Abstract
Background
Sentinel lymph node biopsy has become a standard of care in the treatment of patients with early breast cancer, but clinical guidelines continue to be vague on details of the procedure. We were interested in the results of our 2-day protocol, which includes delayed lymphoscintigraphy at 18 h.
Methods
We reviewed the results of preoperative lymphoscintigrams in patients undergoing surgery for breast cancer. Lymphoscintigraphy was performed 2 h after periareolar injection of 4 × 37 MBq <sup>99m</sup>Tc nanocolloid (early lymphoscintigraphy) and 18 h following injection (delayed lymphoscintigraphy). The early results were compared with the late results.
Results
A total of 238 lymphoscintigraphies were performed in 232 patients (6 bilateral). At 2 h, ≥1 sentinel nodes were visualized in 154/238 (65%) cases; in 84 (35%), no sentinel node was visualized. Delayed lymphoscintigraphy visualized a sentinel node in 40 of 76 (53%) cases with no visualization at 2 h and failed to show a sentinel node in 36 (47%) of these cases (in 8 cases, no delayed lymphoscintigram was obtained).
Conclusions
Delayed lymphoscintigraphy was useful in about 50% of the breast cancer patients in whom immediate scintigraphy failed to demonstrate a sentinel lymph node.
Keywords: Breast cancer, Sentinel node biopsy, Delayed image
Introduction
Sentinel lymph node biopsy (SNB) has been a standard of care in the management of early breast cancer since supplanting routine axillary dissection in the late 1990s [1, 2, 3, 4, 5, 6, 7]. Mapping of sentinel lymph nodes (SLNs) in breast cancer is successful in nearly 95% of cases [8]. Numerous clinical guidelines on the management of breast cancer address technical aspects such as the technique or the location of the application or the type of tracer [1, 9, 10, 11, 12, 13, 14, 15]. Nonetheless, even recent versions of guidelines are consistently vague on the timing of lymphoscintigraphy and on whether repeat or late scintigrams should be obtained [1, 2, 3, 5, 6, 15, 16].
In 2000, our institution established a 2-day protocol for SNB in patients with breast cancer which included a delayed lymphoscintigram at 18 h following injection. The objective of the present study was to review the results of early and delayed lymphoscintigrams and specifically to analyze whether the late images provided clinically relevant information.
Materials and Methods
We reviewed the lymphoscintigrams of all 232 women undergoing SNB for breast cancer at our institution between October 2013 and September 2015 (male patients were excluded). Six patients had bilateral SNB, leading to a total of 238 scintigrams.
Our unit has followed a protocol with early and delayed planar images for SLN detection in early breast cancer since 2000. According to the protocol, the tracer (4 × 37 MBq 99mTc nanocolloid) is injected subcutaneously periareolarly on the afternoon before surgery. Planar static images in the anterior and lateral perspectives are then obtained 2 h following the injection (p.i.). On the following day in the morning, at about 18 h p.i., delayed images are obtained. SPECT-CT has been done since 2011 in most cases.
Patients are operated on the morning after tracer injection. Intraoperatively SLNs are identified with a gamma probe. In cases where scintigraphy failed to identify an SLN, blue dye is injected additionally.
We reviewed patient records for stage of the disease, previous breast surgery, previous (neoadjuvant) chemotherapy, neoadjuvant antihormonal therapy, and breast biopsy within 7 days of scintigraphy. We reviewed the scans to identify how many nodes were seen early and how many were seen late to address the issue whether the late scan provided additional information.
The study protocol was approved by the institutional Ethics Committee; written informed consent was waived because of the retrospective study design.
Results
We analyzed 238 scans in 232 patients (6 women had bilateral SNB). Patient data and clinical and pathological data are summarized in Table 1.
Table 1.
