Abstract
Rising atmospheric oxygen (O2) levels provided a selective pressure for the evolution of O2-dependent micro-organisms that began with the autotrophic eukaryotes. Since these primordial times, the respiring mammalian cell has become entirely dependent on the constancy of electron flow with molecular O2 serving as the terminal electron acceptor in mitochondrial oxidative phosphorylation. Indeed, the ability to “sense” O2 and maintain homeostasis is considered one of the most important roles of the central nervous system (CNS) and likely represented a major driving force in the evolution of the human brain. Today, modern humans have evolved with an oversized brain committed to a continually active state and as a consequence, paradoxically vulnerable to failure if the O2 supply is interrupted. However, our pre-occupation with O2, the elixir of life, obscures the fact that it is a gas with a Janus Face, capable of sustaining life in physiologically controlled amounts yet paradoxically deadly to the CNS when in excess. A closer look at its quantum structure reveals precisely why; the triplet ground state diatomic O2 molecule is paramagnetic and exists in air as a free radical, constrained from reacting aggressively with the brain’s organic molecules due to its “spin restriction”, a thermodynamic quirk of evolutionary fate. By further exploring O2’s free radical “quantum quirkiness” including emergent quantum physiological phenomena, our understanding of precisely how the human brain senses O2 deprivation (hypoxia) and the elaborate redox-signaling defense mechanisms that defend O2 homeostasis has the potential to offer unique insights into the pathophysiology and treatment of human brain disease.
Keywords: Evolution, Oxygen, Brain, Free radicals, Quantum signaling
Background
Astronomers recently detected faint signals of ancient ionized oxygen (O2), the most distant ever discovered, emitted a staggering 13.28 billion years ago (Gya), indicating that stars began forming just 500 million years after the Big Bang when the universe was less than 4% its current age (Hashimoto et al. 2018). Thus, we can thank our (dying) lucky stars, the burning crucibles that convert hydrogen and helium into heavier elements for our O2, the molecule that made our world, our brains and us. As an element, oxygen (O) is unique; it is the third most abundant element in the universe after hydrogen and helium, the second most electronegative element behind fluorine making it an ideal electron acceptor and the most abundant element in the Earth’s crust (Allred and Rochow 1958; Dole 1965). However, while free O2 in the atmosphere distinguishes our planet from all others in the solar system, the early terrestrial atmosphere was not quite so unique.
Coupled evolution of life and O2
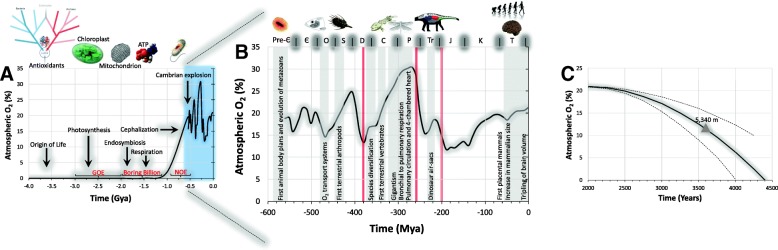
The composition of the ancient atmosphere was largely dictated by volcanic gases and consisted mainly of hydrogen, carbon dioxide (CO2), carbon monoxide, hydrogen sulfide and methane (Holland 2002). Given the ubiquity of the proton gradient in cells, life likely emerged in alkaline thermal vents at the bottom of the oceans, eventually giving rise to two orders of life, archaea and bacteria (Miller and Bada 1988). However, it wasn’t until ~ 1.5 Gya that photosynthesizing blue-green algae (cyanobacteria) began to breathe life into what was effectively a reductive, anerobic atmosphere splitting water to obtain the hydrogen required to drive metabolic reactions (2H2O → 4H + O2↑)(Nisbet and Sleep 2001). The inexorable rise in atmospheric O2 during the Proterozoic Eon of the Pre-Cambrian period ∼2,500–540 million years ago (Myr) signaled a death sentence to anerobes yet sparked an explosion of the planet’s biota and saw the number and diversity of multicellular species expand exponentially (Berner et al. 2007). Figure 1a illustrates the major evolutionary and developmental events that have been inextricably linked to atmospheric O2 “pulses” over two oxidation events, the Great Oxidation Event (GOE) and Neoproterozoic Event (NOE) interspersed by the Boring Billion, though the two-step transition from a virtually anoxic environment to present day conditions has been challenged by a more gradual increase in O2 levels, termed the Great Oxidation Transition (Lyons et al. 2014). Though beyond the remit of the current review, other atmospheric gases, notably carbon dioxide, has also helped shape life on Earth to which the brain has evolved heightened sensitivity (Cummins et al. 2014; Willie et al. 2014; Bailey et al. 2017b).
Fig. 1.
Coupled evolution of life and atmospheric oxygen. Major evolutionary and developmental events that have been linked to “pulses” in the atmospheric oxygen (O2) concentration based on the GEOCARBSULPH model (Berner 2007; Berner 2009). a Note that since the origin of life within 500 million years of Earth’s formation (LUCA, Last Universal Common Ancestor), oxygenic photosynthesis was responsible for the rapid increase in atmospheric O2 levels during the Proterozoic Eon of the Pre-Cambrian period (~ 0–10% in < 1 billion years) preceded by endosymbiosis, emergence of cellular respiration with adenosine triphosphate the universal energy source and cephalization, a characteristic feature of the ancestral bilateria, leading to the first appearance of a central nervous system (Holland et al. 2013). Pre-Є, Pre-Cambrian; Є, Cambrian; O, Ordovician; S, Silurian; D, Devonian; C, Carboniferous; P, Permian; Tr, Triassic; J, Jurassic; K, Cretaceous; T, Tertiary. b Stochastic changes in atmospheric O2 levels during the Phanerozoic eon peaked during the Carboniferous/Permian periods resulting in gigantism subsequent to augmented O2 diffusive capacity and heralded major evolutionary advances that included a 3.5-fold increase in hominin brain volume over ~ 2.75 million years (Seymour et al. 2016). Also note the three major extinction events (red bands) associated with dramatic falls in atmospheric O2 levels. c Parabolic projection of the decline in future atmospheric O2 levels using a stochastic model (Livina et al. 2015) applied to original data obtained from recording stations in the Scripps Programme (Keeling 1988). Note that the model predicts that in ~ 3,600 years, atmospheric O2 levels will be so low that hypoxia will be encountered even at sea-level, equivalent to being exposed to a terrestrial altitude of ~ 5,340 m which represents the highest elevation know to sustain lifelong human habitation with complete (O2) depletion predicted within ~ 4.4 millennia (Martin et al. 2017)
Being surrounded by O2 likely favored the survival of organisms capable of tolerating the toxicity associated with its damaging free radical reactions (see later), specializing in cellular mechanisms that could harness the gas safely to generate energy giving rise to aerobic respiration, central to oxidative phosphorylation and bioenergetic homeostasis following a symbiotic merger with the once free-living α-proteobacteria that subsequently gave way to the more sophisticated mitochondrion (Gray et al. 2001). Chemical reduction by the mitochondrial electron transport chain has since seen O2 become the (ideal) terminal electron acceptor reducing it to water, its thermodynamic “nirvana”, supplying ∼30 molecules of adenosine triphosphate (ATP) per metabolized glucose molecule to the respiring eukaryote. This provided efficient, regulated metabolic support signaling the development of more complex structures such as the early brain in bilateri, conferring a clear evolutionary advantage over the 2 ATP/glucose yield by the more basic anerobic glycolytic reaction.
