Abstract
Prostaglandin E<sub>2</sub> (PGE<sub>2</sub>), an essential endogenous lipid mediator for normal physiological functions, can also act as an inflammatory mediator in pathological conditions. We determined whether Staphylococcus aureus lipoproteins are essential for inducing PGE<sub>2</sub> secretion by immune cells and whether pattern recognition receptors mediate this process. PGE<sub>2</sub> levels secreted by mouse peritoneal macrophages infected with the S. aureus isogenic mutant, lgt::ermB (Δlgt; deficient in lipoprotein maturation), decreased compared with those from macrophages infected with wild-type (WT) S. aureus. Experiments using toll-like receptors 2 (TLR2)-deficient, TLR4-deficient, and NLRP3-deficient mice indicated that these 3 proteins are involved in macrophage PGE<sub>2</sub> secretion in response to S. aureus, and lipoproteins were essential for S. aureus invasion and survival within macrophages. Inhibition of endogenous PGE<sub>2</sub> synthesis had no effect on bacterial invasion. Exogenous PGE<sub>2</sub> inhibited phagocytosis in the WT S. aureus and its isogenic mutant but increased intracellular killing accompanied by enhanced IL-1β secretion. Our data demonstrate that S. aureus can induce macrophage TLR/mitogen-activated protein kinase/NF-κB signaling and that PGE<sub>2</sub> treatment upregulates NLRP3/caspase-1 signaling activation. Thus, macrophage PGE<sub>2</sub> secretion after S. aureus infection depends on bacterial lipoprotein maturation and macrophage receptors TLR2, TLR4, and NLRP3. Moreover, exogenous PGE<sub>2</sub> regulates S. aureus-induced macrophage activation through TLRs and NLRP3 inflammasome signaling.
Keywords: Prostaglandin E2, Staphylococcus aureus, Toll-like receptor 2, Toll-like receptor 4, NLRP3
Introduction
Staphylococcus aureus is a major gram-positive pathogen that causes bacterial infections, bacteremia, and sepsis in mammals [1, 2]. During the infection process, S. aureusactivates immune cells and induces host inflammatory responses, including secretion of proinflammatory cytokines and chemokines and macrophage scavenger receptor A (SR-A)-dependent phagocytosis [3, 4]. The innate immune system is crucial for host defenses against bacterial infection. In early host responses against invading pathogens, specific pathogen-associated molecular patterns or damage-associated molecular patterns are recognized by immune cell pattern recognition receptors (PRRs), including toll-like receptors (TLRs) and NOD-like receptors (NLRs) [5, 6, 7].
Among PRRs, TLR2 is essential for defense against S. aureus infection. TLR2 stimulation by S. aureus results in activation of the transcription factor NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways, which promote secretion of numerous proinflammatory mediators [6, 8, 9]. Substantial evidence supports a broad role for TLR2 as a PRR for a variety of microbes and microbial structures. TLR2 recognizes S. aureus peptidoglycan and lipoteichoic acid [10, 11]; however, S. aureus bacterial lipoproteins (BLPs) are the dominant immunobiologically active compounds that activate cells via TLR2 [12, 13]. Specifically, the TLR2-TLR1 heterodimer recognizes triacylated lipopeptides from gram-negative bacteria, whereas the TLR2-TLR6 heterodimer recognizes diacylated lipopeptides from gram-positive bacteria [6]. Stenzel et al. [14] reported that immune responses to experimental S. aureus-induced brain abscesses not only depended on TLR2 but also required TLR4, a well-known lipopolysaccharide (LPS) sensor, suggesting that TLR4 participates in host defenses against S. aureus infections. S. aureus also activates the NLR pyrin domain-containing 3 (NLRP3) inflammasome, which includes the best-characterized NLR molecule (NLRP3), resulting in caspase-1 activation and mature IL-1β secretion [15]. Hence, multiple PRRs are involved in the host immune response to S. aureus infection and lipoproteins from S. aureus play key roles in immune response modulation, inflammation, and pathogenicity [16, 17]. However, the roles of S. aureus lipoproteins in inducing immune cell secretion of prostaglandin E2 (PGE2) are unknown.
PGE2 is an endogenous lipid mediator that is essential for the normal physiological functions of various organs of the gastrointestinal, cardiovascular, and female reproductive systems, but in pathological conditions, PGE2 can also act as an inflammatory mediator [18, 19]. PGE2 is synthesized in large amounts in response to cell specific trauma, stimuli, pathogen infection, or signaling molecules [20, 21]. Inhibition of PGE2 synthesis is considered an important anti-inflammatory strategy [22]. PGE2 is generated by the conversion of arachidonic acid into the intermediate mediator prostaglandin endoperoxide H2 by 2 different cyclooxygenases (COXs), COX-1 and COX-2 [23]. COX-1 is constitutively expressed and generates prostaglandins (PGs) to contribute to physiological homoeostasis. In contrast, COX-2 is an inducible enzyme responsible for PG production during different pathological processes involving inflammation, such as infectious diseases, cancer, arthritis, and atherosclerosis [18]. Prostaglandin endoperoxide H2 is then transformed into PGE2 by 3 types of PGE synthases (PGESs): cytosolic PGES, microsomal PGES-1 (mPGES-1), and mPGES-2 [21]. mPGES-1 appears to be functionally coupled with COX-2, and its induction is usually coordinated with COX-2 expression [24]. Cyclic AMP (cAMP) is the main intracellular second messenger of PGE2 signaling in macrophages, and PGE2 is crucial for modulating immune cell function via cAMP-protein kinase A (PKA) signaling [25]. A previous study reported that in macrophages activated by the TLR4 agonist LPS, exogenous PGE2 induced the cAMP-dependent PKA signaling pathway, which is important for modulating immune responses and inflammatory processes characterized by increased COX-2 and mPGES-1 expression [18]. COX-2 and mPGES-1 are enzymes upregulated at the site of inflammation and account for the bulk of PGE2 biosynthesis [26]. Granick et al. [27] reported that granulopoiesis in S. aureus-infected wounds is induced by TLR2/MyD88 activation of hematopoietic stem and progenitor cells through a mechanism involving autocrine production and PGE2 activity. Moreover, NLRP3 inflammasome-mediated activation of the COX-2/mPGES-1/PGE2 cascade contributes to albumin-induced proximal tubule cell injury [28]. These findings indicate that PGE2 is closely associated with the innate immune response triggered by PRRs.
Here, we examined the effect of S. aureus lipoproteins and host TLR2, TLR4, and NLRP3 inflammasome on macrophage PGE2 secretion after S. aureus infection. Additionally, the role of PGE2 in regulating the TLR2, TLR4, and NLRP3 inflammasome-mediated innate immune response to S. aureus infection was analyzed.
Materials and Methods
Ethics Statement
All animal experiments were performed according to regulations of the Administration of Affairs Concerning Experimental Animals in China. The experimental protocol was approved by the Animal Welfare and Research Ethics Committee of the Inner Mongolia Agricultural University (approval ID: 20151227-2).
Bacterial Strains and Animals
S. aureus SA113 wild-type strain (WT; ATCC 35558), an S. aureus SA113 isogenic mutant lgt::ermB (Δlgt) deficient in lipoprotein maturation, and its complemented strain SA113 lgt::ermB + pRBlgt (+ pRB) were kindly provided by Prof. Friedrich Götz of Mikrobielle Genetik, Universität Tübingen, Germany [29, 30]. All bacterial strains were cultured in Mueller-Hinton II cation adjusted broth (MH broth, BD Biosciences, Sparks, MD, USA) at 37°C for 16 h with constant shaking to an optical density at 600 nm of 2.0. C57BL/6J WT, TLR2-deficient (TLR2−/–), and TLR4-deficient (TLR4−/–) mice were provided by the Model Animal Research Center of Nanjing University, Nanjing, China. NLRP3-deficient (NLRP3−/–) mice were obtained from the Jackson Laboratory, Bar Harbor, ME, USA.
Experimental Infections and Treatment of Mouse Peritoneal Macrophages
Three days before peritoneal macrophages were extracted, 8-week-old C57BL/6J WT, TLR2−/–, TLR4−/–, and NLRP3−/– mice were injected with 2 mL 3% thioglycolate medium (BD Biosciences, Sparks, MD, USA). Peritoneal macrophages were isolated by washing the peritoneal cavity with endotoxin-free phosphate-buffered saline (PBS, Hyclone, Logen, UT, USA) and cultured at 37°C in 5% CO2 in RPMI 1,640 media supplemented with 10% fetal bovine serum (Hyclone, Logen, UT, USA). Cells (2 × 106 cells/well) were seeded into 6-well culture plates in 1 mL fresh culture medium and washed 3 times with PBS before infection. Macrophages were infected with 6 × 106, 2 × 107, or 6 × 107S. aureus in a total volume of 750 μL of culture medium (final multiplicity of infection [MOI] 3:1, 10:1, or 30:1, respectively). After 1 h of incubation at 37°C, 250 μL of culture medium containing gentamicin (final concentration, 100 μg/mL) was added to kill extracellular bacteria. In the groups treated with the PKA inhibitor H89 (Cayman Chemical Company, Ann Arbor, MI, USA), macrophages were incubated in culture media supplemented with 10 µM H89 for 2 h before infection. In the groups treated with COX-2 selective inhibitors, macrophages were incubated in culture media supplemented with 10 µM NS398 (Cayman Chemical Company, Ann Arbor, MI, USA) or 1 µM CAY10404 (Cayman Chemical Company, Ann Arbor, MI, USA) for 40 min before infection. In PGE2 (Cayman Chemical Company, Ann Arbor, MI, USA) treatment groups, macrophages were incubated in culture media supplemented with 1 µM PGE2 for 24 h before infection. Then, the cells were infected with S. aureus (MOI 10:1 or MOI 30:1) followed by treatment with gentamicin as described above.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated 4, 8, and 12 h post-S. aureus infection using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was treated with DNase I and reverse transcribed using a Revert Aid first-strand cDNA synthesis kit (Thermo Scientific, Waltham, MA, USA). Total cDNA was used as template for real-time PCR with FastStart Universal SYBR Green Master (Roche Applied Science, Mannheim, Germany) on an iCycler iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The primers used in this study are listed in Table 1. PCR conditions were as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of amplification at 95°C for 15 s and 60°C for 60 s. Target gene expression was normalized to that of Gapdh (2[Ct Gapdh − Ct gene]) as previously described [31].
