Abstract
In the National Toxicology Program’s toxicity studies, rats were more sensitive than mice to Bis(2-chloroethoxy)methane (CEM) – induced cardiac toxicity following dermal application to male and female F344/N rats and B6C3F1 mice. Thiodiglycolic acid (TDGA) is a major metabolite of CEM in rats. It has been implicated that chemicals metabolized to TDGA cause cardiac toxicity in humans. Therefore, the toxicokinetics of CEM and TDGA were investigated in male and female F344/N rats and B6C3F1 mice following a single intravenous administration or dermal application of CEM to aid in the interpretation of the toxicity data. Absorption of CEM following dermal application was rapid in both species and genders. Bioavailability following dermal application was low but was higher in rats than in mice with females of both species showing higher bioavailability than males. CEM was rapidly distributed to the heart, thymus, and liver following both routes of administration. Plasma CEM Cmax and AUC∞ increased proportionally with dose, although at the dermal dose of 400 mg/kg in rats and 600 mg/kg in mice non-linear kinetics were apparent. Following dermal application, dose-normalized plasma CEM Cmax and AUC∞ was significantly higher in rats than in mice (p-value < 0.0001 for all comparisons except for Cmax in the highest dose groups where p-value = 0.053). In rats, dose-normalized plasma CEM Cmax and AUC∞ was higher in females than in males: however, the difference was significant only at the lowest dose (p-value = 0.009 for Cmax and 0.056 for AUC∞). Similar to rats, female mice also showed higher Cmax and AUC∞ in females than in male: the difference was significant only for Cmax at the lowest dose (p-value = 0.002). Dose-normalized heart CEM Cmax was higher in rats than in mice and in females than their male counterparts. The liver CEM Cmax was lower compared to that of heart and thymus in both rats and mice following intravenous administration and in rats following dermal application. This is likely due to the rapid metabolism of CEM in the liver as evidenced by the high concentration of TDGA measured in the liver. Dose-normalized plasma and heart TDGA Cmax values were higher in rats compared to mice. In rats, females had higher plasma and heart TDGA Cmax than males; however, there was no gender difference in plasma or heart TDGA Cmax in mice. These findings support the increased sensitivity of rats compared to mice to CEM-induced cardiac toxicity. Data also suggest that, either CEM Cmax or AUC can be used to predict the CEM-induced cardiac toxicity. Although, both plasma and heart TDGA Cmax was consistent with the observed species difference and the gender difference in rats, the gender difference in mice to cardiac toxicity could not be explained based on the TDGA data. This animal study suggests that toxicologically significant concentrations of CEM and TDGA could possibly be achieved in the systemic circulation and/or target tissues in humans as a result of dermal exposure to CEM.
Keywords: bis(2-chloroethoxy)methane, thiodiglycolic acid, bioavailability, toxicokinetics, metabolism
The toxicokinetics of bis(2-chloroethoxy)methane and its metabolite, thiodiglycolic acid, were investigated in male and female F344/N rats and B6C3F1 mice following a single intravenous administration or dermal application of CEM to aid in the interpretation of the toxicity data.
Data suggest that, either CEM Cmax or AUC can be used to predict the CEM-induced cardiac toxicity.
Although, both plasma and heart TDGA Cmax was consistent with the observed species difference and the gender difference in rats, the gender difference in mice to cardiac toxicity could not be explained based on the TDGA data.
This animal study suggests that toxicologically significant concentrations of CEM and TDGA could possibly be achieved in the systemic circulation and/or target tissues in humans as a result of dermal exposure to CEM.
1. Introduction
Bis(2-chloroethoxy)methane (CEM) is used as a starting compound to produce polysulfide elastomers for a variety of sealant applications because of their resistance to degradation by many solvents and resistance to high temperatures (Mutuc et al., 2008; Vietti and Scherrer, 1992). Over 95% of polysulfide polymers are made from CEM and sodium polysulfide (HSDB, 2009). Approximately 10 to 50 million pounds of CEM were produced in the United States in 1977 although no current data on import volumes were found.
Release of the chemical can occur by volatilization during its manufacture, formulation in polysulfides, or use as a solvent. Hence, the occupational exposure, in addition to dermal and oral could also occur by inhalation (HSDB, 2009). The chemical was detected as a subsurface contaminant at a number of industrial sites (Mutuc et al., 2008; Thomann, 1995). CEM was found (levels not reported) in the industrial wastes of metal finishing, plastics and chemical manufacturing, and steam electric power industries that discharge effluents to the combined sewerage system along the lower Passaic River in New Jersey (Shear et al., 1996). Samples of treated effluents from a synthetic rubber manufacturing plant revealed CEM levels of 140 mg/L (USEPA, 1973).
The exposure to CEM of the general population is possible via drinking water and consumption of seafood. CEM has been detected in a variety of environmental locations including sludges and water from inland waterways (McFall et al., 1985; USEPA, 1973; van Steenderen et al., 1987). Clams sampled from the Chef Menteur River that flows from Lake Pontchartrain, Louisiana, to the Gulf of Mexico had wet weight tissue concentrations of 12 ng CEM/g, even though the chemical was not detected in the lake’s sediment samples (McFall et al., 1985). However, CEM was not detected in fish taken from 14 river tributaries of Lake Michigan (Camanzo et al., 1987). The estimated half-life of CEM degradation in a model pond was 11 y. Therefore, when compared to a half-life of 0.5 to 2 y for structurally similar haloethers, CEM presents the potential for persistent environmental exposure (USEPA, 2000).
In 3-month subchronic studies conducted by the National Toxicology Program, CEM caused cardiac toxicity in male and female F344/N rats and B6C3F1 mice following dermal application of doses between 50 and 600 mg/kg to a site minimized from oral grooming. CEM-induced cardiac toxicity was less severe in mice than in rats (NTP, 2009; Nyska et al., 2009). The cardiac toxicity resulted in down regulation of ATP synthase transcripts suggesting reduced capacity for energy production (Dunnick et al., 2006). In two-year dermal toxicity and carcinogenicity studies, no evidence of carcinogenic activity was observed in male or female F344/N rats administered between 75 and 300 mg/kg CEM and in male or female B6C3F1 mice administered between 100 and 600 mg/kg CEM. However, administration of CEM for 2 years resulted in increased incidences of non-neoplastic lesions in the nose of male and female rats, the forestomach of male rats, the heart of male and female mice, and the forestomach and skin of male mice (NTP, 2009). CEM was mutagenic in the presence of exogenous metabolic activation in Salmonella typhimurium strains TA100 and TA1535. However, CEM did not increase the frequency of micronucleated peripheral blood erythrocytes of male and female B6C3F1 mice following dermal exposure to 3 months or micronucleated reticulocytes in bone marrow of male F344/N rats following exposure to three daily treatments by gavage (NTP, 2009).
Disposition and metabolism of CEM was investigated in F344 rats and B6C3F1 mice following intravenous administration (1 mg/kg), gavage administration (0.1 and 10 mg/kg), and dermal application (0.1 and 10 mg/kg) (Black et al., 2007). Following gavage administration, CEM was readily absorbed and rapidly excreted in urine: the disposition was not significantly affected by species, gender or the dose-range studied. Following dermal application (protected from oral grooming), the total absorbed dose ranged from 12 to 18% for F344 rats and 14 to 22% for B6C3F1 mice, thereby indicating similar dermal absorption occurred between genders and species. Following dermal application of a similar dose to a site unprotected from oral grooming, the total dose absorbed over 24 h ranged from 33 to 55% for rats and 17 to 25% for mice (RTI, 2002). As observed following oral administration, most of the dermal absorbed dose was excreted in the urine. Following intravenous, and presumably oral or dermal absorption, CEM-derived radioactivity was rapidly distributed from the blood to the tissues. In the thymus, CEM-derived radioactivity increased over time; at 8 hours post-dosing, the concentration of CEM-derived radioactivity in the thymus was equal to that found in kidney and exceeded that found in all other tissues (Black et al., 2007). Following intravenous administration of CEM in rats, thiodiglycolic acid (TDGA) was identified as the major metabolite in urine and represented approximately 40% of the total administered dose (Black et al., 2007). Thioglycolic acid has been reported to elicit potentially toxic effects in rats and mice (Gan et al., 2003). The proposed metabolic scheme for CEM is given in Figure 1.
Figure 1.
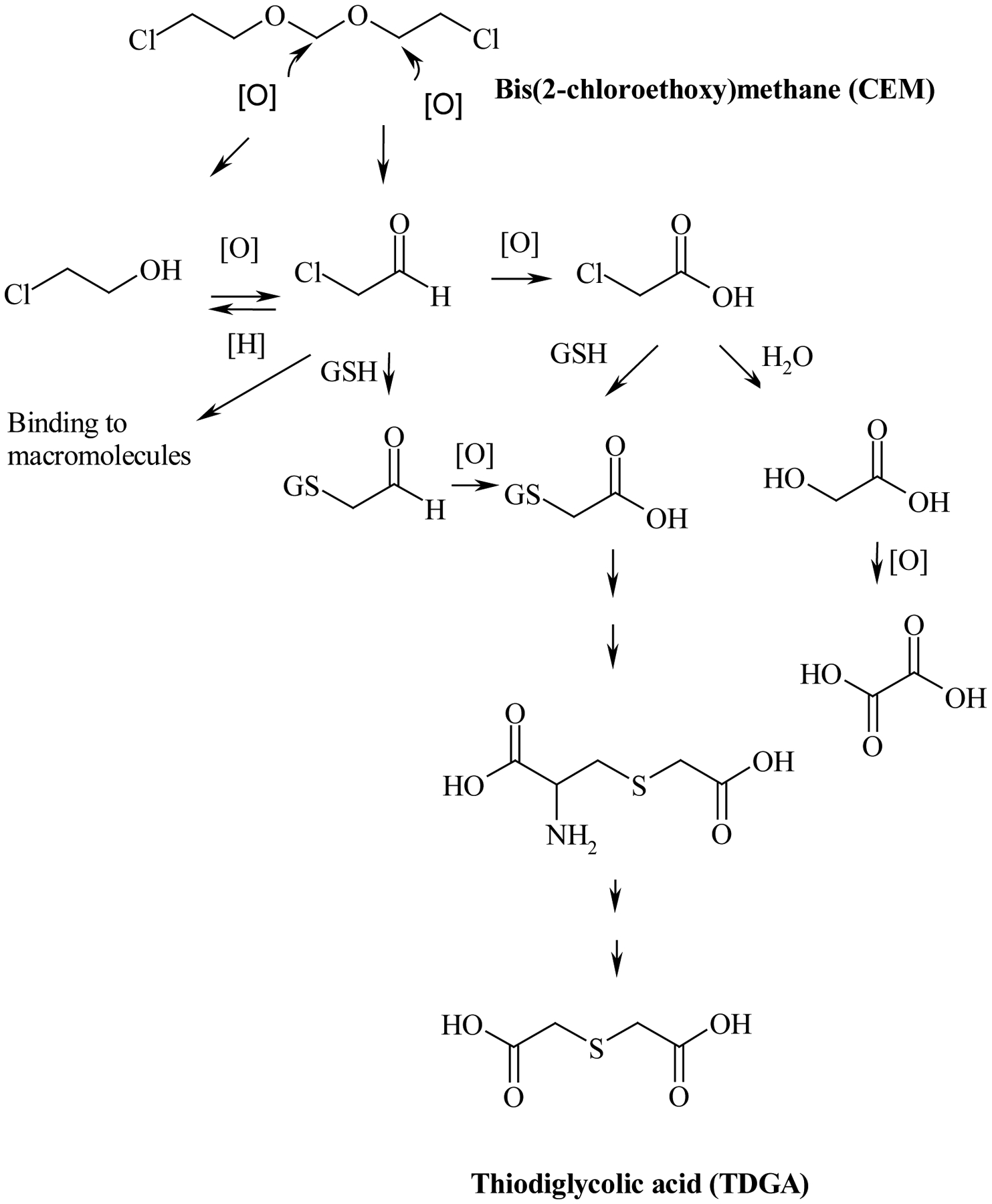
The proposed metabolic scheme of CEM (GSH, Glutathione) (adapted from Black et al., 2007).