The inextricable link between O2 and biological evolution is especially evident over the more recent Phanerozoic Eon (~ 550 Myr) when atmospheric levels increased to between 15 and 20% sparking the first animal body plans marking the advent of metazoan evolution (Fig. 1b) (Berner et al. 2007). Further elaborations to O2 transport systems included the emergence of the parallel pulmonary circulation and the four chambered heart during the Permian when atmospheric O2 levels peaked during the late Carboniferous period reaching a staggering 35%, imposing fewer limits on O2 diffusion allowing the giant Carboniferous dragonfly (Meganeura monyi) with a wing span in excess of 75 cm to flourish (Graham et al. 1995).
It would seem intuitive that further refinements were made to endogenous antioxidant defences to cope with this extra O2 (Halliwell 2006); indeed, some of the plants that evolved at that time are more O2 resistant than more recently evolved plants (Beerling et al. 1998). However, sequence and phylogenetic analyzes suggest that even the Last Universal Common Ancestor (LUCA) was capable of detoxifying reactive oxygen species (ROS) using superoxide dismutase (SOD), catalase, peroxiredoxins and hemoglobin-binding (of the albeit limited O2) a billion years before O2 became abundant in the atmosphere or ocean (Slesak et al. 2012), though this may have evolved in response to localized O2 formation through abiotic sources (e.g. photolysis of water by ultraviolet light given the early lack of an ozone layer) or cohabitation with an oxidative photosynthesizing organism (Case 2017). Furthermore, the anerobic bacterium Chlorobium limicola is capable of generating the potent antioxidant ergothioneine through an enzymatic reaction that differs from all other known (aerobic) pathways (Burn et al. 2017) implying an apparent uncoupling between antioxidant defense and O2 bioavailability, arguing an alternative albeit undefined role for antioxidant defense in early anerobic environments that may have been “repurposed” at a later stage for biological defense against oxidative damage following the inextricable shift towards a more oxidizing atmosphere (Ruszczycky and Liu 2017).
The general consensus is that controlled O2•- scavenging is an essential defense mechanism that serves to minimize oxidative damage in an aerobic world. The early presence of iron (Fe) and manganese (Mn) SOD isoforms among archaea and bacteria coupled with the independent evolution of the copper, zinc (Cu,Zn) isoform stands testament to this (McCord et al. 1971). Interestingly, in eukaryotic species that contain both the Cu,Zn SOD and MnSOD isoforms, the former is localized to the cytosol whereas the latter is constrained to the mitochondrion; a unique distribution of two evolutionary separate yet functionally identical enzymes lending additional support to the endosymbiotic origin of the mitochondrion (Fridovich 1974).
Notwithstanding the finer details, contemporary estimates now suggest that the green plants on earth combine a total of 150 billion tons of carbon (from CO2) with 25 billion tons of H2 (from H2O) to liberate 400 billion tons of O2 each year to maintain O2 at its current atmospheric level (Bailey 2001). However, it is unlikely that O2 is here to stay since there has been an inexorable decline in atmospheric levels over the past 20 years. Originally assumed to be linear (equivalent to ~ 4 ppm/year), more recent estimates suggest that the decline is more likely parabolic (Livina et al. 2015). Application of this parabolic projection to original data (Keeling 1988) makes for some startling if not indeed catastrophic predictions (Martin et al. 2017) as outlined in Fig. 1c notwithstanding the predictive constraints associated with a mathematical (as opposed to a geochemical) model. Within ~ 3,600 years from now, it is predicted that atmospheric O2 levels will become so low that even living at sea-level will feel as hypoxic as living at an equivalent terrestrial altitude of ~ 5,340 m, the highest elevation known to sustain lifelong human habitation with complete depletion predicted within ~ 4.4 millennia (Martin et al. 2017). Global deoxygenation may impact brain morphology and hemodynamic function as humans are likely to undergo further selection for physiological phenotypes that confer improved ability to survive chronic hypoxemic stress, potentially resembling those of well-adapted high-altitude populations like the Tibetans and Sherpa (Gilbert-Kawai et al. 2014).
Evolution of the human brain; size and flow mattered
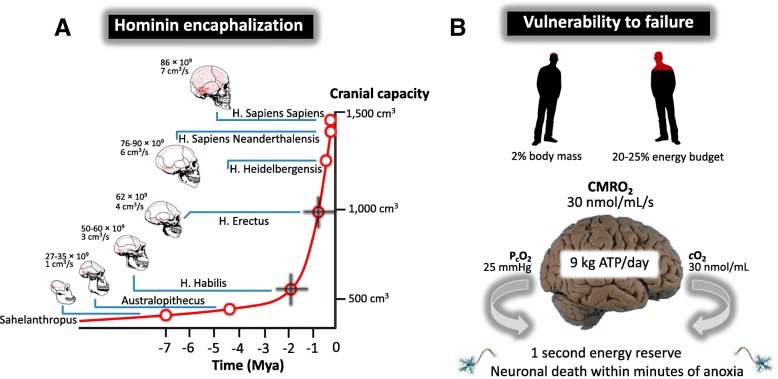
Environmental pressures caused by climatic fluctuations have long been assumed to play a key role in hominin speciation and adaptation (Maslin and Christensen 2007). Not surprisingly, O2 has played an especially important role in the development of the human brain, arguably the most significant event in the evolution of human life. The fossil record and neuroanatomical analysis of closely related species indicates that the hominin brain increased in size by ~ 3.5 fold over a period of ~ 3 million years (from 400 to 600 cm3 to 1,200–1,600 cm3) with a neocortex that has come to constitute 80% of the brain with disproportionate increases observed in the prefrontal and posterior parietal cortex (Fig. 2a) (Semendeferi et al. 2002; Schoenemann 2006; Azevedo et al. 2009). With an encephalization quotient of 7 (seven times larger in relation to our expected brain-to-body mass ratio) the modern human is the most encephalized of all species (Hadjistassou et al. 2015).
Fig. 2.
Evolution of the hominin brain and vulnerability to failure. a Exponential increase in cranial capacity observed in fossil hominids over time beginning with Homo Habilis and marked encaphalization linked to the physically active “Hunter Gatherer”, Homo Erectus (annotated). Data based on the (calculated) mean of published individual data points (Schoenemann 2006). Note also the increase in total number of neurones estimated from separate derivations of cranial capacity and corresponding increases in cerebral blood flow calculated from the size of the internal carotid foramina, in relation to endocranial volume (Seymour et al. 2016). b The human brain’s oxygen (O2) dependence comes at a cost with a corresponding high vulnerability to failure given that it is an entirely aerobic organ characterized by limited energy reserves that becomes evident when confronted by complete oxygen lack (anoxia). CMRO2, cerebral metabolic rate of oxygen; PcO2 (average) cerebral tissue partial pressure of O2; cO2, cerebral oxygen content
Furthermore, recent estimates indicate that unlike primates, the increase in human brain volume was accompanied by an even greater (6-fold) increase in global cerebral blood flow to support rapid development in interneuron connectivity, synaptic activity and cognitive function (Seymour et al. 2016). It would thus seem that we ultimately got smarter through a rush of blood to the head! Thus, the brain did not simply become bigger, but more specialized areas were likely added, providing new functions for more complex analysis including cognitive specialization (Weaver 2005).
Selection acting on physical endurance capacity and subsequent increases in cerebral perfusion and O2 delivery may have been the primordial stimulus for accelerated neurotrophin and growth factor signaling that may have contributed to overall brain growth and development as early as 1.8 Myr when our ancestors in particular Homo Erectus began walking and running longer distances than previous hominin taxa (Raichlen and Polk 2013). Given that brain tissue is metabolically expensive (see below), such disproportional increases in brain volume would not likely have occurred unless they conferred some sort of adaptive (reproductive, social, cognitive, ecological and health) benefits though the finer details remain unresolved. These benefits are especially pertinent in modern times since physical activity maintained across the human lifespan is known to be neuroprotective with the capacity to improve cerebral perfusion, vasoreactivity and thus by consequence O2 and glucose delivery, delaying cognitive decline and dementia in an increasingly aged population (Bailey et al. 2013; Burley et al. 2016; Wolters et al. 2017; Bailey et al. 2018a).