Table 1.
The primers used in this study
| Gene symbol | Accession number | Primer sequence | |
| Gapdh | NM_008085.2 | Forward: 5′-AGGTCGGTGTGAACGGATTTG-3′ | Reverse: 5′-GGGGTCGTTGATGGCAACA-3′ |
| Cox-2 | NM_011198.4 | Forward: 5′-TTCAACACACTCTATCACTGGC-3′ | Reverse: 5′-AGAAGCGTTTGCGGTACTCAT-3′ |
| mPges-1 | NM_022415.3 | Forward: 5′-GGATGCGCTGAAACGTGGA-3′ | Reverse: 5′-CAGGAATGAGTACACGAAGCC-3′ |
| Cox-2, cyclooxygenases 2; mPges-1, microsomal prostaglandin E synthases 1. | |||
Western Blot Analysis
For total cellular protein extraction, macrophages were treated with M-PER mammalian protein extraction reagent (Thermo Scientific, Waltham, MA, USA). The protein sample concentrations were determined using BCA assay kits (Thermo Scientific, Rockford, IL, USA). For western blotting analysis, 10 μg of total protein per lane was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride membranes. Membranes were blocked with StartingBlockTM (TBS) Blocking Buffer (Thermo Scientific, Rockford, IL, USA) for 1 h at room temperature and then incubated with primary antibody for 14 h at 4°C. Rabbit anti-COX-2, anti-mature IL-1β, anti-phospho PKA substrate (A rabbit monoclonal antibody detects peptides and proteins containing a phospho-Ser/Thr residue with arginine at the −3 and −2 positions), anti-phospho-ERK, anti-ERK, anti-phospho-p38, anti-p38, anti-phospho-JNK, anti-JNK, anti-phospho-NF-κB p65, and anti-NF-κB p65 monoclonal antibodies (1:1,000, Cell Signaling Technology, Beverly, MA, USA); rabbit anti-mPGES-1 (1:1,000), anti-GAPDH (1:10,000), and anti-pro-caspase-1 + p10 + p12 monoclonal antibodies (1:2,000, Abcam, Cambridge, UK); and goat anti-pro-IL-1β polyclonal antibody (1:1,000, R&D Systems, Minneapolis, MN, USA) were used for protein detection. Proteins were visualized using secondary horseradish peroxidase-conjugated goat anti-rabbit or donkey anti-goat antibodies (1:10,000, Abcam, Cambridge, UK) and Pierce SuperSignal West Femto chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA). Grayscale values of bands generated by western blotting were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Enzyme-Linked Immunosorbent Assay
The supernatants of macrophages cultured in 6-well plates were centrifuged at 300 g for 8 min at 4°C, then stored at −80°C. The concentrations of PGE2, cytokines, and chemokines were measured using a PGE2 enzyme-linked immunosorbent assay (ELISA) Kit-Monoclonal (Cayman Chemical Company, Ann Arbor, MI, USA) and mouse ELISA kits for Tumor necrosis factor (TNF)-α, interleukin (IL)-1β (Biolegend, San Diego, CA, USA), and RANTES (PeproTech, Rocky Hill, NJ, USA) following the manufacturer's instructions. Three biological replicates were performed.
Effect of PGE2 on Cell Viability and S. aureus Growth
To analyze the effect of PGE2 on cell viability, macrophages (2 × 104) were incubated with PGE2 (0.1–10 µM) for 24 and 48 h at 37°C in 96-well plates, followed by the addition of 10 μL/well CCK-8 solutions (Cell Counting Kit-8, Dojindo Laboratories, Kumamoto, Japan). After incubation at 37°C for 2 h in the dark, absorbance of the samples was measured at 450 nm using a microplate spectrophotometer. To analyze the effect of PGE2 on S. aureus growth, 1.8 × 107 colony forming units (CFU)/mL were cultured at 37°C in MH broth supplemented with different concentrations of PGE2 (0.1–10 µM). S. aureus growth was analyzed turbidimetrically at 600 nm for 24 h.
Analysis of S. aureus Invasion into Macrophages
To analyze S. aureus invasion into and survival within macrophages, cells were cultured in 6-well plates (2 × 106 cells/well). Then, S. aureus bacterial suspensions (MOI 30:1) were added to the culture medium, followed by incubation for 2 h in 5% CO2 at 37°C. Next, cells were washed 3 times with PBS and incubated in culture media containing gentamicin (final concentration, 100 μg/mL) for 1 h in 5% CO2 at 37°C to kill extracellular bacteria. Finally, macrophage monolayers were digested using trypsin (0.25%) supplemented with 0.1% EDTA-2Na (Hyclone, Logen, UT) and lysed with 800 μL sterile distilled water. Lysates were diluted 1,000-fold and plated on MH agar in triplicate at 37°C overnight. Total CFU were determined by colony counting. To analyze the effects of PGE2 on S. aureus invasion into macrophages, cells were incubated with PGE2 (1 µM) for 24 h before infecting with S. aureus at MOI 30:1, followed by the experimental procedure described above.
Microscopy Assay of Bacterial Phagocytosis and Tetrazolium Dye Reduction Assay of Bacterial Killing
To verify the effects of endogenous and exogenous PGE2 on phagocytosis, macrophages were cultured in 35-mm glass bottom dishes (2 × 106 cells/dish). Cells were pretreated with COX-2 selective inhibitors NS398 (10 µM for 40 min), CAY10404 (1 µM for 40 min), PGE2 (1 µM for 24 h), or left untreated. Then, cell membranes were labeled with 8 μM 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, Thermo Scientific, Carlsbad, CA, USA). They were then infected for 30 min with either Hoechst 33258-labeled WT S. aureus or the Δlgt strain (MOI 30:1) and then fixed with 4% paraformaldehyde. Confocal microscopy (LSM 800, Zeiss, Oberkochen, Germany) was used for capturing images (×400 magnification) and analyzing fluorescence intensity. Images from different samples were captured under identical conditions. The ability of bacteria to survive within macrophages (with or without NS398, CAY10404, or PGE2 pretreatment) 3 h after S. aureus infection (MOI 30:1) was quantified using a tetrazolium dye reduction assay as described previously [32, 33, 34]. Results are expressed as the percent survival of ingested S. aureus within macrophages.
Flow Cytometry Analysis
Macrophages untreated or pretreated with NS398 (10 µM), CAY10404 (1 µM), or PGE2 (1 µM) were infected with WT S. aureus or the Δlgt strain (MOI 30:1) for 9 h or were unstimulated. Cells were then stained with PE-conjugated anti-TLR2 (mT2.7, eBioscience, San Diego, CA, USA), anti-TLR4 (SA15–21, Biolegend, San Diego, CA, USA), anti-SR-A (M204PA, eBioscience, San Diego, CA, USA), or Alexa Fluor 488-conjugated anti-NLRP3 (768319, R and D Systems, Minneapolis, MN, USA) antibodies. Macrophages from C57BL/6J WT, NLRP3−/–, TLR2−/–, and TLR4−/– mice were infected with WT S. aureus (MOI 30:1) for 9 h and then stained with PE-conjugated anti-SR-A antibody. Data were analyzed using Flowjo 10.0 software (Treestar, Ashland, OR, USA).
Data Analysis
All data were analyzed using GraphPad Prism 6 (GraphPad InStat Software, San Diego, CA, USA) and are expressed as the mean ± SD. Statistical significance was evaluated by one-way ANOVA followed by Tukey's multiple comparisons test or 2-way ANOVA with Bonferroni's post-test, as appropriate. Differences with p values ≤0.05 were considered statistically significant (* p < 0.05; ** p < 0.01; *** p < 0.001).