It has been suggested that chemicals metabolized to TDGA, such as ifosfamide and 2-chloroacetaldehyde, cause cardiac toxicity in humans (Visarius et al., 1998). Cardiac toxicity was thought to occur via the mitochondria and CEM was shown to cause mitochondrial toxicity in rats and mice within 3 days of dosing (Dunnick et al., 2006), thereby suggesting a role for TDGA in CEM induced-cardiac toxicity in rodents. To the best of our knowledge, there are no studies that have investigated the toxicokinetics of CEM or TDGA in rats and mice. Therefore, the present study was conducted to determine kinetic parameters of CEM and TDGA in plasma and tissues of F344/N rats and B6C3F1 mice following a single dermal dose of CEM and to determine the bioavailability of CEM following dermal application. In addition, groups of animals from each gender and species were dosed intravenously with TDGA to understand the disposition of TDGA in rats and mice. The information from these studies contributed to the interpretation of the toxicity study results. Furthermore, the findings from this investigation will improve the usefulness of toxicity study results in risk assessment of humans to CEM.
2. Materials and Methods
2.1. Chemicals
CEM (CAS #111-91-1) was obtained from Karl Industries (Aurora, OH) and stored frozen at ≤ −20°C. The material was analyzed for identity by infrared and nuclear magnetic resonance spectroscopy, and spectra were consistent with the literature. Purity was assessed by elemental analysis and gas chromatography-flame ionization detection and was approximately 98.6 %. TDGA (CAS# 123-93-3) was obtained from Aldrich (St. Louis, MO) and stored at room temperature. Purity assessed by gas chromatography-flame ionization detection was approximately 99.8 %. The structures of CEM and TDGA are given in Figure 1. Ethyl-3-mercaptopropionate (99%) (Fisher Scientific, Pittsburgh, PA) and [2H8]CEM (Cambridge Isotope Laboratories, Andover, MA) were used as internal standards in the quantitation of TDGA and CEM, respectively.
2.2. Dose Formulation Preparation
Intravenous formulations of CEM or TDGA were prepared by mixing an appropriate amount in a vehicle consisting of Cremophor:ethanol:water (1:1:8, v/v/v). Dermal formulations of CEM were prepared in 95% ethanol. All formulations were stored at ≤ 5°C, analyzed before dosing, and found to be within 10% of the targeted concentration.
2.3. Animals
All studies were conducted at Battelle Memorial Institute (Columbus, OH). F344/N rats and B6C3F1 mice were obtained from Taconic (Germantown, NY). For intravenous studies, jugular vein catheters were implanted by Hilltop (Scottsdale, PA). Animals were quarantined for 4–7 days upon arrival at the study laboratory. Animals were distributed randomly into groups based on body weight and gender using Xybion PATH/TOX SYSTEM (Xybion Medical Systems Corporation, Version 4.2.2). Animals were individually housed in solid bottom polycarbonate cages suspended on stainless steel racks. Food (irradiated NTP-2000 pelleted feed, Zeigler Brothers, Inc. Gardners, PA) and water (City of Columbus tap water) were provided ad libitum. During the quarantine and study periods, room temperature was maintained between 19 to 24 °C and relative humidity was maintained within 31 to 76%. A 12-h light dark cycle was maintained during the quarantine and study periods. On the day of dosing, male and female F344/N rats were 13 ± 1 weeks old and ranged in weight from 221.4 to 306.8 and 103.2 to 212.2 grams, respectively; male and female B6C3F1 mice were 12 ± 1 weeks old and ranged in weight from 15.5 to 36.1 and from 18.6 to 27.8 grams respectively.
All animal use was in accordance with the United States Public Health Service policy on humane care and use of laboratory animals. These studies were conducted in compliance with the Food and Drug Administration Good Laboratory Practice Regulation (21 CFR Part 58).
2.4. Preliminary Toxicokinetic Studies in F344/N Rats and B6C3F1 Mice
Preliminary toxicokinetic studies were conducted to set doses for the full study (data not shown) as well as to obtain samples for bioanalytical method development for CEM and TDGA. Following intravenous administration of 10 mg/kg in rats and mice, CEM profiles were established. However, TDGA levels were below the limit of quantitation of the analytical method (200 ng/mL).
Dermal doses tested for rats were 75 and 300 mg/kg and mice were 100, 150, 400 (female only) and 600 (males only) mg/kg based on the doses used (between 50 and 600 mg/kg) in the National Toxicology Program’s dermal toxicity and carcinogenicity studies (NTP 2009). In rats, CEM profiles were established but TDGA profiles were not well delineated, except the plasma and heart profiles, respectively, of the male and female 300 mg/kg groups. The low dose (100 or 150 mg/kg) in mice did not allow for profiling of CEM as the concentrations were often below the limit of quantitation of the analytical method. At the high dose (400 or 600 mg/kg), concentration-time profiles were well delineated in plasma, heart, and thymus; however, TDGA profiles were not well delineated. Therefore, doses for the definitive study in rats and mice were set as given below.
2.5. Single Intravenous Administration in F344/N Rats B6C3F1 Mice
Male and female F344/N rats (42/gender/group for 20 mg/kg group, and 21 males for 40 mg/kg group) and B6C3F1 mice (30/gender/group for 50 mg/kg group, and 33 males for 100 mg/kg group) were given a single bolus intravenous injection of CEM at 20 or 40 (males only) mg/kg for the rats and 50 or 100 (males only) mg/kg for the mice or of TDGA at dosages of 20 mg/kg for the rats and 50 mg/kg for the mice. The dosing volume was 2 mL/kg for rats and 4 mL/kg for mice. The dosing solution was delivered through the jugular vein catheter.
2.6. Single Dermal Application in F344/N Rats B6C3F1 Mice
Male and female F344/N rats (33/gender/group for 100 mg/kg group, 15/gender/group for 200 mg/kg group, and 36/gender/group for 400 mg/kg group) and B6C3F1 mice (63/gender/group for 300 mg/kg group, 63 males for 450 mg/kg group, and 69/gender/group for 600 mg/kg group) were given a single dermal application of CEM at 100, 200 or 400 mg/kg for the rats and 300, 450 (males only) or 600 mg/kg for the mice. The dermal application site was unprotected from oral grooming to be consistent with National Toxicology Program’s toxicity and carcinogenicity studies (NTP 2009). The oral grooming was kept at a minimum by applying the dosing formulation to a limited, difficult to access, application site. The application site was positioned on the dorsal surface just below the back neck line and between the scapulae (intrascapular region). The dosing volume was 0.5 mL/kg for rats and 2 mL/kg for mice.
2.7. Blood and Tissue Collection
After dosing, animals were anesthetized with ~70 % CO2 (~30 % O2) and blood was collected from rats via the retro orbital sinus and from mice by cardiac puncture. Following administration of CEM, blood samples were obtained up to 900 min (rat, intravenous), 1800 min (rat, dermal), 360 min (mouse, intravenous), or 720 min (mouse, dermal) depending on the gender and the dose. Following intravenous administration of TDGA, blood samples were obtained up to 600 min for rats and 240 min for mice. Three rats and three mice of each dose group were bled at each time point and up to 2 mL of blood were collected. Rats were bled a second time with a minimum of 1 h of recovery time. Blood was collected into sealed glass tubes containing ethylenediaminetetraacetic acid, mixed gently and immediately placed on wet ice. Plasma was separated within 60 min of collection, transferred to the final storage vial and was immediately stored at −70°C until analyzed. The plasma was stored at −70°C until analyzed. Following final blood collection, animals were terminated using 100 % CO2 and the heart, liver, and thymus were collected for all animals, except for the following dose groups: rat intravenous, 40 mg/kg; rat dermal, 200 mg/kg; mouse intravenous, 100 mg/kg; mouse dermal, 450 mg/kg. The tissues were placed in plastic vials, flash frozen using liquid nitrogen, and stored frozen at −70°C until analysis.
2.8. Sample Analysis
For analysis of CEM, samples of 0.2 mL of plasma or 200 mg of minced tissue plus 200 μL of physiological saline was placed in a conical glass tube along with 50 μL of 800 ng/mL [2H8]CEM in deionized water. After adding methyl-t-butyl ether (1 mL for liver or 400 μL for plasma and other tissues), the preparations were vortexed for 10–20 seconds, tumbled for 30 min for plasma or overnight for tissues, and centrifuged at 1500 rpm for approximately 5 min. The ether layer was transferred to an autosampler vial such that headspace was limited and the vials were sealed. Samples were analyzed by gas chromatography-mass spectrometry (GC-MS) (Agilent 6890 Plus GC with 5973N mass spectrometer, Palo Alto, CA) in electron impact ionization mode. The GC injector temperature was 220°C and the injections were made in split mode with a split ratio of 10:1. Chromatography was performed on an RTX-5 MS column (30 m × 0.32 mm ID × 1.0 μm film thickness) (Restek, Bellefonte, PA) with an oven program of 90°C (1 min) to 200°C at 8°C/min, then to 250°C at 20°C/min, and held for 2 min. Ion source and the mass spectrometer transfer line temperatures were 230°C and 210°C, respectively. Ions monitored in selected ion monitoring mode were m/z 93 and m/z 97 for CEM and [2H8]CEM, respectively.
For analysis of TDGA, 0.1 mL of plasma or 100 mg of minced tissue was combined in a conical glass tube with 0.5 mL of boron trifluoride in methanol. The tubes were capped, heated at 60°C for approximately 1 h. Two mililiter of toluene, 5 mL of deionized water, and 100 μL of 31.2 μg/mL solution of ethyl-3-mercaptopropionate in toluene were added to each tube. Tubes were vortexed for 10–20 seconds and then rotated approximately 30 min for plasma or overnight for tissues. The toluene layer was transferred to an autosampler vial such that headspace was limited and the vials were sealed. Analysis was conducted with the same analytical system as described for CEM with a few modifications. Splitless injections were made using Leap CTC Analytics Combipal system (Carrboro, NC) at a GC injector temperature of 230°C. The oven was held at 80°C (2 min) and then ramped to 300°C for 6 min at 10°C/min. Ion source and the mass spectrometer transfer line temperatures were 230°C and 210°C, respectively. Ions monitored in selected ion monitoring mode were m/z 146 and m/z 134 for TDGA and ethyl-3-mercaptopropionate, respectively.