Vulnerability to failure
Today, the “modern” human brain exemplifies our reliance on O2 because, unlike most other organs, this evolutionary “drive for size” has meant that it is now committed to a continually active state and is entirely aerobic since it does not store glucose or much glycogen constrained by a relatively low capillary density and thus relies on a constant blood supply (Bailey 2016; Bailey et al. 2017b). Though it weighs a meagre 2% of our total body mass and demands 15% of the body’s cardiac output, the human brain allocates a disproportionate 20–25% of total resting metabolic rate to brain function (Attwell et al. 2010) compared with 8–10% for non-human primates and 3–5% for most non-primate mammals (Leonard et al. 2003). Assuming an average brain mass of 1.4 kg, O2 is consumed at a rate of ~ 1.5 mmol/min/g tissue or ~ 3 mol of O2/day, generating a staggering ~ 18 mol or ~ 9 kg of ATP/day (Fig. 2b). To put this into clearer perspective, this is roughly equivalent to what a human leg muscle would generate during a marathon (Attwell and Laughlin 2001).
This equates to more than 10 times that expected from its mass alone helping power its ~ 86 billion neurons (Herculano-Houzel 2012) and complex connectome spanning up to 1015 synapses with over 100,000 km of interconnections and ~ 250–300 billion glia capable of storing anywhere between 58 and 580 terabytes of information (Nunn et al. 2016). This obligatory requirement to process large amounts of O2 over a relatively small tissue mass is required to support the high rate of ATP formation to fuel the maintenance of ionic equilibria and uptake of neurotransmitters for synaptic transmission with 40–60% of this energy directed towards moving ions “uphill” with the majority of energy supplied by mitochondria and consumed at the synapses (Alle et al. 2009; Harris et al. 2012). This is even more paradoxical when one considers that lineages with large brains generally exhibit poor hypoxia tolerance, hence one would have expected O2 constraints to have constrained the evolution of large brain size (Sukhum et al. 2016) and indeed average endocranial volume has decreased by 240 mL during the Holocene (past 10,000 years), ~ 36 times greater than the rate of increase observed during the previous 800,000 years (Henneberg 1988).
However, this obligatory high rate of O2 consumption is associated with high “vulnerability for failure” given the brain’s paradoxically limited O2 reserves. Assuming an average cerebral tissue partial pressure of O2 (PcO2) of ~ 25 mmHg and lack of O2-binding proteins, the brain’s O2 content is a meagre ~ 30 nmoL/mL such that given an average cerebral metabolic rate of oxygen (CMRO2) of 30 nmoL/mL/s, the O2 present would sustain metabolism for at best 1 s if blood supply were to be interrupted by anoxia (Leithner and Royl 2014) (Fig. 2b). Unable to compromize on its excessive energy budget, failure of ATP-dependent ion exchangers results in the breakdown of ionic gradients and membrane depolarization triggering a cytotoxic increase in intracellular Ca2+ concentration and uncontrolled release of excitatory neurotransmitters that ultimately converge in neuronal death (Lipton 1999). This can result in devastating consequences, as the clinical complications associated with stroke and head trauma stand testament to.
The paradox of O2; quandry of quantum quirkiness
Despite it’s early appearance, the discovery of O2described as “the most important discovery in the history of science” had to wait until 1774 when Joseph Priestley (1733–1804) first described the existence of “dephlogisticated air” by heating mercuric oxide though this remains a hotly contested topic given that the gas had been purified and used to sustain human life and exercise by both a Polish alchemist (Michał Sędziwój, 1566–1636) and Dutch engineer (Cornelis Jacobszoon Drebbel, 1572–1633) some two centuries earlier. Priestley marvelled at its magical properties, capable of reigniting an ember of wood and increasing the survival of mice in a closed container although the luckless Carl Wilhelm Scheele (1742–1786) had produced the gas (“fire-air”) earlier and Antoine Laurent Lavoisier (1743–1794) provided a more informed description of the true nature of O2 naming it “oxigene” that had eluded Priestley who remained wedded to the “phlogiston theory” (West 2014). But before we consider how the brain senses the “elixir of life” and the neuroprotective mechanisms that collectively serve to preserve homeostasis when faced by the challenge of O2 lack (hypoxia), it is important to remind ourselves that our fundamental need for O2 obscures the fact that it is a toxic, mutagenic gas; deadly to the central nervous system (CNS) when in excess, yet paradoxically capable of sustaining life in physiologically controlled amounts.
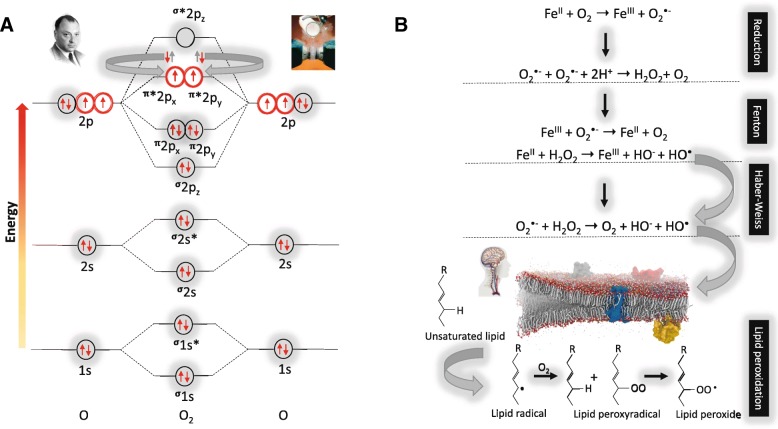
Unlike the majority of stable molecules with all of their electrons housed as “spin opposed” pairs conforming with the Pauli Exclusion Principle (+½ + −½ denoted as ↑↓) (Fig. 3a, upper left insert), a closer examination of its molecular orbital structure, reveals that triplet ground state (most stable) diatomic O2 molecule (3∑g−O2) exists in air as a free (di)radical (Fig. 3a) (Bailey 2003; Bailey et al. 2009). Technically speaking, we should refer to this gas as O2• [superscript dot denotes (2) unpaired electron(s)] and not simply O2 since we’re choosing to ignore its most fascinating attribute! A lone electron is located in separate π*2p antibonding orbitals with the same spin quantum or spin states (+½ or ↑↑) consistent with Hund’s rule (Hund 1925). This molecular peculiarity renders O2 paramagnetic allowing it to respond to a magnetic field, a property routinely exploited in numerous medical devices including oximeters, near infra-red spectrometers, magnetic resonance imaging and laboratory demonstrations whereby liquid O2 is able to hang “suspended” when poured between the poles of a magnet (Fig. 3a, upper right insert).