Results
Lipoproteins Are Essential for COX-2 and mPGES-1 Expression in S. aureus-Infected Macrophages
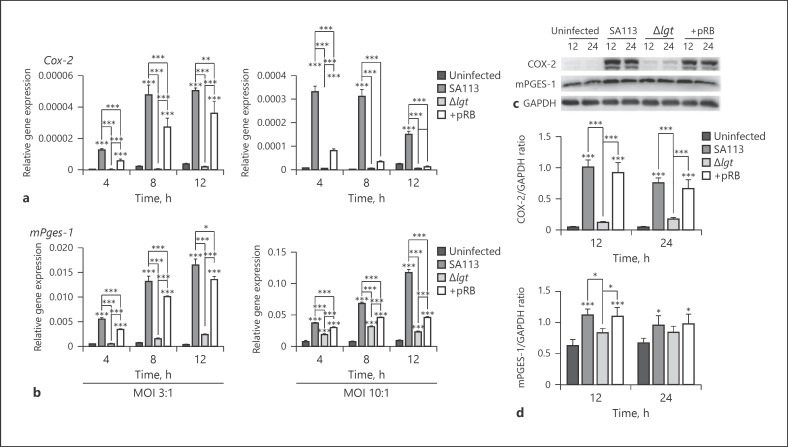
The detailed roles of lipoproteins in COX-2 and mPGES-1 expression in macrophages after S. aureus infection remain unknown. To analyze the role of S. aureus lipoproteins in COX-2 and mPGES-1 expression, their mRNA levels were detected by qRT-PCR, and protein levels were tested by western blotting of extracts from S. aureus-infected macrophages. WT and + pRB S. aureus induced high Cox-2 and mPGES-1 mRNA expression 4, 8, and 12 h post-infection at MOI 3:1 or 10:1 compared with that in uninfected cells or cells infected with Δlgt S. aureus (Fig. 1a, b). Consistent with the qRT-PCR results, COX-2 protein expression increased 12 and 24 h (p < 0.001) and mPGES-1 protein expression increased 12 h (p < 0.05) after infection with WT and + pRB S. aureus (MOI 10:1) compared with that in cells infected with the Δlgt strain or uninfected controls (Fig. 1c, d). These results demonstrate that S. aureus lipoproteins are essential for triggering COX-2 and mPGES-1 expression in macrophages after infection.
Fig. 1.
Lipoproteins are essential for S. aureus-induced COX-2 and mPGES-1 expression in macrophages. Mouse peritoneal macrophages were infected with S. aureus SA113 (WT), isogenic mutant lgt::ermB (Δlgt), or its complemented strain, lgt::ermB + pRBlgt (+ pRB), at MOI 3:1 or 10:1 or not infected. Cox-2 and mPges-1 mRNA expression levels were detected by qRT-PCR and normalized to those of the housekeeping gene Gapdh at the indicated timepoints (a, b). Levels of specific proteins in total macrophage extracts after S. aureus infection at MOI 10:1 were determined by western blotting. GAPDH was used as a loading control (c). Grayscale values were measured using ImageJ software (d). Results are expressed as the mean ± SD of 3 independent experiments and were analyzed by 2-way ANOVA with Bonferroni's post-test. * p < 0.05; ** p < 0.01; *** p < 0.001. COX-2, cyclooxygenases 2; mPGES-1; microsomal prostaglandin E synthases 1; MOI, multiplicity of infection.
TLR2, TLR4, and NLRP3 Affect COX-2 and mPGES-1 Expression in Macrophages after S. aureus Infection
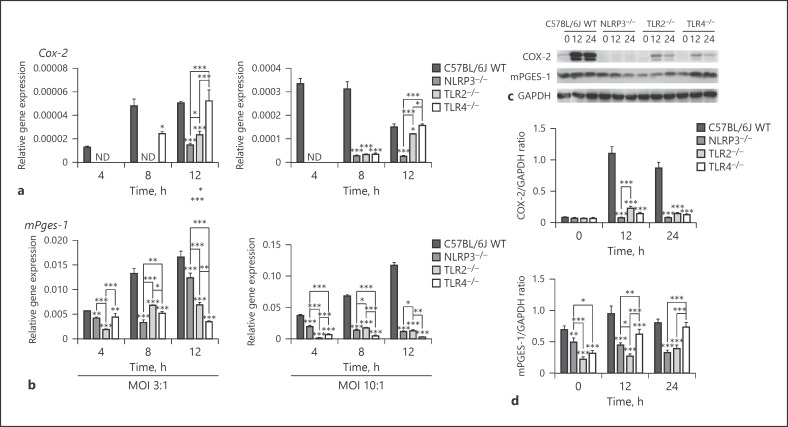
COX-2 and mPGES-1 expression is closely associated with the innate immune response triggered by PRRs [18, 27, 28]. Thus, the detailed roles of TLR2, TLR4, and NLRP3 in COX-2 and mPGES-1 expression in macrophages after S. aureus infection were investigated. To determine the involvement of TLR2, TLR4, and the NLRP3 inflammasome in S. aureus-induced macrophage COX-2 and mPGES-1 expression, we examined macrophages from C57BL/6J WT, NLRP3−/–, TLR2−/–, and TLR4−/– mice. Cox-2 mRNA expression was not detected in macrophages from NLRP3−/– and TLR2−/– mice 4 and 8 h post-infection with WT S. aureusat MOI 3:1 or 4 h post infection at MOI 10:1 (Fig. 2a). Additionally, Cox-2 mRNA expression was not detected in TLR4−/– macrophages 4 h post-infection at MOI 3:1 or 10:1. High Cox-2 mRNA expression levels were observed in WT macrophages compared with those in macrophages from NLRP3−/–, TLR2−/–and TLR4−/– mice at 8 and 12 h (p < 0.05) after S. aureus infection at MOI 3:1 or 10:1. WT S. aureus (MOI 10:1) induced high mPges-1 mRNA expression levels in WT macrophages compared with those in NLRP3−/–, TLR2−/–, and TLR4−/– macrophages (4, 8, and 12 h, p < 0.01; Fig. 2b).
Fig. 2.
TLR2, TLR4, and the NLRP3 inflammasome are involved in S. aureus-induced COX-2 and mPGES-1 expression in macrophages. Macrophages from WT, NLRP3−/–, TLR2−/–, and TLR4−/– mice were infected with WT S. aureus SA113 (SA113) at MOI 3:1 or 10:1. Cox-2 and mPges-1 mRNA expression levels were determined by qRT-PCR and standardized to those of Gapdh at the indicated timepoints (a, b). Levels of the indicated proteins in total macrophage extracts after S. aureus infection at MOI 10:1 were determined by western blotting. GAPDH served as a loading control (c). Grayscale values were measured using ImageJ software (d). Results are expressed as the mean ± SD of 3 independent experiments and were analyzed by 2-way ANOVA with Bonferroni's post-test. * p < 0.05; ** p < 0.01; *** p < 0.001. ND, not detected; TLR, toll-like receptors; WT, wild-type; COX-2, cyclooxygenases-2; mPGES-1; microsomal prostaglandin E synthases-1; MOI, multiplicity of infection.
COX-2 and mPGES-1 protein expression in macrophages after S. aureus infection at MOI 10:1 was analyzed by western blotting. High levels of COX-2 and mPGES-1 protein expression were observed in WT macrophages compared to those in NLRP3−/–, TLR2−/–, and TLR4−/– macrophages 0, 12, and 24 h post-infection, consistent with the mRNA expression data (Fig. 2c, d). These results demonstrate that TLR2, TLR4, and the NLRP3 inflammasome are involved in COX-2 and mPGES-1 expression in S. aureus-infected macrophages.
Induction of PGE2, Proinflammatory Cytokine, and Chemokine Secretion by Macrophages Is Dependent on S. aureus Lipoproteins and Host TLR2, TLR4, and NLRP3
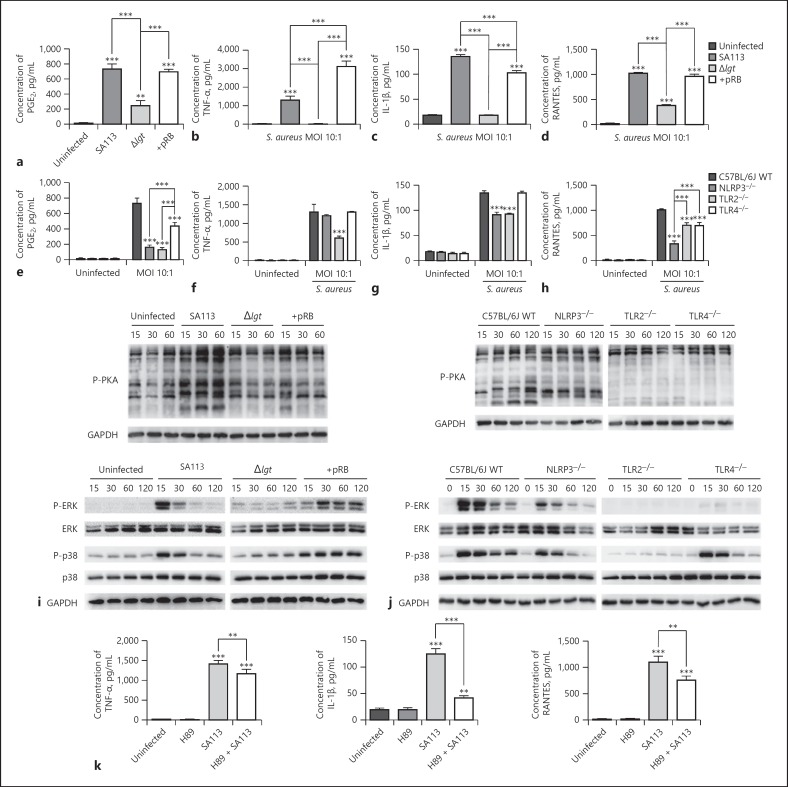
PGE2 biosynthesis depends on COX-2 and mPGES-1 expression during the inflammatory process [26]. Thus, following COX-2 and mPGES-1 expression level analysis, we next determined whether S. aureus lipoproteins and macrophage TLR2, TLR4, and the NLRP3 inflammasome are involved in production of PGE2, proinflammatory cytokines (TNF-α and IL-1β), and RANTES (a chemokine) by S. aureus-infected macrophages using ELISA assays. The results indicated that compared with those produced by WT and + pRB S. aureus, Δlgt S. aureus induced low levels of PGE2, TNF-α, IL-1β, and RANTES secretion into macrophage supernatants after infection of macrophages for 9 h at MOI 10:1 (p < 0.001; Fig. 3a–d).