Calibrations for both analyses were performed using 6-point standard curves prepared in rat plasma. A 1/x weighted quadratic regression equation was calculated for each analyte relating the response ratio of analyte/internal standard (y) to its total mass in ng (x) in the calibration standard. The regression equation, sample response ratio, sample weight or volume and dilution factor were used to calculate sample concentrations. The methods described were found suitable for samples in the range of 2 to 200 ng of CEM per sample for all tissues except liver where the range was 4 to 200 ng and from 20 to 1200 ng of TDGA. Both methods met acceptability criteria for linearity (r2 ≥ 0.98), precision (≤ ± 5%), accuracy (≤ ± 10%) and recovery (≥ 80% for TDGA and ≥ 90% for CEM).”
2.9. Data Analysis
2.9.1. Concentration of CEM and TDGA in Plasma, Thymus, Heart, and Liver
Plasma and tissue measurements that were above the limit of quantitation (LOQ, 2 ng for CEM in plasma, heart, and thymus; 4 ng in liver and 20 ng for TDGA in plasma, heart, thymus, and liver) were used in calculations. Mean concentrations were calculated from the replicate samples at each time point.
2.9.2. Toxicokinetics
WinNonlin (Version 5.0.1, Pharsight Corporation, Mountain View, CA) was used for toxicokinetic analysis. The tissue concentration time data were analyzed using noncompartmental analysis. For the plasma concentration time data, the following two-compartmental models were used for intravenous administration and dermal application, respectively:
Where:
C(t) is the plasma concentration at time t; A0 and B0 are the intercepts of the initial and terminal phases with the concentration axis for intravenous data; A, B, and C are the intercepts of the distribution, elimination, and absorption phases with the concentration axis for dermal data; k is a rate constant (subscripts describe the compartment and direction) (Figure 2); α and β are the first order hybrid rate constants for distributional and terminal elimination phases. Half lives for the absorption and elimination were calculated as 0.693/k01 and 0.693/k10, respectively. The area under the curve was estimated to the last sampling time point using the trapezoidal rule and further extrapolated to infinity (AUC∞) by dividing the last plasma concentration by β. Volume of distribution to the central (V1) and peripheral compartments (V2), clearance (Cl), mean residence time (MRT), and absolute bioavailability following dermal application (F) were calculated using standard equations (Boroujerdi, 2002). For the dermal data, clearance and volume of distribution were adjusted for bioavailability (F) and expressed as Cl/F and V/F, respectively.
Figure 2.
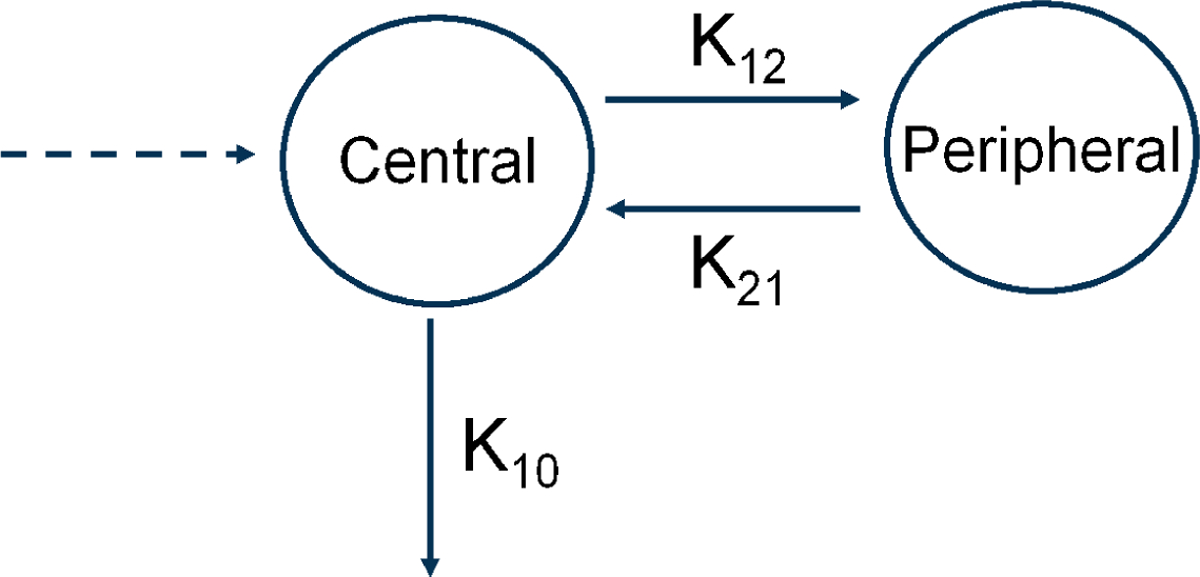
A schematic representation of two-compartmental toxicokinetic model. k10 represents rate of CEM elimination from the central compartment, while k12 and k21 represent rates of distributions from the central to peripheral compartment, and vice versa, respectively.
2.9.3. Statistical Analyses
The statistical comparisons for gender- and species-related differences were performed using a two-sample t-test procedure with the Satterthwaite approximation (statistical significance level set to be p-value = 0.05) using Microsoft® Excel 2007. Systemic exposure parameters (Cmax and AUC∞ values) were dose-normalized for the t-test procedures when the dose levels were different for the comparisons. Only plasma data were used in the statistical comparisons for gender- and species-related differences since the tissue data generated point estimates of PK parameters from the non-compartmental analysis.
3. Results
3.1. Intravenous Administration of CEM
The plasma CEM concentration-versus-time profiles showed a biphasic decline following administration of 20 (males and females) and 40 mg/kg (males) in rats and 50 mg/kg (males and females) and 100 mg/kg (males) in mice. As an example, Figures 3a and 3b show the data from male rats administered 20 mg/kg and male mice administered 50 mg/kg, respectively. Plasma concentration time data were best fitted by a two-compartment model with bolus input, first order output with 1/Ypred 2 weighting and kinetic parameters estimated from this model are presented in Table 1 for rats and mice of both genders. The lack of measureable time points in the terminal phase of the female mice created more uncertainty in these estimates. In male rats and mice, Cmax and AUC∞ increased proportionately with the dose. The estimated V1 exceeds the reported aqueous body water volume in rats (688 mL/kg) and mice (725 mL/kg) (Davies and Morris, 1993) suggesting that CEM has substantive tissue distribution or protein binding. The elimination of CEM from the central compartment was rapid in both species with mice eliminating CEM faster than rats (p-value < 0.0001). Half-lives of elimination were between 5 and 7 min in mice and 16 and 25 min in rats. Other kinetic parameters also showed that a species difference existed between rats and mice, i.e. mice had shorter t1/2α and t1/2β, faster clearance, shorter MRT, and smaller V2 than rats (p-value ≤ 0.0002). For a given species, there were no dose- or gender-related differences in the toxicokinetics parameters following intravenous administration over the dose range tested except in mice where AUC∞ (predicted) was significantly higher in males than in females (p-value = 0.028).
Figure 3.
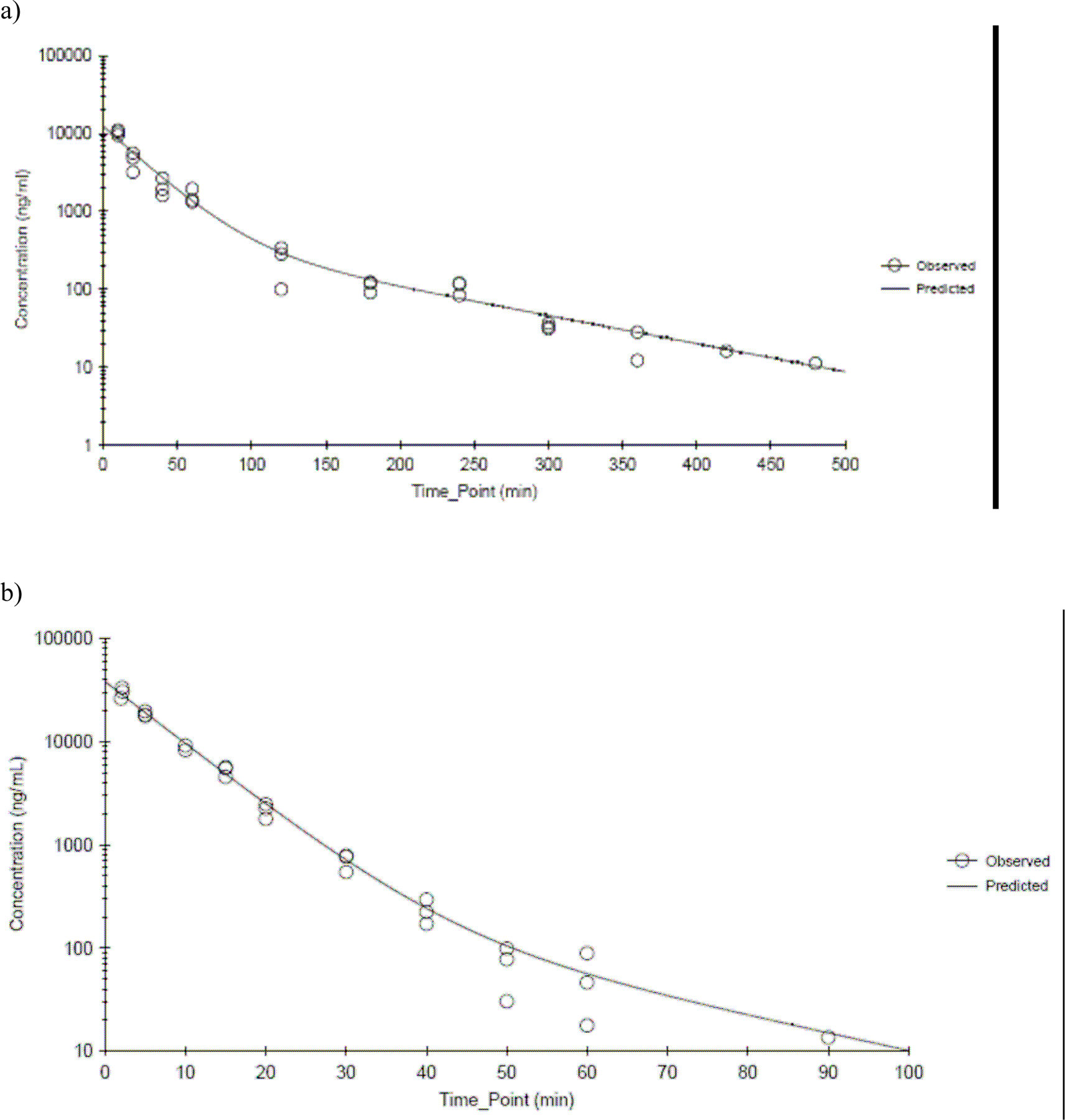
CEM plasma concentration time profiles following a single intravenous administration of CEM at a) 20 mg/kg in male F344/N rats and b) 50 mg/kg in male B6C3F1 mice. Data were fitted by a two-compartment model with bolus input, first order elimination with 1/Ypred 2 weighting. N≤3 per time point.
Table 1.