Fig. 3.
a Molecular orbital diagram of the most stable form (electronic ground state) of the diatomic oxygen molecule (3∑g−O2) and b. Biological reactions underpinning oxygen toxicity. a Each line represents a molecular orbital and the arrows represent electrons, the direction of which indicates their spin quantum number. Note that oxygen (O2) with an electronic structure of 1s22s22p4 qualifies as a di-radical since it contains two unpaired electrons each occupying different π*2p anti-bonding orbitals (highlighted in red) with the same spin quantum number (parallel spin) in accordance with Hund’s rule. It is for this reason that O2 is paramagnetic allowing liquid O2 to hang magically suspended between the poles of a magnet (upper right insert). During the process of oxidation when O2 looks to accept a (spin opposed) pair of electrons (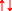 ), only one of the pair (
), only one of the pair ( ) can “fit” into each of the vacant π*2p anti-bonding orbitals to create a spin opposed pair (as indicated). Hence, O2 thermodynamically prefers to accept only one electron at a time to conform with the Pauli Exclusion Principle [named after the Nobel Prize winning work of the Austrian physicist Wolfgang Pauli (1900–1958), photograph upper left insert]. Fortuitously, this “spin restriction” means that O2 reacts “sluggishly” with the brain’s organic compounds with the organic donor having to undergo a “slow spin inversion’” to donate its electrons. b Three types of reactions lead to the superoxide anion (O2•−)-mediated formation of the damaging hydroxyl radical (HO•) capable of causing indiscriminate damage to biological cell membranes that characterizes O2 toxicity; [1] one-electron reduction of molecular O2 to O2•− catalyzed by transition metals including iron (Fe), [2] Fenton reactions that involve metal-catalyzed formation of HO• and [3] Haber-Weiss reaction involving the combination of O2•− and hydrogen peroxide (H2O2) to yield additional HO•
) can “fit” into each of the vacant π*2p anti-bonding orbitals to create a spin opposed pair (as indicated). Hence, O2 thermodynamically prefers to accept only one electron at a time to conform with the Pauli Exclusion Principle [named after the Nobel Prize winning work of the Austrian physicist Wolfgang Pauli (1900–1958), photograph upper left insert]. Fortuitously, this “spin restriction” means that O2 reacts “sluggishly” with the brain’s organic compounds with the organic donor having to undergo a “slow spin inversion’” to donate its electrons. b Three types of reactions lead to the superoxide anion (O2•−)-mediated formation of the damaging hydroxyl radical (HO•) capable of causing indiscriminate damage to biological cell membranes that characterizes O2 toxicity; [1] one-electron reduction of molecular O2 to O2•− catalyzed by transition metals including iron (Fe), [2] Fenton reactions that involve metal-catalyzed formation of HO• and [3] Haber-Weiss reaction involving the combination of O2•− and hydrogen peroxide (H2O2) to yield additional HO•
When O2 attempts to oxidize another atom or molecule by accepting a spin opposed pair of electrons from it (↑↓), one of the electrons in the pair with a spin state opposite to that of the unpaired electron in O2 would “fit” comfortably into the orbital, to create a spin-opposed pair (↑↓, bold arrow denotes the accepted electron). However, this would not be the case with the other electron given its parallel spin state (↑↑), thus preventing it from “pairing up” in accordance with the Pauli Exclusion Principle. Thus, unlike most other oxidizing free radical species, this parallel spin renders O2 less reactive at “normal” concentrations despite its powerful oxidizing nature (Halliwell and Gutteridge 1984; Fridovich 2013). This “spin-restriction” forces O2 to accept its electrons one at a time, a thermodynamic quirk of fate that protects the C − H bonds of the brain’s organic biomolecules from spontaneous combustion (Bailey et al. 2009). It is the unusual combination of strong π bonding (remarkably high resonance stabilization energy of 100 kcal/mol) and weak σ bonding in •OO• that enables this unique molecule to be abundant in Earth’s atmosphere and provide the chemical energy to sustain aerobic life (Borden et al. 2017), safely!
Janus face of O2; too much of a good thing can kill you
Paradoxically however, this gas and products of its metabolism becomes toxic at elevated PO2’s, an original observation credited to Priestley who noted that a candle burned out faster in O2 than in air, speculating that we humans may “...live out too fast, and the animal powers be too soon exhausted in this pure kind of air. A moralist, at least, may say, that the air which nature has provided for us is as good as we deserve” (Priestley 1776). Further elaborations were provided by Paul Bert (1833–1886) who, in 1878, described convulsions in larks when exposed to 15–20 atm, a response that subsequently became known as the “Bert Effect” (Bert 1943). In modern times, supplemental O2 (hyperoxia) is commonly used as part of the therapy of many circulatory disorders yet it is well known that the gas can exert toxic effects when not used judiciously, damaging the CNS, eyes and lungs.
However, it wasn’t until 1954 that the damaging of effects of O2 toxicity were eventually linked to free radical formation (Gerschman et al. 1954), more specifically increased mitochondrial formation of the univalent reductant, the superoxide anion (O2•−) (Chance et al. 1979) (Fig. 3b). Though not especially “super” [one electron reduction potential (EO΄) = + 940 mV], O2•- can be converted to hydrogen peroxide (H2O2) through reduction or dismutation and upon reaction with transition metal ions, ultimately forming the hydroxyl radical (OH•). This species is at the top of the free radical “league of reactivity” (EO΄ = + 2310 mV), thermodynamically capable of oxidizing any biomolecule that it collides with at a rate constant very near the diffusion limit (Buettner 1993).
Contemporary physiology has taught us the conceptual significance of the “O2-cascade”, highlighting that the ever-decreasing PO2 gradient serves to provide a “pressure-head” to maintain diffusive O2 flux driving the gas from the capillary into the (cerebral) mitochondrion (Wagner 1996). But perhaps we need to consider an alternative viewpoint; the endogenous resistances offered to O2 transport (i.e. the sequential, progressive reduction in PO2) may have evolved as an alternative form of endogenous antioxidant defense, limiting the concentration of (toxic) O2 to which the mitochondrion is exposed (P50 for PO2-dependant mitochondrial O2 consumption < 1 mmHg), given its inherent vulnerability to oxidative damage and corresponding respiratory dysfunction (Hill et al. 2018). The fact that the Michaelis constant (Km) of the terminal reductant, cytochrome c oxidase, for O2 is so extraordinarily low (0.03–0.3 mmHg) (Vanderkooi et al. 1991) stands testament to how important it is to harness this molecule and maintain cellular PO2 within “safe” manageable physiological limits. Indeed, increasing O2 levels equivalent to the conditions typically encountered in most isolated mitochondrial studies amplifies uncoupled mitochondrial proton leak and oxidative stress reducing bioenergetic efficiency (Gnaiger et al. 2000).
The brain and oxidative stress; bittersweet balance
Our reliance on this toxic gas is matched by an equally fascinating fact in that despite its limited regenerative capacity, the brain is poorly equipped to cope with these potentially damaging O2-induced free radical reactions. Nervous tissue seems to out-perform other tissues in that it is capable of generating more O2•− with antioxidant defenses that are modest at best and neuronal membrane lipids rich in eicosapentaenoic (C20:5) and docosahexaenoic (C22:6) polyunsaturated fatty-acid side chains are especially susceptible to lipid peroxidation (Bailey 2003; Bailey et al. 2009; Cobley et al. 2018).
Indeed, second to adipose tissue, nerve tissue contains the highest concentration of these highly peroxidizable lipids. Furthermore, a dense network of mitochondria exposed to high mass-specific O2 flux, an abundance of autoxidizable neurotransmitters, cytochrome P450 and reactive microglia also serve to compound O2•− formation. Excitotoxic amino acids, highly active neuronal Ca2+ trafficking, excessive glucose/glutamate uptake and enrichment of redox-active transition metals with the capacity to catalyze Fenton/Haber–Weiss-driven generation of OH• to initiate neuronal apoptosis and further compound membrane destabilization and vascular damage further contribute to the brain’s ‘oxidant burden sensitizing it’s potential to damage (Bailey 2003; Bailey et al. 2009; Cobley et al. 2018). This, however, is not as much of a paradox as was once thought (see later).
Given that its O2 supply is so delicate coupled with its limited ability to contain these potentially damaging free radical chain reactions, it would seem intuitive for evolution to favor feedback mechanisms capable of sensing subtle changes in hypoxia and orchestrating transmission of signals to the cerebrovasculature coupling local cerebral O2 delivery (CDO2) to tissue metabolic demand such that cerebral homeostasis remains preserved consistent with the conservation of mass principle (Bailey et al. 2017b). Indeed, evolution appears to have perfected this millions of years ago with the emergence of anoxia-tolerant vertebrates such as the freshwater turtle (Trachemys scripta and Chrysemys picta) and the crucian carp (Carassius carassius) who can effectively negotiate brain survival through specializations of brain physiology despite days to weeks of anoxia, entering into a state of deep hypometabolism and suppression of cellular injury during anoxia-reoxygenation (Nilsson and Lutz 2004; Larson et al. 2014).