Fig. 3.
S. aureus lipoproteins and host TLR2, TLR4, and NLRP3 are involved in secretion of PGE2 and proinflammatory cytokines and chemokines by macrophages after S. aureus infection. WT and corresponding gene-deficient macrophages were infected with multiple S. aureus strains (SA113, Δlgt, and + pRB) at MOI 10:1 or not infected. PGE2, TNF-α, IL-1β, and RANTES release into the supernatant of macrophage cultures was analyzed by ELISA 9 h after infection (a–h). Activation of the cAMP-PKA (P-PKA) and MAPK (P-ERK and P-p38) pathways was evaluated by western blotting. GAPDH served as a loading control (i, j). WT macrophages (untreated or pre-treated with 10 µM H89 for 2 h) were infected with WT S. aureus strains at MOI 10:1 or not infected. TNF-α, IL-1β, and RANTES production in the supernatants of macrophages was analyzed by ELISA 9 h after infection (k). Results are expressed as the mean ± SD of 3 independent experiments and were analyzed by one-way ANOVA followed by Tukey's multiple comparisons test or 2-way ANOVA with Bonferroni's post-test. * p < 0.05; ** p < 0.01; *** p < 0.001. PKA, protein kinase A; NLRP3, NOD-like receptor P3; TLR, toll-like receptors; WT, wild-type; COX-2, cyclooxygenases-2; mPGES-1; microsomal prostaglandin E synthases-1; MOI, multiplicity of infection.
Subsequently, PGE2 and cytokine release into cultured macrophage (WT, NLRP3−/–, TLR2−/–, or TLR4−/–) supernatants was analyzed after infection with WT S. aureus (MOI 10:1). S. aureus-infected NLRP3−/–, TLR2−/–, and TLR4−/– macrophages exhibited impaired PGE2 and RANTES secretion compared with macrophages from WT mice (p < 0.001; Fig. 3e, h). Moreover, TLR2−/– macrophages exhibited impaired TNF-α secretion, while NLRP3−/– and TLR2−/– macrophages exhibited impaired IL-1β secretion (p < 0.001; Fig. 3f, g).
To investigate the mechanisms of cytokine secretion, activation of the cAMP-PKA and MAPK pathways in macrophages after S. aureus infection (MOI 10:1) was examined by western blotting. In the cAMP-PKA pathway, WT and + pRB S. aureus induced high levels of PKA phosphorylation 15, 30, and 60 min post-infection, compared with those in cells infected with S. aureus Δlgt or uninfected controls (Fig. 3i). WT S. aureus also induced high levels of PKA phosphorylation in WT macrophages 15, 30, 60, and 120 min post-infection compared with those in macrophages from TLR2−/– and TLR4−/– mice. Additionally, the levels of PKA phosphorylation in infected NLRP3−/– macrophages were slightly reduced compared to in WT macrophages (Fig. 3j).
In the MAPK pathway, S. aureus Δlgt-infected macrophages had impaired p38 and ERK phosphorylation relative to that in cells infected by WT and + pRB S. aureus (Fig. 3i). Moreover, NLRP3−/–, TLR2−/–, and TLR4−/– macrophages exhibited impaired p38 and ERK phosphorylation compared with that in macrophages from WT mice after WT S. aureus infection (Fig. 3j). These MAPK pathway activation data are consistent with the results for cytokine release (Fig. 3b–d, f–h).
Subsequently, WT macrophages were pretreated with the PKA inhibitor H89 to further evaluate the roles of the cAMP-PKA pathway in S. aureus-induced cytokine release. The results indicated that TNF-α, IL-1β, and RANTES secretion in WT S. aureus-infected macrophages (MOI 10:1) was partially decreased by H89 treatment (Fig. 3k). Altogether, these findings suggest that macrophage PGE2, proinflammatory cytokine, and chemokine secretion, along with cAMP-PKA and MAPK pathway activation, are dependent on the presence of S. aureus lipoproteins and macrophage TLR2, TLR4, and the NLRP3 inflammasome.
Endogenous PGE2 Regulates WT S. aureus-Induced Macrophage TLR2/NLRP3 Expression and Cytokine Secretion but Does Not Affect Bacterial Phagocytosis and Intracellular Killing
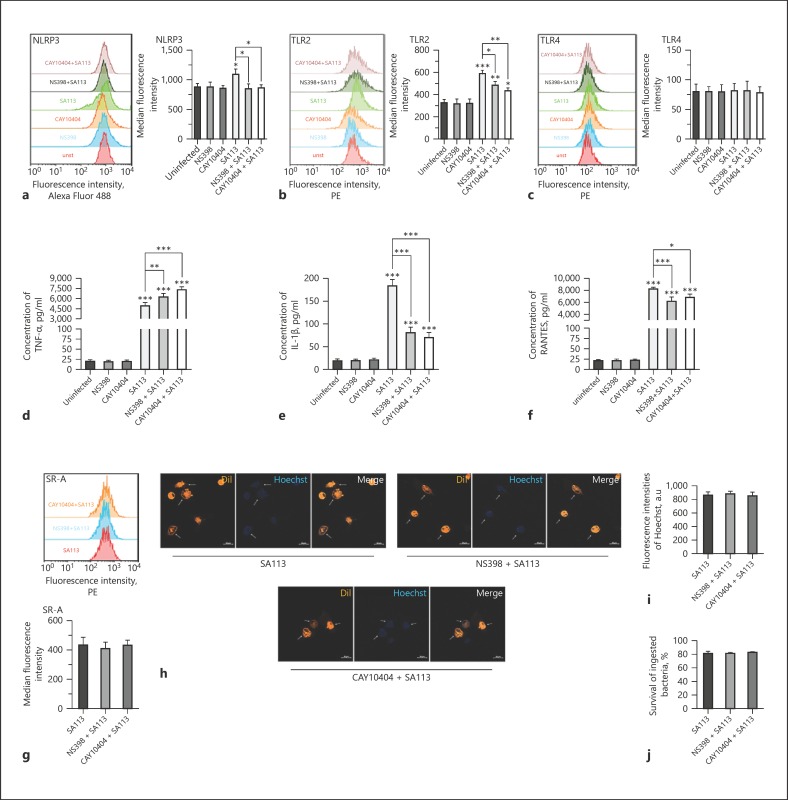
PGE2 has important roles in modulation of the inflammatory and immune responses and participates in the regulation of cytokine production and leucocyte migration, proliferation, and differentiation through autocrine and paracrine signaling [35, 36]. In this study, we analyzed whether endogenous PGE2 regulates TLR/NLRP3 expression and downstream effectors in S. aureus-infected macrophages. The COX-2-selective inhibitors NS398 and CAY10404 were used to block endogenous PGE2 production in macrophages after infection according to previous studies [37, 38]. Based on our preliminary results, 10 µM NS398 or 1 µM CAY10404 was used (online suppl. Fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000499604). Flow cytometry analysis revealed that in WT S. aureus-infected macrophages (MOI 30:1), COX-2 inhibitors decreased S. aureus-induced NLRP3 intracellular expression and TLR2 surface expression but had no effect on TLR4 surface expression (Fig. 4a–c). Additionally, TNF-α secretion was enhanced, while IL-1β and RANTES secretion was decreased by pretreatment with COX-2 inhibitors in macrophages after S. aureus infection for 9 h (MOI 30:1; Fig. 4d–f).
Fig. 4.
Endogenous PGE2 regulates S. aureus-induced TLR2/NLRP3 expression and cytokine secretion in macrophages but not bacterial phagocytosis or intracellular killing. Macrophages (untreated, pre-treated with 10 µM NS398 for 40 min, or pre-treated with 1 µM CAY10404 for 40 min) were infected with WT S. aureus at MOI 30:1 or not infected. NLRP3, TLR2, and TLR4 expression in macrophages was measured by flow cytometry and quantified as the median fluorescence intensity using Flowjo 10.0 software (a–c). TNF-α, IL-1β, and RANTES production in the supernatants of macrophages was analyzed by ELISA 9 h after infection (d–f). SR-A expression in macrophages was measured by flow cytometry and quantified as the median fluorescence intensity using Flowjo 10.0 software (g). Phagocytosis of Hoechst 33258 (blue)-labeled WT S. aureus within DiI-labeled macrophages (orange) was analyzed by microscopy (× 400, h, i). Bacterial intracellular killing was measured by tetrazolium dye reduction assay (j). The results are expressed as the mean ± SD of 3 independent experiments and were analyzed by one-way ANOVA followed by Tukey's multiple comparisons test. * p < 0.05; ** p < 0.01; *** p < 0.001. NLRP3, NOD-like receptor P3; TLR, toll-like receptors.