Plasma CEM Toxicokinetic Parameter Estimates Following Intravenous Administration of CEM to F344/N Rats and B6C3F1 micea
| Parameter | F 344 Rats | B6C3F1 Mice | ||
|---|---|---|---|---|
| 20 mg/kg | 40 mg/kg | 50 mg/kg | 100 mg/kg | |
| Male | ||||
| Cmax (predicted) (μg/mL)b | 12.4 + 2.2 | 24.1 + 2.8 | 38.2 + 4.7 | 63.1 + 10.5 |
| t½α (min) | 17.0 + 2.3 | 22.6 + 2.0 | 4.94 + 0.34 | 6.85 + 0.58 |
| t½β (min) | 83.4 + 10.3 | 86.7 + 7.9 | 17.8 + 5.0 | 28 + 13.2 |
| k10 half-life (min) | 20.0 + 2.4 | 25.4 + 1.9 | 5.11 + 0.30 | 7.01 + 0.52 |
| AUC∞ (predicted)(μg mL−1 min) | 357 + 32 | 882 + 58 | 282 + 23 | 638 + 72 |
| Cl (mL min−1 kg−1) | 55.9 + 5.1 | 45.4 + 3.0 | 178 + 14 | 157 + 18 |
| MRT (min) | 42.5 + 3.3 | 46.4 + 2.3 | 7.97 + 0.41 | 10.8 + 0.7 |
| V1 (mL/kg) | 1620 +290 | 1660 +190 | 1310+160 | 1590+260 |
| V2 (mL/kg) | 762 + 143 | 440 + 71 | 106 + 23 | 109 + 28 |
| Female | ||||
| Cmax (predicted) (μg/mL)b | 13.2 + 2.3 | 30.4 + 5.8 | ||
| t½α (min) | 13.7 + 1.7 | 4.63 + 0.72 | ||
| t½β (min) | 68.5 + 6.9 | 23.7 + 47.7 | ||
| k10 half-life (min) | 16.3 + 1.8 | 4.86 + 0.62 | ||
| AUC∞ (predicted)(μg mL−1 min) | 312 + 27 | 214 + 23 | ||
| Cl (mL min−1 kg−1) | 64.1 + 5.5 | 234 +251 | ||
| MRT (min) | 35.5 + 2.6 | 8.30 + 3.21 | ||
| V1 (mL/kg) | 1510 +260 | 1640+320 | ||
| V2 (mL/kg) | 767 + 126 | 300+585 | ||
Based on a two-compartment model with bolus input, first order elimination, and 1/Yhat2 weighting. Estimate ± SE.
Cmax (predicted) based on the model prediction at time 0.
CEM concentrations were determined in heart, thymus and liver in rats dosed with 20 mg/kg and in mice dosed with 50 mg/kg. In both genders and species, CEM was measureable in all tissues at the earliest time point indicating rapid distribution to tissues. Tissue concentration time data were analyzed using noncompartmental analysis and results are presented in Table 2. As seen with the plasma, mice eliminated CEM from tissues faster than rats. However, in contrast to the plasma, the tissue kinetics showed some differences between genders depending on the species. In rats, the half-life values were similar for males and females with respect to the heart (male, 69.9 min; female, 68.1 min) and liver (male, 39.1 min; female, 25.7 min); however, the half-life of CEM in thymus was 164 min in females and 80.5 min in males. In mice, the half-lives in males and females were similar: the values were between 8 and 9 min in males and between 7 and 15 min in females. The maximum tissue concentration, Cmax, of liver was higher in males (rat, 3.89 μg/g; mouse, 1.65 μg/g) than females (rat, 1.70 μg/g; mouse, 0.164 μg/g) and was lower than that of thymus (rat ≥ 42.7 μg/g, mouse ≥ 30.3 μg/g) and heart (rat ≥ 11.6 μg/g, mouse ≥ 34.6 μg/g) in both genders. The time to reach maximum tissue concentration, Tmax, was shorter for mice (3.52 to 6.96 min) compared to rats (14.8 to 43.7 min) for all tissues in both genders. Tmax of heart and thymus was similar between genders but for the liver was shorter in females compared to males in both species.
Table 2.
Tissue CEM Toxicokinetic Parameter Estimates Following Intravenous Administration of CEM to F344 Rats (20 mg/kg) and B6C3F1 mice (50 mg/kg)a
| Parameter | F344 Rats | B6C3F1 Mice | ||||
|---|---|---|---|---|---|---|
| Heart | Thymus | Liver | Heart | Thymus | Liver | |
| Male | ||||||
| Cmax (observed) (μg/g) | 12.6 | 48.7 | 3.89 | 39.3 | 32.8 | 1.65 |
| Tmax (observed) (min) | 14.9 | 14.8 | 43.7 | 3.90 | 3.90 | 6.96 |
| Half-life (min) | 69.9 | 80.5 | 39.1 | 8.88 | 7.91 | 7.66 |
| Female | ||||||
| Cmax (observed) (μg/g) | 11.6 | 42.7 | 1.70 | 34.6 | 30.3 | 0.164 |
| Tmax (observed) (min) | 15.0 | 14.9 | 14.8 | 4.19 | 4.19 | 3.52 |
| Half-life (min) | 68.1b | 164 | 25.7 | 6.98 | 5.74 | 15.2 |
Based on a non-compartmental analysis.
Non-compartmental analysis gave poor visual fit of terminal phase and hence user defined value is given.
CEM heart:plasma and thymus:plasma ratios tended to be at or above one for both species and genders. However, liver:plasma ratio reached one in male rats only after 40 min. In female rats and both male and female mice, the liver:plasma ratio never exceeded one. There were no clear differences in any of the CEM tissue:plasma ratios between the two genders except in the liver where the liver:plasma ratio was consistently lower in both the female rats and mice.
3.2. Dermal Application of CEM
3.2.1. CEM Toxicokinetics
CEM was applied in a single application, unprotected from oral grooming, to male and female rats at 100, 200, and 400 mg/kg and to male mice at 300, 450, and 600 mg/kg and to female mice at 300 and 600 mg/kg. In both species and genders, CEM was measureable in plasma samples at the earliest post-dose collection time point of 5 min in rats and 2 min in mice. The plasma CEM concentration-versus-time profiles showed a biphasic decline and were best described by a two-compartment model with first order input and first order output and 1/Ypred2 weighting. Figures 4a and 4b show the observed profiles and model-fits for male and female rats administered 100 mg/kg, respectively, and Figures 5a and 5b show the observed profiles and model-fits for male and female mice administered 300 mg/kg CEM, respectively. The corresponding parameter estimates are presented in Table 3 and Table 4 for rats and mice, respectively. In both rats and mice, the absorption of CEM was rapid with half-lives ≤ 11.8 min for rats and ≤ 7.71 min for mice without a dose- or- gender-related effect. As seen following intravenous administration, the estimated V1/F is large in both species and genders suggesting that CEM has substantive tissue distribution or protein binding. In male rats, Cmax increased proportionally to the dose, which was not overtly evident in female rats. Both Cmax and AUC∞ were higher in females than in males although the difference was significant only at the lowest dose (p-value = 0.009 for Cmax and 0.056 for AUC∞). AUC∞ increased greater than dose-proportional to the dose in both genders with the elimination half-life (k10 half-life) from the central compartment increasing from 28.9 and 15.6 min at 100 mg/kg to 66.8 and 75.8 min at 400 mg/kg for males and females, respectively, suggesting that perhaps the saturation of metabolism began to occur closer to 400 mg/kg.
Figure 4.
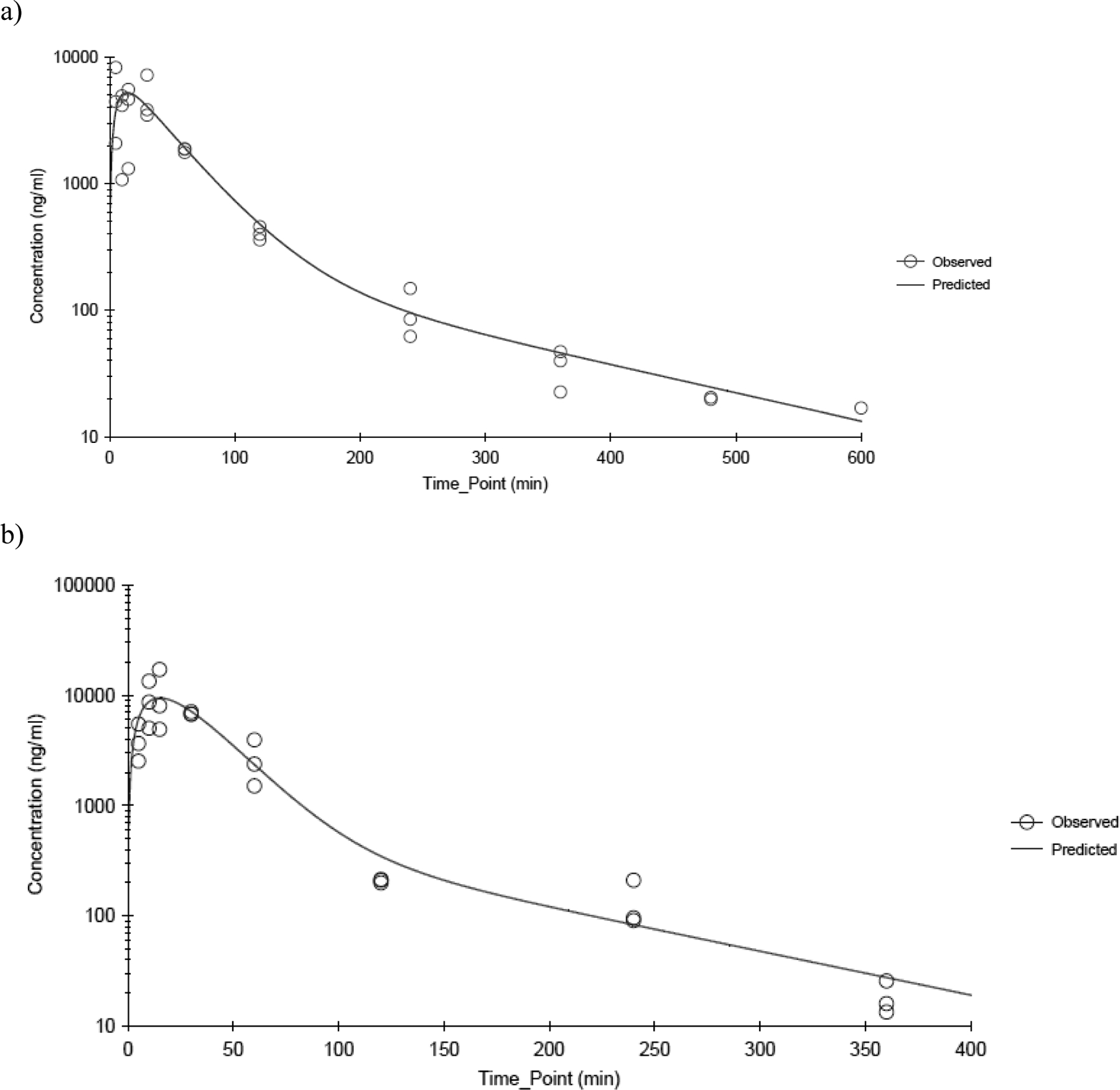
CEM plasma concentration time profiles following a single dermal application of 100 mg/kg CEM in a) male and b) female F344/N rats. Data were fitted using a two-compartment model with first order input, first order elimination and 1/Ypred 2 weighting. N≤3 per time point.
Figure 5.