Patient demographics (n = 232) and tumor characteristics of the overall study cohort
| Mean age, years (range) | 60.l (24–89) |
| Tumor, n (%) | |
| Right side | l24 (52) |
| Left side | ll4 (48) |
| pT stage, n (%) | (n = l97) |
| pT0 | l (0.5) |
| pTis, DCIS | 20 (l0) |
| PT1 | 2 (l) |
| pTla | l6 (8.l) |
| pTlb | 54 (27) |
| pTlc | 76 (39) |
| pT2 | 26 (l3) |
| pT2c | l (0.5) |
| pT3 | l (0.5) |
| yT stage, n (%) | (n = 4l) |
| yT0 | l3 (32) |
| yTis, DCIS | 6 (l5) |
| yT1 | l (2.4) |
| yTla | 5 (l2) |
| yTlb | 4 (l0) |
| yTlc | 8 (20) |
| yT2 | 4 (l0) |
SLN Detection Rate at Scintigraphy
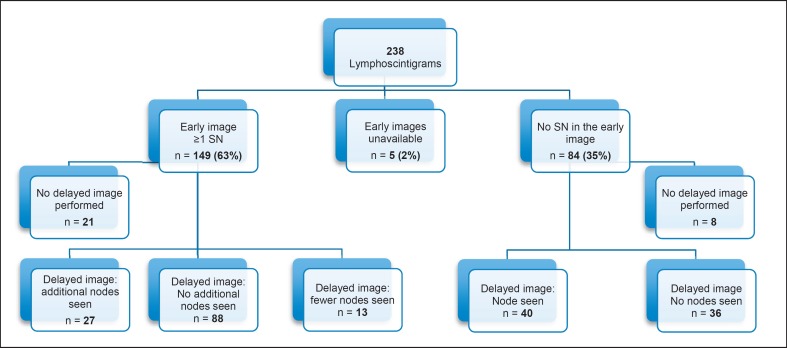
Five early scintigrams (2%) were no longer available for review. SLNs were seen in the early readings in 149 of 238 scintigrams, for an early detection rate of 63%. Of interest are the 84 scintigrams in which the early reading showed no sentinel node (SN). 76 of these patients had late scintigrams, 40 (53%) of which showed a node. Thus, the overall scinitgraphic detection rate was 81% (Fig. 1).
Fig. 1.
Flowchart of 238 scintigrams in 232 patients.
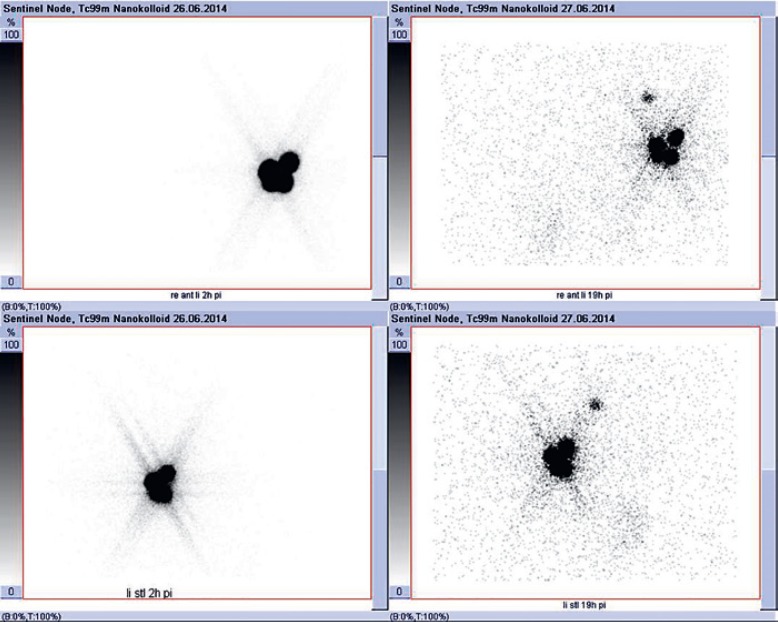
In 124/204 cases (61%), early and late lymphoscintigraphy showed the same number of SNs. In 67/204 cases (33%), delayed images showed more lymph nodes than the early images (Fig. 2). In 13/204 cases (6%), the early images showed more SNs than the delayed images.
Fig. 2.
Early and delayed lymphoscintigraphy pictures showing no SLN in the early images and one obvious SLN in the delayed images.
