Abstract
Background
Different studies suggest that fulvestrant 500 mg every 28 days (HD-FUL) could be an active treatment in HR+ advanced breast cancer (ABC) patients even treated with aromatase inhibitors in the adjuvant setting. The aim of this analysis is to describe the outcome of ABC patients treated with HD-FUL as first-line treatment in terms of median duration of treatment and the overall response rate in a real-world setting.
Methods
For the purpose of the present analysis, we considered two data sets of HR+ ABC patients collected in Italy between 2012 and 2015 (EVA and GIM-13 AMBRA studies).
Results
Eighty-one and 91 patients have been identified from the two data sets. The median age was 63 years (range 35–82) for the EVA and 57.8 years (range 35.0–82.3) for the AMBRA patients. ORRs were 23.5 and 24.3% in the whole population, 26.9% in the patients with bone only, and 21.8 and 21.4% in those with visceral metastases. The median duration of HD-FUL was 11.6 months (range 1–48) and 12.4 months (range 2.9–70.0) in the two data sets, respectively.
Conclusion
These data suggest that HD-FUL should still continue to play a significant role as first-line therapy in HR+ ABC patients.
Keywords: Fulvestrant, First-line setting, Hormone receptor positive, Advanced breast cancer
Introduction
Approximately 250,000 new cases of breast cancer are diagnosed each year, and over two-thirds of these patients will be categorized as having hormone receptor-positive disease (HR+) [1]. For patients with HR+ advanced breast cancer (ABC), clinical guidelines recommend sequential treatment with endocrine therapy, unless they are experiencing visceral crises and/or endocrine resistance is known or suspected [2]. Several hormonal therapeutic options to treat postmenopausal HR+ ABC patients are currently available. The treatment options most extensively studied are selective estrogen receptor (ER) modulators and aromatase inhibitors (AIs). Fulvestrant (FUL) is an ER antagonist indicated for the treatment of postmenopausal women with HR+, locally advanced, or metastatic breast cancer for disease relapse on or after adjuvant antiestrogen therapy or disease progression on therapy with antiestrogen [3]. FUL does not show cross-resistance with tamoxifen (TAM) or the ER agonist activity associated with TAM [3]. Considering that the use of AIs in the adjuvant setting has deeply increased after the results of the ATAC BIG 1–98 trials, the first-line treatment landscape for HR+ ABC has changed as well and requires now to consider alternative strategies to those used in the past. Early studies with FUL have been conducted using the dose of 250 mg once per month [4, 5], which is now known to be less effective than the higher dose (HD-FUL; 500 mg every 2 weeks the first months, then once per month) [6, 7]. More recently, the FALCON study compared HD-FUL to anastrozole as first-line therapy in HR+ ABC with de novo disease: the progression-free survival (PFS) was significantly longer in the HD-FUL group than in the anastrozole group (hazard ratio 0.797, 95% CI 0.637–0.999, p = 0.0486). The median PFS was 16.6 months (95% CI 13.83–20.99) in the HD-FUL group versus 13.8 months (95% CI 11.99–16.59) in the anastrozole group.
The aim of this analysis is to describe the outcome of ABC patients treated with HD-FUL as a first-line treatment in terms of median duration of treatment and the overall response rate (ORR) in a real-world setting.
Materials and Methods
Study Design
For the purpose of the present analysis, we considered two data sets of HR+ ABC patients collected in Italy between 2012 and 2015: the first data set was retrieved by a multicenter, retrospective cohort study [8], which collected data of HR+ ABC patients who received the EVE-EXE combination between July 2013 and December 2015 in 38 oncology centers in Italy, whereas the second data set was identified in the context of the GIM-13 AMBRA study, a retrospective cohort study designed to describe the choice of first and subsequent lines of treatment in HER2-ve ABC patients. In this report, the two data sets have been analyzed separately for the identified measures of outcome. Both studies obtained the approval of all ethical committees of the participating sites. All patients provided written informed consent. Data were collected via an electronic database in both cases. Baseline information included patient's age at metastatic diagnosis, breast cancer history, (date of stage at initial diagnosis, any adjuvant and/or neoadjuvant therapy), hormone and HER2 status, and number and sites of metastases. Due to the observational design of both studies and considering the known toxicity of HD-FUL, no data regarding safety have been collected. Additionally, no adverse events or dropout with HD-FUL were reported by the participating centers.