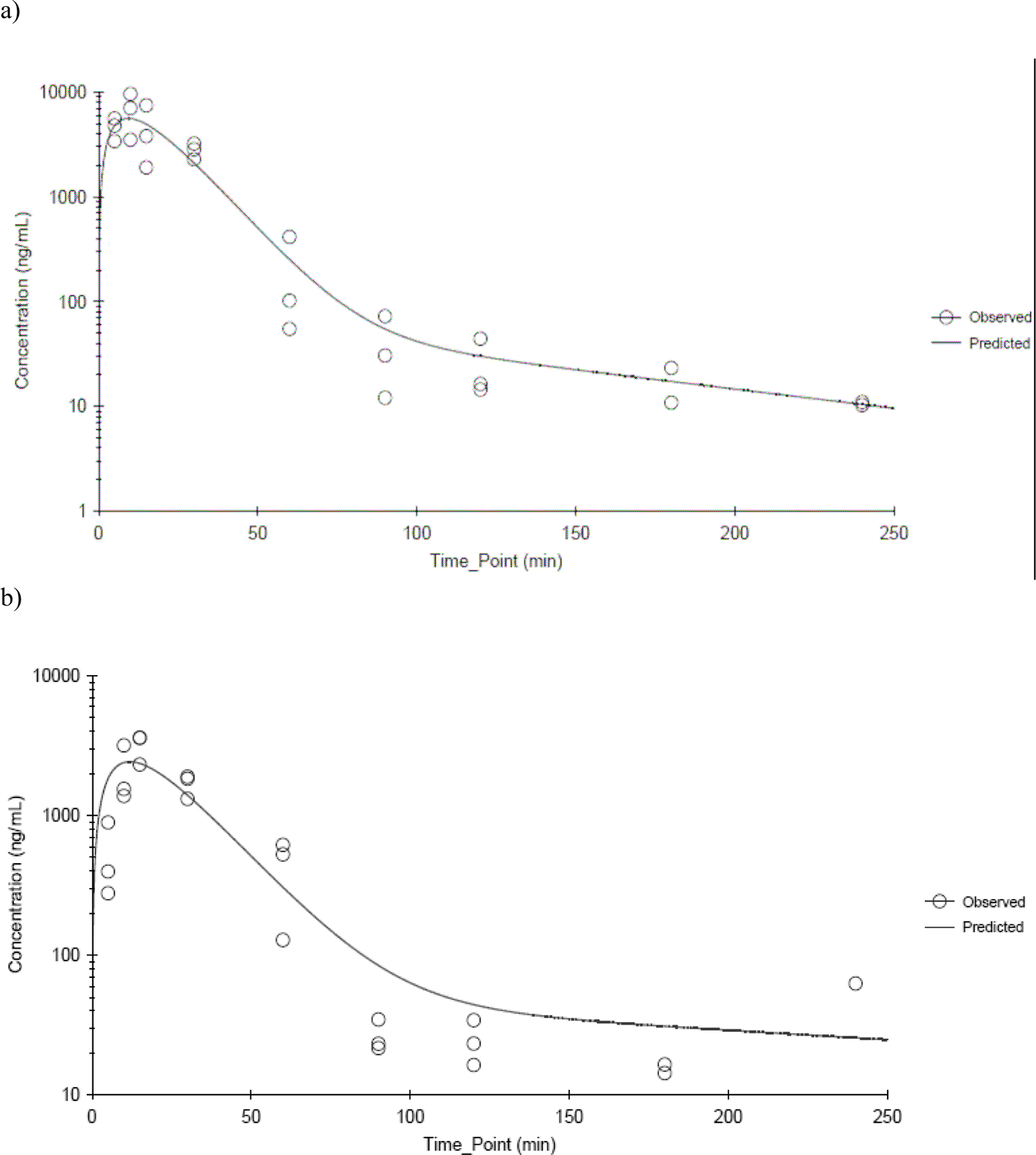
CEM plasma concentration time profiles following a single dermal application of 300 mg/kg CEM in a) male and b) female B6C3F1 mice. Data were fitted using a two-compartment model with first order input, first order elimination and 1/Ypred 2 weighting. N≤3 per time point.
Table 3.
Plasma CEM Toxicokinetic Parameter Estimates Following Dermal Application of CEM to F344 Ratsa
| Parameter | 100 mg/kg | 200 mg/kg | 400 mg/kg | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Absorption half-life (min) | 4.67 + 2.37 | 8.06 + 7.98 | 5.10+ 1.89 | 11.8 + 10.0 | 10.1 + 3.8 | 7.50 + 3.08 |
| Cmax (predicted) (ĝ/mL) | 5.26 + 0.68 | 9.41 + 1.48 | 7.92 + 0.87 | 9.97 + 1.24 | 12.2 + 1.7 | 15.0 + 2.2 |
| Tmax (predicted) (min) | 14.1 + 3.8 | 15.2 + 3.4 | 15.0 + 3.2 | 20.9 + 3.4 | 31.7 + 6.9 | 27.2 + 6.9 |
| V1/F (mL/kg) | 13,000+3100 | 5020+3900 | 17,000 + 2900 | 8950 + 6460 | 23,100 + 5000 | 20,500 + 4300 |
| V2/F (mL/kg) | 6630+1930 | 2590+1170 | 44,300 + 30,100 | 4070 +1840 | 21,700 + 14,900 | 9520+3710 |
| Cl/F (mL min−1 kg−1) | 312 + 34 | 223 + 31 | 403 + 42 | 321 + 43 | 239 + 33 | 188 + 25 |
| k10 half-life (min) | 28.9 + 5.6 | 15.6 + 11.3 | 29.2 + 3.2 | 19.4 + 13.5 | 66.8 + 7.7 | 75.8 + 8.8 |
| t½α (min) | 24.9 + 5.6 | 13.5 + 10.8 | 26.1 + 2.7 | 17.6 + 13.0 | 62.2 + 6.8 | 70.4 + 8.5 |
| t½β (min) | 135 + 40 | 75.7 + 29.3 | 729 + 348 | 114 + 40 | 973 + 501 | 570+205 |
| AUC∞ (predicted)(μg mL−1 min) | 320 + 35 | 448 + 63 | 496 + 52 | 624 + 84 | 1670+230 | 2130 +280 |
| AUC∞ (predicted)/Dose (μg min mL−1 kg mg−1) | 3.20 + 0.35 | 4.48 + 0.63 | 2.48 + 0.26 | 3.12 + 0.42 | 4.2 + 0.6 | 5.3 + 0.7 |
Based on a two-compartment model with first order input, first order elimination, and 1/Yhat2 weighting. Estimate ± SE.
Table 4.
Plasma CEM Toxicokinetic Parameter Estimates Following Dermal Application of CEM to B6C3F1 micea
| Parameter | 300 mg/kg | 450 mg/kg | 600 mg/kg | ||
|---|---|---|---|---|---|
| Male | Female | Male | Male | Female | |
| Absorption half-life (min) | 6.07 + 6.05 | 5.06 + 3.46 | 7.00 + 3.56 | 7.55 + 4.56 | 7.71 + 2.93 |
| Cmax (predicted) (μg/mL) | 2.42 + 0.50 | 5.58 + 0.92 | 4.11 + 0.50 | 12.3 + 2.1 | 15.5 + 1.8 |
| Tmax (predicted) (min) | 11.9 + 3.7 | 9.36 + 2.01 | 13.7 + 2.3 | 14.6 + 3.1 | 14.1 + 1.8 |
| V1/F (mL/kg) | 60,700 + 40,400 | 25,000 + 10,800 | 53,500 + 18,300 | 23,700 + 9400 | 17,900 + 4600 |
| V2/F (mL/kg) | 154,000 + 612,000 | 10,600 + 5100 | 80,600 + 427,000 | 6470 + 33,200 | 3730+2210 |
| Cl/F (mL min−1 kg−1) | 3080 + 970 | 1960+260 | 2590 +480 | 1150 +180 | 959 + 98 |
| k10 half-life (min) | 13.7 + 10.7 | 8.87 + 3.30 | 14.3+ 6.0 | 14.2 + 4.6 | 12.9+ 2.5 |
| t½α (min) | 11.4 + 7.0 | 8.41 + 3.18 | 13.2 + 3.9 | 13.9 + 4.2 | 12.7 + 2.4 |
| t½β (min) | 224 + 645 | 82.4 + 39.7 | 284 +1010 | 198+734 | 153 + 79 |
| AUC∞ (predicted) (μg mL−1 min) | 97.5 + 30.6 | 153 + 20 | 174 + 32 | 520 + 80 | 626 + 64 |
| AUC∞, (predicted)/Dose (μg min mL−1 kg mg−1) | 0.325 + 0.102 | 0.510 + 0.067 | 0.387 + 0.071 | 0.867 + 0.133 | 1.04 + 0.11 |
Based on a two-compartment model with first order input, first order elimination, and 1/Yhat2 weighting.
In mice, the plasma Cmax and AUC∞ increased with dose. Dose-normalized plasma CEM Cmax and AUC∞ was significantly lower in mice than in rats (p-value < 0.0001 for all comparisons except for Cmax in the highest dose groups where p-value = 0.053). The increase was greater than proportional at the highest dose of 600 mg/kg in both genders although the elimination from the central compartment was not significantly affected by the dose. Similar to rats, both Cmax and AUC∞ were higher in females than in males although the difference was significant only for Cmax at the low dose (p-value = 0.002). Mice cleared CEM faster than rats (p-value ≤ 0.0049) with elimination half-lives (k10 half lives) between 8.87 and 14.3 min and Cl/F between 959 and 3080 mL min−1 kg−1. The Cl/F values for rats were between 188 and 403 mL min−1 kg−1. For a given species, there were no gender-related differences in plasma toxicokinetics parameters of CEM following dermal application over the dose range tested except the ones noted above.
CEM concentrations were determined in heart, thymus, and liver in male and female rats dosed with 100 and 400 mg/kg and mice dosed with 300 and 600 mg/kg. In both rats and mice, CEM was measureable in all tissues from the earliest data collection time point. Tissue concentration-versus-time data showed a biphasic decline in both species and genders. The parameter estimates derived from the non-compartmental analysis are shown in Table 5. In rats, Cmax values generally increased with dose but differed between tissues with liver having the lowest concentrations in both genders. Cmax in heart was higher in female rats than male rats at 100 mg/kg although it was not apparent at 400 mg/kg. In mice, Cmax values generally increased with dose and were similar among tissues and genders except in heart where females had higher levels than males at both dose levels. Tmax increased with the dose in female rats and mice whereas such an effect was not observed in male counterparts.
Table 5.
