mann-Whitney U test, Kruskal-Wallis test, and χ2 test. Correlation analysis was performed by Spearman rank-order correlation coefficient. A P value ≤ .05 was considered significant. R values were generated to assess the strength of correlations. Receiver operating characteristic (ROC) curve was constructed to illustrate the sensitivity and specificity of NLR and PLR to predict all-cause mortality. Youden’s J statistic was calculated to determine optimal cutoff values.14 To assess the value of integrating NLR and PLR to the sPESI score to predict all-cause mortality, an NLR or PLR greater than the optimal cutoff value was scored as 1 point and added to the patient’s sPESI score. When both NLR and PLR were greater than the optimal cutoff values, 2 points were added to the sPESI score. No additional points were added to the original sPESI score when both NLR and PLR were below the optimal cutoff values.
Results
We identified 191 patients who met the study inclusion criteria. Twenty-eight patients died during the study period. Table 1 summarizes the baseline clinical characteristics. Patients with acute PE who died had significantly higher PESI score (123 [107-170] vs 90.0 [68.0-121]; P < .01) and sPESI score (2.00 [1.25-3.75] vs 1.00 [0-2.00]; P < .01) but lower lymphocyte count (0.800 [0.400-1.30] vs. 1.50 [1.10-2.20] K/mm3; P < .01) and hemoglobin (11.5 [9.83-13.5] vs 13.1 [11.5-14.7] g/dL; P < .01). The frequency of chronic pulmonary disease, excluding asthma and chronic obstructive pulmonary disease, was higher in nonsurvivors compared to survivors (17.9% vs 3.10%; P < .01). There was no significant difference in age, gender, PE severity, and vitals between survivors and nonsurvivors.
Table 1.
Baseline Clinical Characteristics.a
| Parameter | Survived, n = 163 | Died, n = 28 | P Value |
|---|---|---|---|
| Age, years | 59.5 (16.0) | 62.3 (15.2) | .42 |
| Gender | |||
| Male | 83 (50.9%) | 12 (42.9%) | .43 |
| Female | 80 (49.1%) | 16 (57.1%) | |
| CAD | 16 (9.80%) | 4 (14.3%) | .51 |
| CHF | 20 (12.3%) | 4 (14.3%) | .79 |
| DM | 32 (19.6%) | 6 (21.4%) | .90 |
| HTN | 88 (54.0%) | 16 (57.1%) | .84 |
| Chronic pulmonary disease (not asthma or COPD) | 5 (3.10%) | 5 (17.9%) | <.01 |
| COPD | 10 (6.10%) | 1 (3.60%) | .57 |
| Prior DVT | 24 (14.7%) | 7 (25.0%) | .18 |
| Prior PE | 23 (14.1%) | 8 (28.6%) | .06 |
| Prior stroke | 11 (6.70%) | 4 (14.3%) | .18 |
| PE severity | |||
| Low risk | 54 (33.1%) | 7 (25.0%) | .53 |
| Submassive | 99 (60.7%) | 18 (64.3%) | |
| Massive | 10 (6.10%) | 3 (10.7%) | |
| Vitals | |||
| SBP, mm Hg | 106 (97.0-117) | 101 (88.0-119) | .17 |
| HR, beats/min | 106 (20.9) | 113 (30.0) | .22 |
| RR, breaths/min | 23.0 (20.0-27.0) | 21.0 (18.0-30.0) | .75 |
| PESI score | 90.0 (68.0-121) | 123 (107-170) | <.01 |
| sPESI score | 1.00 (0-2.00) | 2.00 (1.25-3.75) | <.01 |
| WBC, K/µL | 9.20 (7.10-11.2) | 9.40 (5.60-10.9) | .26 |
| Neutrophil count, K/mm3 | 6.40 (5.00-8.40) | 6.60 (4.10-9.20) | .66 |
| Lymphocyte count, K/mm3 | 1.50 (1.10-2.20) | 0.800 (0.400-1.30) | <.01 |
| Hgb, g/dL | 13.1 (11.5-14.7) | 11.5 (9.83-13.5) | <.01 |
| Platelet count, K/µL | 227 (175-284) | 205 (126-272) | .20 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; DM, diabetes mellitus; HTN, hypertension; COPD, chronic obstructive pulmonary disease; DVT, deep venous thrombosis; Hgb, hemoglobin; HR, heart rate; IQR, interquartile range; PE, pulmonary embolism; PESI, Pulmonary Embolism Severity Index; RR, respiratory rate; SBP, systolic blood pressure; SD, standard deviation; sPESI, Simplified Pulmonary Embolism Severity Index; WBC, white blood cells.
a Values are expressed as mean (SD), median (IQR), and individuals (percentage).