Patients
The eligible patients were female, ≥18 years, with documented HR+ ABC, not previously treated for metastatic disease. Other inclusion criteria were HER2-negative disease (IHC 0–1 or IHC 2, confirmed as FISH negative), measurable or evaluable lesions, and availability of all requested data. Data retrieval included disease characteristics, hormone receptor and HER2 status, sites of metastases and tumor biology, as well as previous therapies received in the adjuvant setting. Patients with de novo disease were excluded.
Treatment Plan
No treatment plan was provided a priori due to the observational nature of the study. Physicians were asked to identify all consecutive patients who fit the prespecified criteria of the study and to collect patients' data from the clinical records in an electronic case report form dedicated to the study.
Clinical Outcomes
All measures of clinical outcomes were based on the physician's evaluation, and no central review was planned. The primary endpoint of this analysis was the duration of HD-FUL treatment. Secondary endpoints were: ORR, disease control rate, and HD-FUL treatment according to the disease-free interval (DFI; <24 vs. ≥24 months, calculated from the time to initial diagnosis to the time of first relapse). Time to treatment change (TTC) was defined as the time between the start of the analyzed therapy until the start of a subsequent one. Considering that HR+ patients treated with endocrine treatment are more likely to change the type of treatment in case of disease progression, rather than for toxicity or other reasons, it is reasonable to assume that TTC well describes the time to tumor progression.
Statistical Considerations
Demographic data, baseline characteristics of the patients and disease, and treatment information were summarized with standard summary statistics (mean standard deviation [SD] and range for continuous data, relative and absolute frequencies for categorical data). PFS was calculated using the progression dates declared by researchers. TTC was defined as the time between first-line chemotherapy start and the beginning of the subsequent therapy, if any, or the last observation, or death. Overall survival (OS) was computed from primary tumor diagnosis to the last available follow-up or death.
Categorical variables were summarized by frequency statistics. Contingency tables were evaluated by the χ2 test, and the two-tailed Fisher exact test was used to compare proportions. Continuous variables were summarized by descriptive statistics. Means, medians, and SDs of continuous variables were evaluated by the equal-variance t test and Aspin-Welch Test for Unequal Variance. Time parameters were estimated by the product-limit method, with failed observations censored to “1.” All statistical tests were two sided at the conventional 5% significance level. Analyses were carried out using NCSS® statistical software (V 12; Hintze J., Kaysville, UT, USA).
Results
Patient and Tumor Characteristics
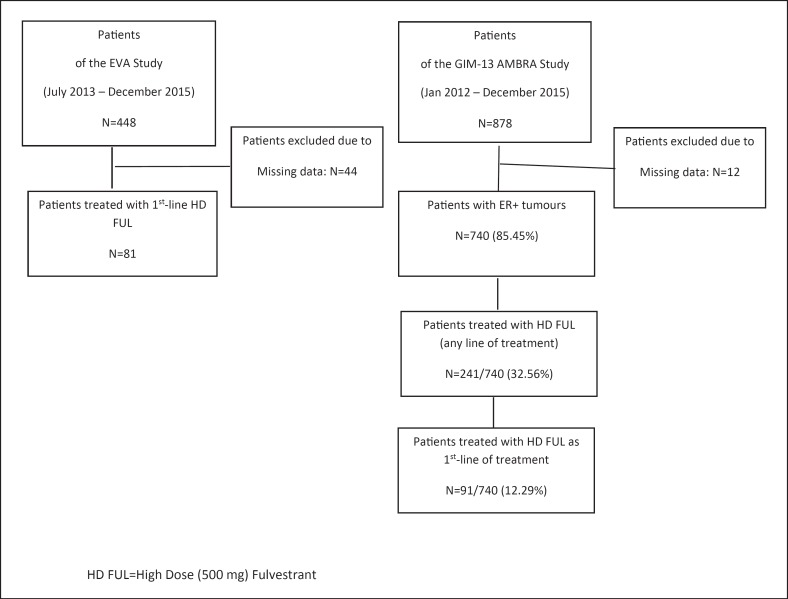
We retrieved clinical data from the database of the EVA and the GIM-13 AMBRA studies: 404 and 878 ABC patients, respectively, have been enrolled into these two trials, of whom 81 (20%) and 91 (10.4%) have received HD-FUL as first-line treatment for their advanced disease, respectively (Fig. 1).