S. aureus can survive and proliferate inside professional phagocytes, such as neutrophils, monocytes, and macrophages, and this phenomenon may even contribute to the dissemination of S. aureus within its host [39]. Additionally, increased SR-A expression is important for S. aureus phagocytosis in macrophages [40]. The data indicate that COX-2 inhibitors did not affect SR-A expression, bacterial phagocytosis, or intracellular killing in S. aureus-infected macrophages (MOI 30:1; Fig. 4g–j). These findings suggest that endogenous PGE2 does not affect bacterial phagocytosis and intracellular killing, although it regulates WT S. aureus-induced TLR2/NLRP3 expression and cytokine secretion in macrophages.
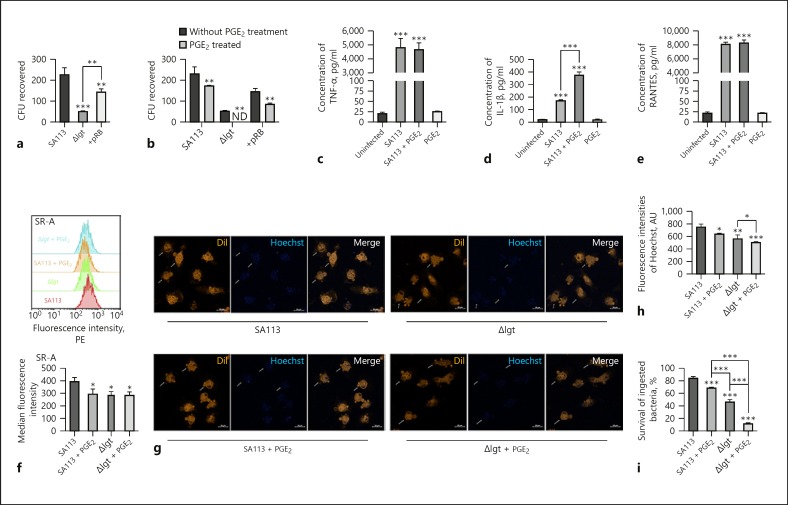
Phagocytosis of S. aureus and Their Survival within Macrophages Are Dependent on S. aureus Lipoproteins and Inhibited by Exogenous PGE2 Treatment
The roles of lipoproteins in the phagocytosis of S. aureus and their survival within macrophages, as well as the influence of exogenous PGE2 on these processes, were unknown. According to the recovered CFUs, compared with WT and + pRB S. aureus, infection with the Δlgt strain (MOI 30:1) induced low levels of bacteria invasion and survival within macrophages (Fig. 5a). To analyze the effect of PGE2 on S. aureus invasion into macrophages, cells were pretreated with PGE2 for 24 h before infection (MOI 30:1). Treatment with 0.1–10 µM PGE2 for 24 and 48 h was not cytotoxic to macrophages and had no effect on S. aureus growth. Furthermore, S. aureus invasion into macrophages was significantly inhibited by 1 and 10 µM PGE2 (online suppl. Fig. S2). Thus, 1 µM PGE2 was used for subsequent experiments. Invasion of WT, + pRB, and Δlgt S. aureus into macrophages was inhibited by PGE2 treatment (Fig. 5b).
Fig. 5.
Phagocytosis of S. aureus and survival within macrophages are dependent on S. aureus lipoproteins, and these processes are inhibited by PGE2. Macrophages (untreated or pre-treated with 1 µM PGE2 for 24 h) were infected using multiple S. aureus strains (SA113, Δlgt, and + pRB) at MOI 30:1 or not infected. To analyze S. aureus invasion into macrophages 3 h after infection, the colony counting technique was used to determine CFU recovered from macrophage lysates (a, b). ND, not detected. TNF-α, IL-1β, and RANTES production in the supernatants of PGE2-pre-treated or untreated macrophages was analyzed by ELISA 9 h after infection (c–e). SR-A expression in macrophages infected with WT or Δlgt S. aureus was measured by flow cytometry and quantified as median fluorescence intensity using Flowjo 10.0 software (f). Phagocytosis of Hoechst 33,258 (blue)-labeled WT or Δlgt S. aureus within DiI-labeled macrophages (Orange) was analyzed by microscopy assay (×400, g, h). Bacterial intracellular killing was measured by tetrazolium dye reduction assay (i). Results are expressed as the mean ± SD of 3 independent experiments and analyzed by one-way ANOVA followed by Tukey's multiple comparisons test or 2-way ANOVA. * p < 0.05; ** p < 0.01; *** p < 0.001. CFU, colony forming units; PGE2, prostaglandin E2; SR-A, scavenger receptor A.
Next, cytokine production by macrophages exposed to WT S. aureus (MOI 30:1) was measured by ELISA. PGE2 had no effect on TNF-α or RANTES secretion by macrophages into culture supernatants after S. aureus infection for 9 h (Fig. 5c, e), whereas IL-1β production was enhanced by PGE2 treatment (Fig. 5d). These results demonstrate that S. aureus invasion into macrophages is dependent on S. aureus lipoproteins and inhibited by PGE2 treatment, which also leads to enhanced IL-1β secretion.
Analysis of flow cytometry data demonstrated that WT S. aureus induced high levels of SR-A expression in peritoneal macrophages compared to in cells infected with Δlgt S. aureus (MOI 30:1, p < 0.05), and this process was inhibited by PGE2 treatment (p < 0.05; Fig. 5f). Consistent with these results, the microscopy assay indicated that compared to infection with the Δlgt strain, infection with Hoechst 33258-labeled WT S. aureus (MOI 30:1) for 30 min induced higher levels of bacteria invasion into macrophages (p < 0.01), and this process was inhibited by PGE2 (p < 0.05; Fig. 5g, h). Meanwhile, percent survival of WT S. aureus within macrophages 3 h post-infection (MOI 30:1) was higher than that of the Δlgt strain (p < 0.001; Fig. 5i). The PGE2 pretreatment increased both WT and Δlgt S. aureus intracellular killing, as evidenced by decreases in the survival of ingested bacteria of approximately 16 and 35%, respectively (p < 0.001; Fig. 5i). Thus, both phagocytosis of S. aureus and their survival within peritoneal macrophages are dependent on the presence of S. aureus lipoproteins, and these processes are inhibited by PGE2 treatment, which limits their invasion.
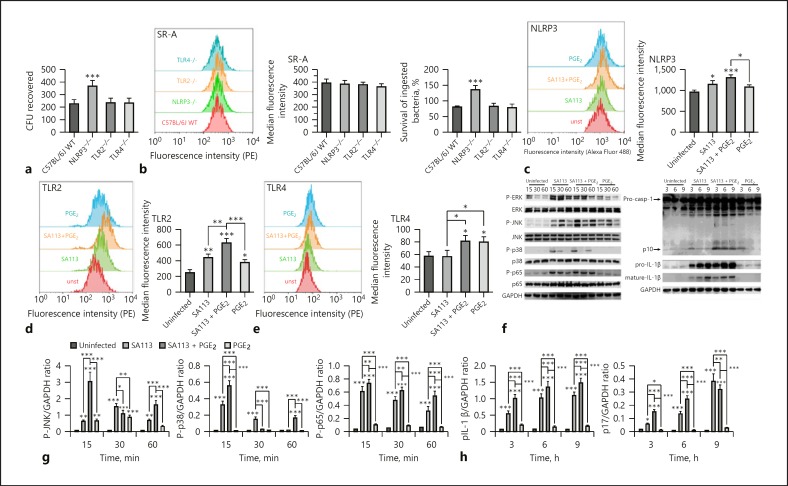
Exogenous PGE2 Regulates TLR2-, TLR4-, and NLRP3-Mediated Signaling Activation in Macrophages after S. aureus Infection
In this study, we further evaluated the roles of TLR2, TLR4, and NLRP3 in S. aureus survival within macrophages. Additionally, whether exogenous PGE2 regulates TLR2-, TLR4-, and NLRP3-mediated signaling activation in macrophages after S. aureus infection was analyzed. WT S. aureus invasion and/or survival increased in NLRP3−/– macrophages compared with that in macrophages from WT, TLR2−/–, and TLR4−/– mice. To clarify the associated mechanisms, we analyzed SR-A expression, which was tightly correlated to S. aureus phagocytosis in macrophages and bacterial intracellular killing after infection (MOI 30:1). There was no significant difference in SR-A expression among the 4 types of macrophages after WT S. aureus infection. However, percent survival of WT S. aureus within NLRP3−/– macrophages after infection increased compared to that of WT, TLR2−/–, and TLR4−/– macrophages (p < 0.001). Thus, NLRP3 inflammasomes may play key roles in bacterial intracellular killing and preventing S. aureus survival within macrophages (Fig. 6a, b).