The median NLR and PLR of the study population were 4.29 (2.52-7.92) and 155.0 (97.0-242.1), respectively. In comparison, the median NLR and PLR of the patients that did not meet the study inclusion criteria were 6.49 (3.33-14.8) and 196.1 (102.9-362.2), respectively. The distribution of NLR and PLR of the study population according to PE severity is summarized in Table 2. We identified 61 patients with low-risk PE, 117 patients with submassive PE, and 13 patients with massive PE. No significant difference in NLR was observed between patients with low-risk, submassive, and massive PE (P = .58, by the Kruskal-Wallis test). However, there was a significant difference in PLR between patients with low-risk, submassive, and massive PE (P = .02, by the Kruskal-Wallis test). Platelet-to-lymphocyte ratio was significantly higher in patients with low-risk PE compared to patients with massive PE (173 [109-145] vs 90.3 [50.4-164]; P < .01, by the Mann-Whitney U test).
Table 2.
Distribution of NLR and PLR According to Pulmonary Embolism Severity.a
| Parameter | Low Risk PE, n = 61 | Submassive PE, n = 117 | Massive PE, n = 13 | P Value |
|---|---|---|---|---|
| NLR | 4.18 (2.58-6.69) | 4.46 (2.58-8.55) | 2.88 (1.71-8.79) | .58 |
| PLR | 173 (109-145) | 154 (96.0-250) | 90.3 (50.4-164)b | .02 |
Abbreviations: IQR, interquartile range; NLR, neutrophil-to-lymphocyte ratio; PE, pulmonary embolism; PLR, platelet-to-lymphocyte ratio.
a Values are expressed as median (IQR).
b P < .01 versus low-risk PE.
Neutrophil-to-lymphocyte ratio was significantly elevated in nonsurvivors compared to survivors (8.10 [4.28-13.7] vs 3.91 [2.46-6.71]; P < .01; Figure 1A). Platelet-to-lymphocyte ratio was also significantly elevated in the nonsurvivors compared to survivors (263 [147-407] vs 148 [95.5-214]; P < .01; Figure 1B).
Figure 1.
A, Comparison of NLR in patients with acute pulmonary embolism based on survival and mortality. B, Comparison of PLR in patients with acute pulmonary embolism based on survival and mortality. NLR indicates neutrophil-to-lymphocyte Ratio; PLR, platelet-to-lymphocyte ratio.
Neutrophil-to-lymphocyte ratio positively correlated with PLR (R = .737; P < .01). Table 3 summarizes the relationships between NLR, PLR, and parameters associated with PE severity. Both NLR and PLR positively correlated with PESI (R = .270, P < .01 and R = 0.176, P = .02) and sPESI (R = .215, P < .01 and R = .147, P = .04). Platelet-to-lymphocyte ratio negatively correlated with RV/left ventricular (LV) ratio (R = −.232; P < .01). Neither NLR nor PLR was associated with brain natriuretic peptide, lactate, or troponin.
Table 3.
Correlations Between NLR, PLR, and Parameters Associated With Severity of Pulmonary Embolism.
| Parameter | NLR | PLR | ||
|---|---|---|---|---|
| R | P Value | R | P Value | |
| PESI score | .270 | <.01 | .176 | .02 |
| sPESI score | .215 | <.01 | .147 | .04 |
| BNP | .018 | .83 | −.033 | .69 |
| Lactate | .013 | .88 | −.049 | .58 |
| Troponin | .019 | .80 | −.132 | .08 |
| RV/LV ratio | −.129 | .09 | −.232 | <.01 |
Abbreviations: BNP, brain natriuretic peptide; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PESI, Pulmonary Embolism Severity Index; RV/LV, right/left ventricular; sPESI, Simplified Pulmonary Embolism Severity Index.