SLN Detection Rate at Surgery
SLNs were found at surgery in all 193 patients with positive early or late scintigraphy. This leaves 45 negative scintigraphies (36 cases with no visualization at early and late scintigraphy, 8 cases with no visualization in early scintigraphy and no late scintigraphy, and 1 case with late scintigraphy only). In 33 of these 45 cases (73%), blue dye was injected before the incision to visualize the SLN. This procedure led to the detection of SNs in 31 (69%) of the 45 axillae with negative scintigraphy. In 12 cases, preoperative lymphoscintigraphy showed no SNs and blue dye was not injected. 6 of these cases had nodes detected with intraoperative scintigraphy and 6 underwent axillary dissection. Altogether, with the combination of lymphoscintigraphy and facultative blue dye, the overall detection rate for SNs at the time of surgery was 94% (224 of 238 axillae).
Patients after Neoadjuvant Chemotherapy
Twenty-seven patients had received neoadjuvant chemotherapy before surgery. 24 (89%) of these patients had both early and delayed scans. 18 of the 24 early scans (75%) showed an SN; the delayed image showed an SN in 1 of the 6 remaining cases (16%). In 2 of the 5 cases which remained negative at 18 h, blue dye was injected and visualized an SN in both cases. 3 of 27 patients only had early images, all of which showed SNs. The overall detection rate after neoadjuvant chemotherapy was 23/27 (85%).
Discussion
Considering the consistency with which guidelines recommend SNB as the standard of care for many patients with early breast cancer, and considering that this is by far the most common cancer in women, the lack of clarity of the guidelines on technical details is surprising. Even very recent updates of widely used German [2], ASCO [4] and NCCN [3] guidelines provide little if any guidance on technique, tracers or timing. In our study, we used 4 × 37 MBq 99mTc nanocolloid, which we applied in a subcutaneous fashion around the areola. 99mTc is a rather small colloid which is quickly transported to the nodes but remains in the nodes long enough to permit a delayed image after 18 h [1, 16]. The dosage of 148 MBq is consistent with guidelines [16]. Because of the sufficiently high amount of activity, we were able to ensure an enrichment of the tracer in the delayed planar images performed 18 h p.i. and also in the SPECT-CT.
Guidelines are vague on the timing of lymphoscintigraphy [1, 2, 3, 5, 6, 16]. Either the possibility of performing an early and a delayed image is left open [1, 6] or there is nothing at all on the specific time of the lymphoscintigraphy [2, 5]. Neither the NCCN breast cancer guidelines [5] nor the German breast cancer guidelines [2] provide detail on the question of when lymphoscintigraphy images should be performed and when a delayed image is necessary. The 2014 interdisciplinary guidelines of the German Society of Nuclear Medicine are the most specific on the timing of lymphoscintigraphy in breast cancer [6]. These guidelines stipulate an early image with the possibility of a delayed image up to 24 h p.i. Furthermore, in case of nonvisualization after 1 h p.i., they suggest a 5-min massage of the injection site or warming the breast. If this is not successful, the German nuclear medicine guidelines recommend a subdermal or subareolar reinjection [6]. Interestingly, the recent German AGO Guidelines [17] are now ambivalent (“+/–”) for the need for preoperative lymphoscintigraphy at all.
The results of our study suggest that delayed images 18 h p.i. are able to show an SLN in 52% of cases where the early images were negative. This represents actionable additional clinical information. This may be due to the dose of the tracer, which was high enough [17], and also to the subcutaneous application of the tracer [18]. With subcutaneous application, the tracer has a more moderate transit time than with intradermal application.
Our early scintigraphic detection rate of 63% is modest. Other studies report early detection rates of 41–65% [19], 68% [20], 73% [15], 91% [21], 93% [22], and 98% [23]. At our unit, only clearly visible lymph nodes are defined as SLN in the early pictures. Enrichments which are low in contrast are normally read as negative, in the knowledge that there will be a later reading. Also, we do not massage or apply heat to the breast after injecting the tracer. Our overall detection rate including the delayed images was 81%, which is similar to the rates reported by others: 96% [15], 98% [21], 96% [23], 88% [22], 72% [20], and 82% [19].
Recent studies comparing 1-day and 2-day protocols continue to be ambivalent. Mount et al. [24] found a 2-day protocol to be “reliable” compared with a 1-day protocol. In contrast, Unkart et al. [25] found similar outcomes between 1-day and 2-day protocols. It is likely that local logistical issues and infrastructure influence practice.
In summary, the results of our series indicate that delayed scintigraphy is potentially useful if early scintigraphy fails to show an SLN [22]. In these patients, we were able to extend the clinical result and gain actionable information. This is consistent with the results of others [19, 24, 25] who also found no significant impact of delayed imaging in patients in whom an SN can be visualized at immediate imaging.