Cerebral O2 sensing
Given the evolutionary importance of O2 for the maintenance of complex life, it is likely that the ability to “sense” subtle changes in PO2 and mount a defense against metabolic compromize and/or structural damage was one of the first roles of the CNS and likely represented a major driving force in the evolution of the human brain, thus providing a selective advantage (Costa et al. 2014). Indeed, the CNS regulates neural activity of the cardiovascular and respiratory systems that are located almost exclusively in the brainstem, one the most primitive neuroanatomical regions of the human brain (~ 300 Mya) that has remained highly conserved across vertebrate evolution (Northcutt 2002). It is becoming increasingly clear that an inability to sense O2 adequately has been implicated in the pathophysiology of a variety of CNS disorders including stroke, head trauma, neoplasia, vascular malformations and neurodegenerative diseases, highlighting its clinical importance (Sharp and Bernaudin 2004).
Systemic hypoxia is acutely sensed by central (carotid body) and peripheral (pulmonary arteries, ductus arteriosus, adrenal medulla, neuroepithelial bodies in the lung) chemoreceptors that initiate cardiorespiratory reflexes that collectively serve to improve pulmonary gas exchange and cerebral O2 delivery (Sharp and Bernaudin 2004; Weir et al. 2005). A key regulatory role has been assigned to the red blood cell including its ability to autonomously regulate its own deformability and flow velocity through capillaries (Wei et al. 2016) with hemoglobin implicated as the hypoxic sensor capable of releasing vasoactive metabolites from neurons, astrocytes, pericytes and smooth muscle cells (Singel and Stamler 2005). While numerous mediators including β-adrenergic receptor activation, prostaglandins, epoxyeicosatrienoic acids, ATP-sensitive potassium channels, adenosine, free radicals and associated reactive oxygen/nitrogen species have been proposed, considerable evidence supports an increasingly important role for nitric oxide with the stable metabolites nitrite and S-nitrosohemoglobin widely contested given their ability to conserve and transfer bioactivity within the microcirculation (Stamler et al. 1997; Cosby et al. 2003; Bailey et al. 2017a).
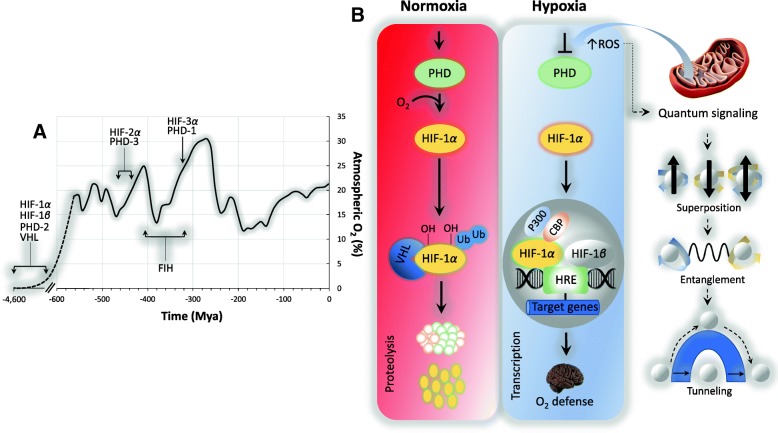
Longer term adjustments are achieved through differential regulation of the highly conserved transcriptional complex hypoxia-inducible factor (HIF), whose complexity has increased in tandem with the evolution of ever-more sophisticated O2 transport systems and rising atmospheric O2 levels (Taylor and McElwain 2010) (Fig. 4a). HIF-1 consists of an oxygen-sensitive HIF-1 alpha (α) subunit that heterodimerizes with the HIF-1beta (β) subunit to bind DNA. In normoxia, HIF-1α is oxidized (hydroxylated) by prolyl hydroxylases (PHDs) using α-ketoglutarate. The hydroxylated HIF-1α subunit interacts with the von Hippel–Lindau protein and is subsequently catabolized by proteasomes, such that HIF-1α is continuously synthesized and degraded. However, in hypoxia, HIF-1α hydroxylation does not occur, stabilizing HIF-1 where it binds to a hypoxia response element leading to the expression of a wide variety of genes involved in angiogenesis, cell proliferation, erythropoiesis, glucose transport, glycolytic metabolism and cell survival (Semenza 2007; Ratcliffe 2013).
Fig. 4.
a Evolution of genes encoding the hypoxia-inducible factor (HIF) pathway and (b). Importance of mitochondrial-generated reactive oxygen species (ROS) stabilization of HIF-1α during hypoxia including emergent quantum signaling aspects. a Appearance of genes based on published approximations (Taylor and McElwain 2010). b During normoxia, hypoxia-inducible factor-1 alpha (HIF-1α) is hydroxylated on prolines by the prolyl hydroxylases (PHD), tagging it for recognition by the von Hippel Lindau tumor suppressor protein (VHL) resulting in the continual ubiquitination and degradation of HIF-1α. During hypoxia, the mitochondrial formation of the superoxide anion from the Qo site of the bc1 complex of Complex III are released into the intermembrane space and enter the cytosol to decrease PHD activity preventing hydroxylation resulting in HIF-1α stabilization and transcription of genes that collectively preserve cerebral oxygen (O2) homeostasis. Note that emerging “quantum” aspects of cerebral O2 sensing are also outlined. FIH, factor inhibiting HIF; CBP, cyclic AMP-response element binding protein; HRE, hypoxia response element
While recent advances have revealed the molecular underpinnings of this highly conserved pathway, a hotly debated topic relates to the precise molecular identity of the O2 sensor (Kemp 2006). While numerous models have been proposed (Neubauer and Sunderram 2004), accumulating evidence suggests a central role for the mitochondrion which makes intuitive sense given its intimate relationship with O2 and fact that cytochrome aa3 represents its terminal acceptor. It has been suggested that cellular hypoxia increases ROS formation due to distal obstruction of the electron transport chain and retrograde accumulation of electrons leading to autoxidation. More specifically, hypoxia triggers O2•− formation from Complex III by increasing ubisemiquinone lifetime at the outer ubiquinone binding site (Qo) site with release to the intermembrane space and subsequent formation of hydrogen peroxide triggering HIF-α stabilization subsequent to PHD inactivation potentially related to phosphorylation or decreased bioavailability of Fe (II) (Chandel et al. 1998; Bell et al. 2007; Smith et al. 2017) (Fig. 4b). However, this theory is not without its critics and remains a source of ongoing debate (Ward 2006; Weir and Archer 2006), considered by some as controversial if not indeed counterintuitive (↓O2 → ↓electron flux/uncoupled leakage) with evidence supporting a more direct link between molecular O2 and PHD inhibition/HIF activation (Dunham-Snary et al. 2016).
Importantly however, the ability to respond to subtle changes in ambient oxygenation using O2•− as an ancient signal transductant in addition to protection against oxidative stress was present even in the last universal common ancestor (LUCA), a genetically and metabolically diverse community containing the molecular origins of all present life forms estimated to have appeared ~ 3.8 Gya (Slesak et al. 2012; Briehl 2015). The evidence that LUCA was able to use O2•− as an ancient signal transductant is based on accumulating gene and protein sequences from organisms that belong to the three domains of life (Archaea, Bacteria, Eukaryotes) allowing for reconstruction of the cellular processes that the protocell likely exploited (Woese et al. 1990). The fact that SOD, catalase and peroxiredoxins have been observed in organisms from all three domains of life (Kornas et al. 2010) combined with ROS-detoxifying reactions identified in strict anerobes (Slesak et al. 2012) implies that LUCA was likely an O2-sensing (potentially via O2•− and H2O2)/ROS-tolerant organism equipped with a primordial enzymatic antioxidant system that evolved prior to the photosynthetic rise in atmospheric O2 (Briehl 2015; Slesak et al. 2016). Combined with the emerging concept of oxidative hormesis, it is becoming increasingly clear that at physiological concentrations, free radicals and associated ROS have the adaptive capacity to preserve cerebral O2 homeostasis through cell-cell communication and should not simply be constrained to toxic, mutagenic “accidents” of in-vivo chemistry limited to cellular oxidative damage and pathophysiology (Bailey et al. 2018b).