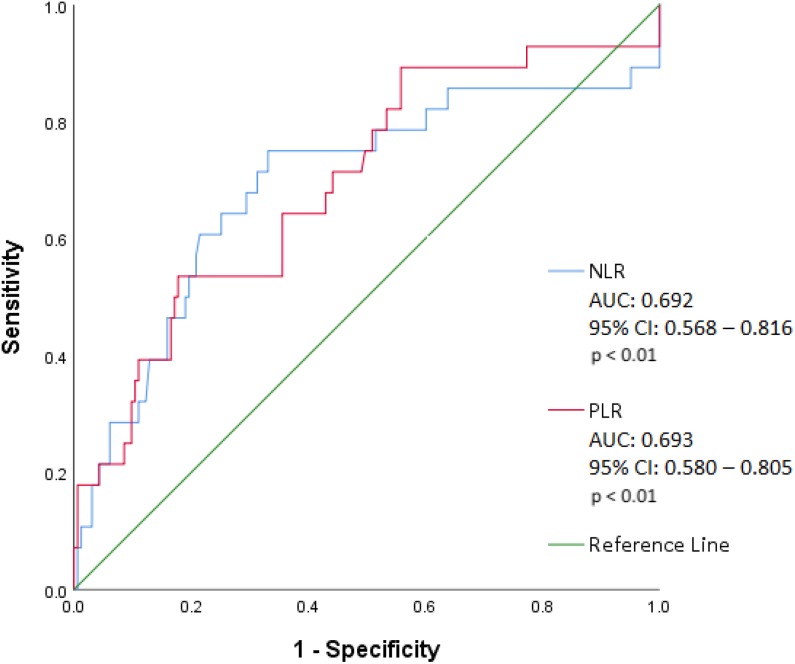
Based on ROC curve analysis (Figure 2), NLR of 5.46 was associated with all-cause mortality with sensitivity of 75.0% and specificity of 66.9% (area under the curve [AUC]: 0.692 [95% confidence interval, CI]: 0.568-0.816; P < .01). Platelet-to-lymphocyte ratio of 256.6 was associated with all-cause mortality with sensitivity of 53.6% and specificity of 82.2% (AUC: 0.693 [95% CI: 0.580-0.805]; P < .01).
Figure 2.
Receiver operating characteristic curve illustrating the sensitivity and specificity of NLR and PLR to predict all-cause mortality in acute pulmonary embolism. AUC indicates area under the curve; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
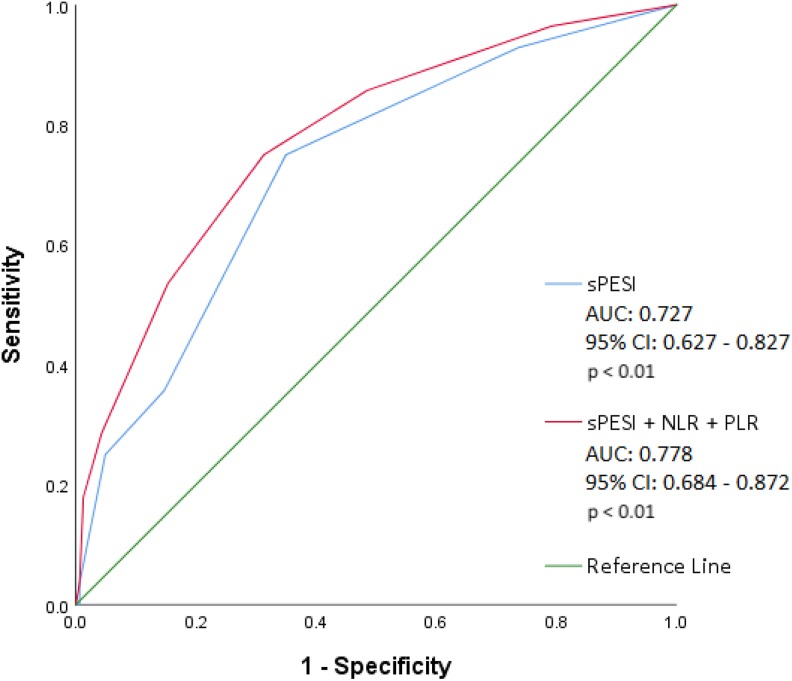
To assess the value of integrating NLR and PLR to the sPESI score to predict all-cause mortality, NLR > 5.46 or PLR > 256.6 was scored as 1 point and added to the patient’s sPESI score. In patients with both NLR > 5.46 and PLR > 256.6, two points were added to the sPESI score. This integrated model demonstrated increased predictive value compared to that of the sPESI score alone (AUC: 0.778 [95% CI: 0.684-0.872]; P < .01 vs AUC: 0.727 [95% CI: 0.627-0.827]; P < .01; Figure 3).
Figure 3.