Statement of Ethics
The study protocol was approved by the Ethics Committee of the Medical University of Graz. Written informed consent was waived as this was a retrospective review.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This project received no external funding.
Author Contributions
Dr. Taumberger wrote the protocol, collected data and wrote the first draft of the manuscript. Dr. Pernthaler cowrote the protocol, collected data, reviewed scans and oversaw all versions of the manuscript. Thomas Schwarz collected data and reviewed scans. Drs. Bjelic-Radisic, Pristauz, Aigner and Tamussino supervised the protocol and data analysis and helped write the paper. All authors reviewed and approved the final version of the manuscript.
References
- 1.Buscombe J, Paganelli G, Burak ZE, Waddington W, Maublant J, Prats E, et al. European Association of Nuclear Medicine Oncology Committee and Dosimetry Committee Sentinel node in breast cancer procedural guidelines. Eur J Nucl Med Mol Imaging. 2007 Dec;34((12)):2154–9. doi: 10.1007/s00259-007-0614-z. [DOI] [PubMed] [Google Scholar]
- 2.Onkologie L. (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4. 1, 2018 AWMF Registernummer: 032–045OL, www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom. [Google Scholar]
- 3.Kuehn T, Bembenek A, Decker T, Munz DL, Sautter-Bihl ML, Untch M, et al. Consensus Committee of the German Society of Senology A concept for the clinical implementation of sentinel lymph node biopsy in patients with breast carcinoma with special regard to quality assurance. Cancer. 2005 Feb;103((3)):451–61. doi: 10.1002/cncr.20786. [DOI] [PubMed] [Google Scholar]
- 4.Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017 Feb;35((5)):561–4. doi: 10.1200/JCO.2016.71.0947. [DOI] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology Breast Cancer − Verson 3. 2018 (October 25, 2018) www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 6.Schmidt M, Bares R, Brenner W, Buck A, Grünwald F, Kopp J, et al. DGN-Handlungsempfehlung (S1-Leitlinie) Verfahrensanweisung für die technische Durchführung der nuklearmedizinischen Wächter-Lymphknoten-Diagnostik. Stand 10/2014 − AWMF-Registernummer 031–033. www.awmf.org/uploads/tx_szleitlinien/031-033l_S1_W%C3%A4chter_Lymphknoten_Diagnostik_2014–10.pdf. [Google Scholar]
- 7.Zurrida S, Veronesi U. Milestones in breast cancer treatment. Breast J. 2015 Jan-Feb;21((1)):3–12. doi: 10.1111/tbj.12361. [DOI] [PubMed] [Google Scholar]
- 8.Kauffmann GW, Moser E, Sauer R. Radiologie. 3rd ed. München: Elsevier, Urban & Fischer Verlag; 2006. p. pp. 772. [Google Scholar]
- 9.Caruso G, Cipolla C, Costa R, Morabito A, Latteri S, Fricano S, et al. Lymphoscintigraphy with peritumoral injection versus lymphoscintigraphy with subdermal periareolar injection of technetium-labeled human albumin to identify sentinel lymph nodes in breast cancer patients. Acta Radiol. 2014 Feb;55((1)):39–44. doi: 10.1177/0284185113493775. [DOI] [PubMed] [Google Scholar]
- 10.Klimberg VS, Rubio IT, Henry R, Cowan C, Colvert M, Korourian S. Subareolar versus peritumoral injection for location of the sentinel lymph node. Ann Surg. 1999 Jun;229((6)):860–4. doi: 10.1097/00000658-199906000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMasters KM, Wong SL, Martin RC, 2nd, Chao C, Tuttle TM, Noyes RD, et al. University of Louisville Breast Cancer Study Group Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg. 2001 May;233((5)):676–87. doi: 10.1097/00000658-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motomura K, Komoike Y, Hasegawa Y, Kasugai T, Inaji H, Noguchi S, et al. Intradermal radioisotope injection is superior to subdermal injection for the identification of the sentinel node in breast cancer patients. J Surg Oncol. 2003 Feb;82((2)):91–6. doi: 10.1002/jso.10200. [DOI] [PubMed] [Google Scholar]