Tissue CEM Toxicokinetic Parameter Estimates Following Dermal Application of CEM to F344 Rats and B6C3F1 micea
| Parameter | Heart | Thymus | Liver | |||
|---|---|---|---|---|---|---|
| 100 mg/kg | 400 mg/kg | 100 mg/kg | 400 mg/kg | 100 mg/kg | 400 mg/kg | |
| Male F344 Rats | ||||||
| Cmax (observed) (μg/g) | 2.95 | 18.2 | 6.07 | 28.7 | 1.66 | 11.1 |
| Tmax (observed) (min) | 34.0 | 34.7 | 34.0 | 34.7 | 34.0 | 64.3 |
| Half-life (min) | 71.5 | 91.0 | 65.5 | 175 | 58.4 | 86.1 |
| Female F344 Rats | ||||||
| Cmax (observed) (μg/g) | 8.87 | 20.4 | 8.37 | 27.6 | 2.26 | 14.5 |
| Tmax (observed) (min) | 15.5 | 34.7 | 15.6 | 34.3 | 34.3 | 64.0 |
| Half-life (min) | 63.0 | 387 | 43.8 | 187 | 46.7 | 199 |
| 300 mg/kg | 600 mg/kg | 300 mg/kg | 600 mg/kg | 300 mg/kg | 600 mg/kg | |
| Male B6C3F1 Mice | ||||||
| Cmax (observed) (μg/g) | 2.51 | 9.95 | 3.00 | 14.0 | 3.15 | 13.2 |
| Tmax (observed) (min) | 18.0 | 14.0 | 13.2 | 13.9 | 17.6 | 32.3 |
| Half-life (min) | 23.5 | 25.5 | 14.7 | 14.5 | 26.4 | 44.3 |
| Female B6C3F1 Mice | ||||||
| Cmax (observed) (μg/g) | 6.63 | 17.5 | 5.88 | 13.7 | 3.49 | 15.6 |
| Tmax (observed) (min) | 14.5 | 33.0 | 14.4 | 18.6 | 14.1 | 32.3 |
| Half-life (min) | 10.8 | 61.9 | 14.3 | 16.7 | 24.0 | 30.7 |
Based on a non-compartmental analysis.
In rats, the half-lives of CEM in tissues were between 58.4 min and 175 min in males and between 43.8 min and 387 min in females with a trend of increase in half-life with increase in dose. In mice, the half-lives of CEM in tissues were between 14.5 min and 44.3 min in males and between 10.8 min and 61.9 min in females showing rapid elimination. In both rats and mice, half-lives in tissues were longer than those observed in plasma indicating slower elimination of CEM from tissues.
CEM heart:plasma and thymus:plasma ratios tended to be above one for both genders and species. With the exception of male mice receiving 600 mg/kg, all rats and mice had a slow increase in CEM liver:plasma ratio, which did not reach equal levels until about 60 to 120 min. The CEM liver:plasma ratios generally remained above 1 for the remaining measurable time points in both species. There were no clear differences in any of the CEM tissue:plasma ratios between the two genders.
3.2.2. Bioavailability of CEM
The CEM AUC∞ (predicted) after dermal application was compared to the CEM AUC∞ (predicted) after intravenous administration and normalized by dose in male and female rats and mice to estimate absolute bioavailability of CEM. Since there were signs of potential saturation of elimination kinetics at the highest dermal doses of 400 mg/kg in rats and 600 mg/kg in mice, the bioavailability was estimated at 100 mg/kg and 200 mg/kg rats and 300 mg/kg and 450 mg/kg in mice. Estimated absolute bioavailabilities for male rats were 11.2% and 17.9% and for female rats were 20.0% and 28.7% for 100 mg/kg and 200 mg/kg, respectively. Estimated absolute bioavailabilities for male mice were 5.1% and 6.6% for 300 mg/kg and 450 mg/kg, respectively. The estimated absolute bioavailability for female mice was 11.9% at 300 mg/kg. The average bioavailability estimated for female rats (24.3%) was higher than for male rats (14.4%). The bioavailability of female mice (11.9%) was higher than the average bioavailability estimated for male mice (5.9%).
3.2.3. TDGA Toxicokinetics
TDGA concentrations were determined in plasma in rats and mice following dermal application of CEM. In rats, TDGA concentrations were above the limit of quantitation only in the 400 mg/kg dose group, whereas in mice, TDGA concentrations were above the limit of quantitation in all dose groups. TDGA was not measurable until 60 min following application of CEM in both species and genders. The plasma concentration-versus-time data exhibited biphasic decline as shown in Figure 6a and 6b for male rats and mice applied 400 and 450 mg/kg CEM, respectively. Toxicokinetic parameters estimated from non-compartmental analysis are provided in Table 7. Cmax increased proportionately with dose in mice. The elimination of TDGA in plasma was slower than that of CEM for both rats and mice. The half-lives of TDGA in plasma were between 322 min and 386 min in rats and between 185 min and 214 min for mice indicating that the elimination of TDGA was faster in mice. Other than higher Cmax in female rats compared to male rats, there were no gender-related differences in toxicokinetics in rats and mice.
Figure 6.
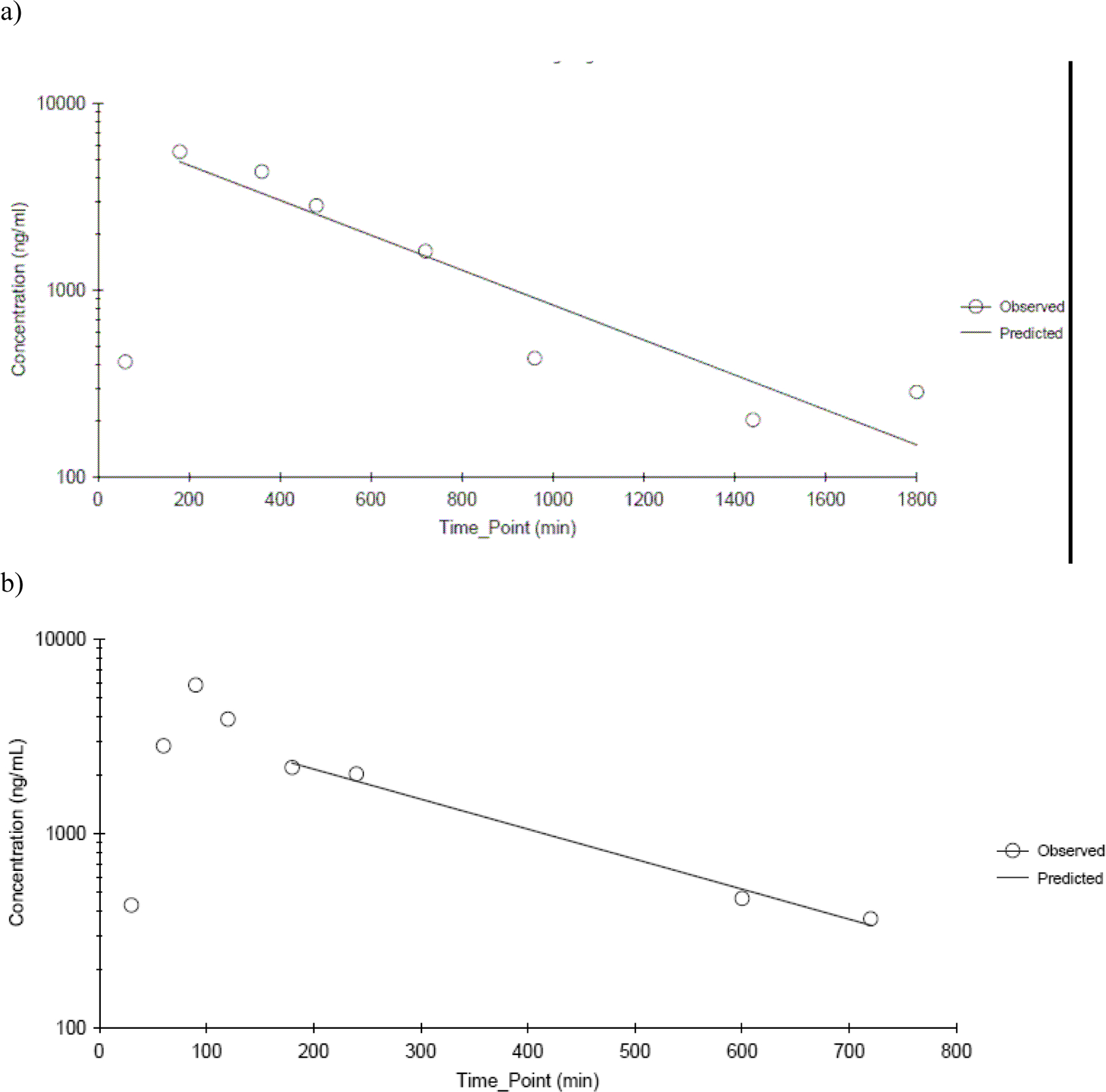
TDGA plasma concentration time profiles following a single dermal application of a) 400 mg/kg CEM in male F344/N rats and b) 450 mg/kg CEM in male B6C3F1 mice. Data were fitted using a non-compartmental analysis. N≤3 per time point.
Table 7.
Tissue TDGA Toxicokinetic Parameter Estimates Following Dermal Application of CEM to F344 Rats and B6C3F1 micea
| Parameter | Heart | Thymus | Liver | |||
|---|---|---|---|---|---|---|
| 400 mg/kg | 400 mg/kg | 400 mg/kg | ||||
| Male F344 Rats | ||||||
| Cmax (observed) (μg/g) | 3.35 | 5.47 | 84.4 | |||
| Tmax (observed) (min) | 360 | 480 | 360 | |||
| Half-life (min) | 422 | 853 | 296 | |||
| Female F344 Rats | ||||||
| Cmax (observed) (μg/g) | 6.80 | 11.0 | 123 | |||
| Tmax (observed) (min) | 360 | 480 | 480 | |||
| Half-life (min) | 548 | 652 | 373 | |||
| Heart | Thymus | Liver | ||||
| 300 mg/kg | 600 mg/kg | 300 mg/kg | 600 mg/kg | 300 mg/kg | 600 mg/kg | |
| Male B6C3F1 Mice | ||||||
| Cmax (observed) (μg/g) | 0.731 | 3.12 | 2.35 | 10.0 | 48.2 | 88.2 |
| Tmax (observed) (min) | 180 | 240 | 180 | 480 | 90 | 120 |
| Half-life (min) | 448 | 169 | 746 | 542 | 140 | 145 |
| Female B6C3F1 Mice | ||||||
| Cmax (observed) (μg/g) | 0.833 | 2.64 | 2.94 | 6.76 | 56.9 | 93.4 |
| Tmax (observed) (min) | 240 | 240 | 240 | 480 | 90 | 120 |
| Half-life (min) | 340 | 218 | 447 | 300 | 133 | 140 |
Based on a non-compartmental analysis.
Heart, thymus, and liver samples from rats applied 400 mg/kg and mice applied 300 and 600 mg/kg of CEM were analyzed for TDGA concentrations. In rats, TDGA was not consistently measureable in heart and thymus until 60 min and in liver until 10 min (in some males and all females) following application. In mice, TDGA in heart samples was measureable from 30 min following administration for both males and females at 300 mg/kg and 600 mg/kg; TDGA was measureable from 60 min in thymus and 15 min in liver at 300 mg/kg and 600 mg/kg in both males and females. Tissue concentration-versus-time data showed biphasic decline in both species and genders. The parameter estimates derived from the non-compartmental analysis are shown in Table 8. In mice, the Cmax in tissues increased proportionately with the dose. In both species and genders, Cmax in the liver was much higher than in the heart and thymus. The elimination of TDGA in tissues was slow and is similar to that in plasma. The kinetic parameters indicated that there were no gender- or dose-related differences in mice. However, in rats, females had higher tissue Cmax than males.
Table 8.