Receiver operating characteristic curve illustrating the sensitivity and specificity of the sPESI score integrated with NLR and PLR to predict all-cause mortality in acute pulmonary embolism. The integrated sPESI score (sPESI + NLR + PLR) was applied by adding points to the original sPESI score if NLR or PLR were above optimal cutoff values. Two points were added if NLR >5.46 and PLR >256.6. One point was added if NLR >5.46 or PLR >256.6. No additional points were added if NLR <5.46 and PLR <256.6. AUC indicates area under the curve; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; sPESI, Simplified Pulmonary Embolism Severity Index.
Of the 28 nonsurvivors, only 5 patients died from PE (17.9%), while 16 patients died from non-PE causes (57.1%) and 7 died from unknown causes (25%). The frequency of in-hospital mortality was 6 (21.4%) deaths, in which 4 deaths were attributed to PE. The median number of days between PE diagnosis and mortality was 56.0 (13.0-124). Further details regarding PE- and non-PE-associated mortality are specified in Table 4.
Table 4.
Etiologies of All-Cause and In-Hospital Mortality.
| n (%) | |
|---|---|
| All-Cause Mortality | |
| Pulmonary embolism | 5 (17.9%) |
| Massive | 2 (7.14%) |
| Submassive | 3 (10.7%) |
| Low risk | 0 |
| Non-pulmonary embolism | 16 (57.1%) |
| Respiratory failure | 1 (3.57%) |
| Hospice | 10 (35.7%) |
| Metastatic cancer | 2 (7.14%) |
| Septic shock | 3 (10.7%) |
| Unknown | 7 (25.0%) |
| In-Hospital Mortality | |
| Pulmonary embolism | 4 (66.6%) |
| Massive | 2 (33.3%) |
| Submassive | 2 (33.3%) |
| Low risk | 0 |
| Septic shock | 1 (16.7%) |
| Unknown | 1 (16.7%) |
Discussion
Our findings demonstrate elevated NLR and PLR are associated with all-cause mortality in acute PE. These markers were significantly elevated in nonsurvivors compared to survivors, which is consistent with prior reports.15,16 Furthermore, we demonstrated that both NLR and PLR correlated with the PESI and sPESI scores. Platelet-to-lymphocyte ratio has been correlated with the sPESI score, but to our knowledge, an association between NLR and the PESI score or sPESI score has not been reported.17 These findings support the role of NLR and PLR as novel prognostic markers that may help identify patients with acute PE who are at higher risk of all-cause mortality upon initial presentation.
The elevation of NLR and PLR in nonsurvivors suggests that acute PE is associated with a pro-inflammatory state. In our study, we found a decrease in lymphocyte count coupled with minimal change in neutrophil and platelet counts influenced the increase in NLR and PLR in nonsurvivors. This finding differs from other studies, which have reported that neutrophil count can be markedly elevated in the setting of decreased lymphocytes in PE nonsurvivors.15,16 The differences may reflect the highly variable state of the inflammatory milieu and the need for markers from multiple cell lines to acquire a global view of the immune response to PE. Both NLR and PLR are promising markers because they integrate cell counts that are routinely measured in clinical practice, offering timely information about inflammation status. The importance of taking inflammation into consideration during initial PE evaluation cannot be overstated, as increased levels of inflammatory mediators are associated with poor clinical outcomes in thromboembolic disease.9,18
Additionally, we sought to integrate NLR and PLR to the sPESI score to determine whether its prognostic utility could be enhanced with additional criteria reflective of inflammation status. Our integrative model demonstrated a small increase in the area under the ROC curve for assessing all-cause mortality, suggesting improved predictive value compared to that of the sPESI score alone. This finding is similar to that of a prior study by Ozcan Cetin et al, which assessed the value of adding PLR to the sPESI score to predict long-term all-cause mortality in acute PE.19 Ozcan Cetin et al added 1 point to patients’ sPESI scores for PLR >149.1 and found a significant increase in the area under the ROC curve.19 We did not observe such a substantial improvement upon integrating NLR and PLR to the sPESI score. This discrepancy may be due to differences between study populations with ours having less exclusion criteria not encompassing conditions such as cardiomyopathy, congenital heart disease, severe valvular heart disease, coronary artery disease, history of PE, renal insufficiency, and hepatic insufficiency.19 Our findings support a role for NLR and PLR as clinical parameters which may add to the predictive value of existing risk stratification tools such as the sPESI score.