Fig. 1.
CONSORT flow chart.
EVA Study Data Set
Median age at the time of study enrolment was 63 years (range 35–82). All patients had HR+ tumors (ER+ = 96%; PgR+ve = 87%). The majority of patients had ≥3 metastatic sites (45, 57%), mainly at bone (55, 70%), with or without other sites, (32, 39.5%). All patients but 6 received adjuvant endocrine therapy, mainly AIs (43, 53.1%) or TAM (32, 39.5%) with or without sequential AIs.
GIM-13 AMBRA Study Data Set
All patients had HR+ tumors (ER+/PgR+: 49%; ER+/PgR–: 51%). Median age at diagnosis was 57.8 years (range 35.0–82.3). Median DFI was 71.3 months (range 6.2–205.9); 88/91 (96.7%) had received adjuvant endocrine treatment, mainly AIs (43, 48.8%) or TAM followed by AIs (32, 36.3%). Only 7 patients (7.7%) relapsed during the first 2 years of adjuvant endocrine therapy, while 36.5% of the patients relapsed while they were on 5-year adjuvant treatment and can therefore be considered primary endocrine-resistant patients. Bone and visceral metastases were present in 52 (57.1%) and 28 patients (30.8%), respectively.
Details are summarized in Table 1.
Table 1.
Patients and tumor characteristics
| EVA study (n = 81) | GIM-13 AMBRA study (n = 91) | |
|---|---|---|
| Median age (range), years | 63 (35–82) | 57.8 (35.0–82.3) |
| ECOG PS | 79/81 | n.a. |
| 0–1 | 95 | |
| ≥2 | 5 | |
| Hormone receptor status | ||
| ER+ve | 96 | 49 |
| PgR+ve | 87 | 51 |
| Metastatic sites | ||
| Bone | 70 | 57.1 |
| Soft tissue | 42 | n.a. |
| Viscera | 41 | 30.8 |
| Number of metastatic sites | ||
| 1 | 10 | |
| 2 | 32 | |
| ≥3 | 57 | |
| Adjuvant endocrine therapy | 57.51 | 96.7 |
| Tamoxifen ± LHRH | 26.73 | 48.8 |
| Als | 30.78 | 36.3 |
Values are presented as percentages, unless otherwise indicated. n.a., not available.
Treatment Exposure and Clinical Activity
EVA Study Data Set
Median follow-up time was 29.2 months (range 26.8–30.8). ORR was 23.5% in the whole population, 26.9% in patients with relapse at bone only, and 21.8% in those with visceral metastases. Median duration of objective response was 12.0 months (range 8–36) in bone-only patients and 20.0 months (range 8–40) in those with visceral involvement. The clinical benefit rate (CBR), defined as the sum of ORR + SD ≥6 months, was 38.3% in the whole population, 57.7 and 53.1%, according to the site of relapse. No difference has been observed in terms of median duration of CB according to the site of metastases (bone only: 15.4 months, range 6–48; viscera: 18.5 months, range 7–40).
Median duration of HD-FUL was 11.6 months (range 1–48) in the whole population, 10.6 months (range 1–48), and 12.2 months (range 2–40) in patients with bone and visceral relapse, respectively.