Redox signaling; quantum in the quotidian
Since the brain’s evolution and ongoing survival depends on its constancy of electron flow, it would be remiss not to make albeit brief reference to quantum neuroscience, an emerging discipline focused at the biological quantum/classical interface, that promises to offer unique insight into the finer details of O2 sensing that classical approaches otherwise fail to explain. Erwin Schrödinger (1887–1961) famous for his wave equation for non-relativistic quantum mechanics (QM) given by: ĤIψ > = EIψ > (time-independent equation where Ĥ = Hamiltonian operator, E = energy and ψ = wave function that describes velocity or location of a particle) was the first to ask if biological systems harness QM to perform a task more efficiently than even the best classical equivalent for selective advantage (Schrödinger 1944). Initially met with fierce resistance given such seemingly counterintuitive concepts as (quantum) superposition (a particle can be in two places at once and exist in different states, both as a particle and a wave), entanglement (two particles at a distance form a relationship) and tunnelling (a particle can pass through a solid object) and challenges posed by the impossibly warm, wet brain that collapses coherence (and hence QM effects), emerging evidence now suggests that there may well be some cases in which QM does indeed provide a biological advantage (Wolynes 2009; Ball 2011; Lambert et al. 2013).
QM appears to be exploited by Nature during avian navigation, olfaction and arguably the best described of all, light harvesting in photosynthesis allowing excitons, generated by ancient green, sulfur-breathing bacteria, to travel as a coordinated wave rather than (classically) as a simple straight line, “feeling out” the most efficient pathway to transport energy to the plant’s reaction center within a staggeringly short, 10− 9 s, achieving close to 100% efficiency (Thyrhaug et al. 2018). Could the mitochondrial formation of free radicals, themselves sub-atomic species, exploit quantum-based signaling to preserve cerebral O2 homeostasis? Preliminary evidence suggests that this may well be the case with formation of “spin-correlated radical pairs” mediated by weak magnetic fields and evidence for mitochondrial electron tunnelling and entanglement (Usselman et al. 2014; Nunn et al. 2016; Usselman et al. 2016) forcing a reappraisal of currently (i.e. classically) accepted concepts revealing more complex cellular and molecular mechanisms than previously thought (Fig. 4b).
Conclusion
The current review has explored the intimate relationship between rising atmospheric O2 levels and evolution of life on Earth and the brain. The modern day human has evolved with an oversized brain exquisitely vulnerable to failure given that it is entirely reliant on O2, a toxic, mutagenic free radical gas that exists in air as a diradical, deadly in excess yet paradoxically capable of sustaining life in controlled physiological amounts. By further exploring O2’s “quantum quirkiness”, our understanding of precisely how the human brain senses hypoxia and the elaborate redox-signaling defense mechanisms that emerging evidence suggests may harness QM to preserve O2 homeostasis has the potential to offer unique insights into the pathophysiology and treatment of human brain disease.
Acknowledgements
I would like to thank Professors Jim Al-Khalili (Department of Physics, University of Surrey, UK), Peter D Wagner (Department of Medicine, University of California at San Diego, California, USA), Joe M McCord (Department of Medicine, Division of Pulmonary Science and Critical Care Medicine, University of Colorado at Denver, Denver, CO, USA) and Irwin Fridovich (Department of Biochemistry, Duke University Medical Center, Durham, NC, USA) for critical discussion.
Funding
Supported by a Royal Society Wolfson Research Fellowship (#WM170007).
Abbreviations
- 3Σg-O2
Triplet ground state oxygen
- ATP
Adenosine triphosphate
- C
Carboniferous
- CBP
Cyclic adenosine monophosphateresponse element binding protein
- CDO2
Cerebral oxygen delivery
- CMRO2
Cerebral metabolic rate of oxygen
- CNS
Central nervous system
- cO2
Cerebral oxygen content
- Cu,Zn/Mn SOD
Copper, zinc/manganese superoxide dismutase
- D
Devonian
- E
Energy
- EO’
One electron reduction potential
- FIH
Factor inhibiting hypoxia-inducible factor
- GOE
Great oxidation event
- Gya
Billion years ago
- H2O2
Hydrogen peroxide
- HIF
Hypoxia-inducible factor
- HIF-1α/β
Hypoxia-inducible factor alpha/beta
- HRE
Hypoxia response element
- J
Jurassic
- K
Cretaceous
- Km
Michaelis constant
- LUCA
Last universal common ancestor
- Mya
Million years ago
- NOE
Neoproterozoic event
- O
Ordovician
- O2•-
Superoxide anion
- O2/O2•
Oxygen
- OH•
Hydroxyl radical
- P
Permian
- P50
Partial pressure of oxygen required to achieve 50% hemoglobin saturation
- PcO2
Cerebral tissue partial pressure of oxygen
- PHD
Prolyl hydroxylase
- Pre-Є
Pre-Cambrian
- QM
Quantum mechanics
- S
Silurian
- T
Tertiary
- Tr
Triassic
- VHL
Von Hippel Lindau tumor suppressor protein
- ψ
Wave function
- Є
Cambrian
- ĤIψ
Hamiltonian operator
Authors’ contributions
The author read and approved the final manuscript.
Authors’ information
DMB embarked on a PhD in human physiology while working as the first research physiologist at the British Olympic Medical Centre, UK in collaboration with the Department of Biochemistry at Oxford University, UK (Prof EA Newsholme). A former international athlete, he was interested in the factors that limit systemic oxygen transport and the implications for human exercise performance. Following training at the Department of Medicine, University of California San Diego, USA (Drs PD Wagner, JB West, RS Richardson) and Departments of Surgery and Anesthesiology, University of Colorado Health Sciences Center, USA (Drs JM McCord, JT Reeves), he returned to the University of South Wales where he is currently employed as a Royal Society Wolfson Research Fellow and Professor of Physiology and Biochemistry leading the Neurovascular Research Laboratory. His research is “quantum superimposed” between two topics; how free radicals control oxygen delivery to the brain and the cerebrovascular complications associated with sedentary ageing.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares he has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
This article has been retracted at the request of the author due to significant overlap with one of his other publications without proper citation. This article is considered redundant. The author agrees to this retraction.
Change history
6/20/2019
This article [1] has been retracted at the request of the author due to significant overlap with one of his other publications [2] without proper citation. This article is considered redundant. The author agrees to this retraction.