Interestingly, we found RV/LV ratio negatively correlated with PLR but did not correlate with NLR. Since increased platelet activation occurs in acute PE, a higher RV/LV ratio reflecting a larger PE burden may correspond to increased platelet consumption and therefore a smaller PLR.20 This hypothesis is consistent with our finding that PLR was significantly lower in patients with massive PE compared to patients with low-risk PE. In contrast, Ates et al found that PLR was significantly elevated in patients with massive PE compared to patients with submassive or low-risk PE.21 It is worth noting that other studies have observed an elevation of PLR in patients with RV dysfunction and a positive correlation between PLR and RV/LV ratio.19,22 In fact, PLR has also been positively correlated with CT pulmonary artery obstruction index, suggesting that higher PLR is associated with increased thrombus burden.19 Correlation of PLR with other platelet indices such as mean platelet volume and platelet distribution width, which have been associated with PE severity, may be useful.23 Neutrophil-to-lymphocyte ratio has been shown to be elevated in patients with RV dysfunction, but a correlation between NLR and RV/LV ratio has not been established to our knowledge.22 Although we did not observe a significant difference in NLR between patients based on PE severity, Ates et al found that NLR was significantly elevated in patients with massive PE compared to patients with submassive or low-risk PE.21 The variations observed may be due to differences between study populations with ours having substantially less patients with massive PE compared to the cohort studied by Ates et al (n = 13 vs n = 218).21 Furthermore, our exclusion criteria did not encompass conditions such as heart failure, RV and LV dysfunction, intracardiac thrombus, pericardial effusion, nephrotic syndrome, acute renal and liver failure, endocrine disorders, major surgical interventions, major trauma, and intubation.21
Future studies in acute PE should consider the prognostic utility of serial NLR and PLR measurements since inflammation is a dynamic process. In experimental models of PE, the levels of neutrophils infiltrating the pulmonary arterial wall peaked at 2 days and returned to baseline by 8 days, while levels of macrophages peaked at 1 day and returned to baseline by 4 days.6 Furthermore, immunohistological analysis of the RV after PE revealed neutrophils invaded between 6 and 18 hours and resolved between day 4 and week 1.7,24,25 Monocytes were also present early in the course but predominantly switched from a pro-inflammatory phenotype to a healing phenotype by week 6.24 These findings suggest that inflammation associated with PE acutely worsens at the onset but may improve during the course of PE. Monitoring the trend of the inflammatory milieu using NLR and PLR may provide insight into response to therapy and as to whether patients with PE are recovering or worsening during hospitalization.
Persistent inflammation in acute PE may reflect underlying diseases. When patients with PE are appropriately diagnosed and treated, the majority of deaths within 1 year are due to underlying conditions such as infection, cancer, congestive heart failure, and chronic lung disease.26 The causes of mortality seen in our cohort are consistent with this observation, which suggests NLR and PLR are less useful for predicting mortality directly attributed to PE. Rather, the elevation of these markers may be more reflective of underlying comorbidities that increase the risk of all-cause mortality in patients with acute PE. Trending NLR and PLR could further our understanding of how systemic inflammation affects acute PE outcomes.
Limitations
This study was a retrospective analysis with a limited sample size at 2 centers. As such, a formal power calculation was not performed. Furthermore, we were unable to determine the NLR and PLR of patients prior to the PE diagnosis. Hence, we could not compare NLR and PLR at the time of PE diagnosis to the values at baseline.
Conclusion
Neutrophil-to-lymphocyte ratio and PLR are simple biomarkers that are readily available from routine laboratory values and may be useful components of PE risk prediction models. Elevated NLR and PLR were associated with all-cause mortality in acute PE. The elevation of NLR and PLR may reflect the presence of systemic inflammation. Further studies are needed to ascertain the pathophysiologic process that underlies the associations with all-cause mortality.
Footnotes
Authors’ Note: Informed consent for patient information to be published in this article was not obtained because the authors had a waiver of informed consent from the institutional review board to conduct this research.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was funded by internal research funds from the Cardiovascular Research Institute, Center for Translational Research and Education, and Loyola University Medical Center.