GIM-13 AMBRA Study Data Set
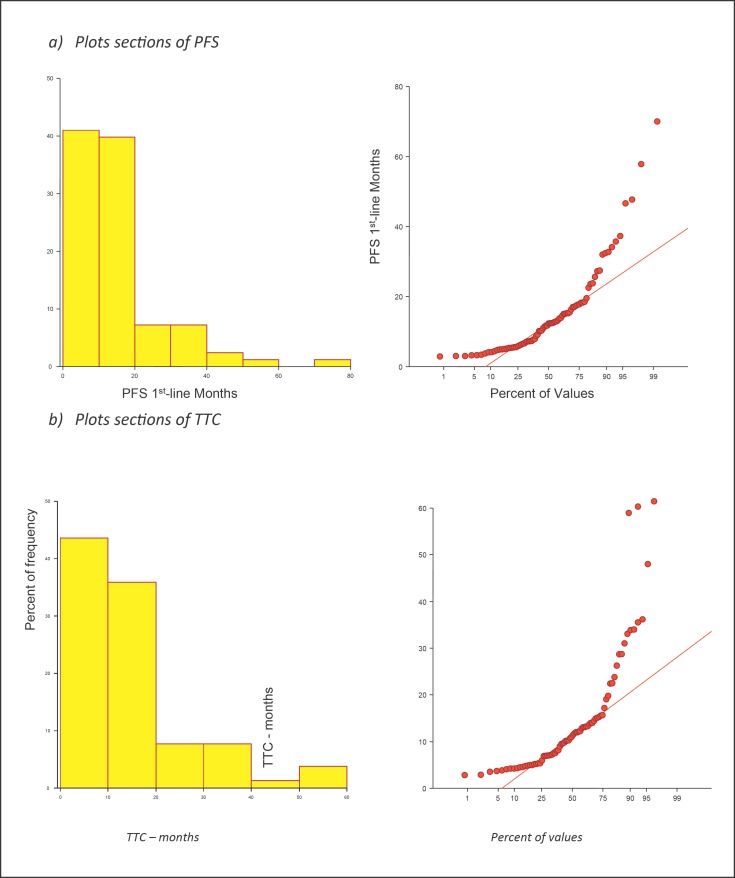
Median follow-up time from the start of the first-line therapy was 21.7 months (range 0–93.3). ORR was 24.3% (26.9 and 21.4 in patients with bone and visceral metastases, respectively). Median PFS was 12.3 months (95% CI: 8.7–13.9) in the whole population (Fig. 2) and 12.8 months (range 2.9–47.7) and 9.2 months (range 3.0–70.0) in patients with bone or visceral disease, respectively. Median TTC was 11.2 months (95% CI: 8.18–13.1). No difference between PFS and TTC was found (p = 0.92). According to the type of endocrine resistance, the median duration of treatment was 6.0 months (range 3.3–12.4) in primary resistant patients and 12.4 months (range 2.9–70.0) in secondary resistant ones. Efficacy data of the two populations are summarized in Table 2.
Fig. 2.
Plot sections of PFS and TTC (AMBRA GIM-13 data set).
Table 2.
Efficacy data of EVA and AMBRA GIM-13 patients
| EVA (n = 81) | AMBRA GIM-13 (n = 91) | |||
|---|---|---|---|---|
| ORR − whole population | 19 | 23.5% | 21/87 | 24.3% |
| ORR − bone only | 7 | 26.9% | 14/52 | 26.9% |
| ORR − visceral ± other | 7 | 21.8% | 6/28 | 21.4% |
| CBR − whole population | 31 | 38.3% | 65/87 | 74.7% |
| CBR − bone only | 15 | 57.7% | 41/52 | 78.8% |
| CBR − visceral ± other | 17 | 53.1% | 19/28 | 67.8% |
| Median duration of treatment (months, range) | 11.6 | 1–48 | NE | |
| Median duration of treatment − bone only (months, range) | 10.6 | 1–48 | NE | |
| Median duration of treatment − visceral ± other (months, range) | 12.2 | 2–40 | NE | |
| Median PFS (months, range) | NE | 12.3 | 8.7–13.9 | |
| Median PFS − bone metastases (months, range) | NE | 12.8 | 2.9–47.7 | |
| Median PFS − visceral metastases (months, range) | NE | 9.2 | 3.0–70.0 | |
ORR, objective response rate; CBR, clinical benefit rate; PFS, progression free survival; NE, not evaluable.