References
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Allred AL, Rochow EG. A scale of electronegativity based on electrostatic force. J Inorg Nucl Chem. 1958;5:264–268. [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REJ, Leite REP, Filho WJ, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Bailey DM. The last “oxygenless” ascent of Mt. Everest. Br J Sports Med. 2001;35:294–296. doi: 10.1136/bjsm.35.5.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM. Radical dioxygen: from gas to (unpaired!) electrons. Adv Exp Med Biol. 2003;543:201–221. [PubMed] [Google Scholar]
- Bailey DM. The brain in hypoxia; curiosity, cause and consequence. Exp Physiol. 2016;101(9):1157–1159. doi: 10.1113/EP085857. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Bartsch P, Knauth M, Baumgartner RW. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: from the molecular to the morphological. Cell Mol Life Sci. 2009;66:3583–3594. doi: 10.1007/s00018-009-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke. 2013;44:3235–3238. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Owens TS, Calverley TA. Letter by bailey et al regarding article, “cerebral perfusion and the risk of dementia: a population-based study”. Circulation. 2018;137:1414–1415. doi: 10.1161/CIRCULATIONAHA.117.030573. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Rasmussen P, Evans KA, Bohm AM, Zaar M, Nielsen HB, Brassard P, Nordsborg NB, Homann PH, Raven PB, McEneny J, Young IS, McCord JM, Secher NH. Hypoxia compounds exercise-induced free radical formation in humans; partitioning contributions from the cerebral and femoral circulation. Free Radic Biol Med. 2018;124:104–113. doi: 10.1016/j.freeradbiomed.2018.05.090. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Rasmussen P, Overgaard M, Evans KA, Bohm AM, Seifert T, Brassard P, Zaar M, Nielsen HB, Raven PB, Secher NH. Nitrite and S-Nitrosohemoglobin exchange across the human cerebral and femoral circulation: relationship to basal and exercise blood flow responses to hypoxia. Circulation. 2017;135:166–176. doi: 10.1161/CIRCULATIONAHA.116.024226. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Willie CK, Hoiland RL, Bain AR, MacLeod DB, Santoro MA, DeMasi DK, Andrijanic A, Mijacika T, Barak OF, Dujic Z, Ainslie PN. Surviving without oxygen: how low can the human brain go? High Alt Med Biol. 2017;18:73–79. doi: 10.1089/ham.2016.0081. [DOI] [PubMed] [Google Scholar]
- Ball P. Physics of life: the dawn of quantum biology. Nature. 2011;474:272–274. doi: 10.1038/474272a. [DOI] [PubMed] [Google Scholar]
- Beerling DJ, Woodward FI, Lomas MR, Wills MA, Qucik MP, Valdes PJ. The influence of carboniferous palaeoatmospheres on plant function: an experimental and modelling assessment. Philos Trans R Soc B Biol Sci. 1998;353:131–139. [Google Scholar]
- Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner RA. GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim Cosmochim Acta. 2007;70:5653–5664. [Google Scholar]
- Berner RA. Phanerozoic atmospheric oxygen: new results using the geocarbsulf model. Am J Sci. 2009;309:603–606. [Google Scholar]
- Berner RA, Vandenbrooks JM, Ward PD. Evolution. Oxygen and evolution. Science. 2007;316:557–558. doi: 10.1126/science.1140273. [DOI] [PubMed] [Google Scholar]
- Bert P. Barometric pressure. Researches in Experimental Physiology. Columbus, Ohio: College Book Company; 1943. [Google Scholar]
- Borden WT, Hoffmann R, Stuyver T, Chen B. Dioxygen: what makes this triplet Diradical kinetically persistent? J Am Chem Soc. 2017;139:9010–9018. doi: 10.1021/jacs.7b04232. [DOI] [PubMed] [Google Scholar]
- Briehl MM. Oxygen in human health from life to death--an approach to teaching redox biology and signaling to graduate and medical students. Redox Biol. 2015;5:124–139. doi: 10.1016/j.redox.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- Burley Claire V., Bailey Damian M., Marley Christopher J., Lucas Samuel J. E. Brain train to combat brain drain; focus on exercise strategies that optimize neuroprotection. Experimental Physiology. 2016;101(9):1178–1184. doi: 10.1113/EP085672. [DOI] [PubMed] [Google Scholar]
- Burn R, Misson L, Meury M, Seebeck FP. Anaerobic origin of Ergothioneine. Angew Chem Int Ed Engl. 2017;56:12508–12511. doi: 10.1002/anie.201705932. [DOI] [PubMed] [Google Scholar]
- Case Adam. On the Origin of Superoxide Dismutase: An Evolutionary Perspective of Superoxide-Mediated Redox Signaling. Antioxidants. 2017;6(4):82. doi: 10.3390/antiox6040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- Costa KM, Accorsi-Mendonca D, Moraes DJ, Machado BH. Evolution and physiology of neural oxygen sensing. Front Physiol. 2014;5:302. doi: 10.3389/fphys.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Selfridge AC, Sporn PH, Sznajder JI, Taylor CT. Carbon dioxide-sensing in organisms and its implications for human disease. Cell Mol Life Sci. 2014;71:831–845. doi: 10.1007/s00018-013-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole M. The natural history of oxygen. J Gen Physiol. 1965;49(Suppl):5–27. doi: 10.1085/jgp.49.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Snary KJ, Hong ZG, Xiong PY, Del Paggio JC, Herr JE, Johri AM, Archer SL. A mitochondrial redox oxygen sensor in the pulmonary vasculature and ductus arteriosus. Pflugers Archiv. 2016;468:43–58. doi: 10.1007/s00424-015-1736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Evidence for the symbiotic origin of mitochondria. Life Sci. 1974;14:819–826. doi: 10.1016/0024-3205(74)90071-x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Oxygen: how do we stand it? Med Princ Pract. 2013;22:131–137. doi: 10.1159/000339212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- Gilbert-Kawai ET, Milledge JS, Grocott MP, Martin DS. King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology (Bethesda) 2014;29:388–402. doi: 10.1152/physiol.00018.2014. [DOI] [PubMed] [Google Scholar]
- Gnaiger E, Mendez G, Hand SC. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci U S A. 2000;97:11080–11085. doi: 10.1073/pnas.97.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JB, Dudley R, Aguilar NM, Gans C. Implications of the late paleozoic oxygen pulse for physiology and evolution. Science. 1995;375:117–120. [Google Scholar]
- Gray Michael W, Burger Gertraud, Lang B Franz. Genome Biology. 2001;2(6):reviews1018.1. doi: 10.1186/gb-2001-2-6-reviews1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjistassou C., Bejan A., Ventikos Y. Cerebral oxygenation and optimal vascular brain organization. Journal of The Royal Society Interface. 2015;12(107):20150245–20150245. doi: 10.1098/rsif.2015.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Laporte N, Mawatari K, Ellis RS, Inoue AK, Zackrisson E, Roberts-Borsani G, Zheng W, Tamura Y, Bauer FE, Fletcher T, Harikane Y, Hatsukade B, Hayatsu NH, Matsuda Y, Matsuo H, Okamoto T, Ouchi M, Pello R, Rydberg CE, Shimizu I, Taniguchi Y, Umehata H, Yoshida N. The onset of star formation 250 million years after the big bang. Nature. 2018;557:392–395. doi: 10.1038/s41586-018-0117-z. [DOI] [PubMed] [Google Scholar]
- Henneberg M. Decrease of human skull size in the Holocene. Hum Biol. 1988;60:395–405. [PubMed] [Google Scholar]
- Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RL, Kulbe JR, Singh IN, Wang JA, Hall ED. Synaptic mitochondria are more susceptible to traumatic brain injury-induced oxidative damage and respiratory dysfunction than non-synaptic mitochondria. Neuroscience. 2018;386:265–283. doi: 10.1016/j.neuroscience.2018.06.028. [DOI] [PubMed] [Google Scholar]
- Holland HD. Volcanic gases, black smokers, and the great oxidation event. Geochim Cosmochim Acta. 2002;66:3811–3826. [Google Scholar]
- Holland LZ, Carvalho JE, Escriva H, Laudet V, Schubert M, Shimeld SM, Yu JK. Evolution of bilaterian central nervous systems: a single origin? Evodevo. 2013;4:27. doi: 10.1186/2041-9139-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund F. Zur deutung verwickelter spektren, insbesondere der elemente scandium bis nickel. Z Phys. 1925;33:855. [Google Scholar]
- Keeling RF. Development of an interferometric oxygen analyzer for precise measurement of the atmospheric O2 mole fraction. Cambridge, Massachusetts: Harvard University; 1988. [Google Scholar]