Fig. 6.
TLR2-, TLR4-, and NLRP3-mediated signaling activation in macrophages after S. aureus infection is regulated by PGE2 treatment. WT and corresponding gene-deficient macrophages were infected using WT S. aureus SA113 at MOI 30:1 for 3 h, and the CFU recovered from lysates was determined (a). SR-A expression in WT and corresponding gene-deficient macrophages after WT S. aureus infection was measured by flow cytometry and quantified as median fluorescence intensity using Flowjo 10.0 software. Bacterial intracellular killing was measured by tetrazolium dye reduction assay to analyze S. aureus invasion into macrophage (b). Macrophages (untreated or pre-treated with 1 µM PGE2 for 24 h) were infected with WT S. aureus SA113 at MOI 30:1 or not infected. NLRP3, TLR2, and TLR4 expression in macrophages was measured by flow cytometry and quantified as median fluorescence intensity using Flowjo 10.0 software (c–e). Activation of MAPK (P-ERK, P-JNK, and P-p38), NF-κB (P-p65), and caspase-1 (p10, pro-IL-1β, and mature IL-1β) pathways were evaluated by western blotting; GAPDH was used as a loading control (f). Grayscale values were measured using ImageJ software (g, h). Results are expressed as the mean ± SD of 3 independent experiments and were analyzed by one-way ANOVA followed by Tukey's multiple comparisons test or 2-way ANOVA with Bonferroni's post-test. * p < 0.05; ** p < 0.01; *** p < 0.001. CFU, colony forming units; SR-A, scavenger receptor A; NLRP3, NOD-like receptor P3; TLR, toll-like receptors; PGE2, prostaglandin E2.
We further examined the effect of exogenous PGE2 on NLRP3, TLR2, and TLR4 expression in macrophages after infection with WT S. aureus (MOI 30:1). The flow cytometry data demonstrated that intracellular expression of NLRP3 in macrophages was induced by S. aureus infection (p < 0.05), whereas PGE2 pretreatment for 24 h had no significant effect on NLRP3 expression in S. aureus-infected macrophages (Fig. 6c). TLR2 surface expression on macrophages increased after S. aureus infection or PGE2 pretreatment relative to that in untreated control cells (p < 0.05). Moreover, S. aureus-induced TLR2 surface expression was enhanced by PGE2 pretreatment relative to that in cells infected with S. aureus alone (p < 0.01; Fig. 6d). Surface expression of TLR4 was also induced by PGE2 treatment, but not by S. aureus infection (Fig. 6e). To evaluate the effect of PGE2 on S. aureus-induced MAPK, NF-κB, and caspase-1 signaling in macrophages, activation of ERK, JNK, p38, p65, and caspase-1 was examined by western blotting. The activation of JNK (15 and 60 min; p < 0.001), p38 (15 and 60 min; p < 0.001), p65 (15, 30, and 60 min; p < 0.01), and caspase-1 (3, 6, and 9 h) was upregulated by PGE2 in macrophages after S. aureus infection (MOI 30:1; Fig. 6f, g). Meanwhile, S. aureus-induced expression of pro-IL-1β (3, 6, and 9 h; p < 0.001) and mature IL-1β (3 and 6 h; p < 0.001) in total macrophage cellular protein was enhanced by PGE2 treatment (Fig. 6f, h). Thus, TLR2-, TLR4-, and NLRP3-mediated signaling activation in macrophages can be regulated by exogenous PGE2.
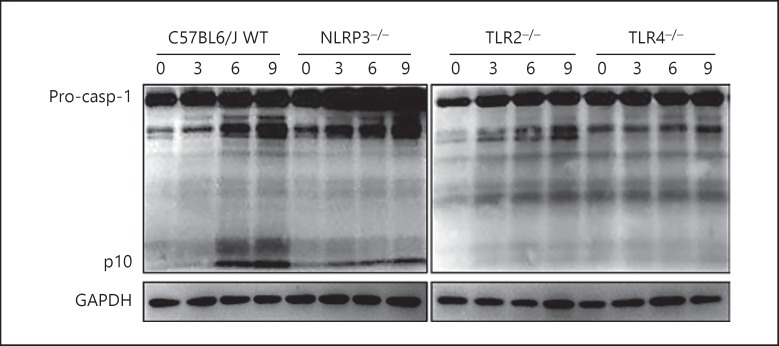
Activation of Caspase-1 in S. aureus-Infected Macrophages Depends on TLR2, TLR4, and NLRP3
To evaluate the influence of TLRs on NLRP3 inflammasome-mediated signaling activation, the roles of TLR2, TLR4, and NLRP3 in caspase-1 activation of macrophages after S. aureus infection (MOI 10:1) were analyzed. The results indicated that S. aureus-induced activation of caspase-1 was decreased in NLRP3−/–, TLR2−/–, and TLR4−/– macrophages at 3, 6, and 9 h post-infection (Fig. 7). Thus, not only NLRP3 but also TLR2 and TLR4 may be involved in S. aureus-induced caspase-1 activation in macrophages.
Fig. 7.
S. aureus-induced activation of caspase-1 was decreased in NLRP3−/–, TLR2−/–, and TLR4−/– macrophages. WT and corresponding gene-deficient macrophages were infected with WT S. aureus SA113 (MOI 10:1). Activation of caspase-1 was examined by western blotting; GAPDH was used as a loading control. WT, wild-type; NLRP3, NOD-like receptor P3; TLR, toll-like receptors.
Discussion
The results of this study indicate a correlation between PGE2, a known endogenous lipid mediator, and PRR-mediated innate immune responses to S. aureus by mouse peritoneal macrophages. It is well-known that COX-2 and mPGES-1 expression and PGE2 biosynthesis are closely associated with the host immune response and inflammatory process [18, 26]. Here, we found that in macrophages, the S. aureusisogenic mutant strain Δlgt deficient in lipoprotein maturation induced low levels of COX-2 and mPGES-1 expression relative to that in cells infected with its complemented strain (+ pRB) or WT S. aureus (Fig. 1). COX-2 and mPGES-1 expressions were also impaired in TLR2−/–, TLR4−/–, and NLRP3−/– macrophages (Fig. 2). These results indicate that in S. aureus-infected macrophages, BLPs are essential for triggering COX-2 and mPGES-1 expression. Additionally, S. aureus-induced COX-2 and mPGES-1 expression was dependent on the presence of TLR2, TLR4, and NLRP3 in macrophages.
Here, secretion of PGE2, proinflammatory cytokines, and chemokines was investigated in S. aureus-infected macrophages. Our results show that the S. aureus Δlgt strain induced reduced levels of PGE2, TNF-α, IL-1β, and RANTES secretion by macrophages compared with the corresponding WT and + pRB strains. Furthermore, PGE2 and RANTES secretion was impaired in TLR2−/–, TLR4−/–, and NLRP3−/– macrophages. Moreover, TNF-α secretion was impaired in TLR2−/– macrophages, while IL-1β secretion was decreased in both NLRP3−/– and TLR2−/– macrophages (Fig. 3). These results, particularly regarding PGE2 secretion by macrophages, are consistent with the COX-2 and mPGES-1 expression data generated in this study.
Our results indicated that in addition to MAPK signaling, activation of the cAMP-PKA pathways was involved in cytokine release in macrophages after S. aureus infection (Fig. 3k). We found that the S. aureus Δlgt strain induced low levels of cAMP-PKA and MAPK pathway activation. Meanwhile, TLR2−/–, TLR4−/–, and NLRP3−/– macrophages exhibited impaired activation of both cAMP-PKA and MAPK signaling, consistent with the data regarding PGE2 and inflammatory cytokine secretion (Fig. 3). These findings suggest that BLPs are required for macrophage activation and PGE2 secretion after S. aureus infection, along with macrophage expression of TLR2, TLR4, and NLRP3. Our findings are consistent with those of previous studies indicating that activation of TLR2 and the NLRP3 inflammasome is induced by S. aureus BLPs [12, 15, 41]. There is no clear evidence that supports direct recognition of BLPs or other components of S. aureus by TLR4; however, while the immune response to experimental S. aureus-induced brain abscesses is reported to crucially depend on the recognition of S. aureus by TLR2, TLR4 is also required for the induction of an optimal intracerebral immune response [14].
The cooperation and cross-talk between multiple PRRs in the innate immune system are essential for host recognition of pathogen-associated molecular patterns/damage-associated molecular patterns and defense against bacterial infection [42, 43]. For example, TLR4 and TLR2 interact functionally in the basolateral membrane of medullary thick ascending limb cells to mediate LPS-induced ERK activation [44]. NLRP3 signaling is regulated by a priming signal that induces expression of NLRP3 and pro-IL-1β and is mediated by NF-κB activation via TLRs and the IL-1 receptor [43]. Thus, we conclude that BLPs are dominant immunobiologically active compounds in S. aureus that activate cells through both TLR2 and NLRP3; however, this process could be optimized via TLR4 to ensure an effective immune response to S. aureus infection.