Discussion
This analysis focuses on HR+ ABC patients retrieved from two large data sets and treated with HD-FUL as first-line treatment; the vast majority of patients considered in these two data sets have received adjuvant endocrine therapy. ORR was observed in 21.9–23.5% of the patients and in 26.9% of those with bone only involvement. Median duration of treatment was 11.6 months in the whole population, 10.6 months in patients with only bone sites, and 12.2 months in those with visceral involvement.
The FIRST trial [9] was a randomized, open-label phase II study comparing HD-FUL with anastrozole 1 mg as first-line endocrine therapy for postmenopausal women with HR+ advanced breast cancer. ORR and CBR were observed in 36 and 72.5% of the patients, respectively.
Similarly, in the FALCON trial [10], a phase 3, randomized, double-blind trial that enrolled endocrine-naive patients, ORR and CBR were 46 and 78%, respectively.
Both ORR and CBR observed in the two data sets analyzed in the present paper are lower than those reported in the FIRST and FALCON trials; however, the populations enrolled in these studies were quite different from the patients considered in this analysis: in the FIRST trial, the majority of patients were endocrine naive (71.6%) at the moment of study enrolment, as well as in the FALCON trial: in both cases, the population has to be considered endocrine sensitive. On the contrary, only 7.4 and 3.3% did not receive any kind of adjuvant endocrine therapy in the EVA and AMBRA data sets.
Non-endocrine-naive patients in the CONFIRM trial [7], a double-blind, parallel-group, multicenter phase III study comparing two different doses of FUL (500 vs. 250 mg), ORR in the HD-FUL arm was 9.1%. This study enrolled patients who experienced relapse on adjuvant endocrine therapy or within 1 year from completion of adjuvant endocrine therapy. For patients who experienced relapse after >1 year from completion of adjuvant endocrine therapy or for patients presenting with de novo advanced disease, eligibility required a previous treatment with either an anti-estrogen or an Al as a first-line therapy. This population should be considered an example of HD-FUL performance in endocrine-resistant patients.
Median duration of HD-FUL was 11.6 months (range 1–48) in the whole population, 10.6 months (range 1–48), and 12.2 months (range 2–40) in patients with bone and visceral relapse, respectively, in the EVA data set. Median TTC, which can be considered a more realistic parameter to be compared with treatment duration, was 11.2 months (95% CI: 8.18–13.1). Median PFS was 12.3 months (95% CI: 8.7–13.9) in the AMBRA data set, without any statistical difference in comparison to TTC (p = 0.92). According to the different trials mentioned above, median PFS ranged between 6.5 [7] and 23.4 months [10]: the various endocrine sensitivity of the patients enrolled should be again advocated to explain the different results, with the lowest one observed in pretreated patients and the highest one in those who were endocrine sensitive.
The fundamental question for clinical and payer decision making is how treatment efficacy measured in clinical trials translates into real-world effectiveness. Most evidence on this relationship relies on comparisons of surrogate and nonsurrogate endpoints within trials themselves as opposed to comparisons with real-world OS.
Lakdawalla et al. [11] examined the relationship between reported randomized clinical trials (RCT) efficacy and real-world effectiveness for oncology treatments and examined whether this relationship varies by RCT endpoint (OS vs. surrogate measures such as PFS or time to tumor progression). The authors found that real-world OS treatment benefits were similar to those observed in RCTs based on OS endpoints, but were 16% less than RCT efficacy estimates based on surrogate endpoints. These results, however, varied by tumor type and line of therapy.
The present analysis has different limitations: (1) the small sample size in both data sets, and (2) the absence of data regarding safety. However, considering the lack of data in this setting, the present data could add in any case some knowledge for the clinical practice.