Plasma TDGA Toxicokinetic Parameter Estimates Following Intravenous Administration of TDGA to F344 rats (20 mg/kg) and B6C3F1 mice (50 mg/kg)a
| Parameter | F344 Rats | B6C3F1 Mice |
|---|---|---|
| Male | ||
| Cmax (predicted) (μg/mL)b | 32.3 ± 6.4 | 124 ± 18 |
| t½α (min) | 22.0 ± 3.0 | 3.80 ± 0.33 |
| t½β (min) | 649 ± 628 | 72.1 ± 8.1 |
| k10 half-life (min) | 30.6 ± 8.0 | 4.97 ± 0.46 |
| AUC∞ (predicted) (μg mL−1 min) | 1420 ± 296 | 888 ± 63 |
| Cl (mL min−1 kg−1) | 14.0 ± 2.9 | 56.3 ± 4.0 |
| MRT (min) | 296 ± 374 | 29.9 ± 3.6 |
| V1 (mL/kg) | 619±123 | 403 ± 58 |
| V2 (mL/kg) | 3530±4490 | 1280±230 |
| Female | ||
| C (predicted) (μg/mL)b | 71.9 ± 19.0 | 111 ± 11 |
| t½α (min) | 6.35 ± 0.90 | 4.43 ± 0.32 |
| t½β (min) | 129 ± 15 | 72.2 ± 5.7 |
| k10 half-life (min) | 9.29 ± 1.55 | 6.23 ± 0.45 |
| AUC∞ (predicted) (μg mL−1 min) | 963 ±107 | 997 ± 46 |
| Cl (mL min−1 kg−1) | 20.8 ± 2.3 | 50.2 ± 2.3 |
| MRT (min) | 67.9 ± 10.4 | 36.6 ± 2.9 |
| V1 (mL/kg) | 278 ± 74 | 451 ± 46 |
| V2 (mL/kg) | 1130±275 | 1380±160 |
Based on a two-compartment model with bolus input, first order elimination, and 1/Yhat2 weighting. Estimate ± SE.
Cmax (predicted) based on the model prediction at time 0.
The TDGA heart:plasma ratio remained close to one for all time points in both rats and mice. In rats, the TDGA thymus:plasma ratio was initially close to one at the first few measureable time points; the ratio exceeded 2 at 720 min and reached a maximum of 9.51 in males at 1440 min and 6.42 in females at 1800 min. In mice, the thymus:plasma ratios reached maxima of 4.08 and 5.29 in the male and female 300 mg/kg group, and 2.11 and 6.36 in the male and female 600 mg/kg group. These data suggest a build-up of TDGA in the thymus in both rats and mice following dermal application. In both species and genders, the TDGA liver:plasma ratio was always much higher than one most likely due to the metabolism of the applied CEM to TDGA by the liver. In rats liver:plasma ratio reached a maximum of 22.7 in males and 41.9 in females. In mice, liver:plasma ratio reached a maxima of 30.3 and 39.7 at 300 mg/kg and 17.1 and 24.9 at 600 mg/kg for males and females, respectively.
3.3. IV Administration of TDGA
To better understand the TDGA kinetics following dermal application of CEM, TDGA was intravenously administered to male and female rats (20 mg/kg) and mice (50 mg/kg). Plasma concentration-versus-time data showed a biphasic decline and was best described by a two-compartment model with bolus input and first order elimination. Parameter estimates using this model are presented in Table 9. The estimated volume of distribution for the central compartment was (≤ 619 mL/kg for rats and ≤ 451 mL/kg for mice) slightly lower than the reported volume of total body water for rats and mice (668 mL/kg for rats and 725 mL/kg for mice, (Davies and Morris, 1993) suggesting that tissue distribution of TDGA may not be that prevalent. Mice cleared TDGA rapidly than rats (p-value < 0.0001). The elimination of TDGA from the central compartment was rapid in both species with mice eliminating faster than rats (p-value = 0.0024). In rats, males eliminated TDGA faster than females (p-value = 0.0096) whereas the opposite was true in mice (p-value = 0.0406). There was no gender difference in other kinetic parameters for TDGA.
Table 9.
Tissue Toxicokinetic Parameter Estimates Following Intravenous Administration of TDGA to F344 Rats (20 mg/kg) and B6C3F1 mice (50 mg/kg)a
| Parameter | F344 Rats | B6C3F1 Mice | ||||
|---|---|---|---|---|---|---|
| Heart | Thymus | Liver | Heart | Thymus | Liver | |
| Male | ||||||
| Cmax (observed) (μg/g) | 8.93 | 8.70 | 83.0 | 40.4 | 23.9 | 78.3 |
| Tmax (observed) (min) | 15.5 | 15.7 | 25.2 | 3.76 | 6.39 | 16.3 |
| Half-life (min) | 129 | 109 | 64.6 | 92.8 | 128 | 51.1 |
| Female | ||||||
| Cmax (observed) (μg/g) | 7.78 | 8.78 | 183 | 37.9 | 38.9 | 96.6 |
| Tmax (observed) (min) | 14.7 | 15.0 | 44.0 | 3.89 | 3.89 | 16.6 |
| Half-life (min) | 147 | 149 | 125 | 73.4 | 181 | 52.0 |
Based on a non-compartmental analysis.
TDGA concentrations were determined in heart, thymus, and liver and were measureable from the earliest sampling time point in both species and genders. The heart and thymus concentration-versus-time data showed a biphasic decline. The liver concentration-versus-time profiles also showed a similar biphasic decline although they appeared to have a lag time before the decline phase. Parameter estimates derived from non-compartmental analysis are presented in Table 10. Cmax values were higher in liver than in heart and thymus in both species and genders. There was no clear gender-related difference in the kinetic parameters, except that Cmax in the liver of female rats was higher than in the male rats.
The TDGA heart:plasma ratio tended to remain above one after the early time points in both rats and mice. In rats, heart:plasma ratio was greater in females than in males. In both rats and mice, thymus:plasma ratio followed the same pattern as in heart tissue. The liver:plasma ratio was always greater than one, even at the first measured time point and tended to be higher in females than males in both species i.e. the ratio reached a maximum of 21 at 120 min in male rats, 134 at 320 minutes in female rats, 30 at 45 min in male mice and 42 at 45 min in female mice. These observations lend further evidence that TDGA is sequestered in the liver.
4. Discussion
Species and gender differences in CEM-induced cardiac toxicity were reported in rodents. Toxicokinetic parameters are critical to understanding dose-response relationships, gender-related differences in toxicological outcomes, and species differences in chemical disposition. It has been suggested that chemicals metabolized to TDGA cause cardiac toxicity in humans (Visarius et al., 1998) and TDGA has been identified as a major metabolite of CEM in rodents (Black et al., 2007). To the best of our knowledge, there is no data in the literature pertaining to the toxicokinetic properties of CEM or TDGA. Therefore, we investigated the plasma and tissue toxicokinetics of CEM and TDGA, following a single dermal application of CEM in male and female F344/N rats and B6C3F1 mice.
Because non-linear kinetics were observed at the highest dermal doses (400 mg/kg in rats and 600 mg/kg in mice), only the lower dermal doses were used to estimate the bioavailability of CEM following dermal application. The absolute bioavailability was low in both species but was about 2-fold higher in rats (14.4% for male rats, 24.4% for female rats) than in mice (5.9% for male mice, 11.9% for female mice). There was also a gender difference in bioavailability where females had approximately 2-fold higher bioavailability than males in both species. Black et al. reported that the total dose absorbed following dermal application of 0.1 and 10 mg/kg [14C]CEM to a site protected from oral grooming was 12 to 18% for F344 rats and 14 to 22% for B6C3F1 mice (Black et al., 2007). This provides evidence that there is substantive absorption of CEM in rats and mice following dermal application. However, unlike the bioavailability estimated in our studies, a species or gender difference in absorption was not observed by Black and coworkers. In the same investigation, they reported that, in males, when the application site was not protected from oral grooming, the total absorbed dose 24 h post-dosing was higher (rats, 33% to 55%; mice, 17% to 25% indicating perhaps some oral administration of the test article in rats but not in mice when the dermal site was not protected (RTI, 2002). Therefore, it is possible that in our studies, the estimated bioavailability may have had some contribution due to oral uptake. This may also provide a partial explanation for the higher bioavailability observed in rats in the current investigation compared to mice of both genders. The absolute bioavailability estimated in our investigation in rats and mice was much lower than the total absorbed dose based on the radioactivity indicating significant metabolism of CEM in the liver and possibly at other sites. It is also worth noting that the doses used in Black et al. (2007) were much lower than the current investigation. In addition, Black et al. used ethanol as the dermal vehicle compared to Cremophor:ethanol:water (1:1:8, v/v/v) used in the current investigation.
When adjusted for bioavailability, the volume of distribution of CEM was similar in both species and genders following dermal application and is significantly higher than the volume of total body water for rats (668 mL/kg) and for mice (725 mL/kg) (Davies and Morris, 1993) suggesting significant tissue distribution or protein binding of CEM. It has been shown that following intravenous administration, and presumably oral or dermal absorption, CEM-derived radioactivity was rapidly distributed from the blood to the tissues (Black et al., 2007). At 0.25 h post administration, the highest concentrations were reported in the organs of primary excretion and metabolism, except for the thymus where CEM-derived radioactivity increased over time, i.e. at 8 h post dosing, the concentration exceeded that found in all other tissues and was equal to that found in kidney. Over 90% of the CEM-derived radioactivity in thymus was found to be bound to macromolecules (Black et al., 2007). In the current study, we analyzed CEM concentrations in thymus, heart and liver. In fact, CEM was detected in heart, liver and thymus ≤ 5 min following dermal application indicating rapid distribution. In both species and genders, heart:plasma and thymus:plasma ratio tended to be at or above one. However, liver:plasma ratio had a slow increase which is likely due to the metabolism of CEM to TDGA in the liver. This was confirmed by the detection of TDGA in plasma and tissues under investigation. The observed liver TDGA Cmax was much higher than heart or thymus in rats and mice. Furthermore, the TDGA liver:plasma ratios were much higher than the heart:plasma or the thymus:plasma ratios. These results indicate that in addition to the metabolism of CEM to TDGA in the liver, TDGA was being sequestered in the liver. This was further confirmed by significantly higher TDGA Cmax in the liver compared to plasma, heart or thymus following intravenous administration of TDGA in rats and mice of both genders (Table 10). Although, the metabolism of CEM to TDGA was known based on the detection of TDGA in urine following intravenous administration of [14C]CEM (Black et al., 2007), this is the first study showing the presence of TDGA in plasma and tissues following dermal application of CEM in rats and mice of both genders. TDGA is a known metabolite of 2-chloroethanol and 2-chloroacetaldehyde (Grunow and Altmann, 1982; Joqueviel et al., 1997). Therefore, it has been postulated that the ether linkage of CEM is cleaved to form 2-chloracetaldehyde which conjugates with glutathione and the glutathione conjugate subsequently undergoes oxidation and metabolism to form TDGA(Black et al., 2007).
The elimination of CEM from the central compartment was rapid in both species; however, the overall elimination was 3- to 5-fold faster in mice than in rats following both routes of administration. Although there was no dose-dependence of elimination in mice, in rats the elimination half-life increased by 2- to 5-fold at the highest dose studied. Elimination of TDGA from plasma and tissues following dermal application, in general, was about 20 times slower than CEM. As seen with CEM, elimination of TDGA was faster in mice than in rats.