ORCID iDs: Trung Phan  https://orcid.org/0000-0003-4472-509X
https://orcid.org/0000-0003-4472-509X
Yevgeniy Brailovsky  https://orcid.org/0000-0002-4811-5267
https://orcid.org/0000-0002-4811-5267
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998. Arch Intern Med. 2003;163(14):1711–1717. [DOI] [PubMed] [Google Scholar]
- 2. Kucher N, Goldhaber S. Risk stratification of acute pulmonary embolism. Semin Thromb Hemost. 2006;32(8):838–847. [DOI] [PubMed] [Google Scholar]
- 3. den Exter PL, van der Hulle T, Klok FA, Huisman MV. Advances in the diagnosis and management of acute pulmonary embolism. Thromb Res. 2014;133(Suppl 2):S10–S16. [DOI] [PubMed] [Google Scholar]
- 4. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718–726. [DOI] [PubMed] [Google Scholar]
- 5. Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–2704. [DOI] [PubMed] [Google Scholar]
- 6. Eagleton MJ, Henke PK, Luke CE, et al. Inflammation and intimal hyperplasia associated with experimental pulmonary embolism. J Vasc Surg. 2002;36(3):581–588. [DOI] [PubMed] [Google Scholar]
- 7. Watts JA, Zagorski J, Gellar MA, Stevinson BG, Kline JA. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol. 2006;41(2):296–307. [DOI] [PubMed] [Google Scholar]
- 8. Diehl P, Aleker M, Helbing T, et al. Increased platelet, leukocyte and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension. J Thromb Thrombolysis. 2011;31(2):173–179. [DOI] [PubMed] [Google Scholar]
- 9. Jo JY, Lee MY, Lee JW, Rho BH, Choi WI. Leukocytes and systemic inflammatory response syndrome as prognostic factors in pulmonary embolism patients. BMC Pulm Med. 2013;13(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12(11):1764–1775. [DOI] [PubMed] [Google Scholar]
- 11. Ince LM, Weber J, Scheiermann C. Control of leukocyte trafficking by stress-associated hormones. Front Immunol. 2018;9:3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172(8):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiménez D, Aujesky D, Moores L, et al. Simplification of the Pulmonary Embolism Severity Index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(15):1383–1389. [DOI] [PubMed] [Google Scholar]
- 14. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 15. Ma Y, Mao Y, He X, Sun Y, Huang S, Qiu J. The values of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in predicting 30 day mortality in patients with acute pulmonary embolism. BMC Cardiovasc Disord. 2016;16(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karataş MB, İpek G, Onuk T, et al. Assessment of prognostic value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with pulmonary embolism. Acta Cardiol Sin. 2016;32(3):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kundi H, Balun A, Cicekcioglu H, et al. The relation between platelet-to-lymphocyte ratio and Pulmonary Embolism Severity Index in acute pulmonary embolism. Heart Lung. 2015;44(4):340–343. [DOI] [PubMed] [Google Scholar]
- 18. Venetz C, Labarère J, Jiménez D, Aujesky D. White blood cell count and mortality in patients with acute pulmonary embolism. Am J Hematol. 2013;88(8):677–681. [DOI] [PubMed] [Google Scholar]
- 19. Ozcan Cetin EH, Cetin MS, Canpolat U, et al. Platelet-to-lymphocyte ratio as a novel marker of in-hospital and long-term adverse outcomes among patients with acute pulmonary embolism: A single center large-scale study. Thromb Res. 2017;150:33–40. [DOI] [PubMed] [Google Scholar]
- 20. Chung T, Connor D, Joseph J, et al. Platelet activation in acute pulmonary embolism. J Thromb Haemost. 2007;5(5):918–924. [DOI] [PubMed] [Google Scholar]
- 21. Ates H, Ates I, Kundi H, Yilmaz FM. Diagnostic validity of hematologic parameters in evaluation of massive pulmonary embolism. J Clin Lab Anal. 2017;31(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jia D, Liu F, Zhang Q, Zeng GQ, Li XL, Hou G. Rapid on-site evaluation of routine biochemical parameters to predict right ventricular dysfunction in and the prognosis of patients with acute pulmonary embolism upon admission to the emergency room. J Clin Lab Anal. 2018;32(4):e22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Günay E, Sarinc Ulasli S, Kacar E, et al. Can platelet indices predict obstruction level of pulmonary vascular bed in patients with acute pulmonary embolism? Clin Respir J. 2014;8(1):33–40. [DOI] [PubMed] [Google Scholar]
- 24. Watts JA, Gellar MA, Obraztsova M, Kline JA, Zagorski J. Role of inflammation in right ventricular damage and repair following experimental pulmonary embolism in rats. Int J Exp Pathol. 2008;89(5):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zagorski J, Gellar MA, Obraztsova M, Kline JA, Watts JA. Inhibition of CINC-1 decreases right ventricular damage caused by experimental pulmonary embolism in rats. J Immunol. 2007;179(11):7820–7826. [DOI] [PubMed] [Google Scholar]
- 26. Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245. [DOI] [PubMed] [Google Scholar]