- 13.Mudun A, Sanli Y, Ozmen V, Turkmen C, Ozel S, Eroglu A, et al. Comparison of different injection sites of radionuclide for sentinel lymph node detection in breast cancer: single institution experience. Clin Nucl Med. 2008 Apr;33((4)):262–7. doi: 10.1097/RLU.0b013e3181662fc7. [DOI] [PubMed] [Google Scholar]
- 14.Pelosi E, Bellò M, Giors M, Ala A, Giani R, Bussone R, et al. Sentinel lymph node detection in patients with early-stage breast cancer: comparison of periareolar and subdermal/peritumoral injection techniques. J Nucl Med. 2004 Feb;45((2)):220–5. [PubMed] [Google Scholar]
- 15.Sadeghi R, Forghani MN, Memar B, Rajabi Mashhadi MT, Dabbagh Kakhki VR, Abdollahi A, et al. How long the lymphoscintigraphy imaging should be continued for sentinel lymph node mapping? Ann Nucl Med. 2009 Aug;23((6)):507–10. doi: 10.1007/s12149-009-0284-y. [DOI] [PubMed] [Google Scholar]
- 16.Giammarile F, Alazraki N, Aarsvold JN, Audisio RA, Glass E, Grant SF, et al. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur J Nucl Med Mol Imaging. 2013 Dec;40((12)):1932–47. doi: 10.1007/s00259-013-2544-2. [DOI] [PubMed] [Google Scholar]
- 17.Kommission Mamma der Argbeitsgemeinschaft Gynäkologische Onkologie in der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe 2018 www.ago-online.defileadmin/downloads/leitlinien/mamma/2018–03. [Google Scholar]
- 18.Tanis PJ, Nieweg OE, Valdés Olmos RA, Kroon BB. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg. 2001 Mar;192((3)):399–409. doi: 10.1016/s1072-7515(00)00776-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Heck K, Pruitt SK, Wong TZ, Scheri RP, Georgiade GS, et al. Impact of delayed lymphoscintigraphy for sentinel lymphnode biopsy for breast cancer. J Surg Oncol. 2015 Jun;111((8)):931–4. doi: 10.1002/jso.23915. [DOI] [PubMed] [Google Scholar]
- 20.Yeung HW, Cody HS, 3rd, Turlakow A, Riedel ER, Fey J, Gonen M, et al. Lymphoscintigraphy and sentinel node localization in breast cancer patients: a comparison between 1-day and 2-day protocols. J Nucl Med. 2001 Mar;42((3)):420–3. [PubMed] [Google Scholar]
- 21.Gutman F, Sanson A, Piquenot JM, Hitzel A, Ladonne JM, Dessogne P, et al. Intra-individual comparison of sentinel lymph node scintigraphy on the day of injection and on the following day in breast cancer. Nucl Med Commun. 2006 Jan;27((1)):5–9. doi: 10.1097/01.mnm.0000189781.62282.0d. [DOI] [PubMed] [Google Scholar]
- 22.Wang HY, Tsai CC, Hung GU, Lin WY. Effectiveness of delayed 2-day lymphoscintigraphy on sentinel lymph node detection in patients with breast cancer with negative early lymphoscintigraphy. Clin Nucl Med. 2006 Sep;31((9)):523–6. doi: 10.1097/01.rlu.0000233905.72033.cb. [DOI] [PubMed] [Google Scholar]
- 23.Jangjoo A, Forghani MN, Mehrabibahar M, Rezapanah A, Kakhki VR, Zakavi SR, et al. Comparison of early and delayed lymphoscintigraphy images of early breast cancer patients undergoing sentinel node mapping. Nucl Med Commun. 2010 Jun;31((6)):521–5. [PubMed] [Google Scholar]
- 24.Mount MG, White NR, Nguyen CL, Orr RK, Hird RB. Evaluating one day versus two days preoperative lymphoscintigraphy protocols for sentinel lymph node biopsy in breast cancer. Am Surg. 2015 May;81((5)):454–7. [PubMed] [Google Scholar]
- 25.Unkart JT, Proudfoot J, Wallace AM. Outcomes of “one-day” vs “two-day” injection protocols using Tc-99m tilmanocept for sentinel lymph node biopsy in breast cancer. Breast J. 2018 Jul;24((4)):526–30. doi: 10.1111/tbj.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]