In the 3-month toxicity study in rats, all 600 mg/kg males and females and two 400 mg/kg females died before the end of the study (NTP, 2009). The cause of death was considered to be related to the cardiotoxic effect of CEM. Chemical-related cardiac lesions occurred in both male and female rats, primarily in the 400 and 600 mg/kg groups, and consisted of cardiomyocytic cytoplasmic vacuolization and interstitial mononuclear cell infiltration of minimal to moderate severity. In our studies, non linear kinetics were apparent in rats at around 400 mg/kg indicating that perhaps the toxicity of CEM is severe at doses that produce non-linear kinetics. At 400 mg/kg, Cmax in plasma were 12.2 μg/mL and 15 μg/mL and heart were 18.2 μg/mL and 20.4 μg/mL, in males and females, respectively. In the 3-month toxicity study in mice, except for three 600 mg/kg females, all mice survived to the end of the study; chemical-related myocardial vacuolization consisted of widespread cardiomyocytes exhibiting vacuolization of the sarcoplasm and was seen primarily in 400 and 600 mg/kg female mice (NTP, 2009). Since toxicokinetics were not evaluated at 400 mg/kg in female mice, it is not clear whether the non linear kinetics has begun to occur at or above this dose. However, at 600 mg/kg, both male and female mice showed non-linear kinetics. At 600 mg/kg, Cmax in plasma were 12.3 μg/mL and 15.5 μg/mL and heart were 9.95 μg/mL and 17.5 μg/mL, in males and females, respectively.
Rats were more sensitive to CEM-induced cardiac toxicity than mice. Furthermore, in both species females were more sensitive than males. Our data clearly showed that rats underwent higher systemic exposure to CEM than mice when normalized to the dose. There was a trend that females of both species underwent higher systemic exposure to CEM than the corresponding males although the difference was not significant. Also, there was a trend that the dose-normalized plasma and heart CEM Cmax was higher in rats than in mice and in females than the corresponding males. These data suggest that, either CEM Cmax or AUC can be used to predict the toxicity associated with CEM. It has been suggested that chemicals metabolized to TDGA in humans, such as ifosfamide and 2-chloroacetaldehyde, cause cardiac toxicity via mitochondrial toxicity (Hofmann et al., 1991; Joqueviel et al., 1997; Joqueviel et al., 1998; Visarius et al., 1998) and CEM similarly showed mitochondrial toxicity in rats and mice within 3 days of dosing (Dunnick et al., 2006). In our investigation, dose-normalized plasma and heart TDGA Cmax was higher in rats compared to mice. Also, both plasma and heart TDGA Cmax was higher in female rats compared to male rats. However, there was no gender difference in plasma or heart TDGA Cmax in mice. Therefore, if one were to assume that the mechanism of CEM-induced cardiac toxicity is due to TDGA, the observed gender-difference in cardiac toxicity in mice cannot be explained based on the toxicokinetics of TDGA. However, plasma and heart toxicokinetic parameters of CEM are consistent with both species and gender difference in toxicity and hence may be a more important predictor of CEM-induced toxicity. In toxicity studies, in addition to heart toxicity, CEM increased incidences of atrophy in the thymus in 600 mg/kg male and female rats and of lymphoid necrosis in the thymus of 600 mg/kg female mice. In our studies, thymus:plasma ratios of CEM and TDGA were ≥1 indicating sequestration of CEM and TDGA.
In conclusion, CEM is quickly absorbed after dermal application to a site minimized from oral grooming, distributed from the central compartment, and metabolized to TDGA, which is cleared slowly from the plasma and tissues compared to the parent compound. Rats underwent higher exposure to CEM and the metabolite TDGA than mice when normalized to the administered dose. This supports the data from toxicity studies where rats were more sensitive than mice to CEM-induced toxicity. In rats, female rats had higher exposure to CEM and TDGA compared to males, supporting the increased sensitivity in female rats compared to male rats to toxicity of CEM. In mice, females had higher exposure than males to CEM but not to TDGA. Therefore, these data indicates that toxicologically significant concentrations of CEM and TDGA are likely to be achieved in the systemic circulation and/or target tissues in humans as a result of dermal exposure to CEM.
Table 6.
Plasma TDGA Toxicokinetic Parameter Estimates Following Dermal Application of CEM to F344 Rats and B6C3F1 micea
| Parameter | F344 Rats | B6C3F1 Mice | ||
|---|---|---|---|---|
| 400 mg/kg | 300 mg/kg | 450 mg/kg | 600 mg/kg | |
| Male | ||||
| Cmax (observed) (μg/g) | 5.51 | 2.32 | 5.82 | 7.78 |
| Tmax (observed) (min) | 180 | 240 | 90 | 240 |
| Half-life (min) | 322 | 188 | 195 | 205 |
| Female | ||||
| Cmax (observed) (μg/g) | 10.8 | 1.95 | NAb | 4.33 |
| Tmax (observed) (min) | 360 | 90 | NA | 90 |
| Half-life (min) | 386 | 185 | NA | 214 |
Based on a non-compartmental analysis.
NA, Not applicable
Acknowledgements
1. This study was conducted for the National Toxicology Program, National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health, Department of Health and Human Services, under Contract No. N01-ES-55551.
3. The authors are grateful to Drs. J. Michael Sanders and Michael DeVito for their review of this manuscript.
Abbreviations
- CEM
bis(2-chloroethoxy)methane
- TDGA
thiodiglycolic acid
- GC
gas chromatography
- MS
mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH, or the United States government.
References
- Black SR, Decosta KS, Patel PR and Mathews JM (2007). [14C]bis(2-chloroethoxy)methane: comparative absorption, distribution, metabolism and excretion in rats and mice. Xenobiotica 37, 427–40. [DOI] [PubMed] [Google Scholar]
- Boroujerdi M (2002). Pharmacokinetics: Principles and applications. McGraw-Hill Medical Publishing Division. [Google Scholar]
- Camanzo J, Rice CP, Jude DJ and Rossmann R (1987). Organic priority pllutants in nearshore fish from 14 Lake Michigan tributaries and embayments. J. Great Lakes Res 13, 296–309. [Google Scholar]
- Davies B and Morris T (1993). Physiological parameters in laboratory animals and humans. Pharm Res 10, 1093–5. [DOI] [PubMed] [Google Scholar]
- Dunnick J, Blackshear P, Kissling G, Cunningham M, Parker J and Nyska A (2006). Critical pathways in heart function: bis(2-chloroethoxy)methane-induced heart gene transcript change in F344 rats. Toxicol Pathol 34, 348–56. [DOI] [PubMed] [Google Scholar]
- Gan HF, Meng XS, Song CH and Li BX (2003). A survey on health effects in a human population exposed to permanent-waving solution containing thioglycolic acid. J Occup Health 45, 400–4. [DOI] [PubMed] [Google Scholar]
- Grunow W and Altmann HJ (1982). Toxicokinetics of chloroethanol in the rat after single oral administration. Arch Toxicol 49, 275–84. [DOI] [PubMed] [Google Scholar]
- Hofmann U, Eichelbaum M, Seefried S and Meese CO (1991). Identification of thiodiglycolic acid, thiodiglycolic acid sulfoxide, and (3-carboxymethylthio)lactic acid as major human biotransformation products of S-carboxymethyl-L-cysteine. Drug Metab Dispos 19, 222–6. [PubMed] [Google Scholar]
- (Hazardous Substance Data Base (HSDB) (2009) National Institute for Occupational Safety and Health, HSDB database available through the National Library of Medicine MEDLARS System. [Google Scholar]
- Joqueviel C, Gilard V, Martino R, Malet-Martino M and Niemeyer U (1997). Urinary stability of carboxycyclophosphamide and carboxyifosfamide, two major metabolites of the anticancer drugs cyclophosphamide and ifosfamide. Cancer Chemother Pharmacol 40, 391–9. [DOI] [PubMed] [Google Scholar]
- Joqueviel C, Martino R, Gilard V, Malet-Martino M, Canal P and Niemeyer U (1998). Urinary excretion of cyclophosphamide in humans, determined by phosphorus-31 nuclear magnetic resonance spectroscopy. Drug Metab Dispos 26, 418–28. [PubMed] [Google Scholar]
- McFall JA, Antoine SR and DeLeon IR (1985). Base-neutral extractable organic pollutants in biota and sediments from Lake Ponchartrain. Chemosphere 14, 1561–1569. [Google Scholar]
- Mutuc MD, Love NG and Vikesland PJ (2008). Surface catalyzed Fenton treatment of bis(2-chloroethyl) ether and bis(2-chloroethoxy) methane. Chemosphere 70, 1390–8. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (NTP (2009). Toxicology and carcinogenesis studies of Bis(2-chloroethoxy)methane (CAS No. 111-91-1) in F344/N rats and B6C3F1 mice (dermal studies) [PubMed] [Google Scholar]
- Nyska A, Cunningham M, Snell M, Malarkey D, Sutton D and Dunnick J (2009). The pivotal role of electron microscopic evaluation in investigation of the cardiotoxicity of bis(2-chloroethoxy)methane in rats and mice. Toxicol Pathol 37, 873–7. [DOI] [PubMed] [Google Scholar]
- RTI International (RTI) (2002) Bis(2-chloroethoxy)methane: comparative metabolism and excretion in rats and mice RTI Report No. RTI/64U-6855/14P. NIEHS Contract No. N01-ES-75407 RTI International, Research Triangle Park, NC. [Google Scholar]
- Shear NM, Schmidt CW, Huntley SL, Crawford DW and Fineley BL (1996). Evaluation of the factors relating combined sewer overflows with sediment contamination of the lower Passaic River. Marine Pollut. Bull 32, 288–304. [Google Scholar]
- Thomann RV (1995). Modeling organic chemical fate in aquatic systems: significance of bioaccumulation and relevant time-space scales. Environ Health Perspect 103 Suppl 5, 53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA) (1973) Current Practice in GC-MS Analysis of Organics in Water. EPA Project 16020 GHP. EPA Report No. EPA-R2-73-277 Southeast Environmental Research Laboratory, Athens, GA [Google Scholar]
- United States Environmental Protection Agency (USEPA) (2000). Economics Background Document. USEPA Final Rule Listing Certain Industrial Wastewater Sludges Generated by Chlorinated Aliphatic Chemical Manufacturing Facilities, as RCRA Hazardous Wastecodes K174 & K175: Industry Profile and Estimation of Regulatory Costs. Office of Solid Waste, U.S. Environmental Protection Agency, Ed.^ Eds.), Washington, DC. [Google Scholar]
- van Steenderen RA, Theron SJ and Hassett AJ (1987). The occurrence of organic micro-pollutants in the Vaal River between Grootraai Dam and Parys. Water SA (Pretoria) 13, 209–214. [Google Scholar]
- Vietti D and Scherrer M (1992). Polysulfides In Kirk-Othmer Encyclopedia of Chemical Technology 3ed John Wiley and Sons, Inc., New York [Google Scholar]
- Visarius TM, Bahler H, Kupfer A, Cerny T and Lauterburg BH (1998). Thiodiglycolic acid is excreted by humans receiving ifosfamide and inhibits mitochondrial function in rats. Drug Metab Dispos 26, 193–6. [PubMed] [Google Scholar]