- Kemp PJ. Detecting acute changes in oxygen: will the real sensor please stand up? Exp Physiol. 2006;91:829–834. doi: 10.1113/expphysiol.2006.034587. [DOI] [PubMed] [Google Scholar]
- Kornas A, Kuzniak E, Slesak I, Miszalski Z. The key role of the redox status in regulation of metabolism in photosynthesizing organisms. Acta Biochim Pol. 2010;57:143–151. [PubMed] [Google Scholar]
- Lambert N, Chen YN, Cheng YC, Li CM, Nori F. Quantum biology. Nat Phys. 2013;9:10–18. [Google Scholar]
- Larson J, Drew KL, Folkow LP, Milton SL, Park TJ. No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. J Exp Biol. 2014;217:1024–1039. doi: 10.1242/jeb.085381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithner C, Royl G. The oxygen paradox of neurovascular coupling. J Cereb Blood Flow Metab. 2014;34:19–29. doi: 10.1038/jcbfm.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WR, Robertson ML, Snodgrass JJ, Kuzawa CW. Metabolic correlates of hominid brain evolution. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:5–15. doi: 10.1016/s1095-6433(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Livina VN, Martins TM, Forbes AB. Tipping point analysis of atmospheric oxygen concentration. Chaos. 2015;25:036403. doi: 10.1063/1.4907185. [DOI] [PubMed] [Google Scholar]
- Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth's early ocean and atmosphere. Nature. 2014;506:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- Martin D, McKenna H, Livina V. The human physiological impact of global deoxygenation. J Physiol Sci. 2017;67:97–106. doi: 10.1007/s12576-016-0501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslin MA, Christensen B. Tectonics, orbital forcing, global climate change, and human evolution in Africa: introduction to the African paleoclimate special volume. J Hum Evol. 2007;53:443–464. doi: 10.1016/j.jhevol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- McCord JM, Keele BB, Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971;68:1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Bada JL. Submarine hot springs and the origin of life. Nature. 1988;334:609–611. doi: 10.1038/334609a0. [DOI] [PubMed] [Google Scholar]
- Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol. 2004;96:367–374. doi: 10.1152/japplphysiol.00831.2003. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Lutz PL. Anoxia tolerant brains. J Cereb Blood Flow Metab. 2004;24:475–486. doi: 10.1097/00004647-200405000-00001. [DOI] [PubMed] [Google Scholar]
- Nisbet EG, Sleep NH. The habitat and nature of early life. Nature. 2001;409:1083–1091. doi: 10.1038/35059210. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Understanding vertebrate brain evolution. Integr Comp Biol. 2002;42:743–756. doi: 10.1093/icb/42.4.743. [DOI] [PubMed] [Google Scholar]
- Nunn AV, Guy GW, Bell JD. The quantum mitochondrion and optimal health. Biochem Soc Trans. 2016;44:1101–1110. doi: 10.1042/BST20160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley J. Experiments and observations on different kinds of air. London: Johnson; 1776. [Google Scholar]
- Raichlen DA, Polk JD. Linking brains and brawn: exercise and the evolution of human neurobiology. Proc Biol Sci. 2013;280:20122250. doi: 10.1098/rspb.2012.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol. 2013;591:2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszczycky MW, Liu HW. Biochemistry: the surprising history of an antioxidant. Nature. 2017;551:37–38. doi: 10.1038/551037a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenemann PT. Evolution of the size and functional areas of the human brain. Annu Rev Anthropol. 2006;35:379–406. [Google Scholar]
- Schrödinger EM. What is life? The physical aspect of the living cell. Cambridge: Cambridge University Press; 1944. [Google Scholar]
- Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat Neurosci. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Bosiocic V, Snelling EP. Fossil skulls reveal that blood flow rate to the brain increased faster than brain volume during human evolution. R Soc Open Sci. 2016;3:160305. doi: 10.1098/rsos.160305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- Slesak I, Slesak H, Kruk J. Oxygen and hydrogen peroxide in the early evolution of life on earth: in silico comparative analysis of biochemical pathways. Astrobiology. 2012;12:775–784. doi: 10.1089/ast.2011.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesak I, Slesak H, Zimak-Piekarczyk P, Rozpadek P. Enzymatic antioxidant Systems in Early Anaerobes: theoretical considerations. Astrobiology. 2016;16:348–358. doi: 10.1089/ast.2015.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Waypa GB, Schumacker PT. Redox signaling during hypoxia in mammalian cells. Redox Biol. 2017;13:228–234. doi: 10.1016/j.redox.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J, Jia L, Eu J, McMahon T, Demchenko I, Bonaventura J, Gernet K, Piantadosi C. Blood flow regulation by S-Nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- Sukhum KV, Freiler MK, Wang R, Carlson BA. The costs of a big brain: extreme encephalization results in higher energetic demand and reduced hypoxia tolerance in weakly electric African fishes. Proc Biol Sci. 2016;283. [DOI] [PMC free article] [PubMed]
- Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology. 2010;25:272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- Thyrhaug E, Tempelaar R, Alcocer MJP, Zidek K, Bina D, Knoester J, Jansen TLC, Zigmantas D. Identification and characterization of diverse coherences in the Fenna-Matthews-Olson complex. Nat Chem. 2018;10:780–786. doi: 10.1038/s41557-018-0060-5. [DOI] [PubMed] [Google Scholar]
- Usselman RJ, Chavarriaga C, Castello PR, Procopio M, Ritz T, Dratz EA, Singel DJ, Martino CF. The quantum biology of reactive oxygen species partitioning impacts cellular bioenergetics. Sci Rep. 2016;6:38543. doi: 10.1038/srep38543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usselman RJ, Hill I, Singel DJ, Martino CF. Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLoS One. 2014;9:e93065. doi: 10.1371/journal.pone.0093065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi JM, Erecinska M, Silver IA. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol Cell Physiol. 1991;260:C1131–C1150. doi: 10.1152/ajpcell.1991.260.6.C1131. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol. 1996;58:21–50. doi: 10.1146/annurev.ph.58.030196.000321. [DOI] [PubMed] [Google Scholar]
- Ward JP. Point: hypoxic pulmonary vasoconstriction is mediated by increased production of reactive oxygen species. J Appl Physiol. 2006;101:993–995. doi: 10.1152/japplphysiol.00480.2006. [DOI] [PubMed] [Google Scholar]
- Weaver AH. Reciprocal evolution of the cerebellum and neocortex in fossil humans. Proc Natl Acad Sci U S A. 2005;102:3576–3580. doi: 10.1073/pnas.0500692102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei HS, Kang H, Rasheed ID, Zhou S, Lou N, Gershteyn A, McConnell ED, Wang Y, Richardson KE, Palmer AF, Xu C, Wan J, Nedergaard M. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron. 2016;91:851–862. doi: 10.1016/j.neuron.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EK, Archer SL. Counterpoint: hypoxic pulmonary vasoconstriction is not mediated by increased production of reactive oxygen species. J Appl Physiol. 2006;101:995–998. doi: 10.1152/japplphysiol.00480a.2006. [DOI] [PubMed] [Google Scholar]
- Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB. Joseph Priestley, oxygen, and the enlightenment. Am J Physiol Lung Cell Mol Physiol. 2014;306:L111–L119. doi: 10.1152/ajplung.00310.2013. [DOI] [PubMed] [Google Scholar]
- Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592:841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, Ikram MA, Heart-Brain Connection Collaborative Research G Cerebral perfusion and the risk of dementia: a population-based study. Circulation. 2017;136:719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- Wolynes PG. Some quantum weirdness in physiology. PNAS. 2009;106:17247–17248. doi: 10.1073/pnas.0909421106. [DOI] [PMC free article] [PubMed] [Google Scholar]