It is well-known that PGE2 has important roles in regulating inflammatory and immune responses [35, 36]. Thus, the roles of endogenous PGE2 in regulating TLR/NLRP3 expression and downstream effectors in S. aureus-infected macrophages were analyzed. We found that TNF-α secretion was enhanced, while IL-1β secretion was decreased in S. aureus-infected macrophages when endogenous PGE2 production was blocked (Fig. 4d, e). These results, particularly those regarding TNF-α secretion by macrophages, are consistent with the results of a previous study showing that TNF-α production in macrophages after stimulation is enhanced by a COX-2 inhibitor indomethacin treatment [45]. Subsequent analysis suggested that enhanced IL-1β production is closely related to inhibition of intracellular invasion by S. aureus. Although IL-1β was decreased, pretreatment with COX-2 inhibitors had no effect on S. aureus phagocytosis and survival within macrophages (Fig. 4g–j). Previous findings demonstrated that the regulation of TNF-α secretion can increase the rate of bacterial clearance [46]. Thus, the constant S. aureus phagocytosis and intracellular killing in macrophages with blockage of endogenous PGE2 production may be dependent on enhanced TNF-α production. According to these results, a high concentration of exogenous PGE2 was used to analyze its roles in macrophages in response to S. aureus infection.
In previous studies, 1 µM exogenous PGE2 was used for analyzing its effects in alveolar macrophages during bacterial infection, suggesting that this concentration of PGE2 was acceptable in this study [32, 40, 47]. Here, based on preliminary results, a high concentration of PGE2 (1 µM) was used. We found that BLPs were essential for S. aureus invasion and/or survival within macrophages and that this process was inhibited by exogenous PGE2 (Fig. 5a, b). To understand the mechanisms underlying this process, bacterial phagocytosis and intracellular killing were analyzed. Our results showed that WT S. aureus induced higher levels of SR-A expression and bacterial invasion and lower levels of intracellular killing in peritoneal macrophages compared to those in cells infected with the Δlgt strain, suggesting that S. aureus lipoproteins are responsible for both bacterial phagocytosis and survival within macrophages (Fig. 5f–i). Meanwhile, SR-A expression, bacterial phagocytosis, and survival within macrophages after S. aureus infection were downregulated by PGE2 (Fig. 5f–i); however, these results are surprisingly different than those found in previous studies on the effects of PGE2 on alveolar macrophages [32, 40, 47]. We suggest that these differences could be explained by tissue-specific differences in the macrophage populations.
A previous study indicated that in mouse bone marrow-derived macrophages, PGE2-induced IL-1β production was dependent on activation of the transcription factor CREB via increased levels of cAMP [48]. We found that production of the proinflammatory cytokine IL-1β by S. aureus-infected macrophages was enhanced by PGE2 treatment (Fig. 5d), consistent with our observation of enhanced activation of MAPK, NF-κB, and caspase-1 signaling (Fig. 6). NLRP3 inflammasomes are responsible for activation of caspase-1 and expression of mature IL-1β, and the inflammasome priming signal is promoted by TLR-mediated activation [43]. We also found that S. aureus-induced activation of caspase-1 was decreased in not only NLRP3−/– macrophages but also TLR2−/– and TLR4−/– macrophages 3, 6, and 9 h post-infection (Fig. 7). Furthermore, surface expression of TLR2 and TLR4, but not intracellular expression of NLRP3, increased in S. aureus-infected macrophages treated with PGE2 (Fig. 6). These results suggest that priming of NLRP3 inflammasomes in macrophages after S. aureus infection may be associated with activation of TLR2 and TLR4. Although IL-1β secretion is upregulated by PGE2, there is no direct evidence to support a role for IL-1β in suppressing S. aureus macrophage invasion. Interestingly, S. aureus invasion was only enhanced in NLRP3−/– macrophages, suggesting that NLRP3 inflammasomes, but not TLR2 and TLR4, may play a key role in preventing S. aureus survival within macrophages (Fig. 6a, b); therefore, we infer that there is a tight relationship between NLRP3-mediated IL-1β production and inhibition of intracellular invasion by S. aureus. This will be investigated further in our future studies.
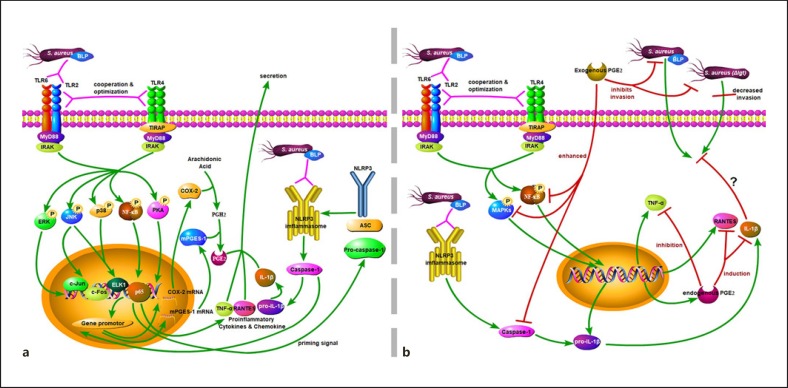
Based on the findings of previous studies and our results, although not all of the results in the figure have been experimentally confirmed, we infer that BLPs in S. aureus are essential immunobiologically active compounds that activate mouse peritoneal macrophages via both TLR2 and NLRP3 signaling. This process can be optimized by TLR4 to ensure an effective immune response, inducing release of a series of inflammatory mediators, including proinflammatory cytokines (TNF-α and IL-1β), a chemokine (RANTES), and PGE2 (Fig. 8a). Hence, lipoproteins play a crucial role in S. aureus phagocytosis and their survival within peritoneal macrophages. These processes can be suppressed by exogenous PGE2 treatment, in addition to enhanced activation of TLR-mediated MAPK and NF-κB signaling, and can promote NLRP3 inflammasome priming and IL-1β secretion. Endogenous PGE2 regulates S. aureus-induced cytokine secretion from macrophages but has no effect on bacterial phagocytosis or intracellular killing (Fig. 8b). These results suggest that TLR2-, TLR4-, and NLRP3-mediated activation of macrophages after S. aureus infection can be regulated by PGE2. However, the detailed role of NLRP3-mediated IL-1β secretion in inhibiting S. aureus survival within macrophages was not analyzed in this study. Furthermore, whether PGE2 and its analogs can be used as a medicine or immunomodulator to protect against infectious diseases caused by S. aureus remains unknown. Thus, additional studies, especially clinical tests, are needed.
Fig. 8.
The relationship between PGE2 and TLR2-, TLR4-, and NLRP3-mediated activation of macrophages after S. aureus infection. BLP in general of S. aureus is essential immunobiologically active compounds that activate mouse peritoneal macrophages via TLR2 and NLRP3 inflammasomes. This process can be optimized by TLR4 to ensure an effective immune response, which leads to the secretion of a series of inflammatory mediators inducing TNF-α, IL-1β, RANTES, and PGE2 through MAPK, NF-κB, cAMP-PKA, and caspase-1 signaling (a). Meanwhile, BLP is required for S. aureus phagocytosis and their survival within peritoneal macrophages. These processes can be modified by exogenous PGE2 treatment, but not endogenous PGE2, which can decrease bacterial phagocytosis while also increasing intracellular killing, activation of TLR-mediated MAPK, and NF-κB signaling and can promote NLRP3 inflammasome priming and IL-1β secretion. The relationship between NLRP3-mediated IL-1β production and inhibition of intracellular invasion by S. aureus is unknown (b). The green arrows indicate activation, induction, or invasion; the pink arrows indicate recognition or interaction; the red arrows indicate modulation. COX-2, cyclooxygenases-2; TLR, toll-like receptors; PGE2, prostaglandin E2.
Statement of Ethics
All animal experiments were performed according to regulations of the Administration of Affairs Concerning Experimental Animals in China. The experimental protocol was approved by the Animal Welfare and Research Ethics Committee of the Inner Mongolia Agricultural University (approval ID: 20151227–2).
Disclosure Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Sources
This work was supported by Science Foundation for Distinguished and Outstanding Young Scholars of Inner Mongolia Agricultural University (2017XYQ-2), the National Natural Science Foundation of China (No. 31560714; 31672603), and Initial Scientific Research Foundation of Inner Mongolia Agricultural University (No. YJ2014-10).
Author Contributions
B.L. and J.W.: conceptualization. J.W., W.M., S.F., and Y.Y.: data curation. J.W. and B.L.: formal analysis. B.L., J.C., and SF.: funding acquisition. F.B. and Y.S.: Investigation. A.G. and B.J.: methodology. B.L. and J.C.: Project administration.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgments
We thank Prof. Friedrich Götz (Mikrobielle Genetik, Universität Tübingen, Germany) for providing the S. aureus strains.