The clinical landscape of first-line treatment for HR+ ABC patients deeply changed in the last 3 years due to the release of results concerning CDK 4/6 inhibitors [12, 13] in association with AIs: however, it is our opinion that HD-FUL should still continue to play a significant role in this changing scenario.
One potential advantage of HD-FUL is that it may improve treatment compliance due its monthly parenteral administration compared with daily oral intake of other endocrine therapies. Adherence to oral long-term treatments is a major problem that should be considered [14]. It is estimated that around 20% of the breast cancer patients receiving oral endocrine therapy do not take their medication regularly, primarily in the adjuvant setting where benefit of treatment is not clearly perceived by the patients [15]. Parenteral administration of HD-FUL provides greater control over endocrine treatment compliance, reducing oral absorption and pharmacokinetic interactions with food or other drugs, which are important aspects to be considered in patients with breast cancer who usually are receiving multiple medications.
A second but not negligible aspect to consider is drug cost, finance being the new dose-limiting toxicity. It has been estimated that breast cancer expenditures in 2010 have been 16.5 billion USD annually, which represents 13% of all cancer spending, more than any other cancer type, distributed across all phases of care [16]. The mean annual spending during the year after an advanced breast cancer diagnosis is 69,492 USD; chemotherapy adds 7,301 USD on average.
The American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) have developed frameworks that quantify survival gains in light of toxicity and quality of life to assess the benefits of cancer therapies. Del Paggio et al. [17] applied these frameworks to a cohort of contemporary randomized controlled trials to explore agreement between the two approaches and to assess the relation between treatment benefit and cost. They found that there is only fair correlation between these two major value frameworks and negative correlations between framework outputs and drug costs. In conclusion, this retrospective analysis of two different data sets of HR+ MBC patients previously treated in the adjuvant setting with AIs or TAM, suggests some evidence that HD-FUL could be a potential first-line treatment in these patients: these results should be put into the actual clinical landscape, and moreover, predictive biomarkers are strongly awaited to better select the patients who should derive a benefit from therapies different from CDK 4/6 inhibitors.
Appendix 1
List of Co-Authors
Cicchiello F., Oncology Unit, ASST Monza, Monza, Italy; Riva F., Oncology Unit, ASST Monza, Monza, Italy; Ballerio A., Oncology Unit, ASST della Valle Olona − Presidio Ospedaliero di Saronno, Saronno, Italy; Coccato S., Oncology Unit, AULSS 3, Mirano, Italy; Vaccaro G., Oncology Department, Policlinico “Paolo Giaccone”, Palermo, Italy; De Martini P., Oncology Unit 2 − Città della Salute e della Scienza di Torino, Turin, Italy; Cocciolone V., Dipartimento di Scienze Cliniche Applicate e Biotecnologiche (DISCAB) − Università Degli Studi Dell'Aquila, L'Aquila, Italy; Arcangeli V., Oncology Unit Rimini, Azienda USL Romagna, Rimini, Italy; Diodati L., Oncology Unit 2, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy; Campidoglio S., Oncology Unit, Ospedale Sacro Cuore di Gesù, Fatebenefratelli, Benevento, Italy; Dester M., Breast Unit, ASST di Cremona, Cremona, Italy; Pizzuti L., Oncology Unit 2, Istituto Nazionale Tumori Regina Elena − IFO, Rome, Italy; Caggia F., Oncology Unit, Policlinico University Hospital of Modena, Modena, Italy; Ferrari L., Oncology Unit, ASL di Frosinone Osp. “SS. Trinità,” Sora, Italy; Girelli S., Oncology Unit, ASST Fatebenefratelli Sacco Presidio Ospedaliero Fatebenefratelli, Milan, Italy; Bedolis R., Oncology Departement, Ospedale di Gallarate, ASST Valle Olona, Gallarate, Italy; Alù M., Oncology Unit, ARNAS Civico Palermo, Palermo, Italy; Piezzo M., National Cancer Institute “Fondazione Giovanni Pascale”, Naples, Italy; Bordin E., Ospedale Martini della ASL “Città di Torino”, Turin, Italy; Benedetto C., Dipartimento Universitario Ginecologia e Ostetricia 1, Ospedale S. Anna, Turin, Italy; Cursano M., Oncology Unit, Università Campus Bio-Medico, Rome, Italy; Bozzoli E., Oncology Unit 1, Istituto Regina Elena − IFO, Rome, Italy; Marchese A., Oncology Unit, Ospedale Sacro Cuore di Gesù, Fatebenefratelli, Benevento, Italy; Latini L., Osp. di Macerata, Macerata, Italy.