References
- 1.Archer GL. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis. 1998 May;26((5)):1179–81. doi: 10.1086/520289. [DOI] [PubMed] [Google Scholar]
- 2.Vautor E, Magnone V, Rios G, Le Brigand K, Bergonier D, Lina G, et al. Genetic differences among Staphylococcus aureus isolates from dairy ruminant species: a single-dye DNA microarray approach. Vet Microbiol. 2009 Jan;133((1-2)):105–14. doi: 10.1016/j.vetmic.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Bubeck Wardenburg J, Williams WA, Missiakas D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Natl Acad Sci USA. 2006 Sep;103((37)):13831–6. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas CA, Li Y, Kodama T, Suzuki H, Silverstein SC, El Khoury J. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med. 2000 Jan;191((1)):147–56. doi: 10.1084/jem.191.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004 Jul;4((7)):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010 May;11((5)):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 7.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014 Jan;14((1)):9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 8.Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005 Jul;18((3)):521–40. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang L, Wu HM, Ding PS, Liu RY. TLR2 mediates phagocytosis and autophagy through JNK signaling pathway in Staphylococcus aureus-stimulated RAW264.7 cells. Cell Signal. 2014 Apr;26((4)):806–14. doi: 10.1016/j.cellsig.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Schröder NW, Morath S, Alexander C, Hamann L, Hartung T, Zähringer U, et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003 May;278((18)):15587–94. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 11.Dziarski R, Gupta D. Staphylococcus aureus peptidoglycan is a toll-like receptor 2 activator: a reevaluation. Infect Immun. 2005 Aug;73((8)):5212–6. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol. 2006 Feb;18((2)):355–62. doi: 10.1093/intimm/dxh374. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Tawaratsumida K, Kariya H, Kiyohara A, Suda Y, Krikae F, et al. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol. 2006 Sep;177((5)):3162–9. doi: 10.4049/jimmunol.177.5.3162. [DOI] [PubMed] [Google Scholar]
- 14.Stenzel W, Soltek S, Sanchez-Ruiz M, Akira S, Miletic H, Schlüter D, et al. Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am J Pathol. 2008 Jan;172((1)):132–45. doi: 10.2353/ajpath.2008.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz-Planillo R, Franchi L, Miller LS, Núñez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009 Sep;183((6)):3942–8. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen MT, Götz F. Lipoproteins of gram-positive bacteria: key players in the immune response and virulence. Microbiol Mol Biol Rev. 2016 Aug;80((3)):891–903. doi: 10.1128/MMBR.00028-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito S, Quadery AF. Staphylococcus aureus lipoprotein induces skin inflammation, accompanied with ifn-gamma-producing t cell accumulation through dermal dendritic cells. Pathogens. 2018 Jul;7((3)):7. doi: 10.3390/pathogens7030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Díaz-Muñoz MD, Osma-García IC, Fresno M, Iñiguez MA. Involvement of PGE2 and the cAMP signalling pathway in the up-regulation of COX-2 and mPGES-1 expression in LPS-activated macrophages. Biochem J. 2012 Apr;443((2)):451–61. doi: 10.1042/BJ20111052. [DOI] [PubMed] [Google Scholar]
- 19.Wånggren K, Lalitkumar PG, Stavreus-Evers A, Ståbi B, Gemzell-Danielsson K. Prostaglandin E2 and F2alpha receptors in the human Fallopian tube before and after mifepristone treatment. Mol Hum Reprod. 2006 Sep;12((9)):577–85. doi: 10.1093/molehr/gal058. [DOI] [PubMed] [Google Scholar]
- 20.Serhan CN, Levy B. Success of prostaglandin E2 in structure-function is a challenge for structure-based therapeutics. Proc Natl Acad Sci USA. 2003 Jul;100((15)):8609–11. doi: 10.1073/pnas.1733589100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006 Jun;119((3)):229–40. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003 Jun;110((5-6)):255–8. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 23.Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:165–75. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 24.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007 Sep;59((3)):207–24. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 25.Su Y, Huang X, Raskovalova T, Zacharia L, Lokshin A, Jackson E, et al. Cooperation of adenosine and prostaglandin E2 (PGE2) in amplification of cAMP-PKA signaling and immunosuppression. Cancer Immunol Immunother. 2008 Nov;57((11)):1611–23. doi: 10.1007/s00262-008-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogdan D, Falcone J, Kanjiya MP, Park SH, Carbonetti G, Studholme K, et al. Fatty acid-binding protein 5 controls microsomal prostaglandin E synthase 1 (mPGES-1) induction during inflammation. J Biol Chem. 2018 Apr;293((14)):5295–306. doi: 10.1074/jbc.RA118.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granick JL, Falahee PC, Dahmubed D, Borjesson DL, Miller LS, Simon SI. Staphylococcus aureus recognition by hematopoietic stem and progenitor cells via TLR2/MyD88/PGE2 stimulates granulopoiesis in wounds. Blood. 2013 Sep;122((10)):1770–8. doi: 10.1182/blood-2012-11-466268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang Y, Zhao F, Liang J, Deng X, Zhang Y, Ding G, et al. Activation of cox-2/mpges-1/pge2 cascade via nlrp3 inflammasome contributes to albumin-induced proximal tubule cell injury. Cell Physiol Biochem. 2017;42((2)):797–807. doi: 10.1159/000478070. [DOI] [PubMed] [Google Scholar]
- 29.Stoll H, Dengjel J, Nerz C, Götz F. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect Immun. 2005 Apr;73((4)):2411–23. doi: 10.1128/IAI.73.4.2411-2423.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmaler M, Jann NJ, Ferracin F, Landmann R. T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88-dependent T cell activation. J Immunol. 2011 Jan;186((1)):443–52. doi: 10.4049/jimmunol.1001407. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25((4)):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007 Nov;37((5)):562–70. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005 Aug;106((3)):1067–75. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods. 1985 Sep;82((1)):131–40. doi: 10.1016/0022-1759(85)90232-7. [DOI] [PubMed] [Google Scholar]
- 35.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001 Jul;108((1)):15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakata D, Yao C, Narumiya S. Prostaglandin E2, an immunoactivator. J Pharmacol Sci. 2010;112((1)):1–5. doi: 10.1254/jphs.09r03cp. [DOI] [PubMed] [Google Scholar]
- 37.Eisengart CA, Mestre JR, Naama HA, Mackrell PJ, Rivadeneira DE, Murphy EM, et al. Prostaglandins regulate melanoma-induced cytokine production in macrophages. Cell Immunol. 2000 Sep;204((2)):143–9. doi: 10.1006/cimm.2000.1686. [DOI] [PubMed] [Google Scholar]
- 38.Tajima T, Murata T, Aritake K, Urade Y, Michishita M, Matsuoka T, et al. EP2 and EP4 receptors on muscularis resident macrophages mediate LPS-induced intestinal dysmotility via iNOS upregulation through cAMP/ERK signals. Am J Physiol Gastrointest Liver Physiol. 2012 Mar;302((5)):G524–34. doi: 10.1152/ajpgi.00264.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horn J, Stelzner K, Rudel T, Fraunholz M. Inside job: staphylococcus aureus host-pathogen interactions. Int J Med Microbiol. 2018 Aug;308((6)):607–24. doi: 10.1016/j.ijmm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Domingo-Gonzalez R, Katz S, Serezani CH, Moore TA, Levine AM, Moore BB. Prostaglandin E2-induced changes in alveolar macrophage scavenger receptor profiles differentially alter phagocytosis of Pseudomonas aeruginosa and Staphylococcus aureus post-bone marrow transplant. J Immunol. 2013 Jun;190((11)):5809–17. doi: 10.4049/jimmunol.1203274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller P, Müller-Anstett M, Wagener J, Gao Q, Kaesler S, Schaller M, et al. The Staphylococcus aureus lipoprotein SitC colocalizes with Toll-like receptor 2 (TLR2) in murine keratinocytes and elicits intracellular TLR2 accumulation. Infect Immun. 2010 Oct;78((10)):4243–50. doi: 10.1128/IAI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007 Mar;7((3)):179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe Y, Nagai Y, Takatsu K. Activation and regulation of the pattern recognition receptors in obesity-induced adipose tissue inflammation and insulin resistance. Nutrients. 2013 Sep;5((9)):3757–78. doi: 10.3390/nu5093757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Good DW, George T, Watts BA., 3rd Toll-like receptor 2 is required for LPS-induced Toll-like receptor 4 signaling and inhibition of ion transport in renal thick ascending limb. J Biol Chem. 2012 Jun;287((24)):20208–20. doi: 10.1074/jbc.M111.336255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caughey GE, Pouliot M, Cleland LG, James MJ. Regulation of tumor necrosis factor-alpha and IL-1 beta synthesis by thromboxane A2 in nonadherent human monocytes. J Immunol. 1997 Jan;158((1)):351–8. [PubMed] [Google Scholar]
- 46.Skerry C, Harper J, Klunk M, Bishai WR, Jain SK. Adjunctive TNF inhibition with standard treatment enhances bacterial clearance in a murine model of necrotic TB granulomas. PLoS One. 2012;7((6)):e39680. doi: 10.1371/journal.pone.0039680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salina AC, Souza TP, Serezani CH, Medeiros AI. Efferocytosis-induced prostaglandin E2 production impairs alveolar macrophage effector functions during Streptococcus pneumoniae infection. Innate Immun. 2017 Apr;23((3)):219–27. doi: 10.1177/1753425916684934. [DOI] [PubMed] [Google Scholar]
- 48.Martínez-Colón GJ, Taylor QM, Wilke CA, Podsiad AB, Moore BB. Elevated prostaglandin E2 post-bone marrow transplant mediates interleukin-1β-related lung injury. Mucosal Immunol. 2018 Mar;11((2)):319–32. doi: 10.1038/mi.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data