Statement of Ethics
The EVA and the GIM-13 AMBRA studies obtained the approval of all ethical committees of the participating sites. All patients provided written informed consent.
Disclosure Statement
The Authors declare that they have no conflict of interest.
Funding Sources
The two studies were supported by an unrestricted grant from A and Q − Consorzio per la riqualificazione agro-alimentare (EVA) and from Celgene srl (GIM-13 AMBRA).
Author Contributions
Marina E. Cazzaniga, Giorgio Mustacchi, Paolo Pronzato, and Valter Torri: study concept, study design, data analysis and interpretation.
Valter Torri and Luca Clivio: statistical analysis.
All authors: manuscript preparation and review.
Acknowledgements
The authors would like to thank Dr. Monica Perez Gila for data management of the EVA Study and for linguistic revision and Oncotech for the data management of GIM-13 AMBRA Study.
References
- 1.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park) 2012;26:688–694. 96. [PubMed] [Google Scholar]
- 2.Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3) Ann Oncol. 2017 Dec;28((12)):3111. doi: 10.1093/annonc/mdx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991 Aug;51((15)):3867–73. [PubMed] [Google Scholar]
- 4.Howell A, Robertson JF, Vergote I. A review of the efficacy of anastrozole in postmenopausal women with advanced breast cancer with visceral metastases. Breast Cancer Res Treat. 2003 Dec;82((3)):215–22. doi: 10.1023/B:BREA.0000004375.17920.0b. [DOI] [PubMed] [Google Scholar]
- 5.Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002 Aug;20((16)):3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 6.Robertson JF, Nicholson RI, Bundred NJ, Anderson E, Rayter Z, Dowsett M, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 2001 Sep;61((18)):6739–46. [PubMed] [Google Scholar]
- 7.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010 Oct;28((30)):4594–600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 8.Cazzaniga ME, Airoldi M, Arcangeli V, Artale S, Atzori F, Ballerio A, et al. on behalf of. EVA Study Group Efficacy and safety of Everolimus and Exemestane in hormone-receptor positive (HR+) human-epidermal-growth-factor negative (HER2-) advanced breast cancer patients: new insights beyond clinical trials. The EVA study. Breast. 2017 Oct;35:115–21. doi: 10.1016/j.breast.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 9.Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiówka M, Hewson N, et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II FIRST Study. J Clin Oncol. 2015 Nov;33((32)):3781–7. doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson JF, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016 Dec;388((10063)):2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 11.Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood) 2012 Apr;31((4)):676–82. doi: 10.1377/hlthaff.2011.1300. [DOI] [PubMed] [Google Scholar]
- 12.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017 Nov;35((32)):3638–46. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 13.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016 Nov;375((18)):1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 14.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71((1-2)):1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 15.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010 Sep;28((27)):4120–8. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yabroff KR, Lund J, Kepka D, Mariotto A. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev. 2011 Oct;20((10)):2006–14. doi: 10.1158/1055-9965.EPI-11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Paggio JC, Sullivan R, Schrag D, Hopman WM, Azariah B, Pramesh CS, et al. Delivery of meaningful cancer care: a retrospective cohort study assessing cost and benefit with the ASCO and ESMO frameworks. Lancet Oncol. 2017 Jul;18((7)):887–94. doi: 10.1016/S1470-2045(17)30415-1. [DOI] [PubMed] [Google Scholar]